Effect of Exogenous Ketones as an Adjunct to Low-Calorie Diet on Metabolic Markers
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
2.3. Dietary Intake and Control
2.4. Anthropometrics and Resting Metabolic Rate
2.5. Body Composition
2.6. Blood Collection and Analyses
2.7. Supplementation
2.8. Statistical Analyses
3. Results
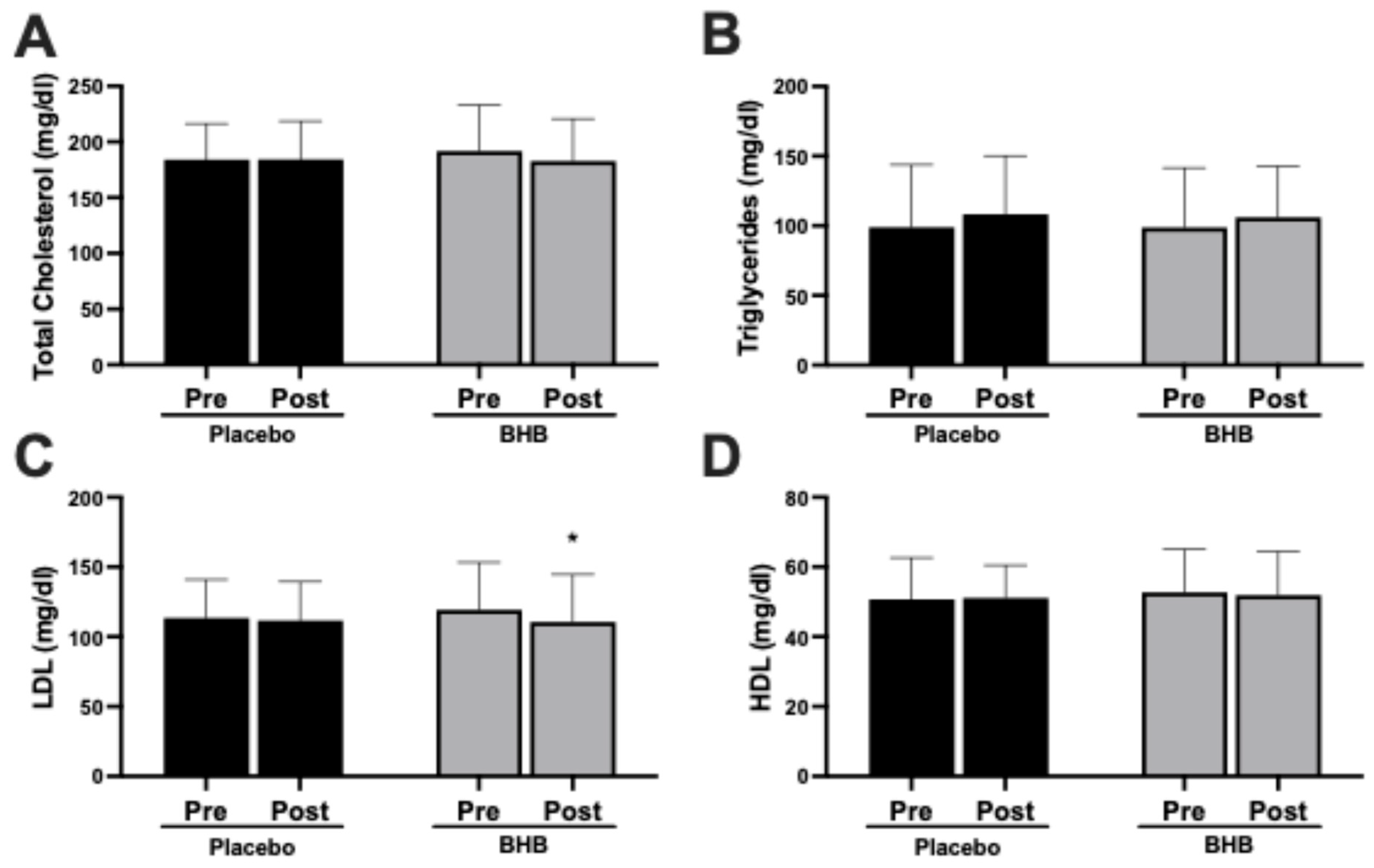
3.1. Cardiometabolic Markers
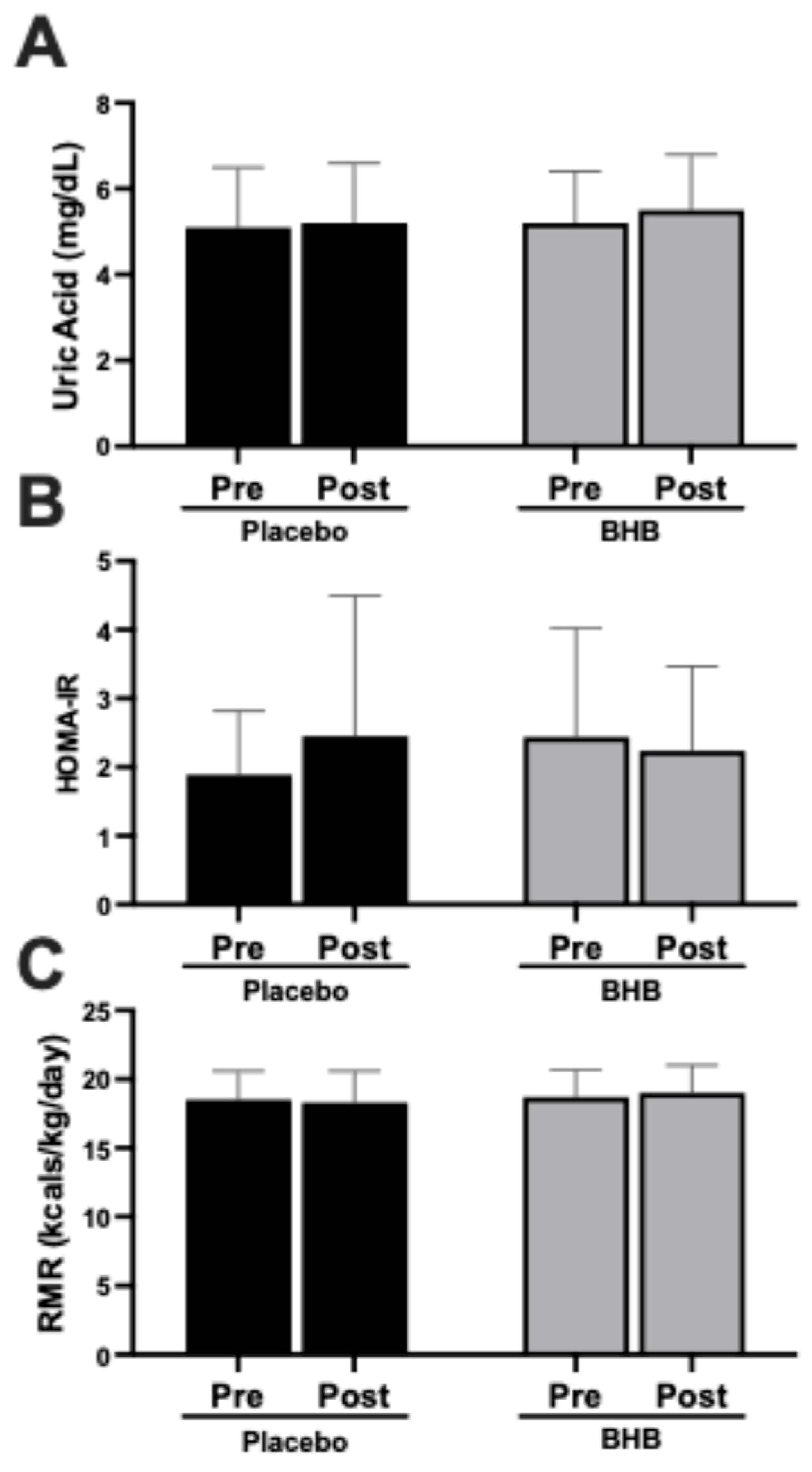
3.2. Metabolic and Renal Markers
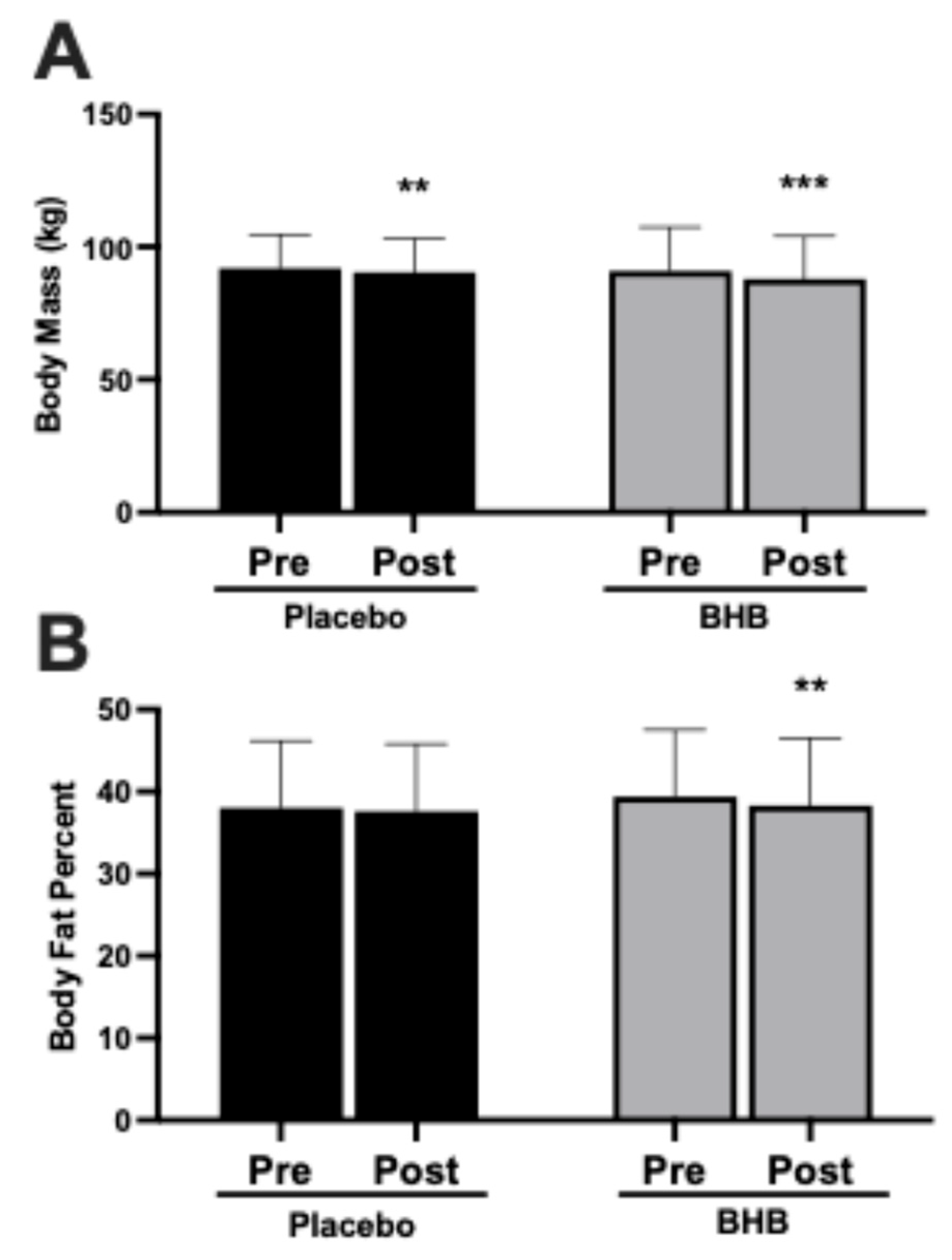
3.3. Body Mass and Body Fat Percentage
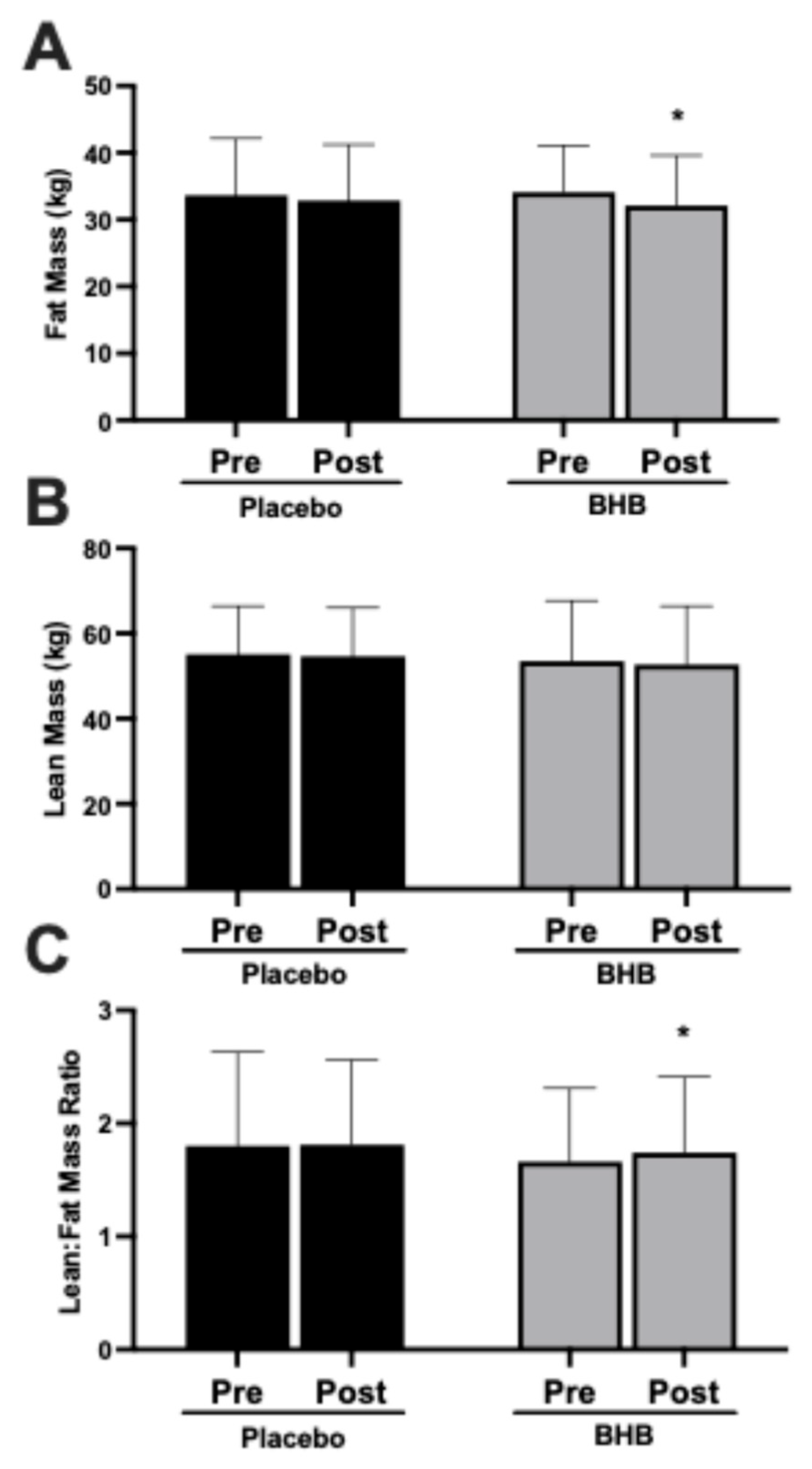
3.4. Fat Mass, Lean Mass, and Lean-to-Fat Mass Ratio
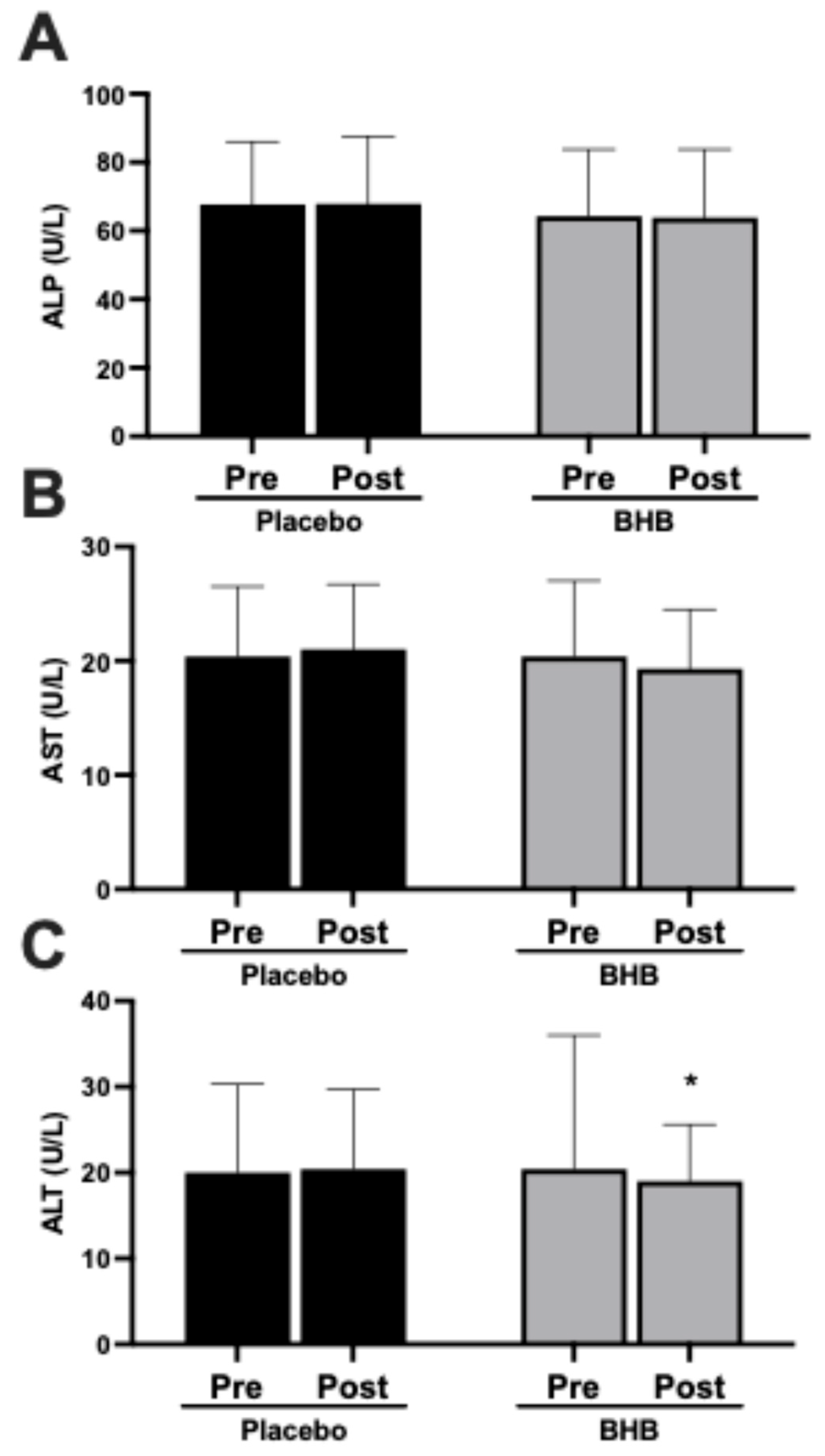
3.5. Liver Enzymes
4. Discussion
4.1. Summary of Main Findings
4.2. Cardiometabolic Outcomes and Metabolic Stability
4.3. Mechanistic Insights: Potential Positive Effects of BHB
4.4. Relevance to GLP-1 Agonist Therapies
4.5. Liver Health and Formulation Safety
4.6. Strengths and Limitations
4.7. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Group | Mean ± SD | p-Value * | |
|---|---|---|---|
| Age (years) | PLA QBHB | 34.9 ± 6.1 33.9 ± 35.9 | 0.57 |
| Height (centimeters) | PLA QBHB | 172.2 ± 9.5 168.9 ± 12.6 | 0.81 |
| Body Mass (kilograms) | PLA QBHB | 91.7 ± 12.5 90.8 ± 16.5 | 0.57 |
| Body Mass Index (kg/m2) | PLA QBHB | 30.8 ± 2.7 31.7 ± 2.9 | 0.64 |
| DEXA % Fat | PLA QBHB | 38.0 ± 8.2 39.4 ± 8.1 | 0.90 |
| Allocated Subjects (n = 51) | ||
|---|---|---|
| Placebo (n = 27) | BHB (n = 24) | |
| Severity | ||
| Mild | 2 | 5 |
| Moderate | ||
| Severe | ||
| Relationship to Study Treatment | ||
| Not related | 1 | |
| Possible | 2 | 2 |
| Definite | ||
| Relationship to Test Article | ||
| Not related | ||
| Possible | 2 | 2 |
