Effects of Cocoa Consumption on Cardiometabolic Risk Markers: Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Inclusion and Exclusion Criteria
2.3. Search Strategy
2.4. Data Extraction and Management
2.5. Risk of Bias and Strength of Evidence
2.6. Data-Analysis Strategy
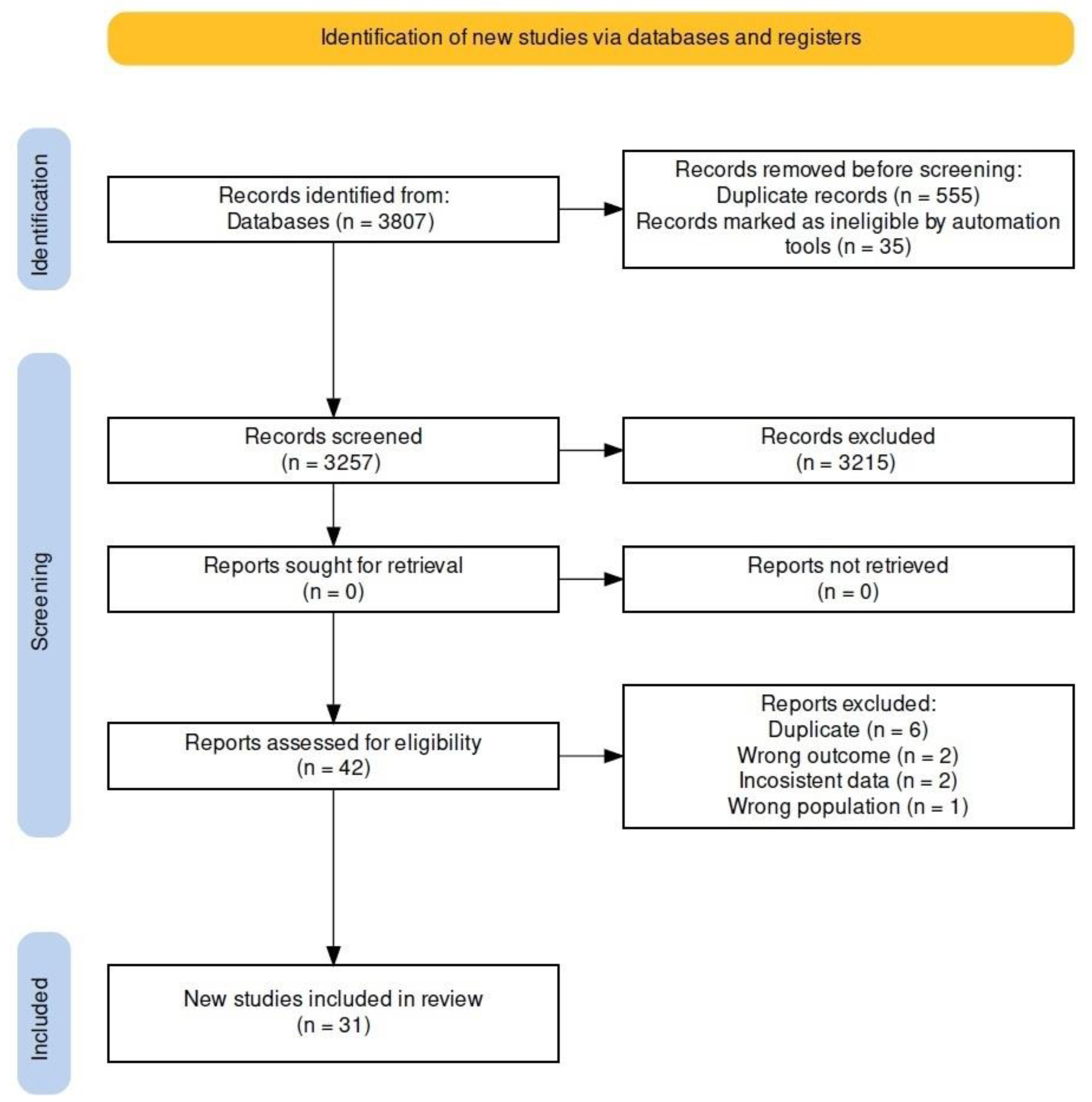
3. Results
3.1. Characteristics of the Studies
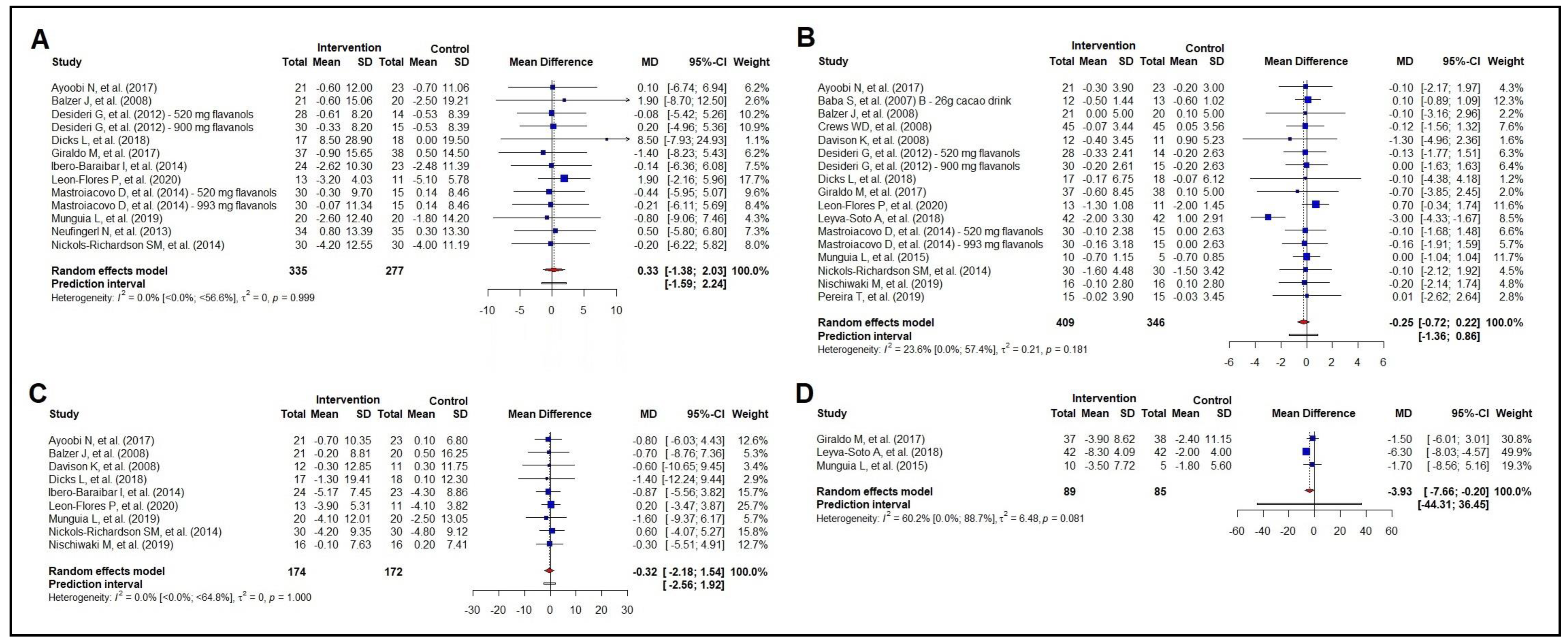
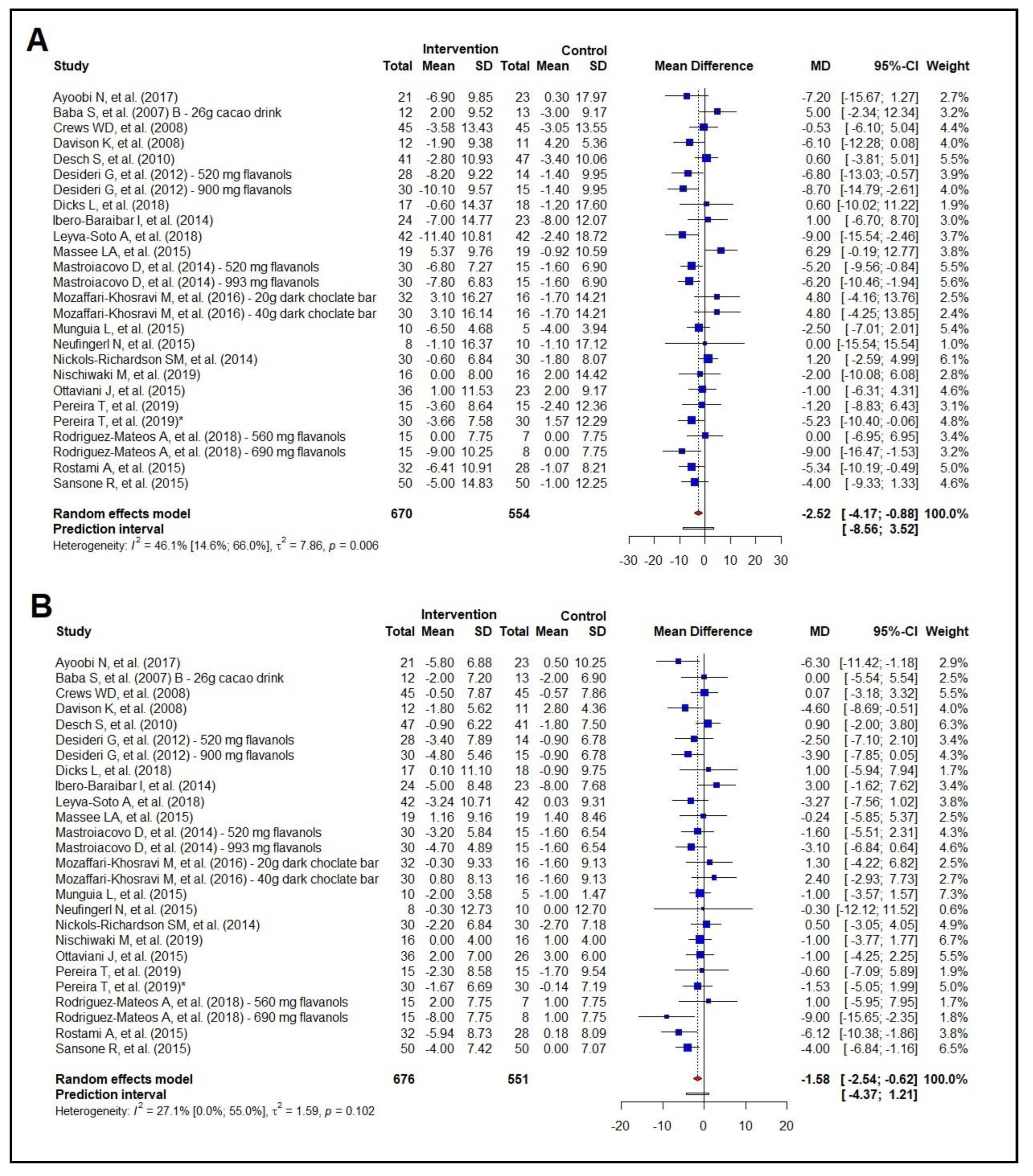
3.2. Meta-Analysis of the Effect on Anthropometric Measurements
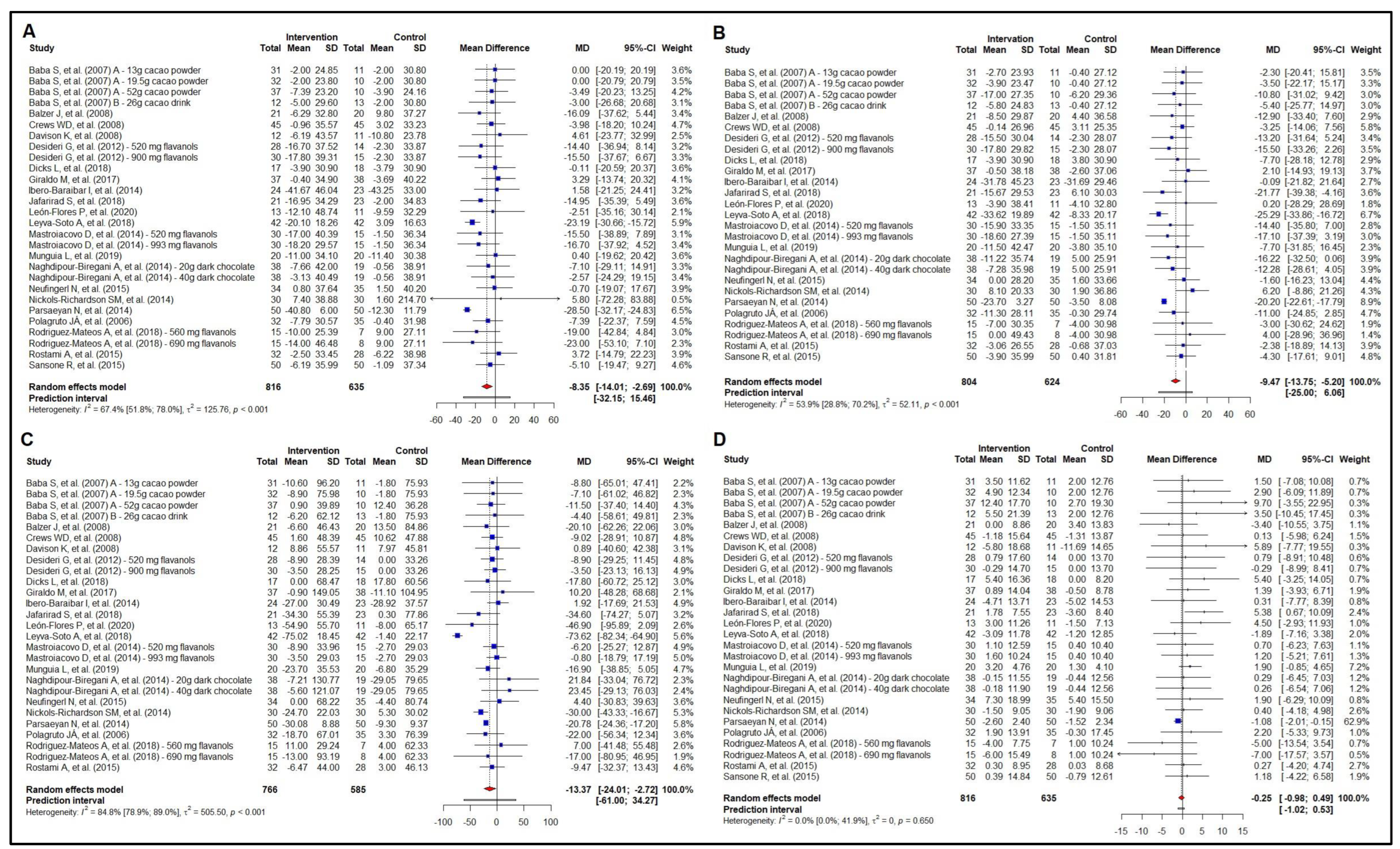
3.3. Meta-Analysis of the Effect on Lipid Profile
3.4. Meta-Analysis of the Effect on Glycemic Profile
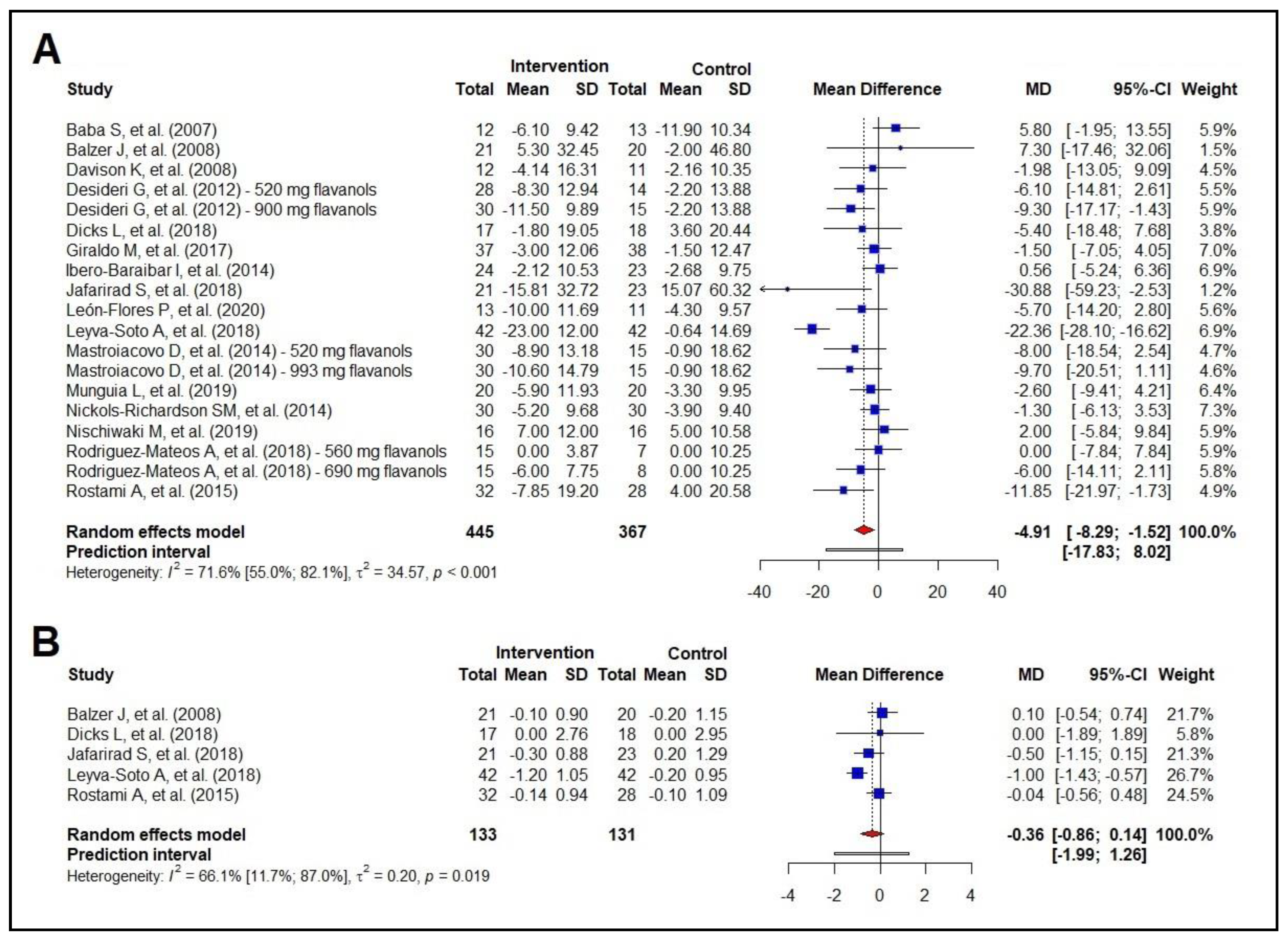
3.5. Meta-Analysis of the Effect on Blood Pressure
3.6. Risk of Bias Assessment (RoB 2) and Strength of Evidence (GRADE)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oliveira, G.; Brant, L.; Polanczyk, C.; Malta, D.; Biolo, A.; Nascimento, B.; Souza, M.; Lorenzo, A.; Fagundes Júnior, A.; Schaan, B.; et al. Estatística Cardiovascular–Brasil 2021. Arq. Bras. Cardiol. 2022, 118, 115–373. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Parretti, H.M.; Jebb, S.A.; Johns, D.J.; Lewis, A.L.; Christian-Brown, A.M.; Aveyard, P. Clinical effectiveness of very-low-energy diets in the management of weight loss: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2016, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Gori, A.M.; Sereni, A.; Becatti, M.; Fiorillo, C.; Marcucci, R.; Casini, A. Low-Calorie Vegetarian Versus Mediterranean Diets for Reducing Body Weight and Improving Cardiovascular Risk Profile: CARDIVEG Study (Cardiovascular Prevention With Vegetarian Diet). Circulation 2018, 137, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Miller, E.R., 3rd; Weaver, C.M.; Appel, L.J. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. J. Am. Coll. Cardiol. 2017, 70, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remon, A.; Martinez-Gonzalez, M.A.; Lopez-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Lapetra, J.; et al. Polyphenol intake and mortality risk: A re-analysis of the PREDIMED trial. BMC Med. 2014, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Ebrahimi, F.; Farzaei, M.H.; Baratpourmoghaddam, A.; Ahmadi, P.; Rostamiasrabadi, P.; Rasouli Amirabadi, A.H.; Rahimi, R. Dietary polyphenols for atherosclerosis: A comprehensive review and future perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Tressera-Rimbau, A.; Arranz, S.; Eder, M.; Vallverdú-Queralt, A. Dietary Polyphenols in the Prevention of Stroke. Oxid. Med. Cell Longev. 2017, 2017, 7467962. [Google Scholar] [CrossRef]
- Meng, C.C.; Jalil, A.M.; Ismail, A. Phenolic and theobromine contents of commercial dark, milk and white chocolates on the Malaysian market. Molecules 2009, 14, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Rudra, A.; Arvind, I.; Mehra, R. Polyphenols: Types, sources and therapeutic applications. Int. J. Home Sci. 2021, 7, 69–75. [Google Scholar] [CrossRef]
- Corti, R.; Flammer, A.J.; Hollenberg, N.K.; Lüscher, T.F. Cocoa and cardiovascular health. Circulation 2009, 119, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; 694p. [Google Scholar]
