Abstract
Background: Neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease, are among the leading causes of disability and mortality worldwide. Dietary patterns have emerged as modifiable risk factors that may influence disease onset and progression. The Mediterranean diet (MedDiet), rich in fruits, vegetables, whole grains, legumes, fish, and extra virgin olive oil, has been consistently associated with better cognitive outcomes and reduced risk of neurodegeneration. Aim: This narrative review summarizes current evidence on the role of the MedDiet in slowing the progression of neurodegenerative diseases, with a particular focus on polyphenols such as resveratrol and oleuropein as key bioactive mediators. Methods: We synthesized findings from epidemiological studies, clinical trials, and mechanistic research to provide an integrated overview of how adherence to the MedDiet and its polyphenol components affects neurodegenerative disease trajectories. Results: Epidemiological studies suggest that higher MedDiet adherence is associated with slower cognitive decline, reduced conversion from mild cognitive impairment to Alzheimer’s disease, and better motor and non-motor outcomes in Parkinson’s disease. Mechanistically, the MedDiet modulates oxidative stress, neuroinflammation, mitochondrial function, vascular health, and the gut–brain axis. Polyphenols such as resveratrol and oleuropein exert neuroprotective effects through antioxidant activity, modulation of amyloid aggregation, mitochondrial biogenesis, and activation of signaling pathways (e.g., SIRT1). Clinical studies, although limited, indicate beneficial effects of polyphenol-rich interventions on cognitive and metabolic biomarkers. Conclusions: Current evidence supports the Mediterranean diet as a promising dietary strategy to slow the progression of neurodegenerative diseases. Polyphenols, including resveratrol and oleuropein, may play a role in mediating these effects. Further well-designed, long-term clinical trials are needed to establish causal relationships, optimize dosage, and explore biomarker-driven personalized nutrition approaches.
1. Introduction
Neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease, are among the most pressing public health challenges worldwide [1,2,3,4,5]. Their prevalence increases steeply with age, and they contribute substantially to disability, loss of independence, and premature mortality [2]. Although pharmacological therapies continue to advance, current treatments have only modest effects on slowing disease progression, and none provide meaningful disease modification [6,7,8,9,10]. As a result, there is growing emphasis on identifying preventive and adjunctive strategies that may preserve cognitive function and delay neurodegenerative processes across the lifespan [11,12,13,14].
Dietary patterns have emerged as one of the most influential and modifiable determinants of brain aging [3,15,16,17,18,19,20,21,22,23,24,25]. Among them, the Mediterranean diet (MedDiet) has received particular attention [3,15,17,19,20,23,26,27]. Characterized by high intake of fruits, vegetables, legumes, whole grains, nuts, fish, and extra virgin olive oil; moderate consumption of dairy products and wine; and low intake of red and processed meats, the MedDiet represents a nutrient-dense, anti-inflammatory, and metabolically favorable dietary pattern [14,27,28,29,30,31,32,33,34,35,36]. Numerous epidemiological and interventional studies have linked the MedDiet to improved cardiovascular and metabolic health and to better cognitive performance in older adults [15,27,29,37,38,39,40,41,42].
Within the MedDiet, polyphenols have been proposed as key contributors to neuroprotection [13]. Among these, resveratrol—abundant in grapes, berries and red wine [43,44,45]—and oleuropein—characteristic of olives and olive oil [46,47]—have attracted particular interest. Experimental and clinical studies suggest that these compounds exert antioxidant [48,49,50,51,52,53], anti-inflammatory [43,54,55,56,57], and neuroprotective effects, influence mitochondrial function [58,59,60,61,62], and modulate protein aggregation pathways relevant to Alzheimer’s and Parkinson’s disease [63,64,65]. They also activate key signaling cascades involved in cellular stress resistance and brain aging, including SIRT1 [66,67,68,69,70,71].
The aim of this narrative review is to summarize current evidence on the Mediterranean diet and the progression of neurodegenerative diseases, with particular focus on polyphenols—especially resveratrol and oleuropein—as potential mediators of these associations. By integrating epidemiological findings, clinical trial data, and mechanistic insights, the review highlights how the MedDiet may influence neurodegenerative trajectories and discusses key areas where further research is needed to refine preventive and therapeutic applications.
2. Methods
2.1. Literature Search
The literature search was conducted in the PubMed, Scopus, and Web of Science databases, covering the period from January 2010 to August 2025. The following keywords and their combinations were used: “Mediterranean diet,” “neurodegenerative diseases,” “Alzheimer’s disease,” “Parkinson’s disease,” “cognitive decline,” “polyphenols,” “resveratrol,” “oleuropein,” “oxidative stress,” and “neuroprotection.” This search initially yielded 5732 records (PubMed: 1856; Scopus: 1944; Web of Science: 1932). After removing 1324 duplicates, 4408 records remained for screening. Titles and abstracts were screened for relevance, leading to the exclusion of 4212 studies that did not meet the inclusion criteria. The full texts of 196 studies were assessed for eligibility, and 70 studies were ultimately included in the qualitative synthesis. The search was further supplemented by manual screening of references cited in relevant reviews and clinical studies.
2.2. Inclusion Criteria
Studies were considered eligible if they met the following criteria:
- Population: Human studies related to neurodegenerative diseases or cognitive decline.
- Intervention/exposure: Examination of the Mediterranean diet as a whole or its key bioactive components [e.g., resveratrol, oleuropein, other polyphenols].
- Outcomes: Incidence or progression of neurodegenerative diseases, changes in cognitive function, or biomarkers [e.g., oxidative stress, inflammatory markers, amyloid and tau pathology].
- Study types: Randomized controlled trials (RCTs), prospective and retrospective cohort studies, and cross-sectional studies, but not experimental [in vivo or in vitro] research.
2.3. Exclusion Criteria
The following were excluded:
- Publications without original data [editorials, letters, conference abstracts].
- Studies not related to neurodegenerative diseases or cognitive outcomes.
- Studies investigating isolated nutrients outside the context of the Mediterranean diet.
- Animal or other preclinical studies.
2.4. Data Extraction and Synthesis
From each eligible study, we extracted information on authorship, publication year, study population, sample size, exposure or intervention, outcomes, and key results. Evidence was synthesized narratively and grouped into the following themes:
- Epidemiological associations between the Mediterranean diet and cognitive decline or neurodegenerative diseases.
- Findings from clinical trials of Mediterranean diet-based interventions.
- Mechanistic insights focusing on polyphenols, particularly resveratrol and oleuropein.
- Remaining knowledge gaps and directions for future research.
2.5. Quality Assessment and Limitations
As a narrative review, no formal quality appraisal tool was applied. However, when interpreting the literature, we prioritized studies with strong methodological design, adequate sample size, and longer follow-up. Potential biases, heterogeneity across studies, and publication bias were taken into consideration.
2.6. Aim of the Review
The aim of this narrative review is to synthesize current evidence on how the Mediterranean diet may influence the progression of neurodegenerative diseases, with particular emphasis on the potential contributions of polyphenols such as resveratrol and oleuropein.
It is important to emphasize that focusing on resveratrol and oleuropein does not imply that these compounds are the sole or principal drivers of the Mediterranean diet’s neuroprotective effects. Dietary intake levels of both molecules are generally very low, and the concentrations used in mechanistic studies—particularly in vitro and animal models—often exceed those achievable through habitual Mediterranean dietary patterns. Their inclusion in this review reflects their status as two of the most extensively investigated bioactive constituents of the diet, rather than exclusive mediators of its benefits. Accordingly, the relevance of these mechanistic pathways to human physiology is limited by substantial dose discrepancies, and the evidence presented should be interpreted as supporting biological plausibility rather than direct proof of efficacy at dietary exposure levels. The overall neuroprotective potential of the Mediterranean diet is more likely to stem from synergistic interactions among multiple dietary components—including other olive-derived phenolics, monounsaturated fatty acids, antioxidant- and fiber-rich plant foods, and gut–brain axis-mediated metabolic effects. Therefore, the discussion of resveratrol and oleuropein serves primarily as an illustrative framework for understanding specific mechanistic pathways, without overstating their isolated importance.
3. Mechanistic Basis: How the Mediterranean Diet May Slow Neurodegeneration
The neuroprotective effects of the MedDiet arise from a constellation of biological mechanisms supported by its nutrient composition and bioactive compounds. These mechanisms provide a rationale for epidemiological observations linking the MedDiet to slower cognitive decline and reduced neurodegenerative risk. Table 1 provides a comparative overview of key dietary patterns, highlighting the nutrients and bioactive compounds that may influence neurodegenerative processes.

Table 1.
Comparative overview of Mediterranean, DASH, MIND, and Western dietary patterns.
3.1. Anti-Inflammatory Effects
Chronic low-grade inflammation is a central driver of neurodegenerative diseases [72,73,74,75], and several components of the MedDiet act to counter this process [23,76,77,78,79]. The diet’s high content of omega-3 fatty acids, olive oil phenolics, polyphenols, carotenoids, and dietary fiber contributes to lower circulating levels of proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). Additional micronutrients—including zinc, magnesium, and calcium—support immune regulation. Extra virgin olive oil provides phenolic compounds such as oleocanthal, oleacein, and oleic acid, which inhibit nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-related inflammatory pathways and platelet activation. By engaging these complementary pathways, the MedDiet reduces systemic inflammation and may thereby mitigate neuroinflammatory cascades implicated in cognitive decline and neurodegeneration [80].
3.2. Antioxidant Effects and Protection Against Protein Misfolding
Oxidative stress is a key mediator of neuronal injury, protein misfolding, and synaptic dysfunction in Alzheimer’s and Parkinson’s disease [81,82,83,84]. The MedDiet’s rich supply of polyphenols, vitamins E and C, and carotenoids enhances cellular antioxidant capacity and reduces reactive oxygen species (ROS) [85,86,87,88]. Polyphenols play a particularly important role, as their antioxidant and anti-inflammatory properties counteract protein aggregation processes linked to the formation of amyloid-β and α-synuclein deposits, which are central features of Alzheimer’s and Parkinson’s disease pathophysiology. Experimental models have demonstrated that resveratrol promotes amyloid-β degradation through proteasomal and autophagosomal pathways, increases the activity of degrading enzymes such as neprilysin, and inhibits the pathological hyperphosphorylation of tau protein. Furthermore, resveratrol directly interferes with Aβ aggregation and facilitates the formation of non-toxic conformations. Similarly, quercetin enhances AMPK activity and reduces tau hyperphosphorylation, while other flavonoids—including anthocyanins and caffeic acid derivatives—exert neuroprotective effects by attenuating oxidative stress and protein aggregation. Collectively, these mechanisms enhance neuronal resilience against toxic protein accumulation and ROS-induced damage, thereby highlighting the antioxidant components of the MedDiet as key contributors to the slowing of neurodegenerative processes [89].
3.3. Support of Mitochondrial Function
Mitochondrial dysfunction is a recognized early event in the pathogenesis of Alzheimer’s and Parkinson’s disease, contributing to impaired energy metabolism, increased oxidative stress, and heightened neuronal vulnerability [90,91,92,93,94,95,96,97,98,99]. Bioactive components of the Mediterranean diet—including resveratrol and major polyphenols from extra virgin olive oil (EVOO), such as hydroxytyrosol, oleuropein, and oleocanthal—play a crucial role in maintaining mitochondrial health [76,100,101]. These compounds activate key energy-regulating pathways, including AMP-activated protein kinase (AMPK), sirtuin-1 (SIRT1), and the mechanistic target of rapamycin (mTOR), which converge on transcriptional regulators of mitochondrial biogenesis such as peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α), nuclear respiratory factor-1 (NRF1), and mitochondrial transcription factor A (TFAM) [66,102,103,104,105,106]. Through these pathways, they stimulate the formation of new mitochondria, enhance ATP production, and help maintain mitochondrial DNA stability [59,60,61,62,67,107,108,109]. Experimental studies consistently show that EVOO polyphenols increase ATP levels, improve respiratory chain complex activity, and restore oxidative phosphorylation efficiency. In parallel, EVOO-derived phenolics reduce mitochondrial ROS generation, strengthen endogenous antioxidant defenses, and activate the Nrf2-dependent vitagene network, leading to increased expression of cytoprotective enzymes such as superoxide dismutase and catalase. By also suppressing NF-κB-mediated inflammatory signaling, they blunt both oxidative and inflammatory stress in cells of the neurovascular unit [43,58,66,105,110,111,112,113,114]—two interlinked drivers of neurodegenerative pathology. Beyond these effects, EVOO polyphenols promote mitophagy, stabilize mitochondrial membrane structure and fluidity, and support a healthy balance between mitochondrial fusion and fission. Together, these actions counteract age- and metabolism-related mitochondrial dysfunction. Because neurons depend heavily on efficient mitochondrial function, these mechanisms are likely to contribute substantially to the neuroprotective effects of the Mediterranean diet [115]. It is important to note that certain mechanistic elements presented in this section overlap with those discussed in Section 3.4, as mitochondrial and vascular processes are biologically tightly interconnected.
3.4. Improvement of Vascular and Metabolic Health
Vascular dysfunction [116,117,118,119,120,121] and metabolic impairment [23,72,122,123] are major contributors to Alzheimer’s disease and cognitive decline [124,125,126,127,128], influencing cerebral perfusion, blood–brain barrier integrity, insulin signaling, and the accumulation of amyloid and tau pathology. The MedDiet favorably modulates glucose and insulin metabolism [23,35,87], enhances endothelial and neurovascular function [129,130,131,132,133,134], and reduces the risk of atherosclerosis. These improvements support cerebral blood flow and neuronal energy supply and mitigate vascular contributions to cognitive impairment and dementia. The MedDiet’s emphasis on plant-based foods—fruits, vegetables, legumes, whole grains, and nuts—provides antioxidants, anti-inflammatory nutrients, and fiber that collectively reduce insulin resistance, endothelial dysfunction, and cardiovascular risk. Polyphenols play a particularly important role: compounds such as resveratrol, quercetin, catechins, and ellagic acid help maintain vascular homeostasis by increasing endothelial nitric oxide availability, reducing adhesion molecule expression (ICAM-1, VCAM-1), and lowering inflammatory cytokines, including TNF-α and IL-6 [61,110,111,112,114,129,135,136,137,138,139,140,141,142,143,144,145,146]. Together, these mechanisms slow vascular aging by limiting oxidative stress, reducing endothelial senescence and telomere-related damage, and dampening vascular inflammation. By preserving endothelial function, improving metabolic control, and lowering cardiometabolic risk factors, the MedDiet supports cardiovascular health and, indirectly, contributes to reducing neurodegenerative risk and progression [25,147].
Functional brain connectivity is increasingly recognized as a key mechanistic component of Alzheimer’s disease and age-related cognitive decline [148,149,150,151,152,153,154]. Neurodegeneration disrupts large-scale neural networks—particularly the default mode, frontoparietal, and hippocampal networks—leading to impaired synchronization between regions that support memory, executive function, and attention. Resting-state fMRI studies consistently show reduced connectivity within the default mode network and altered hippocampal–prefrontal coupling even in early or preclinical AD stages [148,149,151,152,153]. Emerging evidence suggests that dietary patterns such as the Mediterranean diet may influence these network-level alterations [155,156]. Interventions enriched with polyphenols have been shown to enhance hippocampal functional connectivity [157] and improve cerebrovascular responsiveness and neurovascular coupling [51]. These findings raise the possibility that diet-induced modulation of neurovascular and metabolic pathways may partly restore or preserve functional network integrity, offering a complementary mechanism through which the Mediterranean diet could slow cognitive decline.
