Vitamin D in Atopic Dermatitis: Role in Disease and Skin Microbiome
Abstract
1. Introduction
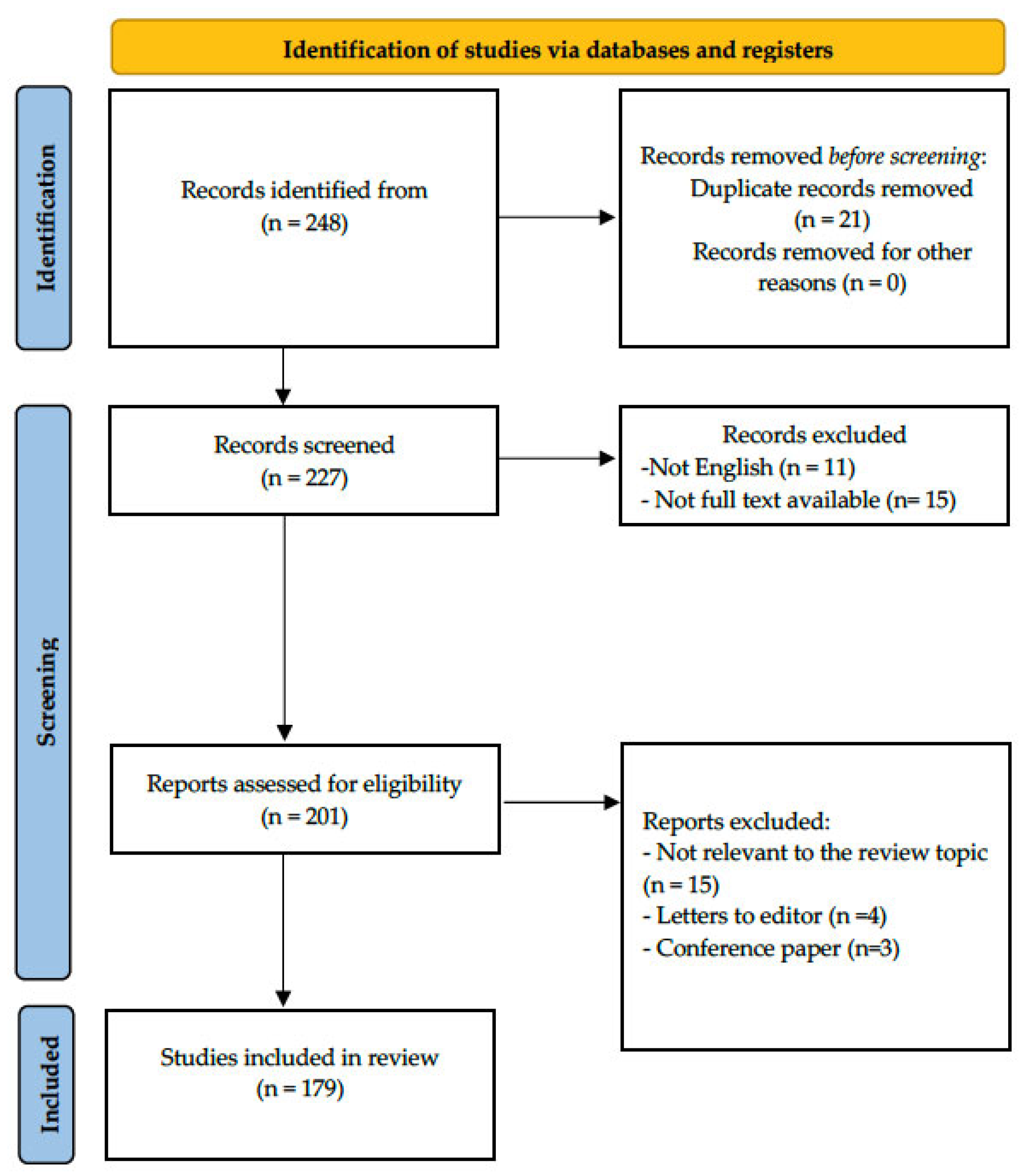
2. Materials and Methods
3. Vitamin D Metabolism and Skin Immune Mechanisms
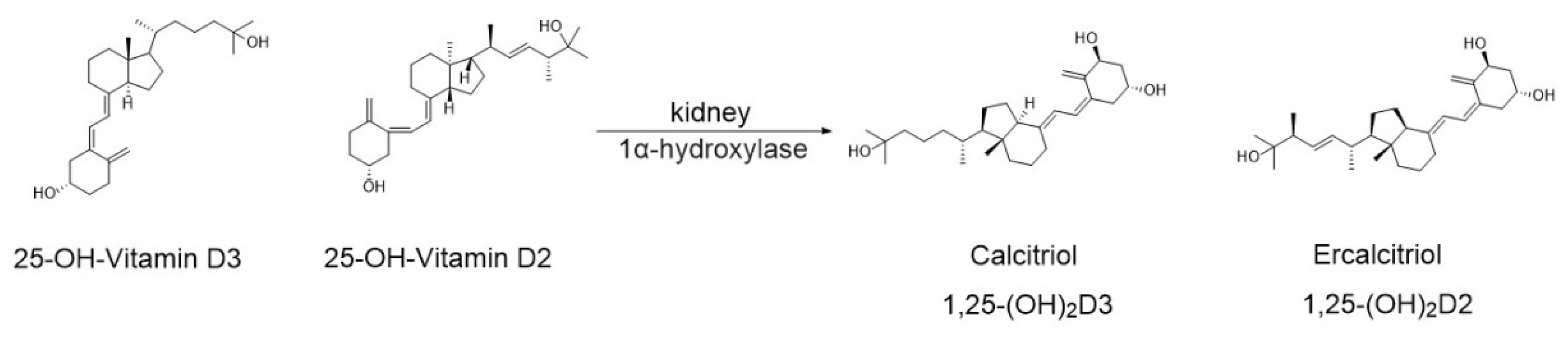
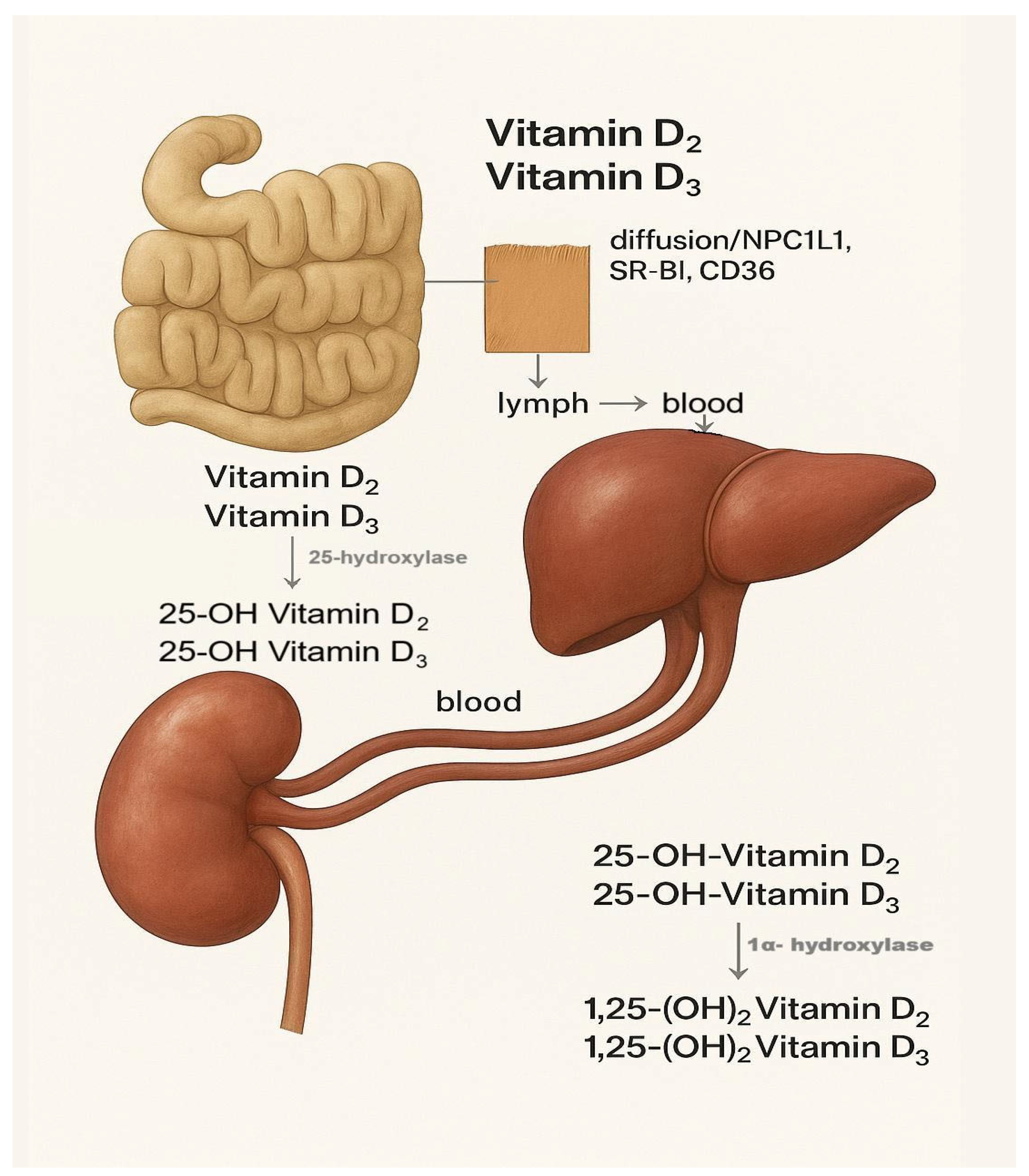
3.1. Vitamin D Metabolism
3.2. Skin Immune Mechanisms
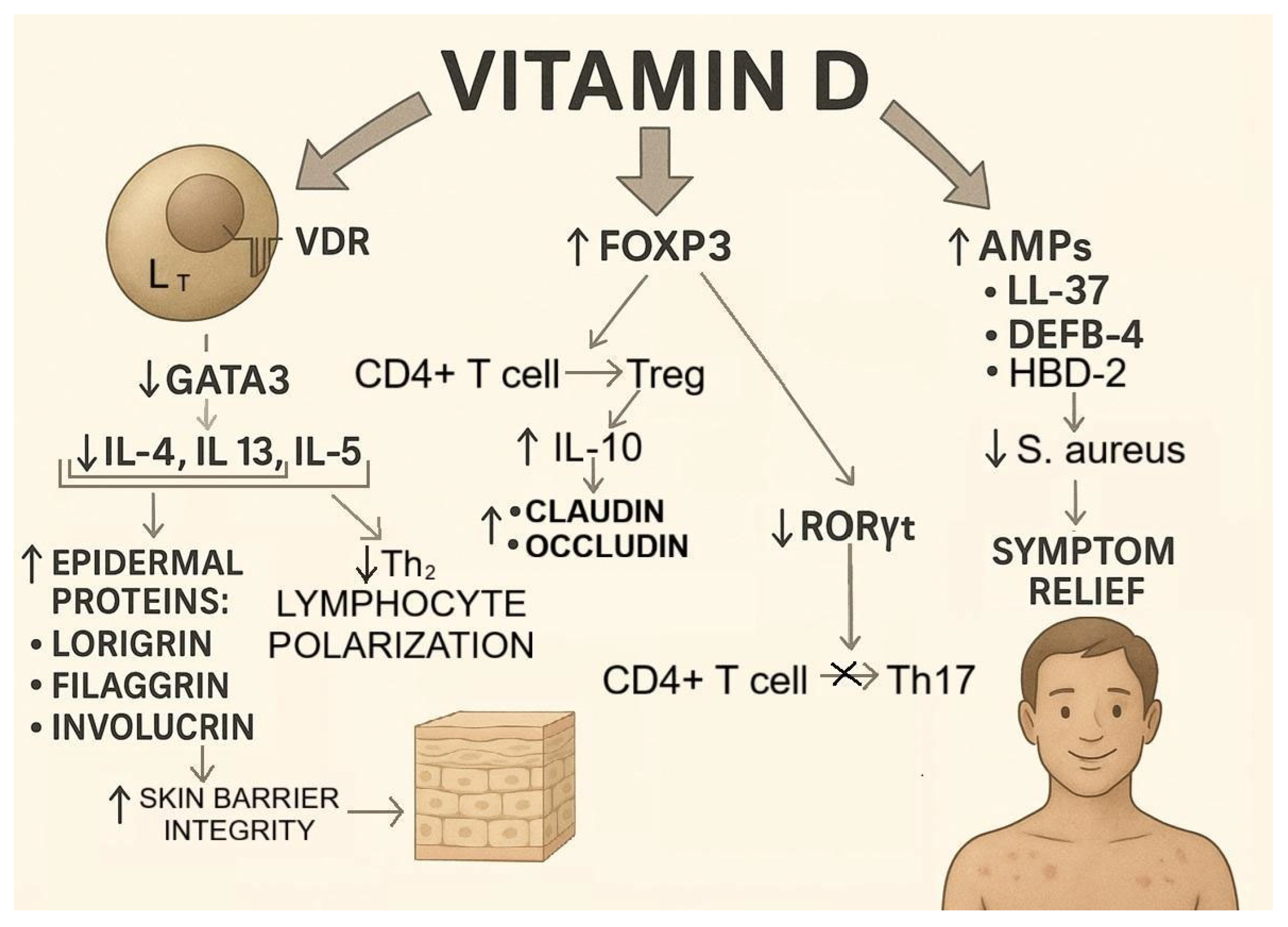
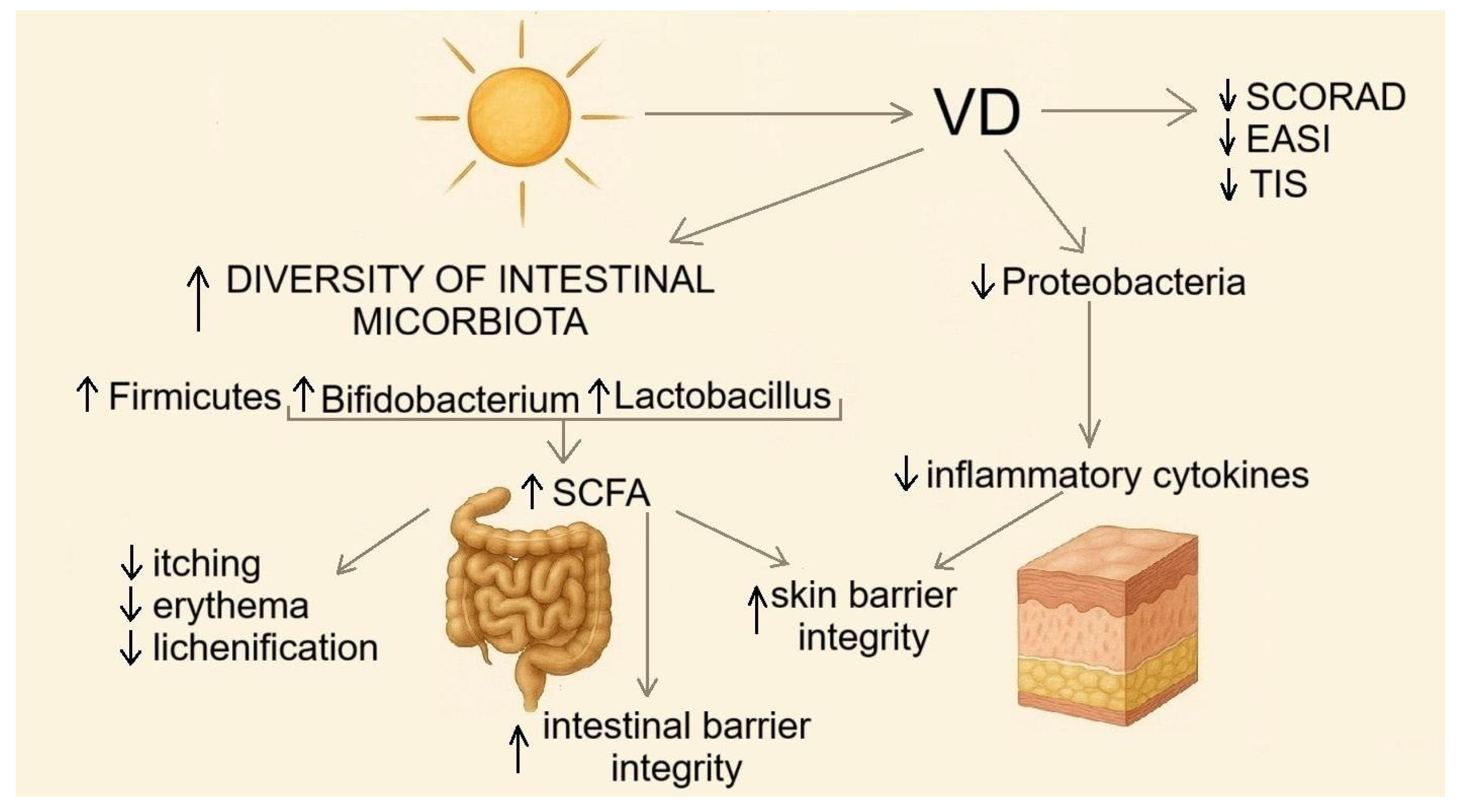
4. The Role of Vitamin D in the Pathogenesis and Treatment of Atopic Dermatitis/Immune Pathways Linking the Microbiota and Atopic Dermatitis
- Inhibition of Th2 polarization: By binding to the VDR in the nucleus of T lymphocytes, VD downregulates the transcription factor GATA3 [77], which is critical for the polarization of naive CD4+ T cells into Th2 cells. This reduces the production of cytokines (IL-4, IL-5, and IL-13) essential for Th2 polarization, thereby preventing excessive pathological Th2 differentiation, which is a hallmark of autoimmune diseases, including AD [78].
- Promotion of regulatory T cell (Treg) differentiation: VD enhances the expression of the transcription factor forkhead box P3 (FOXP3), the master regulator of Treg development, in naive T cells [79]. This leads to both increased Treg differentiation and suppression of Th17 differentiation, since FOXP3 inhibits the expression of RORγt, the transcription factor required for Th17 lineage commitment [80].
- Induction of AMPs: In AD, 70–90% of lesions are colonized by S. aureus, which exacerbates inflammation and impairs barrier function [81]. VD increases the production of AMPs, including LL-37 [82] and human β-defensin 4 (DEFB-4) [83], which exert strong antimicrobial effects against S. aureus. By upregulating genes encoding AMPs, VD enhances their production and limits the pathogenic role of S. aureus in AD [84].