- Robinson, K.A.; Dickersin, K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int. J. Epidemiol. 2002, 31, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Glanville, J.; Foxlee, R.; Wisniewski, S.; Noel-Storr, A.; Edwards, M.; Dooley, G. Translating the Cochrane EMBASE RCT filter from the Ovid interface to Embase.com: A case study. Health Info Libr. J. 2019, 36, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.; Savović, J.; Higgins, J.P.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R.; Group, R. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Schunemann, H.J.; Brennan, S.; Akl, E.A.; Hultcrantz, M.; Alonso-Coello, P.; Xia, J.; Davoli, M.; Rojas, M.X.; Meerpohl, J.J.; Flottorp, S.; et al. The development methods of official GRADE articles and requirements for claiming the use of GRADE—A statement by the GRADE guidance group. J. Clin. Epidemiol. 2023, 159, 79–84. [Google Scholar] [CrossRef] [PubMed]
- IntHout, J.; Loannidis, J.; Rovers, M.; Goeman, J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2015, 6, e010247. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Sutton, A.; Welton, N.; Ades, A. Evidence synthesis for decision making 3: Heterogeneity--subgroups, meta-regression, bias, and bias-adjustment. Med. Decis. Making 2013, 33, 618–640. [Google Scholar] [CrossRef] [PubMed]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M. Quantifying the risk of error when interpreting funnel plots. Syst. Rev. 2015, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Page, M.; Higgins, J.; Sterne, J. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; Wiley Online Libraty: Minneapolis, MN, USA, 2021. [Google Scholar]
- Higgins, J.; Li, T.; Deeks, J. Choosing effect. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023); Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; Cochrane: London, UK, 2023. [Google Scholar]
- Mozaffari-Khosravi, H.; Naghdipour-Biregani, A.; Zavar-Reza, J.; Poursoleiman, F. The effects of dark chocolate consumption on oxidative stress and blood pressure in patients with metabolic syndrome: A randomized clinical trial. J. Nutr. Food Secur. 2016, 1, 1–8. [Google Scholar]
- Massee, L.A.; Ried, K.; Pase, M.; Travica, N.; Yoganathan, J.; Scholey, A.; Macpherson, H.; Kennedy, G.; Sali, A.; Pipingas, A. The acute and sub-chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: A randomized, controlled trial. Front. Pharmacol. 2015, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, J.I.; Balz, M.; Kimball, J.; Ensunsa, J.L.; Fong, R.; Momma, T.Y.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. Safety and efficacy of cocoa flavanol intake in healthy adults: A randomized, controlled, double-masked trial. Am. J. Clin. Nutr. 2015, 102, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.; Kirch, N.; Gronwald, D.; Wernken, K.; Zimmermann, B.F.; Helfrich, H.P.; Ellinger, S. Regular Intake of a Usual Serving Size of Flavanol-Rich Cocoa Powder Does Not Affect Cardiometabolic Parameters in Stably Treated Patients with Type 2 Diabetes and Hypertension-A Double-Blinded, Randomized, Placebo-Controlled Trial. Nutrients 2018, 10, 1435. [Google Scholar] [CrossRef]
- Pereira, T.; Bergqvist, J.; Vieira, C.; Grüner Sveälv, B.; Castanheira, J.; Conde, J. Randomized study of the effects of cocoa-rich chocolate on the ventricle-arterial coupling and vascular function of young, healthy adults. Nutrition 2019, 63–64, 175–183. [Google Scholar] [CrossRef]
- Ibero-Baraibar, I.; Abete, I.; Navas-Carretero, S.; Massis-Zaid, A.; Martinez, J.A.; Zulet, M.A. Oxidised LDL levels decreases after the consumption of ready-to-eat meals supplemented with cocoa extract within a hypocaloric diet. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Ayoobi, N.; Jafarirad, S.; Haghighizadeh, M.; Jahanshahi, A. Protective Effect of Dark Chocolate on Cardiovascular Disease Factors and Body Composition in Type 2 Diabetes: A Parallel, Randomized, Clinical Trial. Iran. Red. Crescent Med. J. 2017, 19, e21644. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Kato, Y.; Natsume, M.; Yasuda, A.; Kido, T.; Fukuda, K.; Muto, Y.; Kondo, K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2007, 85, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Natsume, M.; Yasuda, A.; Nakamura, Y.; Tamura, T.; Osakabe, N.; Kanegae, M.; Kondo, K. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J. Nutr. 2007, 137, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: The Cocoa, Cognition, and Aging (CoCoA) study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study--a randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.; Coates, A.M.; Buckley, J.D.; Howe, P.R. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int. J. Obes. 2008, 32, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, M.; Nakano, Y.; Matsumoto, N. Effects of regular high-cocoa chocolate intake on arterial stiffness and metabolic characteristics during exercise. Nutrition 2019, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Khalili, M.; Haghighat, N.; Eghtesadi, S.; Shidfar, F.; Heidari, I.; Ebrahimpour-Koujan, S.; Eghtesadi, M. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015, 11, 21–29. [Google Scholar] [PubMed]
- Desch, S.; Kobler, D.; Schmidt, J.; Sonnabend, M.; Adams, V.; Sareban, M.; Eitel, I.; Blüher, M.; Schuler, G.; Thiele, H. Low vs. higher-dose dark chocolate and blood pressure in cardiovascular high-risk patients. Am. J. Hypertens. 2010, 23, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Munguía, L.; Gutiérrez-Salmeán, G.; Hernández, M.; Ortiz, A.; Sánchez, M.; Nájera, N.; Meaney, E.; Rubio-Gayosso, I.; Ceballos, G. Beneficial effects of a flavanol-enriched cacao beverage on anthropometric and cardiometabolic risk profile in overweight subjects. Rev. Mex. Cardiol. 2015, 26, 78–86. [Google Scholar]
- Munguia, L.; Rubio-Gayosso, I.; Ramirez-Sanchez, I.; Ortiz, A.; Hidalgo, I.; Gonzalez, C.; Meaney, E.; Villarreal, F.; Najera, N.; Ceballos, G. High Flavonoid Cocoa Supplement Ameliorates Plasma Oxidative Stress and Inflammation Levels While Improving Mobility and Quality of Life in Older Subjects: A Double-Blind Randomized Clinical Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Nickols-Richardson, S.M.; Piehowski, K.E.; Metzgar, C.J.; Miller, D.L.; Preston, A.G. Changes in body weight, blood pressure and selected metabolic biomarkers with an energy-restricted diet including twice daily sweet snacks and once daily sugar-free beverage. Nutr. Res. Pract. 2014, 8, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Weber, T.; Skene, S.S.; Ottaviani, J.I.; Crozier, A.; Kelm, M.; Schroeter, H.; Heiss, C. Assessing the respective contributions of dietary flavanol monomers and procyanidins in mediating cardiovascular effects in humans: Randomized, controlled, double-masked intervention trial. Am. J. Clin. Nutr. 2018, 108, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.; Rodriguez-Mateos, A.; Heuel, J.; Falk, D.; Schuler, D.; Wagstaff, R.; Kuhnle, G.G.; Spencer, J.P.; Schroeter, H.; Merx, M.W.; et al. Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: A randomised, controlled, double-masked trial: The Flaviola Health Study. Br. J. Nutr. 2015, 114, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Neufingerl, N.; Zebregs, Y.E.; Schuring, E.A.; Trautwein, E.A. Effect of cocoa and theobromine consumption on serum HDL-cholesterol concentrations: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, M.; Toro, J.; Arango, C.; Posada, L.; HI, G. Ensayo clínico aleatorizado y controlado del efecto del consumo de cacao en pacientes con resistencia a la insulina resistance. Acta Medica Colomb. 2017, 42, 90–96. [Google Scholar]