3.5. Gut–Brain Axis and Microbiome Modulation
The Mediterranean diet, rich in fiber, prebiotic compounds, and polyphenols, promotes a favorable gut microbial profile characterized by greater microbial diversity and increased production of short-chain fatty acids (SCFAs), especially butyrate [77,158]. These metabolites exert systemic effects that extend to the central nervous system by strengthening blood–brain barrier function, modulating innate and adaptive immune responses, and supporting neuronal energy metabolism. Butyrate and propionate, in particular, reduce inflammatory signaling, promote microglial homeostasis, and enhance mitochondrial efficiency. The MedDiet is associated with a microbiota composition enriched in beneficial butyrate-producing species such as Faecalibacterium prausnitzii, Eubacterium rectale, and Roseburia spp., while reducing proinflammatory or dysbiotic taxa [77,158,159,160,161,162,163,164]. Functional microbial outputs are equally important: SCFAs generated from the fermentation of dietary fibers and complex carbohydrates enhance regulatory T cell activity, lower systemic low-grade inflammation, and attenuate neuroinflammatory processes that contribute to neurodegenerative progression. Polyphenols and other plant-derived compounds further shape microbiome composition and activity. Phenolic constituents of olive oil, nuts, and red wine are metabolized by gut bacteria into bioactive derivatives with antioxidant and neuromodulatory effects. These metabolites promote the growth of Bifidobacterium and Akkermansia species and inhibit opportunistic pathogens such as Ruminococcus gnavus, which has been linked to increased gut permeability, metabolic endotoxemia, and systemic inflammation [77]. Overall, the MedDiet exerts a dual influence on the gut–brain axis: it shifts microbiome composition toward anti-inflammatory, SCFA-producing communities and increases the generation of metabolites that support neuronal health. These interrelated effects contribute to improved neuroprotection, preserved cognitive function, and a slower trajectory of neurodegenerative processes. It is important to note that much of the mechanistic evidence comes from in vitro or animal models, where the concentrations of polyphenols—particularly resveratrol and oleuropein—often exceed levels achievable through a typical Mediterranean diet. Direct biomarker-level evidence from human studies is limited, and daily dietary intake is far lower than experimental doses. Therefore, these mechanisms should be interpreted as providing biological plausibility rather than direct proof of effects at physiologically relevant dietary levels. Taken together, these mechanisms highlight the central role of the gut–brain axis in mediating the neuroprotective effects of the Mediterranean diet, and Figure 1 summarizes these interconnected pathways.
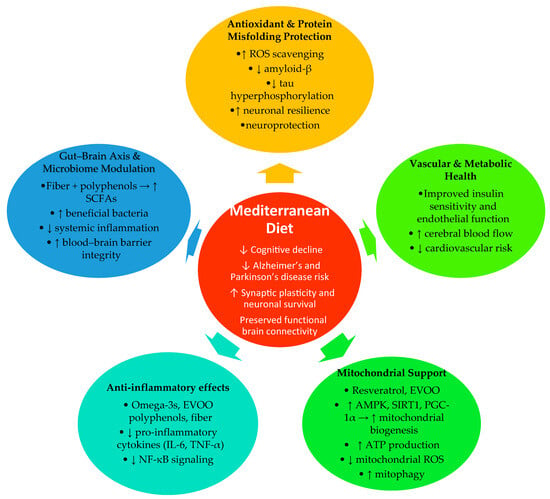
Figure 1.
Mechanistic overview: how the Mediterranean diet may slow neurodegeneration. Abbreviations EVOO—Extra Virgin Olive Oil; IL-6—Interleukin-6; TNF-α—Tumor Necrosis Factor-alpha; NF-κB—Nuclear Factor kappa-light-chain-enhancer of activated B cells; ROS—Reactive Oxygen Species; AMPK—AMP-activated Protein Kinase; SIRT1—Sirtuin-1; PGC-1α—Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 Alpha; SCFAs—Short-Chain Fatty Acids; ↑ indicates an increase in the corresponding variable; ↓ indicates a decrease in the corresponding variable.
4. Polyphenols as Key Mediators
A defining feature of the Mediterranean diet is its high content of polyphenol-rich foods. Polyphenols influence several biological pathways relevant to neurodegeneration, including oxidative stress responses, inflammatory signaling, mitochondrial function, and protein aggregation dynamics. Below, we summarize the most extensively studied representatives.
4.1. Resveratrol
Resveratrol, a stilbenoid polyphenol abundant in grapes, berries and red wine, is among the most widely studied natural compounds in the context of Alzheimer’s disease [165,166,167,168,169,170,171,172,173]. Its neuroprotective actions are mediated through multiple interconnected mechanisms [174,175,176,177,178,179,180]. Its neuroprotective effects are mediated through multiple mechanisms: it reduces ROS through antioxidant and redox-regulating activity, restores glutathione levels, and activates antioxidant enzymes; SIRT1 activation inhibits NF-κB signaling, attenuates microglial overactivation, and supports neuronal survival; additionally, it modulates the AMPK/PGC-1α/mTOR pathways, enhances mitochondrial biogenesis, and optimizes ATP production. Resveratrol also reduces amyloid-β aggregation, mitigates tau hyperphosphorylation, regulates Cu2+, Zn2+, and Fe2+ homeostasis, and stimulates the expression of neurotrophic factors such as BDNF, NGF, and NT-3. Its anti-inflammatory effects are mediated through inhibition of MAPK and STAT1/3 signaling and suppression of iNOS and COX-2, while additional mechanisms include the induction of autophagy, activation of the Nrf2/HO-1/NQO1 pathway, and reduction in cholinesterase activity [71].
In cellular models, resveratrol attenuates Aβ1-42-induced mitochondrial damage and oxidative stress, promotes PGC-1α deacetylation via AMPK-dependent mechanisms, and activates the SIRT1 pathway, which inhibits NF-κB signaling, reduces proinflammatory cytokine (TNF-α, IL-1β, IL-6) production, and protects against microglial overactivation [181,182]. In animal studies, resveratrol has demonstrated multiple neuroprotective effects, including reduction in Aβ accumulation, decreased lipid peroxidation, enhanced expression of antioxidant enzymes, and improvement of spatial memory [183,184,185]. Furthermore, it stimulates the expression of neurotrophic factors in the nervous system, such as BDNF, NGF, and NT-3, promoting neurogenesis, neuronal survival, and cognitive function [186,187].
Although clinical studies have been limited by small sample sizes and short durations, they suggest that resveratrol can cross the blood–brain barrier and favorably modulate oxidative stress and inflammatory biomarkers, while potentially reducing Aβ accumulation [188,189,190]. Some studies, however, reported adverse effects such as weight loss, nausea, or diarrhea. Confirmation of long-term cognitive benefits requires further investigation. Due to its low bioavailability, pharmacokinetics, drug interactions, and formulation are critical factors for the clinical efficacy of resveratrol.
4.2. Olive Oil Polyphenols and Their Neuroprotective Effects
The extra virgin olive oil (EVOO) contains several potent phenolic compounds, most notably oleuropein (OLE), hydroxytyrosol (HT), and oleocanthal (OLC) [191]. These molecules exhibit strong antioxidant and anti-inflammatory properties and influence multiple pathways implicated in neurodegeneration [192,193]. Preclinical studies show that HT and OLE aglycone can cross the blood–brain barrier, while OLC enhances Aβ clearance and reduces neurotoxic aggregation [11,194,195].
Mechanistically, EVOO polyphenols activate the Nrf2/ARE antioxidant signaling pathway, increase expression of neurotrophic factors (BDNF, NGF), inhibit inflammatory mediators (NF-κB, iNOS, COX-2), attenuate mitochondrial dysfunction, and prevent apoptosis in oxidative stress-induced injury models [64]. These mechanisms parallel, and in some cases complement, those observed for resveratrol.
In vitro and in vivo studies across models of Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis consistently demonstrate reduced oxidative stress, less neuronal damage, and decreased inflammation associated with these compounds [64]. Clinical data—including findings from PREDIMED [196] and the MICOIL pilot study [197]—suggest that high-polyphenol EVOO improves cognitive performance, reduces blood–brain barrier permeability, lowers neurotoxic Aβ levels, and may slow cognitive decline. These results support the concept that regular EVOO consumption meaningfully contributes to the neuroprotective profile of the Mediterranean diet.
4.3. Other Polyphenols and Bioactive Compounds in the Mediterranean Diet
Beyond olive oil and resveratrol, the Mediterranean diet incorporates a wide array of other polyphenol-rich foods, including citrus fruits, berries, pomegranate, grapes, spices containing curcumin, and green tea rich in catechins [198,199]. These compounds exhibit antioxidant and anti-inflammatory effects and can modulate microglial activation, thereby reducing neuroinflammatory processes.
Many polyphenols also support synaptic function and cognitive resilience. Preclinical studies indicate that flavonoids, catechins, and curcumin enhance neuronal antioxidant defenses—often via Nrf2/ARE pathway activation—and increase levels of neurotrophic factors such as BDNF and NGF. These mechanisms promote synaptic plasticity, neuronal survival, and regeneration [200].
While clinical evidence in humans is still emerging, epidemiological data and animal experiments consistently support the neuroprotective potential of these bioactive compounds. Long-term consumption of polyphenol-rich foods within the framework of the Mediterranean diet may therefore contribute to healthy cognitive aging and reduce the risk of neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease [201].
5. Mediterranean Diet and Neurodegeneration: Epidemiological Evidence
5.1. Cognitive Decline and Global Cognitive Performance
The relationship between the Mediterranean diet and cognitive function has been investigated in numerous epidemiological and interventional studies over the past decades [202,203,204,205,206,207,208,209,210,211,212]. Several prospective cohort studies have examined the associations between dietary patterns and the risk of dementia, mild cognitive impairment, or general cognitive decline in adults of various ages and sexes [210,211,212]. The results are mixed: some studies did not find a significant association between adherence to the Mediterranean diet and the risk of cognitive decline [210,213], whereas others reported beneficial effects, particularly for memory and global cognition [208,209].
Studies using a metabolomics approach, which assessed adherence to the Mediterranean diet based on biomarkers, also support its potential neuroprotective effects [214]. In cohort studies, higher adherence to the Mediterranean diet was especially associated with a reduced risk of cognitive decline in older women [215,216,217]. Clinical interventions, such as the PREDIMED and PREDIMED-NAVARRA trials, have demonstrated in randomized controlled settings that supplementing the Mediterranean diet with extra virgin olive oil or nuts significantly improves memory, executive functions, and overall cognitive performance compared to control groups [196,218]. Similarly, studies evaluating Mediterranean diet adherence have reported beneficial effects on memory, language, and visuospatial abilities, as well as mental health and quality of life [31,208,219].
Overall, current evidence suggests that the Mediterranean diet, particularly when enriched with antioxidant-rich supplements, may help maintain cognitive function in older age and reduce the risk of dementia. However, the effects appear to be population- and context-dependent, and further long-term randomized trials are needed to clarify the underlying mechanisms [218]. The main characteristics of the key studies are summarized in the table below (Table 2).

Table 2.
Mediterranean dietary patterns and cognitive decline or mild cognitive impairment.
5.2. The Mediterranean Diet and the Risk of Alzheimer’s Disease: Summary of Epidemiological Evidence
Numerous prospective and cross-sectional studies have demonstrated that greater adherence to the Mediterranean diet is associated with better cognitive health and a reduced risk of developing Alzheimer’s disease. Early influential cohort studies, such as those by Gu et al. [23] and Scarmeas et al. [222], showed that higher MeDi scores were linked to a 30–50% lower risk of AD or conversion from MCI to AD, with evidence of a dose–response relationship. Similarly, Morris et al. [22] reported that higher adherence to both the Mediterranean and MIND diets was significantly associated with a lower incidence of AD.
Cross-sectional data support these findings: in the Australian study by Gardener et al. [26], individuals with AD or MCI exhibited significantly lower MeDi scores compared to healthy controls, while higher adherence was related to less decline in MMSE performance over follow-up. In very old adults, Nicoli et al. [223] found that greater adherence to the Mediterranean diet and higher consumption of plant-based foods were associated with lower prevalence and incidence of AD and dementia.
Conversely, some more recent long-term investigations—such as Glans et al. [211]—did not observe significant associations between Mediterranean diet adherence and the risk of AD or overall dementia. These inconsistencies may partly reflect methodological differences in dietary assessment and changes in lifestyle behaviors over time. Overall, current epidemiological evidence suggests that higher adherence to the Mediterranean diet—particularly greater intake of vegetables, fruits, fish, and olive oil—is associated with a significantly lower risk of Alzheimer’s disease (Table 3).

Table 3.
Summary of epidemiological studies on Mediterranean and related dietary patterns in relation to Alzheimer’s disease and dementia risk.
5.3. Mediterranean Diet and Parkinson’s Disease Risk
Epidemiological evidence increasingly indicates that higher adherence to the Mediterranean diet is associated with a lower risk of Parkinson’s disease and a reduced likelihood of developing prodromal PD features. Most cohort and case–control studies report relative risk reductions of 20–50%, with the largest benefits generally observed in European and Mediterranean populations. In Asian populations, risk reductions were somewhat smaller, although the direction of the effect—reduced Parkinson’s disease risk—remained consistent. Overall, the strongest associations were observed in Mediterranean, Caucasian, and Latin American cohorts, whereas effects in Asian and African American populations tended to be smaller or less consistent, potentially reflecting differences in baseline dietary patterns, cultural eating habits, and gut microbiome profiles.
In a U.S. cohort of 706 participants, Agarwal et al. [231] found that greater adherence to the MeDi, alongside the MIND diet, was associated with a lower risk of developing parkinsonism (HR = 0.89; 95% CI 0.83–0.96). In a case–control study of 455 individuals, Alcalay et al. [232] reported that high MeDi adherence was linked to a 14% reduction in PD risk (OR = 0.86; 95% CI 0.77–0.97; p = 0.01) and delayed disease onset.
Findings from the Greek HELIAD cohort further support a neuroprotective role of the MeDi. Each one-point increase in MeDi score was associated with a 2% reduction in prodromal PD probability (p < 0.001), and participants in the highest adherence quartile had a ~21% lower risk than those in the lowest quartile [233,234]. Longitudinal analyses showed that higher MeDi adherence reduced the likelihood of developing possible or probable prodromal PD by 60–70% (p-trend = 0.003) and lowered the incidence of PD/dementia with Lewy bodies by approximately 9–10% (HR = 0.906; 95% CI 0.823–0.997).
Consistent findings have been observed in Scandinavian populations. In a study of over 47,000 Swedish women, Yin et al. [235] reported that the highest MeDi adherence was associated with a 46% lower risk of PD (HR = 0.54; 95% CI 0.30–0.98), while each unit increase in MeDi score conferred a 29% lower PD probability among women aged ≥65 years (95% CI 0.57–0.89).