- Upregulation of epidermal structural proteins: VD indirectly increases the expression of the key epidermal proteins filaggrin, involucrin, and loricrin by suppressing IL-4 and IL-13, which otherwise downregulate their synthesis [85]. A proper balance of these proteins ensures epidermal barrier integrity and contributes to improved AD outcomes [86] (Figure 7).
5. Evidence from Animal Models and Mechanistic Studies
- (I).
- strengthens barrier structure (filaggrin, loricrin, and TJ proteins);
- (II).
- limits Th2/Th17 inflammation and alarmins (TSLP/IL-33);
- (III).
- enhances antimicrobial defense (AMPs, especially LL-37).
6. Vitamin D as a Modulator of the Skin Microbiome in Atopic Dermatitis
7. Potential Therapeutic Approaches, Clinical Implications, and Future Directions
8. Strengths and Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,25(OH)2D3 | 1,25-Dihydroxycholecalciferol, calcitriol |
| 1,25(OH)2D2 | 1,25-Dihydroxyergocalciferol, ercalcitriol |
| 25(OH)D | 25-Hydroxyvitamin D |
| 7-DHC | 7-dehydrocholesterol |
| 1,25(OH)2D | 1,25-Dihydroxyvitamin D |
| AD | Atopic dermatitis |
| AhR | Aryl hydrocarbon receptor |
| AMPs | Antimicrobial peptides |
| AQP3 | Aquaporin 3 |
| BMI | Body mass index |
| CD | Cluster of differentiation |
| CLDN1 | Claudin-1 |
| CXCL1 | C-X-C motif chemokine ligand 1 |
| CXCR2 | C-X-C motif chemokine receptor 2 |
| DBP | Vitamin D-binding protein |
| DEFB-4 | Human β-defensin 4 |
| DLQI | Dermatology Life Quality Index |
| DMOG | Dimethyloxalylglycine |
| EASI | Eczema Area and Severity Index |
| FGF7 | Fibroblast growth factor 7 |
| FLG | Filaggrin |
| FOXP3 | Forkhead box P3 |
| HBD-2 | Human β-defensin 2 |
| HIF-ROS | Hypoxia-inducible factor—reactive oxygen species |
| IAld | Indole-3-aldehyde |
| IAA | Indole-3-acetic acid |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IL | Interleukin |
| JAK1 | Janus kinase 1 |
| LL-37 | Human cathelicidin antimicrobial peptide |
| MC903 (Calcipotriol) | Vitamin D analog used experimentally in dermatology |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| NPC1L1 | Niemann-Pick C1-like protein 1 |
| POEM | Patient-Oriented Eczema Measure |
| RCT | Randomized controlled trial |
| Raptor-KO | Regulatory-associated protein of mTOR knockout |
| SCFA | Short-chain fatty acids |
| SCORAD | Scoring Atopic Dermatitis |
| SR-BI | Scavenger receptor class B type I |
| STAT6 | Signal transducer and activator of transcription 6 |
| TEWL | Transepidermal water loss |
| Th | T helper cells |
| TIS | Three-Item Severity |
| TJs | Tight junctions |
| TLR3 | Toll-like receptor 3 |
| tol-DCs | Tolerogenic dendritic cells |
| TNF-α | Tumor necrosis factor alpha |
| TRMs | Tissue-resident macrophages |
| Treg | Regulatory T cells |
| TSLP | Thymic stromal lymphopoietin |
| UVB | Ultraviolet B radiation |
| VD | Vitamin D |
| VD2 | Vitamin D2 (ergocalciferol) |
| VD3 | Vitamin D3 (cholecalciferol) |
| VDR | Vitamin D receptor |
| ZO | Zonula occludens |
References
- Borrego-Ruiz, A.; Borrego, J.J. Nutritional and Microbial Strategies for Treating Acne, Alopecia, and Atopic Dermatitis. Nutrients 2024, 16, 3559. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Walton, J.; Roberts, N.; Hussain, K. Viral infections in atopic dermatitis. Clin. Exp. Dermatol. 2024, 50, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, G.; Guo, Q.; Yang, Y.; Yang, J.; Feng, X.; Yao, Z. Allergens in Atopic Dermatitis. Clin. Rev. Allergy Immunol. 2025, 68, 11. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Su, J.; Tian, F.; Zhou, Y.; Liu, S.; Lou, F. Risk of depression in patients with atopic dermatitis: An updated systematic review and meta-analysis of children, adolescent and adult groups. J. Paediatr. Child Health 2024, 60, 640–647. [Google Scholar] [CrossRef]
- Fitzmaurice, W.; Silverberg, N.B. Long-Term Impact of Atopic Dermatitis on Quality of Life. Dermatol. Clin. 2024, 42, 549–557. [Google Scholar] [CrossRef]
- Chatrath, S.; Lei, D.; Yousaf, M.; Chavda, R.; Gabriel, S.; Silverberg, J.I. Longitudinal course and predictors of depressive symptoms in atopic dermatitis. J. Am. Acad. Dermatol. 2022, 87, 582–591. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Renert-Yuval, Y.; Brunner, P.M. Atopic dermatitis. Lancet 2025, 405, 583–596. [Google Scholar] [CrossRef]
- Joshi, A.A.; Vocanson, M.; Nicolas, J.F.; Wolf, P.; Patra, V. Microbial derived antimicrobial peptides as potential therapeutics in atopic dermatitis. Front. Immunol. 2023, 14, 1125635. [Google Scholar] [CrossRef]
- Tokura, Y.; Hayano, S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol. Int. 2022, 71, 14–24. [Google Scholar] [CrossRef]
- Rusu, E.; Enache, G.; Cursaru, R.; Alexescu, A.; Radu, R.; Onila, O.; Cavallioti, T.; Rusu, F.; Posea, M.; Jinga, M.; et al. Prebiotics and probiotics in atopic dermatitis. Exp. Ther. Med. 2019, 18, 926–931. [Google Scholar] [CrossRef]
- Fyhrquist, N.; Yang, Y.; Karisola, P.; Alenius, H. Endotypes of atopic dermatitis. J. Allergy Clin. Immunol. 2025, 156, 24–40.e4. [Google Scholar] [CrossRef]
- Wienholtz, N.K.F.; Vestergaard, C.; Deleuran, M.; Drljevic-Nielsen, A.; Andersson, A.M. Atopisk eksem [Atopic dermatitis]. Ugeskr Laeger 2025, 187, V10240722. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, D.; Feng, Z.; Zhu, X.; Yang, F.; Zhang, B.; Hu, M.; Wang, Y.; Feng, H.; Yu, Y.; et al. Diterpenoid DGT alleviates atopic dermatitis-like responses in vitro and in vivo via targeting IL-4Rα. Biomed. Pharmacother. 2024, 179, 117321. [Google Scholar] [CrossRef] [PubMed]
- Afshari, M.; Kolackova, M.; Rosecka, M.; Čelakovská, J.; Krejsek, J. Unraveling the skin; a comprehensive review of atopic dermatitis, current understanding, and approaches. Front. Immunol. 2024, 15, 1361005. [Google Scholar] [CrossRef] [PubMed]
- Nevid, M.; Boguniewicz, M. Current and Emerging Biologics for Atopic Dermatitis. Immunol. Allergy Clin. N. Am. 2024, 44, 577–594. [Google Scholar] [CrossRef]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Gallagher, K.; Halperin-Goldstein, S.; Paller, A.S. New treatments in atopic dermatitis update. Ann. Allergy Asthma Immunol. 2025, 135, 498–510.e10. [Google Scholar] [CrossRef]
- Elizalde-Jiménez, I.G.; Ruiz-Hernández, F.G.; Carmona-Cruz, S.A.; Pastrana-Arellano, E.; Aquino-Andrade, A.; Romo-González, C.; Arias-de la Garza, E.; Álvarez-Villalobos, N.A.; García-Romero, M.T. Global Antimicrobial Susceptibility Patterns of Staphylococcus aureus in Atopic Dermatitis: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2024, 160, 1171–1181. [Google Scholar] [CrossRef]
- Qian, X.; Tong, M.; Zhang, T.; Li, Q.; Hua, M.; Zhou, N.; Zeng, W. IL-24 promotes atopic dermatitis-like inflammation through driving MRSA-induced allergic responses. Protein Cell 2025, 16, 188–210. [Google Scholar] [CrossRef]
- Saheb Kashaf, S.; Harkins, C.P.; Deming, C.; Joglekar, P.; Conlan, S.; Holmes, C.J.; NISK Comparative Sequencing Program; Almeida, A.; Finn, R.D.; Segre, J.A.; et al. Staphylococcal diversity in atopic dermatitis from an individual to a global scale. Cell Host Microbe 2023, 31, 578–592.e6. [Google Scholar] [CrossRef]
- Demessant-Flavigny, A.L.; Connétable, S.; Kerob, D.; Moreau, M.; Aguilar, L.; Wollenberg, A. Skin microbiome dysbiosis and the role of Staphylococcus aureus in atopic dermatitis in adults and children: A narrative review. J. Eur. Acad. Dermatol. Venereol. 2023, 37 (Suppl. S5), 3–17. [Google Scholar] [CrossRef]
- Meledathu, S.; Naidu, M.P.; Brunner, P.M. Update on atopic dermatitis. J. Allergy Clin. Immunol. 2025, 155, 1124–1132. [Google Scholar] [CrossRef]
