Abstract
Background/Objectives: Emerging evidence suggests that hippocampal neuroinflammation (HNF) drives cognitive decline via dysregulation of the microbiota-gut-brain axis. Corylus heterophylla Fisch. male flower extract (CFE), a flavonoid-rich by-product of hazelnut processing, presents a promising yet unexplored neuroprotective candidate. This study investigated the preventive effects and mechanisms of CFE against HNF-induced cognitive decline. Methods: In the present study, mice were pretreated with CFE (200 mg/kg) before the Lipopolysaccharide (LPS) administration. Cognitive function, inflammation, core pathology, neuroplasticity, gut microbiota and serum metabolites were assessed. The chemical composition of CFE was analyzed by UHPLC-MS and its direct immunomodulatory effects were investigated in BV2 cells. Results: Behavioral assessments demonstrated significant therapeutic efficacy. This was evidenced by the recovery from hippocampal damage, accompanied by reduced levels of core pathological markers (Aβ1–42, Tau, p-Tau (Ser404), GSK-3β), decreased expression of pro-inflammatory mediators including IL-33, elevated levels of neurotrophic factors (BDNF and MAP2), and attenuated abnormal activation of astrocytes and microglia. The 16S rRNA analysis confirmed that CFE ameliorated gut microbial dysbiosis. Notably, CFE significantly increased the relative abundance of Muribaculaceae and Lachnospiraceae, while significantly decreased Staphylococcus and Helicobacter. Metabolomics revealed enhanced levels of α-linolenic acid (ALA), serotonin (5-HT) and acetic acid, which correlated positively with Muribaculaceae and Lachnospiraceae. Phytochemical analysis identified luteolin and kaempferol as the predominant flavonoids in CFE. In BV2 cells, CFE, luteolin and kaempferol shifted microglial polarization from the M1 phenotype toward the M2 phenotype. Conclusions: CFE alleviated HNF-induced cognitive decline by regulating microbiota-gut-brain axis and microglial M1/M2 polarization.
1. Introduction
Cognitive decline is increasingly observed in younger populations, a trend accelerated by the COVID-19 pandemic, with hippocampal neuroinflammation (HNF) recognized as a pivotal driver in its pathogenesis [1,2]. Lipopolysaccharide (LPS)-induced HNF impairs neuronal synaptic plasticity and contributes to dysregulation across microbial, metabolic and microglial systems [3,4]. This inflammatory state facilitates the accumulation of amyloid-beta 1–42 (Aβ1–42) and promotes the aberrant phosphorylation of Tau protein [5,6]. A central kinase in this process, glycogen synthase kinase-3β (GSK-3β), drives the formation of phosphorylated Tau (P-Tau) and neurofibrillary tangles (NFTs) [7]. Concurrently, HNF triggers an upregulation of interleukin-33 (IL-33), leading to synaptic damage and a downregulation of brain-derived neurotrophic factor (BDNF) [8,9,10]. The resultant reduction in BDNF exacerbates neuronal vulnerability. Furthermore, inflammatory mediators compromise the integrity of microtubule-associated protein 2 (MAP2), disrupting neuronal structure and function, which culminates in dendritic damage and cognitive decline [11,12,13].
Peripheral inflammation can penetrate a compromised blood-brain barrier (BBB) to enter the central nervous system, thereby activating microglia [14]. Once aberrantly activated, microglia release pro-inflammatory factors (e.g., IL-1β, TNF-α, IL-33), generate reactive oxygen species (ROS), and further stimulate astrocytes, collectively amplifying HNF and driving cognitive decline [15]. The surface receptor cluster of differentiation 11b (CD11b), critical for microglial migration and phagocytosis, is significantly elevated during activation and serves as a biomarker for HNF severity [4]. As the primary immune effector cells in the brain, activated microglia polarize into pro-inflammatory (M1) and anti-inflammatory (M2) phenotypes. An imbalance in M1/M2 polarization is strongly implicated in the pathogenesis of HNF [16]. Notably, flavonoid compounds such as luteolin and kaempferol can promote a shift from the M1 to the M2 phenotype in microglial cells, characterized by the downregulation of M1 markers (iNOS, CD86, TNF-α) and upregulation of M2 markers (Arg-1, CD206, IL-10) [17,18].
The microbiota-gut-brain axis plays a significant role in modulating brain function [19]. LPS-induced gut microbial dysbiosis, marked by reduced diversity and abundance, exacerbates HNF [20]. Specifically, HNF correlates negatively with beneficial bacteria (e.g., Muribaculaceae, Lachnospiraceae) and positively with pathogenic genera (e.g., Staphylococcus, Helicobacter) [21,22,23]. Meanwhile, gut microbial metabolites, such as short-chain fatty acids (SCFAs), can cross the BBB and influence brain function, with evidence suggesting they ameliorate cognitive decline by elevating levels of BDNF and serotonin (5-HT) [24,25].
Accumulating evidence highlights the potential of floral extracts as natural neuroprotective agents against cognitive decline. For example, Daphne genkwa flower extract attenuated LPS-induced hippocampal neuronal loss and microglial activation by inhibiting TNF-α and IL-1β and enhancing BDNF expression [26]. Abelmoschus Manihot Medicus flower extract reversed cognitive decline in mice by elevating hippocampal levels of BDNF/TrkB/GluR1 [27]. Hibiscus sabdariffa extract improved cognitive decline by inhibiting cerebral P-Tau formation [28]. Corylus heterophylla Fisch., a wild hazelnut species of the family Betulaceae primarily distributed in Northeast China, notably in the Changbai Mountain region of Jilin Province, has been studied for its bioactive properties [29]. Preclinical studies indicate that the nut kernel possesses neuroprotective effects, including inhibition of Aβ generation and reduction in pro-inflammatory cytokines in the mouse hippocampus [30,31]. Pollen extracts from the male flowers of Corylus heterophylla Fisch. demonstrate potent antibacterial and antioxidant activities [32]. Corylus heterophylla Fisch. male flower extract (CFE), used traditionally for cirrhosis, demonstrates significant hepatoprotective activity against CCl4-induced liver injury in mice [33]. Furthermore, the total flavonoids from CFE protect against ischemic renal injury by mitigating oxidative stress, inhibiting profibrotic signaling, and reducing renal interstitial fibrosis [34]. However, the effects of CFE on HNF and associated cognitive decline remain unexplored. Therefore, we aimed to investigate the protective effect of CFE against cognitive decline by examining its role in regulating microbiota-gut-brain axis and microglial polarization. This research seeks to elucidate the underlying mitigating mechanisms and provide novel therapeutic strategies for cognitive decline.
2. Materials and Methods
2.1. Preparation of Flower Extract
Corylus heterophylla Fisch. male flowers were collected in Yanqing, Beijing. Flower samples (0.5 g) were dried in an oven, pulverized using an electric blender, and sieved (80-mesh). Ultrasonic-assisted extraction was performed using four solvent systems: (A) Methanol-water (4:6, v/v), (B) Methanol-water (8:2, v/v), (C) Chloroform-methanol (6:4, v/v), and (D) Chloroform-methanol-water (6:3:1, v/v/v) (10 mL each) [35,36]. Subsequently, extracts were centrifuged (10 min, 8000 rpm, 4 °C), filtered through 0.22-μm membranes, and lyophilized. The dried extracts were stored at −20 °C until further use. Three independent extractions per solvent system were conducted under identical conditions.
2.2. Culture and Treatment of HT22 Cells
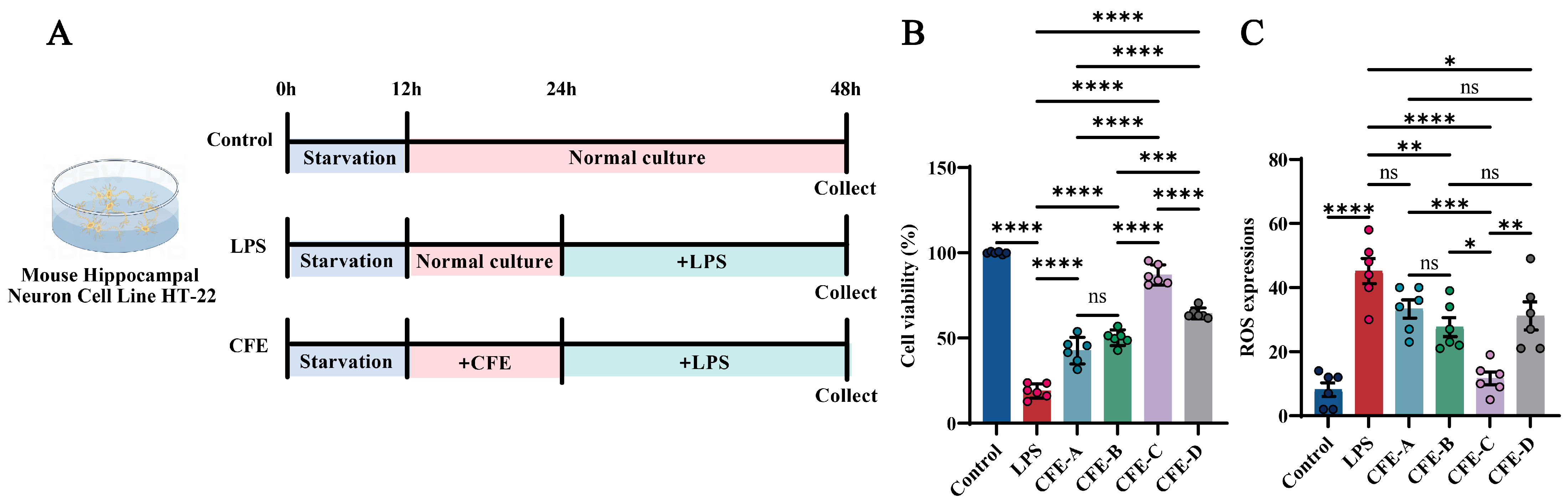
The mouse hippocampal neuronal cell line HT22 (iCell Bioscience Inc., Shanghai, China) was cultured in Dulbecco’s Modified Eagle Medium (DMEM; cat. #11965092, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; cat. #SX1101, SORFA, Deqing, China) and 1% penicillin-streptomycin (P/S; cat. #15140122, Gibco, Grand Island, NY, USA), and maintained at 37 °C in a 5% CO2 atmosphere. Cells were passaged using 0.25% trypsin-0.02% EDTA (cat. #C0201, Beyotime, Shanghai, China). For viability and ROS assays, cells were seeded in 96-well plates at a density of 5 × 104 cells/mL. Upon reaching 80% confluency, cells were serum-starved for 12 h and then assigned to the following groups (Figure 1A, N = 6 per group): Control: normal culture for 36 h; LPS: treated with 1 μg/mL LPS (cat. #L2880, Sigma, Kawasaki, Japan) for 24 h after 12 h of normal culture; CFE: pretreated with 200 μg/mL of solvent A/B/C/D extracts for 12 h, followed by 24 h LPS exposure. Cell viability was assessed using a CCK-8 kit (Meilunbio, Shanghai, China) by measuring the absorbance at 450 nm with an Infinite M200 microplate reader (TECAN, Männedorf, Switzerland). ROS levels were quantified with an assay kit (cat. #S0033S, Beyotime Institute of Biotechnology, Haimen, China). Briefly, cells were incubated with 10 μM 2′,7′-dichlorofluorescin diacetate (DCFH-DA) at 37 °C for 30 min. The suspensions were then centrifuged at 1000× g for 5 min and resuspended in fresh medium for fluorescence measurement [37].
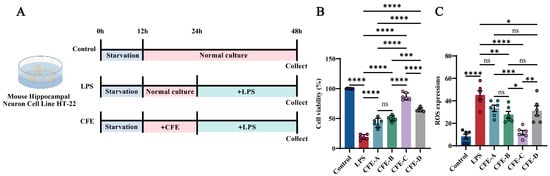
Figure 1.
Effects of CFE on HT22 cells with lipopolysaccharide (LPS) administration. The colored dots represent data points from different sample groups. (A) Schematic of the experimental timeline for HT22 cell treatments. Cells were assigned to the Control, LPS and CFE groups. (B) Cell viability measured by CCK-8 assay. (C) Quantitative analysis of intracellular reactive oxygen species (ROS) levels. Data presented as means ± SEM (N = 6). Statistically significant differences were indicated: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns p > 0.05, compared to the LPS group.
2.3. The Chemical Composition of CFE Obtained with Solvent C Was Analyzed by UHPLC-MS
The chemical profile of CFE, the most effective extract obtained using solvent C, was characterized by UHPLC-MS. Chromatographic separation was carried out on a Hi Q Sil C18 column (250 mm × 4.6 mm, 5 μm) maintained at 40 °C. A gradient elution was applied over 35 min using 0.1% formic acid in water (mobile phase A) and acetonitrile (mobile phase B), at a flow rate of 0.3 mL/min. The injection volume was set to 4 μL. Mass spectrometry detection was performed in positive/negative polarity switching mode with the following parameters: sheath gas, 40 arb; auxiliary gas, 5 arb; spray voltage, 3500 V (positive) and 3000 V (negative); capillary temperature, 320 °C. Full-scan mass spectra were acquired across the m/z range of 80–1200 at a resolution of 70,000, with an AGC target of 3 × 106 and a maximum injection time of 200 ms. Quality control samples were analyzed periodically to ensure system stability. Quantification of the major flavonoid compounds was conducted using the external standard method.