Large U.S. datasets provide further support. Molsberry et al. [236] examined 47,679 participants and found that individuals in the highest aMED quintile had 18–33% lower odds of exhibiting three or more prodromal PD features (OR = 0.82–0.67; p-trend < 0.001). Similarly, Xu et al. [237], using NHANES data, observed that high MeDi adherence was associated with a 22% reduction in PD odds (OR = 0.78; 95% CI 0.65–0.93), whereas adherence to a Western dietary pattern more than doubled PD risk (OR = 2.19; 95% CI 1.16–4.14).
Taken together, these findings consistently support an inverse association between Mediterranean diet adherence and Parkinson’s disease risk, as summarized in Table 4.

Table 4.
Summary of observational studies investigating adherence to Mediterranean diet and Parkinson’s disease risk or prodromal features.
6. Cognitive Effects of Trans-Resveratrol
Trans-resveratrol has been investigated for its effects on cerebral perfusion, neurovascular function, and cognition in both acute and chronic settings. Acute supplementation studies [242,243,244] consistently demonstrate increased cerebral blood flow and enhanced oxygen extraction in the frontal cortex, accompanied by improved neurovascular coupling. These physiological changes were observed in healthy older adults and individuals with type 2 diabetes. Although acute administration generally did not yield significant improvements on short-term cognitive tests, it reliably supported cerebrovascular responsiveness during cognitive tasks.
Chronic supplementation trials provide stronger evidence for cognitive benefits. Longer-term administration of trans-resveratrol [157,245,246,247,248,249] has been shown to improve memory performance, particularly in domains related to retention and consolidation, while enhancing hippocampal functional connectivity. Several studies also documented increased cerebrovascular responsiveness to cognitive and hypercapnic stimulation, alongside improvements in metabolic parameters such as HbA1c and insulin resistance—factors relevant to neurodegenerative risk. Importantly, most human trials used resveratrol doses in the range of 150–1000 mg/day, which are several hundred-fold higher than the amounts obtainable from a Mediterranean dietary pattern, and therefore the clinical relevance of these findings to dietary intake is limited.
In individuals with mild cognitive impairment or Alzheimer’s disease [189,250,251,252,253,254], trans-resveratrol reduced markers of neuroinflammation, activated SIRT1-mediated neuroprotective signaling, and partially preserved hippocampal volume or structure. Cognitive gains in these populations were generally modest, but biological and neuroimaging markers suggest a slowing of neuropathological processes.
Natural dietary sources of resveratrol have also been examined. Grape-based formulations and resveratrol-enriched wine improved attention, working memory, and regional brain metabolism in both younger and older adults [255], supporting the concept that whole-food sources may exert synergistic effects.
Overall, available evidence indicates that trans-resveratrol enhances cerebrovascular function and supports neuroprotective pathways, with the most consistent benefits emerging from chronic supplementation in older adults and individuals at increased risk of cognitive decline. It is important to note that the doses of trans-resveratrol used in clinical supplementation trials (ranging from ~150 mg/day to 1000 mg/day) are substantially higher than the amounts typically obtained from a Mediterranean diet, where average daily intake through natural sources such as red grapes, red wine, and berries is estimated at approximately 1–5 mg/day. Therefore, while supplementation studies provide mechanistic and proof-of-concept evidence for cognitive and cerebrovascular benefits, these effects may not be fully achievable through habitual dietary intake alone. Future research should aim to clarify whether long-term adherence to a polyphenol-rich Mediterranean diet can produce comparable outcomes at physiologically attainable resveratrol levels. Key findings from these studies are summarized in Table 5.

Table 5.
Summary of human clinical trials investigating resveratrol and cognitive or cerebrovascular outcomes.
7. Olive Oil-Derived Polyphenols and Their Role in Cognitive Health
7.1. Cognitive Outcomes in MCI and Mild AD Populations Following Olive Oil or Olive Extract Interventions
Several clinical trials have investigated the effects of high-polyphenol extra virgin olive oil and other olive-derived extracts on cognitive performance in adults with MCI and mild AD. Overall, regular consumption of high-polyphenol EVOO or olive extracts has been associated with improvements in cognitive function in these populations. Tsolaki et al. [197] reported that in 50 MCI participants, 12 months of high-polyphenol early harvest EVOO combined with a Mediterranean diet significantly improved MMSE, ADAS-Cog, Digit Span, and Letter Fluency scores compared to moderate-polyphenol EVOO and Mediterranean diet alone (p < 0.05). Dimitriadis et al. [258] found in 43 MCI participants that high-polyphenol EVOO reduced EEG-measured over-excitation, decreased the theta/beta ratio, and enhanced integrated dynamic functional connectivity (p < 0.001).
Kaddoumi et al. [259] conducted a 6-month study with 25 MCI participants and observed that EVOO reduced blood–brain barrier permeability, increased both resting-state and task-based functional connectivity, and improved CDR and behavioral scores. Refined olive oil (ROO) improved CDR scores and task-based activation but did not affect BBB permeability or functional connectivity. Both EVOO and ROO lowered plasma Aβ42/Aβ40 and p-tau/t-tau ratios. Since refined olive oil contains only negligible amounts of oleuropein and other polyphenols, the observed cognitive effects cannot be attributed to oleuropein, and alternative mechanisms—such as the high monounsaturated fatty acid (MUFA) content—are more likely to account for these findings. This distinction should be considered when interpreting the results.
In mild AD populations, olive leaf extract (OLE) and oleuropein + S-acetyl-glutathione supplementation preserved or improved cognitive and functional scores over 6 months [260,261]. Additionally, low-dose EVOO integrated into the Mediterranean diet improved ADAS-Cog scores over 12 months [262]. Collectively, these findings support the cognition-enhancing effects of high-polyphenol EVOO and olive-derived extracts in MCI and mild AD populations (Table 6).

Table 6.
Human randomized controlled trials using olive-derived interventions and cognitive outcomes.
7.2. Olive Oil Polyphenols and Cognitive Function: Evidence from Mediterranean Diet Studies
Among epidemiological and intervention studies examining the effects of the Mediterranean diet on cognitive function, several highlight the role of extra virgin olive oil and olive polyphenols, including oleuropein, in improving memory performance and global cognitive function. In the PREDIMED trial, participants consuming EVOO showed significant improvements in the MMSE and Clock Drawing Test (CDT) compared with the control group [196]. Similarly, Valls-Pedret et al. [218,263] reported that a polyphenol-rich Mediterranean diet, including olive oil, was positively associated with enhanced verbal memory, working memory, and frontal cognitive components.
Cross-sectional studies, such as Anastasiou et al. [219] and Andreu-Reinón et al. [264], have shown that higher Mediterranean diet adherence—particularly with olive oil consumption—is associated with reduced dementia risk and better memory performance. Further evidence from Bajerska et al. [265] and Talhaoui et al. [266] indicates that specific olive oil intake, independent of total diet scores, supports cognitive domains such as global cognition, visual memory, and executive function, highlighting the neuroprotective potential of olive oil and its polyphenols, including oleuropein, in older adults.
It is important to note that refined olive oil contains negligible amounts of oleuropein and other polyphenols; thus, any cognitive improvements observed with ROO are likely mediated by other components, such as monounsaturated fatty acids or minor bioactive compounds, rather than the polyphenols responsible for the effects of high-polyphenol extra virgin olive oil. While the Mediterranean diet naturally provides EVOO and polyphenols, additional supplementation can deliver higher doses of bioactive compounds, potentially enhancing neuroprotective effects, improving bioavailability, or more effectively targeting cognitive pathways.
Overall, current evidence suggests that incorporating olive oil and polyphenol-rich foods into the Mediterranean diet may be critical for preventing Alzheimer’s disease and other cognitive impairments, particularly in aging populations (Table 7).

Table 7.
Studies on Mediterranean diet, olive-derived polyphenols [including oleuropein], and cognitive function.
8. Non-Olive Polyphenols and Cognitive Function: Evidence from Flavonoids, Catechins, and Cocoa Flavanols
This review does not provide an exhaustive analysis of trials focusing on general polyphenol supplementation or broader polyphenol subclasses (e.g., Ginkgo biloba, soy isoflavones, anthocyanins, cocoa flavanols, flavonoid extracts, chlorogenic acids, curcuminoids). These areas encompass more than one hundred clinical trials and have been comprehensively reviewed elsewhere. Within the scope of this article, these compounds are briefly summarized to contextualize the wider evidence base linking polyphenol intake to cognitive aging.
Multiple prospective cohort studies suggest that higher dietary intake of flavonoids and other polyphenols is associated with slower age-related cognitive decline. In the SU.VI.MAX cohort, Kesse-Guyot et al. [271] reported that higher intakes of total polyphenols—particularly catechins, theaflavins, and flavonols—were associated with better verbal memory and language performance in middle-aged adults. Similarly, in the Nurses’ Health Study, greater midlife flavonoid consumption was linked to a higher likelihood of healthy aging, including preserved cognitive and mental function [272].
These findings are consistent with results from the Framingham Offspring Study, where Shishtar et al. [273] observed a trend toward slower cognitive decline with higher flavanol intake. Data from the Memory and Aging Project further showed that dietary patterns rich in green leafy vegetables and their bioactive components [e.g., lutein, folate, vitamin K] were associated with significantly slower cognitive decline [274]. Collectively, this evidence supports the hypothesis that flavonoid- and polyphenol-rich diets during midlife may confer protection against late-life cognitive deterioration through antioxidant, anti-inflammatory, and neuroprotective mechanisms.
Human observational and interventional studies consistently indicate that flavonoids found in berries and grapes—particularly anthocyanins and flavanols—are associated with beneficial cognitive effects. In the Nurses’ Health Study, Devore et al. [275] reported that higher blueberry and strawberry consumption was linked to slower cognitive aging in older women, corresponding to approximately 2–2.5 years of cognitive “youthfulness.”
Interventional evidence complements these findings. Krikorian et al. [276] showed that 16 weeks of Concord grape juice supplementation improved memory and increased neural activation in older adults with MCI. In a six-month placebo-controlled trial, Lee et al. [254] found that freeze-dried grape powder (FDGP), rather than isolated grape polyphenols, preserved metabolic integrity in brain regions vulnerable in early Alzheimer’s disease. Additionally, long-term data from the Framingham Offspring Study revealed that higher intakes of flavonols, anthocyanins, and flavonoid polymers were associated with significantly lower risk of Alzheimer’s disease and related dementias (HR 0.24–0.58) [277]. Together, these results suggest that regular consumption of flavonoid-rich berries and grapes may slow cognitive decline and reduce neurodegenerative risk.
Catechins—major polyphenols in green tea—exhibit well-documented antioxidant, anti-inflammatory, and neuroprotective properties [278,279,280]. Clinical trials support cognitive benefits in midlife and older adulthood. In a double-blind, randomized, placebo-controlled trial, Baba et al. [281] found that 12 weeks of daily supplementation with decaffeinated green tea catechins (336 mg) improved cognitive performance in Japanese adults aged 50–69 years. Participants exhibited reduced error rates after a single dose and faster response times on working memory tasks after 12 weeks.
Consistent results were reported by Ide et al. [282], who observed improvements in overall cognitive performance—particularly attention and memory—in elderly Japanese individuals with MCI. These findings suggest that habitual intake of green tea catechins may confer modest but measurable cognitive benefits, likely mediated by antioxidant and neuroprotective mechanisms.
A growing body of evidence also supports the cognitive benefits of cocoa-derived flavanols, particularly in older adults. In the Cocoa, Cognition and Aging (CoCoA) studies, daily consumption of cocoa flavanols for eight weeks improved attention, executive function, and verbal fluency in older individuals with normal cognition and in those with MCI [283,284]. These improvements were accompanied by reductions in insulin resistance, blood pressure, and oxidative stress, suggesting that vascular and metabolic pathways may mediate the observed cognitive effects.
A retrospective clinical study in MCI patients found that higher cocoa polyphenol intake was associated with slower progression of cognitive decline [285]. Long-term cohort data also show that regular chocolate consumption is inversely correlated with cognitive decline risk, independently of age, education, and cardiovascular factors [286]. Together, these findings indicate that habitual cocoa flavanol consumption may help preserve cognitive function and support healthy brain aging.
9. Discussion and Conclusions
This review integrates epidemiological, clinical, and mechanistic evidence linking adherence to the Mediterranean diet with healthier cognitive aging and a reduced risk of neurodegenerative diseases. Prospective cohort studies consistently show that individuals who closely follow the Mediterranean diet experience slower cognitive decline and lower incidence of Alzheimer’s disease, Parkinson’s disease, and related dementias. Although population-specific variability exists, the overall evidence supports a protective association. Clinical interventions, particularly those incorporating high-polyphenol extra virgin olive oil, have demonstrated improvements in memory, executive function, cerebrovascular responsiveness, and disease-relevant biomarkers.
Mechanistic data provide a coherent biological rationale for these observations. Mediterranean diet components modulate multiple pathways central to neurodegenerative processes, including oxidative stress, chronic inflammation, mitochondrial dysfunction, cerebrovascular impairment, and gut–brain axis regulation. Polyphenols—especially resveratrol, oleuropein, and hydroxytyrosol—appear particularly relevant due to their roles in modulating amyloid and tau pathology, activating SIRT1, supporting mitochondrial and endothelial integrity, and regulating neuroinflammatory signaling.
It should be noted that much of the mechanistic evidence derives from in vitro studies, animal models, or metabolic biomarkers, whereas human, long-term neural outcomes (cognition, neurodegeneration, protein aggregation) remain limited. Daily intake of resveratrol and oleuropein from a typical Mediterranean diet is extremely low (~1 mg/day), suggesting that these compounds alone are unlikely to account for the observed cognitive benefits. Therefore, mechanistic and intervention studies must clearly specify the model system (cellular, animal, or human) and compare administered doses with achievable dietary intake levels. Moreover, extra virgin olive oil contains a complex mixture of polyphenols, and cognitive benefits likely reflect synergistic effects between polyphenols and other bioactive components, such as monounsaturated fatty acids. Overall, the cognitive benefits of the Mediterranean diet are probably not attributable to a single polyphenol, but rather to the combined action of multiple dietary components.
The clinical relevance of the Mediterranean diet extends beyond prevention. Its multidimensional biological effects suggest potential as a complementary therapeutic strategy for individuals with mild cognitive impairment or early-stage neurodegenerative disease. Polyphenols may also serve as biomarkers of dietary exposure and biological effect, and targeted supplementation could be considered for individuals with insufficient habitual intake. Responses to dietary and polyphenol-based interventions are likely individual-specific, influenced by genetics, comorbidities, physical activity, smoking, sleep patterns, and regional dietary habits. Collectively, the evidence supports the Mediterranean diet as a biologically plausible, feasible approach to promote cognitive health, with mechanistic and clinical data highlighting the importance of the synergistic action of multiple dietary components rather than any single polyphenol.
Interpretation of the literature must also take into account several limitations. Considerable variability exists in the scoring systems used to assess Mediterranean diet adherence, as well as in the dietary assessment tools themselves, which complicates the comparison of results across studies. Many cohort studies rely on self-reported dietary intake, which introduces recall bias. Interindividual differences in polyphenol absorption, metabolism, and bioavailability introduce further heterogeneity, as do differences in gut microbiome composition and lifestyle habits. Randomized controlled trials remain relatively few, often involve modest sample sizes and short intervention periods, and employ diverse formulations of polyphenol-rich foods or supplements. Importantly, the bioavailability of resveratrol in dietary sources is low and highly variable, and intervention doses used in mechanistic studies often exceed what can be achieved through habitual dietary intake, highlighting the need for careful interpretation of translatability. Furthermore, the studies summarized in the tables exhibit considerable heterogeneity, not only in dietary assessment methods but also due to geographical and cultural differences, which complicates direct comparison and synthesis of results. These limitations underscore the need for additional mechanistic and translational research in humans.