- Paternoster, L. Genetic landscape of atopic dermatitis. Curr. Opin. Allergy Clin. Immunol. 2024, 24, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.P.; Bikle, D.D.; Elias, P.M. Evidence that loss-of-function filaggrin gene mutations evolved in Northern Europeans to favor intracutaneous vitamin D3 production. Evol. Biol. 2014, 41, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Criado, P.R.; Miot, H.A.; Bueno-Filho, R.; Ianhez, M.; Criado, R.F.J.; de Castro, C.C.S. Update on the pathogenesis of atopic dermatitis. An. Bras. Dermatol. 2024, 99, 895–915. [Google Scholar] [CrossRef] [PubMed]
- Sidbury, R.; Sullivan, A.F.; Thadhani, R.I.; Camargo, C.A. Randomized controlled trial of vitamin D supplementation for winter related atopic dermatitis in Boston: A pilot study. Br. J. Dermatol. 2008, 159, 245–247. [Google Scholar] [CrossRef]
- Sullivan, E.; Firnhaber, J. Atopic and Contact Dermatitis. Prim. Care 2025, 52, 553–566. [Google Scholar] [CrossRef]
- Bath-Hextall, F.J.; Jenkinson, C.; Humphreys, R.; Williams, H.C. Dietary supplements for established atopic eczema. Cochrane Database Syst. Rev. 2012, 2015, CD005205. [Google Scholar] [CrossRef]
- Hattangdi-Haridas, S.R.; Lanham-New, S.A.; Wong, W.H.S.; Ho, M.H.K.; Darling, A.L. Vitamin D deficiency and effects of vitamin D supplementation on disease severity in patients with atopic dermatitis: A systematic review and meta-analysis in adults and children. Nutrients 2019, 11, 1854. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, M.; Celiberto, L.S.; Yang, H.; Sham, H.P.; Vallance, B.A. The gut-skin axis: A bi-directional, microbiota-driven relationship with therapeutic potential. Gut Microbes 2025, 17, 2473524. [Google Scholar] [CrossRef]
- Grieco, T.; Paolino, G.; Moliterni, E.; Chello, C.; Sernicola, A.; Egan, C.G.; Morelli, M.; Nannipieri, F.; Battaglia, S.; Accoto, M.; et al. Transcriptomic profiling of lesional and perilesional skin in atopic dermatitis suggests barrier dysfunction, inflammatory activation, and alterations to vitamin D metabolism. Int. J. Mol. Sci. 2025, 26, 6152. [Google Scholar] [CrossRef]
- Samanta, S. Vitamin D and immunomodulation in the skin: A useful affirmative nexus. Explor. Immunol. 2021, 1, 90–111. [Google Scholar] [CrossRef]
- Nielsen, A.Y.; Høj, S.; Thomsen, S.F.; Meteran, H. Vitamin D supplementation for treating atopic dermatitis in children and adults: A systematic review and meta-analysis. Nutrients 2024, 16, 4128. [Google Scholar] [CrossRef]
- Li, Q.; Chan, H. Vitamin D and skin disorders: Bridging molecular insights to clinical innovations. Mol. Med. 2025, 31, 259. [Google Scholar] [CrossRef] [PubMed]
- Przechowski, K.; Krawczyk, M.N.; Krasowski, R.; Pawliczak, R.; Kleniewska, P. Vitamin D and atopic dermatitis—A mere correlation or a real supportive treatment option? Nutrients 2025, 17, 2582. [Google Scholar] [CrossRef]
- Udompataikul, M.; Huajai, S.; Chalermchai, T.; Taweechotipatr, M.; Kamanamool, N. The effects of oral vitamin D supplement on atopic dermatitis: A clinical trial with Staphylococcus aureus colonization determination. J. Med. Assoc. Thai 2015, 98 (Suppl. S9), S23–S30. [Google Scholar]
- Cabalín, C.; Pérez-Mateluna, G.; Iturriaga, C.; Camargo, C.A.J.r.; Borzutzky, A. Oral vitamin D modulates the epidermal expression of the vitamin D receptor and cathelicidin in children with atopic dermatitis. Arch. Dermatol. Res. 2023, 315, 761–770. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, M.M. Vitamin D and vitamin D-binding protein in health and disease. Int. J. Mol. Sci. 2023, 24, 4642. [Google Scholar] [CrossRef]
- Cashman, K.D.; Kiely, M. Chapter 58-Vitamin D and Food Fortification. W: Feldman & Pike’s Vitamin D; Elsevier: Amsterdam, The Netherlands, 2024; pp. 135–160. [Google Scholar] [CrossRef]
- Crafa, A.; Cannarella, R.; Cannarella, V.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Retrospective real world study on vitamin D supplementation: Looking for the most effective molecule and its frequency of use. Clin. Nutr. 2025, 47, 265–274. [Google Scholar] [CrossRef]
- Wu, S.E.; Chen, W.L. Moderate sun exposure is the complementor in insufficient vitamin D consumers. Front. Nutr. 2022, 9, 832659. [Google Scholar] [CrossRef]
- Kallioğlu, M.A.; Sharma, A.; Kallioğlu, A.; Kumar, S.; Khargotra, R.; Singh, T. UV index-based model for predicting synthesis of (pre-)vitamin D3 in the Mediterranean basin. Sci. Rep. 2024, 14, 3541. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Proteins involved in fat-soluble vitamin and carotenoid transport across the intestinal cells: New insights from the past decade. Prog. Lipid Res. 2023, 92, 101166. [Google Scholar] [CrossRef] [PubMed]
- Kiourtzidis, M. Modifiers of Absorption, Tissue Distribution and Activation of Vitamin D: The Role of NPC1L1, SR-B1, CD36, and ABC-G5/G8 Transporters. Ph.D. Thesis, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany, 2023. Available online: https://d-nb.info/134023386X/34 (accessed on 3 September 2025).
- Bendotti, G.; Biamonte, E.; Leporati, P.; Goglia, U.; Ruggeri, R.M.; Gallo, M. Vitamin D supplementation: Practical advice in different clinical settings. Nutrients 2025, 17, 783. [Google Scholar] [CrossRef] [PubMed]
- Kampka, Z.; Czapla, D.; Wojakowski, W.; Stanek, A. Vitamin D supplementation in heart failure—Confusion without a cause? Nutrients 2025, 17, 1839. [Google Scholar] [CrossRef]
- Kise, S.; Iijima, A.; Nagao, C.; Okada, T.; Mano, H.; Nishikawa, M.; Ikushiro, S.; Kanemoto, Y.; Kato, S.; Nakanishi, T.; et al. Functional analysis of vitamin D receptor (VDR) using adenovirus vector. J. Steroid Biochem. Mol. Biol. 2023, 230, 106275. [Google Scholar] [CrossRef]
- Casella, A.; Long, C.; Zhou, J.; Lai, M.; O’Lear, L.; Caplan, I.; Levine, M.A.; Roizen, J.D. Differential frequency of CYP2R1 variants across populations reveals pathway selection for vitamin D homeostasis. J. Clin. Endocrinol. Metab. 2020, 105, 1302–1315. [Google Scholar] [CrossRef]
- Baroncelli, G.I.; Comberiati, P.; Aversa, T.; Baronio, F.; Cassio, A.; Chiarito, M.; Cosci o di Coscio, M.; De Sanctis, L.; Di Iorgi, N.; Faienza, M.F.; et al. Diagnosis, treatment, and management of rickets: A position statement from the Bone and Mineral Metabolism Group of the Italian Society of Pediatric Endocrinology and Diabetology. Front. Endocrinol. 2024, 15, 1383681. [Google Scholar] [CrossRef]
- Wang, L.K.; Shanmugasundaram, M.; Cooney, E.; Lee, P.D.K. Siblings with vitamin D-dependent rickets type 1A: Importance of genetic testing and a review of genotype-phenotype correlations. Am. J. Med. Genet. A 2024, 194, e63780. [Google Scholar] [CrossRef]
- Bin Rubaian, N.F.; Al-Awam, B.S.; Aljohani, S.M.; Almuhaidib, S.R. Alopecia with vitamin D-dependent rickets type 2A: A case report. Clin. Cosmet. Investig. Dermatol. 2024, 17, 13–16. [Google Scholar] [CrossRef]
- Jiao, Q.; Zhi, L.; You, B.; Wang, G.; Wu, N.; Jia, Y. Skin homeostasis: Mechanism and influencing factors. J. Cosmet. Dermatol. 2024, 23, 1518–1526. [Google Scholar] [CrossRef]
- Kwiecien, K.; Zegar, A.; Jung, J.; Brzoza, P.; Kwitniewski, M.; Godlewska, U.; Grygier, B.; Kwiecinska, P.; Morytko, A.; Cichy, J. Architecture of antimicrobial skin defense. Cytokine Growth Factor Rev. 2019, 49, 70–84. [Google Scholar] [CrossRef]
- Agarwal, S.; Krishnamurthy, K. Histology, Skin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Zwirner, J.; Hammer, N. Anatomy and physiology of the skin. In Scars; Nischwitz, S.P., Kamolz, L.P., Branski, L.K., Eds.; Springer: Cham, Switzerland, 2024; pp. 1–16. [Google Scholar] [CrossRef]
