Nutritional Behavior of Patients with Bone Diseases: A Cross-Sectional Study from Austria
Abstract
1. Introduction
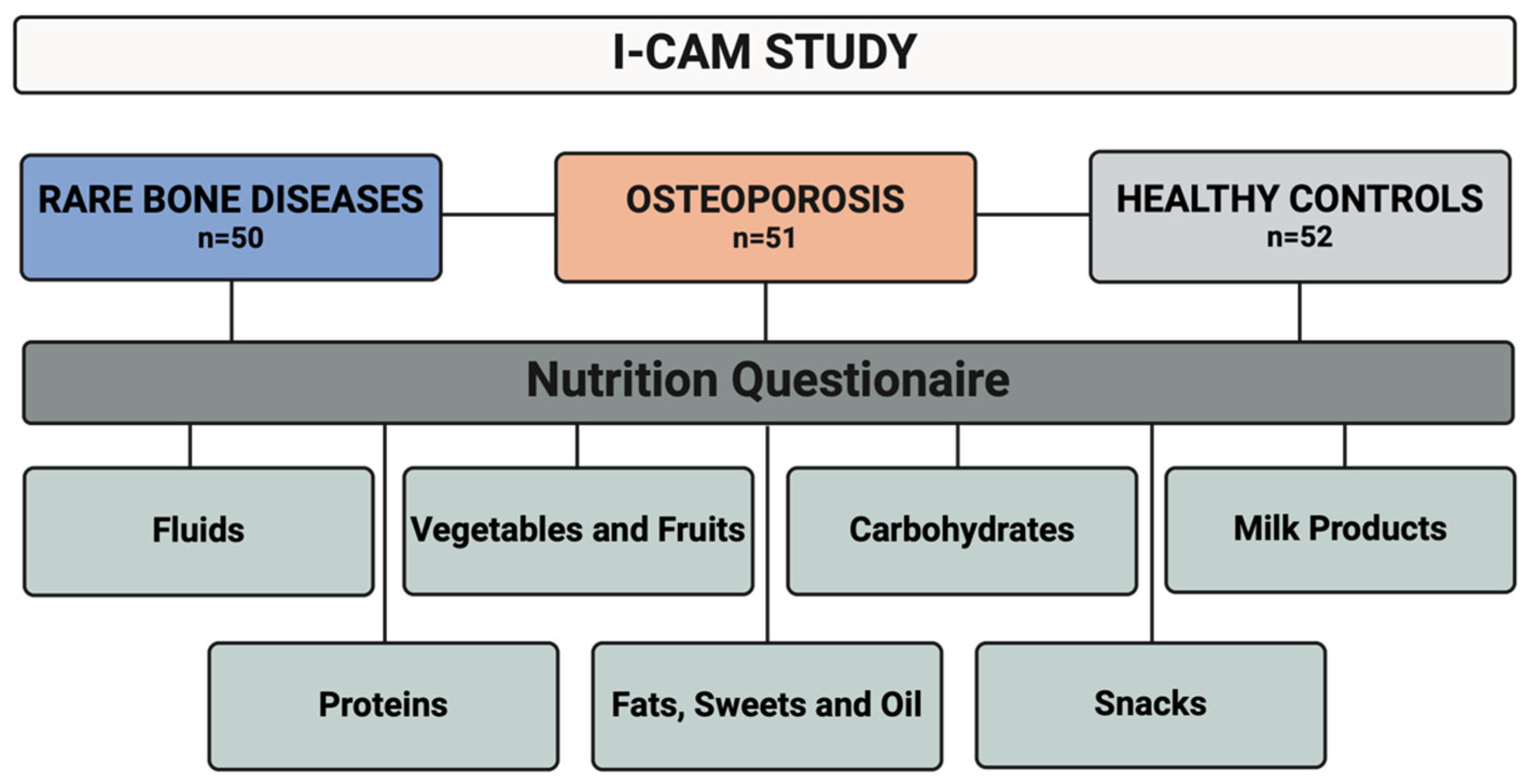
2. Methods
Statistics
3. Results
3.1. Demographic Characteristics
3.2. Nutritional Categories
3.2.1. Fluids
3.2.2. Vegetables and Fruits
3.2.3. Carbohydrates
3.2.4. Milk Products
3.2.5. Proteins
3.2.6. Fat, Sweets and Oil
3.2.7. Snacks
3.3. Effects of Socioeconomic Status Indicators on the BMI Level
3.3.1. Effect of Educational Level
3.3.2. Effect of Marital Status
3.3.3. Effect of Employment Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Javaid, M.K.; Ward, L.; Pinedo-Villanueva, R.; Rylands, A.J.; Williams, A.; Insogna, K.; Imel, E.A. Musculoskeletal Features in Adults with X-linked Hypophosphatemia: An Analysis of Clinical Trial and Survey Data. J. Clin. Endocrinol. Metab. 2022, 107, e1249–e1262. [Google Scholar] [CrossRef] [PubMed]
- Genest, F.; Rak, D.; Petryk, A.; Seefried, L. Physical Function and Health-Related Quality of Life in Adults Treated with Asfotase Alfa for Pediatric-Onset Hypophosphatasia. J. Bone Miner. Res. Plus 2020, 4, e10395. [Google Scholar] [CrossRef] [PubMed]
- Forlino, A.; Marini, J.C. Osteogenesis imperfecta. Lancet 2016, 387, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Behanova, M.; Medibach, A.; Haschka, J.; Kraus, D.; Raimann, A.; Mindler, G.T.; Zwerina, J.; Kocijan, R. Health-related quality of life and fatigue in adult rare bone disease patients: A cross-sectional study from Austria. Bone 2024, 181, 117034. [Google Scholar] [CrossRef] [PubMed]