Future studies should aim to include larger, multicenter clinical trials with harmonized definitions of MedDiet adherence and standardized intervention protocols. The incorporation of biomarker-based endpoints—such as measures of amyloid and tau pathology, neuroinflammation, vascular function, and microbiome composition—will be crucial to clarifying causal pathways and strengthening the clinical relevance of findings. Integrating neuroimaging, metabolomics, and vascular assessments will help elucidate how specific components of the MedDiet contribute to neuroprotection and which individuals are most likely to benefit from dietary modification or targeted polyphenol supplementation. Such work is essential to advance the development of personalized nutritional strategies for neurodegenerative disease prevention and management.
In conclusion, although important gaps in our understanding remain, the collective evidence suggests that the Mediterranean diet is a promising, feasible, and biologically plausible approach to support cognitive health and reduce the burden of neurodegenerative diseases. Polyphenols—particularly those derived from extra virgin olive oil and grapes—likely contribute to these benefits through their combined antioxidant, anti-inflammatory, mitochondrial, and anti-amyloid actions, but the overall protective effect of the MedDiet is likely due to the synergistic interaction of multiple dietary components rather than the effect of any single polyphenol. Continued research focusing on biomarker-based outcomes, dose–response relationships, and precision nutrition strategies will be essential to translate these insights into targeted dietary recommendations and clinically meaningful interventions for individuals at risk of cognitive decline and neurodegeneration.
Author Contributions
A.L., T.J., D.M., P.V., E.M.P., V.F.-P., Á.S., Á.L., T.C., J.T.V., and M.F. contributed to the conceptualization and design of the study, drafted the manuscript, critically revised it for important intellectual content, and approved the final version for publication. All authors have read and agreed to the published version of the manuscript.
Funding
Project no. TKP2021-NKTA-47 was funded by the National Research, Development and Innovation Fund of Hungary under the TKP2021-NKTA scheme, with support from the Ministry of Innovation and Technology. The research was also supported by the Ministry of Innovation and Technology under the National Cardiovascular Laboratory Program (RRF-2.3.1-21-2022-00003) from the National Research, Development and Innovation Fund. In addition, this work received funding from the European University for Well-Being (EUniWell) program [grant agreement number: 101004093/EUniWell/EAC-A02-2019/EAC-A02-2019-1]. MF and VFP received funding from the Semmelweis University Excellence Research Program (KTKP), funded by the Faculty of Medicine, Semmelweis University. The funding bodies had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the article for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gao, Y.; Liu, X. Secular Trends in the Incidence of and Mortality Due to Alzheimer’s Disease and Other Forms of Dementia in China From 1990 to 2019: An Age-Period-Cohort Study and Joinpoint Analysis. Front. Aging Neurosci. 2021, 13, 709156. [Google Scholar] [CrossRef]
- 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef]
- Peng, S.; Liu, P.; Wang, X.; Li, K. Global, regional and national burden of Parkinson’s disease in people over 55 years of age: A systematic analysis of the global burden of disease study, 1991–2021. BMC Neurol. 2025, 25, 178. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Li, Q. Global trends and projections of Parkinson’s disease incidence: A 30-year analysis using GBD 2021 data. J. Neurol. 2025, 272, 286. [Google Scholar] [CrossRef]
- Arroyo-Pacheco, N.; Sarmiento-Blanco, S.; Vergara-Cadavid, G.; Castro-Leones, M.; Contreras-Puentes, N. Monoclonal therapy with lecanemab in the treatment of mild Alzheimer’s disease: A systematic review and meta-analysis. Ageing Res. Rev. 2025, 104, 102620. [Google Scholar] [CrossRef] [PubMed]
- Sugandhi, V.V.; Gadhave, D.G.; Ugale, A.R.; Kulkarni, N.; Nangare, S.N.; Patil, H.P.; Rath, S.; Saxena, R.; Lavate, A.; Patel, A.T.; et al. Advances in Alzheimer’s therapy: Exploring neuropathological mechanisms to revolutionize the future therapeutic landscape. Ageing Res. Rev. 2025, 109, 102775. [Google Scholar] [CrossRef] [PubMed]
- Preethy, H.A.; Rajendran, K.; Sukumar, A.J.; Krishnan, U.M. Emerging paradigms in Alzheimer’s therapy. Eur. J. Pharmacol. 2024, 981, 176872. [Google Scholar] [CrossRef]
- Fekete, M.; Balazs, P.; Lehoczki, A.; Forrai, J.; Dosa, N.; Fazekas-Pongor, V.; Feher, A.; Madarasz, B.; Varga, J.T. The role of gut microbiome and its modification while regulating the defence mechanisms, particularly in severe COVID-19 cases. Med. Int. Rev. 2023, 30, 154–166. [Google Scholar]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Csipo, T.; Forrai, J.; Dosa, N.; Peterfi, A.; Lehoczki, A.; Tarantini, S. Nutrition strategies promoting healthy aging: From improvement of cardiovascular and brain health to prevention of age-associated diseases. Nutrients 2022, 15, 47. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; de la Torre, R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef]
- Ontario, M.L.; Siracusa, R.; Modafferi, S.; Scuto, M.; Sciuto, S.; Greco, V.; Bertuccio, M.P.; Trovato Salinaro, A.; Crea, R.; Calabrese, E.J.; et al. Potential prevention and treatment of neurodegenerative disorders by olive polyphenols and hidrox. Mech. Ageing Dev. 2022, 203, 111637. [Google Scholar] [CrossRef]
- Grabska-Kobylecka, I.; Szpakowski, P.; Krol, A.; Ksiazek-Winiarek, D.; Kobylecki, A.; Glabinski, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef]
- Nagpal, D.; Nema, S.; Nagpal, S.; Pandey, M.M.; Kaushik, D.; Kathuria, H. Management and Prevention of Neurodegenerative Disorders: Can Antioxidant-Rich Dietary Interventions Help? Antioxidants 2025, 14, 1078. [Google Scholar] [CrossRef]
- Fekete, M.; Varga, P.; Ungvari, Z.; Fekete, J.T.; Buda, A.; Szappanos, A.; Lehoczki, A.; Mozes, N.; Grosso, G.; Godos, J.; et al. The role of the Mediterranean diet in reducing the risk of cognitive impairement, dementia, and Alzheimer’s disease: A meta-analysis. Geroscience 2025, 47, 3111–3130. [Google Scholar] [CrossRef]
- Dilmore, A.H.; Martino, C.; Neth, B.J.; West, K.A.; Zemlin, J.; Rahman, G.; Panitchpakdi, M.; Meehan, M.J.; Weldon, K.C.; Blach, C.; et al. Effects of a ketogenic and low-fat diet on the human metabolome, microbiome, and foodome in adults at risk for Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 4805–4816. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Leurgans, S.E.; Agrawal, S.; Aggarwal, N.T.; Cherian, L.J.; James, B.D.; Dhana, K.; Barnes, L.L.; Bennett, D.A.; Schneider, J.A. Association of Mediterranean-DASH Intervention for Neurodegenerative Delay and Mediterranean Diets With Alzheimer Disease Pathology. Neurology 2023, 100, e2259–e2268. [Google Scholar] [CrossRef] [PubMed]
- Hoscheidt, S.; Sanderlin, A.H.; Baker, L.D.; Jung, Y.; Lockhart, S.; Kellar, D.; Whitlow, C.T.; Hanson, A.J.; Friedman, S.; Register, T.; et al. Mediterranean and Western diet effects on Alzheimer’s disease biomarkers, cerebral perfusion, and cognition in mid-life: A randomized trial. Alzheimer’s Dement. 2022, 18, 457–468. [Google Scholar] [CrossRef]
- Roman, G.C.; Jackson, R.E.; Gadhia, R.; Roman, A.N.; Reis, J. Mediterranean diet: The role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Power, R.; Prado-Cabrero, A.; Mulcahy, R.; Howard, A.; Nolan, J.M. The Role of Nutrition for the Aging Population: Implications for Cognition and Alzheimer’s Disease. Annu. Rev. Food Sci. Technol. 2019, 10, 619–639. [Google Scholar] [CrossRef] [PubMed]
- Berti, V.; Walters, M.; Sterling, J.; Quinn, C.G.; Logue, M.; Andrews, R.; Matthews, D.C.; Osorio, R.S.; Pupi, A.; Vallabhajosula, S.; et al. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology 2018, 90, e1789–e1798. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Luchsinger, J.A.; Stern, Y.; Scarmeas, N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer’s disease. J. Alzheimers Dis. 2010, 22, 483–492. [Google Scholar] [CrossRef]
- Trabado-Fernandez, A.; Garcia-Colomo, A.; Cuadrado-Soto, E.; Peral-Suarez, A.; Salas-Gonzalez, M.D.; Lorenzo-Mora, A.M.; Aparicio, A.; Delgado-Losada, M.L.; Maestu-Unturbe, F.; Lopez-Sobaler, A.M. Association of a DASH diet and magnetoencephalography in dementia-free adults with different risk levels of Alzheimer’s disease. Geroscience 2024, 47, 1747–1759. [Google Scholar] [CrossRef]
- Lehoczki, A.; Csípő, T.; Lipécz, Á.; Major, D.; Fazekas-Pongor, V.; Csík, B.; Mózes, N.; Fehér, Á.; Dósa, N.; Árva, D.; et al. Western Diet and Cognitive Decline: A Hungarian Perspective-Implications for the Design of the Semmelweis Study. Nutrients 2025, 17, 2446. [Google Scholar] [CrossRef]
- Gardener, S.; Gu, Y.; Rainey-Smith, S.R.; Keogh, J.B.; Clifton, P.M.; Mathieson, S.L.; Taddei, K.; Mondal, A.; Ward, V.K.; Scarmeas, N.; et al. Adherence to a Mediterranean diet and Alzheimer’s disease risk in an Australian population. Transl. Psychiatry 2012, 2, e164. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Varga, P.; Fekete, J.T.; Buda, A.; Szappanos, A.; Lehoczki, A.; Mozes, N.; Grosso, G.; Menyhart, O.; et al. Impact of adherence to the Mediterranean diet on stroke risk. Geroscience 2025, 47, 3565–3581. [Google Scholar] [CrossRef]
- Vinciguerra, F.; Graziano, M.; Hagnäs, M.; Frittitta, L.; Tumminia, A. Influence of the mediterranean and ketogenic diets on cognitive status and decline: A narrative review. Nutrients 2020, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Fekete, M.; Fekete, J.T.; Grosso, G.; Ungvari, A.; Gyorffy, B. Adherence to the Mediterranean diet and its protective effects against colorectal cancer: A meta-analysis of 26 studies with 2,217,404 participants. Geroscience 2025, 47, 1105–1121. [Google Scholar] [CrossRef]
- Godos, J.; Scazzina, F.; Paterno Castello, C.; Giampieri, F.; Quiles, J.L.; Briones Urbano, M.; Battino, M.; Galvano, F.; Iacoviello, L.; de Gaetano, G.; et al. Underrated aspects of a true Mediterranean diet: Understanding traditional features for worldwide application of a “Planeterranean” diet. J. Transl. Med. 2024, 22, 294. [Google Scholar] [CrossRef]
- Godos, J.; Grosso, G.; Ferri, R.; Caraci, F.; Lanza, G.; Al-Qahtani, W.H.; Caruso, G.; Castellano, S. Mediterranean diet, mental health, cognitive status, quality of life, and successful aging in southern Italian older adults. Exp. Gerontol. 2023, 175, 112143. [Google Scholar] [CrossRef]
- Godos, J.; Castellano, S.; Ferri, R.; Caraci, F.; Lanza, G.; Scazzina, F.; Alanazi, A.M.; Marx, W.; Galvano, F.; Grosso, G. Mediterranean diet and chronotype: Data from Italian adults and systematic review of observational studies. Exp. Gerontol. 2023, 181, 112284. [Google Scholar] [CrossRef]
- Marventano, S.; Godos, J.; Platania, A.; Galvano, F.; Mistretta, A.; Grosso, G. Mediterranean diet adherence in the Mediterranean healthy eating, aging and lifestyle (MEAL) study cohort. Int. J. Food Sci. Nutr. 2018, 69, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Pajak, A.; Mistretta, A.; Marventano, S.; Raciti, T.; Buscemi, S.; Drago, F.; Scalfi, L.; Galvano, F. Protective role of the Mediterranean diet on several cardiovascular risk factors: Evidence from Sicily, southern Italy. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Mistretta, A.; Marventano, S.; Purrello, A.; Vitaglione, P.; Calabrese, G.; Drago, F.; Galvano, F. Beneficial effects of the Mediterranean diet on metabolic syndrome. Curr. Pharm. Des. 2014, 20, 5039–5044. [Google Scholar] [CrossRef]
- Grosso, G.; Mistretta, A.; Frigiola, A.; Gruttadauria, S.; Biondi, A.; Basile, F.; Vitaglione, P.; D’Orazio, N.; Galvano, F. Mediterranean diet and cardiovascular risk factors: A systematic review. Crit. Rev. Food Sci. Nutr. 2014, 54, 593–610. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Madarász, B.; Fazekas-Pongor, V.; Szarvas, Z.; Fekete, M.; Varga, J.T.; Tarantini, S.; Csiszar, A.; Lionetti, V.; Tabák, A.G.; Ungvari, Z. Survival and longevity of European rulers: Geographical influences and exploring potential factors, including the Mediterranean diet—A historical analysis from 1354 to the twentieth century. GeroScience 2024, 46, 3801–3818. [Google Scholar] [CrossRef]
- Gensous, N.; Garagnani, P.; Santoro, A.; Giuliani, C.; Ostan, R.; Fabbri, C.; Milazzo, M.; Gentilini, D.; di Blasio, A.M.; Pietruszka, B.; et al. One-year Mediterranean diet promotes epigenetic rejuvenation with country- and sex-specific effects: A pilot study from the NU-AGE project. Geroscience 2020, 42, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, I.; Marston, L.; Mukadam, N. Which components of the Mediterranean diet are associated with dementia? A UK Biobank cohort study. Geroscience 2022, 44, 2541–2554. [Google Scholar] [CrossRef]
- Tognon, G.; Rothenberg, E.; Eiben, G.; Sundh, V.; Winkvist, A.; Lissner, L. Does the Mediterranean diet predict longevity in the elderly? A Swedish perspective. Age 2011, 33, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Hernández-Cacho, A.; Nishi, S.K.; Babio, N.; Belzer, C.; Konstati, P.; Vioque, J.; Corella, D.; Castañer, O.; Vidal, J.; et al. Mediterranean diet, gut microbiota, and cognitive decline in older adults with obesity/overweight and metabolic syndrome: A prospective cohort study. BMC Med. 2025, 23, 669. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S. Resveratrol: From grapevines to mammalian biology. FASEB J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef]
- Murtaza, G.; Latif, U.; Najam-Ul-Haq, M.; Sajjad, A.; Karim, S.; Akhtar, M.; Hussain, I. Resveratrol: An active natural compound in red wines for health. J. Food Drug Anal. 2013, 21, 12. [Google Scholar]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133. [Google Scholar] [CrossRef]
- Calabro, A.; Aiello, A.; Silva, P.; Caruso, C.; Candore, G.; Accardi, G. Geroprotective applications of oleuropein and hydroxytyrosol through the hallmarks of ageing. Geroscience 2025. [Google Scholar] [CrossRef]
- Terracina, S.; Petrella, C.; Francati, S.; Lucarelli, M.; Barbato, C.; Minni, A.; Ralli, M.; Greco, A.; Tarani, L.; Fiore, M.; et al. Antioxidant Intervention to Improve Cognition in the Aging Brain: The Example of Hydroxytyrosol and Resveratrol. Int. J. Mol. Sci. 2022, 23, 15674. [Google Scholar] [CrossRef]
- Wiedenhoeft, T.; Tarantini, S.; Nyul-Toth, A.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Kiss, T.; Csiszar, A.; Csiszar, A.; et al. Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience 2019, 41, 711–725. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Springo, Z.; Tucsek, Z.; Gautam, T.; Giles, C.B.; Wren, J.D.; Koller, A.; Sonntag, W.E.; Csiszar, A.; et al. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: Role of resveratrol treatment in vasoprotection. Aging Cell 2015, 14, 400–408. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Tucsek, Z.; Ashpole, N.M.; Sosnowska, D.; Gautam, T.; Ballabh, P.; Koller, A.; Sonntag, W.E.; Csiszar, A.; et al. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H299–H308. [Google Scholar] [CrossRef]
- Carrizzo, A.; Puca, A.; Damato, A.; Marino, M.; Franco, E.; Pompeo, F.; Traficante, A.; Civitillo, F.; Santini, L.; Trimarco, V.; et al. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension 2013, 62, 359–366. [Google Scholar] [CrossRef]
- Li, H.; Yan, Z.; Zhu, J.; Yang, J.; He, J. Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology 2011, 60, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Wang, M.; Bernier, M.; Zhang, J.; Park, S.S.; Maudsley, S.; An, S.S.; Santhanam, L.; Martin, B.; Faulkner, S.; et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014, 20, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef]
- Ghanim, H.; Sia, C.L.; Korzeniewski, K.; Lohano, T.; Abuaysheh, S.; Marumganti, A.; Chaudhuri, A.; Dandona, P. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J. Clin. Endocrinol. Metab. 2011, 96, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Johnson, R.W. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009, 12, 445–453. [Google Scholar] [CrossRef]
- Bernier, M.; Wahl, D.; Ali, A.; Allard, J.; Faulkner, S.; Wnorowski, A.; Sanghvi, M.; Moaddel, R.; Alfaras, I.; Mattison, J.A.; et al. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging 2016, 8, 899–916. [Google Scholar] [CrossRef]
- Ungvari, Z.; Sonntag, W.E.; de Cabo, R.; Baur, J.A.; Csiszar, A. Mitochondrial Protection by Resveratrol. Exerc. Sport. Sci. Rev. 2011, 39, 128–132. [Google Scholar] [CrossRef]
- Yousuf, S.; Atif, F.; Ahmad, M.; Hoda, N.; Ishrat, T.; Khan, B.; Islam, F. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res. 2009, 1250, 242–253. [Google Scholar] [CrossRef]
- Ungvari, Z.; Labinskyy, N.; Mukhopadhyay, P.; Pinto, J.T.; Bagi, Z.; Ballabh, P.; Zhang, C.; Pacher, P.; Csiszar, A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1876–H1881. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Tariq, U.; Naz, A.; Rizwan, M. Neuroprotective effects of oleuropein: Recent developments and contemporary research. J. Food Biochem. 2021, 45, e13967. [Google Scholar] [CrossRef]
- Gonçalves, M.; Vale, N.; Silva, P. Neuroprotective effects of olive oil: A comprehensive review of antioxidant properties. Antioxidants 2024, 13, 762. [Google Scholar] [CrossRef]
- Di Risola, D. Use of Olive Oil Polyphenols to Counteract Neuroinflammation and Neurodegenerative Diseases. 2023. Available online: https://iris.uniroma1.it/handle/11573/1693261 (accessed on 11 October 2025).