- León-Flores, P.; Nájera, N.; Pérez, E.; Pardo, B.; Jimenez, F.; Diaz-Chiguer, D.; Villarreal, F.; Hidalgo, I.; Ceballos, G.; Meaney, E. Effects of Cacao By-Products and a Modest Weight Loss Intervention on the Concentration of Serum Triglycerides in Overweight Subjects: Proof of Concept. J. Med. Food 2020, 23, 745–749. [Google Scholar] [CrossRef]
- Jafarirad, S.; Ayoobi, N.; Karandish, M.; Jalali, M.T.; Haghighizadeh, M.H.; Jahanshahi, A. Dark Chocolate Effect on Serum Adiponectin, Biochemical and Inflammatory Parameters in Diabetic Patients: A Randomized Clinical Trial. Int. J. Prev. Med. 2018, 9, 86. [Google Scholar] [CrossRef]
- Balzer, J.; Rassaf, T.; Heiss, C.; Kleinbongard, P.; Lauer, T.; Merx, M.; Heussen, N.; Gross, H.B.; Keen, C.L.; Schroeter, H.; et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J. Am. Coll. Cardiol. 2008, 51, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Naghdipour-Biregani, A.; Mozaffari-Khosravi, H.; Poursoleiman, F.; Zavar-Reza, J.; Rahmanian, M.; Dehghani, A. The Effect of Dark Chocolate Consumption on Lipid Profile in Patients with Metabolic Syndrome: A Randomized Clinical Trial. Iran. J. Diabetes Obes. 2014, 6, 9–13. [Google Scholar]
- Polagruto, J.A.; Wang-Polagruto, J.F.; Braun, M.M.; Lee, L.; Kwik-Uribe, C.; Keen, C.L. Cocoa flavanol-enriched snack bars containing phytosterols effectively lower total and low-density lipoprotein cholesterol levels. J. Am. Diet. Assoc. 2006, 106, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Parsaeyan, N.; Mozaffari-Khosravi, H.; Absalan, A.; Mozayan, M.R. Beneficial effects of cocoa on lipid peroxidation and inflammatory markers in type 2 diabetic patients and investigation of probable interactions of cocoa active ingredients with prostaglandin synthase-2 (PTGS-2/COX-2) using virtual analysis. J. Diabetes Metab. Disord. 2014, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Crews, W.; Harrison, D.; Wright, J. A double-blind, placebo-controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: Clinical findings from a sample of healthy, cognitively intact older adults. Am. J. Clin. Nutr. 2008, 87, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Soto, A.; Chavez-Santoscoy, R.A.; Lara-Jacobo, L.R.; Chavez-Santoscoy, A.V.; Gonzalez-Cobian, L.N. Daily Consumption of Chocolate Rich in Flavonoids Decreases Cellular Genotoxicity and Improves Biochemical Parameters of Lipid and Glucose Metabolism. Molecules 2018, 23, 2220. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Maldonado, J.; Laranjeiro, M.; Coutinho, R.; Cardoso, E.; Andrade, I.; Conde, J. Central arterial hemodynamic effects of dark chocolate ingestion in young healthy people: A randomized and controlled trial. Cardiol. Res. Pract. 2014, 2014, 945951. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, S.; Lambert, J.D. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. Eur. J. Nutr. 2014, 53, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; Macgregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Zhao, P.; Tian, H.B.; Chen, L.H.; Cui, L.Q. Effect of Grape Polyphenols on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2015, 10, e0137665. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Kord-Varkaneh, H.; Ghaedi, E.; Nazary-Vanani, A.; Mohammadi, H.; Shab-Bidar, S. Does cocoa/dark chocolate supplementation have favorable effect on body weight, body mass index and waist circumference? A systematic review, meta-analysis and dose-response of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Yu, I.A.; Garcia-Ortiz, L.; Gomez-Marcos, M.A.; Rodriguez-Sanchez, E.; Lugones-Sanchez, C.; Maderuelo-Fernandez, J.A.; Recio-Rodriguez, J.I. Cocoa-rich chocolate and body composition in postmenopausal women: A randomised clinical trial. Br. J. Nutr. 2021, 125, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A., Sr.; Flack, J.M. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: A Mendelian randomization analysis. J. Am. Coll. Cardiol. 2012, 60, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Faludi, A.A.; Izar, M.C.O.; Saraiva, J.F.K.; Chacra, A.P.M.; Bianco, H.T.; Afiune, A.N.; Bertolami, A.; Pereira, A.C.; Lottenberg, A.M.; Sposito, A.C.; et al. Atualização da Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose—2017. Arq. Bras. Cardiol. 2017, 109, 1–76. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, M.; Conti, A. Theobroma cacao L., the Food of the Gods: A scientific approach beyond myths and claims. Pharmacol. Res. 2010, 61, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Tokede, O.A.; Gaziano, J.M.; Djoussé, L. Effects of cocoa products/dark chocolate on serum lipids: A meta-analysis. Eur. J. Clin. Nutr. 2011, 65, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Matsui, N.; Ito, R.; Nishimura, E.; Yoshikawa, M.; Kato, M.; Kamei, M.; Shibata, H.; Matsumoto, I.; Abe, K.; Hashizume, S. Ingested cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition 2005, 21, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Xavier, H.T.; Izar, M.; Faria Neto, J.; Assad, M.; Rocha, V.; Sposito, A.; Fonseca, F.; Dos Santos, J.; Santos, R.; Bertolami, M.; et al. V Diretriz brasileira de dislipidemias e prevenção da aterosclerose. Arq. Bras. Cardiol. 2013, 101, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Desch, S.; Schmidt, J.; Kobler, D.; Sonnabend, M.; Eitel, I.; Sareban, M.; Rahimi, K.; Schuler, G.; Thiele, H. Effect of cocoa products on blood pressure: Systematic review and meta-analysis. Am. J. Hypertens. 2010, 23, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Fakler, P.; Stocks, N.P. Effect of cocoa on blood pressure. Cochrane Database Syst. Rev. 2017, 4, Cd008893. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Arisi, T.O.P.; Gorski, F.; Eibel, B.; Barbosa, E.; Boll, L.; Waclawovsky, G.; Lehnen, A.M. Dietary intake of anthocyanins improves arterial stiffness, but not endothelial function, in volunteers with excess weight: A randomized clinical trial. Phytother. Res. 2023, 37, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Actis-Goretta, L.; Ottaviani, J.I.; Fraga, C.G. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J. Agric. Food Chem. 2006, 54, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.A.; Persson, K.; Hägg, S.; Andersson, R.G. Effects of cocoa extract and dark chocolate on angiotensin-converting enzyme and nitric oxide in human endothelial cells and healthy volunteers--a nutrigenomics perspective. J. Cardiovasc. Pharmacol. 2011, 57, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schömig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA 2007, 298, 49–60. [Google Scholar] [CrossRef] [PubMed]
- McInnes, G.T. Lowering blood pressure for cardiovascular risk reduction. J. Hypertens. Suppl. 2005, 23, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Vian, I.; Zielinsky, P.; Zilio, A.M.; Schaun, M.I.; Brum, C.; Lampert, K.V.; De Avila, N.; Baldissera, G.; Klanovicz, T.M.; Zenki, K.; et al. Increase of prostaglandin E2 in the reversal of fetal ductal constriction after polyphenol restriction. Ultrasound Obstet. Gynecol. 2018, 52, 617–622. [Google Scholar] [CrossRef]
