Abstract
Micronutrient deficiencies are not always present in individuals independently and may occur in association with other deficiency processes. Objective: Verify the association between the nutritional status of iodine and that of iron, selenium, and zinc in population studies. Methods: A bibliographic search was carried out in Medline, Web of Science, and CINAHL databases, without date and language restrictions, using English search terms and their synonyms. The search terms were joined by the Boolean operator AND while the respective synonyms were connected by OR following the PRISMA guidelines. Results: A total of 40 articles were included. The studies were published between 1993 and 2025, mostly involving both sexes and the main age groups were children and adolescents. Among the micronutrients analyzed, selenium stood out, being evaluated in 55.0% (n = 22) of the studies, followed by iron in 37.5% (n = 15) and zinc in 27.5% (n = 11). The most commonly used methods for assessing nutritional status were serum selenium, followed by urinary selenium. For iron, hemoglobin, ferritin, and serum iron were used in 73.3% (n = 11), 60.0% (n = 9), and 46.7% (n = 7) of the studies, respectively. For zinc, serum concentration was the most frequently used method; however, in one study, urinary zinc was evaluated. Overall, the nutritional status of iodine was associated with that of selenium, iron, and zinc, although this trend was not observed in some studies. Conclusions: The coexistence of deficiency processes in an individual still needs to be further elucidated. Combined strategies that effectively combat, prevent, and treat these micronutrient deficiencies must consider the possible interactions between them.
1. Introduction
Micronutrient deficiencies, especially of minerals such as iodine, iron, and zinc, are recognized as worldwide public health problems and are termed “Generalized Global Micronutrient Deficiencies”. At regional levels, selenium deficiency can be included among other deficiency processes [1,2].
Iodine deficiency is mainly monitored in school-age children, but it can affect individuals of all ages. Its nutritional status is influenced by dietary, socioeconomic, demographic, and lifestyle factors. Iodine is the micronutrient involved in the formation of thyroid hormones, triiodothyronine (T3) and Thyroxine (T4), which are essential for proper thyroid function. This micronutrient can be found in foods such as oysters, shellfish, and saltwater fish, and its concentration in foods may be affected by environmental factors [3].
Micronutrient deficiencies in an individual are not always present in isolation. In many cases, they occur in association with other deficiency processes. Therefore, understanding the metabolic pathways of mineral absorption (iodine, iron, selenium, and zinc) can enable the establishment of possible associations between deficiency processes. Also, the pathway of iodine absorption and formation of thyroid hormones can provide insight into the relationship between the nutrients covered in this systematic review. Iodine is highlighted because approximately 2 billion people worldwide suffer from iodine deficiency. Among adults, it can cause infertility, thyroid cancer, hypothyroidism, cognition deficiency, goiter, and reduced productivity, otherwise known as Iodine Deficiency Disorders (IDDs) [4,5].
Iron deficiency can impair oxygen transport and cognitive development, among other factors. Zinc plays a role in the immune response, inflammation, and oxidative stress control, which can increase an individual’s vulnerability to infectious diseases. Selenium’s antioxidant role is crucial for thyroid metabolism, working synergistically with iodine [6].
Thyroid hormone formation occurs on the apical surface of the cell. Iodide is oxidized by thyroperoxidase (TPO), an iron (Fe)-dependent enzyme, and in the presence of hydrogen peroxide (H2O2) to bind to thyroglobulin (Tg). Hydrogen peroxide is dangerous for thyrocyte and is therefore controlled by Glutathione Peroxidase (GPx), a selenoprotein (Se). The union of oxidized iodine with Tg generates the monoiodotyrosine (MIT) and diiodotyrosine (DIT) complexes. When MIT and DIT combine, triiodothyronine (T3) is formed, while the union of two DIT molecules forms Thyroxine (T4). For them to be expelled from the thyrocyte and for the inactive thyroid hormone (T4) to transform into the active hormone (T3), the presence of deiodinases I and II (DI and DII) is necessary, which are selenoproteins and also depend on zinc (Zn) for proper functioning [6].
Thus, iodine deficiency in association with other deficiency processes can cause a further decline in an individual’s health status. In relation to thyroid hormone formation, the contributions of minerals (iodine, iron, selenium, and zinc) and associated enzymes are very crucial.
In this context, this systematic review aimed to verify the association between the nutritional status of iodine and that of iron, selenium, and zinc in population studies.
2. Materials and Methods
The systematic review was conducted according to PRISMA guidelines—Preferred Reporting Items for Systematic Reviews and Meta-Analysis [7] and based on the following research question: “Is the nutritional status of iodine associated with that of iron, selenium, and zinc in population studies?” (Supplementary S1). Part of the content of this work, including data, analyses and figures, was presented in the doctoral thesis of Lopes, S.O [8].
2.1. Registration of Review
The systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42021295475).
2.2. Inclusion and Exclusion Criteria
Inclusion criteria were human studies and population-based studies that investigated the correlation between iodine status and selenium, iron, or zinc status using biochemical assessments. There were no restrictions on gender, age, or study design. For longitudinal studies, baseline data were considered. There were no restrictions on the date, place, or language of publication.
Review studies and book chapters were excluded in addition to studies with pregnant women and subjects with genetic diseases such as Down syndrome, sickle cell anemia, HIV, and cancer (Supplementary S2).
2.3. Search and Selection of Articles
The search strategy followed the recommendations of the Peer Review of Electronic Search Strategies (PRESS) [9] (Supplementary S2). Three databases were used, Medline (via Pubmed), Web of Science, and CINAHL. Index terms and synonyms were selected with the aid of DECs and MESH terms coupled with Boolean operators OR for synonyms and AND for supplemental terms. The terms used were “iodine deficiency”, “iodine” with “famine, iron”, “anemia, iron-deficiency”, “growth disorders”, “iodine”, “malnutrition” (The full search strategy can be seen in Supplementary S2). The search for publications occurred on 2 August 2025. The search string used in Medline (via Pubmed) is available in the Supplementary Information (Supplementary S3).
2.4. Selection of Studies
The study selection was performed independently by a pair of researchers (SOL and EMM) using the Rayyan Software. Duplicates were identified and affected using the Rayyan software. Titles and abstracts were read first, followed by full articles. A third researcher (JMB) was asked to conduct an evaluation in the event of an impasse.