| Definite | ||
| Body System and AEs | ||
| Gastrointestinal | ||
| abdominal distension; bloating | 2 | |
| nonspecific; diarrhea | ||
| GI inflammatory disorder; abdominal pain | ||
| motility; constipation | 1 | |
| motility; defecation frequency decreased | ||
| motility; frequent bowel movements | 2 | |
| nausea | ||
| oral dryness & salivary; dry mouth | ||
| Hematology Investigations | ||
| liver function investigation; increased AST & ALT | ||
| Immune | ||
| Hypersensitivity; urticaria (hives) | ||
| Nervous System | ||
| headache | ||
| Renal & Urinary | ||
| dysuria; micturition burning | ||
| Surgical & Medical Procedures | ||
| venipuncture; diaphoresis | ||
| Total Number of AE Experienced During Study | 2 | 3 |
| Total Number of Subjects Experiencing AE: n | 2 | 3 |
| Variables | n | Baseline (Week 0) | Post (Week 8) | Delta | Within (p) | Group × Time (p) |
|---|---|---|---|---|---|---|
| Body Mass (kg) | ||||||
| PLA | 27 | 91.9 ± 12.8 | 90.4 ± 12.8 | −1.51 ± 2.61 † | 0.006 | 0.09 |
| BHB | 24 | 90.9 ± 16.5 | 87.9 ± 16.2 | −3.05 ± 2.53 † | <0.001 | |
| Body Mass Index (kg/m2) | ||||||
| PLA | 27 | 30.9 ± 2.8 | 30.4 ± 2.6 | −0.52 ± 0.88 | 0.005 | 0.08 |
| BHB | 24 | 31.7 ± 2.8 | 30.7 ± 2.8 | −1.07 ± 0.90 | <0.001 | |
| Waist Circumference (cm) | ||||||
| PLA | 27 | 98.2 ± 9.3 | 96.1 ± 9.4 | −2.1 ± 6.3 | 0.09 | 0.69 |
| BHB | 24 | 96.4 ± 11.1 | 94.9 ± 10.9 | −1.5 ± 5.9 | 0.23 | |
| Hip Circumference (cm) | ||||||
| PLA | 27 | 110.6 ± 7.5 | 109.8 ± 7.7 | −0.8 ± 4.0 † | 0.29 | 0.05 |
| BHB | 24 | 110.5 ± 7.2 | 105.9 ± 5.7 | −4.6 ± 5.4 † | <0.001 | |
| DEXA Fat Mass (kg) | ||||||
| PLA | 27 | 33.6 ± 8.5 | 32.8 ± 8.4 | −0.82 ± 2.39 † | 0.09 | 0.22 |
| BHB | 24 | 34.1 ± 7.0 | 32.1 ± 7.5 | −1.97 ± 2.32 †† | <0.001 | |