- Hahn, M.; Baierle, M.; Charao, M.F.; Bubols, G.B.; Gravina, F.S.; Zielinsky, P.; Arbo, M.D.; Cristina Garcia, S. Polyphenol-rich food general and on pregnancy effects: A review. Drug Chem. Toxicol. 2017, 40, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Zielinsky, P.; Piccoli, A.L., Jr.; Manica, J.L.; Nicoloso, L.H.; Menezes, H.; Busato, A.; Moraes, M.R.; Silva, J.; Bender, L.; Pizzato, P.; et al. Maternal consumption of polyphenol-rich foods in late pregnancy and fetal ductus arteriosus flow dynamics. J. Perinatol. 2010, 30, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Pedra, S.; Zielinsky, P.; Binotto, C.; Martins, C.; Fonseca, E.; Guimarães, I.; Corrêa, I.; Pedrosa, K.; Lopes, L.; Nicoloso, L.; et al. Brazilian Fetal Cardiology Guidelines—2019. Arq. Bras. Cardiol. 2019, 112, 600–648. [Google Scholar] [CrossRef] [PubMed]
| Reference | Population | n M/F | Groups | Mean Age (Years) | Duration (Weeks) | Outcomes | Intervention (Daily) | Comparator (Control Group) |
|---|---|---|---|---|---|---|---|---|
| Ayoobi et al. [35] | Type 2 diabetes mellitus | Total: 44 Sex: 17/27 | INT: 21 CG: 23 | INT: 50.6 CG: 50.7 | 8 | SBP; DBP; BMI; WC; body weight | 84% cocoa beverage (30 g) | Therapeutic lifestyle changes guidelines |
| Baba et al. [37] | Healthy and mild hypercholesterolemia | Total: 25 Sex: 25 M | INT: 13 CG: 12 | INT: 38.0 CG: 38.0 | 12 | SBP; DBP; glucose; BMI; HDL; LDL; cholesterol; triglycerides | 26 g cocoa beverage (199 mg of total polyphenols) | Placebo beverage |
| Baba et al. [36] | Healthy and hypercholesterolemia | Total: 131 Sex: NA | INT 1: 31 INT 2: 32 INT 3: 37 CG: 31 | INT 1: 49.0 INT 2: 49.0 INT 3: 49.0 CG: 49.0 | 4 | HDL; LDL; cholesterol; triglycerides | Cocoa beverage INT: 13 g cocoa powder (140.9 mg polyphenols) INT 2: 419.5 g cocoa powder (211.2 mg of polyphenols) INT 3: 26 g cocoa powder (281.5 mg of polyphenols) | Placebo beverage |
| Balzer et al. [53] | Type 2 diabetes mellitus/drug therapy | Total: 41 Sex: 12/29 | INT: 21 CG: 20 | INT: 63.1 CG: 64.4 | 4 | Body weight; BMI; WC; glucose; HbA1c. HDL; LDL; cholesterol; triglycerides | Cocoa beverage = mix of cocoa powder + milk powder (963 mg of flavanols) | Cocoa beverage = cocoa extract + milk powder (75 mg of flavanols) |
| Crews et al. [57] | Healthy elderly | Total: 90 Sex: 38/52 | INT: 45 CG: 45 | INT: 68.8 CG: 68.7 | 6 | SBP; DBP; BMI; HDL; LDL; cholesterol; triglycerides | One dark-chocolate bar (37.0 g containing 60% cocoa, 11 g natural cocoa, and 397.30 mg of total proanthocyanins/g) and one 8-ounce (237 mL)11 g natural cocoa beverage containing 357.41 mg of total proanthocyanins/g) | Placebo bar and beverage |
| Davison et al. [40] | Overweight and obese | Total: 49 Sex: 17/32 | INT: 25 CG: 24 | INT: 45.2 CG: 44.4 | 12 | SBP; DBP; glucose; WC; body weight; BMI; HDL; LDL; cholesterol; triglycerides | High-flavanol cocoa beverage (902 mg of flavanols) | Low-flavanol cocoa beverage (36 mg of flavanols) |
| Desch et al. [43] | Type 2 diabetes mellitus; hypertension | Total: 91 Sex: 71/20 | INT: 43 CG: 48 | INT: 62.5 CG: 66.8 | 24 | SBP; DBP | 25 g dark-chocolate bar (21 mg of flavanol-epicatechin) | 6 g dark-chocolate bar (5 mg of flavanol-epicatechin) |
| Desideri et al. [38] | Elderly with mild cognitive impairment | Total: 90 Sex: 43/47 | INT 1: 30 INT 2: 30 CG: 30 | INT 1: 71.3 INT 2: 71.2 CG: 71.0 | 8 | SBP; DBP; glucose; BMI; body weight; HDL; LDL; cholesterol; triglycerides | INT 1: cocoa beverage (520 mg of flavanols) INT 2: cocoa beverage (990 mg of flavanols) | Cocoa beverage (45 mg of flavanols) |
| Dicks et al. [32] | Type 2 diabetes mellitus; hypertension (stable w/treatment) | Total: 35 Sex: 18/17 | INT: 17 CG: 18 | INT: 62.8 CG: 65.6 | 12 | SBP; DBP; glucose; HbA1c; BMI; HDL; LDL; cholesterol; triglycerides; WC, body weight | 2.5 g cocoa capsules (207 mg of flavanols) | Placebo capsule |