2.4. Animal Treatment
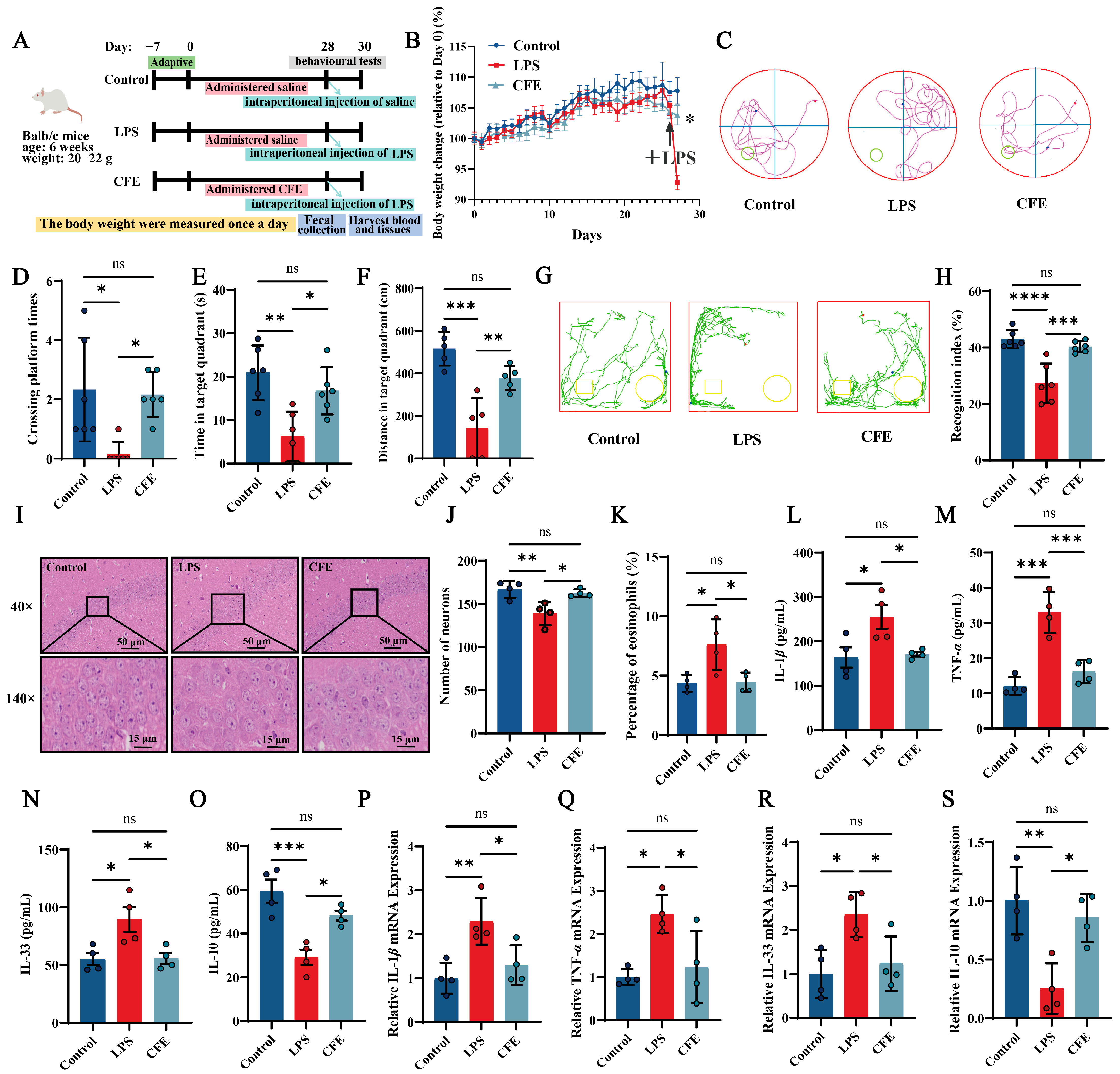
All animal procedures were approved by the Animal Experimentation Ethics Committee of China Agricultural University (Protocol Aw71705202-6-04, 17 July 2025). As outlined in Figure 2A, a total of 30 male Balb/c mice (6 weeks old, 20–22 g; Beijing Sibeifu Biotechnology Co., Ltd., Beijing, China) were randomly assigned to three groups: Control, LPS and CFE. Following a 7-day acclimatization period, mice received daily intragastric gavage for 28 consecutive days. The Control and LPS groups were administered 0.2 mL of saline per day, whereas the CFE group received 0.2 mL of CFE (extracted with solvent C, 200 mg/kg) daily. The CFE dose was selected based on preliminary experiments and previous literature [38]. Upon completion of the 28-day gavage regimen, all mice except the Control group received a single intraperitoneal injection of LPS (1 mg/kg). Control mice were injected with an equal volume of saline. Body weight was recorded daily throughout the study.
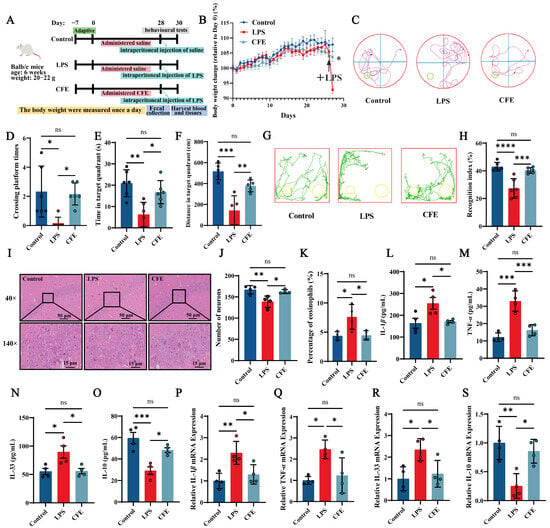
Figure 2.
Pretreatment with Corylus heterophylla Fisch. male flower extract (CFE) ameliorated pathological outcomes in a mouse model of hippocampal neuroinflammation (HNF). The colored dots represent data points from different sample groups. (A) Schematic of the experimental timeline. (B) Rate of body weight change from Day 0 in mice. The arrows indicate intraperitoneal injection of LPS in mice on day 28. (C) Representative swimming trajectories from the Morris Water Maze test (N = 6). (D) Number of platform crossings within 60 s. (E) Time spent in the target quadrant within 60 s. (F) Distance traveled in the target quadrant within 60 s. (G) Representative exploration curves from the novel object recognition test (N = 6). (H) Recognition index for each group. (I) Representative hematoxylin and eosin (H&E)-stained sections of the hippocampus. (J) Quantification of neuronal density in the hippocampal cornu ammonis 1 (CA1) region. (K) Percentage of eosinophilic cells in the CA1 region. (L–O) Serum levels of IL-1β, TNF-α, IL-33, and IL-10 measured by ELISA. (P–S) Hippocampal mRNA expression of IL-1β, TNF-α, IL-33, and IL-10 assessed by RT-qPCR. Data are presented as mean ± SEM (N = 4). Statistically significant differences were indicated: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns p > 0.05, compared to the LPS group.
2.5. Behavioral Assessments
2.5.1. Morris Water Maze (MWM)
Spatial learning and memory were assessed using the MWM test [37]. The apparatus consisted of a circular pool (120 cm in diameter) filled with water maintained at 25 °C. A submerged escape platform (10 cm in diameter, positioned 2 cm below the water surface) was placed in the center of quadrant III. During the training phase, mice that failed to locate the platform within 60 s were gently guided to it and allowed to remain there for 15 s. For the probe test, the platform was removed and each mouse was released into quadrant I. The following parameters were recorded over a 60 s period: the number of platform crossings, and the time spent and distance traveled in the target quadrant.
2.5.2. Novel Object Recognition Experiment (NOR)
In the NOR test, during the training phase, two identical rectangular objects (A and B) were placed in opposite corners of the arena. Mice were positioned facing away from the objects to avoid initial bias and allowed to explore freely for 5 min. In the subsequent test phase, object B was replaced with a novel cylindrical object (C). Mice were reintroduced into the arena, and their exploration was recorded for 5 min. The following parameters were quantified: movement trajectories, exploration time directed toward the familiar object A (TA), and exploration time directed toward the novel object C (TC) [39]. The recognition index was calculated as follows: Recognition Index (%) = [TC/(TA + TC)] × 100%.
2.6. Hematoxylin and Eosin (H&E) Staining
Following behavioral testing, mice were transcardially perfused with saline followed by 4% paraformaldehyde. Brains were promptly dissected and further fixed by immersion in 4% PFA for 24 h at 4 °C. After graded ethanol dehydration, the tissues were paraffin embedded using a tissue embedding station. Subsequently, 4-μm-thick sections were cut and prepared for H&E staining. To evaluate neuronal damage in the hippocampal cornu ammonis 1 (CA1) region, we quantified the density of surviving neurons and the proportion of eosinophilic cells [40]. Analysis was conducted on four randomly selected 40× fields per section using ImageJ2x software (Rawak Software, Stuttgart, Germany).
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
Blood samples were collected from all mice (Control, LPS and CFE groups) via retro-orbital bleeding upon completion of behavioral testing, which was performed 2 days after the final LPS challenge and pretreatment administration. Samples were centrifuged at 3000× g for 15 min at 4 °C, and the serum was collected for analysis. Serum concentrations of IL-1β (Cat. #RX203063M, RUIXIN, Yueqing, China), TNF-α (Cat. #EK282, RUIXIN), IL-33 (Cat. #RX203053M, RUIXIN), IL-10 (Cat. #EK210, RUIXIN), 5-HT (Cat. #MM-0443M2, Jiangsu Meimian Industrial Co., Ltd., Yancheng, China), and ALA (Cat. #MM-926718O2, Jiangsu Meimian Industrial Co., Ltd.) were quantified using commercially available ELISA kits [41]. For hippocampal tissue analysis, 20 mg of tissue was homogenized in 1 mL of RIPA buffer (MA0151, Meilunbio) supplemented with protease inhibitors. The homogenate was centrifuged at 10,000× g for 5 min at 4 °C, and the resulting supernatant was collected. Hippocampal levels of 5-HT and ALA were measured using specific ELISA kits, with absorbance read on a microplate reader (model 3903 2010, Bio Rad, Hercules, CA, USA).
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from hippocampal tissue using TRIzol™ Reagent (Cat. #15596026, Invitrogen, Carlsbad, CA, USA). RNA quality and concentration were assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Shanghai, China). Complementary DNA (cDNA) was synthesized using the PrimeScript RT Reagent Kit (Cat. #DRR037A, TaKaRa, Osaka, Japan). qRT-PCR was then performed with TB Green Premix Ex Taq (Cat. #RR420A, Takara) on a real-time PCR system. Gene expression levels were calculated using the 2−ΔΔCt method and normalized to the housekeeping gene GAPDH. The primer sequences used are listed in Supplementary Table S1.
2.9. Immunohistochemistry (IHC)
IHC staining of brain tissue sections was performed using primary antibodies against the following targets: Aβ1–42 (cat. #A9810, Sigma Aldrich, St. Louis, MO, USA), Tau (cat. #10274-1-AP, Proteintech, Rosemont, IL, USA), P-Tau (Ser404) (cat. #35834, Cell Signaling Technology, Danvers, MA, USA), BDNF (cat. #25699-1-AP, Proteintech), MAP2 (cat. #17490-1-AP, Proteintech) and CD11b (cat. #66519-1-IG, Proteintech). Antigen antibody complexes were detected using a horseradish peroxidase-conjugated secondary antibody followed by diaminobenzidine chromogenic development with a Vectastain ABC kit [42]. The average optical density (AOD) of immunopositive regions was quantified in four randomly selected fields within the hippocampal CA1 subregion using ImageJ2x software (Rawak Software, Germany).
2.10. Western Blot Analysis (WB)
Western blot analysis was performed following a previously described protocol with slight modifications [43]. Hippocampal tissues were homogenized on ice for 30 min in RIPA lysis buffer supplemented with 1% PMSF and phosphatase inhibitors (Beyotime, Shanghai, China). After centrifugation at 12,000× g for 20 min at 4 °C, the supernatant was collected, and total protein concentration was determined using a bicinchoninic acid (BCA) assay kit (Beyotime, China). Protein samples were denatured by heating at 95 °C for 20 min, separated on 10% SDS-polyacrylamide gels, and transferred onto polyvinylidene fluoride (PVDF) membranes (cat. #IPVH00010, Millipore, Burlington, MA, USA). Membranes were blocked with 5% bovine serum albumin (BSA) in PBS for 1 h at room temperature and then incubated overnight at 4 °C with the following primary antibodies: Aβ1–42 (cat. #A9810, Sigma-Aldrich), Tau (cat. #10274-1-AP, Proteintech), P-Tau (Ser404) (cat. #35834, Cell Signaling Technology, Danvers, MA, USA), GSK-3β (cat. #22104-1-AP, Proteintech), BDNF (cat. #25699-1-AP, Proteintech), MAP2 (cat. #17490-1-AP, Proteintech), CD11b (cat. #ab184307, Abcam, Cambridge, UK), and GAPDH (cat. #10494-1-AP, Proteintech). After washing, membranes were incubated for 2 h at room temperature with an HRP-conjugated secondary antibody (cat. #SA00001 2, Proteintech). Protein bands were visualized using enhanced chemiluminescence (ECL) substrate on an Image Quant LAS4000 system (GE Healthcare, Chicago, IL, USA). Band intensities were quantified using ImageJ2x software (National Institutes of Health, Bethesda, MD, USA) and normalized to GAPDH.
2.11. Immunofluorescence (IF)
Brain tissues were fixed in 4% paraformaldehyde, embedded, and sectioned to a thickness of 40 μm using a cryostat. After three brief washes with PBS, sections were blocked with 5% BSA for 1 h at room temperature and then incubated overnight at 4 °C with the following primary antibodies: rabbit anti-Iba1 (1:500, ab178846, Abcam) and rabbit anti-GFAP (1:500, ab7260, Abcam). Following three PBS washes, sections were incubated with appropriate fluorescent dye conjugated secondary antibodies for 2 h at room temperature in the dark. After a final PBS wash, sections were mounted, air dried, and imaged using a laser scanning confocal microscope (Thermo Fisher Scientific, Waltham, MA, USA).
2.12. Metabolomic Profiling of Fecal Short-Chain Fatty Acids (SCFAs)
Quantitative analysis of fecal SCFAs (acetic, propionic and butyric acids) was performed by targeted metabolomics following an established methodology [44]. Fecal samples (25 mg) were subjected to acid extraction using 0.5% phosphoric acid (H3PO4), followed by cryogenic homogenization, sonication in an ice bath for 10 min, and centrifugation. The supernatant was derivatized with butanol containing 2-ethylbutyric acid as an internal standard, then vortexed, sonicated, and centrifuged. GC-MS analysis was carried out on an Agilent 8890B/5977B system equipped with an HP-FFAP column (30 m × 0.25 mm × 0.25 μm). The operating conditions were as follows: splitless injection at 260 °C; helium carrier gas at a constant flow of 1.0 mL/min; oven temperature programmed from 80 °C (held for 1 min) to 120 °C at 40 °C/min, then to 200 °C at 10 °C/min, and finally to 230 °C (held for 3 min). Detection was performed in selected ion monitoring (SIM) mode using electron ionization (70 eV). The ion source and transfer line temperatures were set to 230 °C, and the quadrupole temperature was 150 °C. Quantification was based on external calibration curves generated with MassHunter software (v10.0).
2.13. Sequencing of the Microbial 16S Ribosomal RNA (16S rRNA)
Fecal samples collected from the experimental mice were immediately frozen at −80 °C and processed within 3 h. DNA extraction and 16S rRNA gene sequencing were subsequently conducted by Majorbio Bio Pharm Technology Co., Ltd. (Shanghai, China). Briefly, the V3–V4 hypervariable regions of the bacterial 16S rRNA gene were amplified and sequenced on an Illumina NextSeq 2000 platform. After DNA extraction and PCR amplification, the raw sequencing reads were subjected to quality filtering, paired end assembly, and clustering into operational taxonomic units (OTUs). Microbial diversity was analyzed with beta diversity assessed via principal coordinate analysis (PCoA) based on Bray-Curtis distances. Differentially abundant bacterial genera were identified using linear discriminant analysis effect size (LEfSe) with thresholds of LDA score > 3 and p < 0.05.
2.14. Serum Metabolomics Analysis
Untargeted metabolomic profiling of serum samples was conducted following an adapted protocol [45]. Briefly, serum proteins were precipitated using cold methanol acetonitrile (1:1, v/v). After centrifugation, the supernatant was collected, dried under nitrogen, reconstituted in an appropriate solvent, and analyzed on a UHPLC-MS/MS system (Thermo Scientific Q Exactive, Waltham, MA, USA). Chromatographic separation was performed on an HSS T3 column using a gradient of water and acetonitrile, each containing 0.1% formic acid. Mass spectrometry analysis was carried out in data dependent acquisition (DDA) mode with electrospray ionization in both positive and negative polarities at a resolution of 70,000.