| DEXA Lean Mass (kg) | ||||||
| PLA | 27 | 55.1 ± 11.3 | 54.6 ± 11.4 | −0.45 ± 1.95 | 0.25 | 0.58 |
| BHB | 24 | 53.5 ± 14.2 | 52.7 ± 13.7 | −0.85 ± 2.09 | 0.06 | |
| DEXA Percent Fat (%) | ||||||
| PLA | 27 | 38.0 ± 8.2 | 37.6 ± 8.2 | −0.38 ± 2.07 | 0.35 | 0.54 |
| BHB | 24 | 39.4 ± 8.1 | 38.3 ± 8.2 | −1.11 ± 2.03 | 0.01 | |
| DEXA Lean–Fat Ratio | ||||||
| PLA | 27 | 1.80 ± 0.83 | 1.81 ± 0.75 | 0.013 ± 0.20 † | 0.75 | 0.43 |
| BHB | 24 | 1.66 ± 0.65 | 1.74 ± 0.67 | 0.09 ± 0.19 | 0.04 | |
| Variables | n | Baseline (Week 0) | Post (Week 8) | Within (p) | Delta | Between-Group p-Value * |
|---|---|---|---|---|---|---|
| Glucose (grams/dL) | ||||||
| PLA | 27 | 90.2 ± 5.9 | 89.0 ± 9.1 | −1.22 ± 9.86 | 0.53 | 0.09 |
| BHB | 24 | 87.5 ± 6.7 | 89.0 ± 9.3 | 1.50 ± 10.69 | 0.50 | |
| Insulin (μIU/mL) | ||||||
| PLA | 27 | 8.4 ± 4.0 | 11.2 ± 9.6 | 2.7 ± 9.1 | 0.13 | 0.34 |
| BHB | 24 | 11.1 ± 6.7 | 10.0 ± 4.9 | −1.2 ± 4.6 | 0.22 | |
| Uric Acid (mg/dL) | ||||||
| PLA | 27 | 5.1 ± 1.4 | 5.2 ± 1.4 | 0.05 ± 0.86 | 0.76 | 0.77 |
| BHB | 24 | 5.2 ± 1.2 | 5.5 ± 1.3 | 0.28 ± 0.93 | 0.16 | |
| Alkaline Phosphatase (U/L) | ||||||
| PLA | 27 | 67.7 ± 18.3 | 67.9 ± 19.7 | 0.19 ± 8.16 | 0.91 | 0.41 |
| BHB | 24 | 64.3 ± 19.5 | 63.9 ± 19.9 | 0.42 ± 7.23 | 0.78 | |
| AST (U/L) | ||||||
| PLA | 27 | 20.4 ± 6.1 | 21.0 ± 5.7 | 0.63 ± 5.20 | 0.54 | 0.04 |
| BHB | 24 | 20.4 ± 6.6 | 19.3 ± 5.2 | −1.08 ± 6.92 | 0.45 | |
| ALT (U/L) | ||||||
| PLA | 27 | 20.0 ± 10.3 | 20.4 ± 9.3 | 0.41 ± 5.50 | 0.70 | 0.24 |
| BHB | 24 | 23.8 ± 15.6 | 19.0 ± 6.6 | −4.75 ± 11.0 | 0.05 | |
| Total Cholesterol (mg/dL) | ||||||
| PLA | 27 | 184.0 ± 32.4 | 184.3 ± 34.4 | 0.3 ± 21.0 | 0.95 | 0.24 |