- Kocijan, R.; Medibach, A.; Lechner, L.; Haschka, J.; Kocijan, A.; Kraus, D.A.; Zwerina, J.; Behanova, M. Use of Complementary and Alternative Medicine in Patients with Rare Bone Diseases and Osteoporosis. Nutrients 2024, 16, 816. [Google Scholar] [CrossRef] [PubMed]
- Dinulescu, A.; Păsărică, A.-S.; Carp, M.; Dușcă, A.; Dijmărescu, I.; Pavelescu, M.L.; Păcurar, D.; Ulici, A. New Perspectives of Therapies in Osteogenesis Imperfecta-A Literature Review. J. Clin. Med. 2024, 13, 1065. [Google Scholar] [CrossRef] [PubMed]
- Seefried, L.; Duplan, M.B.; Briot, K.; Collins, M.T.; Evans, R.; Florenzano, P.; Hawkins, N.; Javaid, M.K.; Lachmann, R.; Ward, L.M. Anticipated effects of burosumab treatment on long-term clinical sequelae in XLH: Expert perspectives. Front. Endocrinol. 2023, 14, 1211426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- e.V DdDWOG. Prophylaxe, Diagnostik und Therapie der Osteoporose bei Postmenopausalen Frauen und bei Männern ab Dem 50. Lebensjahr. 2023. Available online: https://leitlinien.dv-osteologie.org/wp-content/uploads/2024/02/DVO-Leitlinie-zur-Diagnostik-und-Therapie-der-Osteoporose-Version-2.1.-2023-002.pdf (accessed on 24 January 2024).