- Rogina, B.; Tissenbaum, H.A. SIRT1, resveratrol and aging. Front. Genet. 2024, 15, 1393181. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Danz, E.D.; Skramsted, J.; Henry, N.; Bennett, J.A.; Keller, R.S. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic. Biol. Med. 2009, 46, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef]
- Micheli, L.; Bertini, L.; Bonato, A.; Villanova, N.; Caruso, C.; Caruso, M.; Bernini, R.; Tirone, F. Role of hydroxytyrosol and oleuropein in the prevention of aging and related disorders: Focus on neurodegeneration, skeletal muscle dysfunction and gut microbiota. Nutrients 2023, 15, 1767. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: Role of SIRT1. Oxidative Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Wieckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western diet as a trigger of Alzheimer’s disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Sanfilippo, C.; Castrogiovanni, P.; Vinciguerra, M.; Imbesi, R.; Ulivieri, M.; Fazio, F.; Blennow, K.; Zetterberg, H.; Di Rosa, M. A sex-stratified analysis of neuroimmune gene expression signatures in Alzheimer’s disease brains. Geroscience 2023, 45, 523–541. [Google Scholar] [CrossRef]
- Kellogg, C.M.; Pham, K.; Machalinski, A.H.; Porter, H.L.; Blankenship, H.E.; Tooley, K.B.; Stout, M.B.; Rice, H.C.; Sharpe, A.L.; Beckstead, M.J.; et al. Microglial MHC-I induction with aging and Alzheimer’s is conserved in mouse models and humans. Geroscience 2023, 45, 3019–3043. [Google Scholar] [CrossRef]
- Pollicino, F.; Veronese, N.; Dominguez, L.J.; Barbagallo, M. Mediterranean diet and mitochondria: New findings. Exp. Gerontol. 2023, 176, 112165. [Google Scholar] [CrossRef]
- Gundogdu, A.; Nalbantoglu, O.U. The role of the Mediterranean diet in modulating the gut microbiome: A review of current evidence. Nutrition 2023, 114, 112118. [Google Scholar] [CrossRef] [PubMed]
- Itsiopoulos, C.; Mayr, H.L.; Thomas, C.J. The anti-inflammatory effects of a Mediterranean diet: A review. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 415–422. [Google Scholar] [CrossRef]
- Camargo, A.; Delgado-Lista, J.; Garcia-Rios, A.; Cruz-Teno, C.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Gutierrez-Mariscal, F.M.; Lora-Aguilar, P.; Rodriguez-Cantalejo, F.; Fuentes-Jimenez, F.; et al. Expression of proinflammatory, proatherogenic genes is reduced by the Mediterranean diet in elderly people. Br. J. Nutr. 2012, 108, 500–508. [Google Scholar] [CrossRef]
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines 2020, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Sen, A.; Hongpaisan, J. Hippocampal microvasculature changes in association with oxidative stress in Alzheimer’s disease. Free Radic. Biol. Med. 2018, 120, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Mota, S.I.; Costa, R.O.; Ferreira, I.L.; Santana, I.; Caldeira, G.L.; Padovano, C.; Fonseca, A.C.; Baldeiras, I.; Cunha, C.; Letra, L.; et al. Oxidative stress involving changes in Nrf2 and ER stress in early stages of Alzheimer’s disease. Biochim. Biophys. Acta 2015, 1852, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Jones, D.P.; Goldberg, J.; Ziegler, T.R.; Bostick, R.M.; Wilson, P.W.; Manatunga, A.K.; Shallenberger, L.; Jones, L.; Vaccarino, V. Association between adherence to the Mediterranean diet and oxidative stress. Am. J. Clin. Nutr. 2008, 88, 1364–1370. [Google Scholar] [CrossRef]
- Ambring, A.; Friberg, P.; Axelsen, M.; Laffrenzen, M.; Taskinen, M.R.; Basu, S.; Johansson, M. Effects of a Mediterranean-inspired diet on blood lipids, vascular function and oxidative stress in healthy subjects. Clin. Sci. 2004, 106, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Chatzianagnostou, K.; Del Turco, S.; Pingitore, A.; Sabatino, L.; Vassalle, C. The Mediterranean Lifestyle as a Non-Pharmacological and Natural Antioxidant for Healthy Aging. Antioxidants 2015, 4, 719–736. [Google Scholar] [CrossRef]
- Silva, P.; Vauzour, D. Wine polyphenols and neurodegenerative diseases: An update on the molecular mechanisms underpinning their protective effects. Beverages 2018, 4, 96. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Shah, R.; Alford, N.; Mishra, S.P.; Jain, S.; Hansen, B.; Sanberg, P.; Molina, A.J.A.; Yadav, H. The Triple Alliance: Microbiome, Mitochondria, and Metabolites in the Context of Age-Related Cognitive Decline and Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 2187–2202. [Google Scholar] [CrossRef]
- Agnihotri, A.; Aruoma, O.I. Alzheimer’s Disease and Parkinson’s Disease: A Nutritional Toxicology Perspective of the Impact of Oxidative Stress, Mitochondrial Dysfunction, Nutrigenomics and Environmental Chemicals. J. Am. Coll. Nutr. 2020, 39, 16–27. [Google Scholar] [CrossRef]
- Long, A.N.; Owens, K.; Schlappal, A.E.; Kristian, T.; Fishman, P.S.; Schuh, R.A. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurol. 2015, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef] [PubMed]
- Corral-Debrinski, M.; Horton, T.; Lott, M.T.; Shoffner, J.M.; McKee, A.C.; Beal, M.F.; Graham, B.H.; Wallace, D.C. Marked changes in mitochondrial DNA deletion levels in Alzheimer brains. Genomics 1994, 23, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio Tejo, F.; Quintanilla, R.A. Contribution of the Nrf2 Pathway on Oxidative Damage and Mitochondrial Failure in Parkinson and Alzheimer’s Disease. Antioxidants 2021, 10, 1069. [Google Scholar] [CrossRef]
- Brahadeeswaran, S.; Lateef, M.; Calivarathan, L. An insight into the molecular mechanism of mitochondrial toxicant-induced neuronal apoptosis in Parkinson’s disease. Curr. Mol. Med. 2022, 23, 63–75. [Google Scholar]
- Lopert, P.; Patel, M. Mitochondrial mechanisms of redox cycling agents implicated in Parkinson’s disease. J. Neural Transm. 2016, 123, 113–123. [Google Scholar] [CrossRef]
- Pszczolowska, M.; Walczak, K.; Miskow, W.; Mroziak, M.; Chojdak-Lukasiewicz, J.; Leszek, J. Mitochondrial disorders leading to Alzheimer’s disease-perspectives of diagnosis and treatment. Geroscience 2024, 46, 2977–2988. [Google Scholar]
- Chen, Y.; Zhang, Y.; Yang, H.; Li, H.; Zhou, L.; Zhang, M.; Wang, Y. Associations of sugar-sweetened, artificially sweetened, and naturally sweet juices with Alzheimer’s disease: A prospective cohort study. Geroscience 2024, 46, 1229–1240. [Google Scholar] [CrossRef]
- Caplliure-Llopis, J.; Peralta-Chamba, T.; Carrera-Julia, S.; Cuerda-Ballester, M.; Drehmer-Rieger, E.; Lopez-Rodriguez, M.M.; de la Rubia Orti, J.E. Therapeutic alternative of the ketogenic Mediterranean diet to improve mitochondrial activity in Amyotrophic Lateral Sclerosis (ALS): A Comprehensive Review. Food Sci. Nutr. 2020, 8, 23–35. [Google Scholar] [CrossRef]
- Amick, K.A.; Mahapatra, G.; Bergstrom, J.; Gao, Z.; Craft, S.; Register, T.C.; Shively, C.A.; Molina, A.J.A. Brain region-specific disruption of mitochondrial bioenergetics in cynomolgus macaques fed a Western versus a Mediterranean diet. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E652–E664. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef]
- Sorrenti, V.; Benedetti, F.; Buriani, A.; Fortinguerra, S.; Caudullo, G.; Davinelli, S.; Zella, D.; Scapagnini, G. Immunomodulatory and Antiaging Mechanisms of Resveratrol, Rapamycin, and Metformin: Focus on mTOR and AMPK Signaling Networks. Pharmaceuticals 2022, 15, 912. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, Q.; Xu, X.; Xing, Y.; Liang, J.; Lin, Q.; Huang, M.; Chen, Y.; Lin, B.; Chen, W. Resveratrol Ameliorates Systemic Sclerosis via Suppression of Fibrosis and Inflammation Through Activation of SIRT1/mTOR Signaling. Drug Des. Devel Ther. 2020, 14, 5337–5348. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, J.; Qin, S.; Zhou, Z.; Zeng, Q.; Long, R.; Mao, Z.; Dong, X.; Zhao, R.; Zhang, R.; et al. Resveratrol induces autophagy impeding BAFF-stimulated B-cell proliferation and survival by inhibiting the Akt/mTOR pathway. Biochem. Pharmacol. 2022, 202, 115139. [Google Scholar] [CrossRef]
- Biala, A.; Tauriainen, E.; Siltanen, A.; Shi, J.; Merasto, S.; Louhelainen, M.; Martonen, E.; Finckenberg, P.; Muller, D.N.; Mervaala, E. Resveratrol induces mitochondrial biogenesis and ameliorates Ang II-induced cardiac remodeling in transgenic rats harboring human renin and angiotensinogen genes. Blood Press 2010, 19, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Labinskyy, N.; Pinto, J.T.; Ballabh, P.; Zhang, H.; Losonczy, G.; Pearson, K.; de Cabo, R.; Pacher, P.; Zhang, C.; et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H13–H20. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, J.K.; de Cabo, R.; Mattison, J.A. Resveratrol Blunts Mitochondrial Loss in Slow and Mixed Skeletal Muscle Phenotypes of Non-Human Primates following a Long-Term High Fat/Sugar Diet. J. Diet. Suppl. 2022, 20, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.A.; Baccalini, R.; Battaglia, E.; Falchi, M.; Ferrero, M.E. Resveratrol inhibits TNF alpha-induced endothelial cell activation. Therapie 2001, 56, 613–616. [Google Scholar]
- Csiszar, A.; Smith, K.; Labinskyy, N.; Orosz, Z.; Rivera, A.; Ungvari, Z. Resveratrol attenuates TNF-{alpha}-induced activation of coronary arterial endothelial cells: Role of NF-{kappa}B inhibition. Am. J. Physiol. 2006, 291, H1694–H1699. [Google Scholar]
- Gracia-Sancho, J.; Villarreal, G., Jr.; Zhang, Y.; Garcia-Cardena, G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc. Res. 2009, 85, 514–519. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Jin, X.; Liang, X.Y.; Zhou, Y.; Zhang, T.; Xie, Q.; Zhou, X.; Chang, H.; Fu, Y.J.; et al. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy 2013, 9, 2033–2045. [Google Scholar] [CrossRef]
- Silva-Soto, M.; Carrillo-Fernández, P.; Saez Lancellotti, E.T.; Medina-Jiménez, E.; Mogaburo Alba, J.F.; Catena-Granados, N.; López-Carmona, M.D.; Pérez-Belmonte, L.M.; Prieto Lain, N.; Gómez Hernández, A.I.; et al. Extra Virgin Olive Oil Phenolic Compounds: Modulating Mitochondrial Function and Protecting Against Chronic Diseases-A Narrative Review. Nutrients 2025, 17, 1443. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Tarantini, S.; Fulop, G.A.; Kiss, T.; Valcarcel-Ares, M.N.; Galvan, V.; Ungvari, Z.; Yabluchanskiy, A. Hypertension impairs neurovascular coupling and promotes microvascular injury: Role in exacerbation of Alzheimer’s disease. Geroscience 2017, 39, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Girouard, H.; Iadecola, C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. (1985) 2006, 100, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef]
- Nicolakakis, N.; Hamel, E. Neurovascular function in Alzheimer’s disease patients and experimental models. J. Cereb. Blood Flow. Metab. 2011, 31, 1354–1370. [Google Scholar] [CrossRef]
- Tarantini, S.; Fulop, G.A.; Kiss, T.; Farkas, E.; Zolei-Szenasi, D.; Galvan, V.; Toth, P.; Csiszar, A.; Ungvari, Z.; Yabluchanskiy, A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience 2017, 39, 465–473. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Martire, S.; Fuso, A.; Mosca, L.; Forte, E.; Correani, V.; Fontana, M.; Scarpa, S.; Maras, B.; d’Erme, M. Bioenergetic Impairment in Animal and Cellular Models of Alzheimer’s Disease: PARP-1 Inhibition Rescues Metabolic Dysfunctions. J. Alzheimers Dis. 2016, 54, 307–324. [Google Scholar] [CrossRef]
- Misiak, B.; Leszek, J.; Kiejna, A. Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease--the emerging role of systemic low-grade inflammation and adiposity. Brain Res. Bull. 2012, 89, 144–149. [Google Scholar] [CrossRef]
- van Dinther, M.; Voorter, P.H.M.; Zhang, E.; van Kuijk, S.M.J.; Jansen, J.F.A.; van Oostenbrugge, R.J.; Backes, W.H.; Staals, J. The neurovascular unit and its correlation with cognitive performance in patients with cerebral small vessel disease: A canonical correlation analysis approach. Geroscience 2024, 46, 5061–5073. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Border, J.J.; Rivers, P.L.; Zhang, H.; Williams, J.M.; Fan, F.; Roman, R.J. Amyloid beta accumulation in TgF344-AD rats is associated with reduced cerebral capillary endothelial Kir2.1 expression and neurovascular uncoupling. Geroscience 2023, 45, 2909–2926. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, A.; Nyul-Toth, A.; Patai, R.; Csik, B.; Gulej, R.; Nagy, D.; Shanmugarama, S.; Benyo, Z.; Kiss, T.; Ungvari, Z.; et al. Cerebromicrovascular senescence in vascular cognitive impairment: Does accelerated microvascular aging accompany atherosclerosis? Geroscience 2025, 47, 5511–5524. [Google Scholar] [CrossRef] [PubMed]