| BHB | 24 | 191.8 ± 41.2 | 182.8 ± 37.9 | −9.0 ± 19.5 | 0.03 | |
| Triglycerides (mg/dL) | ||||||
| PLA | 27 | 98.9 ± 44.9 | 108.3 ± 42.0 | 9.37 ± 48.9 | 0.33 | 0.95 |
| BHB | 24 | 98.8 ± 42.9 | 111.9 ± 66.8 | 13.1 ± 52.9 | 0.24 | |
| HDL Cholesterol (mg/dL) | ||||||
| PLA | 27 | 50.7 ± 11.9 | 51.1 ± 9.4 | 0.41 ± 7.0 | 0.77 | 0.07 |
| BHB | 24 | 52.7 ± 12.4 | 49.9 ± 12.5 | −2.79 ± 3.7 | 0.001 | |
| LDL Cholesterol (mg/dL) | ||||||
| PLA | 27 | 113.4 ± 27.5 | 111.5 ± 27.9 | −1.89 ± 20.9 | 0.64 | 0.53 |
| BHB | 24 | 119.3 ± 33.9 | 110.5 ± 34.1 | −8.79 ± 19.3 | 0.04 | |
| Homeostatic Model Assessment of Insulin Resistance (HOMA−IR) | ||||||
| PLA | 27 | 1.89 ± 0.93 | 2.45 ± 2.05 | 0.56 ± 0.46 | 0.14 | 0.59 |
| BHB | 24 | 2.44 ± 1.59 | 2.24 ± 1.23 | 0.20 ± 0.40 | 0.42 | |
| Total Cholesterol:HDL Ratio | ||||||
| PLA | 27 | 3.75 ± 0.80 | 3.65 ± 0.60 | 0.10 ± 0.20 | 0.38 | 0.85 |
| BHB | 24 | 3.74 ± 0.85 | 3.78 ± 0.82 | 0.04 ± 0.23 | 0.70 | |
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief 2020, 1–8. [Google Scholar]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2018, 102, 183–197. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Farhana, A.; Rehman, A. Metabolic Consequences of Weight Reduction; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Dai, S.; Wellens, J.; Yang, N.; Li, D.; Wang, J.; Wang, L.; Yuan, S.; He, Y.; Song, P.; Munger, R.; et al. Ultra-processed foods and human health: An umbrella review and updated meta-analyses of observational evidence. Clin. Nutr. 2024, 43, 1386–1394. [Google Scholar] [CrossRef]