2.5. Data Extraction
Data extraction was performed independently by two researchers (SOL and EMM). The following data were extracted from articles into an Excel spreadsheet: author, year of publication, location, type of study, sample (number of participants, sex, and age), objective, method of assessing nutritional status of iodine and other micronutrients, association between iodine and other micronutrients, statistical test, p-value, and adjustment variables.
2.6. Evaluation of the Methodological Quality of the Selected Articles
To assess the risk of bias, tools recommended by the Joanna Briggs Institute were utilized, considering the study design and the following protocols: “Checklist for Analytical Cross-sectional Studies”, “Checklist for Case Control Studies”, “Checklist for Cohort Studies” and “Checklist for Randomized Controlled Trials” [10].
This instrument scores key aspects of each study: inclusion criteria, study description, exposure measurement, objectives, confounding factors, strategies used to tackle confounding factors, results measures, and statistical adequacy, among others, based on the study’s design [10].
The risk of bias was classified according to the percentage of affirmative responses (“yes”) being as follows: ≥70% considered low risk, between 50% and 69% moderate, and ≤49% high [11] (Supplementary S4). This assessment was not an exclusion criterion for articles in the review.
2.7. Data Synthesis and Analysis
Measures of correlation between the nutritional status of iodine (T3, T4, TSH, and UIC) and that of iron (serum iron, ferritin, and hemoglobin), selenium, and zinc were obtained from each study.
Meta-analysis was only performed on studies that had correlation. Adjustment for the calculations was considered. Age and sex stratification, when possible, did not influence the final result of the meta-analysis. The biomarkers used/type of analysis were organized according to the method used.
Data were loaded into an Excel spreadsheet and later exported to RStudio software, version 4.2.0, for meta-analysis. The metacor function of the meta package was used to summarize the correlation coefficients. To assess publication bias, the funnel symmetry test was applied, performed by the funnel function [12]. Heterogeneity among studies was assessed by Cochrane’s Q (χ2 p < 0.10) and quantified according to the proportion variance (I2). Values above 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively. For all analyses, the random effect was considered, considering the moderate or high heterogeneity observed (I2 > 50%). All results are summarized in the forest plot graph, using the forest function of the metafor package [13]. To assess the impact of excluding each study individually, influence analysis (leave one out) was applied.
3. Results
A total of 2367 articles were identified in three databases. After removing duplicates and reading titles, abstracts, and full texts, 40 articles were included in the systematic review (Supplementary S3). Figure 1 shows the selection processes.

Figure 1.
Scheme of the methodology adopted for the systematic review [7].
Regarding the assessment of risk of bias, according to the study design, cross-sectional studies [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] presented a risk of bias. Studies with a control scheme [42,43,44,45,46,47] presented a risk of bias in relation to issues related to the control of confounding factors, and strategies to address confounding factors for other issues were considered to have a low risk of bias. In basic clinical trials [48,49,50] and cohorts [51,52,53], there was also a risk of bias (Figure 2).Among cross-sectional studies, 85.7% were classified as low risk, and the remainder as moderate risk. Among case–control studies, 83.3% were classified as low risk. All randomized clinical trials and cohort studies had a low risk of bias.
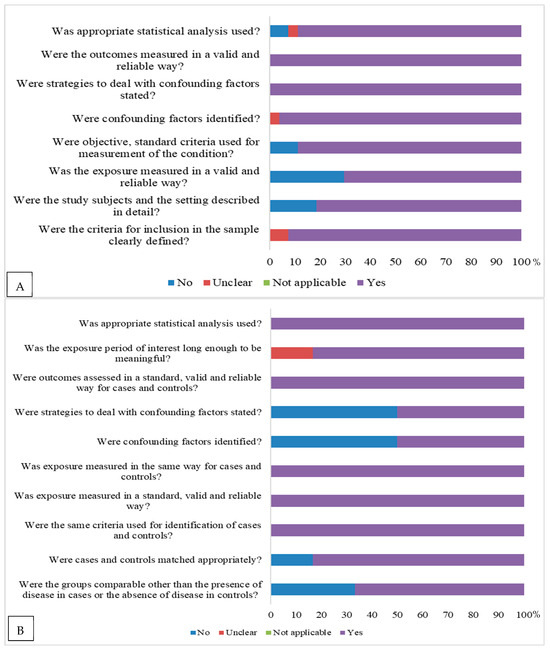
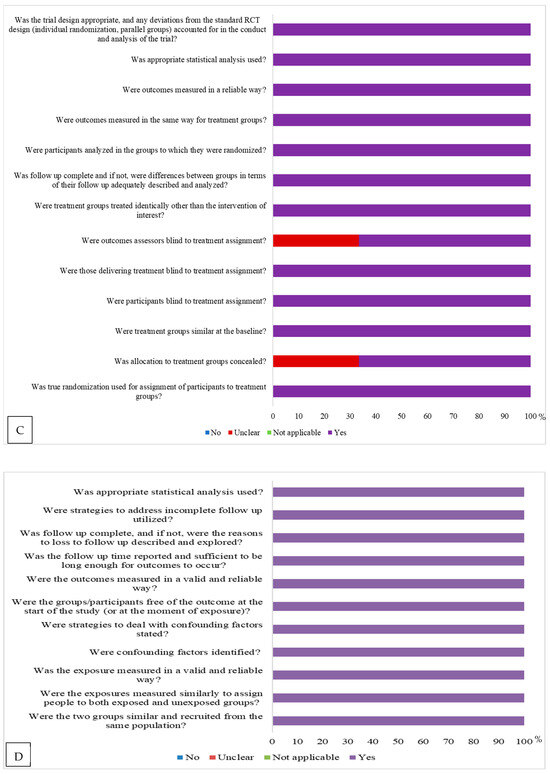
Figure 2.
Risk of bias assessment according to the Joanna Briggs Institute’s risk of bias assessment tool (2020) [10] according to study design: (A) = for cross-sectional studies; (B) = control case; (C) = randomized clinical trial, and (D) = cohort.