- Gulej, R.; Nyul-Toth, A.; Csik, B.; Petersen, B.; Faakye, J.; Negri, S.; Chandragiri, S.S.; Mukli, P.; Yabluchanskiy, A.; Conley, S.; et al. Rejuvenation of cerebromicrovascular function in aged mice through heterochronic parabiosis: Insights into neurovascular coupling and the impact of young blood factors. Geroscience 2024, 46, 327–347. [Google Scholar] [CrossRef]
- Gulej, R.; Nyul-Toth, A.; Csik, B.; Patai, R.; Petersen, B.; Negri, S.; Chandragiri, S.S.; Shanmugarama, S.; Mukli, P.; Yabluchanskiy, A.; et al. Young blood-mediated cerebromicrovascular rejuvenation through heterochronic parabiosis: Enhancing blood-brain barrier integrity and capillarization in the aged mouse brain. Geroscience 2024, 46, 4415–4442. [Google Scholar] [CrossRef]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; De Caterina, R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: Antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 622–629. [Google Scholar] [CrossRef]
- Rallidis, L.S.; Lekakis, J.; Kolomvotsou, A.; Zampelas, A.; Vamvakou, G.; Efstathiou, S.; Dimitriadis, G.; Raptis, S.A.; Kremastinos, D.T. Close adherence to a Mediterranean diet improves endothelial function in subjects with abdominal obesity. Am. J. Clin. Nutr. 2009, 90, 263–268. [Google Scholar] [CrossRef]
- Shannon, O.M.; Mendes, I.; Kochl, C.; Mazidi, M.; Ashor, A.W.; Rubele, S.; Minihane, A.M.; Mathers, J.C.; Siervo, M. Mediterranean Diet Increases Endothelial Function in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2020, 150, 1151–1159. [Google Scholar] [CrossRef]
- Torres-Pena, J.D.; Garcia-Rios, A.; Delgado-Casado, N.; Gomez-Luna, P.; Alcala-Diaz, J.F.; Yubero-Serrano, E.M.; Gomez-Delgado, F.; Leon-Acuna, A.; Lopez-Moreno, J.; Camargo, A.; et al. Mediterranean diet improves endothelial function in patients with diabetes and prediabetes: A report from the CORDIOPREV study. Atherosclerosis 2018, 269, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Torres-Pena, J.D.; Rangel-Zuniga, O.A.; Alcala-Diaz, J.F.; Lopez-Miranda, J.; Delgado-Lista, J. Mediterranean Diet and Endothelial Function: A Review of its Effects at Different Vascular Bed Levels. Nutrients 2020, 12, 2212. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Fernandez-Gandara, C.; Garcia-Rios, A.; Rangel-Zuniga, O.A.; Gutierrez-Mariscal, F.M.; Torres-Pena, J.D.; Marin, C.; Lopez-Moreno, J.; Castano, J.P.; Delgado-Lista, J.; et al. Mediterranean diet and endothelial function in patients with coronary heart disease: An analysis of the CORDIOPREV randomized controlled trial. PLoS Med. 2020, 17, e1003282. [Google Scholar] [CrossRef]
- Nicholson, S.K.; Tucker, G.A.; Brameld, J.M. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc. Nutr. Soc. 2008, 67, 42–47. [Google Scholar] [CrossRef]
- Ou, H.C.; Chou, F.P.; Sheen, H.M.; Lin, T.M.; Yang, C.H.; Huey-Herng Sheu, W. Resveratrol, a polyphenolic compound in red wine, protects against oxidized LDL-induced cytotoxicity in endothelial cells. Clin. Chim. Acta Int. J. Clin. Chem. 2006, 364, 196–204. [Google Scholar] [CrossRef]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Forstermann, U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.G.; Wang, Z.R.; Huang, Y.Z.; Cao, K.J.; Wu, J.M. Effect of red wine and wine polyphenol resveratrol on endothelial function in hypercholesterolemic rabbits. Int. J. Mol. Med. 2003, 11, 317–320. [Google Scholar] [CrossRef]
- Bhatt, S.R.; Lokhandwala, M.F.; Banday, A.A. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur. J. Pharmacol. 2011, 667, 258–264. [Google Scholar] [CrossRef]
- Chow, S.E.; Hshu, Y.C.; Wang, J.S.; Chen, J.K. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J. Appl. Physiol. 2007, 102, 1520–1527. [Google Scholar] [CrossRef]
- Csiszar, A.; Pinto, J.T.; Gautam, T.; Kleusch, C.; Hoffmann, B.; Tucsek, Z.; Toth, P.; Sonntag, W.E.; Ungvari, Z. Resveratrol Encapsulated in Novel Fusogenic Liposomes Activates Nrf2 and Attenuates Oxidative Stress in Cerebromicrovascular Endothelial Cells From Aged Rats. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 70, 303–313. [Google Scholar] [CrossRef]
- Moon, S.O.; Kim, W.; Sung, M.J.; Lee, S.; Kang, K.P.; Kim, D.H.; Lee, S.Y.; So, J.N.; Park, S.K. Resveratrol suppresses tumor necrosis factor-alpha-induced fractalkine expression in endothelial cells. Mol. Pharmacol. 2006, 70, 112–119. [Google Scholar] [CrossRef]
- Pendurthi, U.R.; Rao, L.V. Resveratrol suppresses agonist-induced monocyte adhesion to cultured human endothelial cells. Thromb. Res. 2002, 106, 243–248. [Google Scholar] [CrossRef]
- Micek, A.; Godos, J.; Giampieri, F.; Battino, M.; Quiles, J.L.; Del Rio, D.; Mena, P.; Caruso, G.; Frias-Toral, E.; Azpíroz, I.D.; et al. The effect of anthocyanins and anthocyanin-rich foods on cognitive function: A meta-analysis of randomized controlled trials. GeroScience 2025. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H18–H24. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Habermeier, A.; Closs, E.I.; Thum, T.; Spanier, G.; Lu, Q.; Oelze, M.; Torzewski, M.; Lackner, K.J.; et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J. Pharmacol. Exp. Ther. 2010, 335, 149–154. [Google Scholar] [CrossRef]
- Stromsnes, K.; Mas-Bargues, C.; Gambini, J.; Gimeno-Mallench, L. Protective Effects of Polyphenols Present in Mediterranean Diet on Endothelial Dysfunction. Oxid. Med. Cell Longev. 2020, 2020, 2097096. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Dong, F.; Sheng, C.; Wu, J.; Han, Y.; Jiang, J.; Alzheimer’s Disease Neuroimaging, I.; Weiner, M.W.; Aisen, P.; et al. Dynamic proportional loss of functional connectivity revealed change of left superior frontal gyrus in subjective cognitive decline: An explanatory study based on Chinese and Western cohorts. Geroscience 2025, 47, 5619–5634. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Colomo, A.; Lopez-Sanz, D.; Carrasco-Gomez, M.; Ramirez-Torano, F.; Alfonsin, S.; Spuch, C.; Comis-Tuche, M.; Maestu, F. Plasma p-tau231 and NfL differently associate with functional connectivity patterns in cognitively unimpaired individuals. Geroscience 2025. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, P.; Mak, H.K.F.; Hui, E.S. The structural-functional-connectivity coupling of the aging brain. Geroscience 2024, 46, 3875–3887. [Google Scholar] [PubMed]
- Williamson, J.N.; James, S.A.; Mullen, S.P.; Sutton, B.P.; Wszalek, T.; Mulyana, B.; Mukli, P.; Yabluchanskiy, A.; Alzheimer’s Disease Neuroimaging Initiative Consortium; Yang, Y. Sex differences in interacting genetic and functional connectivity biomarkers in Alzheimer’s disease. Geroscience 2024, 46, 6071–6084. [Google Scholar] [CrossRef]
- Williamson, J.; James, S.A.; Mukli, P.; Yabluchanskiy, A.; Wu, D.H.; Sonntag, W.; Alzheimer’s Disease Neuroimaging Initiative, C.; Yang, Y. Sex difference in brain functional connectivity of hippocampus in Alzheimer’s disease. Geroscience 2024, 46, 563–572. [Google Scholar] [CrossRef]
- Garcia-Colomo, A.; Nebreda, A.; Carrasco-Gomez, M.; de Frutos-Lucas, J.; Ramirez-Torano, F.; Spuch, C.; Comis-Tuche, M.; Bruna, R.; Alfonsin, S.; Maestu, F. Longitudinal changes in the functional connectivity of individuals at risk of Alzheimer’s disease. Geroscience 2024, 46, 2989–3003. [Google Scholar] [CrossRef]
- Kraft, J.N.; Hausman, H.K.; Hardcastle, C.; Albizu, A.; O’Shea, A.; Evangelista, N.D.; Boutzoukas, E.M.; Van Etten, E.J.; Bharadwaj, P.K.; Song, H.; et al. Task-based functional connectivity of the Useful Field of View (UFOV) fMRI task. Geroscience 2023, 45, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Casares, N.; Bernal-Lopez, M.R.; Roe-Vellve, N.; Gutierrez-Bedmar, M.; Fernandez-Garcia, J.C.; Garcia-Arnes, J.A.; Ramos-Rodriguez, J.R.; Alfaro, F.; Santamaria-Fernandez, S.; Steward, T.; et al. Brain Functional Connectivity Is Modified by a Hypocaloric Mediterranean Diet and Physical Activity in Obese Women. Nutrients 2017, 9, 685. [Google Scholar] [CrossRef]
- Karavasilis, E.; Balomenos, V.; Christidi, F.; Velonakis, G.; Angelopoulou, G.; Yannakoulia, M.; Mamalaki, E.; Drouka, A.; Brikou, D.; Tsapanou, A.; et al. Mediterranean diet and brain functional connectivity in a population without dementia. Front. Neuroimaging 2024, 3, 1473399. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Floel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Abreu, M.T.; Devkota, S.; Issokson, K. A Mediterranean Diet for Crohn’s Disease: Embracing Colorful Diversity to Improve the Microbiome. Gastroenterology 2025, 168, 872–874. [Google Scholar] [CrossRef]
- Abrignani, V.; Salvo, A.; Pacinella, G.; Tuttolomondo, A. The Mediterranean Diet, Its Microbiome Connections, and Cardiovascular Health: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4942. [Google Scholar] [CrossRef]
- Jennings, A.; Kuhn, T.; Bondonno, N.P.; Waniek, S.; Bang, C.; Franke, A.; Kassubek, J.; Muller, H.P.; Both, M.; Weber, K.S.; et al. The gut microbiome modulates associations between adherence to a Mediterranean-style diet, abdominal adiposity, and C-reactive protein in population-level analysis. Am. J. Clin. Nutr. 2024, 119, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kranyak, A.; Haran, K.; Smith, P.; Johnson, C.; Liao, W.; Bhutani, T. The Mediterranean Diet as a Potential Solution to the Gut Microbiome Dysbiosis in Psoriasis Patients. J. Psoriasis Psoriatic Arthritis 2024, 9, 69–81. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Parise, A.; Mena, P.; Meschi, T. The interaction between Mediterranean diet and intestinal microbiome: Relevance for preventive strategies against frailty in older individuals. Aging Clin. Exp. Res. 2024, 36, 58. [Google Scholar] [CrossRef]
- Vazquez-Cuesta, S.; Lozano Garcia, N.; Rodriguez-Fernandez, S.; Fernandez-Avila, A.I.; Bermejo, J.; Fernandez-Aviles, F.; Munoz, P.; Bouza, E.; Reigadas, E. Impact of the Mediterranean Diet on the Gut Microbiome of a Well-Defined Cohort of Healthy Individuals. Nutrients 2024, 16, 793. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhu, Q.; Zhang, S.; OuYang, D.; Lu, J.H. Resveratrol in experimental Alzheimer’s disease models: A systematic review of preclinical studies. Pharmacol. Res. 2019, 150, 104476. [Google Scholar] [CrossRef] [PubMed]
- Albani, D.; Polito, L.; Signorini, A.; Forloni, G. Neuroprotective properties of resveratrol in different neurodegenerative disorders. Biofactors 2010, 36, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Bartra, C.; Yuan, Y.; Vuraic, K.; Valdes-Quiroz, H.; Garcia-Baucells, P.; Slevin, M.; Pastorello, Y.; Sunol, C.; Sanfeliu, C. Resveratrol Activates Antioxidant Protective Mechanisms in Cellular Models of Alzheimer’s Disease Inflammation. Antioxidants 2024, 13, 177. [Google Scholar] [CrossRef]
- Liu, X.; Baxley, S.; Hebron, M.; Turner, R.S.; Moussa, C. Resveratrol Attenuates CSF Markers of Neurodegeneration and Neuroinflammation in Individuals with Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 5044. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Y.; Cao, Z.; Ji, Y.; Cheng, X.; Zheng, X. The Multi-Dimensional Action Map of Resveratrol Against Alzheimer’s Disease: Mechanism Integration and Treatment Strategy Optimization. Nutrients 2025, 17, 3451. [Google Scholar] [CrossRef]
- Patel, S.; Thornton, A.; Parmar, M.S. Resveratrol’s Multifaceted Potential in Alzheimer’s Disease: Insights from Preclinical and Clinical Evidence. Mol. Neurobiol. 2025, 62, 16229–16260. [Google Scholar] [CrossRef] [PubMed]
- Puranik, N.; Kumari, M.; Tiwari, S.; Dhakal, T.; Song, M. Resveratrol as a Therapeutic Agent in Alzheimer’s Disease: Evidence from Clinical Studies. Nutrients 2025, 17, 2557. [Google Scholar] [CrossRef]
- Surya, K.; Rathinam, A.; Abubakkar, M.N.; Jayachandran, K.S.; Kandasamy, M.; Anusuyadevi, M. Resveratrol mitigates activated astrocytes and microglia preventing Alzheimer’s Disease (AD) progression and facilitates neuronal communication in Amyloid-beta25-35 induced rat model for AD: A special emphasis on non-neuronal involvement in AD pathophysiology. Psychopharmacology 2025, 242, 2529–2545. [Google Scholar]
- Tao, G.; Wang, X.; Wang, J.; Ye, Y.; Zhang, M.; Lang, Y.; Ding, S. Dihydro-resveratrol ameliorates NLRP3 inflammasome-mediated neuroinflammation via Bnip3-dependent mitophagy in Alzheimer’s disease. Br. J. Pharmacol. 2025, 182, 1005–1024. [Google Scholar] [CrossRef]
- Wicinski, M.; Domanowska, A.; Wodkiewicz, E.; Malinowski, B. Neuroprotective Properties of Resveratrol and Its Derivatives-Influence on Potential Mechanisms Leading to the Development of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2749. [Google Scholar] [PubMed]