2.15. Culture and Treatment of BV2 Cells
BV2 microglial cells were maintained in complete DMEM and seeded in 6 well plates at a density of 5 × 104 cells/mL. Upon reaching 80% confluency, cells were serum starved for 12 h. In a preliminary experiment, the optimal CFE concentration was determined using the following groups: Control (normal culture), LPS (1 μg/mL, 12 h normal culture + 24 h LPS) and CFE (200, 400, or 600 μg/mL; 12 h pretreatment with CFE + 24 h LPS). Based on cell viability results, 400 μg/mL CFE was selected for subsequent polarization studies. For M1 polarization, the experimental groups included: Control (normal culture), LPS (1 μg/mL, 12 h normal culture + 24 h LPS), CFE (400 μg/mL, 12 h pretreatment with CFE + 24 h LPS), luteolin (14 μM, CAS: 491-70-3, cat. #B20888, Shanghai Yuanye BioTechnology Co., Ltd., Shanghai, China, 12 h pretreatment with luteolin + 24 h LPS) and kaempferol (10 μM, CAS: 520-18-3, cat. #YZ-110861, Solarbio, Beijing, China, 12 h pretreatment with kaempferol + 24 h LPS). For M2 polarization, groups included: Control (normal culture), IL-4 (20 ng/mL, cat. #P00196, Solarbio, 12 h normal culture + 24 h IL-4), CFE (400 μg/mL, 12 h pretreatment with CFE+ 24 h IL-4), luteolin (14 μM, 12 h pretreatment with luteolin + 24 h IL-4) and kaempferol (10 μM, 12 h pretreatment with kaempferol + 24 h IL-4). Total RNA was extracted using TRIzol reagent, and cDNA was synthesized. mRNA expression levels of M1 markers (iNOS, CD86, TNF-α) and M2 markers (Arg1, CD206, IL-10) were analyzed by quantitative PCR using the 2−ΔΔCt method (primer sequences are listed in Supplementary Table S1).
2.16. Data Processing
Data are presented as mean ± standard error of the mean (SEM). Statistical analyses were performed with Prism 9.5 (GraphPad Software, San Diego, CA, USA). Specifically, one-way analysis of variance (ANOVA) was applied, followed by Tukey’s post hoc test for multiple comparisons. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. CFE Enhanced Cell Viability and Attenuates ROS Production in HT22 Cells
We evaluated the effects of CFE on cell viability and ROS levels in HT22 cells using an LPS-induced model of HNF (Figure 1A–C). The CCK-8 assay revealed that LPS treatment significantly decreased HT22 cell viability compared to the Control group (p < 0.0001). This decrease was significantly mitigated by pretreatment with CFE-A, CFE-B, CFE-C and CFE-D (200 μg/mL, all p < 0.0001). Furthermore, CFE exhibited antioxidant activity, as LPS-induced ROS overproduction (p < 0.0001) was significantly suppressed by CFE-B (p < 0.001), CFE-C (p < 0.0001) and CFE-D (p < 0.05), with the solvent C extract showing the most pronounced reduction. Based on the combined neuroprotective and antioxidant effects, CFE extracted with solvent C was selected for all subsequent experiments.
3.2. Metabolite Profiling of CFE Extracted with Solvent C
Metabolite analysis of the solvent C extract of CFE tentatively identified 274 compounds, which were categorized into the following chemical classes: 91 terpenoids, 75 flavonoids, 52 phenylpropanoids, 20 alkaloids, 9 polyketides, 9 shikimate/acetate-malonate pathway derived compounds, 7 fatty acid-related compounds, 7 amino acid-related compounds, and 4 others. The ten most abundant and biologically relevant compounds from each major class are listed in Table 1, along with their tentative identities, molecular formulas, retention times, and m/z values. Subsequently, targeted quantitative analysis of the ten principal flavonoids was performed by HPLC-MS/MS (Table 2). Among these, luteolin and kaempferol were the most abundant, with quantified concentrations of 10,796.37 ± 65.53 ng/mL and 3082.77 ± 110.12 ng/mL, respectively.

Table 1.
Metabolites Identified in CFE.

Table 2.
Quantification of major identified flavonoids compounds in the CFE.
3.3. CFE Attenuated Cognitive Decline in HNF Mice
To evaluate the potential of CFE in mitigating LPS-induced HNF and cognitive decline, mice were administered CFE for four weeks (Figure 2A). No significant differences in body weight were observed among groups prior to LPS challenge. Following LPS administration, mice in the LPS group exhibited significant weight loss, confirming successful model induction. Notably, CFE pretreatment significantly attenuated this LPS-induced weight reduction (p < 0.05), indicating its ability to counteract inflammation-associated metabolic changes (Figure 2B).
Spatial learning and memory were assessed using the MWM (Figure 2C–F). Compared with the Control group, LPS-treated mice showed significant decreases in the number of platform crossings (p < 0.05), time spent in the target quadrant (p < 0.01), and distance traveled in the target quadrant (p < 0.001), indicating marked spatial memory deficits. CFE pretreatment significantly improved these parameters, increasing platform crossings (p < 0.05), time in the target quadrant (p < 0.05), and distance traveled in the target quadrant (p < 0.01) relative to the LPS group, demonstrating that CFE alleviates spatial memory impairment.
These findings were further supported by the NOR test (Figure 2G,H). Mice pretreated with CFE displayed a significantly higher recognition index than LPS-treated mice (p < 0.001), reflecting enhanced exploratory behavior and novel-object discrimination. Collectively, these results indicate that CFE pretreatment effectively ameliorates the severe cognitive decline associated with HNF in mice.
3.4. CFE Alleviated Hippocampal Damage in HNF Mice
Hippocampal histopathological changes are presented in Figure 2I–K. The LPS group showed a disrupted cytoarchitecture characterized by disorganized neuronal arrangement, widened intercellular spaces, and irregular morphology. Nucleolar condensation, dissolution, or loss was also evident. Quantitative analysis revealed a significant reduction in neuronal density (p < 0.01) and in the proportion of eosinophilic neurons (p < 0.05) compared to the Control group. In contrast, CFE intervention effectively restored hippocampal histology, significantly increasing both neuronal density (p < 0.05) and the percentage of eosinophilic neurons (p < 0.05) relative to the LPS group. Together, these results demonstrate that CFE pretreatment confers substantial protection against HNF-induced structural damage in the hippocampus.
3.5. CFE Modulated Inflammatory Cytokine Levels in Serum and Hippocampus of HNF Mice
ELISA analysis of serum (Figure 2L–O) showed that LPS administration significantly increased the levels of the pro-inflammatory cytokines IL-1β (p < 0.05), TNF-α (p < 0.001), and IL-33 (p < 0.05), while reducing the anti-inflammatory cytokine IL-10 (p < 0.001). In contrast, CFE pretreatment significantly lowered serum IL-1β (p < 0.05), TNF-α (p < 0.001), and IL-33 (p < 0.05), and elevated IL-10 (p < 0.05) compared to the LPS group. Similarly, RT-qPCR analysis of hippocampal tissue (Figure 2P–S) indicated that LPS challenge markedly upregulated mRNA expression of IL-1β (p < 0.01), TNF-α (p < 0.05), and IL-33 (p < 0.05), and downregulated IL-10 (p < 0.01). CFE intervention significantly reversed these changes, reducing IL-1β, TNF-α, and IL-33 expression and enhancing IL-10 levels (all p < 0.05). Together, these data demonstrate that CFE effectively mitigates systemic and hippocampal inflammation in HNF mice.
3.6. CFE Alleviated the Expression of Aβ1–42 Protein in the Hippocampus of HNF Mice
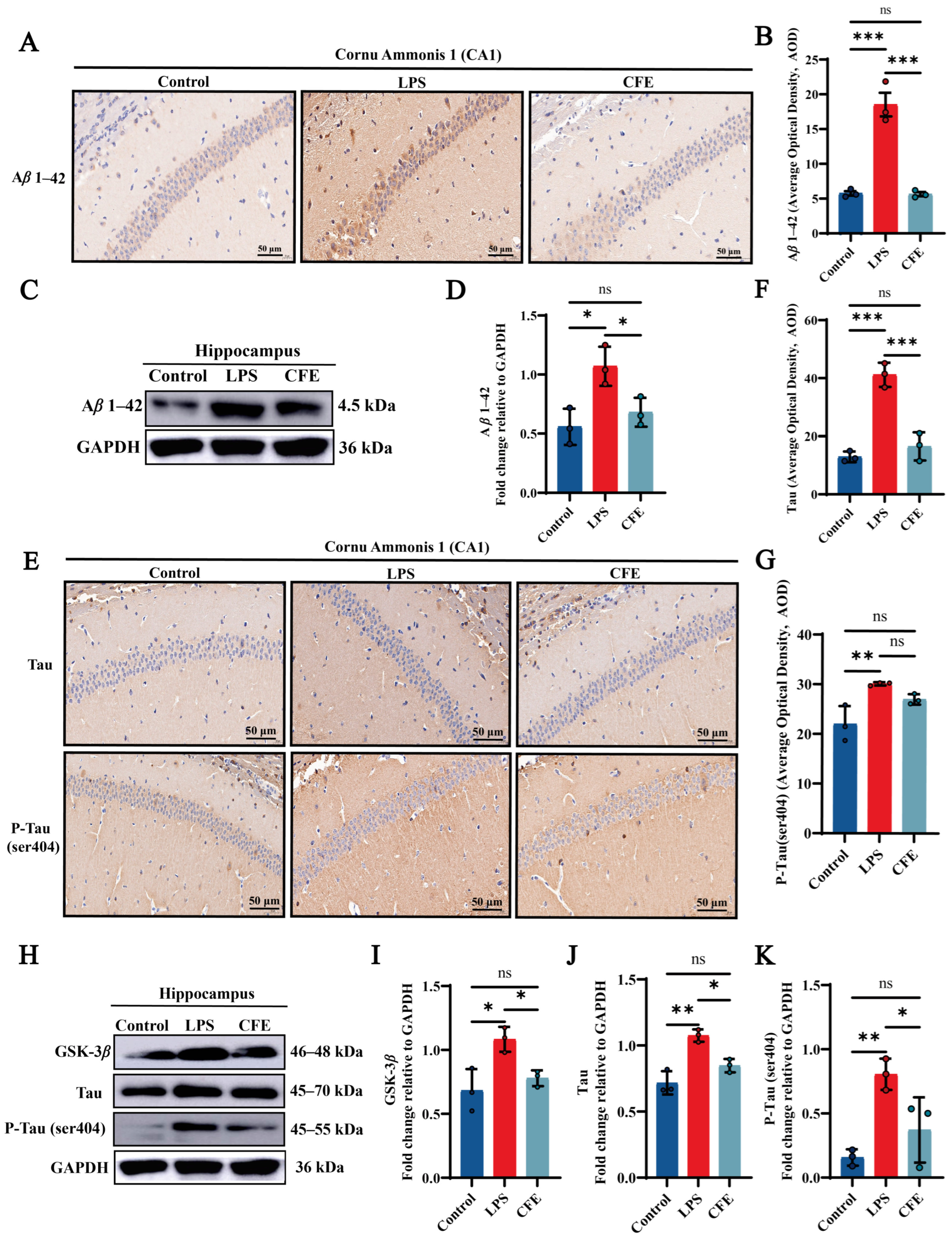
To assess the impact of CFE on Aβ1–42 levels, IHC (Figure 3A,B) and WB (Figure 3C,D) were performed. IHC analysis confirmed that the LPS group exhibited significantly elevated AOD values for Aβ1–42 compared to the Control group (p < 0.001), as evidenced by widespread brown-yellow deposition throughout the hippocampus. CFE intervention markedly reduced Aβ1–42 AOD relative to the LPS group (p < 0.001). WB analysis confirmed this reduction (p < 0.05). The results suggested that the amelioration of cognitive decline by CFE in HNF mice is associated with reduced hippocampal Aβ1–42 deposition and subsequent improvement in the brain microenvironment.
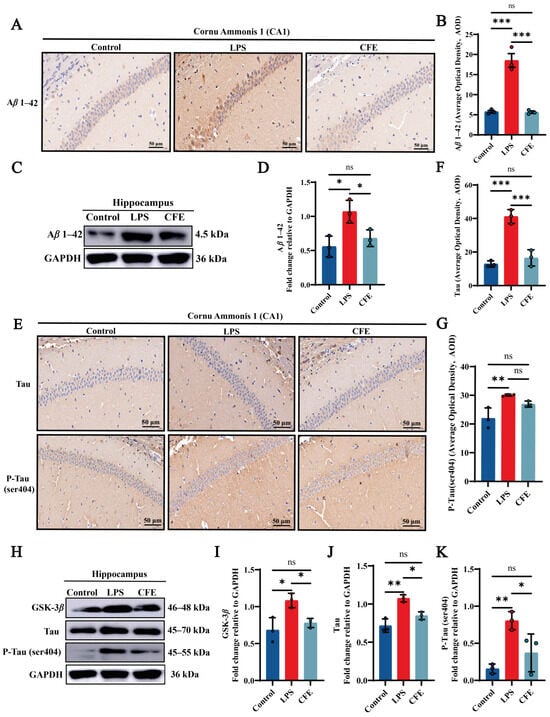
Figure 3.
CFE downregulated hippocampal levels of Aβ1–42, GSK-3β, Tau and P-Tau in HNF mice. The colored dots represent data points from different sample groups. (A) Representative immunohistochemical (IHC) staining images of Aβ1–42 (40× magnification). (B) Quantification of Aβ1–42 IHC staining intensity expressed as average optical density (AOD). (C) Representative Western blot images of hippocampal Aβ1–42. (D) Quantitative analysis of Aβ1–42 protein levels from Western blot, normalized to GAPDH. (E) Representative IHC staining images of total Tau and P-Tau (Ser404) (40×). (F,G) Quantification of Tau and P-Tau (Ser404) IHC staining intensity (AOD). (H) Representative Western blot images of GSK-3β, total Tau and P-Tau (Ser404). (I–K) Quantitative analysis of GSK-3β, total Tau and P-Tau (Ser404) protein levels from Western blot, normalized to GAPDH. Data are presented as the mean ± SEM (N = 3). Statistically significant differences were indicated: * p < 0.05, ** p < 0.01, *** p < 0.001, ns p > 0.05, compared to the LPS group.
3.7. CFE Alleviated the Expressions of GSK-3β, Tau and P-Tau Protein in the Hippocampus of HNF Mice
IHC and WB analysis of CA1 hippocampal regions quantified GSK-3β, Tau and P-Tau (ser404) immunoreactivity (Figure 3E–K). Compared to the Control group, the LPS group demonstrated a marked increase in AOD values for Tau (p < 0.001) and P-Tau (ser404) (p < 0.01), characterized by extensive brown-yellow deposits diffusely distributed across the hippocampal formation. CFE intervention significantly lowered Tau (p < 0.001) and P-Tau (ser404) AOD value compared to the LPS group. Furthermore, WB analysis revealed a decrease in GSK-3β, Tau and P-Tau (ser404) (all p < 0.05) expression which is consistent with the IHC results. Our results show that CFE reduces hippocampal GSK-3β, Tau and P-Tau (ser404) expression, suggesting a potential mechanism for neuronal cytoskeletal stabilization.