The studies were published between 1993 and 2025, with 70.0% (n = 28) [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41], 15.0% (n = 6) [42,43,44,45,46,47], 7.5% (n = 3) [48,49,50], and 7.5% (n = 3) [51,52,53] corresponding to cross-sectional design, case–control, clinical trial, and cohort studies, respectively. In relation to sample composition, 15.0% (n = 6) [24,27,33,41,43,47] of the studies were conducted with women only, 2.5% (n = 1) [17] with men, and the remaining with both sexes. The countries with the highest number of works were Turkey [17,19,20,34,40,43,44,47] and Iran [21,22,24,27,28,29,30,32], with each country representing 20.0% (n = 8) of the studies. The age groups addressed in majority of the studies were children and adolescents 50.0% (n = 20), followed by adults and the elderly 32.5% (n = 13), with others 17.5% (n = 7) covering different ages (Table 1).

Table 1.
Description of the included studies organized according to study type and year of publication.
Table 2 presents the assessment methods of nutritional status and iodine association within iron, selenium, and zinc in population studies. The most used indicators for the direct or indirect assessment of nutritional status of iodine were as follows: Thyroid Stimulating Hormone (TSH) [14,15,17,18,19,21,22,24,25,26,27,29,30,33,34,35,36,37,38,39,41,42,43,44,45,48,49,50,52,53] and Thyroxine (T4) or free T4 [14,15,17,18,19,21,22,24,25,26,27,28,29,30,33,34,35,39,41,42,43,44,45,48,49,50,52,53] were utilized in 75.0% (n = 30) of the studies. The urinary iodine concentration (UIC) accounts for 62.5% (n = 25) [14,16,17,18,19,21,22,23,26,27,28,29,30,31,33,35,36,38,41,43,44,47,50,51,52], triiodothyronine (T3) represents -60.0% (n = 24) [14,15,17,19,21,22,24,25,26,33,34,35,36,37,41,42,43,44,45,48,50,51,53], ultrasonography and thyroid palpation account for 27.5% (n = 11) [14,19,20,25,26,31,33,40,43,44,51] and 20.0% (n = 8) [14,16,21,24,28,32,46,47], respectively. TSH, T3, and T4 are direct indicators of thyroid function, but also serve as indirect indicators of iodine nutritional status [54].

Table 2.
Methods employed for the assessment of nutritional status and association of iodine, iron, selenium, and zinc in population studies.
Among the micronutrients analyzed, selenium was most prominent, being evaluated in 55.0% (n = 22) [15,17,18,19,20,25,26,30,33,38,40,41,42,43,44,46,47,48,49,50,51] of the studies, iron 37.5% (n = 15) [14,21,22,23,29,31,33,34,35,36,37,39,45,49,52], and zinc 27.5% (n = 11) [16,17,23,24,27,28,32,40,42,45,47]. The nutritional status of selenium was mainly assessed through serum levels, however four articles evaluated urinary selenium [15,38,41,47] and Glutathione Peroxidase, reflecting selenium intake over 2 to 3 months [18,48,49]. To assess iron, 73.3% (n = 11) [14,22,23,31,34,35,37,39,45,52,53] of the articles employed hemoglobin, ferritin 60.0% (n = 9) [14,21,22,23,29,34,36,39,52], and serum iron 46.7% (n = 7) [14,34,35,36,37,39,45]. For zinc, serum level was mainly utilized, however a study employed urinary zinc [47] (Table 2).
TSH had both positive [14] and negative [35,37,42] correlations with hemoglobin. Furthermore, TSH presented a negative correlation with ferritin [22,37], serum iron [36,37,45,52], urinary iron [45], red blood cell count [52], and selenium [50]. T4 had a negative correlation with hemoglobin [14] and serum selenium [15,48]; it also had a positive correlation with hemoglobin [37,53], ferritin [22], serum iron [37], and transferrin saturation [37], and selenium [50] (Table 2).
Figure 3 shows the markers related to the nutritional status of iodine and micronutrients. For 30.0% (n = 12) [15,18,22,23,24,25,27,30,31,32,33,43] of the studies, no statistical relationship was found between the nutritional status of iodine and selenium, iron, or zinc.
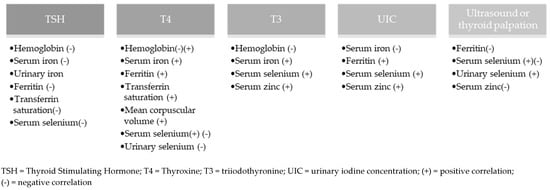
Figure 3.
Relationship between methods for assessing nutritional status of iodine with selenium, iron, and zinc.
Related to meta-analysis, there was a positive correlation between some markers of iodine nutrition and the evaluated micronutrients. For T3 and T4 there was a positive correlation with selenium [0.34 (0.11 to 0.54)], as well as the relationship between T4 and ferritin [0.37 (0.05 to 0.62)]. Also, there was a negative correlation between TSH and ferritin [−0.53 (−0.80 to −0.09)], serum iron [−0.53 (−0.69 to −0.32)], and Hemoglobin [−0.33 (−0.42 to −0.24)] (Figure 4). For the other markers of the nutritional status of iodine and other micronutrients, the correlation was not significant (Supplementary Figure S1).
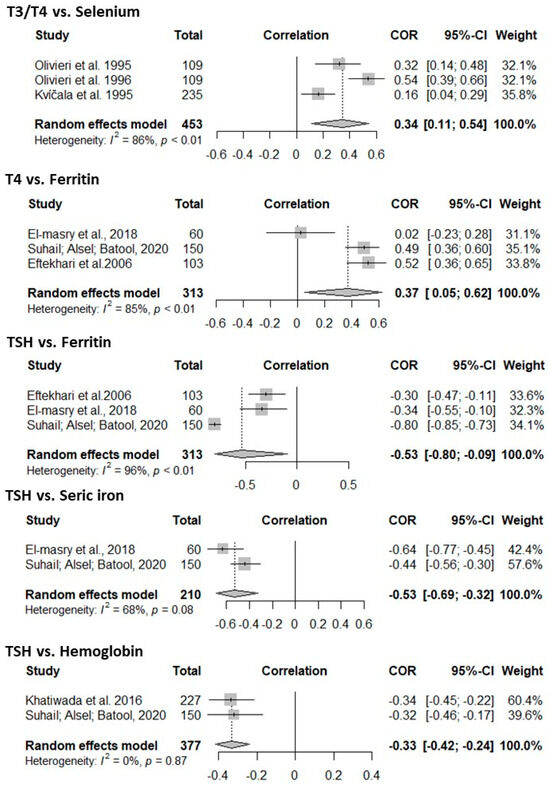
Figure 4.