- MacGibeny, M.A.; Adjei, S.; Pyle, H.; Bunick, C.G.; Ghannoum, M.; Grada, A.; Harris-Tryon, T.; Tyring, S.K.; Kong, H.H. The human skin microbiome in health. J. Am. Acad. Dermatol. 2025, 93, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ahle, C.M.; Stødkilde, K.; Poehlein, A.; Bömeke, M.; Streit, W.R.; Wenck, H.; Reuter, J.H.; Hüpeden, J.; Brüggemann, H. Interference and co-existence of staphylococci and Cutibacterium acnes within the healthy human skin microbiome. Commun. Biol. 2022, 5, 923. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, G.; Abudouwanli, A.; Yang, M.; Sun, Q.; Zhao, W.; Ikeda, A.; Tan, Y.; Ma, L.; Ogawa, H.; et al. The interaction between the skin microbiome and antimicrobial peptides within the epidermal immune microenvironment: Bridging insights into atopic dermatitis. Allergol. Int. 2025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.E.; Zheng, P.; Ye, S.Z.; Ma, X.; Liu, E.; Pang, Y.B.; He, Q.Y.; Zhang, Y.X.; Li, W.Q.; Zeng, J.H.; et al. Microbiome: Role in Inflammatory Skin Diseases. J. Inflamm. Res. 2024, 17, 1057–1082. [Google Scholar] [CrossRef]
- Puls, J.-S.; Winnerling, B.; Power, J.J.; Krüger, A.M.; Brajtenbach, D.; Johnson, M.; Bilici, K.; Camus, L.; Fließwasser, T.; Schneider, T.; et al. Staphylococcus epidermidis bacteriocin A37 kills natural competitors with a unique mechanism of action. ISME J. 2024, 18, wrae044. [Google Scholar] [CrossRef]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef]
- Lima, R.D.; Hajiarbabi, K.; Ng, B.D.; Sood, A.; Ferreira, R.B.R. Skin-associated commensal microorganisms and their metabolites. J. Appl. Microbiol. 2025, 136, lxaf111. [Google Scholar] [CrossRef]
- Zheng, Y.; Hunt, R.L.; Villaruz, A.E.; Fisher, E.L.; Liu, R.; Liu, Q.; Cheung, G.Y.C.; Li, M.; Otto, M. Commensal Staphylococcus epidermidis contributes to skin barrier homeostasis by generating protective ceramides. Cell Host Microbe 2022, 30, 301–313.e9. [Google Scholar] [CrossRef]
- Wang, W.-L.; Lai, Y.-H.; Huang, C.-H.; Lai, J.-Y.; Yao, C.-H. Lumbrokinase-containing gelatin nanofibers with multiple bioactivities for effective skin wound healing. Mater. Today Bio. 2025, 32, 101713. [Google Scholar] [CrossRef]
- Boraldi, F.; Lofaro, F.D.; Bonacorsi, S.; Mazzilli, A.; Garcia-Fernandez, M.; Quaglino, D. The role of fibroblasts in skin homeostasis and repair. Biomedicines 2024, 12, 1586. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Duan, Z.; Chen, X.; Li, M. The immune function of dermal fibroblasts in skin defence against pathogens. Exp. Dermatol. 2023, 32, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Fleckner, M.; Döhmen, N.K.; Salz, K.; Christophers, T.; Windolf, J.; Suschek, C.V.; Oezel, L. Exposure of primary human skin fibroblasts to carbon dioxide-containing solution significantly reduces TGF-β-induced myofibroblast differentiation in vitro. Int. J. Mol. Sci. 2024, 25, 13013. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Duan, Z.; Zeng, R.; Yang, L.; Liu, W.; Liu, Y.; Yao, Q.; Chen, X.; Zhang, L.-J.; Li, M. Antimicrobial peptide-producing dermal preadipocytes defend against Candida albicans skin infection via the FGFR-MEK-ERK pathway. PLoS Pathog. 2023, 19, e1011754. [Google Scholar] [CrossRef]
- Lee, S.H.; Sacks, D.L. Resilience of dermis resident macrophages to inflammatory challenges. Exp. Mol. Med. 2024, 56, 2105–2112. [Google Scholar] [CrossRef]
- Guan, F.; Wang, R.; Yi, Z.; Luo, P.; Liu, W.; Xie, Y.; Liu, Z.; Xia, Z.; Zhang, H.; Cheng, Q. Tissue macrophages: Origin, heterogeneity, biological functions, diseases and therapeutic targets. Signal Transduct. Target. Ther. 2025, 10, 93. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.; Li, S. Heterogeneity and plasticity of tissue-resident memory T cells in skin diseases and homeostasis: A review. Front. Immunol. 2024, 15, 1378359. [Google Scholar] [CrossRef]
- Ruchti, F.; LeibundGut-Landmann, S. New insights into immunity to skin fungi shape our understanding of health and disease. Parasite Immunol. 2023, 45, e12948. [Google Scholar] [CrossRef]
- Reider, I.E.; Lin, E.; Krouse, T.E.; Parekh, N.J.; Nelson, A.M.; Norbury, C.C. γδ T cells mediate a requisite portion of a wound healing response triggered by cutaneous poxvirus infection. Viruses 2024, 16, 425. [Google Scholar] [CrossRef]
- Lujan, R.A.; Pei, L.; Shannon, J.P.; Dábilla, N.; Dolan, P.T.; Hickman, H.D. Widespread and dynamic expression of granzyme C by skin-resident antiviral T cells. Front. Immunol. 2023, 14, 1236595. [Google Scholar] [CrossRef]
- Ziadlou, R.; Pandian, G.N.; Hafner, J.; Akdis, C.A.; Stingl, G.; Maverakis, E.; Brüggen, M.C. Subcutaneous adipose tissue: Implications in dermatological diseases and beyond. Allergy 2024, 79, 3310–3325. [Google Scholar] [CrossRef]
- Guan, J.; Wu, C.; He, Y.; Lu, F. Skin-associated adipocytes in skin barrier immunity: A mini-review. Front. Immunol. 2023, 14, 1116548. [Google Scholar] [CrossRef] [PubMed]
- Erfanian, S.; Jafaripour, S.; Jokar, M.H.; Sedighi, S.; Jahromi, A.S.; Razavi, F.; Zargar, M.F.; Moradzadeh, M. The effect of vitamin D on GATA3 gene expression in peripheral blood mononuclear cells in allergic asthma. Adv. Respir. Med. 2022, 90, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.; Lee, S.K.; Jeong, S.-Y.; Jung, M.; Han, S.-D.; Lee, J.; You, H.; Kim, H.; Kim, T.M. Amelioration of atopic dermatitis via suppression of Th2 receptor expression by miR-22-3p and HSP70 in extracellular vesicles. J. Allergy Clin. Immunol. 2025, 155, AB222. [Google Scholar] [CrossRef]
- Li, D.; Ma, X.; Zhang, W.; Zhong, P.; Li, M.; Liu, S. Impact of vitamin D3 supplementation on motor functionality and the immune response in Parkinson’s disease patients with vitamin D deficiency. Sci. Rep. 2025, 15, 25154. [Google Scholar] [CrossRef]
- Cartes-Velásquez, R.; Vera, A.; Torres-Quevedo, R.; Medrano-Díaz, J.; Pérez, A.; Muñoz, C.; Carrillo-Bestagno, H.; Nova-Lamperti, E. The immunomodulatory role of vitamin D in regulating the Th17/Treg balance and epithelial-mesenchymal transition: A hypothesis for gallbladder cancer. Nutrients 2024, 16, 4134. [Google Scholar] [CrossRef]
- Wang, Z.; Hülpüsch, C.; Traidl-Hoffmann, C.; Reiger, M.; Schloter, M. Understanding the role of Staphylococcus aureus in atopic dermatitis: Strain diversity, microevolution, and prophage influences. Front. Med. 2024, 11, 1480257. [Google Scholar] [CrossRef]
- Aidoukovitch, A.; Bankell, E.; Svensson, D.; Nilsson, B.O. Vitamin D triggers hCAP18/LL-37 production: Implications for LL-37-induced human osteoblast cytotoxicity. Biochem. Biophys. Res. Commun. 2024, 712–713, 149962. [Google Scholar] [CrossRef]
- Bishop, L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and immune regulation: Antibacterial, antiviral, anti-inflammatory. JBMR Plus 2020, 5, e10405. [Google Scholar] [CrossRef]
- Artusa, P.; White, J.H. Vitamin D and its analogs in immune system regulation. Pharmacol. Rev. 2025, 77, 100032. [Google Scholar] [CrossRef]
- Napolitano, M.; di Vico, F.; Ruggiero, A.; Fabbrocini, G.; Patruno, C. The hidden sentinel of the skin: An overview on the role of interleukin-13 in atopic dermatitis. Front. Med. 2023, 10, 1165098. [Google Scholar] [CrossRef]
- Moosbrugger-Martinz, V.; Leprince, C.; Méchin, M.C.; Simon, M.; Blunder, S.; Gruber, R.; Dubrac, S. Revisiting the roles of filaggrin in atopic dermatitis. Int. J. Mol. Sci. 2022, 23, 5318. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.T.; Chiu, C.J.; Tsai, C.Y.; Lee, Y.-R.; Liu, W.-L.; Chuang, H.-L.; Huang, M.-T. Short-chain fatty acids ameliorate allergic airway inflammation via sequential induction of PMN-MDSCs and Treg cells. J. Allergy Clin. Immunol. Glob. 2023, 2, 100163. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, K.; Kim, W. Gut microbiota restoration through fecal microbiota transplantation: A new atopic dermatitis therapy. Exp. Mol. Med. 2021, 53, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Shao, J.H.; Liao, Y.T.; Wang, L.-N.; Jia, Y.; Dong, P.-J.; Liu, Z.-Z.; He, D.-D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Kim, K.; Jang, H.; Kim, E.; Kim, H.; Sung, G.Y. Recent advances in understanding the role of the skin microbiome in the treatment of atopic dermatitis. Exp. Dermatol. 2023, 32, 2048–2061. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.G. Vitamin D receptor influences intestinal barriers in health and disease. Cells 2022, 11, 1129. [Google Scholar] [CrossRef]