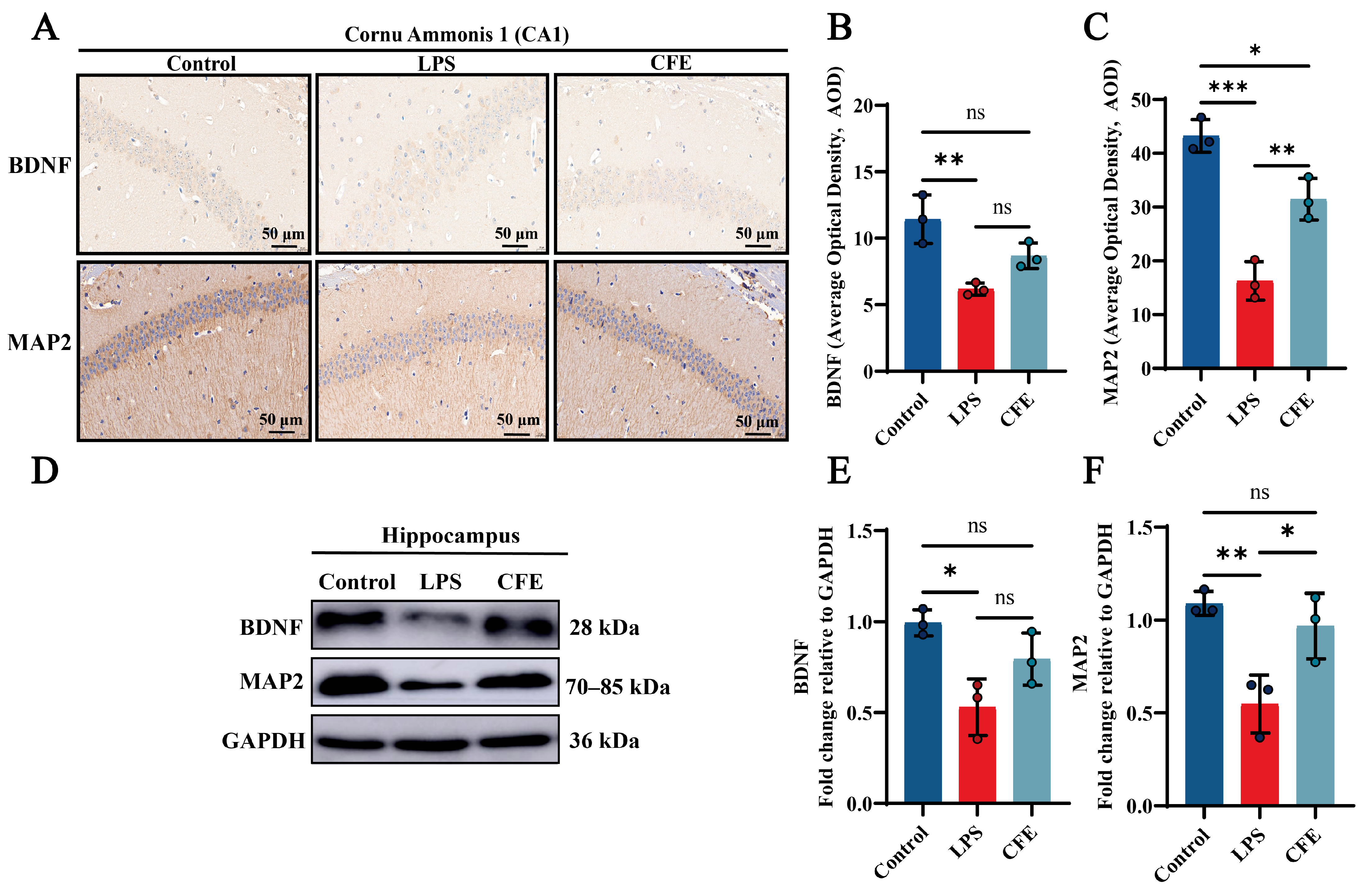
3.8. CFE Enhanced the Expressions of BDNF and MAP2 Protein in the Hippocampus of HNF Mice
IHC examination of CA1 hippocampal subfields was performed to quantify BDNF and MAP2 expression (Figure 4A–C). In the Control group, BDNF and MAP2 high expressions were a brown-yellow sediment, which was widely distributed in the hippocampus. CFE supplementation significantly increased BDNF and MAP2 AOD value compared to the LPS group (p < 0.01). Notably, WB analysis (Figure 4D–F) revealed enhanced expression of both BDNF and MAP2 in the CFE group compared to the LPS group (p < 0.05). Our findings indicated that CFE enhanced neuronal synaptic plasticity by modulating the BDNF signaling pathway.
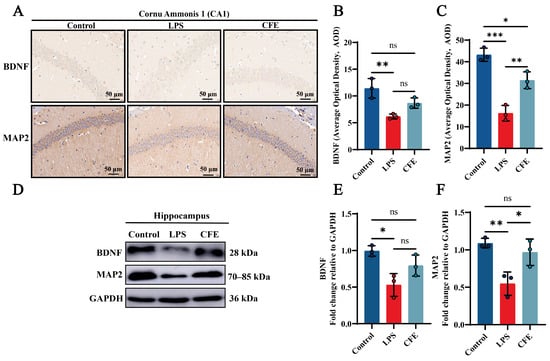
Figure 4.
CFE increased hippocampal levels of BDNF and MAP2 in HNF mice. The colored dots represent data points from different sample groups. (A) Representative immunohistochemical (IHC) staining images of BDNF and MAP2. (B,C) Quantification of BDNF and MAP2 IHC staining intensity expressed as AOD. (D) Representative Western blot images of hippocampal BDNF and MAP2. (E,F) Quantitative analysis of BDNF and MAP2 protein levels from Western blot, normalized to GAPDH. Data are presented as the mean ± SEM (N = 3). Statistically significant differences were indicated: * p < 0.05, ** p < 0.01, *** p < 0.001, ns p > 0.05, compared to the LPS group.
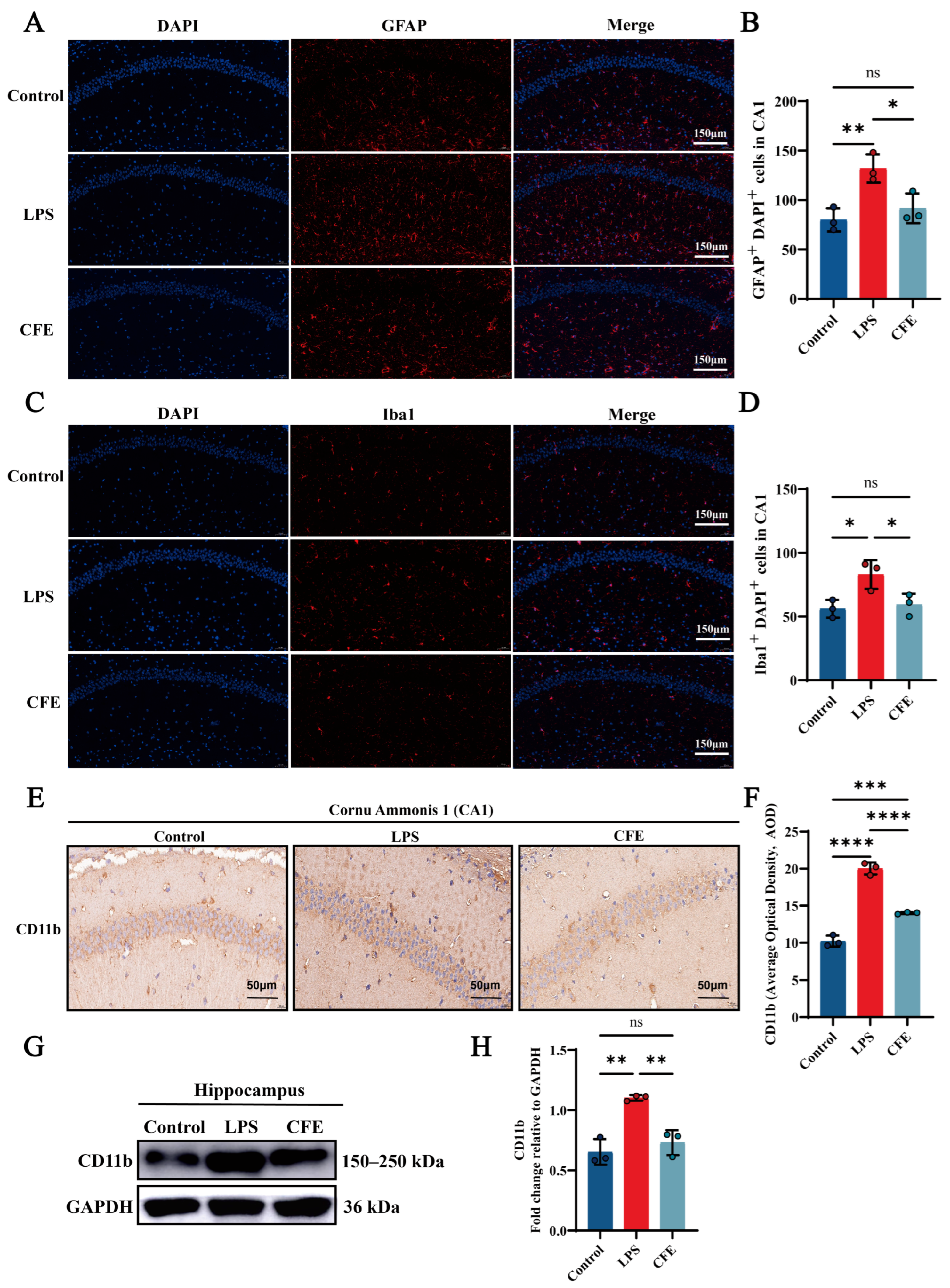
3.9. CFE Inhibited the Abnormal Activation of Microglia and Astrocytes in the Hippocampus of HNF Mice
IF analysis revealed that the activation of microglia and astrocytes, as indicated by Iba1 and GFAP labeling, respectively, was significantly attenuated in the CFE group compared to the LPS group (all p < 0.05) (Figure 5A–D). Additionally, IHC analysis demonstrated that CFE intervention markedly reduced CD11b AOD compared to the LPS group (p < 0.0001) (Figure 5E,F). Concordantly, WB analysis (Figure 5G,H) confirmed decreased CD11b protein expression in the CFE group (p < 0.01). These data collectively indicated that CFE inhibited microglial and astrocyte activation.
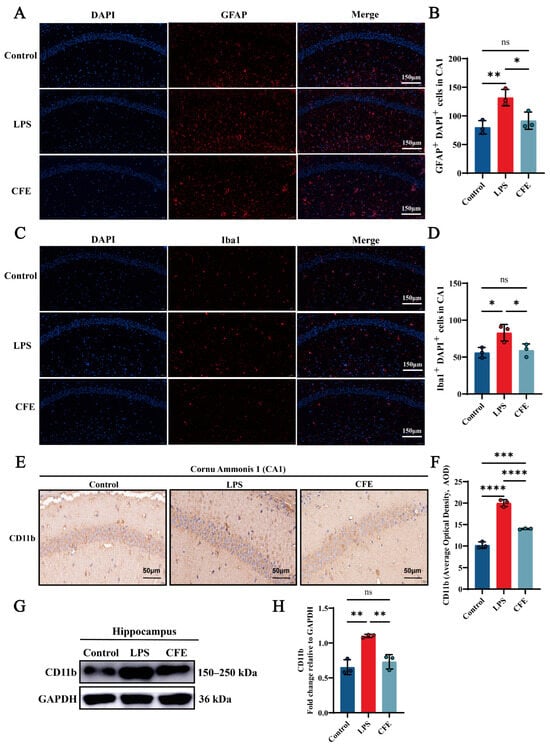
Figure 5.
CFE suppressed glial cell activation in the hippocampus of HNF mice. The colored dots represent data points from different sample groups. (A) Representative immunofluorescence (IF) image of GFAP (astrocyte marker) in the hippocampal CA1 region. (B) Quantitative analysis of GFAP immunofluorescence intensity. (C) Representative immunofluorescence image of Iba1 (microglial marker) in the hippocampal CA1 region. (D) Quantitative analysis of Iba1 immunofluorescence intensity. (E) Representative immunohistochemical (IHC) staining of CD11b. (F) Quantification of CD11b IHC intensity expressed as AOD. (G) Representative Western blot images of hippocampal CD11b. (H) Quantitative analysis of CD11b protein levels from Western blot, normalized to GAPDH. Data are presented as the mean ± SEM (N = 3). Statistically significant differences were indicated: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns p > 0.05, compared to the LPS group.