- Haffner, D.; Emma, F.; Eastwood, D.M.; Duplan, M.B.; Bacchetta, J.; Schnabel, D.; Wicart, P.; Bockenhauer, D.; Santos, F.; Levtchenko, E.; et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat. Rev. Nephrol. 2019, 15, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Tournis, S.; Yavropoulou, M.P.; Polyzos, S.A.; Doulgeraki, A. Hypophosphatasia. J. Clin. Med. 2021, 10, 5676. [Google Scholar] [CrossRef]
- Liu, W.; Lee, B.; Nagamani, S.C.S.; Nicol, L.; Rauch, F.; Rush, E.T.; Sutton, V.R.; Orwoll, E. Approach to the Patient: Pharmacological Therapies for Fracture Risk Reduction in Adults with Osteogenesis Imperfecta. J. Clin. Endocrinol. Metab. 2023, 108, 1787–1796. [Google Scholar] [CrossRef]
- LoMauro, A.; Landoni, C.V.; Fraschini, P.; Molteni, F.; Aliverti, A.; Bertoli, S.; De Amicis, R. Eat, breathe, sleep with Osteogenesis Imperfecta. Orphanet J. Rare Dis. 2021, 16, 435. [Google Scholar] [CrossRef] [PubMed]
- Wilsford, L.D.; Sullivan, E.; Mazur, L.J. Risk factors for vitamin D deficiency in children with osteogenesis imperfecta. J. Pediatr. Orthop. 2013, 33, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Gnoli, M.; Brizola, E.; Tremosini, M.; Di Cecco, A.; Sangiorgi, L. Vitamin D and Bone fragility in Individuals with Osteogenesis Imperfecta: A Scoping Review. Int. J. Mol. Sci. 2023, 24, 9416. [Google Scholar] [CrossRef] [PubMed]
- Coccia, F.; Pietrobelli, A.; Zoller, T.; Guzzo, A.; Cavarzere, P.; Fassio, A.; Flodmark, C.-E.; Gatti, D.; Antoniazzi, F. Vitamin D and Osteogenesis Imperfecta in Pediatrics. Pharmaceuticals 2023, 16, 690. [Google Scholar] [CrossRef]
- Wiedemann, P.; Schmidt, F.N.; Amling, M.; Yorgan, T.A.; Barvencik, F. Zinc and vitamin D deficiency and supplementation in hypophosphatasia patients—A retrospective study. Bone 2023, 175, 116849. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.M.; Eslick, G.D.; Nowson, C.; Smith, C.; Bensoussan, A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: A meta-analysis. Lancet 2007, 370, 657–666. [Google Scholar] [CrossRef] [PubMed]
- The Federal Ministry of social affairs, health, care and consumer protection. Nutr. Recomm. 2019. Available online: https://www.sozialministerium.at/Themen/Gesundheit/Lebensmittel-Ernaehrung.html (accessed on 5 February 2024).
- Wenkert, D. Food for Thought: Dietary Guidelines for Patients with Hypophosphatasia (HPP); Publisher: 2021. Available online: https://softbones.org/wp-content/uploads/2021/03/SoftBones_Nutrition_Guide-DR-2021.03.24_JS-1.pdf (accessed on 25 March 2024).