- Wang, J.; Mei, L.; Hao, Y.; Xu, Y.; Yang, Q.; Dai, Z.; Yang, Y.; Wu, Z.; Ji, Y. Contemporary perspectives on the role of vitamin D in enhancing gut health and its implications for preventing and managing intestinal diseases. Nutrients 2024, 16, 2352. [Google Scholar] [CrossRef]
- Grieco, T.; Moliterni, E.; Paolino, G.; Chello, C.; Sernicola, A.; Egan, C.G.; Nannipieri, F.; Battaglia, S.; Accoto, M.; Tirotta, E.; et al. Association between vitamin D receptor polymorphisms, tight junction proteins and clinical features of adult patients with atopic dermatitis. Dermatol. Pract. Concept. 2024, 14, e2024214. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Aljamaei, H.M.; Stadnyk, A.W. The production and function of endogenous interleukin-10 in intestinal epithelial cells and gut homeostasis. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1343–1352. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Moore, B.B.; Zhang, S.; Xiao, P.; Decker, A.M.; Wang, H.-L. IL-17: Balancing protective immunity and pathogenesis. J. Immunol. Res. 2023, 2023, 3360310. [Google Scholar] [CrossRef]
- Davydova, A.; Kurochkina, Y.; Goncharova, V.; Vorobyeva, M.; Korolev, M. The interleukin-17 cytokine family: Role in development and progression of spondyloarthritis, current and potential therapeutic inhibitors. Biomedicines 2023, 11, 1328. [Google Scholar] [CrossRef]
- Porbahaie, M.; Hummel, A.; Saouadogo, H.; Coelho, R.M.L.; Savelkokoul, H.F.J.; Teodorowicz, M.; van Neerven, R.J.J. Short-chain fatty acids inhibit the activation of T lymphocytes and myeloid cells and induce innate immune tolerance. Benef. Microbes 2023, 14, 401–419. [Google Scholar] [CrossRef]
- Hatano, Y.; Elias, P.M. “Outside-to-inside,” “inside-to-outside,” and “intrinsic” endogenous pathogenic mechanisms in atopic dermatitis: Keratinocytes as the key functional cells involved in both permeability barrier dysfunction and immunological alterations. Front. Immunol. 2023, 14, 1239251. [Google Scholar] [CrossRef] [PubMed]
- Berdyshev, E. Skin lipid barrier: Structure, function and metabolism. Allergy Asthma Immunol. Res. 2024, 16, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Bahman, F.; Choudhry, K.; Al-Rashed, F.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. Aryl hydrocarbon receptor: Current perspectives on key signaling partners and immunoregulatory role in inflammatory diseases. Front. Immunol. 2024, 15, 1421346. [Google Scholar] [CrossRef] [PubMed]
- Rakateli, L.; Huchzermeier, R.; van der Vorst, E.P.C. AhR, PXR and CAR: From xenobiotic receptors to metabolic sensors. Cells 2023, 12, 2752. [Google Scholar] [CrossRef]
- Han, H.; Safe, S.; Jayaraman, A.; Chapkin, R.S. Diet-host-microbiota interactions shape aryl hydrocarbon receptor ligand production to modulate intestinal homeostasis. Annu. Rev. Nutr. 2021, 41, 455–478. [Google Scholar] [CrossRef]
- Wang, M.; Guo, J.; Hart, A.L.; Li, J.V. Indole-3-aldehyde reduces inflammatory responses and restores intestinal epithelial barrier function partially via aryl hydrocarbon receptor (AhR) in experimental colitis models. J. Inflamm. Res. 2023, 16, 5845–5864. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.S. Microbiome of the skin and gut in atopic dermatitis (AD): Understanding the pathophysiology and finding novel management strategies. J. Clin. Med. 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, Y.; Wang, L.; Lin, J.; Xu, C.; Zhao, X.; Zhang, H. TolDC restores the balance of Th17/Treg via aryl hydrocarbon receptor to attenuate colitis. Inflamm. Bowel Dis. 2024, 30, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Taitz, J.; Sun, S.M.; Langford, L.; Ni, D.; Macia, L. Your regulatory T cells are what you eat: How diet and gut microbiota affect regulatory T cell development. Front. Nutr. 2022, 9, 878382. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hener, P.; Zhang, Z.; Kato, S.; Metzger, D.; Chambon, P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. USA 2006, 103, 11736–11741. [Google Scholar] [CrossRef]
- Landheer, J.; Giovannone, B.; Sadekova, S.; Tjabringa, S.; Hofstra, C.; Dechering, K.; Bruijnzeel-Koomen, C.; Chang, C.; Ying, Y.; de Waal Malefyt, R.; et al. TSLP is differentially regulated by vitamin D3 and cytokines in human skin. Immun. Inflamm. Dis. 2015, 3, 32–43. [Google Scholar] [CrossRef]
- Sakamoto, K.; Nagao, K. Mouse models for atopic dermatitis. Curr. Protoc. 2023, 3, e709. [Google Scholar] [CrossRef]
- Hoshino, Y.; Kirima, K.; Arichika, N.; Kakumoto, Y.; Shibamori, M.; Matsumoto, S.; Hiyama, H. Long-term application of MC903 in mice prolongs the characteristic symptoms of atopic dermatitis, such as inflammation, skin barrier dysfunction, and itching. Exp. Anim. 2025, 74, 276–285. [Google Scholar] [CrossRef]
- Choi, J.; Sutaria, N.; Roh, Y.S.; Bordeaux, Z.; Alphonse, M.P.; Kwatra, S.G.; Kwatra, M.M. Translational relevance of mouse models of atopic dermatitis. J. Clin. Med. 2021, 10, 613. [Google Scholar] [CrossRef]
- Antal, A.S.; Dombrowski, Y.; Koglin, S.; Ruzicka, T.; Schaubre, J. Impact of vitamin D3 on cutaneous immunity. J. Investig. Dermatol. 2011, 131, 194–204. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef]
- Hata, T.R.; Kotol, P.; Jackson, M.; Nguyen, M.; Paik, A.; Udall, D.; Kanada, K.; Yamasaki, K.; Alexandrescu, D.; Gallo, R.L. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J. Allergy Clin. Immunol. 2008, 122, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Umehara, Y.; Trujillo-Paez, J.V.; Yue, H.; Peng, G.; Nguyen, H.L.T.; Okumura, K.; Ogawa, H.; Niyonsaba, F. Calcitriol, an active form of vitamin D3, mitigates skin barrier dysfunction in atopic dermatitis NC/Nga mice. Int. J. Mol. Sci. 2023, 24, 9347. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Lee, K.; Oh, K. mTORC1 deficiency prevents the development of MC903-induced atopic dermatitis through the downregulation of type 2 inflammation. Int. J. Mol. Sci. 2023, 24, 5968. [Google Scholar] [CrossRef]
- Gupta, A.; Song, M.H.; Youn, D.H.; Ku, D.; Sasidharan Nair, V.; Oh, K. Prolyl hydroxylase inhibition protects against murine MC903-induced skin inflammation by downregulating TSLP. Front. Immunol. 2024, 15, 1330011. [Google Scholar] [CrossRef]
- Wan, H.; Yang, H.; Wei, M.; Chen, W. Polyinosinic:polycytidylic acid aggravates calcipotriol-induced atopic dermatitis-like skin lesions in mice by increasing the expression of thymic stromal lymphopoietin. Ann. Transl. Med. 2022, 10, 209. [Google Scholar] [CrossRef]
- Alam, M.J.; Xie, L.; Yap, Y.A.; Robert, R. A mouse model of MC903-induced atopic dermatitis. Curr. Protoc. 2023, 3, e695. [Google Scholar] [CrossRef]
- Reinholz, M.; Ruzicka, T.; Schauber, J. Vitamin D and its role in allergic disease. Clin. Exp. Allergy 2012, 42, 817–826. [Google Scholar] [CrossRef]
- Gallieni, M.; Cozzolino, M.; Fallabrino, G.; Pasho, S.; Olivi, L.; Brancaccio, D. Vitamin D: Physiology and pathophysiology. Int. J. Artif. Organs 2009, 32, 87–94. [Google Scholar] [CrossRef]
- Pike, J.; Meyer, M.B. The vitamin D receptor: New paradigms for the regulation of gene expression by 1, 25-dihydroxyvitamin D3. Endocrinol. Metab. Clin. 2010, 39, 255–269. [Google Scholar] [CrossRef]
- Luderer, H.F.; Demay, M.B. The vitamin D receptor, the skin and stem cells. J. Steroid Biochem. Mol. Biol. 2010, 121, 314–316. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Geng, C.; Song, S.; Xie, X.; Wang, C. Vitamin D receptor involves in the protection of intestinal epithelial barrier function via up-regulating SLC26A3. J. Steroid Biochem. Mol. Biol. 2023, 227, 106231. [Google Scholar] [CrossRef]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2010, 127, 773–786. [Google Scholar] [CrossRef]