3.10. CFE Increased SCFAs in Fecal of HNF Mice
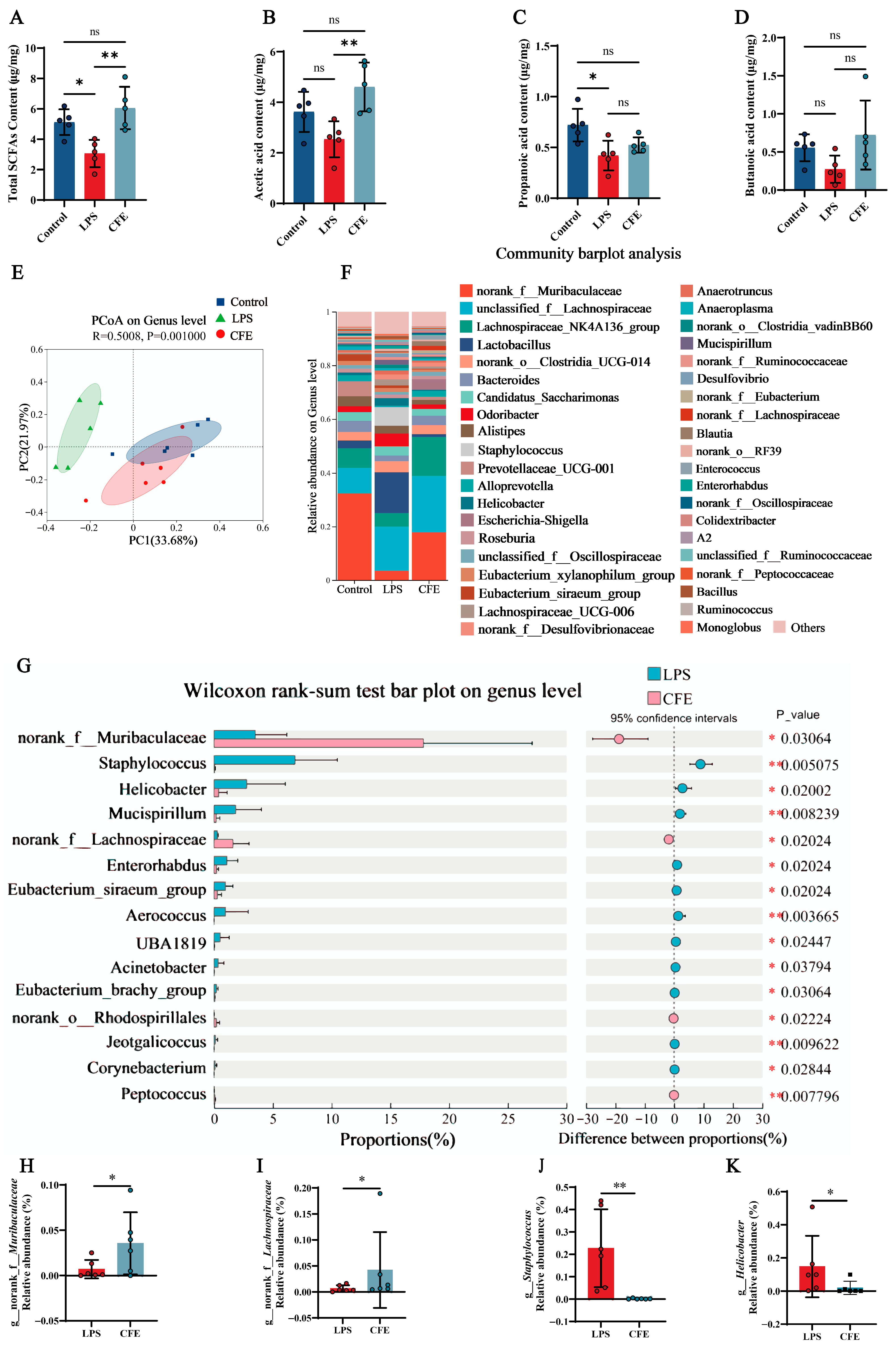
Given the established role of SCFAs in microbiota-gut-brain axis signaling, we measured fecal SCFAs levels to evaluate the effect of CFE on gut microbiota metabolism (Figure 6A–D). Notably, CFE intervention elevated total SCFAs (p < 0.01), acetic acid (p < 0.01), propanoic acid and butanoic acid compared to the LPS group. The results indicated that CFE reversed the reduction in fecal SCFAs levels induced by HNF.
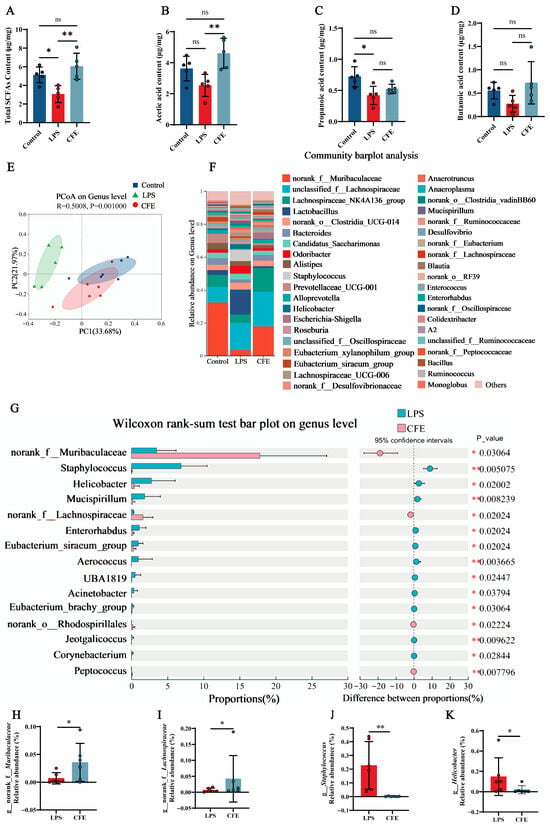
Figure 6.
CFE modulates the gut microbiome and enhances SCFAs production in HNF mice. The colored dots represent data points from different sample groups. (A) Fecal concentration of total SCFAs (N = 5). (B) Fecal concentration of acetic acid (N = 5). (C) Fecal concentration of propionic acid (N = 5). (D) Fecal concentration of butyric acid (N = 5). (E) Beta diversity of the gut microbiota visualized by PCoA based on Bray-Curtis distances at the genus level. The plot shows the microbial community composition of samples from the Control (blue), LPS (green), and CFE (red) groups. Each dot represents an individual sample. The background color illustrates the clustering of samples within the ordination space. (F) Bar plot showing the relative abundance of bacterial genera. (G) Differential abundance analysis between the CFE and LPS groups (Wilcoxon rank-sum test). (H) Relative abundance of the genus Muribaculaceae across groups. (I) Relative abundance of the genus Lachnospiraceae across groups. (J) Relative abundance of the genus Staphylococcus across groups. (K) Relative abundance of the genus Helicobacter across groups. Data are presented as the mean ± SEM (N = 6). Statistically significant differences were indicated: * p < 0.05, ** p < 0.01, ns p > 0.05, compared to the LPS group.
3.11. CFE Reshaped the Gut Microbiota of HNF Mice
Fecal 16S rRNA gene sequencing was performed to evaluate CFE’s impact on gut microbiota in HNF mice. PCoA based on Bray-Curtis distances (Figure 6E) revealed distinct inter-group clustering. PC1 and PC2 accounted for 33.68% and 21.97% of variance, respectively. Notably, reduced compositional overlap between the LPS and CFE groups indicated microbiota restructuring. At the genus level (Figure 6F), CFE administration shifted dominant bacterial flora from Lactobacillus and Staphylococcus (LPS group) to norank_f__Muribaculaceae and unclassified_f__Lachnospiraceae (CFE group). Wilcoxon tests (Figure 6G–K) confirmed CFE significantly increased norank_f__Muribaculaceae (p < 0.05) and norank_f__Lachnospiraceae (p < 0.05), while decreasing Staphylococcus (p < 0.01) and Helicobacter (p < 0.05) compared to the LPS group. Collectively, the findings proved that CFE supplementation renovated LPS-induced gut microbiota disturbances.
3.12. CFE Regulated Serum Metabolites in HNF Mice
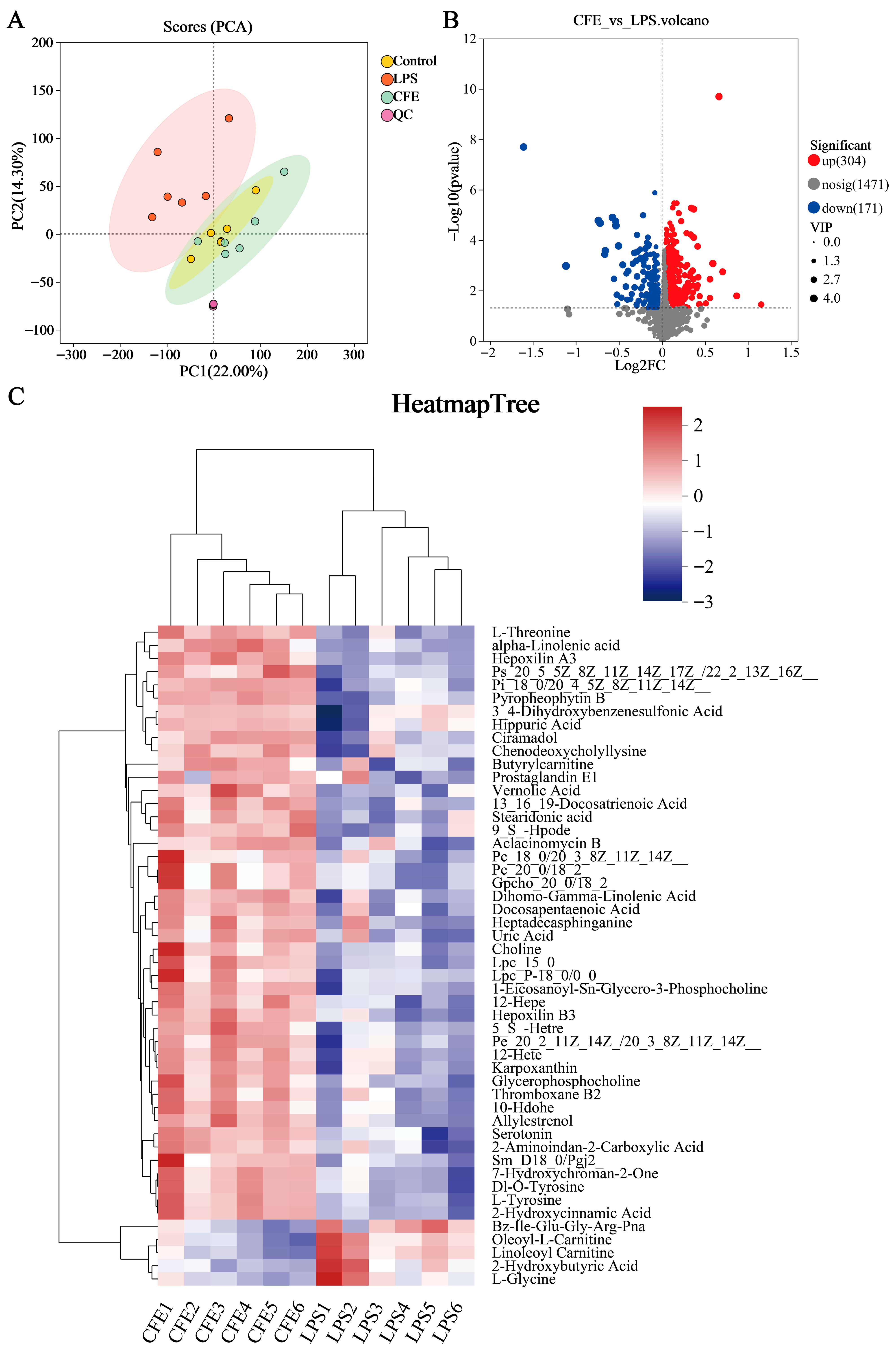
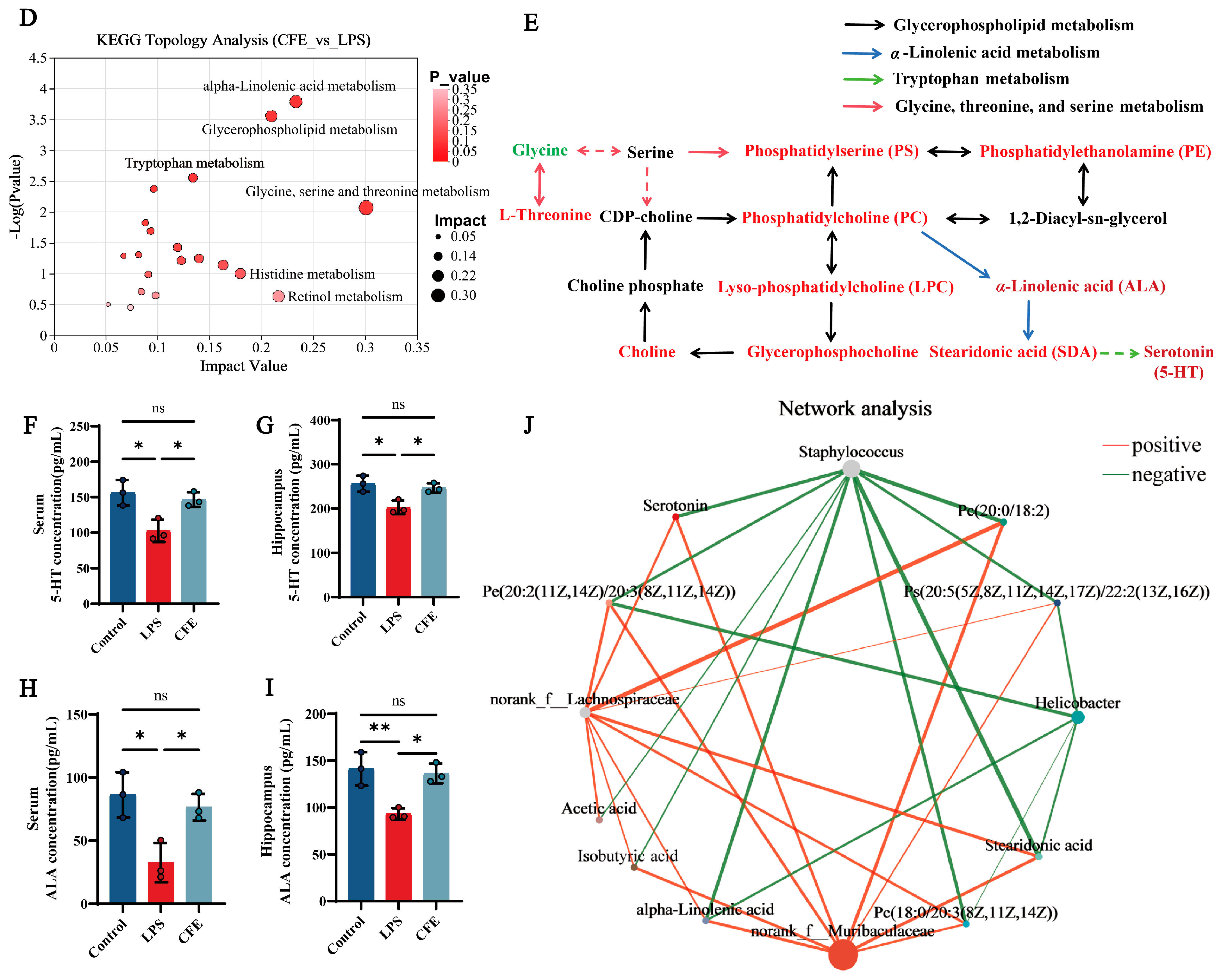
Serum untargeted metabolomic profiling was employed to characterize systemic metabolic alterations during HNF progression. The clear separation between the LPS and control groups in the PCA score plot (Figure 7A) confirmed distinct metabolic profiles and verified successful model induction. Differential metabolites were identified using dual thresholds of variable importance in projection (VIP) > 1 and statistical significance (p < 0.05), with fold-change (FC) values determining regulation directionality (FC < 1: downregulation; FC > 1: upregulation; Figure 7B). 475 metabolites exhibited significant abundance changes between the LPS and CFE groups (304 upregulated, 171 downregulated). Hierarchical clustering analysis revealed that the CFE group substantially increased concentrations of phosphatidylserine [PS (20:5 (5Z,8Z,11Z,14Z,17Z)/22:1 (13Z))], phosphatidylcholines [PC (18:0/20:3 (8Z,11Z,14Z), PC (20:0/18:2)], phosphatidylethanolamine [PE (20:2 (11Z,14Z)/20:3 (8Z,11Z,14Z))], lysophosphatidylcholines [LPC (15:0), LPC (P-18:0/0:0)], choline, ALA, stearidonic acid (SDA), and 5-HT compared to the LPS group (Figure 7C). KEGG Topological analysis further identified 20 significantly enriched metabolic routes, notably encompassing glycerophospholipid metabolism, α-linolenic acid metabolism, tryptophan metabolism, and glycine-serine-threonine metabolism (Figure 7D). Metabolomic interaction networks revealed the pathway interconnections and underlying mechanisms (Figure 7E). ELISA quantification confirmed CFE group elevations of 5-HT (p < 0.05) and ALA (p < 0.05) in both serum and hippocampal tissues compared to the LPS group (Figure 7F–I). For establishing gut-microbiota-metabolite crosstalk, Pearson correlation-based networks were generated and genus-level networks demonstrated significant associations (Figure 7J): g__norank_f__Muribaculaceae and g__norank_f__Lachnospiraceae abundances showed positive correlations with PC (15:0/18:2), PC (20:0/18:2), PE (20:2/20:3), PS (20:5/22:1), 5-HT, ALA, SDA, acetate, and isobutyrate, while Staphylococcus abundance was inversely correlated with these metabolites. Similarly, Helicobacter exhibited negative correlations specifically with PC (18:0/20:3), PE (20:2/20:3), PS (20:5/22:1), ALA, and SDA. Collectively, CFE mediated microbiota remodeling characterized by enrichment of Muribaculaceae and Lachnospiraceae alongside suppression of Staphylococcus and Helicobacter potentiated beneficial metabolite production.