| Giraldo et al. [50] | Insulin resistance | Total: 75 Sex: 10/65 | INT: 37 CG: 38 | INT: 49.3 CG: 51.1 | 8 | body weight; BMI; AC; glucose; HDL; LDL; cholesterol; triglycerides | 70% dark-chocolate bar (50 g) (430 mg of total polyphenols) | White chocolate with colorant |
| Ibero-Baraibar et al. [34] | Healthy middle-aged | Total: 47 Sex: 22/25 | INT: 24 CG: 23 | INT: 57.0 CG: 57.0 | 4 | SBP; DBP, glucose; WC; body weight; HDL; LDL; cholesterol; triglycerides | Meals supplemented with 1.4 g cocoa extract (414.3 mg of flavanols) | Control meals without polyphenols |
| Jafarirad et al. [52] | Type 2 diabetes mellitus/drug therapy (metformin or glibenclamide) | Total: 44 Sex: 30/14 | INT: 21 CG: 23 | INT: 52.3 CG: 52.3 | 8 | HbA1c; glucose; HDL; LDL; cholesterol; triglycerides | 84% dark chocolate (30 g) | Therapeutic lifestyle changes guidelines |
| León-Flores et al. [51] | Overweight. serum triglycerides levels of 150–350 mg/dL | Total: 24 Sex: NA | INT: 13 CG: 11 | INT: 48.3 CG: 42.0 | 8 | body weight; BMI; WC; Glucose; HDL; LDL; cholesterol; triglycerides | cocoa cookie (25 mg of flavonoids) | Placebo cookie (without cocoa) |
| Leyva-Soto et al. [58] | Young with 3 cardiovascular risk factors | Total: 84 Sex: 47/37 | INT: 42 CG: 42 | INT: 23.8 CG: 23.6 | 24 | SBP; DBP; glucose; WC; BMI; HbA1c; HDL; LDL; cholesterol; triglycerides | 70% dark-chocolate bar (2 g) (127 mg of total polyphenols/70 mg of flavonoids) | 2 g milk chocolate bar (21 mg of flavonoids) |
| Massee et al. [30] | Healthy young | Total: 40 Sex: 13/27 | INT: 20 CG: 20 | INT: 24.4 CG: 23.9 | 4 | SBP; DBP | 3.05 g cocoa extract (standardized tablet) (250 mg of catechin polyphenol) | Placebo tablet |
| Mastroiacovo et al. [39] | Healthy elderly | Total: 90 Sex: 37/53 | INT 1: 30 INT 2: 30 CG: 30 | INT 1: 68.7 INT 2: 70.0 CG: 70.0 | 8 | SBP; DBP; glucose; HbA1c; BMI; HDL; LDL; cholesterol; triglycerides | INT 1: cocoa beverage (520 mg of flavanols) INT 2: cocoa beverage (993 mg of flavanols) | Cocoa beverage (45 mg of flavanols) |
| Mozaffari-Khosravi et al. [29] | Metabolic syndrome | Total: 94 Sex: 45/49 | INT 1: 32 INT 2: 30 CG: 32 | INT 1: 49.6 INT 2: 51.7 CG: 52.8 | 8 | SBP; DBP | INT 1: 76% dark-chocolate bar (20 g) (2.46 mg of total polyphenols) INT 2: 76% dark-chocolate bar (40 g) (4.92 mg of total polyphenols) | No placebo |
| Munguía et al. [44] | Overweight | Total: 15 Sex: 4/11 | INT: 5 CG: 10 | INT: 41.0 CG: 48.8 | 4 | SBP; DBP, glucose; WC; body weight; BMI; HDL; LDL; cholesterol; triglycerides | Cocoa extract during morning fasting (80 mg of flavonoids) | Placebo powder (without flavonoids) |
| Munguia et al. [45] | Elderly | Total: 61 Sex: 13/48 | INT: 34 CG: 27 | INT: 76.1 CG: 75.6 | 8 | body weight, WC. Glucose. HDL; LDL; cholesterol; triglycerides | 22 g cocoa beverage (179 mg of flavonoids) | Placebo beverage |
| Naghdipour-Biregani et al. [54] | Middle-aged/metabolic syndrome | Total: 114 Sex: NA | INT 1: 38 INT 2: 38 CG: 38 | INT 1: 49.6 INT 2: 51.8 CG: 52.9 | 8 | HDL; LDL; cholesterol; triglycerides | INT: 76% dark chocolate (20 g) (2.46 mg of total polyphenols) INT 2: 76% dark chocolate (40 g) (4.92 mg of total polyphenols) | No placebo |
| Neufingerl et al. [49] | Healthy men and postmenopausal women | Total: 69 Sex: 34/35 | INT: 35 CG: 34 | INT: 55.2 CG: 55.4 | 4 | SBP; DBP; body weight; HDL; LDL; cholesterol; triglycerides | 6 g cocoa extract (325 mg of flavonoids) | Placebo beverage |
| Nickols-Richardson et al. [46] | Overweight and obese premenopausal women | Total: 60 Sex: 60F | INT: 30 CG: 30 | INT: 36.0 CG: 36.0 | 18 | SBP; DBP; glucose; WC; BMI; body weight; HDL; LDL; cholesterol; triglycerides | Cocoa beverage + chocolate bar (270 mg of flavonoids) | Placebo snack and beverage (without flavonoids) |