- Stefanakis, K.; Kokkorakis, M.; Mantzoros, C.S. The impact of weight loss on fat-free mass, muscle, bone and hematopoiesis health: Implications for emerging pharmacotherapies aiming at fat reduction and lean mass preservation. Metab. Clin. Exp. 2024, 161, 156057. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Gower, B.A.; Hunter, G.R. Metabolic adaptation delays time to reach weight loss goals. Obesity 2022, 30, 400–406. [Google Scholar] [CrossRef]
- Athinarayanan, S.J.; Volek, J.S. Mitigating muscle loss during weight loss: Can nutritional ketosis make a difference? A call for more research. Obesity 2025, 33, 431–434. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Koutnik, A.P.; Poff, A.M.; Ford, K.M.; D’Agostino, D.P. Commentary: Ketone Diester Ingestion Impairs Time-Trial Performance in Professional Cyclists. Front. Physiol. 2018, 9, 279. [Google Scholar] [CrossRef]
- Falkenhain, K.; Daraei, A.; Forbes, S.C.; Little, J.P. Effects of Exogenous Ketone Supplementation on Blood Glucose: A Systematic Review and Meta-analysis. Adv. Nutr. 2022, 13, 1697–1714. [Google Scholar] [CrossRef]
- Poff, A.M.; Koutnik, A.P.; Egan, B. Nutritional Ketosis with Ketogenic Diets or Exogenous Ketones: Features, Convergence, and Divergence. Curr. Sports Med. Rep. 2020, 19, 251–259. [Google Scholar] [CrossRef]
- Ari, C.; D’Agostino, D.P. Divergent Hepatic Outcomes of Chronic Ketone Supplementation: Ketone Salts Preserve Liver Health While Ketone Esters and Precursors Drive Inflammation and Steatosis. Pharmaceuticals 2025, 18, 1436. [Google Scholar] [CrossRef] [PubMed]
- Cahill, G.F., Jr.; Herrera, M.G.; Morgan, A.P.; Soeldner, J.S.; Steinke, J.; Levy, P.L.; Reichard, G.A., Jr.; Kipnis, D.M. Hormone-fuel interrelationships during fasting. J. Clin. Investig. 1966, 45, 1751–1769. [Google Scholar] [CrossRef]
- Owen, O.E.; Morgan, A.P.; Kemp, H.G.; Sullivan, J.M.; Herrera, M.G.; Cahill, G.F., Jr. Brain metabolism during fasting. J. Clin. Investig. 1967, 46, 1589–1595. [Google Scholar] [CrossRef]