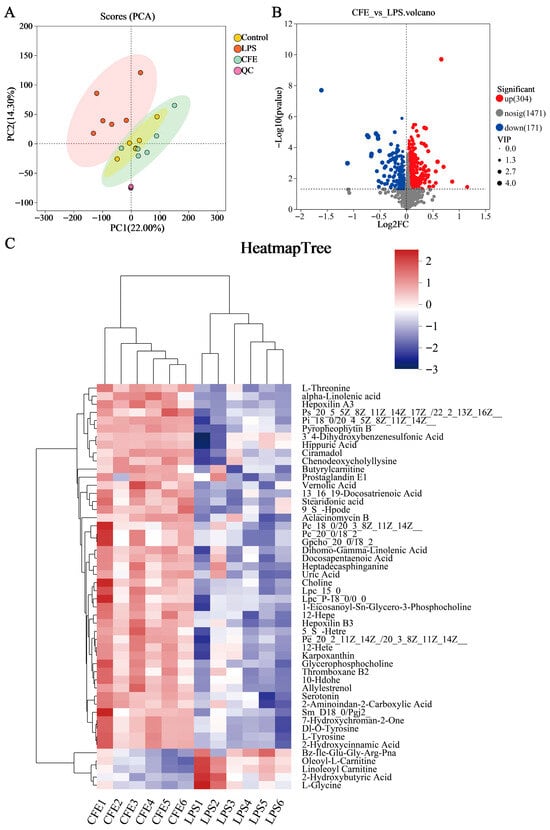
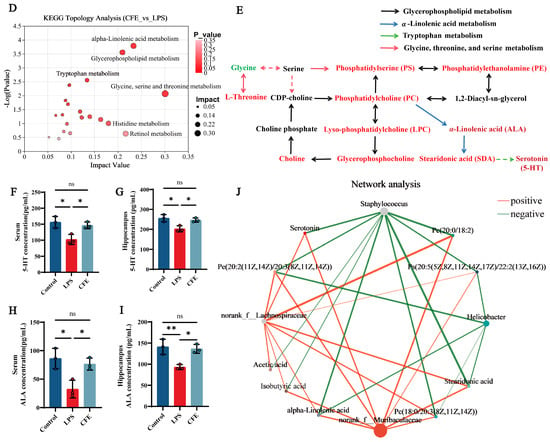
Figure 7.
CFE modulated host systemic metabolism. The colored dots represent data points from different sample groups. (A) Principal component analysis (PCA) score chart. (B) The volcano plots of significantly differential metabolic components in the CFE group compared to the LPS group (p < 0.05). (C) Hierarchical cluster analysis of differential metabolites between the CFE group and the LPS group. (D) Potential target metabolic pathways of CFE in the pretreatment of HNF mice. (E) A schematic map depicting the enriched key pathways and the targeted compounds regulated by CFE in HNF mice; Red represents the upregulation of metabolites and green represents the downregulation of metabolites. (F,G) 5-HT concentrations in serum and hippocampus (N = 3). (H,I) ALA concentrations in serum and hippocampus (N = 3). (J) Integrated analysis reveals that the gut microbiome is associated with changes in metabolites. Red edges indicate a positive correlation and green edges indicate a negative correlation. Data are presented as the means ± SEM (N = 6). Statistically significant differences were indicated: * p < 0.05, ** p < 0.01, ns p > 0.05, compared to the LPS group.
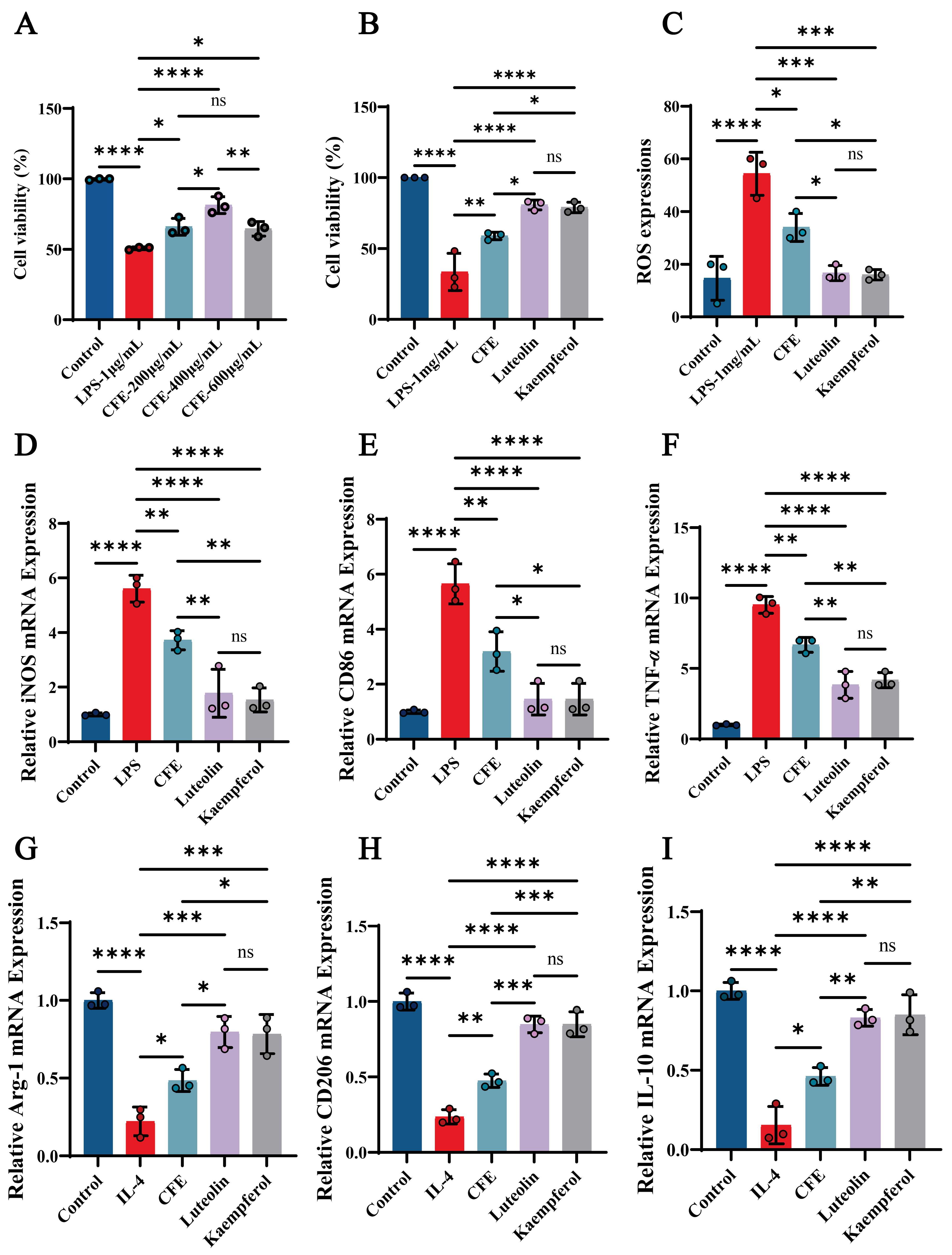
3.13. CFE, Luteolin, and Kaempferol Significantly Improved Cell Viability and Reduced ROS Levels in BV2 Cells
We evaluated the impact of CFE, luteolin and kaempferol on cell viability and ROS levels in BV2 cells. The CCK-8 assay revealed that CFE significantly attenuated LPS-induced cytotoxicity in a concentration-dependent manner, with the most pronounced improvement in BV2 cell viability observed at 400 μg/mL (p < 0.01), prompting the selection of this concentration for further experiments (Figure 8A). Both luteolin (14 μM) and kaempferol (10 μM) also enhanced BV2 cells viability (p < 0.001, Figure 8B). Moreover, CFE, luteolin, and kaempferol significantly suppressed ROS generation (Figure 8C, p < 0.05, p < 0.01, and p < 0.01, respectively). The findings indicated that CFE, Luteolin, and Kaempferol confer protection against inflammatory and oxidative stress in BV2 cells.
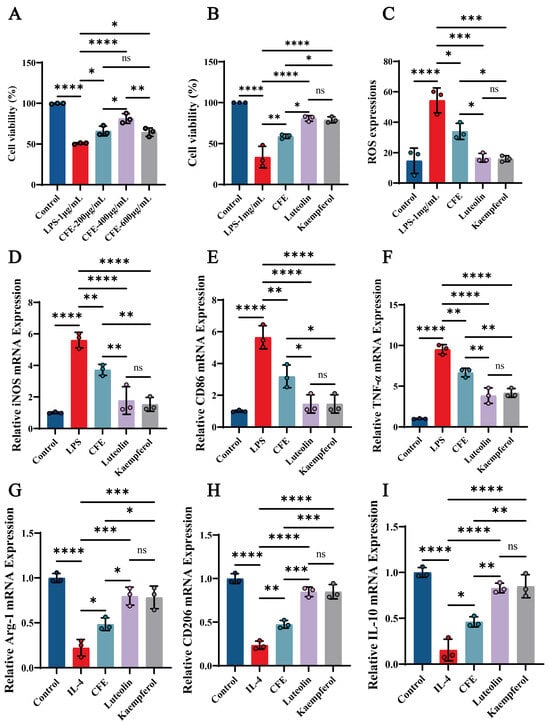
Figure 8.
CFE regulated microglial polarization in LPS-induced BV2 cells. The colored dots represent data points from different sample groups. (A) Cell viability assessed by CCK-8 assay following pretreatment with increasing concentrations of CFE. (B) Cell viability measured by CCK-8 after pretreatment with CFE, luteolin or kaempferol. (C) Quantitative analysis of intracellular ROS levels. (D–F) mRNA expression of M1 polarization markers (iNOS, CD86, TNF-α) determined by qPCR. (G–I) mRNA expression of M2 polarization markers (Arg-1, CD206, IL-10) determined by qPCR. Data are presented as the means ± SEM (N = 3). Statistically significant differences were indicated: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns p > 0.05, compared to the LPS group.
3.14. CFE, Luteolin and Kaempferol Suppressed M1 Polarization and Promoted M2 Polarization in BV2 Microglial Cells
RT-qPCR analysis showed that CFE, luteolin and kaempferol significantly suppressed M1 marker expression (Figure 8D–F) while elevating M2 marker expression (Figure 8G–I). Specifically, CFE downregulated iNOS, CD86, and TNF-α (all p < 0.05) while concurrently upregulating Arg-1, CD206, and IL-10 (all p < 0.05). Luteolin significantly decreased iNOS (p < 0.01), CD86 (p < 0.001), and TNF-α (p < 0.01), and increased Arg-1 (p < 0.01), CD206 (p < 0.001), and IL-10 (p < 0.01). Kaempferol downregulated iNOS (p < 0.01), CD86 (p < 0.001), and TNF-α (p < 0.01), and upregulated Arg-1 (p < 0.01), CD206 (p < 0.001), and IL-10 (p < 0.001). Compared to the CFE group, the Luteolin and Kaempferol groups demonstrated superior regulatory effects on M1/M2 polarization of BV2 microglial cells. The results showed that the CFE, Luteolin and Kaempferol attenuated macrophage inflammatory responses in BV2 macrophages by suppressing M1 polarization and promoting M2 polarization.
4. Discussion
The rising incidence of cognitive decline, which increasingly affects younger demographics and severely compromises quality of life, identifies HNF as a central driver in its pathogenesis and progression [46,47,48]. However, the development of therapeutics specifically targeting HNF remains limited. In this context, the growing recognition of plant-derived natural products as potential treatments for neurological disorders has opened a new avenue for HNF intervention [26,49]. Our study demonstrated that CFE confers significant protection against cognitive decline in a mouse model of HNF. This protective efficacy is underpinned by a multi-faceted mechanism involving direct anti-neuroinflammatory action, remodeling of the gut microbiota and associated metabolites, modulation of microglial polarization, and subsequent activation of the BDNF/5-HT pathway. Phytochemical analysis further revealed that CFE possesses a rich flavonoid profile, with luteolin and kaempferol identified as the predominant constituents, which are considered primary contributors to its observed therapeutic effects.
Our initial experiments used in vitro models to assess the neuroprotective and antioxidant potential of CFE. Pretreatment with CFE demonstrated substantial efficacy in protecting HT22 cells against HNF, with the solvent C extract (chloroform-methanol, 6:4, v/v) exhibiting the strongest activity and therefore being selected for subsequent investigation. The marked recovery in cell viability and pronounced reduction in ROS levels confirmed that CFE confers protection against the intertwined pathologies of inflammatory and oxidative stress in HNF [50]. To identify the active constituents responsible for these effects, we characterized the chemical composition of CFE. HPLC-MS analysis revealed a complex profile with multiple identified compounds. Based on their established bioavailability, BBB permeability, and documented anti-inflammatory and neuroimmunomodulatory properties [51], flavonoids were prioritized as the likely active fraction. Among these, luteolin and kaempferol were the most abundant, quantified at concentrations of 10,796.37 ng/mL and 3082.77 ng/mL, respectively.
The neuroprotective potential of CFE observed in cellular models was further validated in a mouse model of LPS-induced HNF and cognitive decline. As reported in previous studies [52], HNF mice in our model exhibited significant cognitive impairment. Following CFE intervention, behavioral performance improved notably: in the Morris water maze test, increased platform crossings, longer time spent, and greater distance traveled in the target quadrant indicated enhanced spatial learning and memory. In the novel object recognition test, an elevated recognition index reflected improved exploratory behavior and discrimination of novel objects. These behavioral benefits were accompanied by amelioration of hippocampal histopathology. CFE intervention effectively reversed key LPS-induced structural alterations in the CA1 region, including disorganized neuronal arrangement, widened intercellular spaces, nucleolar condensation, and significant reductions in neuronal density and eosinophilic proportion. Together, these histopathological findings demonstrate that CFE effectively preserves hippocampal structural integrity under neuroinflammatory conditions.
