Abstract
Background/Objectives: Vitamin B12 (B12) is essential for the provision of methyl groups for numerous essential pathways. Infant B12 deficiency (B12D) can lead to severe, even irreversible neurological abnormalities. Maternal B12 status in pregnancy and during the breastfeeding period correlates significantly with the child’s B12 status. B12D is a target disease in some newborn screening (NBS) programs. This study investigates whether infants that were clinically symptomatic and diagnosed with B12D in their first year of life could be retrospectively detected by the Austrian NBS algorithm. Methods: Data from infants with clinically diagnosed B12D in their first year of life between 2012 and 2022 were retrospectively collected in Austria (B12-related NBS implemented in 2018) and Switzerland (B12-related NBS not implemented). NBS data were retrospectively analysed, and clinical information was collected by a survey. Correlations between clinical symptoms, NBS data, biochemical parameters at diagnosis, maternal medical history and B12 status were analysed. Results: Four/forty-eight cases were retrospectively detected by the first-tier NBS parameters. From two children material for second-tier testing was available and B12D was confirmed by elevated total homocysteine (tHcy), resulting in a detection rate between 4.3 and 9.3%. The numbers of neurological and haematological symptoms correlated with low B12 and elevated levels of tHcy and methylmalonic acid. Although the detection rate of symptomatic B12D by NBS was low, fewer infants with symptomatic B12D were observed in the period after implementation of B12-related NBS (Austria). A history of B12D-relevant maternal disease such as pernicious anaemia was reported in 12 cases. Conclusions: B12D causes severe clinical symptoms in infants. NBS has a very limited retrospective detection rate of infants with severe B12D but seems to correlate with a reduction in cases due to not yet precisely quantified mechanisms. The workup triggered by NBS recalls is costly and often challenging for families. Maternal B12D increases the risk of infant B12D but also of other pregnancy-related health risks. To increase the efficacy of the prevention of infant B12D, to promote a healthy pregnancy and breastfeeding period, and to reduce the frequency of NBS recalls, pregnant women should be screened for B12D to be counselled and treated.
1. Introduction
Vitamin B12, or cobalamin (Cbl), is an essential micronutrient that plays an important role in cellular metabolism acting as cofactor for two metabolic enzymes. After gastrointestinal absorption and complex intracellular processing steps, Cbl as methylcobalamin is a cofactor of the cytosolic methionine synthase (MTR), and Cbl as adenosylcobalamin is a cofactor for the mitochondrial methylmalonyl-CoA mutase (MMUT) [1]. MTR remethylates methionine from homocysteine (Hcy) and provides methyl groups for numerous metabolic reactions such as DNA, RNA, phospholipid and neurotransmitter synthesis. This reaction depends on the provision of methyl groups via the folate cycle [2].
Homocysteine remethylation to methionine is additionally and independently from the B12 pathway performed by the enzyme betaine-homocysteine-S-methyltransferase via betaine as the methyl donor and with choline as a substrate [3]. Choline is frequently a critical nutrient in pregnant women [4].
MMUT is needed for the intramitochondrial conversion of methylmalonic acid (MMA) into succinyl-coA (Figure 1) [2].
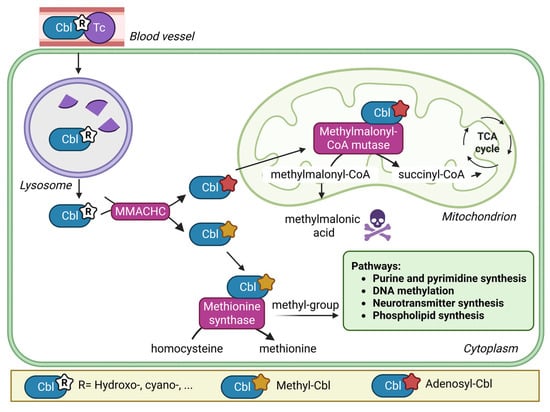
Figure 1.
Simplified pathway of intracellular cobalamin processing. Legend: In blood Cbl is bound to the transport protein transcobalamin (Tc). The Cbl/Tc complex (holoTC) is taken up by the cells via receptor-mediated endocytosis. In the lysosome Tc is degraded and Cbl is liberated. In the cytoplasm, functional groups are removed, Cbl is reduced and the bioactive forms methyl-Cbl and adenosyl-Cbl are synthesised. Created in BioRender. Forny, P. (2025) https://BioRender.com/6443h9d (accessed on 20 August 2025).
The gastrointestinal absorption of Cbl from food is impaired with the chronic use of proton pump inhibitors [5] and in acquired disorders such as pernicious anaemia, chronic inflammatory bowel or coeliac disease, post bariatric surgery [6] or in genetic conditions such as congenital intrinsic factor deficiency [7]. The intracellular processing of Cbl is disrupted in several rare inherited metabolic disorders (e.g., the cblC-MMACHC defect) [8].
Food of animal origin is the primary source of vitamin B12 in humans [9], and insufficient intake is the most prevalent origin of B12 deficiency (B12D) in healthy individuals [10,11,12,13].
The focus of this study is severe, symptomatic B12D in the first year of life caused by insufficient maternal and nutritional Cbl supply. In the first weeks and months of life, infant and maternal B12-related metabolites correlate strongly. Children born to mothers adhering to a vegan or vegetarian diet without supplementing B12 or with unknown Cbl malabsorption or trafficking disorders have a significantly increased risk of B12D due to low stores at birth and impaired B12 supply during breastfeeding [14,15].
Holo-transcobalamin (holoTC) is the active form of Cbl that enters the cells. As explained by its intracellular targets, impaired Cbl bioavailability causes elevated MMA and total Hcy (tHcy) blood concentrations. B12 is almost always assessed, but MMA, tHcy, and holoTC are not as widely used [16]. Methionine is the weakest parameter, as it may be low or remain normal [1].
The optimal indicators and their cutoffs for the diagnosis of B12D have so far not been uniformly defined, especially not for infants. The interpretation of reference intervals or cutoffs must not only be age-related but must consider differences in measurement methods, population sizes, genetic and nutritional backgrounds and calculation models. In clinical practice, B12 < 148 pmol/L is often used as a cutoff for B12D, and 149–221 pmol/L for a low B12 status. However, these reference values have been established in adults [17] and are not without controversy [18]. Reference ranges and cutoffs have so far only been investigated in small and age-diverse paediatric populations. In a Norwegian population the 2.5 percentile of total B12 was 295 (pmol/L in six- to eight-year-old and 249 pmol/L in 9- to 12-year-old children) [19]. The extent to which such references can be transferred to other populations and age groups is unclear. The assessment of plasma B12 does not differentiate between haptocorrin-bound cobalamin and holoTC [17]. For adults, a normal range for holo-TC has been established at 20–125 pmol/L [20]. In the Norwegian sample 56 and 37 pmol/L represented the 2.5th percentile [19]; in a small cohort of younger children, the 2.5th percentile varied with age between about 30 and 45 pmol/L [21].