- Nair, K.S.; Welle, S.L.; Halliday, D.; Campbell, R.G. Effect of beta-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J. Clin. Investig. 1988, 82, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Vandoorne, T.; De Smet, S.; Ramaekers, M.; Van Thienen, R.; De Bock, K.; Clarke, K.; Hespel, P. Intake of a Ketone Ester Drink during Recovery from Exercise Promotes mTORC1 Signaling but Not Glycogen Resynthesis in Human Muscle. Front. Physiol. 2017, 8, 310. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Zhang, Y.; Zhang, X.; Zhang, S.; Liu, Z.; Yuan, H.; Pang, X.; Liu, Y.; Tao, W.; et al. Mechanism of reduced muscle atrophy via ketone body (D)-3-hydroxybutyrate. Cell Biosci. 2022, 12, 94. [Google Scholar] [CrossRef]
- Bielohuby, M.; Sawitzky, M.; Stoehr, B.J.; Stock, P.; Menhofer, D.; Ebensing, S.; Bjerre, M.; Frystyk, J.; Binder, G.; Strasburger, C.; et al. Lack of dietary carbohydrates induces hepatic growth hormone (GH) resistance in rats. Endocrinology 2011, 152, 1948–1960. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Santer, P.; Miller, J.J.; Faull, O.K.; Magor-Elliott, S.; Hiyama, S.; Stirling, M.; Clarke, K. On the Metabolism of Exogenous Ketones in Humans. Front. Physiol. 2017, 8, 848. [Google Scholar] [CrossRef]
- Yang, M.U.; van Itallie, T.B. Variability in body protein loss during protracted, severe caloric restriction: Role of triiodothyronine and other possible determinants. Am. J. Clin. Nutr. 1984, 40, 611–622. [Google Scholar] [CrossRef]
- Biolo, G.; Ciocchi, B.; Stulle, M.; Bosutti, A.; Barazzoni, R.; Zanetti, M.; Antonione, R.; Lebenstedt, M.; Platen, P.; Heer, M.; et al. Calorie restriction accelerates the catabolism of lean body mass during 2 wk of bed rest. Am. J. Clin. Nutr. 2007, 86, 366–372. [Google Scholar] [CrossRef]