Meta-analysis of the correlation coefficients between the nutritional status of iodine (T3, T4, TSH, and UIC) and that of iron (serum iron, ferritin, and hemoglobin), selenium, and zinc. References [15,22,35,37,48,49,51].
Publication bias was observed due to the asymmetry of the funnel plots, as shown in Supplementary Figure S2. To assess the impact of excluding each study individually, influence analysis (leave one out) was applied (Supplementary Figure S3). For the meta-analysis assessing the correlation between T4 and ferritin, it was found that excluding one study would reduce heterogeneity (I2) to 0%. Similarly, in the correlation between TSH and ferritin, one of the studies contributed significantly to greater heterogeneity. For both, the methodological quality was assessed, and they were not excluded from the analysis.
4. Discussion
Micronutrient deficiencies are a health problem that must be addressed, especially because they can affect cognitive and motor development; additionally, in women of reproductive age, they can cause spontaneous abortions, fetal malformations, and other problems [54]. The causal factors for micronutrient deficiencies include low intake and/or absorption, the presence of diseases and changes in physiological status, such as pregnancy, breastfeeding, and varying micronutrient requirements by age [6].
Iodine nutritional status showed a positive correlation (direct or indirect) with T3/T4, selenium, and ferritin, and a negative correlation with ferritin, serum iron, and hemoglobin, as synthesized in the meta-analysis.
Low iodine intake can impair thyroid function and result in Iodine Deficiency Disorders (IDDs), goiter, and difficulties in cognitive development, among other problems. This micronutrient plays a role in the formation of the thyroid hormones T3 and T4, and deficiency processes can lead to decreased TSH levels and increased production of T3 compared to T4 [55].
Iodine is a chemical element absorbed primarily in the stomach and small intestine in the form of iodide. After absorption, most of it goes to the thyroid gland, where it participates in the synthesis of thyroid hormones T3 and T4. These hormones play a role in energy metabolism, growth and development, body temperature regulation, cardiac function, and central nervous system development. The negative feedback mechanism controls hormone production, ensuring balanced concentrations in the body [56].
The recommended daily iodine intake is 90 µg for preschool children (0 to 59 months), 120 µg for schoolchildren (6 to 12 years), 150 µg for adolescents (above 12 years) and adults, and 250 µg for pregnant and lactating women. The main biochemical marker for monitoring iodine nutritional status in populations is urinary iodine concentration, assessed by the median value. It is important to emphasize that this indicator applies to the population level, not the individual. The cutoff points are as follows: insufficient intake (≤99.0 µg/L), adequate (100–199 µg/L), more than adequate (200–299 µg/L), and excessive (≥300 µg/L), considering non-pregnant women. For pregnant women, <150 µg/L is considered insufficient, 150–249 is considered adequate, 250–499 µg/L is considered above requirements, and ≥500 µg/L is considered excessive [57].
The IUC marker may be influenced by the hydration status of the individual being analyzed, especially in random urine samples. However, the coefficient of variation is generally less than 10%. However, it is important to sample the population where this test is being performed to determine the varying levels of hydration in this group [57].
When iodine intake is insufficient, the thyroid gland activates adaptive mechanisms, such as increased TSH secretion, leading to hyperplasia and goiter formation. This process can maintain T3 production but compromises T4 levels, altering the individual’s metabolic function and overall health [57].
Excess iodine is also a significant health problem that must be addressed. High intake can cause bone changes, especially in menopausal women, and increase the risk of cardiovascular dysfunction associated with iodine-induced hyperthyroidism. The body has an adaptation mechanism to excess iodine, known as the Wolff–Chaikoff effect, in which there is a transient reduction in hormone synthesis through the inhibition of thyroid peroxidase (an iron-dependent enzyme). However, when this adaptation does not occur properly, clinical complications can arise [53,58].
Understanding the context of iodine deficiency or excess requires devising strategies. The main intervention strategy for preventing Iodine Deficiency Disorders (IDDs) is universal salt iodization, implemented worldwide since the 1990s. This measure is considered effective due to its low cost, broad coverage, and process safety. Iodine intake among the population can be classified as adequate in most cases, but in some countries, this remains a significant problem to be addressed. The continuous monitoring of iodine nutritional status is essential to prevent both deficiency and excess. Furthermore, process, impact, and sustainability indicators, such as assessment of urinary iodine concentration, goiter analysis, and monitoring of fortification policies, are essential to ensure the effectiveness of actions and protect the population’s health [59].
Some indicators used in the direct and indirect assessments of iodine nutritional status are UIC and assessment of thyroid function (TSH, TG, T3, T4, free T4), respectively. TSH is more sensitive for diagnosing iodine deficiency in newborns, although studies report difficulties in result interpretation [60]. In addition to these methods, the T3/T4 ratio can be mentioned, which also allows the inference of iodine nutritional status based on the proper functioning of the thyroid gland.
In newborns, as part of a physiological response, TSH peaks at birth, stimulating the production of the other thyroid hormones, T3 and T4. This assessment is considered a public health measure, as it can identify congenital hypothyroidism. This condition is one of the leading causes of intellectual disability, and if diagnosed early, it can help prevent permanent health effects [61]. In children and adolescents, TSH levels may remain higher than in adults and decrease with age, until they reach a “balance” and become more stable in adulthood [62]. The negative relationship between TSH and micronutrient status found in this review highlights the relationship between iron nutritional status and thyroid function [63] in different population groups.
Iodine deficiency, as well as other micronutrients, can be associated with food insecurity, making public health interventions important to help manage this. Because it has a cyclical effect, food insecurity can be caused by rising costs of healthy eating, low family education and income, and difficulties in accessing health services, among other factors. This contributes to an increased risk of nutritional deficiencies and, consequently, an increase in diseases associated with this deficiency [64].