Serum/plasma MMA concentrations have been considered elevated from >260, >300 or >350 nmol/L [17]. The 97.5th percentile value in a Danish sample of 393 children was 360 nmol/L [18]. Plasma tHcy concentrations of >6.5 [22], >10 [23] and >13 [17] have been suggested as upper cutoffs. Given this variability and lack of clarity as to which cutoffs are best suited to the population under investigation, the clinical diagnosis of B12 deficiency was set as the criterion for inclusion in this study.
In accordance with the significance of the remethylation pathway as the main provider of methyl groups for numerous essential pathways, infant B12D can lead to severe neurological abnormalities such as irritability, feeding difficulties, failure to thrive, developmental delay or loss of achievements [10,24]. Infant B12D may even result in irreversible nervous system damage and brain atrophy. Beyond the neurological symptoms (macrocytic), anaemia is frequent [11,25].
Since June 2018 the Austrian neonatal screening programme (NBS) targets inborn errors of the Cbl pathway (Figure 2). To identify these, methionine, propionylcarnitine (C3) and ratios such as C3/methionine and C3/acetylcarnitine are used as first-tier parameters [12,26]. THcy is the second-tier parameter [27]. These parameters can also be out of range when B12 availability is low.
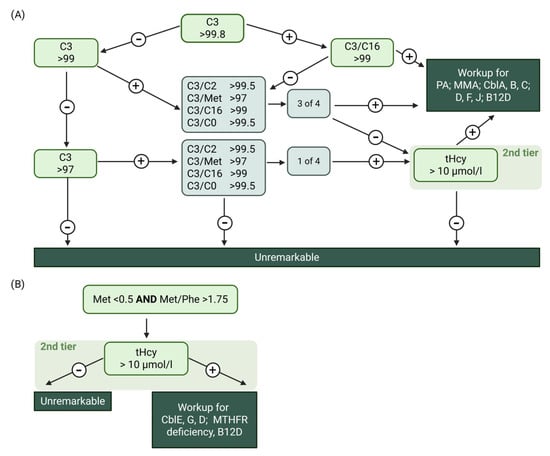
Figure 2.
The Austrian first- and second-tier NBS algorithm to identify B12D. (A) Propionylcarnitine (C3) path, (B) Methionine path. Created in BioRender. Forny, P. (2025) https://BioRender.com/6443h9d (accessed on 20 August 2025).
It is so far unclear whether newborns with clinically diagnosed B12D in infancy are a subgroup of the neonates detected by NBS with low B12 concentrations. Consequently, it is unclear whether early treatment of NBS-identified neonates with low B12 status prevents severe clinical B12D. Thus, it is still under discussion whether detecting and treating children with suboptimal neonatal B12 status is a valuable additional NBS benefit preventing serious harm [28,29].
To address this topic, this study focuses on the question of whether infants that were clinically symptomatic in their first year of life and diagnosed with B12D would have been detected by the Austrian NBS algorithm to allow for preventive treatment. To this purpose, data from infants with clinically diagnosed B12D in their first year of life between 2012 and 2022 were retrospectively collected in Switzerland and Austria and their NBS dried blood spots (DBS)/data were analysed according to the algorithm of the Austrian NBS.
2. Methods
The study was conducted in compliance with ethical standards and received approval from the Ethics Committee of the Medical University of Innsbruck (Austria; EK Nr: 1149/2022) and the cantonal Ethics Committee Zurich (Switzerland; BASEC-Nr: 2022-01725). Due to the retrospective, pseudonymized character of the study, no individual consent was obtained. If in the Swiss centres general consent forms had been in use at the time of patient admission, they had to have been signed, which may result in a selection bias. Patients diagnosed with B12D before implementation of the general consent in the reporting institution were included based on the parents’ consent to NBS, which includes permission to perform research to improve and further develop the Swiss NBS. Data were pseudonymized to protect patient confidentiality. The study group only had access to pseudonymized data provided by the reporting centres and the NBS programme.
3. Patient Cohort
Data from infants clinically diagnosed with B12D in the first 12 months of life and confirmed by biochemical testing according to local standards between January 2012 and December 2022 were included in the study. Exclusion criteria were absence of clinical symptoms (e.g., diagnosis by family screening), unverified B12D or alternative diagnoses such as inborn errors of Cbl metabolism.
In Switzerland, certified paediatric training centres listed on “Schweizerisches Institut für ärztliche Weiter- und Fortbildung” (https://www.siwf.ch/; accessed on 1 November 2022) were contacted. In Austria, paediatric clinics and departments listed on the website of the Austrian Society of Paediatrics (https://www.paediatrie.at/; accessed on 1 June 2022) were contacted. Private practices or other institutions were not contacted. A total of 20 of 86 contacted paediatric units responded to our enquiry. A total 5 of 57 paediatric departments and clinics in Austria and 8 of 29 in Switzerland agreed to contribute clinical and biochemical data from 21 and 31 patients, respectively, with B12D diagnosed during the past 10 years. Four patients did not fulfil the inclusion criteria and were excluded, resulting in a study sample of 48 cases. Using a pseudonymized case questionnaire information on age, sex, anthropometric data at birth and at diagnosis, symptoms at diagnosis and outcome was collected. Percentiles were determined according to Fenton et al. [30] at birth and according to Kromeyer-Hauschild et al. [31] at diagnosis. If the gestational age for term-born children was unknown, it was set at 40 weeks’ gestation for percentile calculation.
Values of B12, holoTC, MMA, tHcy and methionine in blood and MMA in urine were obtained from tests performed in the laboratories of the reporting centres and were collected in their local units (local reference ranges were not collected). The units were converted by the study team for data harmonisation for further analysis (see Table 2). Additionally, type of diet and B12 supplementation at diagnosis were collected for both mother and child. Relevant medical history data of the mother (previous illnesses, bariatric surgery) were also obtained. The centres contacted their respective NBS centre directly to request the B12-related NBS raw data (Austria) or the re-evaluation of available raw data according to the current Austrian NBS algorithm (Switzerland). Notably, the use of percentile cut offs allows the utilisation of the algorithm in different labs independent of absolute values, which may differ from lab to lab due to technical reasons. Percentile distributions for the Swiss cohort were derived separately for each calendar year from all initial newborn screening card values to minimise inter-year variation and long-term drift. Analogous to the Austrian algorithm, screenings that would have triggered a second-tier tHcy measurement were considered positive if predefined percentile thresholds were exceeded. For the acylcarnitine-based criterion, results were classified as positive when C3 exceeded the 99th percentile, or when C3 was above the 95th percentile in combination with at least one of the following ratios above their respective thresholds: C3/Met (>99.5th percentile), C3/C16 (>99.5th percentile), C3/C2 (>99.8th percentile) or C3/C0 (>99.5th percentile). For the methionine-based criterion, screens were considered positive when methionine was below the 0.2nd percentile or when the methionine/phenylalanine ratio was below the 0.2nd percentile.