- Friedlander, A.L.; Braun, B.; Pollack, M.; MacDonald, J.R.; Fulco, C.S.; Muza, S.R.; Rock, P.B.; Henderson, G.C.; Horning, M.A.; Brooks, G.A.; et al. Three weeks of caloric restriction alters protein metabolism in normal-weight, young men. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E446–E455. [Google Scholar] [CrossRef] [PubMed]
- Phinney, S.D.; Tang, A.B.; Waggoner, C.R.; Tezanos-Pinto, R.G.; Davis, P.A. The transient hypercholesterolemia of major weight loss. Am. J. Clin. Nutr. 1991, 53, 1404–1410. [Google Scholar] [CrossRef]
- Hartman, A.L.; Rho, J.M. The New Ketone Alphabet Soup: BHB, HCA, and HDAC. Epilepsy Curr. 2014, 14, 355–357. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Dearlove, D.J.; Faull, O.K.; Clarke, K. Context is key: Exogenous ketosis and athletic performance. Curr. Opin. Physiol. 2019, 10, 81–89. [Google Scholar] [CrossRef]
- Kushner, R.F.; Calanna, S.; Davies, M.; Dicker, D.; Garvey, W.T.; Goldman, B.; Lingvay, I.; Thomsen, M.; Wadden, T.A.; Wharton, S.; et al. Semaglutide 2.4 mg for the Treatment of Obesity: Key Elements of the STEP Trials 1 to 5. Obesity 2020, 28, 1050–1061. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.; Tchabanenko, K.; Pawlosky, R.; Carter, E.; Todd King, M.; Musa-Veloso, K.; Ho, M.; Roberts, A.; Robertson, J.; Vanitallie, T.B.; et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. RTP 2012, 63, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.J.; Kirk, T.; Ashmore, T.; Willerton, K.; Evans, R.; Smith, A.; Murray, A.J.; Stubbs, B.; West, J.; McLure, S.W.; et al. Nutritional Ketosis Alters Fuel Preference and Thereby Endurance Performance in Athletes. Cell Metab. 2016, 24, 256–268. [Google Scholar] [CrossRef]