As key pathological drivers in HNF, both the accumulation of Aβ1–42 and the aberrant hyperphosphorylation of Tau protein are directly promoted by the inflammatory process. Consequently, strategies aimed at inhibiting Aβ1–42 accumulation and P-Tau formation, while mitigating their neurotoxicity, are crucial for preventing and treating HNF [5]. Accumulating evidence suggests that certain plant-derived natural products and their bioactive components can counteract HNF by suppressing Aβ1–42 aggregation and Tau hyperphosphorylation. For instance, Scrophularia buergeriana extract was shown to reduce Aβ1–42 deposition and Tau hyperphosphorylation in a mouse model of cognitive decline [53]. In line with this, our data from IHC and Western blot analyses demonstrated that CFE significantly suppressed the expression of Aβ1–42, the kinase GSK-3β, total Tau, and Tau phosphorylated at Ser404. GSK-3β is a pivotal kinase that, upon activation, potently drives Tau phosphorylation, leading to neurofibrillary tangle formation and subsequent neuronal dysfunction [7]. Our results suggest that CFE reduces the expression of Aβ1–42 and GSK-3β, thereby inhibiting p-Tau (Ser404) formation by blocking Aβ1–42-mediated aggregation. This mechanism likely contributes to the protection of neuronal and synaptic structure, ultimately improving HNF and cognitive decline, which is consistent with previous research [54].
Synaptic plasticity, which underlies learning and memory, depends critically on neurotrophic support and the maintenance of neuronal structural integrity; thus, enhancing synaptic function represents a promising therapeutic strategy for HNF [55]. BDNF acts as a master regulator of neuronal survival, differentiation, and synaptic function, and its expression in the hippocampus is particularly vital for cognitive processes [56]. Similarly, MAP2 is essential for dendritic arborization and stability, which are fundamental for synaptic connectivity and information integration [57]. Previous studies have shown that luteolin significantly restored the BDNF-TrkB signaling axis and increased MAP2 protein levels, thereby attenuating HNF in mice [58]. In the present study, we found that CFE significantly upregulated hippocampal levels of both BDNF and MAP2, indicating its role in promoting a neuroplastic milieu. This enhancement of neurotrophic signaling and structural support likely represents a key mechanism through which CFE facilitates the recovery of cognitive function, as observed in our behavioral tests.
The neuroprotective and synaptoprotective effects of CFE were accompanied by a marked suppression of HNF. Neuroinflammatory responses are typically driven by aberrant activation of glial cells [59]. Pathological activation of microglia and astrocytes triggers the release of pro-inflammatory cytokines, ultimately contributing to neuronal damage and synaptic loss [14]. In our study, CFE significantly reduced both the protein and mRNA levels of key pro-inflammatory cytokines in the hippocampus and in systemic circulation. Furthermore, CFE markedly downregulated hippocampal expression of the surface receptor CD11b, a marker of microglial activation that is upregulated during HNF and contributes to pro-inflammatory signaling [4]. Immunofluorescence analysis further demonstrated that CFE reduced the activation of both microglia (Iba1+) and astrocytes (GFAP+), indicating that CFE exerts an anti-inflammatory effect on hippocampal glial cells.
The microbiota-gut-brain axis functions as a key bidirectional communication network, linking the gut microbiota with the central nervous system and maintaining a dynamic equilibrium among the gut, brain, and microbial communities [60]. Homeostasis of the gut microbiota, characterized by balanced composition and diversity, is essential for overall health [61]. Studies on the therapeutic application of natural products have shown that plant-derived floral extracts can modulate intestinal microecology and exert prebiotic effects. For example, flavonoids from Dendrobium officinale flowers alleviated cognitive decline by reshaping the gut microbiota and suppressing microglial activation, which in turn upregulated BDNF and synaptic proteins and improved synaptic plasticity [49]. In the present study, 16S rRNA sequencing revealed that CFE effectively counteracted LPS-induced gut dysbiosis. It significantly increased the abundance of beneficial bacterial families with known SCFAs-producing capabilities, such as Muribaculaceae and Lachnospiraceae, while suppressing potential pathobionts like Staphylococcus and Helicobacter. These microbial changes are functionally important: Muribaculaceae and Lachnospiraceae are consistently associated with anti-inflammatory activity, gut barrier integrity, and SCFAs production [62,63], whereas Staphylococcus and Helicobacter have been positively correlated with systemic inflammation, increased gut permeability, and risk for hippocampal neuroinflammation and cognitive decline [64,65]. These results confirm that CFE can restore intestinal microbial health by modulating gut flora structure. The regulatory influence of gut microbiota metabolites on the gut-brain axis is largely mediated by SCFAs. Produced through microbial fermentation of dietary fiber, major SCFAs-acetate, propionate, and butyrate-cross the BBB and perform critical functions such as modulating microglial activity, suppressing HNF, and supporting cognitive processes [66]. Our targeted metabolomics data showed that CFE significantly restored fecal SCFAs levels, with acetate exhibiting a particularly marked increase. This rise in SCFAs provides a mechanistic link between CFE-induced gut microbiota remodeling and the alleviation of brain pathology, likely through microglial modulation and reinforcement of the BBB.
To characterize the systemic metabolic changes induced by CFE, we performed untargeted metabolomic profiling of serum. The analysis indicated that CFE administration altered the host metabolome, upregulating key metabolites involved in glycerophospholipid metabolism, α-linolenic acid (ALA) metabolism, and tryptophan metabolism. ELISA confirmed a central finding: elevated levels of ALA and 5-HT in both serum and hippocampal tissues. ALA, a plant-derived omega-3 fatty acid, exhibits multiple neuroprotective properties, including direct inhibition of Tau aggregation, attenuation of Aβ-induced neurotoxicity, and potent anti-inflammatory and antioxidant activities [67,68]. 5-HT, a key monoamine neurotransmitter, is integral not only to regulating mood, sleep, and cognition but also to modulating neuroimmune responses, as its receptors are expressed on microglia and other immune cells [69]. A Pearson correlation-based network further reinforced the gut-brain link. It showed that the abundances of beneficial bacteria (Lachnospiraceae, Muribaculaceae) were positively correlated with ALA, 5-HT, and SCFAs, whereas pathogenic genera (Staphylococcus, Helicobacter) exhibited strong negative correlations. These results suggest that CFE facilitates a coherent gut microbiota-metabolite-brain signaling axis, whereby the restructured gut microbial community enhances the production of beneficial systemic metabolites that can cross the BBB to support brain health and function.
Luteolin treatment has been shown to shift microglial polarization toward the anti-inflammatory M2 phenotype in both in vitro and in vivo studies [17]. Kaempferol, a major flavonoid in edible plants, has been shown to modulate microglial M1/M2 polarization, thereby alleviating neuroinflammatory dyshomeostasis [18]. To determine how the phytochemical profile of CFE influences brain immunity, we examined its direct effects on microglia, the principal cellular mediators of HNF. In BV2 microglial cells, a key finding was that both CFE and its principal flavonoid constituents, luteolin and kaempferol, significantly shifted polarization from the pro-inflammatory M1 phenotype toward the anti-inflammatory M2 state. At the mRNA level, this shift was characterized by downregulation of M1 markers (iNOS, CD86, TNF-α) and upregulation of M2 markers (Arg-1, CD206, IL-10). Notably, luteolin and kaempferol alone induced these changes more potently than the complete CFE extract. These results establish a direct link between the chemical constituents of CFE and the suppression of HNF and glial activation in vitro. We conclude that luteolin and kaempferol mediate the effects of CFE by driving microglial phenotype switching, which in turn disrupts persistent neuroinflammatory signaling and helps establish a microenvironment conducive to neural repair and plasticity.
5. Conclusions
In summary, this study demonstrates that CFE is a promising therapeutic candidate for mitigating HNF and cognitive decline. The protective efficacy of CFE is mediated through a multi-level mechanism: it provides direct neuroprotection against oxidative and inflammatory stress; inhibits core neuropathology by reducing Aβ1–42 and P-Tau levels; promotes neuroplasticity via upregulation of BDNF and MAP2; attenuates neuroinflammation by suppressing microglial and astrocyte activation while modulating cytokine networks; remodels the microbiota-gut-brain axis to enhance production of beneficial metabolites such as SCFAs, ALA and 5-HT; and facilitates microglial polarization toward the anti-inflammatory M2 phenotype, an effect likely attributable to its flavonoid constituents, especially luteolin and kaempferol. Collectively, these findings validate the neuroprotective role of CFE via the microbiota-gut-brain axis and provide novel perspectives for preventing and managing cognitive decline with natural compounds. A limitation of the current study lies in its reliance on animal models, which may not fully recapitulate human disease. Thus, these promising preclinical results warrant further validation in clinical trials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17243958/s1, Table S1: Primer sequence.
Author Contributions
Conceptualization, H.G. and H.H.; methodology, H.G. and L.L.; software, Y.L.; validation, W.L., Y.L. and X.L.; formal analysis, W.L.; investigation, Y.L.; resources, H.G. and B.Z.; data curation, W.L.; writing—original draft preparation, W.L.; writing—review and editing, Y.L.; visualization, H.H.; supervision, Y.L.; project administration, H.G.; funding acquisition, H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China, grant number 2022YFD1600404-04; the National Natural Science Foundation of China, grant number 32201951; the National Center of Technology Innovation for Dairy, grant number 2024-QNJJ-014.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Experimentation Ethics Committee of China Agricultural University (Protocol Aw71705202-6-04, 17 July 2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
We permit unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Sequencing data generated in this study were deposited in the Sequence Read Archive (SRA) (https://www.ncbi.nlm.nih.gov/sra/, accessed on 19 November 2025) with the BioProject ID: PRJNA1366079 and MTBLS12026.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HNF | Hippocampal Neuroinflammation |
| CFE | Corylus heterophylla Fisch. Male Flower Extract |
| LPS | Lipopolysaccharide |
| Aβ1–42 | Amyloid-beta 1–42 |
| P-Tau | Phosphorylated Tau Protein |
| GSK-3β | Glycogen Synthase Kinase-3β |
| IL-33 | Interleukin-33 |
| BDNF | Brain-derived Neurotrophic Factor |
| MAP2 | Microtubule-associated Protein 2 |
| ALA | α-linolenic Acid |
| 5-HT | Serotonin |
| NFTs | Neurofibrillary Tangles |
| BBB | Blood-Brain Barrier |
| CD11b | Cluster of Differentiation 11b |
| ROS | Reactive Oxygen Species |
| SCFAs | Short-chain Fatty Acids |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
| P/S | Penicillin-streptomycin |
| CA1 | Cornu Ammonis 1 |
| OTUs | Operational Taxonomic Units |
| DCFH-DA | 2′,7′-dichlorofluorescin Diacetate |
References
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive deficits in people who have recovered from COVID-19. eClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.B.; Lee, H.; Ji, M.G.; Ngo Hoang, L.; Lee, S.J. The synergistic extract of Zophobas atratus and Tenebrio molitor regulates neuroplasticity and oxidative stress in a scopolamine-induced cognitive impairment model. Front. Aging Neurosci. 2025, 17, 1566621. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflamm. 2008, 5, 37. [Google Scholar] [CrossRef]
- Thapa, A.; Jett, S.D.; Chi, E.Y. Curcumin Attenuates Amyloid-β Aggregate Toxicity and Modulates Amyloid-β Aggregation Pathway. ACS Chem. Neurosci. 2016, 7, 56–68. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, Z.; Wang, D.; Xiao, J. Glycogen synthase kinase-3 as a key regulator of cognitive function. Acta Biochim. Biophys. Sin. 2020, 52, 219–230. [Google Scholar] [CrossRef]
- Decandia, D.; Gelfo, F.; Landolfo, E.; Balsamo, F.; Petrosini, L.; Cutuli, D. Dietary Protection against Cognitive Impairment, Neuroinflammation and Oxidative Stress in Alzheimer’s Disease Animal Models of Lipopolysaccharide-Induced Inflammation. Int. J. Mol. Sci. 2023, 24, 5921. [Google Scholar] [CrossRef]
- Fu, Y.; Jie, J.; Lei, L.; Liu, M.; Wang, J.; Lei, L.; Liu, H. Exploring the destructive synergy between IL-33 and Suilysin hemolysis on blood-brain barrier stability. Microbiol. Spectr. 2024, 12, e0061224. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Peng, F.; Cheng, Z.; Su, J.; Song, J.; Han, X.; Li, R.; Li, X.; Cui, R.; Li, B. Astrocytic FABP7 Alleviates Depression-Like Behaviors of Chronic Unpredictable Mild Stress Mice by Regulating Neuroinflammation and Hippocampal Spinogenesis. FASEB J 2025, 39, e70606. [Google Scholar] [CrossRef] [PubMed]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, R.A.; Ghosh, P.; Pesapane, A.; Taktaz, F.; Puocci, A.; Franzese, M.; Feliciano, M.F.; Tortorella, G.; Scisciola, L.; Sommella, E.; et al. Tirzepatide prevents neurodegeneration through multiple molecular pathways. J. Transl. Med. 2024, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Zuo, Z.-F.; Meng, L.; Yang, Q.; Lv, P.; Zhao, L.-P.; Wang, X.-B.; Wang, Y.-F.; Huang, Y.; Fu, C.; et al. Neuroprotective effect of salidroside on hippocampal neurons in diabetic mice via PI3K/Akt/GSK-3β signaling pathway. Psychopharmacology 2023, 240, 1865–1876. [Google Scholar] [CrossRef]