Pseudonymized Austrian and Swiss data sets were collected and managed using REDCap electronic data capture tools hosted at University Children’s Hospital of Zurich [32,33].
4. Statistical Analysis
First, descriptive analysis (mean, median, SD, IQR, range) of the study parameters (NBS data, age at first symptoms, anthropometric data at birth and at diagnosis; age, symptoms, nutrition and biochemical parameters of the child at diagnosis; treatment of the child, maternal diet at diagnosis, maternal health problems) was performed. Data were checked for content plausibility (CK, MH); outliers were identified during the descriptive analyses.
One-sided correlations were calculated between all biochemical parameters at diagnosis. Two-sided correlation analyses were performed between (a) NBS numerical data and biochemical parameters at diagnosis, (b) age at first symptoms and NBS numerical data, (c) biochemical parameters at diagnosis and total number of symptoms at diagnosis, (d) biochemical parameters at diagnosis and number of symptoms in each symptom category (haematological, failure to thrive, acute/chronic neurologic, movement, cognition) and (e) total number of symptoms and time between first symptoms and diagnosis.
Two-sided Median Test was used to compare the time between first symptoms and diagnosis between groups of children formed based on specific symptoms.
To compare the anthropometric data of the children between birth and time of diagnoses, z-scores for weight, length and head circumference were calculated. Paired T-Test (two-sided) was applied to compare these z-scores between the two time points.
Normality of the data was checked via Kolmogorov–Smirnov test, and linearity was explored via scatterplots. If the data were not normally distributed, the assumption of linearity was not tenable (could not be confirmed) or the variables were ordinal scaled, non-parametric methods (Spearman rank correlation, median test) were used. Missing cases were excluded pairwise. For the Spearman correlations, 95% CIs were computed using the Bonett and Wright method.
To investigate whether the number of children clinically diagnosed with B12D in their first year of life has decreased in Austria since the introduction of NBS for B12D, the observation period was divided into before (1 January 2011–31 May 2018) and after the introduction of NBS for B12D (1 June 2018–31 August 2023). The actual numbers of symptomatic children observed in the two periods was compared with the expected numbers (assuming an unchanged frequency of B12D before and after implementation of NBS for B12D) using one-tailed Chi-square test. For the Swiss cohort this calculation was performed with a two-sided p-value, as a decrease in the number of cases was not expected in this group. The Chi-square test was also used to explore whether the number of cases of symptomatic children in Austria and Switzerland had developed differently between the two study periods.
p-values ≤ 0.05 were considered statistically significant. p-values for correlations between biochemical parameters were adjusted using the Bonferroni–Holm method. Since the other analyses were of explorative character, correction for multiple testing was not applied, which might enhance the potential for type I errors. All calculations were performed with SPSS (IBM SPSS Statistics 29.0.2.0). Graphs were designed using Graph Pad Prism (Version 10.1.2 for Windows, GraphPad Software, Boston, MA, USA, www.graphpad.com; accessed on 15 June 2025). Figures were created with BioRender, (2025, Toronto, ON, Canada), www.biorender.com, accessed on 20 August 2025.
5. Results
Forty-eight cases (42 born at term, 3 preterm, 3 missing data; 23 males) were included. At the time of B12D diagnosis, one infant was supplemented with oral 2 mg/d methylcobalamin, and three received iron preparations. One infant was treated with melatonin.
Mean age at first symptoms of B12D was 5.2 months (median 6, range 0–12 months, n = 45) and age at diagnosis was 5.7 months (median 6, range 0–17 months, n = 43). The diagnosis of B12D was established in 85% of cases within two months from symptom onset. The distribution of clinical symptoms at presentation and the time to diagnosis are shown in Figure 3. Information on auxological parameters, infant and maternal diet and history data on maternal health problems are summarised in Table 1. Four children had further medical conditions that were not considered to cause B12D by the clinicians.
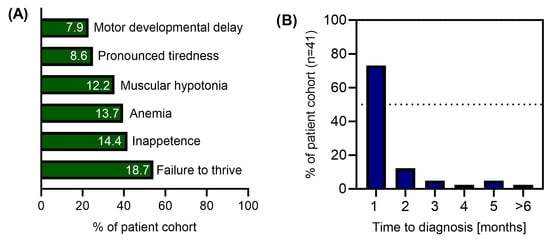
Figure 3.
Major clinical symptoms in infants with vitamin B12 deficiency at presentation (A) and time between first symptoms and diagnosis (B).

Table 1.
Sample characteristics.
Numerical NBS parameters were available for 17 Austrian children and percentiles of NBS parameters for 26 Swiss children (n = 43). Age at first symptoms and NBS parameters did not correlate. Three Austrian children (born before B12D-NBS implementation) and one Swiss child would have been detected by the current Austrian NBS first-tier algorithm resulting in a first-tier diagnostic yield of 9.3% (n = 4/43 cases). Three cases were detected via the C3 path, one by low Met and Met/Phe. Material for retrospective second-tier testing was available for 2/4 first-tier positive cases and, in both, tHcy was above the cutoff.
Case 1: This female term-born child was detected by elevated C3 and the respective ratios and by positive second-tier testing (tHcy 10.8 µmol/L). She had a birthweight of 2840 g (−1.2 z), length of 49 cm (−0.95 z) and a head circumference of 33 cm (−1.23 z). At the age of 6 months, the infant presented with inappetence, repetitive vomiting and failure to thrive with percentile crossing (body weight: −1.87 z; head circumference: −1.62 z). Additionally, muscular hypotonia, lack of movement and a resulting developmental delay were noted. The diagnosis was confirmed with low B12 at 50 ng/L and elevated MMA in urine at 253 mmol/mol creatinine. At the time of diagnosis, the infant was exclusively breastfed and refused solid foods. The cause of the infant’s B12D was traced to a maternal deficiency caused by autoimmune gastritis.
Case 2: This male term-born was detected by elevated C3 and the respective ratios (material for second-tier testing not available). He had a birthweight of 2560g (−1.62 z), length of 49 cm (−1.06 z) and a head circumference of 34 cm (−0.9 z). At the age of 6 months, he presented with muscular hypotonia and anaemia. Auxological parameters were stable: weight: −1.64 z, length −0.96 z, head circumference 0.26. Vitamin B12 levels were very low (<22 ng/L) and urinary MMA was elevated (1220 mmol/mL creatinine). At the time of diagnosis, the boy was breastfed and received solid food. Maternal B12 was low with 178 ng/L.
Case 3: This term-born female was detected by elevated C3 and the respective ratios (material for second-tier testing not available). Her birthweight was 2635g (−1.45 z), and her weight at diagnosis was 4600g (−1.13 z). At the age of three months, she presented with vomiting, anaemia and fatigue. B12D was confirmed by low vitamin B12 levels of 50 ng/L. The girl was exclusively breastfed. The infant’s B12D was attributed to maternal pernicious anaemia.