- Osteogenesis Imperfecta Foundation. Nutrition and OI; Osteogenesis Imperfecta Foundation: Gaithersburg, MD, USA, 2022. [Google Scholar]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus statement from 2(nd) International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Diethelm, K.; Huybrechts, I.; Moreno, L.; De Henauw, S.; Manios, Y.; Beghin, L.; González-Gross, M.; Le Donne, C.; Cuenca-García, M.; Castillo, M.J.; et al. Nutrient intake of European adolescents: Results of the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr. 2014, 17, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Soininen, S.; Eloranta, A.M.; Schwab, U.; Lakka, T.A. Sources of vitamin D and determinants of serum 25-hydroxyvitamin D in Finnish adolescents. Eur. J. Nutr. 2023, 62, 1011–1025. [Google Scholar] [CrossRef]
- Petra Rust, V.H.; König, J. Österreichischer Ernährungsbericht; University of Vienna: Vienna, Austria, 2017. [Google Scholar]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.-K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.-L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.L.; Millward, D.J.; Torgerson, D.J.; Hewitt, C.E.; Lanham-New, S.A. Dietary protein and bone health: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2009, 90, 1674–1692. [Google Scholar] [CrossRef]
- Johansson, H.; Kanis, J.A.; Odén, A.; McCloskey, E.; Chapurlat, R.D.; Christiansen, C.; Cummings, S.R.; Diez-Perez, A.; Eisman, J.A.; Fujiwara, S.; et al. A meta-analysis of the association of fracture risk and body mass index in women. J. Bone Miner. Res. 2014, 29, 223–233. [Google Scholar] [CrossRef]
- Chagas, C.E.; Roque, J.P.; Santarosa Emo Peters, B.; Lazaretti-Castro, M.; Martini, L.A. Do patients with osteogenesis imperfecta need individualized nutritional support? Nutrition 2012, 28, 138–142. [Google Scholar] [CrossRef]
- Mindler, G.T.; Kranzl, A.; Stauffer, A.; Kocijan, R.; Ganger, R.; Radler, C.; Haeusler, G.; Raimann, A. Lower Limb Deformity and Gait Deviations among Adolescents and Adults with X-Linked Hypophosphatemia. Front. Endocrinol. 2021, 12, 754084. [Google Scholar] [CrossRef]
- Lespessailles, E.; Hammoud, E.; Toumi, H.; Ibrahim-Nasser, N. Consequences of bariatric surgery on outcomes in rheumatic diseases. Arthritis Res. Ther. 2019, 21, 83. [Google Scholar] [CrossRef]
- Muschitz, C.; Kocijan, R.; Haschka, J.; Zendeli, A.; Pirker, T.; Geiger, C.; Müller, A.; Tschinder, B.; Kocijan, A.; Marterer, C.; et al. The Impact of Vitamin D, Calcium, Protein Supplementation, and Physical Exercise on Bone Metabolism after Bariatric Surgery: The BABS Study. J. Bone Miner. Res. 2016, 31, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Hallström, H.; Byberg, L.; Glynn, A.; Lemming, E.W.; Wolk, A.; Michaëlsson, K. Long-term coffee consumption in relation to fracture risk and bone mineral density in women. Am. J. Epidemiol. 2013, 178, 898–909. [Google Scholar] [CrossRef]
- Rychter, A.M.; Ratajczak, A.E.; Szymczak-Tomczak, A.; Michalak, M.; Eder, P.; Dobrowolska, A.; Krela-Kaźmierczak, I. Associations of Lifestyle Factors with Osteopenia and Osteoporosis in Polish Patients with Inflammatory Bowel Disease. Nutrients 2021, 13, 1863. [Google Scholar] [CrossRef]