- Shivva, V.; Cox, P.J.; Clarke, K.; Veech, R.L.; Tucker, I.G.; Duffull, S.B. The Population Pharmacokinetics of D-beta-hydroxybutyrate Following Administration of (R)-3-Hydroxybutyl (R)-3-Hydroxybutyrate. AAPS J. 2016, 18, 678–688. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roeth, E.J.; Parker, G.; Cooper-Leavitt, E.F.; Beus, C.G.; Braithwaite, C.R.; Morris, M.D.; Reynolds, A.P.; Evans, E.P.; Radford, J.H.; Davis, F.D.; et al. Effect of Exogenous Ketones as an Adjunct to Low-Calorie Diet on Metabolic Markers. Nutrients 2025, 17, 3582. https://doi.org/10.3390/nu17223582
Roeth EJ, Parker G, Cooper-Leavitt EF, Beus CG, Braithwaite CR, Morris MD, Reynolds AP, Evans EP, Radford JH, Davis FD, et al. Effect of Exogenous Ketones as an Adjunct to Low-Calorie Diet on Metabolic Markers. Nutrients. 2025; 17(22):3582. https://doi.org/10.3390/nu17223582
Chicago/Turabian StyleRoeth, Eliza J., Genevieve Parker, Ella F. Cooper-Leavitt, Colson G. Beus, Cameron R. Braithwaite, Madeline D. Morris, Asher P. Reynolds, Ethan P. Evans, Jack H. Radford, Fischer D. Davis, and et al. 2025. "Effect of Exogenous Ketones as an Adjunct to Low-Calorie Diet on Metabolic Markers" Nutrients 17, no. 22: 3582. https://doi.org/10.3390/nu17223582
APA StyleRoeth, E. J., Parker, G., Cooper-Leavitt, E. F., Beus, C. G., Braithwaite, C. R., Morris, M. D., Reynolds, A. P., Evans, E. P., Radford, J. H., Davis, F. D., Reynolds, P. R., Parrish, R. R., & Bikman, B. T. (2025). Effect of Exogenous Ketones as an Adjunct to Low-Calorie Diet on Metabolic Markers. Nutrients, 17(22), 3582. https://doi.org/10.3390/nu17223582