Case 4: This male term-born with a birthweight of 3500g (0.42 z) and head circumference of 36cm (1.65 z) was detected by decreased Met and Met/Phe ratio. Material for second-tier testing was available and tHcy (15.4 µmol/L) was elevated. He developed muscular hypotonia, reduced appetite, failure to thrive (weight −1.49 z) and anaemia in the first month of life. Diagnosis was confirmed by low levels of vitamin B12 in blood (117 ng/L) and elevated MMA in urine (600 mmol/mol creatinine). The child received formula milk and breastmilk. His mother was diagnosed with autoimmune gastritis, and her B12 (61 ng/L) and holo-TC (6.1 pmol/L) concentrations were low.
The biochemical parameters at diagnosis are shown in Table 2. Two-tailed correlation analyses of NBS numerical data and parameters at diagnosis in the Austrian cohort (n = 17) showed significant correlations between higher C3, C3/Met, C3/C2 and C3/C0 in NBS and lower vitamin B12 in blood at diagnosis (Rho(15) = −0.53, p = 0.03, 95% CI [−0.82, −0.03]; Rho(15) = −0.57, p = 0.02, 95% CI [−0.84, −0.08]; Rho(15) = −0.62, p = 0.01, 95% CI [−0.86, −0.15]; Rho(15) = −0.55, p = 0.02, 95% CI [−0.83, −0.05]).

Table 2.
Biochemical parameters at diagnosis.

Table 3.
Correlations of biochemical parameters at diagnosis and clinical symptoms (two-tailed p).
Table 3.
Correlations of biochemical parameters at diagnosis and clinical symptoms (two-tailed p).
| Biochemical Parameters at Diagnosis | |||||
|---|---|---|---|---|---|
| tHcy | MMA in Blood | MMA in Urine | Vit B12 | holoTC | |
| Total number of symptoms | ρ(23) = 0.71 (p = < 0.001) 95% CI [0.40, 0.88] | ρ(19) = 0.57 (p = 0.01) 95% CI [0.15, 0.82] | ρ(34) = 0.39 (p = 0.02) 95% CI [0.06, 0.64] | ρ(40) = −0.33 (p = 0.03) 95% CI [−0.58, −0.02] | |
| Acute neurological symptoms | ρ(23) = 0.54 (p = 0.01) 95% CI [0.15, 0.78] | ||||
| Chronic neurological symptoms | ρ(23) = 0.43 (p = 0.03) 95% CI (0.02, 0.72] | ||||
| Impaired motor performance | ρ(23) = 0.67 (p < 0.001) 95% CI [0.33, 0.85] | ρ(19) = 0.59 (p = 0.01) 95% CI [0.18, 0.83] | ρ(34) = 0.38 (p = 0.02) 95% CI [0.04, 0.63] | ρ(40) = −0.36 (p = 0.02) 95% CI [−0.60, −0.05] | |
| Cognitive impairment | ρ(12) = 0.61 (p = 0.02) 95% CI [0.06, 0.87] | ||||
| Haematological symptoms | ρ(23) = 0.83 (p < 0.001) 95% CI [0.61, 0.93] | ρ(34) = 0.67 (p < 0.001) 95% CI [0.41, 0.83] | ρ(40) = −0.55 (p < 0.001) 95% CI [−0.74, −0.28] | ||
Higher MMA in urine correlated significantly with higher C3 (Rho(10) = 0.9, p < 0.001, 95% CI [0.59, 0.98]; C3/C2 Rho(10) = 0.71, p = 0.01, 95% CI [0.15, 0.92]; and C3/C0 Rho(10) = 0.75, p = 0.01, 95% CI [0.23, 0.94]). Higher C3/C2, C3/C0 at birth correlated significantly with higher tHcy at diagnosis (Rho(8) = 0.78, p = 0.01, 95% CI [0.20, 0.96]; Rho(8) = 0.65, p = 0.04, 95% CI [−0.05, 0.92]. Correlations between the NBS parameters and MMA in blood and holoTC were not evaluated due to the small numbers of cases (n = 4; n = 3).
One-tailed correlation between the parameters measured at diagnosis was calculated for the complete cohort of Austrian and Swiss children. As expected, low vitamin B12 correlated significantly with high tHcy (Rho(21) = −0.54, padj. = 0.01, 95% CI [−1.00, −0.20]), MMA in blood (Rho(15) = −0.51, padj.= 0.04, 95% CI [−1.00; −0.09]) and MMA in urine (Rho(28) = −0.68, padj. = 0.01, 95% CI [−1.00, −0.44]). While blood and urinary MMA correlated significantly (Rho(14) = 0.69, padj. = 0.01, 95% CI [0.33, 1.00]), tHcy correlated only with urinary MMA (Rho(20) = 0.46, padj. = 0.04, 95% CI [0.10, 1.00]). HoloTC did not correlate with any of the other parameters.
The total number of symptoms did not correlate with the time between first symptoms and diagnosis. The time between first symptoms and diagnosis did not differ when groups of children with versus without a specific symptom (e.g., anaemia or muscular hypotonia; calculated for all symptoms) were compared. Weight z-scores were significantly lower at diagnosis than at birth (t(41) = 2.93, p = 0.01). This corresponds with the reported high frequency of failure to thrive and inappetence at diagnosis.
At diagnosis, 28 infants were fully and 14 partially breastfed. The suspected aetiology of infant B12 deficiency was reported for 21/48 cases. Maternal autoimmune/pernicious anaemia (n = 3), vegan nutrition with insufficient maternal B12 supply (n = 3) and unspecified maternal B12D (n = 15) were considered causal by the clinicians.
The Austrian NBS algorithm for B12-related inborn errors of metabolism was implemented in June 2018. Birth rates in Austria and Switzerland were not significantly different between the periods from January 2011 to June 2018 and from July 2018 to August 2023. Assuming that the NBS algorithm does not prevent infants from developing clinically symptomatic B12D, that factors influencing B12 status do not differ significantly between the two countries and that the incidence of B12D remains stable, numbers of cases should be equally distributed before and after NBS implementation. However, the numbers of cases with symptomatic B12D before (1 January 2011–31 May 2018) and after (1 June 2018–31 August 2023) implementation of B12D-NBS in Austria (Figure 4) developed significantly different between the countries (Chi2 (1, N = 46) =10.01, two-sided p < 0.001). In the Austrian cohort the expected counts (11 before, 8 after implementation) and the observed counts (14 before, 5 after implementation; 95% CI [7.65–23.49]; 95% CI [1.62–11.67]) were not significantly different (Chi2 (1, N = 19= 1.79, one-sided p = 0.09, d = 0.65). In Switzerland the expected (16 before, 11 after June 2018) and observed counts (6 before, 21 after June 2018) were significantly different (Chi2 (1, N = 27) = 14.69, two-sided p < 0.001), indicating more observed cases in recent years.