| Patient Type | RBD (N = 50) | OPO (N = 51) | CTRL (N = 52) | ||||
|---|---|---|---|---|---|---|---|
| OI (N = 17) | HPP (N = 17) | XLH (N = 16) | Total | Group Differences (RBD vs. OPO vs. CTRL) | |||
| Age, mean (SD) | 47.6 (±15.6) | 55.9 (±13.9) | 42.5 (±16.0) | 48.8 (±15.9) | 66.6 (±10.0) | 50.8 (±16.3) | <0.001 |
| Gender, male N (%) | 5 (29.4) | 7 (41.2) | 1 (6.3) | 13 (26.0) | 5 (9.8) | 14 (26.9) | 0.06 |
| Family status, N (%) | * | * | * | * | * | * | 0.09 |
| Single | 4 (23.5) | 1 (5.9) | 3 (18.8) | 8 (16.0) | 4 (7.8) | 13 (25.0) | |
| Married or cohabiting | 9 (52.9) | 10 (58.8) | 9 (56.3) | 28 (56.0) | 25 (49.0) | 30 (57.7) | |
| Divorced | 2 (11.8) | 3 (17.6) | 4 (25.0) | 9 (18) | 13 (25.5) | 5 (9.6) | |
| Widowed | 0 (0.0) | 1 (5.9) | 0 (0.0) | 1 (2.0) | 5 (9.8) | 4 (7.7) | |
| Educational level, N (%) | * | * | * | * | * | * | 0.07 |
| Basic | 9 (52.9) | 7 (41.2) | 10 (62.5) | 26 (52.0) | 19 (37.3) | 28 (53.8) | |
| Secondary | 3 (17.6) | 2 (11.8) | 0 (0.0) | 5 (10.0) | 16 (31.4) | 8 (15.4) | |
| Tertiary | 3 (17.6) | 6 (35.3) | 6 (37.5) | 15 (30.0) | 13 (25.5) | 16 (30.8) | |
| Employment status, employed, N (%) | 9 (52.9) | 8 (47.1) | 12 (75.0) | 29 (58.0) | 22 (43.1) | 41 (78.8) | <0.001 |
| BMI, mean (SD) | 25.4 (±6.2) | 27.2 (±5.1) | 25.8 (±5.7) | 26.2 (±5.6) | 24.2(±3.9) | 26.4 (±4.7) | 0.16 |
| BMI category, N (%) | * | * | * | * | * | * | 0.20 |
| Underweight (BMI < 18.5) | 1 (5.9) | 0 (0.0) | 0 (0.0) | 1 (2.0) | 3 (5.9) | 1 (1.9) | |
| Normal (BMI 18.5–24.9) | 7 (41.2) | 5 (29.4) | 7 (43.8) | 19 (38.0) | 27 (52.9) | 20 (38.5) | |
| Overweight (BMI 25.0–29.9) | 4 (23.5) | 5 (29.4) | 5 (31.3) | 14 (28.0) | 14 (27.5) | 14 (26.9) | |
| Obese (BMI ≥ 30) | 2 (11.8) | 6 (35.3) | 4 (25.0) | 12 (24.0) | 3 (5.9) | 10 (19.2) | |
| RBD | OPO | CTRL | Group Differences (RBD vs. OPO vs. CTRL) | |||||
|---|---|---|---|---|---|---|---|---|
| OI | HPP | XLH | Overall | |||||
| Number of daily meals, N (%) | 1–2 meals | 4 (26.7) | 3 (20.0) | 3 (18.8) | 10 (21.7) | 9 (18.0) | 9 (17.3) | p = 0.840 |
| 3–4 meals | 8 (53.3) | 12 (80.0) | 8 (50.0) | 28 (60.9) | 34 (68.0) | 33 (63.5) | ||
| 5–6 meals | 3 (20.0) | 0 (0.0) | 5 (31.3) | 8 (17.4) | 6 (12.0) | 8 (15.4) | ||
| 6 ≥ meals | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) | 2 (3.8) | ||
| Water/unsweetened drinks per day, N (%) | ≤1 glass (up to 200 mL) | 0 (0.0) | 1 (6.7) | 0 (0.0) | 1 (2.2) | 0 (0.0) | 0 (0.0) | p = 0.124 |
| 2 glasses (300–500 mL) | 1 (6.7) | 0 (0.0) | 2 (12.5) | 3 (6.5) | 3 (6.0) | 4 (7.7) | ||
| 3–5 glasses (600–1000 mL) | 7 (46.7) | 3 (20.0) | 4 (25.0) | 14 (30.4) | 14 (28.0) | 5 (9.6) | ||
| >1 L | 7 (46.7) | 11 (73.3) | 10 (62.5) | 28 (60.9) | 33 (66.0) | 43 (82.7) | ||
| Sweetened drinks per day, N (%) | ≤1 glass (up to 200 mL) | 13 (86.7) | 13 (86.7) | 13 (81.3) | 39 (84.8) | 42 (84.0) | 44 (84.6) | p = 0.841 |