Using iron deficiency anemia as an example, a study by Lopes et al. [64] found that individuals experiencing food insecurity were more likely to be anemic and have low ferritin levels, regardless of their level of insecurity. Thus, iron deficiency, in addition to causing anemia, can cause changes in iodine metabolism, since the thyroid hormone production pathway relies on this mineral for the action of peroxidase.
The interaction between micronutrient deficiencies is not well understood, which further reinforces the need for studies like this. One example is the heterogeneity of the studies included in this review, covering different population groups and the sample representativeness of the studies, as well as the difficulty in finding associations in humans [62]. Establishing associations is easier when using animal models [4]. It is known that deficiency processes can interact with each other because metabolic pathways are interrelated and adequate levels of micronutrients (iodine, iron, selenium, and zinc) are crucial for proper thyroid function [6].
Animal models have demonstrated the effect of selenium on iodine status and thyroid metabolism, but this relationship is not always confirmed when extrapolated to human studies. It is also suggested that combined iodine and iron interventions contribute to improved thyroid volume and function. Regarding zinc, there is limited evidence of its relationship with iodine status, although zinc is essential for proper thyroid function [65,66].
In an attempt to understand the relationship between iodine and selenium, iron, and zinc, hormonal production and the role of micronutrients in hormonal production pathways must be considered. Hormones are classified into three types: steroid hormones, tyrosine compounds, and peptide hormones. Iodine is a structural component of thyroid hormones.
In a study by Hortz et al. [66], combined iodine and selenium deficiency did not increase serum TSH concentrations in comparison to hypothyroidism. This finding was confirmed by other studies [67,68], in which partial selenium deficiency had no effect on hypothyroid symptoms. On the other hand [69,70], found higher TSH concentrations in selenium deficiency compared to isolated iodine deficiency. These discrepancies require further human studies to understand the coexistence of these processes. In addition to selenium and iron, zinc is a micronutrient needed for proper thyroid function. Studies in rats and humans have shown that zinc deficiency reduces iodothyronine levels, corroborating the findings of Ozata et al. [17], who found significantly lower zinc levels in people with endemic goiter, thus justifying a possible zinc–iodine relationship [71].
Many metabolic processes involved in human growth and development are under the direct or indirect control of thyroid hormones. The full functioning of thyroid hormones requires not only adequate levels of iodine but also other nutrients that are important for their formation. This review sought to encompass all biomarkers of the nutritional status for iodine and other nutrients (iron, selenium, and zinc). The age range and study design of the studies to be included were not restricted; furthermore, the review highlights the importance of not only assessing the nutritional status of one micronutrient but also considering other micronutrients that may be related to the deficiency process. This combined approach can contribute to effective programs to eradicate nutritional deficiencies.
One limitation of this review was the lack of restrictions on the publication period of the included studies, which resulted in the inclusion of work over 25 years old. This demonstrates that the discussion on the topic has spanned decades, but it also highlights that significant knowledge gaps remain. Despite the passage of time, new, well-designed research is still needed to deepen our understanding of this issue. Furthermore, understanding the interaction between micronutrient deficiency processes is essential, as synergism between different diseases can occur. A better understanding of these mechanisms, both individually and collectively, is crucial to guiding more effective health and nutrition interventions. Therefore, it is essential that new studies address this issue and contribute to the systematization of more consistent information.
5. Conclusions
The combination of strategies to help combat micronutrient deficiencies (iodine, iron, selenium, and zinc) must consider the possible interactions between them. There is a need to clarify the effects of iodine deficiency combined with other micronutrient deficiencies, the influence of food intake on the metabolism of thyroid hormones, and the best form of mineral supplementation to combat the established deficiency.
Furthermore, working with joint public policies to monitor and prevent these deficiencies is an important tool for maintaining the population’s health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17213432/s1, Supplementary S1. Prisma 2020 Checklist; Supplementary S2. Peer review of electronic search strategies; Supplementary S3. Search strategies in the databases used; Supplementary S4. Risk of bias for each individual study assessed by the Joanna Briggs Institute critical appraisal checklist for studies; Supplementary Figure S1. Meta-analysis of the correlation coefficients between the nutritional status of iodine (T3, T4, TSH, and UIC) and that of iron (serum iron, ferritin and hemoglobin), selenium and zinc; Supplementary Figure S2. Funnel plot of meta-analysis of the correlation coefficients between the nutritional status of iodine (T3, T4, TSH, and UIC) and that of iron (serum iron, ferritin and hemoglobin), selenium and zinc; Supplementary Figure S3. Influence analysis was applied.
Author Contributions
Conceptualization S.O.L. and S.E.P.; methodology S.O.L., E.M.M., A.C.C., S.E.P. and S.d.C.C.F.; validation, S.O.L.; formal analysis, S.O.L., F.M.A., D.d.C.M. and S.E.P.; investigation, S.O.L., E.M.M. and J.M.B.; resources, S.O.L. and F.M.A.; data curation, S.O.L. and E.M.M.; writing—original draft preparation, S.O.L. and F.M.A.; writing—review and editing, S.O.L., S.E.P. and S.d.C.C.F.; visualization, S.O.L.; supervision, S.O.L. and E.M.M.; project administration, S.O.L. and S.E.P.; funding acquisition, S.O.L. and S.E.P. All authors have read and agreed to the published version of the manuscript.
Funding
To the Postgraduate Program in Agroecology and the Postgraduate Program in Nutritional Sciences. Sources of Support: CAPES Foundation (Ministry of Education, Brazil, Financial Code 001), Minas Gerais State Research Support Foundation (FAPEMIG, State of Minas Gerais, Brazil BPD 01017-22), and the National Council for Scientific and Technological Development (CNPq, Ministry of Science and Technology, Brazil (439075/2018-1)).