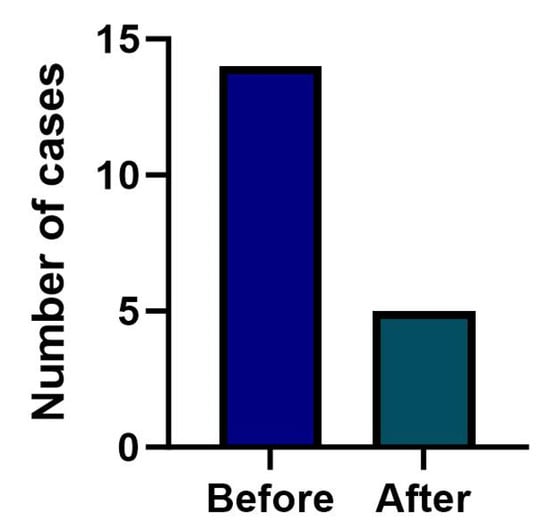
Figure 4.
Numbers of clinically diagnosed infants with B12D before and after the implementation of B12-related NBS in Austria in June 2018.
Information on the treatment of the children with B12D was available for 45 cases. Forty children were treated with Cbl, most of them parenterally (n = 14 cyanocobalamin, n = 26 hydroxocobalamin). Oral cyanocobalamin was administered in two cases, and oral and intranasal methylcobalamin were used in one case each. Doses, application frequency and duration of treatment varied widely from a single dose to 2–7 doses of Cbl in the first week. Cbl treatment was repeated weekly or monthly in some children, and in single cases given for up to one year. In one case, only a switch from breast milk to formula was initiated. Treatment of the mothers was reported for eight cases (three parenterally, one oral, four missing information), no treatment for two cases and for the remaining cases, treatment status was unknown (n = 27) or data were missing (n = 11). Thirty-eight children recovered fully after treatment. Persistent developmental impairment was observed in four, persistent failure to thrive in one, inadequate length gain in one and muscular hypotonia in one patient(s) were noted (n = 3 missing data). No dose–effect or route of application–effect ratios were observed.
6. Discussion
In an earlier publication we reported on the positive predictive value of the Austrian NBS Algorithm for the detection of suboptimal neonatal B12 status in the Austrian population. Of 203.440 screened neonates, 7127 (3.5%) had a positive first-tier test, and in 0.079% (161 cases) the second-tier test tHcy was also above norms. Of these, 34% (55 cases) were diagnosed as B12D if they fulfilled at least one of the following criteria: serum B12 < 150 pmol/L or holoTC < 25 pmol/L or serum MMA > 600 nmol/L [16].
The current study was conducted to explore whether infants that were clinically symptomatic in their first year of life and diagnosed with B12D would have been detected by the Austrian NBS algorithm to allow for preventive treatment. The related question of whether the detection of newborns using this algorithm makes a significant contribution to the advantages of NBS in relation to resource consumption is also discussed.
In a similar study, DBS from 70 Norwegian children with B12D were retrospectively analysed according to the Austrian and the Heidelberg (Germany) algorithms [29]. Our study was conducted because the comparability of the Norwegian and the here-reported cohort must be considered limited, not only due to different genetic backgrounds, but also since in Norway, nitrous oxide (N2O) is frequently used for pain relief in mothers during labour. N2O is not widely used for pain relief in mothers during labour in Austria, but is used by some centres in Switzerland. However, exact information on its use is not available.
Through oxidation of the cobalt ion contained in cobalamin from its functional +1 oxidation state to a non-functional +3 state by N2O, the intracellular functions of methyl- and adenosylmethionine come to a standstill [34], causing an increase in MMA but—probably more importantly—an arrest of the Hcy-methionine cycle, the main donor of methyl groups that are crucial for the synthesis of myelin or the post-translational methylation of the DNA [35]. Thus, maternal N2O use during labour affects B12 bioavailability and functionality in both mother and child [36] and might result in earlier (infant age of 6 months in our cohort versus 10.9 weeks in Norway at workup for B12D) or more pronounced B12D. With the Austrian first-tier test set, four (5.7%) of the seventy Norwegian B12D cases could retrospectively be identified, one by the C3 and three by the Met algorithm. Two of these four cases were also second-tier test positive (tHcy > 6.3 µmol/L at the time). This diagnostic yield of 2.9% in this Norwegian population resembles the performance of the Heidelberg algorithm (4.3%) that had a higher rate of positive first-tier tests (18.6%), requiring tHcy assessment [29]. In this study, three cases were identified by the C3 and one by the Met algorithm. The different distribution of cases detected by the C3 and the Met algorithm between this and the Norwegian study may reflect the effect of N2O, which primarily inhibits methionine synthesis [34]. THcy was above norm in both cases, in which second-tier testing could retrospectively be conducted. This results in a retrospectively confirmed first- and second-tier detection rate of 4.3%. Since the retrospective detection rate of the first-tier test was 9.3% (4 out of 42 cases, with NBS data or material missing in 6 cases), this value corresponds to the maximum possible first and second-tier detection rate for this population, which is like the Norwegian study [29].
We studied the differences between the number of expected and the observed cases before and after implementation of B12D-targeting NBS and found a rise in case numbers in Switzerland, where NBS for B12D is not performed. Although this pattern is consistent with the possibility that B12D-targeting NBS may reduce the reporting of symptomatic B12D, interpretation must remain cautious. Limitations to this conclusion include probable ascertainment bias, different states of B12-related awareness over time and between countries, changing referral patterns, demographic shifts or missing information on the dietary habits and their changes in both countries, as well as small sample sizes. While the observation that clinically diagnosed B12D in the first year of life was reported four times more frequently in German populations without compared to populations with B12-related NBS [37] supports this hypothesis, it does not establish causality.
The balance between resource use and effect of a preventive measure becomes increasingly important in times of dwindling resources and rising costs in healthcare systems, and it must be carefully considered whether B12D should be included as a target disease in NBS programmes in future. Our data and the retrospective analysis of 70 Norwegian cases suggest that NBS has some, albeit very limited, effectiveness in identifying children who developed severe, clinically diagnosed B12D in their first year of life [29]. It was confirmed in this cohort that B12D is a treatable condition, although in the absence of standardised treatment protocols a substantial variability regarding dose, route and duration of B12 treatment was observed.
The many newborns also identified by the algorithm and not affected by inborn errors of the pathways seem to have a suboptimal B12 status with a not yet precisely quantified risk for health impairment.
The costs for an individual case workup and treatment triggered by NBS results indicative of B12D include state-of-the-art biochemical testing, successful treatment with hydroxocobalamin (and folate) and follow-up blood testing, which need to be considered and may vary between different countries or health systems. In an Italian cohort costs per case were calculated to be € 3578 [38]. As it is advised to perform reverse cascade testing of the mother following a NBS recall indicating the possibility of an underlying maternal disease or increased health risk [39], even higher costs per mother–child dyad must be assumed. Not only must the cost–benefit analysis be considered, but the acceptance of NBS and the protection of families from avoidable stress are also highly valued. A comprehensive psychological study used standardised methods to examine the emotional reactions and assessments of parents regarding their child’s health after they had been called back to one of the Italian metabolic centres to be informed of a positive NBS result. In this study, 80% of 169 fathers and 171 mothers reported a clinical level of distress and anxiety, some even experienced post-traumatic symptoms and a majority wished for psychosocial support without differences between cases later confirmed as true or false positive [40].