| 2 glasses (300–500 mL) | 1 (6.7) | 1 (6.7) | 3 (18.8) | 5 (10.9) | 7 (14.0) | 6 (11.5) | ||
| 3–5 glasses (600–1000 mL) | 1 (6.7) | 1 (6.7) | 0 (0.0) | 2 (4.3) | 1 (2.0) | 1 (1.9) | ||
| >1 L | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.9) | ||
| Light drinks per day, N (%) | ≤1 glass (up to 200 mL) | 13 (86.7) | 15 (100) | 15 (93.8) | 43 (93.5) | 47 (94.0) | 50 (96.2) | p = 0.699 |
| 2 glasses (300–500 mL) | 2 (13.3) | 0 (0.0) | 1 (6.3) | 3 (6.5) | 2 (4.0) | 1 (1.9) | ||
| 3–5 glasses (600–1000 mL) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) | 1 (1.9) | ||
| More than 1 L | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Sugar per hot drink, N (%) | none | 11 (73.3) | 12 (80.0) | 14 (87.5) | 37 (80.4) | 43 (86.0) | 40 (76.9) | p = 0.876 |
| 1 teaspoon | 3 (20.0) | 2 (13.3) | 1 (6.3) | 6 (13.0) | 6 (12.0) | 8 (15.4) | ||
| 2 teaspoons | 1 (6.7) | 0 (0.0) | 1 (6.3) | 2 (4.3) | 1 (2.0) | 3 (5.8) | ||
| >2 teaspoons | 0 (0.0) | 1 (6.7) | 0 (0.0) | 1 (2.2) | 0 (0.0) | 1 (1.9) | ||
| Caffeine consumption, N (%) | daily | 13 (76.5) | 13 (76.5) | 14 (87.5) | 40 (80.0) | 49 (96.1) | 45 (86.5) | p = 0.016 * |
| not daily | 4 (23.5) | 4 (23.5) | 2 (12.5) | 10 (20.0) | 2 (3.9) | 7 (13.5) | ||
| Fruit/vegetable juice, N (%) | never | 2 (13.3) | 6 (42.9) | 4 (25.0) | 12 (26.7) | 13 (26.0) | 24 (46.2) | p = 0.034 * |
| not daily | 7 (46.7) | 4 (28.6) | 11 (68.8) | 22 (48.9) | 30 (60.0) | 23 (44.2) | ||
| 1 glass daily (200 mL) | 3 (20.0) | 3 (21.4) | 0 (0.0) | 6 (13.3) | 6 (12.0) | 5 (9.6) | ||
| >1 glass daily | 3 (20.0) | 1 (7.1) | 1 (6.3) | 5 (11.1) | 1 (2.0) | 0 (0.0) | ||
| Portions of vegetables per day, N (%) | <1 portion | 5 (33.3) | 2 (13.3) | 1 (6.3) | 8 (17.4) | 6 (12.0) | 7 (13.5) | p = 0.982 |
| 1 portion | 4 (26.7) | 10 (66.7) | 6 (37.5) | 20 (43.5) | 20 (40.0) | 21 (40.4) | ||
| 2 portions | 3 (20.0) | 3 (20.0) | 5 (31.3) | 11 (23.9) | 15 (30.0) | 15 (28.8) | ||
| >2 portions | 3 (20.0) | 0 (0.0) | 4 (25.0) | 7 (15.2) | 9 (18.0) | 9 (17.3) | ||
| One portion of legumes, N (%) | never | 4 (26.7) | 4 (28.6) | 3 (18.8) | 11 (24.4) | 9 (18.0) | 14 (26.9) | p = 0.838 |
| once a week | 8 (53.3) | 5 (35.7) | 10 (62.5) | 23 (51.1) | 29 (58.0) | 25 (48.1) | ||
| ≥2 times a week | 3 (20.0) | 5 (35.7) | 3 (18.8) | 11 (24.4) | 12 (24.0) | 13 (25.0) | ||
| Portions of fruits per day, N (%) | <1 portion | 5 (33.3) | 3 (20.0) | 1 (6.3) | 9 (19.6) | 6 (12.0) | 12 (23.1) | p = 0.371 |
| 1 portion | 4 (26.7) | 8 (53.3) | 10 (62.5) | 22 (47.8) | 18 (36.0) | 20 (38.5) | ||
| 2 portions | 3 (20.0) | 3 (20.0) | 3 (18.8) | 9 (19.6) | 19 (38.0) | 16 (30.8) | ||
| >2 portions | 3 (20.0) | 1 (6.7) | 2 (12.5) | 6 (13.0) | 7 (14.0) | 4 (7.7) | ||
| Starch productions/cereal products per day, N (%) | never/not daily | 3 (20.0) | 2 (13.3) | 2 (12.5) | 7 (15.2) | 5 (10.0) | 4 (7.7) | p = 0.801 |