Data Availability Statement
Analyses are available in the Supplementary Materials and information was taken from articles included in this review and referenced in the analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The Epidemiology of Global Micronutrient Deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef]
- Soares, M.S. Nutritional Assessment of Selenium in Adults from the City of Manaus, Amazonas. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2018. [Google Scholar] [CrossRef]
- Krela-Kaźmierczak, I.; Czarnywojtek, A.; Skoracka, K.; Rychter, A.M.; Ratajczak, A.E.; Szymczak-Tomczak, A.; Ruchała, M.; Dobrowolska, A. Is there an ideal diet to protect against iodine deficiency? Nutrients 2021, 13, 513. [Google Scholar] [CrossRef]
- Francis, A.K.; Tayie, K.J. Hypertension, Dietary Salt Restriction, and Iodine Deficiency Among Adults. Am. J. Hypertens. 2010, 23, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Biban, B.G.; Lichiardopol, C. Iodine Deficiency, Still a Global Problem? Curr. Health Sci. J. 2017, 43, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J.; Gärtner, R. Selenium and thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Aki, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lopes, S.O. Iodine Nutritional Status and Associated Socioeconomic, Demographic, Health and Nutritional Factors in Family Farmers in the Immediate Geographic Region of Viçosa-MG. Ph.D. Thesis, University of Viçosa, Viçosa, Brazil, 2023. [Google Scholar]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS peer review of electronic search strategies: 2015 guideline statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Joanna Briggs Institute. Critical Appraisal tools for use in JBI Systematic Reviews. 2020. Available online: https://jbi.global/critical-appraisal-tools (accessed on 2 April 2025).
- Costa, A.B.; Zoltowski, A.P.C.; Koller, S.H.; Teixeira, M.A.P. Construção de uma escala para avaliar a qualidade metodológica de revisões sistemáticas. Ciênc. Saúde Coletiva 2015, 20, 2441–2452. [Google Scholar] [CrossRef]
- BalduzzI, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Wolde-Gebriel, Z.; West, C.E.; Gebru, H.; Tadesse, A.S.; Fisseha, T.; Gabre, P.; Aboye, C.; Ayana, G.; Hautvast, J.G.A.J. Interrelationship between vitamin A, iodine and iron status in schoolchildren in Shoa Region, central Ethiopia. Br. J. Nutr. 1993, 70, 593–607. [Google Scholar] [CrossRef]
- Kvícala, J.; Zamrazil, V.; Soutorová, M.; Tomíska, F. Correlations between parameters of body selenium status and peripheral thyroid parameters in the low selenium region. Analyst 1995, 120, 959–965. [Google Scholar] [CrossRef]
- Hampel, R.; Kühlberg, T.; Schneider, K.P.; Glass, A.; Zöllner, H. Serum zinc levels and goitre epidemiology in Germany. Z. Ernahrungswissenschaft 1997, 36, 12–15. [Google Scholar] [CrossRef]
- Ozata, M.; Salk, M.; Aydin, A.; Sayin, S.; Oktenli, C.; Beyham, Z.; Isimer, A.; Ozdemir, I.C. Iodine and zinc, but not selenium and copper, deficiency exists in a male Turkish population with endemic goiter. Biol. Trace Elem. Res. 1999, 69, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Zagrodzki, P.; Szmigiel, H.; Ratajczak, R.; Szybinski, Z.; Zachwieja, Z. The role of selenium in iodine metabolism in children with goiter. Environ. Health Perspect. 2000, 108, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, M.F.; Erdoğan, G.; Sav, H.; Güllü, S.; Kamel, N. Endemic goiter, thiocyanate overload, and selenium status in school-age children. Biol. Trace Elem. Res. 2001, 79, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Aydin, K.; Kendirci, M.; Kurtoğlu, S.; Karaküçük, E.I.; Kiriş, A. Iodine and selenium deficiency in school-children in an endemic goiter area in Turkey. J. Pediatr. Endocrinol. Metab. 2002, 15, 1027–1031. [Google Scholar] [CrossRef]
- Azizi, F.; Mirmiran, P.; Sheikholeslam, R.; Hedayati, M.; Rastmanesh, R. The relation between serum ferritin and goiter, urinary iodine and thyroid hormone concentration. Int. J. Vitam. Nutr. Res. 2002, 72, 296–299. [Google Scholar] [CrossRef]
- Eftekhari, M.H.; Keshavarz, S.A.; Jalali, M.; Elguero, E.; Eshraghian, M.R.; Simondon, K.B. The relationship between iron status and thyroid hormone concentration in iron-deficient adolescent Iranian girls. Asia Pac. J. Clin. Nutr. 2006, 15, 50. [Google Scholar]
- Thurlow, R.A.; Winichagoon, P.; Pongcharoen, T.; Gowachirapant, S.; Boonpraderm, A.; Manger, M.S.; Bailey, K.B.; Wasantwisut, E.; Gibson, R.S. Risk of zinc, iodine and other micronutrient deficiencies among school children in North East Thailand. Eur. J. Clin. Nutr. 2006, 60, 623–632. [Google Scholar] [CrossRef]
- Dabbaghmanesh, M.H.; Sadegholvaad, A.; Zarei, F.; Omrani, G. Zinc Status and Relation to Thyroid Hormone Profile in Iranian Schoolchildren. J. Trop. Pediatr. 2008, 54, 58–61. [Google Scholar] [CrossRef]
- Zagrodzki, P.; Ratajczak, R. Selenium status, sex hormones, and thyroid function in young women. J. Trace Elem. Med. Biol. 2008, 22, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Doupis, J.; Stavrianos, C.; Saltiki, K.; Mantzou, E.; Mastrokostopoulos, A.; Philippou, G.; Alevizaki, M. Thyroid volume, selenium levels and nutritional habits in a rural region in Albania. Hormones 2009, 8, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Keshteli, A.H.; Hashemipour, M.; Siavash, M.; Kelishadi, R.; Amini, M. High prevalence of goiter in schoolchildren in Isfahan; zinc deficiency does not play a role. Endokrynol. Pol. 2010, 61, 287–290. [Google Scholar] [PubMed]
- Moaddab, M.H.; Keshteli, A.H.; Dastjerdi, M.S.; Rezvanian, H.; Aminorroaya, A.; Amini, M.; Kachuei, A.; Hashemipour, M. Zinc status in goitrous school children of Semirom, Iran. J. Res. Med. Sci. 2009, 14, 165–170. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3129056/ (accessed on 3 April 2025).