The incidence of neonatal B12D associated with maternal B12D has been estimated to be 32% in Western populations [41,42]. Three mothers from our cohort of children with severe, symptomatic B12D in infancy were diagnosed with autoimmune gastritis or pernicious anaemia, and one with a not further specified gastritis. The prevalence of autoimmune gastritis is estimated at 1–2% in the general population [43], but the condition is considered underdiagnosed [44]. Women are affected more frequently. Not only is autoimmune gastritis associated with other autoimmune diseases [43], but it impairs the functioning and distribution of immune cells. These dysfunctions can be corrected by treatment with Cbl [45]. Insufficient maternal B12 nutritional intake was the most frequent cause to which infant B12D was attributed by the clinicians. Insufficient B12 intake and declining B12 levels during pregnancy have been described in many populations [46]. In an Italian cohort with mixed ethnic background, B12 intake during pregnancy did not reach the here recommended 2.6 µmol/d in 59% of women [38].
Maternal B12D is not only associated with the risk for consecutive B12D in neonates or infants but with adverse pregnancy outcomes and maternal health risks. Low maternal B12 levels (<148 pmol/L) are associated with preterm birth, low birth weight [47], higher maternal blood glucose levels and increased risk for gestational diabetes [48]. B12D is an important cause of maternal anaemia during pregnancy and anaemia in pregnancy is associated with an increased risk of maternal morbidity, preterm birth and low birth weight [49].
Taking a medical history with a focus on diseases predisposing to or causing B12D and assessing B12 status as part of routine care during early pregnancy would open opportunities for interventions that might benefit both mother and child. Women with low B12 concentrations in early pregnancy should receive diagnostic workup for B12 resorption disorders or low nutritional supply. Prospective maternal screening for B12D during pregnancy would allow healthcare providers to inform about the importance of B12, to provide nutritional counselling and to prescribe B12 supplements during pregnancy and breastfeeding for mothers with B12D-relevant health issues or nutritional habits.
The B12 status of the mother, umbilical cord blood [50] and newborn B12-related parameters [16] correlate strongly with each other, but not every newborn of a B12-deficient mother will have B12D, and some newborns may have B12D despite adequate maternal B12 status [50]. These data suggest that the transplacental maternal-to-foetus transport rate of B12 is variable [51].
The efficacy of oral vitamin B12 supplementation during pregnancy has recently been reviewed. Optimisation of the maternal B12 status can reduce maternal and neonatal B12D, but the evidence for short- and long-term effects of B12 supplementation on mother and child is still uncertain and may vary significantly between populations [52].
Our data tentatively indicate that NBS for B12D may contribute to preventing an increase in clinically diagnosed severe B12D. However, the interpretation of this trend is limited by the incomplete reporting of cases, which precludes an accurate estimate of the true number of affected individuals. Future studies should therefore assess whether the observed pattern persists once data reflecting the full case spectrum become available. In addition, it deserves to be investigated whether the approach to supplement women at risk or with manifest B12D during pregnancy would, together with NBS, be more effective and economical in preventing severe, symptomatic B12D in infants and in reducing the rate of burdensome NBS recalls and workups.
This study has several limitations. The diagnosis of B12D was made by clinicians and did not follow a uniform algorithm, and the diagnostic criteria for B12D in children are generally not sufficiently standardised. The variability in diagnostic criteria as well as of the assays used in different institutions might undermine the comparability of cases, and potentially bias prevalence estimates and conclusions about NBS effectiveness. Furthermore, we must assume selection bias because it remains uncertain whether all cases of severe B12D have been ascertained by our retrospective questionnaire approach dating back over ten years, given the limited response rate from the centres. A reporting bias towards more memorable severe cases cannot be excluded. Material for second-tier testing was not available for all first-tier positives and second-tier tHcy testing was performed on DBS after variable times of storage.
Conclusions: B12 is related to multiple important methylation reactions. B12D can cause severe clinical symptoms, especially in infants, and should be prevented. NBS has a very limited detection rate of newborns who develop severe B12D during infancy; most of them have a normal NBS. However, it appears that NBS may contribute to a reduction in the incidence of cases through other mechanisms that have not yet been precisely quantified. The workup triggered by NBS recalls is costly and may place a strain on families during a particularly sensitive period. Maternal B12D is associated with pregnancy-related and general health risks for mother and child. The B12 status of newborns and their mothers are highly associated. Women should be screened for B12D routinely during pregnancy to be counselled and treated to promote a healthy pregnancy and breastfeeding period and to reduce the frequency of NBS recalls.
Author Contributions
Conceptualization, M.H. (Martina Huemer); Formal Analysis, M.H. (Marion Herle); Data Curation, C.K. and J.M.; Original Draft Preparation, M.H. (Marion Herle), C.K. and M.H. (Martina Huemer); Writing—Review and Editing, all authors; Investigation, C.K., J.M., M.R.B., W.B., C.D., B.H., D.K., H.K., V.K., A.L., R.M., P.M., M.P., V.P., F.R.-G., R.K.S., S.S., N.v.d.W. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Medical University of Innsbruck (Austria, 7 July 2022) and the cantonal Ethics Committee Zurich (Switzerland; BASEC-Nr: 2022-01725; 15 November 2024).
Informed Consent Statement
Due to the retrospective, pseudonymized character of the study, no individual consent was obtained. If in the Swiss centres general consent forms had been in use at the time of patient admission, they had to have been signed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
M.R. Baumgartner is supported by the Swiss National Science Foundation [310030_175779 and 310030_212505] and the University Research Priority Program of the University of Zurich ITINERARE—Innovative Therapies in Rare Diseases. C. Kaufman is supported by the Children’s Research Center (FZK)-Protected Time Grant from the University Children’s Hospital Zurich sponsored by the Claus Cramer Stiftung.
Conflicts of Interest
M Huemer has received project-related research funding from Nutricia Metabolics, Travere, Sanofi, and honoraria for scientific advice from Nutricia Metabolics, Recordati, Chiesi, Immedica Pharma, and Sanofi (all unrelated to this work).