- Bergmann, S.; von Buenau, B.; Vidal-Y-Sy, S.; Haftek, M.; Wladykowski, E.; Houdek, P.; Lezius, S.; Duplan, H.; Bäsler, K.; Dähnhardt-Pfeiffer, S.; et al. Claudin-1 decrease impacts epidermal barrier function in atopic dermatitis lesions dose-dependently. Sci. Rep. 2020, 145, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Cao, H.; Zheng, J.; Chen, L. Claudin-1 Mediated Tight Junction Dysfunction as a Contributor to Atopic March. Front Immunol. 2022, 13, 927465. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.B.; Aitkens, L.; White, J.; Hyndman, K.A. Aquaporin-3 in the epidermis: More than skin deep. Am. J. Physiol. Cell Physiol. 2020, 318, C1144–C1153. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Aquaporin-3 functions as a glycerol transporter in mammalian skin. Biol. Cell 2005, 97, 479–486. [Google Scholar] [CrossRef]
- Tricarico, P.M.; Mentino, D.; De Marco, A.; Del Vecchio, C.; Garra, S.; Cazzato, G.; Foti, C.; Crovella, S.; Calamita, G. Aquaporins are one of the critical factors in the disruption of the skin barrier in inflammatory skin diseases. Int. J. Mol. Sci. 2022, 23, 4020. [Google Scholar] [CrossRef]
- Wang, S.-H.; Zuo, Y.-G. Thymic stromal lymphopoietin in cutaneous immune-mediated diseases. Front. Immunol. 2021, 12, 698522. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.-N.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Vitamin D status and efficacy of supplementation in atopic dermatitis: A systematic review and meta-analysis. Nutrients 2016, 8, 789. [Google Scholar] [CrossRef]
- Çiçek, F.; Köle, M.T. Evaluation of the impact of serum vitamin D levels on the scoring atopic dermatitis index in pediatric atopic dermatitis. Children 2023, 10, 1522. [Google Scholar] [CrossRef]
- McCarthy, R.L.; Tawfik, S.S.; Theocharopoulos, I.; Atkar, R.; McDonald, B.; Dhoat, S.; Hughes, A.; Thomas, B.R.; O’Toole, E.A. Vitamin D deficiency and atopic dermatitis severity in a Bangladeshi population living in East London: A cross-sectional study. Skin Health Dis. 2024, 4, e358. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, A.; Ali, A.; Zahedi, F.D.; Ismail, N.A.S. Immunomodulatory role of vitamin D on gut microbiome in children. Biomedicines 2023, 11, 1441. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, A.C.; Nori, W.; Kassim, M.A.K.; Balasa, A.L.; Mihai, C.M.; Chisnoiu, T.; Mihai, L.; Ungureanu, A.; Frecus, C.E.; Chirila, S.I.; et al. Gut microbiota profile and atopic dermatitis in the first year of life. J. Med. Life 2024, 17, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef]
- Davis, E.C.; Castagna, V.P.; Sela, D.A.; Hillard, M.A.; Lindberg, S.; Mantis, N.J.; Seppo, A.E.; Järvinen, K.M. Gut microbiome and breast-feeding: Implications for early immune development. J. Allergy Clin. Immunol. 2022, 150, 523–534. [Google Scholar] [CrossRef]
- Ojaroodi, A.F.; Jafarnezhad, F.; Eskandari, Z.; Keramat, S.; Stanek, A. Recent updates and advances in the association between vitamin D deficiency and risk of thrombotic disease. Nutrients 2024, 17, 90. [Google Scholar] [CrossRef]
- Zhao, H.; Luo, Y.; Li, W.; Jiang, C.; Jin, E.; Xu, Z. Association between antibiotic exposure and childhood atopic dermatitis: A systematic review and meta-analysis. EClinicalMedicine 2025, 84, 103296. [Google Scholar] [CrossRef]
- Tang, H.; Li, W.; Xu, Y.; Zhou, Y.; Hamblin, M.R.; Wen, X. Gut microbiota modulation: A key determinant of atopic dermatitis susceptibility in children. Front. Microbiol. 2025, 16, 1549895. [Google Scholar] [CrossRef]
- Thangamuni, A.; Fathimathul Harshiba, H.; Muhammed Rafi, N.; Nitol, A.; Mohan, J.; Korrapati, N. Beauty from within: A comprehensive review on interplay between gut health and skin. CosmoDerma 2024, 4, 97. [Google Scholar] [CrossRef]
- Hidayati, A.N.; Sawitri, S.; Sari, D.W.; Prakoeswa, C.R.S.; Indramaya, D.M.; Damayanti, D.; Zulkarnain, I.; Citrashanty, I.; Widia, Y.; Anggraeni, S. Efficacy of vitamin D supplementation on the severity of atopic dermatitis in children: A systematic review and meta-analysis. F1000Research 2023, 11, 274. [Google Scholar] [CrossRef]
- Amestejani, M.; Seyed Salehi, B.; Vasigh, M.; Sobhkhiz, A.; Karami, M.; Alinia, H.; Kamrava, S.K.; Shamspour, N.; Ghalehbaghi, B.; Heshmatzade Behzadi, A. Vitamin D supplementation in the treatment of atopic dermatitis: A clinical trial study. J. Drugs Dermatol. 2012, 11, 327–330. [Google Scholar]
- Park, J.S.; Kim, S.; Sol, I.S.; Lee, K.S.; Park, S.; Yang, H.J.; Lee, E. Effect of vitamin D on the treatment of atopic dermatitis with consideration of heterogeneities: Meta-analysis of randomized controlled trials. Allergy Asthma Immunol. Res. 2023, 15, 262–270. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Foolad, N.; Maarouf, M.; Tran, K.A.; Shi, V.Y. Micronutrients in atopic dermatitis: A systematic review. J. Altern. Complement. Med. 2019, 25, 567–577. [Google Scholar] [CrossRef]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus statement on vitamin D status assessment and supplementation: Whys, whens, and hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef] [PubMed]
- Ziada, S.; Wishahe, A.; Mabrouk, N.; Sahtout, S. Vitamin D deficiency and oral health: A systematic review of literature. BMC Oral Health 2025, 25, 468. [Google Scholar] [CrossRef] [PubMed]
- Venter, F.C.; Ghitea, T.C.; Venter, A.N.; El-Kharoubi, A.F.; El-Kharoubi, M.; Ghitea, E.C.; Ghitea, M.C.; Venter, A. Correlation between vitamin D deficiency (25(OH)D3) and the severity of purulent oropharyngeal infections. J. Clin. Med. 2025, 14, 2410. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cao, Y. Association between atopic dermatitis with hyperparathyroidism not mediated by vitamin D in the United States (NHANES 2005–2006). Arch. Dermatol. Res. 2024, 317, 100. [Google Scholar] [CrossRef]
- Grant, W.B.; Wimalawansa, S.J.; Pludowski, P.; Cheng, R.Z. Vitamin D: Evidence-based health benefits and recommendations for population guidelines. Nutrients 2025, 17, 277. [Google Scholar] [CrossRef]
- Ohya, Y. Prevention of atopic dermatitis: What are we missing? Allergy Asthma Immunol. Res. 2025, 17, 433–446. [Google Scholar] [CrossRef]
- Pludowski, P.; Marcinowska-Suchowierska, E.; Togizbayev, G.; Belaya, Z.; Grant, W.B.; Pilz, S.; Holick, M.F. Daily and weekly “high doses” of cholecalciferol for the prevention and treatment of vitamin D deficiency for obese or multi-morbidity and multi-treatment patients requiring multi-drugs—A narrative review. Nutrients 2024, 16, 2541. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I.; NIHR RESPIRE Global Respiratory Health Unit. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Borzutzky, A.; Iturriaga, C.; Pérez-Mateluna, G.; Cristi, F.; Cifuentes, L.; Silva-Valenzuela, S.; Vera-Kellet, C.; Cabalin, C.; Hoyos-Bachiloglu, R.; Navarrete-Dechent, C.; et al. Effect of weekly vitamin D supplementation on the severity of atopic dermatitis and type 2 immunity biomarkers in children: A randomized controlled trial. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, Y.; Huang, H.; Wang, D. Serum vitamin D level and efficacy of vitamin D supplementation in children with atopic dermatitis: A systematic review and meta-analysis. J. Immunol. Res. 2022, 2022, 9407888. [Google Scholar] [CrossRef]
- Vasdeki, D.; Tsamos, G.; Dimakakos, E.; Patriarcha, V.; Koufakis, T.; Kotsa, K.; Cholewka, A.; Stanek, A. Vitamin D Supplementation: Shedding Light on the Role of the Sunshine Vitamin in the Prevention and Management of Type 2 Diabetes and Its Complications. Nutrients 2024, 16, 3651. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Physiology of vitamin D—Focusing on disease prevention. Nutrients 2024, 16, 1666. [Google Scholar] [CrossRef]
- Chauhan, K.; Shahrokhi, M.; Huecker, M.R. Vitamin D. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441912/ (accessed on 3 September 2025).