- Hashemipour, M.; Soheilipour, F.; Keshteli, A.H.; Siavash, M.; Amini, M.; Kelishadi, R. Association between serum ferritin and goitre in Iranian school children. J. Health Popul. Nutr. 2010, 28, 137–142. [Google Scholar] [CrossRef][Green Version]
- Keshteli, A.H.; Hashemipour, M.; Siavash, M.; Amini, M. Selenium deficiency as a possible contributor of goiter in schoolchildren of Isfahan, Iran. Biol. Trace Elem. Res. 2009, 129, 70–77. [Google Scholar] [CrossRef]
- Henjum, S.; Barikm, I.; Strand, T.A.; Oshaug, A.; Torheim, L.E. Iodine-induced goitre and high prevalence of anaemia among Saharawi refugee women. Public Health Nutr. 2012, 15, 1512–1518. [Google Scholar] [CrossRef]
- Sanjari, M.; Gholamhoseinian, A.; Nakhaee, A. Serum zinc levels and goiter in Iranian school children. J. Trace Elem. Med. Biol. 2012, 26, 42–45. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Zeng, J.; Sun, C. Thyroid volume, goiter prevalence, and selenium levels in an iodine-sufficient area: A cross-sectional study. BMC Public Health 2013, 13, 1153. [Google Scholar] [CrossRef]
- Yavuz, Ö.; Yavuz, T.; Kahraman, C.; Yeşildal, N.; Bundak, R. The relationship between iron status and thyroid hormones in adolescents living in an iodine deficient area. J. Pediatr. Endocrinol. Metab. 2004, 17, 1443–1450. [Google Scholar] [CrossRef]
- Khatiwada, S.; Gelal, B.; Baral, N.; Lamsal, M. Association between iron status and thyroid function in Nepalese children. Thyroid. Res. 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hendryx, M.; Dinh, P.; He, K. Association of iodine and iron with thyroid function. Biol. Trace Elem. Res. 2017, 179, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Suhail, N.; Alsel, B.T.A.; Batool, S. Prevalence and association of thyroid dysfunction with anemia/body iron status among northern Border Saudi population. Int. J. Med. Res. Health Sci. 2020, 9, 1–7. Available online: https://www.ijmrhs.com/medical-research/prevalence-and-association-of-thyroid-dysfunction-with-anemiabody-iron-status-among-northern-border-saudi-population.pdf (accessed on 3 April 2025).
- Campos, R.O.; de Jesus, L.M.; Morais, D.A.; Júnior, W.T.d.S.; Souza, V.C.d.O.; Oliveira, C.A.; Júnior, F.B.; Macedo, M.; Hegedüs, L.; Ramos, H.E. Low urinary selenium levels are associated with iodine deficiency in Brazilian schoolchildren and adolescents. Endocrine 2021, 73, 609–616. [Google Scholar] [CrossRef]
- Islam, R.; Akter, K.M.; Rahman, A.; Khanam, N.N.; Al Azad, S.; Islam, M.R.; Farjana, M.; Rahman, M.H.; Badal, M.N.U.; Ahmed, S. The Serological Basis of the Correlation between Iron Deficiency Anemia and Thyroid Disorders in Women: A Community Based Study. J. Pharm. Res. Int. 2021, 33, 69–81. [Google Scholar] [CrossRef]
- Turan, E.; Turksoy, V.A. Selenium, zinc, and copper status in euthyroid nodular goiter: A cross-sectional study. Int. J. Prev. Med. 2021, 12, 46. [Google Scholar] [CrossRef]
- Berger, J.; Finlayson, J.; von Hurst, P.R.; Brough, L. Iodine and selenium intakes and status and thyroid function in midlife women with low bread intakes in New Zealand. Nutr. Diet. 2025; ahead of print. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Maioli, F.; Nesi, B.; Pratelli, L.; Savarino, L.; Cucinotta, D.; Cavalli, G. Blood micronutrient and thyroid hormone concentrations in the oldest-old. J. Clin. Endocrinol. Metab. 2000, 85, 2260–2265. [Google Scholar] [CrossRef]
- Cinaz, P.; Karakas, D.S.; Camurdan, M.O.; Bideci, A.; Ayvali, E.D.; Yücel, C. Goiter prevalence, serum selenium, and urine iodine status in a previously iodine-deficient area in Turkey. Biol. Trace Elem. Res. 2004, 100, 185–194. [Google Scholar] [CrossRef]
- Hekimsoy, Z.; Biberoglu, S.; Kirkali, G.; Bicer, N.; Erbayraktar, Z. Plasma selenium and urinary iodine in patients with goiter. Trace Elem. Electrolytes 2004, 21, 145–149. [Google Scholar] [CrossRef]
- Kandhro, G.A.; Kazi, T.G.; Afridi, H.I.; Kazi, N.; Arain, M.B.; Sarfraz, R.A.; Sirajuddin; Syed, N.; Baig, J.A.; Shah, A.Q. Evaluation of iron in serum and urine and their relation with thyroid function in female goitrous patients. Biol. Trace Elem. Res. 2008, 125, 203–212. [Google Scholar] [CrossRef]
- Kishosha, P.A.; Galukande, M.; Gakwaya, A.M. Selenium deficiency a factor in endemic goiter persistence in sub-Saharan Africa. World J. Surg. 2011, 35, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Çelik, T.; Savaş, N.; Kurtoğlu, S.; Sangün, Ö.; Aydın, Z.; Mustafa, D.; Öztürk, O.H.; Mısırlıoğlu, S.; Öktem, M. Iodine, copper, zinc, selenium and molybdenum levels in children aged between 6 and 12 years in the rural area with iodine deficiency and in the city center without iodine deficiency in Hatay. Turk. Arch. Pediatr. 2014, 49, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, O.; Girelli, D.; Azzini, M.; Stanzial, A.M.; Russo, C.; Ferroni, M.; Corrocher, R. Low selenium status in the elderly influences thyroid hormones. Clin. Sci. 1995, 89, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, O.; Girelli, D.; Stanzial, A.M.; Rossi, L.; Bassi, A.; Corrocher, R. Selenium, zinc, and thyroid hormones in healthy subjects. Biol. Trace Elem. Res. 1996, 51, 31–41. [Google Scholar] [CrossRef]