- Wang, Z.G.; Yang, C.; Zhu, B.; Hua, F. AMPK-dependent autophagic activation is probably involved in the mechanism of resveratrol exerting therapeutic effects for Alzheimer’s disease. Rejuvenation Res. 2015, 18, 101–102. [Google Scholar] [CrossRef]
- Surya, K.; Manickam, N.; Jayachandran, K.S.; Kandasamy, M.; Anusuyadevi, M. Resveratrol Mediated Regulation of Hippocampal Neuroregenerative Plasticity via SIRT1 Pathway in Synergy with Wnt Signaling: Neurotherapeutic Implications to Mitigate Memory Loss in Alzheimer’s Disease. J. Alzheimers Dis. 2023, 94, S125–S140. [Google Scholar]
- Rege, S.D.; Geetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef]
- Martin, I. Resveratrol for Alzheimer’s disease? Sci. Transl. Med. 2017, 9, eaam6055. [Google Scholar] [CrossRef] [PubMed]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef]
- Arbo, B.D.; Andre-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, S.; Qian, Y.; Xiao, Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav. Immun. 2017, 64, 162–172. [Google Scholar] [CrossRef]
- Huang, T.C.; Lu, K.T.; Wo, Y.Y.; Wu, Y.J.; Yang, Y.L. Resveratrol protects rats from Aβ-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS ONE 2011, 6, e29102. [Google Scholar] [CrossRef] [PubMed]
- Venigalla, M.; Sonego, S.; Gyengesi, E.; Sharman, M.J.; Münch, G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2016, 95, 63–74. [Google Scholar] [CrossRef]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J. Physiol. Pharmacol. 2009, 60, 111–116. [Google Scholar]
- Wang, X.; Ma, S.; Yang, B.; Huang, T.; Meng, N.; Xu, L.; Xing, Q.; Zhang, Y.; Zhang, K.; Li, Q.; et al. Resveratrol promotes hUC-MSCs engraftment and neural repair in a mouse model of Alzheimer’s disease. Behav. Brain Res. 2018, 339, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Tajes, M.; Gutierrez-Cuesta, J.; Folch, J.; Ortuño-Sahagun, D.; Verdaguer, E.; Jiménez, A.; Junyent, F.; Lau, A.; Camins, A.; Pallàs, M. Neuroprotective role of intermittent fasting in senescence-accelerated mice P8 (SAMP8). Exp. Gerontol. 2010, 45, 702–710. [Google Scholar] [CrossRef]
- Imamura, H.; Yamaguchi, T.; Nagayama, D.; Saiki, A.; Shirai, K.; Tatsuno, I. Resveratrol Ameliorates Arterial Stiffness Assessed by Cardio-Ankle Vascular Index in Patients With Type 2 Diabetes Mellitus. Int. Heart J. 2017, 58, 577–583. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflammation 2017, 14, 1. [Google Scholar] [CrossRef]
- Thordardottir, S.; Kinhult Ståhlbom, A.; Almkvist, O.; Thonberg, H.; Eriksdotter, M.; Zetterberg, H.; Blennow, K.; Graff, C. The effects of different familial Alzheimer’s disease mutations on APP processing in vivo. Alzheimers Res. Ther. 2017, 9, 9. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Silenzi, A.; Giovannini, C.; Scazzocchio, B.; Varì, R.; D’Archivio, M.; Santangelo, C.; Masella, R. Extra virgin olive oil polyphenols: Biological properties and antioxidant activity. In Pathology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–233. [Google Scholar]
- Rodríguez-López, P.; Lozano-Sanchez, J.; Borrás-Linares, I.; Emanuelli, T.; Menéndez, J.A.; Segura-Carretero, A. Structure–biological activity relationships of extra-virgin olive oil phenolic compounds: Health properties and bioavailability. Antioxidants 2020, 9, 685. [Google Scholar] [CrossRef] [PubMed]
- Abuznait, A.H.; Qosa, H.; Busnena, B.A.; El Sayed, K.A.; Kaddoumi, A. Olive-oil-derived oleocanthal enhances β-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: In vitro and in vivo studies. ACS Chem. Neurosci. 2013, 4, 973–982. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhang, L.; Yin, H.; Shen, L.; Zheng, W.; Zhang, K.; Zeng, J.; Hu, C.; Liu, Y. Hydroxytyrosol alleviates oxidative stress and neuroinflammation and enhances hippocampal neurotrophic signaling to improve stress-induced depressive behaviors in mice. Food Funct. 2021, 12, 5478–5487. [Google Scholar] [CrossRef]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvadó, J.; San Julián, B.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef]
- Tsolaki, M.; Lazarou, E.; Kozori, M.; Petridou, N.; Tabakis, I.; Lazarou, I.; Karakota, M.; Saoulidis, I.; Melliou, E.; Magiatis, P. A Randomized Clinical Trial of Greek High Phenolic Early Harvest Extra Virgin Olive Oil in Mild Cognitive Impairment: The MICOIL Pilot Study. J. Alzheimers Dis. 2020, 78, 801–817. [Google Scholar] [CrossRef]
- Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 2564. [Google Scholar] [CrossRef]
- Bellavite, P. Neuroprotective Potentials of Flavonoids: Experimental Studies and Mechanisms of Action. Antioxidants 2023, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Meymand, A.Z.; Khodagholi, F.; Kamsorkh, H.M.; Asadi, E.; Noori, M.; Rahimian, K.; Shahrbabaki, A.S.; Talebi, A.; Parsaiyan, H.; et al. A role for flavonoids in the prevention and/or treatment of cognitive dysfunction, learning, and memory deficits: A review of preclinical and clinical studies. Nutr. Neurosci. 2023, 26, 156–172. [Google Scholar] [CrossRef]
- Gardener, H.; Caunca, M.R. Mediterranean Diet in Preventing Neurodegenerative Diseases. Curr. Nutr. Rep. 2018, 7, 10–20. [Google Scholar] [CrossRef]
- Stepaniak, U.; Grosso, G.; Polak, M.; Gradowicz-Prajsnar, B.; Kozela, M.; Bobak, M.; Sanchez-Niubo, A.; Stefler, D.; Haro, J.M.; Pająk, A. Association between dietary (poly)phenol intake and the ATHLOS Healthy Ageing Scale in the Polish arm of the HAPIEE study. Geroscience 2025, 47, 3241–3253. [Google Scholar] [CrossRef] [PubMed]
- Flensted-Jensen, M.; Weinreich, C.M.; Kleis-Olsen, A.S.; Hansen, F.; Skyggelund, N.S.; Pii, J.R.; Whitlock, R.; Abrahamsen, M.B.; Petersen, T.I.; Karlsen, A.; et al. Effects of resistance-based training and polyphenol supplementation on physical function, metabolism, and inflammation in aging individuals. Geroscience 2025. [Google Scholar] [CrossRef]
- Landsberger, T.; Amit, I.; Alon, U. Geroprotective interventions converge on gene expression programs of reduced inflammation and restored fatty acid metabolism. Geroscience 2024, 46, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Molina, R.F.; Riestra-Ayora, J.I.; Vasallo, I.J.T.; Sanz-Fernández, R.; Sánchez-Rodríguez, C. Sirtuins mediate the reduction of age-related oxidative damage in the cochlea under a cocoa-rich diet. Geroscience 2025. [Google Scholar] [CrossRef] [PubMed]
- Gkotzamanis, V.; Panagiotakos, D. Dietary interventions and cognition: A systematic review of clinical trials. Psychiatriki 2020, 31, 248–256. [Google Scholar] [CrossRef]
- Daffner, K.R. Promoting successful cognitive aging: A comprehensive review. J. Alzheimer’s Dis. 2010, 19, 1101–1122. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Kyrozis, A.; Rossi, M.; Katsoulis, M.; Trichopoulos, D.; La Vecchia, C.; Lagiou, P. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur. J. Nutr. 2015, 54, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, A.; Fondell, E.; Ascherio, A.; Yuan, C.; Grodstein, F.; Willett, W. Adherence to Mediterranean diet and subjective cognitive function in men. Eur. J. Epidemiol. 2018, 33, 223–234. [Google Scholar] [CrossRef]
- Haring, B.; Wu, C.; Mossavar-Rahmani, Y.; Snetselaar, L.; Brunner, R.; Wallace, R.B.; Neuhouser, M.L.; Wassertheil-Smoller, S. No Association between Dietary Patterns and Risk for Cognitive Decline in Older Women with 9-Year Follow-Up: Data from the Women’s Health Initiative Memory Study. J. Acad. Nutr. Diet. 2016, 116, 921–930.e921. [Google Scholar] [CrossRef]
- Glans, I.; Sonestedt, E.; Nägga, K.; Gustavsson, A.M.; González-Padilla, E.; Borne, Y.; Stomrud, E.; Melander, O.; Nilsson, P.M.; Palmqvist, S.; et al. Association Between Dietary Habits in Midlife With Dementia Incidence Over a 20-Year Period. Neurology 2023, 100, e28–e37. [Google Scholar]
- Tsivgoulis, G.; Judd, S.; Letter, A.J.; Alexandrov, A.V.; Howard, G.; Nahab, F.; Unverzagt, F.W.; Moy, C.; Howard, V.J.; Kissela, B. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology 2013, 80, 1684–1692. [Google Scholar] [CrossRef]
- Hosking, D.E.; Eramudugolla, R.; Cherbuin, N.; Anstey, K.J. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimer’s Dement. 2019, 15, 581–589. [Google Scholar] [CrossRef]
- Tor-Roca, A.; Sánchez-Pla, A.; Korosi, A.; Pallàs, M.; Lucassen, P.J.; Castellano-Escuder, P.; Aigner, L.; González-Domínguez, R.; Manach, C.; Carmona, F. A Mediterranean Diet-Based Metabolomic Score and Cognitive Decline in Older Adults: A Case–Control Analysis Nested within the Three-City Cohort Study. Mol. Nutr. Food Res. 2023, 68, e2300271. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Zhang, R.; Wang, Y.; Wang, J.; Meng, H.; Cheng, H.; Zhang, J. Mediterranean diet related to 3-year incidence of cognitive decline: Results from a cohort study in Chinese rural elders. Nutr. Neurosci. 2024, 27, 1351–1362. [Google Scholar] [CrossRef]
- Chan, R.; Chan, D.; Woo, J. A cross sectional study to examine the association between dietary patterns and cognitive impairment in older Chinese people in Hong Kong. J. Nutr. Health Aging 2013, 17, 757–765. [Google Scholar] [CrossRef]
- Allcock, L.; Mantzioris, E.; Villani, A. Adherence to a Mediterranean Diet is associated with physical and cognitive health: A cross-sectional analysis of community-dwelling older Australians. Front. Public Health 2022, 10, 1017078. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef]
- Anastasiou, C.A.; Yannakoulia, M.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Arampatzi, X.; Bougea, A.; Labropoulos, I.; Scarmeas, N. Mediterranean diet and cognitive health: Initial results from the Hellenic Longitudinal Investigation of Ageing and Diet. PLoS ONE 2017, 12, e0182048. [Google Scholar] [CrossRef]
- Olsson, E.; Karlström, B.; Kilander, L.; Byberg, L.; Cederholm, T.; Sjögren, P. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J. Alzheimers Dis. 2015, 43, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Geda, Y.E.; Cerhan, J.R.; Knopman, D.S.; Cha, R.H.; Christianson, T.J.; Pankratz, V.S.; Ivnik, R.J.; Boeve, B.F.; O’Connor, H.M.; et al. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2010, 29, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, C.; Galbussera, A.A.; Bosetti, C.; Franchi, C.; Gallus, S.; Mandelli, S.; Marcon, G.; Quadri, P.; Riso, P.; Riva, E.; et al. The role of diet on the risk of dementia in the oldest old: The Monzino 80-plus population-based study. Clin. Nutr. 2021, 40, 4783–4791. [Google Scholar] [CrossRef]
- de Crom, T.O.E.; Mooldijk, S.S.; Ikram, M.K.; Ikram, M.A.; Voortman, T. MIND diet and the risk of dementia: A population-based study. Alzheimers Res. Ther. 2022, 14, 8. [Google Scholar] [CrossRef]
- Mamalaki, E.; Charisis, S.; Anastasiou, C.A.; Ntanasi, E.; Georgiadi, K.; Balomenos, V.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; et al. The Longitudinal Association of Lifestyle with Cognitive Health and Dementia Risk: Findings from the HELIAD Study. Nutrients 2022, 14, 2818. [Google Scholar] [CrossRef]
- Shannon, O.M.; Ranson, J.M.; Gregory, S.; Macpherson, H.; Milte, C.; Lentjes, M.; Mulligan, A.; McEvoy, C.; Griffiths, A.; Matu, J. Mediterranean diet adherence is associated with lower dementia risk, independent of genetic predisposition: Findings from the UK Biobank prospective cohort study. BMC Med. 2023, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Nieves, J.W.; Stern, Y.; Luchsinger, J.A.; Scarmeas, N. Food combination and Alzheimer disease risk: A protective diet. Arch. Neurol. 2010, 67, 699–706. [Google Scholar] [CrossRef]
- Kheirouri, S.; Valiei, F.; Taheraghdam, A.-A. Association of plant-rich dietary patterns of mediterranean and MIND with risk of alzheimer disease. Human. Nutr. Metab. 2024, 37, 200283. [Google Scholar] [CrossRef]
- Charisis, S.; Ntanasi, E.; Yannakoulia, M.; Anastasiou, C.A.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Veskoukis, A.S.; Kouretas, D.; et al. Plasma GSH levels and Alzheimer’s disease. A prospective approach.: Results from the HELIAD study. Free Radic. Biol. Med. 2021, 162, 274–282. [Google Scholar] [CrossRef]
- Calil, S.R.B.; Brucki, S.M.D.; Nitrini, R.; Yassuda, M.S. Adherence to the Mediterranean and MIND diets is associated with better cognition in healthy seniors but not in MCI or AD. Clin. Nutr. ESPEN 2018, 28, 201–207. [Google Scholar] [CrossRef]