References
- McCorvie, T.J.; Ferreira, D.; Yue, W.W.; Froese, D.S. The complex machinery of human cobalamin metabolism. J. Inherit. Metab. Dis. 2023, 46, 406–420. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B(12), folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Sternbach, S.; West, N.; Singhal, N.K.; Clements, R.; Basu, S.; Tripathi, A.; Dutta, R.; Freeman, E.J.; McDonough, J. The BHMT-betaine methylation pathway epigenetically modulates oligodendrocyte maturation. PLoS ONE 2021, 16, e0250486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McGee, M.; Bainbridge, S.; Fontaine-Bisson, B. A crucial role for maternal dietary methyl donor intake in epigenetic programming and fetal growth outcomes. Nutr. Rev. 2018, 76, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Maideen, N.M.P. Adverse Effects Associated with Long-Term Use of Proton Pump Inhibitors. Chonnam Med. J. 2023, 59, 115–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guéant, J.-L.; Guéant-Rodriguez, R.-M.; Alpers, D.H. Chapter Nine—Vitamin B12 absorption and malabsorption. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: New York, NY, USA, 2022; Volume 119, pp. 241–274. [Google Scholar]
- Watkins, D.; Rosenblatt, D.S. Inherited defects of cobalamin metabolism. Vitam. Horm. 2022, 119, 355–376. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, R.; Baruteau, J. Inherited and acquired vitamin B12 deficiencies: Which administration route to choose for supplementation? Front. Pharmacol. 2022, 13, 972468. [Google Scholar] [CrossRef] [PubMed]
- Niklewicz, A.; Hannibal, L.; Warren, M.; Ahmadi, K.R. A systematic review and meta-analysis of functional vitamin B12 status among adult vegans. Nutr. Bull. 2024, 49, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Bito, T. Vitamin B(12) sources and microbial interaction. Exp. Biol. Med. (Maywood) 2018, 243, 148–158. [Google Scholar] [CrossRef]
- Langan, R.C.; Goodbred, A.J. Vitamin B12 Deficiency: Recognition and Management. Am. Fam. Physician 2017, 96, 384–389. [Google Scholar]
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693s–696s. [Google Scholar] [CrossRef] [PubMed]
- Devalia, V.; Hamilton, M.S.; Molloy, A.M.; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 2014, 166, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Wirthensohn, M.; Wehrli, S.; Ljungblad, U.W.; Huemer, M. Biochemical, Nutritional, and Clinical Parameters of Vitamin B12 Deficiency in Infants: A Systematic Review and Analysis of 292 Cases Published between 1962 and 2022. Nutrients 2023, 15, 4960. [Google Scholar] [CrossRef]
- Bjørkevoll, S.M.G.; O’Keeffe, M.; Konijnenberg, C.; Solvik, B.S.; Sødal, A.F.; Kaldenbach, S.; McCann, A.; Ueland, P.M.; Kvestad, I.; Ersvær, E.; et al. Infant vitamin B12 status and its predictors—Cross-sectional baseline results from an ongoing randomized controlled trial. Am. J. Clin. Nutr. 2025, 122, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Knopfli, S.; Goeschl, B.; Zeyda, M.; Baghdasaryan, A.; Baumgartner-Kaut, M.; Baumgartner, M.R.; Herle, M.; Margreitter, J.; Poms, M.; Wortmann, S.B.; et al. The Benefit of Detecting Reduced Intracellular B12 Activity through Newborn Screening Remains Unclear. Int. J. Neonatal Screen. 2024, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, L.; Lysne, V.; Bjørke-Monsen, A.L.; Behringer, S.; Grünert, S.C.; Spiekerkoetter, U.; Jacobsen, D.W.; Blom, H.J. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front. Mol. Biosci. 2016, 3, 27, Erratum in Front. Mol. Biosci. 2017, 4, 53. [Google Scholar] [CrossRef]
- Abildgaard, A.; Knudsen, C.S.; Hoejskov, C.S.; Greibe, E.; Parkner, T. Reference intervals for plasma vitamin B12 and plasma/serum methylmalonic acid in Danish children, adults and elderly. Clin. Chim. Acta 2022, 525, 62–68. [Google Scholar] [CrossRef]
- Strand, M.F.; Hager, H.B.; Fredriksen, P.M.; Lindberg, M. Reference intervals for holotranscobalamin and total vitamin B12 in 6-12-year-old children in Norway: The Health Oriented Pedagogical Project (HOPP). Scand. J. Clin. Lab. Invest. 2025, 85, 138–142. [Google Scholar] [CrossRef]
- Valente, E.; Scott, J.M.; Ueland, P.M.; Cunningham, C.; Casey, M.; Molloy, A.M. Diagnostic accuracy of holotranscobalamin, methylmalonic acid, serum cobalamin, and other indicators of tissue vitamin B12 status in the elderly. Clin. Chem. 2011, 57, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Karlaftis, V.; Hearps, S.; Attard, C.; Savoia, H.; Campbell, J.; Monagle, P.; HAPPI Kids Study Team. Continuous reference intervals for holotranscobalamin, homocysteine and folate in a healthy paediatric cohort. Ann. Clin. Biochem. 2024, 61, 469–473. [Google Scholar] [CrossRef]
- Bjørke-Monsen, A.L.; Torsvik, I.; Saetran, H.; Markestad, T.; Ueland, P.M. Common metabolic profile in infants indicating impaired cobalamin status responds to cobalamin supplementation. Pediatrics 2008, 122, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Refsum, H.; Smith, A.D.; Ueland, P.M.; Nexo, E.; Clarke, R.; McPartlin, J.; Johnston, C.; Engbaek, F.; Schneede, J.; McPartlin, C.; et al. Facts and recommendations about total homocysteine determinations: An expert opinion. Clin. Chem. 2004, 50, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Effect of vitamin B12 deficiency on neurodevelopment in infants: Current knowledge and possible mechanisms. Nutr. Rev. 2008, 66, 250–255. [Google Scholar] [CrossRef]
- Huemer, M.; Baumgartner, M.R. The clinical presentation of cobalamin-related disorders: From acquired deficiencies to inborn errors of absorption and intracellular pathways. J. Inherit. Metab. Dis. 2019, 42, 686–705. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulou, V.; Greber-Platzer, S.; Zeyda, M. Österreichisches Neugeborenen-Screening—Früherkennung von Vitamin-B12-Mangel im Fokus. Pädiatrie Pädologie 2021, 56, 163–167. [Google Scholar] [CrossRef]