- Gashu, D.; Marquis, G.S.; Bougma, K.; Stoecker, B.J. Selenium inadequacy hampers thyroid response of young children after iodine repletion. J. Trace Elem. Med. Biol. 2018, 50, 291–295. [Google Scholar] [CrossRef]
- Rasmussen, L.B.; Schomburg, L.; Köhrle, J.; Pedersen, I.B.; Hollenbach, B.; Hög, A.; Ovesen, L.; Perrild, H.; Laurberg, P. Selenium status, thyroid volume, and multiple nodule formation in an area with mild iodine deficiency. Eur. J. Endocrinol. 2011, 164, 585–590. [Google Scholar] [CrossRef]
- El-Masry, H.; Hamed, A.M.M.; Hassan, M.H.; Abdelzaher, M.H. Thyroid Function among Children with Iron Deficiency Anaemia: Pre and Post Iron Replacement Therapy. J. Clin. Diagn. Res. 2018, 12, BC1–BC5. [Google Scholar] [CrossRef]
- Gu, Y.; Chi, V.T.Q.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Bao, X.; Zhang, S.; Sun, S.; Wang, X.; et al. Low-Normal thyroid function predicts incident Anemia in the general population with Euthyroid status. J. Clin. Endocrinol. Metab. 2019, 104, 5693–5702. [Google Scholar] [CrossRef]
- Candido, A.C.; Azevedo, F.M.; Ribeiro, S.A.V.; Navarro, A.M.; Macedo, M.d.S.; Fontes, E.A.F.; Crispim, S.P.; de Carvalho, C.A.; Pizato, N.; da Silva, D.G.; et al. Iodine Deficiency and Excess in Brazilian Pregnant Women: A Multicenter Cross-Sectional Study (EMDI-Brazil). Nutrients 2025, 17, 2753. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Boelaert, K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015, 3, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’orazi, V.; Tartaglia, F.; Tripodi, D.; Lori, E.; Gagliardi, F.; Praticò, M.; et al. Iodine: Its role in thyroid hormone biosynthesis and beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization—WHO. Assessment of the Iodine Deficiency Disorders and Monitoring Their Elimination; WHO: Geneva, Switzerland, 2007; Available online: https://iris.who.int/bitstream/handle/10665/43781/9789241595827_eng.pdf?sequence=1 (accessed on 16 September 2025).
- Leung, A.M.; Braverman, L.E. Consequences of excess iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef]
- Rigutto-Farebrother, J.; Zimmermann, M.B. Salt reduction and iodine fortification policies are compatible: Perspectives for public health advocacy. Nutrients 2024, 16, 2517. [Google Scholar] [CrossRef]
- Candido, A.C.; Azevedo, F.M.; Macedo, M.S.; Priore, S.E.; Franceschini, S.C.C. Critical analysis of indicators of iodine nutritional status in individuals and populations: A systematic review. Sci. Public Health 2021, 26, 4859–4870. [Google Scholar] [CrossRef]
- Jayasuriya, M.S.; Choy, K.W.; Chin, L.K.; Doery, J.; Stewart, A.; Bergman, P.; Lu, Z.X. Reference intervals for neonatal thyroid function tests in the first 7 days of life. J. Pediatr. Endocrinol. Metab. 2018, 31, 1113–1116. [Google Scholar] [CrossRef]
- Segni, M. Disorders of the Thyroid Gland in Infancy, Childhood and Adolescence in Comprehensive Free Online Endrocrinology Book. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279032/ (accessed on 14 September 2025).
- Garofalo, V.; Condorelli, R.A.; Cannarella, R.; Aversa, A.; Calogero, A.E.; La Vignera, S. Relationship between iron deficiency and thyroid function: A systematic review and meta-analysis. Nutrients 2023, 15, 4790. [Google Scholar] [CrossRef]
- Lopes, S.O.; Abrantes, L.C.S.; Azevedo, F.M.; Morais, N.S.; Morais, D.C.; Gonçalves, V.S.S.; Fontes, E.A.F.; Franceschini, S.C.C.; Priore, S.E. Food insecurity and micronutrient deficiency in adults: A systematic review and meta-analysis. Nutrients 2023, 15, 1074. [Google Scholar] [CrossRef]
- Hess, S.Y. The impact of common micronutrient deficiencies on iodine and thyroid metabolism: The evidence from human studies. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 117–132. [Google Scholar] [CrossRef]
- Hotz, C.S.; Fitzpatrick, D.W.; Trick, K.D.; L’Abbe, M.R. Dietary iodine and selenium interact to affect thyroidhormone metabolism of rats. J. Nutr. 1997, 127, 1214–1218. [Google Scholar] [CrossRef]
- Beckett, G.J.; Nicol, F.; Proudfoot, D.; Dyson, K.; Loucaides, G.; Arthur, J.R. The changes in hepatic enzyme expression caused by selenium deficiency and hypothyroidism in rats are produced by independent mechanisms. Biochem. J. 1990, 266, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Meinhold, H.; Campos-Barros, A.; Walzog, B.; Köhler, R.; Müller, F.; Behne, D. Effects of selenium and iodine deficiency on type I, type II and type III iodothyronine deiodinases and circulating thyroid hormones in the rat. Exp. Clin. Endocrinol. Diabetes 1993, 101, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.R.; Nicol, F.; Beckett, G.J. The role of selenium in thyroid hormone metabolism and effects of selenium deficiency on thyroid hormone and iodine metabolism. Biol. Trace Elem. Res. 1992, 33, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Beckett, G.J.; Nicol, F.; Rae, P.W.; Beech, S.; Guo, Y.; Arthur, J.R. Effects of combined iodine and selenium deficiency on thyroid hormone metabolism in rats. Am. J. Clin. Nutr. 1993, 57, 240S–243S. [Google Scholar] [CrossRef]
- Ruz, M.; Codoceo, J.; Galgani, J.; Muñoz, L.; Gras, N.; Muzzo, S.; Leiva, L.; Bosco, C. Single and multiple selenium-zinc-iodine deficiencies affect rat thyroid metabolism and ultrastructure. J. Nutr. 1999, 129, 174–180. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