- Connell, T.; Seidler, K.; Neil, J. Cathelicidin expression in the pathogenesis of atopic dermatitis and the therapeutic potential of vitamin D. Nutr. Res. 2025, 139, 113–123. [Google Scholar] [CrossRef]
- Rizzoli, R. Vitamin D supplementation: Upper limit for safety revisited? Aging Clin. Exp. Res. 2021, 33, 19–24. [Google Scholar] [CrossRef]
- Diotallevi, F.; Campanati, A.; Martina, E.; Radi, G.; Paolinelli, M.; Marani, A.; Molinelli, E.; Candelora, M.; Taus, M.; Galeazzi, T.; et al. The role of nutrition in immune-mediated, inflammatory skin disease: A narrative review. Nutrients 2022, 14, 591. [Google Scholar] [CrossRef]
- Port, L.R.; Brunner, P.M. Management of atopic hand dermatitis. Dermatol. Clin. 2024, 42, 619–623. [Google Scholar] [CrossRef]
- 1Yang, L.; Zhang, Y.; Wu, J.; Wang, L.; Liu, S.; Zhou, L.; Zhang, J.; Li, C. Calcipotriol inhibits the proliferation of psoriasis HaCaT cells by activating the ferroptosis pathway. Acta Histochem. 2025, 127, 152274. [Google Scholar] [CrossRef]
- Vähävihu, K.; Ala-Houhala, M.; Peric, M.; Karisola, P.; Kautiainen, H.; Hasan, T.; Snellman, E.; Alenius, H.; Schauber, J.; Reunala, T. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br. J. Dermatol. 2010, 163, 321–328. [Google Scholar] [CrossRef]
- Lossius, A.H.; Sundnes, O.; Ingham, A.C.; Edslev, S.M.; Bjornholt, J.V.; Lilje, B.; Bradley, M.; Asad, S.; Haraldsen, G.; Skytt-Andersen, P.; et al. Shifts in the Skin Microbiota after UVB Treatment in Adult Atopic Dermatitis. Dermatology 2022, 238, 109–120. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Vitamin D Treatment Effect for Atopic Dermatitis in Children; Identifier NCT05523986. U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/study/NCT05523986 (accessed on 3 September 2025).
- Navarro-López, V.; Ramírez-Boscá, A.; Ramón-Vidal, D.; Ruzafa-Costas, B.; Genovés-Martínez, S.; Chenoll-Cuadros, E.; Carrión-Gutiérrez, M.; Horga de la Parte, J.; Prieto-Merino, D.; Codoñer-Cortés, F.M. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2018, 154, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.W.; Ong, M.T.; Man, G.C.; Yeung, Y.M.; He, X.; Choi, B.C.; Ng, J.P.; Mok, D.K.; Lam, T.P.; Yung, P.S. The effectiveness of vitamin D supplementation in patients with end-stage knee osteoarthritis: Study protocol for a double-blinded, randomized controlled trial. PLoS ONE 2024, 19, e0309610. [Google Scholar] [CrossRef] [PubMed]
- González-Tarancón, R.; Goñi-Ros, N.; Salvador-Rupérez, E.; Hernández-Martín, Á.; Izquierdo-Álvarez, S.; Puzo-Foncillas, J.; Gilaberte-Calzada, Y. Association between VDR and CYP24A1 polymorphisms, atopic dermatitis, and biochemical lipid and vitamin D profiles in Spanish population: Case-control study. JMIR Dermatol. 2023, 6, e39567. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Lee, S.M.; Towne, J.M.; Cichanski, S.R.; Kaufmann, M.; Jones, G.; Pike, J.W. In vivo contribution of Cyp24a1 promoter vitamin D response elements. Endocrinology 2024, 165, bqae134. [Google Scholar] [CrossRef]
- Milan, K.L.; Ramkumar, K.M. Regulatory mechanisms and pathological implications of CYP24A1 in vitamin D metabolism. Pathol. Res. Pract. 2024, 264, 155684. [Google Scholar] [CrossRef]
- Brustad, N.; Wang, T.; Chen, L.; Kaiser, H.; Gomes, B.; Klein, A.; Vahman, N.; Skov, L.; Stokholm, J.; Schoos, A.M.; et al. Effect of prenatal high-dose vitamin D on childhood atopic dermatitis is modified by maternal cotinine metabolome: A secondary analysis of a randomized clinical trial. J. Am. Acad. Dermatol. 2025. [Google Scholar] [CrossRef]
- Bukvić Mokos, Z.; Tomić Krsnik, L.; Harak, K.; Marojević Tomić, D.; Tešanović Perković, D.; Vukojević, M. Vitamin D in the prevention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2025, 26, 5005. [Google Scholar] [CrossRef]
- Camargo, C.A.; Ganmaa, D.; Sidbury, R.; Erdenedelger, K.; Radnaakhand, N.; Khandsuren, B. Randomized trial of vitamin D supplementation for winter-related atopic dermatitis in children. J. Allergy Clin. Immunol. 2014, 134, 831–835.e1. [Google Scholar] [CrossRef]
- Patchen, B.K.; Best, C.M.; Boiteau, J.; Solvik, B.S.; Vonderschmidt, A.; Xu, J.; Cohen, R.T.; Cassano, P.A. Vitamin D supplementation in pregnant or breastfeeding women or young children for preventing asthma. Cochrane Database Syst. Rev. 2025, 8, CD013396. [Google Scholar] [CrossRef]



| Model | Experimental Conditions | AD-like Phenotype/Main Readouts | Effect of VD/VDR |
|---|---|---|---|
| Ex vivo/in vitro [92,116] | Human keratinocytes/skin; stimulation with 1,25(OH)2D3 | Analysis of filaggrin, loricrin, claudin-1, AMP (LL-37) | VD/VDR strengthens barrier, AMP expression, and limits alarmins |
| MC903 (calcipotriol) [120,121] | C57BL/6 mice; topical application of MC903 (0.05–0.1 µg/day for 5–10 days) | Eosinophilic infiltration, Th2 inflammation, pruritus, ↑TSLP/IL-33 | Excessive local VDR activation → induction of TSLP and inflammation |
| NC/Nga (spontaneous AD) [117] | NC/Nga mice; topical calcitriol (1–2 µg/g/day, 2–3 weeks) | Chronic lesions, ↑TEWL, barrier defects, Th2 | Calcitriol ↓TEWL, ↑filaggrin/loricrin, ↓IL-13/IL-33, ↑β-defensins |
| Allergen model (Dermatophagoides farinae) [117] | BALB/c mice; sensitization + challenge with mite allergen; topical calcitriol | Th2 response, barrier dysfunction, pruritus | Calcitriol reduces inflammation and improves barrier function |
| MC903 + poly(I:C) [120] | As above, + poly(I:C) (TLR3 agonist) topically | Exacerbation of inflammation and pruritus, ↑TSLP | Model sensitive to environmental/infectious factors |
| VDR knockout [123,125] | VDR-/- mice (various genetic backgrounds) | Alopecia, severe barrier defects, impaired keratinocyte differentiation | Demonstrates importance of VDR independent of 1,25(OH)2D3 |
| Area | Key Insights | Clinical Implications | Future Directions |
|---|---|---|---|
| Serum 25(OH)D monitoring | Lower VD levels correlate with AD severity [148] | Routine testing recommended; most studies define <30 ng/mL as deficient [150,151] | Determine optimal therapeutic thresholds (e.g., ≥50–80 ng/mL) [152,153] |
| Oral supplementation | Daily cholecalciferol most effective [149] | Individualized dosing; obesity requires higher intake [154,155,156] | Establish optimal dose and duration; long-term trials needed [153] |
| Topical VD analogs | Calcipotriol validated in psoriasis; limited AD data [165,166,167] | May improve barrier function and reduce inflammation [166,167] | Conduct controlled studies to confirm efficacy and safety [165,166,167] |
| Phototherapy (NB-UVB) | NB-UVB increases VD levels and improves clinical scores [168] | Effective adjunctive therapy for moderate AD [168] | Assess combination strategies (UVB + oral VD) |
| Microbiome modulation | VD reduces S. aureus overgrowth and increases diversity [36] | Supports microbiome restoration and barrier repair [36] | Explore mechanistic pathways affecting skin microbiome |
| Probiotics | Lactobacillus/Bifidobacterium synergize with VD [169,170] | Combined supplementation may reduce inflammation [169] | Clarify which strains and doses are optimal |
| Genetic factors (VDR, CYP24A1) | Polymorphisms influence VD metabolism and response [172,173,174] | Potential for personalized dosing [172] | Pharmacogenomic profiling recommended |
| Pregnancy and early development | Low maternal VD linked with AD risk [168] | Supplementation in early pregnancy may reduce risk [169,170] | Further studies needed due to conflicting evidence [171] |
| Safety | Adverse effects are rare; toxicity >150 ng/mL [153,154,155] | Monitoring recommended, especially with higher doses [156] | Studies on long-term safety required |
| Relapse after discontinuation | Flares may return when VD is stopped [157] | Consider continuous maintenance supplementation [157] | Compare continuous vs. intermittent treatment regimens |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blady, K.; Pomianowski, B.; Strugała, M.; Smółka, L.; Kursa, K.; Stanek, A. Vitamin D in Atopic Dermatitis: Role in Disease and Skin Microbiome. Nutrients 2025, 17, 3584. https://doi.org/10.3390/nu17223584
Blady K, Pomianowski B, Strugała M, Smółka L, Kursa K, Stanek A. Vitamin D in Atopic Dermatitis: Role in Disease and Skin Microbiome. Nutrients. 2025; 17(22):3584. https://doi.org/10.3390/nu17223584
Chicago/Turabian StyleBlady, Karolina, Bartosz Pomianowski, Miłosz Strugała, Leon Smółka, Karolina Kursa, and Agata Stanek. 2025. "Vitamin D in Atopic Dermatitis: Role in Disease and Skin Microbiome" Nutrients 17, no. 22: 3584. https://doi.org/10.3390/nu17223584
APA StyleBlady, K., Pomianowski, B., Strugała, M., Smółka, L., Kursa, K., & Stanek, A. (2025). Vitamin D in Atopic Dermatitis: Role in Disease and Skin Microbiome. Nutrients, 17(22), 3584. https://doi.org/10.3390/nu17223584