| 1–2 times per day | 9 (60.0) | 9 (60.0) | 11 (68.8) | 29 (63.0) | 35 (70.0) | 36 (69.2) | ||
| >2 times per day | 3 (20.0) | 4 (26.7) | 3 (18.8) | 10 (21.7) | 10 (20.0) | 12 (23.1) | ||
| Whole grain products, N (%) | never/once a week | 5 (33.3) | 9 (60.0) | 6 (37.5) | 20 (43.5) | 16 (31.4) | 13 (25.0) | p = 0.203 |
| 2–6 times a week | 4 (26.7) | 3 (20.0) | 5 (31.3) | 12 (26.1) | 11 (21.6) | 20 (38.5) | ||
| once daily | 5 (33.3) | 2 (13.3) | 5 (31.3) | 12 (26.1) | 20 (39.2) | 18 (34.6) | ||
| several times a day | 1 (6.7) | 1 (6.7) | 0 (0.0) | 2 (4.3) | 4 (7.8) | 1 (1.9) | ||
| Portions of milk and milk products per day, N (%) | <1 portion | 1 (6.7) | 5 (33.3) | 4 (25.0) | 10 (21.7) | 5 (9.8) | 12 (23.1) | p = 0.453 |
| 1–2 portions | 10 (66.7) | 9 (60.0) | 9 (56.3) | 28 (60.9) | 38 (74.5) | 34 (65.4) | ||
| 3 portions | 0 (0.0) | 0 (0.0) | 2 (12.5) | 2 (4.3) | 4 (7.8) | 3 (5.8) | ||
| >3 portions | 4 (26.7) | 1 (6.7) | 1 (6.3) | 6 (13.0) | 4 (7.8) | 3 (5.8) | ||
| Eggs per week, N (%) | none | 2 (13.3) | 5 (33.3) | 2 (12.5) | 9 (19.6) | 6 (11.8) | 14 (26.9) | p = 0.489 |
| 1–2 | 6 (40.0) | 6 (40.0) | 12 (75.0) | 24 (52.2) | 33 (64.7) | 24 (46.2) | ||
| 3 | 4 (26.7) | 3 (20.0) | 0 (0.0) | 7 (15.2) | 5 (9.8) | 6 (11.5) | ||
| >3 | 3 (20.0) | 1 (6.7) | 2 (12.5) | 6 (13.0) | 7 (13.7) | 8 (15.4) | ||
| Portions of meet per week (excl. sausage products), N (%) | none | 0 (0.0) | 1 (6.7) | 2 (12.5) | 3 (6.5) | 5 (9.8) | 7 (13.5) | p = 0.350 |
| 1–2 portions | 6 (40.0) | 7 (46.7) | 9 (56.3) | 22 (47.8) | 27 (52.9) | 24 (46.2) | ||
| 3 portions | 7 (46.7) | 4 (26.7) | 3 (18.8) | 14 (30.4) | 13 (25.5) | 8 (15.4) | ||
| >3 portions | 2 (13.3) | 3 (20.0) | 2 (12.5) | 7 (15.2) | 6 (11.8) | 13 (25.0) | ||
| Sausage products per week, N (%) | none | 3 (20.0) | 2 (13.3) | 7 (43.8) | 12 (26.1) | 24 (47.1) | 15 (28.8) | p = 0.262 |
| 1–2 portions | 8 (53.3) | 10 (66.7) | 5 (31.3) | 23 (50.0) | 17 (33.3) | 21 (40.4) | ||
| 3 portions | 1 (6.7) | 1 (6.7) | 3 (18.8) | 5 (10.9) | 5 (9.8) | 10 (19.2) | ||
| >3 portions | 3 (20.0) | 2 (13.3) | 1 (6.3) | 6 (13.0) | 5 (9.8) | 6 (11.5) | ||
| Offal products, N (%) | none | 8 (53.3) | 8 (53.3) | 11 (68.8) | 27 (58.7) | 34 (66.7) | 38 (73.1) | p = 0.349 |
| ≤1 portion per month | 7 (46.7) | 6 (40.0) | 5 (31.3) | 18 (39.1) | 14 (27.5) | 11 (21.2) | ||
| several portions per month | 0 (0.0) | 1 (6.7) | 0 (0.0) | 1 (2.2) | 3 (5.9) | 3 (5.8) | ||
| 1 portion or more per week | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.9) | ||
| Portions of fish per week, N (%) | none | 1 (6.7) | 0 (0.0) | 3 (18.8) | 4 (8.7) | 3 (5.9) | 7 (13.5) | p = 0.044 * |
| <1 portion | 5 (33.3) | 5 (33.3) | 10 (62.5) | 20 (43.5) | 13 (25.5) | 12 (23.1) | ||
| 1–2 portions | 9 (60.0) | 9 (60.0) | 3 (18.8) | 21 (45.7) | 33 (64.7) | 26 (50.0) | ||
| >2 portions | 0 (0.0) | 1 (6.7) | 0 (0.0) | 1 (2.2) | 2 (3.9) | 7 (13.5) | ||