| Nishiwaki et al. [41] | Healthy young | Total: 32 Sex: 24/8 | INT: 16 CG: 16 | INT: 20.8 CG: 20.7 | 4 | SBP; DBP; glucose; WC; BMI | 72% chocolate bar (20 g) (508 mg of polyphenols) | Without placebo |
| Ottaviani et al. [31] | Healthy middle-aged | Total: 74 Sex: 33/41 | INT: 46 CG: 28 | INT: 41.0 CG: 41.0 | 6 | SBP; DBP | 1000 mg (2 cocoa-extract capsules) for 2 wks 1500 mg (3 cocoa-extract capsules) for 2 wks 2000 mg (4 cocoa-extract capsules) for 2 wks (500 mg of flavanols/capsule) | Placebo capsule |
| Parsaeyan et al. [56] | Type 2 diabetes mellitus | Total: 100 Sex: 50/50 | INT: 50 CG: 50 | INT: 54.0 CG: 54.0 | 6 | HDL; LDL; cholesterol; triglycerides | 20 g cocoa extract + 20 g milk powder | Placebo beverage (10 g milk powder) |
| Pereira et al. [59] | Healthy young | Total: 60 Sex: 20/40 | INT: 30 CG: 30 | INT: 19.2 CG: 20.7 | 4 | SBP; DBP; BMI | 75% chocolate bar (10 g) | No placebo |
| Pereira et al. [33] | Healthy young | Total: 30 Sex: 4/26 | INT: 15 CG: 15 | INT: 19.5 CG: 20.4 | 4 | SBP; DBP | 90% dark chocolate (20 g) (364 mg of flavanols) | 55% chocolate bar (20 g) (252 mg of flavanols) |
| Polagruto et al. [55] | Hypercholesterolemia | Total: 67 Sex: 20/47 | INT: 32 CG: 35 | INT: 49.0 CG: 56.0 | 6 | HDL; LDL; cholesterol; triglycerides | Cocoa snack bars (256 mg of flavanols + 3 g sterol esters) | Placebo snack bars (21 mg of flavanols + 0 g sterol esters) |
| Rodriguez-Mateos et al. [47] | Healthy men | Total: 45 Sex: 45 M | INT 1: 15 INT 2: 15 CG: 15 | INT 1: 25.0 INT 2: 23.0 CG: 23.0 | 4 | SBP, DBP; glucose; HDL; LDL; cholesterol; triglycerides | INT 1: cocoa-extract capsules (560 mg of flavanols) INT 2: cocoa-extract capsules (690 mg of flavanols) | Placebo capsule |
| Rostami et al. [42] | Type 2 diabetes mellitus (stable)/drug therapy; hypertension (stable) | Total: 60 Sex: 24/36 | INT: 32 CG: 28 | INT: 58.7 CG: 57.2 | 8 | SBP; DBP; glucose; HbA1c; HDL; LDL; cholesterol; triglycerides | 83% dark-chocolate bar (25 g) (450 mg of flavonoids) | White chocolate (with colorant) |
| Sansone et al. [48] | Healthy middle-aged | Total: 105 Sex: 55/50 | INT: 55 CG: 50 | INT: 45.0 CG: 44.0 | 4 | SBP; DBP, HDL; LDL; cholesterol; triglycerides | cocoa beverage (14 g) (900 mg of flavanols) | Placebo beverage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arisi, T.O.P.; da Silva, D.S.; Stein, E.; Weschenfelder, C.; de Oliveira, P.C.; Marcadenti, A.; Lehnen, A.M.; Waclawovsky, G. Effects of Cocoa Consumption on Cardiometabolic Risk Markers: Meta-Analysis of Randomized Controlled Trials. Nutrients 2024, 16, 1919. https://doi.org/10.3390/nu16121919
Arisi TOP, da Silva DS, Stein E, Weschenfelder C, de Oliveira PC, Marcadenti A, Lehnen AM, Waclawovsky G. Effects of Cocoa Consumption on Cardiometabolic Risk Markers: Meta-Analysis of Randomized Controlled Trials. Nutrients. 2024; 16(12):1919. https://doi.org/10.3390/nu16121919
Chicago/Turabian StyleArisi, Tainah O. P., Diego Silveira da Silva, Elana Stein, Camila Weschenfelder, Patrícia Caetano de Oliveira, Aline Marcadenti, Alexandre Machado Lehnen, and Gustavo Waclawovsky. 2024. "Effects of Cocoa Consumption on Cardiometabolic Risk Markers: Meta-Analysis of Randomized Controlled Trials" Nutrients 16, no. 12: 1919. https://doi.org/10.3390/nu16121919
APA StyleArisi, T. O. P., da Silva, D. S., Stein, E., Weschenfelder, C., de Oliveira, P. C., Marcadenti, A., Lehnen, A. M., & Waclawovsky, G. (2024). Effects of Cocoa Consumption on Cardiometabolic Risk Markers: Meta-Analysis of Randomized Controlled Trials. Nutrients, 16(12), 1919. https://doi.org/10.3390/nu16121919