- Agarwal, P.; Wang, Y.; Buchman, A.S.; Holland, T.M.; Bennett, D.A.; Morris, M.C. MIND Diet Associated with Reduced Incidence and Delayed Progression of ParkinsonismA in Old Age. J. Nutr. Health Aging 2018, 22, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Alcalay, R.N.; Gu, Y.; Mejia-Santana, H.; Cote, L.; Marder, K.S.; Scarmeas, N. The association between Mediterranean diet adherence and Parkinson’s disease. Mov. Disord. 2012, 27, 771–774. [Google Scholar] [CrossRef]
- Maraki, M.I.; Yannakoulia, M.; Stamelou, M.; Stefanis, L.; Xiromerisiou, G.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Anastasiou, C.A.; et al. Mediterranean diet adherence is related to reduced probability of prodromal Parkinson’s disease. Mov. Disord. 2019, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Maraki, M.I.; Yannakoulia, M.; Xiromerisiou, G.; Stefanis, L.; Charisis, S.; Giagkou, N.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; et al. Mediterranean diet is associated with a lower probability of prodromal Parkinson’s disease and risk for Parkinson’s disease/dementia with Lewy bodies: A longitudinal study. Eur. J. Neurol. 2023, 30, 934–942. [Google Scholar] [CrossRef]
- Yin, W.; Löf, M.; Pedersen, N.L.; Sandin, S.; Fang, F. Mediterranean Dietary Pattern at Middle Age and Risk of Parkinson’s Disease: A Swedish Cohort Study. Mov. Disord. 2021, 36, 255–260. [Google Scholar] [CrossRef]
- Molsberry, S.; Bjornevik, K.; Hughes, K.C.; Healy, B.; Schwarzschild, M.; Ascherio, A. Diet pattern and prodromal features of Parkinson disease. Neurology 2020, 95, e2095–e2108. [Google Scholar] [CrossRef]
- Xu, S.; Li, W.; Di, Q. Association of Dietary Patterns with Parkinson’s Disease: A Cross-Sectional Study Based on the United States National Health and Nutritional Examination Survey Database. Eur. Neurol. 2023, 86, 63–72. [Google Scholar] [CrossRef]
- Keramati, M.; Kheirouri, S.; Etemadifar, M. Dietary approach to stop hypertension (DASH), but not Mediterranean and MIND, dietary pattern protects against Parkinson’s disease. Food Sci. Nutr. 2024, 12, 943–951. [Google Scholar] [CrossRef]
- Okubo, H.; Miyake, Y.; Sasaki, S.; Murakami, K.; Tanaka, K.; Fukushima, W.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; et al. Dietary patterns and risk of Parkinson’s disease: A case-control study in Japan. Eur. J. Neurol. 2012, 19, 681–688. [Google Scholar] [CrossRef]
- Strikwerda, A.J.; Dommershuijsen, L.J.; Ikram, M.K.; Voortman, T. Diet Quality and Risk of Parkinson’s Disease: The Rotterdam Study. Nutrients 2021, 13, 3970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, J.; Liu, Y.; Chen, S.; Wu, S.; Gao, X. Diet Quality is Associated with Prodromal Parkinson’s Disease Features in Chinese Adults. Mov. Disord. 2022, 37, 2367–2375. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Reay, J.L.; Haskell, C.F.; Williamson, G.; Dew, T.P.; Kennedy, D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef]
- Wong, R.H.; Raederstorff, D.; Howe, P.R. Acute Resveratrol Consumption Improves Neurovascular Coupling Capacity in Adults with Type 2 Diabetes Mellitus. Nutrients 2016, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M.; Howe, P.R.; Wong, R.H. Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; A 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Reay, J.L.; Williamson, G.; Dew, T.; Zhang, W.; Kennedy, D.O. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 2015, 114, 1427–1437. [Google Scholar] [CrossRef]
- Huhn, S.; Beyer, F.; Zhang, R.; Lampe, L.; Grothe, J.; Kratzsch, J.; Willenberg, A.; Breitfeld, J.; Kovacs, P.; Stumvoll, M.; et al. Effects of resveratrol on memory performance, hippocampus connectivity and microstructure in older adults—A randomized controlled trial. Neuroimage 2018, 174, 177–190. [Google Scholar] [CrossRef]
- Thaung Zaw, J.J.; Howe, P.R.; Wong, R.H. Sustained cerebrovascular and cognitive benefits of resveratrol in postmenopausal women. Nutrients 2020, 12, 828. [Google Scholar] [CrossRef] [PubMed]
- Zaw, J.J.T.; Howe, P.R.; Wong, R.H. Long-term effects of resveratrol on cognition, cerebrovascular function and cardio-metabolic markers in postmenopausal women: A 24-month randomised, double-blind, placebo-controlled, crossover study. Clin. Nutr. 2021, 40, 820–829. [Google Scholar]
- Turner, R.S.; Thomas, R.G.; Craft, S.; Van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Köbe, T.; Witte, A.V.; Schnelle, A.; Tesky, V.A.; Pantel, J.; Schuchardt, J.-P.; Hahn, A.; Bohlken, J.; Grittner, U.; Flöel, A. Impact of resveratrol on glucose control, hippocampal structure and connectivity, and memory performance in patients with mild cognitive impairment. Front. Neurosci. 2017, 11, 105. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Chen, H.; Xu, X.; Li, Y.; Gui, Y. Neuroprotective effect of trans-resveratrol in mild to moderate Alzheimer disease: A randomized, double-blind trial. Neurol. Ther. 2021, 10, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.W.; Grossman, H.; Neugroschl, J.; Parker, S.; Burden, A.; Luo, X.; Sano, M. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 609–616. [Google Scholar] [CrossRef]
- Lee, J.; Torosyan, N.; Silverman, D.H. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double-blinded placebo controlled pilot study. Exp. Gerontol. 2017, 87, 121–128. [Google Scholar] [CrossRef]
- Scholey, A.; Benson, S.; Stough, C.; Stockley, C. Effects of resveratrol and alcohol on mood and cognitive function in older individuals. Nutr. Aging 2014, 2, 133–138. [Google Scholar] [CrossRef]
- Moran, C.; Scotto di Palumbo, A.; Bramham, J.; Moran, A.; Rooney, B.; De Vito, G.; Egan, B. Effects of a Six-Month Multi-Ingredient Nutrition Supplement Intervention of Omega-3 Polyunsaturated Fatty Acids, vitamin D, Resveratrol, and Whey Protein on Cognitive Function in Older Adults: A Randomised, Double-Blind, Controlled Trial. J. Prev. Alzheimers Dis. 2018, 5, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Berry, N.M.; Coates, A.M.; Buckley, J.D.; Bryan, J.; Kunz, I.; Howe, P.R. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J. Hypertens. 2013, 31, 1819–1827. [Google Scholar] [CrossRef]
- Dimitriadis, S.I.; Lyssoudis, C.; Tsolaki, A.C.; Lazarou, E.; Kozori, M.; Tsolaki, M. Greek high phenolic early harvest extra virgin olive oil reduces the over-excitation of information-flow based on Dominant Coupling Mode (DoCM) model in patients with mild cognitive impairment: An EEG resting-state validation approach. J. Alzheimer’s Dis. 2021, 83, 191–207. [Google Scholar]
- Kaddoumi, A.; Denney, T.S., Jr.; Deshpande, G.; Robinson, J.L.; Beyers, R.J.; Redden, D.T.; Praticò, D.; Kyriakides, T.C.; Lu, B.; Kirby, A.N.; et al. Extra-Virgin Olive Oil Enhances the Blood-Brain Barrier Function in Mild Cognitive Impairment: A Randomized Controlled Trial. Nutrients 2022, 14, 5102. [Google Scholar] [CrossRef]
- Loukou, S.; Papantoniou, G.; Pantazaki, A.; Tsolaki, M. The Role of Greek Olive Leaf Extract in Patients with Mild Alzheimer’s Disease (the GOLDEN Study): A Randomized Controlled Clinical Trial. Neurol. Int. 2024, 16, 1247–1265. [Google Scholar] [CrossRef]
- Marianetti, M.; Pinna, S.; Venuti, A.; Liguri, G. Olive polyphenols and bioavailable glutathione: Promising results in patients diagnosed with mild Alzheimer’s disease. Alzheimers Dement. 2022, 8, e12278. [Google Scholar] [CrossRef]
- Mazza, E.; Fava, A.; Ferro, Y.; Rotundo, S.; Romeo, S.; Bosco, D.; Pujia, A.; Montalcini, T. Effect of the replacement of dietary vegetable oils with a low dose of extravirgin olive oil in the Mediterranean Diet on cognitive functions in the elderly. J. Transl. Med. 2018, 16, 10. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Lamuela-Raventós, R.M.; Medina-Remón, A.; Quintana, M.; Corella, D.; Pintó, X.; Martínez-González, M.; Estruch, R.; Ros, E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J. Alzheimers Dis. 2012, 29, 773–782. [Google Scholar] [CrossRef]
- Andreu-Reinón, M.E.; Chirlaque, M.D.; Gavrila, D.; Amiano, P.; Mar, J.; Tainta, M.; Ardanaz, E.; Larumbe, R.; Colorado-Yohar, S.M.; Navarro-Mateu, F.; et al. Mediterranean Diet and Risk of Dementia and Alzheimer’s Disease in the EPIC-Spain Dementia Cohort Study. Nutrients 2021, 13, 700. [Google Scholar]
- Bajerska, J.; Woźniewicz, M.; Suwalska, A.; Jeszka, J. Eating patterns are associated with cognitive function in the elderly at risk of metabolic syndrome from rural areas. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3234–3245. [Google Scholar]
- Talhaoui, A.; Aboussaleh, Y.; Bikri, S.; Rouim, F.Z.; Ahami, A. The relationship between adherence to a Mediterranean diet and cognitive impairment among the elderly in Morocco. Acta Neuropsychol. 2023, 21, 125–138. [Google Scholar] [CrossRef]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; San Julián, B.; Sanchez-Tainta, A.; Corella, D.; Lamuela-Raventós, R.M.; Martínez, J.A.; Martínez-Gonzalez, M. Virgin olive oil supplementation and long-term cognition: The PREDIMED-NAVARRA randomized, trial. J. Nutr. Health Aging 2013, 17, 544–552. [Google Scholar] [CrossRef]
- Galbete, C.; Toledo, E.; Toledo, J.B.; Bes-Rastrollo, M.; Buil-Cosiales, P.; Marti, A.; Guillén-Grima, F.; Martínez-González, M.A. Mediterranean diet and cognitive function: The SUN project. J. Nutr. Health Aging 2015, 19, 305–312. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Andreeva, V.A.; Lassale, C.; Ferry, M.; Jeandel, C.; Hercberg, S.; Galan, P. Mediterranean diet and cognitive function: A French study. Am. J. Clin. Nutr. 2013, 97, 369–376. [Google Scholar] [CrossRef]
- Fischer, K.; Melo van Lent, D.; Wolfsgruber, S.; Weinhold, L.; Kleineidam, L.; Bickel, H.; Scherer, M.; Eisele, M.; van den Bussche, H.; Wiese, B.; et al. Prospective Associations between Single Foods, Alzheimer’s Dementia and Memory Decline in the Elderly. Nutrients 2018, 10, 852. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Fezeu, L.; Andreeva, V.A.; Touvier, M.; Scalbert, A.; Hercberg, S.; Galan, P. Total and specific polyphenol intakes in midlife are associated with cognitive function measured 13 years later. J. Nutr. 2012, 142, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Sun, Q.; Townsend, M.K.; Rimm, E.B.; Grodstein, F. Dietary flavonoid intake at midlife and healthy aging in women. Am. J. Clin. Nutr. 2014, 100, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Shishtar, E.; Rogers, G.T.; Blumberg, J.B.; Au, R.; Jacques, P.F. Long-term dietary flavonoid intake and change in cognitive function in the Framingham Offspring cohort. Public Health Nutr. 2020, 23, 1576–1588. [Google Scholar] [CrossRef]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology 2018, 90, e214–e222. [Google Scholar] [CrossRef]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef]
- Krikorian, R.; Boespflug, E.L.; Fleck, D.E.; Stein, A.L.; Wightman, J.D.; Shidler, M.D.; Sadat-Hossieny, S. Concord grape juice supplementation and neurocognitive function in human aging. J. Agric. Food Chem. 2012, 60, 5736–5742. [Google Scholar] [CrossRef]
- Shishtar, E.; Rogers, G.T.; Blumberg, J.B.; Au, R.; Jacques, P.F. Long-term dietary flavonoid intake and risk of Alzheimer disease and related dementias in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2020, 112, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.A.; Avramovich-Tirosh, Y.; Reznichenko, L.; Zheng, H.; Weinreb, O.; Amit, T.; Youdim, M.B. Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress and PKC signaling pathway. Neurosignals 2005, 14, 46–60. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Baba, Y.; Inagaki, S.; Nakagawa, S.; Kaneko, T.; Kobayashi, M.; Takihara, T. Effect of daily intake of green tea catechins on cognitive function in middle-aged and older subjects: A randomized, placebo-controlled study. Molecules 2020, 25, 4265. [Google Scholar] [CrossRef]
- Ide, K.; Yamada, H.; Takuma, N.; Kawasaki, Y.; Harada, S.; Nakase, J.; Ukawa, Y.; Sagesaka, Y.M. Effects of green tea consumption on cognitive dysfunction in an elderly population: A randomized placebo-controlled study. Nutr. J. 2015, 15, 49. [Google Scholar] [CrossRef]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: The Cocoa, Cognition, and Aging (CoCoA) study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; De Cola, M.C.; Gervasi, G.; Portaro, S.; Naro, A.; Accorinti, M.; Manuli, A.; Marra, A.; De Luca, R.; Bramanti, P. The efficacy of cocoa polyphenols in the treatment of mild cognitive impairment: A retrospective study. Medicina 2019, 55, 156. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Diógenes, M.J.; De Mendonça, A.; Lunet, N.; Barros, H. Chocolate consumption is associated with a lower risk of cognitive decline. J. Alzheimer’s Dis. 2016, 53, 85–93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).