- Barbati, S.A.; D’Amelio, C.; Feroleto, C.; Morotti, M.; Sarrapochiello, I.N.; Natale, F.; Puma, D.D.L.; Gomez-Galvez, Y.; Blanco-Suarez, E.; Iacovitti, L.; et al. Intranasal delivery of extracellular vesicles derived from human bone marrow mesenchymal stem cells dampens neuroinflammation and ameliorates motor deficits in a mouse model of cortical stroke. Exp. Neurol. 2025, 396, 115540. [Google Scholar] [CrossRef]
- Nam, H.Y.; Nam, J.H.; Yoon, G.; Lee, J.-Y.; Nam, Y.; Kang, H.-J.; Cho, H.-J.; Kim, J.; Hoe, H.-S. Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J. Neuroinflamm. 2018, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Tang, S.; Feng, W.; Li, Z.; Liu, X.; Cheng, C.; Wei, F.; Ding, G. Dental pulp stem cells promote the recovery of spinal cord injury by regulating microglia polarization via JAK2/STAT3 signaling pathway. Int. Immunopharmacol. 2025, 164, 115395. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Chen, J.; Xu, S.; Luo, Y. Luteolin Mitigates Dopaminergic Neuron Degeneration and Restrains Microglial M1 Polarization by Inhibiting Toll Like Receptor 4. J. Integr. Neurosci. 2024, 23, 185. [Google Scholar] [CrossRef]
- Su, P.; Liu, L.; Gong, Y.; Peng, S.; Yan, X.; Bai, M.; Xu, E.; Li, Y. Kaempferol improves depression-like behaviors through shifting microglia polarization and suppressing NLRP3 via tilting the balance of PPARγ and STAT1 signaling. Int. Immunopharmacol. 2024, 143, 113538. [Google Scholar] [CrossRef]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer’s disease mouse model via regulating the gut microbiota-brain axis. Brain Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef]
- Zeyneb, H.; Song, Y.; Wang, L.; Zheng, J.; Wang, W.; Pei, H.; Cao, X. Preventive effect of quinoa polysaccharides on lipopolysaccharide-induced inflammation in mice through gut microbiota regulation. Int. J. Biol. Macromol. 2025, 307, 141899. [Google Scholar] [CrossRef]
- Izzy, S.; Yahya, T.; Albastaki, O.; Cao, T.; Schwerdtfeger, L.A.; Abou-El-Hassan, H.; Chopra, K.; Ekwudo, M.N.; Kurdeikaite, U.; Verissimo, I.M.; et al. High-salt diet induces microbiome dysregulation, neuroinflammation and anxiety in the chronic period after mild repetitive closed head injury in adolescent mice. Brain Commun. 2024, 6, fcae147. [Google Scholar] [CrossRef]
- Zou, J.; Gao, J.; Shang, W.; Fan, X. Minocycline Ameliorates Staphylococcus aureus-Induced Neuroinflammation and Anxiety-like Behaviors by Regulating the TLR2 and STAT3 Pathways in Microglia. Brain Sci. 2025, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Wei, F.; Wang, Y.D.; Liu, J.; Xu, B.L.; Ma, S.C.; Yang, J.B. Ginseng and Polygonum multiflorum formula protects brain function in Alzheimer’s disease. Front. Pharmacol. 2025, 16, 1461177. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Gupta, D.P.; Park, S.H.; Lee, Y.S.; Lee, S.; Lim, S.; Byun, J.; Cho, I.H.; Song, G.J. Daphne genkwa flower extract promotes the neuroprotective effects of microglia. Phytomedicine 2023, 108, 154486. [Google Scholar] [CrossRef]
- Zhou, H.R.; Wu, J.R.; Bei, L.; Wang, B.X.; Xu, H.; Wang, J.T.; Ma, S.X. Hydroalcoholic extract from Abelmoschus manihot (Linn.) Medicus flower reverses sleep deprivation-evoked learning and memory deficit. Food Funct. 2020, 11, 8978–8986. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Ekundayo, T.C.; Okaiyeto, K.; Olaniran, A.O. Hibiscus sabdariffa (Roselle) calyx: A systematic and meta-analytic review of memory-enhancing, anti-neuroinflammatory and antioxidative activities. Inflammopharmacology 2023, 31, 231–240. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, M.; Wu, T.; Fang, L.; Liu, C.; Min, W. Novel anti-obesity peptide (RLLPH) derived from hazelnut (Corylus heterophylla Fisch) protein hydrolysates inhibits adipogenesis in 3T3-L1 adipocytes by regulating adipogenic transcription factors and adenosine monophosphate-activated protein kinase (AMPK) activation. J. Biosci. Bioeng. 2020, 129, 259–268. [Google Scholar] [CrossRef]
- Zhao, T.; Ma, W.; Yang, Z.; Liang, L.; Chen, X.; Wang, G.; Ma, Q.; Wang, L. A chromosome-level reference genome of the hazelnut, Corylus heterophylla Fisch. GigaScience 2021, 10, giab027. [Google Scholar] [CrossRef]
- Talebi, S.; Khodagholi, F.; Bahaeddin, Z.; Ansari Dezfouli, M.; Zeinaddini-Meymand, A.; Berchi Kankam, S.; Foolad, F.; Alijaniha, F.; Fayazi Piranghar, F. Does hazelnut consumption affect brain health and function against neurodegenerative diseases? Nutr. Neurosci. 2024, 27, 1008–1024. [Google Scholar] [CrossRef]
- Nikolaieva, N.; Kačániová, M.; González, J.C.; Grygorieva, O.; Nôžková, J. Determination of microbiological contamination, antibacterial and antioxidant activities of natural plant hazelnut (Corylus avellana L.) pollen. J. Environ. Sci. Health B 2019, 54, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Gao, J. Study on Chemical Constituents and Hepatoprotective Activity of Male Flowers of Corylus heterophylla. Master’s Thesis, Yanbian University, Yanji, China, 2018. [Google Scholar] [CrossRef]
- Wu, S.B.; Pu, H.; Cao, Z.D.; Zhang, W.; Qi, Q.Q. Therapeutic Effect of Total Flavones from Hazel’s Flower on Chronic Ischemic Nephropathy Rats Models. Mod. J. Integr. Tradit. Chin. West. Med. 2012, 21, 1164–1166. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; Abdel-Baki, P.M.; El-Rashedy, A.A.; Mahdy, N.E. LC-MS/MS profiling of Tipuana tipu flower, HPLC-DAD quantification of its bioactive components, and interrelationships with antioxidant, and anti-inflammatory activity: In vitro and in silico approaches. BMC Complement. Med. Ther. 2024, 24, 176. [Google Scholar] [CrossRef]
- Akkol, E.K.; Süntar, I.; Keles, H.; Yesilada, E. The potential role of female flowers inflorescence of Typha domingensis Pers. in wound management. J. Ethnopharmacol. 2011, 133, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Alleviates Acute Sleep Deprivation-Induced Memory Loss in Mice by Suppressing Hippocampal Ferroptosis. Front. Pharmacol. 2021, 12, 708645. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, H.T. Ethanolic extract of Sophora japonica flower buds alleviates cognitive deficits induced by scopolamine in mice. Orient. Pharm. Exp. Med. 2017, 17, 337–344. [Google Scholar] [CrossRef]
- Jiao, Q.; Dong, X.; Guo, C.; Wu, T.; Chen, F.; Zhang, K.; Ma, Z.; Sun, Y.; Cao, H.; Tian, C.; et al. Effects of sleep deprivation of various durations on novelty-related object recognition memory and object location memory in mice. Behav. Brain Res. 2022, 418, 113621. [Google Scholar] [CrossRef]
- Hilvering, B.; Koenderman, L. Quality over quantity; eosinophil activation status will deepen the insight into eosinophilic diseases. Respir. Med. 2023, 207, 107094. [Google Scholar] [CrossRef]
- Park, S.H.; Jang, S.; An, J.E.; Choo, B.k.; Kim, H.K. I. inflexus (Thunb.) Kudo extract improves atopic dermatitis and depressive-like behavior in DfE-induced atopic dermatitis-like disease. Phytomedicine 2020, 67, 153137. [Google Scholar] [CrossRef]
- Oliveros, E.; Ramirez, M.; Vazquez, E.; Barranco, A.; Gruart, A.; Delgado-Garcia, J.M.; Buck, R.; Rueda, R.; Martin, M.J. Oral supplementation of 2′-fucosyllactose during lactation improves memory and learning in rats. J. Nutr. Biochem. 2016, 31, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, Q.; Jiang, T.; Li, S.; Ye, J.; Zheng, J.; Wang, X.; Liu, Y.; Deng, M.; Ke, D.; et al. A novel small-molecule PROTAC selectively promotes tau clearance to improve cognitive functions in Alzheimer-like models. Theranostics 2021, 11, 5279–5295. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Yang, D.; Bai, F.; Wang, Q.; Zhang, C.; Yan, Y.; Li, Z.; Li, Y.; Chen, Z.; Li, J.; et al. Spinal cord injury-induced gut dysbiosis influences neurological recovery partly through short-chain fatty acids. npj Biofilms Microbiomes 2023, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhao, W.; Xu, Y.; Wang, Y.; Meng, X.; Xiao, H.; Zhuang, X.; Pan, C.; Mo, T.; Wang, B.; et al. Shenling Kaixin granules attenuate depression via multi-target mechanisms: Evidence from CSDS model and cellular studies. Phytomedicine 2025, 149, 157547. [Google Scholar] [CrossRef]
- Chun, C.T.; Seward, K.; Patterson, A.; Melton, A.; MacDonald-Wicks, L. Evaluation of Available Cognitive Tools Used to Measure Mild Cognitive Decline: A Scoping Review. Nutrients 2021, 13, 3974. [Google Scholar] [CrossRef]
- Fu, J.; Li, J.; Sun, Y.; Liu, S.; Song, F.; Liu, Z. In-depth investigation of the mechanisms of Schisandra chinensis polysaccharide mitigating Alzheimer’s disease rat via gut microbiota and feces metabolomics. Int. J. Biol. Macromol. 2023, 232, 123488. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Kang, Y.; Qin, S.; Chai, J. Neuroinflammation as the Underlying Mechanism of Postoperative Cognitive Dysfunction and Therapeutic Strategies. Front. Cell. Neurosci. 2022, 16, 843069. [Google Scholar] [CrossRef]
- Hu, N.; Wang, K.; Ge, X.; Zhang, X.; Zheng, X.; Sun, S. Effects of Dendrobium officinale Kimura & Migo flower flavonoids on cognitive function by regulating gut microbiota. Front. Pharmacol. 2025, 16, 1562775. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Xie, B.; Abdelgawad, A.; Chen, X.; Han, M.; Shang, Y.; Yuan, S.; Zhang, J. RhANP attenuates endotoxin-derived cognitive dysfunction through subdiaphragmatic vagus nerve-mediated gut microbiota-brain axis. J. Neuroinflamm. 2021, 18, 300. [Google Scholar] [CrossRef]
- Cheng, S.; Gao, H.; Yang, Y.; Zhang, M.; Wang, J.; Li, N.; Ji, Y.; Wang, H.; Zhou, Y. Pharmacological Diversity of Flavonoids and Their Clinical Application Prospects in Neurological Disorders. Phytother. Res. 2025, 39, 5222–5248. [Google Scholar] [CrossRef]
- Mohamed Yusof, N.I.S.; Ramasamy, K.; Mohd Fauzi, F. Elucidating the Neuroprotective Mechanism of 5,7-Dimethoxyflavone and 5,7’,4’-Trimethoxyflavone Through In Silico Target Prediction and in Memory-Impaired Mice. Neurochem. Res. 2025, 50, 274. [Google Scholar] [CrossRef]
- Kim, H.L.; Lee, S.K.; Min, D.E.; Bastola, T.; Chang, B.Y.; Bae, J.H.; Lee, D.R. Effects of Scrophularia buergeriana Extract (Brainon®) on Aging-Induced Memory Impairment in SAMP8 Mice. Curr. Issues Mol. Biol. 2023, 45, 1287–1305. [Google Scholar] [CrossRef]
- Gao, H.; Fang, B.; Sun, Z.; Du, X.; Guo, H.; Zhao, L.; Zhang, M. Effect of Human Milk Oligosaccharides on Learning and Memory in Mice with Alzheimer’s Disease. J. Agric. Food Chem. 2024, 72, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Peng, J.; Wang, H.; Wang, L.; Su, Y.; Ding, L.; Cao, B.; Zhao, Y.; Xing, Q.; Yang, J.J. Modulating neuroinflammation and cognitive function in postoperative cognitive dysfunction via CCR5-GPCRs-Ras-MAPK pathway targeting with microglial EVs. CNS Neurosci. Ther. 2024, 30, e14924. [Google Scholar] [CrossRef]
- Kang, E.J.; Cha, M.G.; Kwon, G.H.; Han, S.H.; Yoon, S.J.; Lee, S.K.; Ahn, M.E.; Won, S.M.; Ahn, E.H.; Suk, K.T. Akkermansia muciniphila improve cognitive dysfunction by regulating BDNF and serotonin pathway in gut-liver-brain axis. Microbiome 2024, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; DeMarco, A.G.; Sweet, R.A.; Grubisha, M.J. MAP2 phosphorylation: Mechanisms, functional consequences, and emerging insights. Front. Cell. Neurosci. 2025, 19, 1610371. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zeng, L.; Zhou, Y.; Yang, C.; Sheng, R.; Liu, X.; Wen, W.; Yang, S.; Zhang, S.; Xu, S. Ethyl Acetate Fraction of Zanthoxylum bungeanum Ameliorates Cognitive Deficit by Facilitating the PHGDH-Dependent Astrocyte-Neuron Serine Shuttle in Aging Mice. J. Agric. Food Chem. 2025, 73, 16301–16316. [Google Scholar] [CrossRef]
- Ge, T.; Chen, X.; Liu, C.; Huang, S. Involvement of CXCR2 in chronic postsurgical pain occurrence through ERK/p38 activation. Neurosci. Lett. 2025, 870, 138440. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Sharma, V.K. Microbiota-Derived SCFAs in Multiple Sclerosis: From Immune Priming to Neurodegeneration. Mol. Neurobiol. 2025, 63, 31. [Google Scholar] [CrossRef]
- Smith, B.J.; Miller, R.A.; Ericsson, A.C.; Harrison, D.C.; Strong, R.; Schmidt, T.M. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019, 19, 130. [Google Scholar] [CrossRef]
- Gu, X.; Fan, M.; Zhou, Y.; Zhang, Y.; Wang, L.; Gao, W.; Li, T.; Wang, H.; Si, N.; Wei, X.; et al. Intestinal endogenous metabolites affect neuroinflammation in 5×FAD mice by mediating “gut-brain” axis and the intervention with Chinese Medicine. Alzheimer’s Res. Ther. 2024, 16, 222. [Google Scholar] [CrossRef]
- Zou, J.; Shang, W.; Yang, L.; Liu, T.; Wang, L.; Li, X.; Zhao, J.; Rao, X.; Gao, J.; Fan, X. Microglia activation in the mPFC mediates anxiety-like behaviors caused by Staphylococcus aureus strain USA300. Brain Behav. 2022, 12, e2715. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Han, L.; Wang, B.; Shi, R.; Ye, J.; Xia, B.; Zhao, Z.; Zhao, B.; Liu, X. Leucine-Restricted Diet Ameliorates Obesity-Linked Cognitive Deficits: Involvement of the Microbiota–Gut–Brain Axis. J. Agric. Food Chem. 2023, 71, 9404–9418. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, H.; Ju, Y.; Liu, J.; Wang, M.; Liu, B.; Zhang, Y. Gut microbiota-derived short-chain fatty acids and depression: Deep insight into biological mechanisms and potential applications. Gen. Psychiatr. 2024, 37, e101374. [Google Scholar] [CrossRef]
- Desale, S.E.; Dubey, T.; Chinnathambi, S. α-Linolenic acid inhibits Tau aggregation and modulates Tau conformation. Int. J. Biol. Macromol. 2021, 166, 687–693. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Shen, W.; Liu, J.; Liu, Y.; Li, Y.; Zhang, F.; Li, T.; Zhang, X.; Tian, W.; et al. α-linolenic acid mitigates microglia-mediated neuroinflammation of schizophrenia in mice by suppressing the NF-κB/NLRP3 pathway via binding GPR120-β-arrestin 2. Int. Immunopharmacol. 2024, 142, 113047. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Yang, J.J.; Zhao, D.M.; Chen, B.; Zhang, G.Q.; Chen, S.; Cao, R.F.; Yu, H.; Zhao, C.Y.; et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol. Res. 2020, 157, 104784. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).