- Rozmaric, T.; Mitulovic, G.; Konstantopoulou, V.; Goeschl, B.; Huemer, M.; Plecko, B.; Spenger, J.; Wortmann, S.B.; Scholl-Bürgi, S.; Karall, D.; et al. Elevated Homocysteine after Elevated Propionylcarnitine or Low Methionine in Newborn Screening Is Highly Predictive for Low Vitamin B12 and Holo-Transcobalamin Levels in Newborns. Diagnostics 2020, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Gramer, G.; Hoffmann, G.F. Vitamin-B12-Mangel im Neugeborenen- und Säuglingsalter—Ursachen, Früherkennung, Diagnostik und Vorstellung eines primär oralen Behandlungsschemas. Monatsschrift Kinderheilkd. 2023, 171, 717–725. [Google Scholar] [CrossRef]
- Ljungblad, U.W.; Lindberg, M.; Eklund, E.A.; Sæves, I.; Sagredo, C.; Bjørke-Monsen, A.-L.; Tangeraas, T. A Retrospective Evaluation of the Predictive Value of Newborn Screening for Vitamin B12 Deficiency in Symptomatic Infants Below 1 Year of Age. Int. J. Neonatal Screen. 2022, 8, 66. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschrift Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Zou, X.; Yi, F.; Zhou, W.; Dong, Y.; Alhaskawi, A.; Zhou, H.; Ezzi, S.H.A.; Kota, V.G.; Abdulla, M.H.A.H.; Alenikova, O.; et al. Mechanisms and recent advances in the diagnosis and treatment of nitrous oxide-induced peripheral neuropathy: A narrative review. Front. Neurol. 2024, 15, 1381938. [Google Scholar] [CrossRef] [PubMed]
- Badshah, A.; Aijaz, A.; Bhat, A.; Babar, A. Nitrous Oxide: No More a Laughing Matter. Cureus 2025, 17, e85467. [Google Scholar] [CrossRef] [PubMed]
- Ljungblad, U.W.; Lindberg, M.; Eklund, E.A.; Saeves, I.; Bjorke-Monsen, A.L.; Tangeraas, T. Nitrous oxide in labour predicted newborn screening total homocysteine and is a potential risk factor for infant vitamin B12 deficiency. Acta Paediatr. 2022, 111, 2315–2321. [Google Scholar] [CrossRef]
- Mütze, U.; Gleich, F.; Haas, D.; Urschitz, M.M.S.; Röschinger, W.; Janzen, N.; Hoffmann, G.F.; Garbade, S.F.; Syrbe, S.; Kölker, S. Vitamin B12 Deficiency Newborn Screening. Pediatrics 2024, 154, e2023064809. [Google Scholar] [CrossRef]
- Ferraro, S.; Lucchi, S.; Montanari, C.; Magnani, L.; Tosi, M.; Biganzoli, D.; Lugotti, A.; Cappelletti, L.; Poli, A.; Pratiffi, E.; et al. Vitamin B12 deficiency in newborns: Impact on individual’s health status and healthcare costs. Clin. Chem. Lab. Med. 2025, 63, 559–571. [Google Scholar] [CrossRef]
- Gaviglio, A.; Petritis, K.; Tagi, V.; Vasco, A.; Mauri, A.; Zuccotti, G.; Verduci, E.; Cereda, C.; Ferraro, S. Beyond newborn screening: The role of reverse cascade testing in familial disease detection. Crit. Rev. Clin. Lab. Sci. 2025, 1–11. [Google Scholar] [CrossRef]
- Bani, M.; Caviglia, S.; Bensi, G.; Carcereri, M.S.; Greco, B.; Lastrucci, E.; Massa, P.; Vissani, S.; Cazzorla, C. Availability of psychological resources for parents receiving communication of positivity at newborn screening for metabolic diseases in Italy. Eur. J. Pediatr. 2024, 183, 965–969. [Google Scholar] [CrossRef]
- Hinton, C.F.; Ojodu, J.A.; Fernhoff, P.M.; Rasmussen, S.A.; Scanlon, K.S.; Hannon, W.H. Maternal and neonatal vitamin B12 deficiency detected through expanded newborn screening--United States, 2003-2007. J. Pediatr. 2010, 157, 162–163. [Google Scholar] [CrossRef]
- Tan, A.; Sinclair, G.; Mattman, A.; Vallance, H.D.; Lamers, Y. Maternal vitamin B12 status in early pregnancy and its association with birth outcomes in Canadian mother–newborn Dyads. Br. J. Nutr. 2021, 126, 1823–1831. [Google Scholar] [CrossRef]
- Rustgi, S.D.; Bijlani, P.; Shah, S.C. Autoimmune gastritis, with or without pernicious anemia: Epidemiology, risk factors, and clinical management. Therap. Adv. Gastroenterol. 2021, 14, 17562848211038771. [Google Scholar] [CrossRef]
- Thain, A.; Hart, K.; Ahmadi, K.R. Addressing the Gaps in the Vitamin B12 Deficiency 2024 NICE Guidelines: Highlighting the Need for Better Recognition, Diagnosis, and Management of Pernicious Anaemia. Eur. J. Clin. Nutr. 2025, 79, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Habtie, T.E.; Zemariam, A.B.; Dagnaw, B.W.; Alamaw, A.W.; Feleke, S.F.; Adisu, M.A. The Immunomodulatory Effect of Vitamin B12 in Pernicious Anemia: A Systematic Review. Oxid. Med. Cell. Longev. 2025, 2025, 8463993. [Google Scholar] [CrossRef] [PubMed]
- Venkatramanan, S.; Armata, I.E.; Strupp, B.J.; Finkelstein, J.L. Vitamin B-12 and Cognition in Children. Adv. Nutr. 2016, 7, 879–888. [Google Scholar] [CrossRef]
- Rogne, T.; Tielemans, M.J.; Chong, M.F.; Yajnik, C.S.; Krishnaveni, G.V.; Poston, L.; Jaddoe, V.W.V.; Steegers, E.A.P.; Joshi, S.; Chong, Y.-S.; et al. Associations of Maternal Vitamin B12 Concentration in Pregnancy With the Risks of Preterm Birth and Low Birth Weight: A Systematic Review and Meta-Analysis of Individual Participant Data. Am. J. Epidemiol. 2017, 185, 212–223. [Google Scholar] [CrossRef]
- Saravanan, P.; Sukumar, N.; Adaikalakoteswari, A.; Goljan, I.; Venkataraman, H.; Gopinath, A.; Bagias, C.; Yajnik, C.S.; Stallard, N.; Ghebremichael-Weldeselassie, Y.; et al. Association of maternal vitamin B(12) and folate levels in early pregnancy with gestational diabetes: A prospective UK cohort study (PRiDE study). Diabetologia 2021, 64, 2170–2182. [Google Scholar] [CrossRef]
- Sifakis, S.; Pharmakides, G. Anemia in pregnancy. Ann. N. Y. Acad. Sci. 2000, 900, 125–136. [Google Scholar] [CrossRef]
- Yıldırmak, Z.Y.; Genç, D.B.; Kural, A.; Mıhmanlı, V.; Salman, S.; Doğan, K.; Çiftçi, M.A.; Efeoğlu, N.D.; Erdoğan, A.; Çaltek, N.Ç.; et al. Assessment of vitamin B12 and homocysteine levels in pregnant women admitted for delivery and cord blood samples of their newborn babies: A multicenter study. Turk. J. Pediatr. 2024, 66, 429–438. [Google Scholar] [CrossRef]
- Joshi, N.; Sahay, A.; Mane, A.; Sundrani, D.; Randhir, K.; Wagh, G.; Thornburg, K.; Powell, T.; Yajnik, C.; Joshi, S. Altered expression of nutrient transporters in syncytiotrophoblast membranes in preeclampsia placentae. Placenta 2023, 139, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.L.; Fothergill, A.; Venkatramanan, S.; Layden, A.J.; Williams, J.L.; Crider, K.S.; Qi, Y.P. Vitamin B12 supplementation during pregnancy for maternal and child health outcomes. Cochrane Database Syst. Rev. 2024, 1, Cd013823. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).