| Butter/margarine daily, N (%) | none/not daily | 6 (42.9) | 6 (40.0) | 3 (18.8) | 15 (33.3) | 18 (36.7) | 24 (48.0) | p = 0.429 |
| <2 teaspoons | 5 (35.7) | 6 (40.0) | 3 (18.8) | 14 (31.1) | 15 (30.6) | 8 (16.0) | ||
| 2 teaspoons (=10 g) | 2 (14.3) | 3 (20.0) | 9 (56.3) | 14 (31.1) | 11 (22.4) | 14 (28.0) | ||
| >2 teaspoons | 1 (7.1) | 0 (0.0) | 1 (6.3) | 2 (4.4) | 5 (10.2) | 4 (8.0) | ||
| Types of mainly used oil (e.g., salad dressing, not for cooking/roasting), N (%) | olive oil | 12 (80.0) | 11 (73.3) | 14 (87.5) | 37 (80.4) | 34 (68.0) | 39 (78.0) | p = 0.208 |
| rapeseed oil | 5 (33.3) | 6 (40.0) | 7 (43.8) | 18 (39.1) | 19 (38.0) | 11 (22.0) | ||
| safflower oil | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.0) | 3 (6.0) | ||
| corn oil | 0 (0.0) | 0 (0.0) | 3 (18.8) | 3 (6.5) | 2 (4.0) | 4 (8) | ||
| sunflower oil | 1 (6.7) | 3 (20.0) | 1 (6.3) | 5 (10.9) | 9 (18.0) | 10 (20.0) | ||
| peanut oil | 1 (6.7) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 2 (4.0) | 2 (4.0) | ||
| other oils | 0 (0.0) | 4 (26.7) | 0 (0.0) | 4 (8.7) | 11 (22.0) | 15 (30.0) | ||
| Types of mainly used oil/fat for cooking/roasting, N (%) | olive oil | 6 (40.0) | 4 (26.7) | 7 (43.8) | 17 (37.0) | 20 (40.0) | 17 (34.0) | p = 0.098 |
| rapeseed oil | 6 (40.0) | 10 (66.7) | 8 (50.0) | 24 (52.2) | 28 (56.0) | 26 (52.0) | ||
| safflower oil | 0 (0.0) | 1 (6.7) | 0 (0.0) | 1 (2.2) | 1 (2.0) | 0 (0.0) | ||
| corn oil | 4 (26.7) | 1 (6.7) | 6 (37.5) | 11 (23.9) | 5 (10.0) | 6 (12.0) | ||
| sunflower oil | 2 (13.3) | 4 (26.7) | 4 (25.0) | 10 (21.7) | 11 (22.0) | 21 (42.0) | ||
| peanut oil | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.0) | 1 (2.0) | ||
| margarine | 0 (0.0) | 2 (13.3) | 1 (6.3) | 3 (6.5) | 2 (4.0) | 2 (4.0) | ||
| coconut fat | 0 (0.0) | 1 (6.7) | 4 (25.0) | 5 (10.9) | 3 (6.0) | 1 (2.0) | ||
| others | 0 (0.0) | 0 (0.0) | 2 (12.5) | 2 (4.3) | 5 (10.0) | 3 (6.0) | ||
| Consumption of unsalted nuts or seeds, N (%) | never/rarely | 8 (57.1) | 12 (80.0) | 3 (18.8) | 23 (51.1) | 25 (52.1) | 34 (68.0) | p = 0.168 |
| daily—less than 1 handful | 5 (35.7) | 2 (13.3) | 12 (75.0) | 19 (42.2) | 16 (33.3) | 14 (28.0) | ||
| daily—1 handful | 1 (7.1) | 1 (6.7) | 1 (6.3) | 3 (6.7) | 7 (14.6) | 2 (4.0) | ||
| daily—more than 1 handful | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| High-fat meals per week, N (%) | never/rarely | 7 (50.0) | 6 (40.0) | 7 (43.8) | 20 (44.4) | 33 (68.8) | 22 (44.0) | p = 0.015 * |
| 1–2 times | 6 (42.9) | 8 (53.3) | 9 (56.3) | 23 (51.1) | 15 (31.3) | 22 (44.0) | ||
| ≥3 times | 1 (7.1) | 1 (6.7) | 0 (0.0) | 2 (4.4) | 0 (0.0) | 6 (12.0) | ||
| Consumption of sweets or desserts, N (%) | never/rarely | 4 (28.6) | 3 (20.0) | 6 (37.5) | 13 (28.9) | 15 (31.3) | 12 (24.0) | p = 0.680 |
| 1–6 portions per week | 7 (50.0) | 5 (33.3) | 5 (31.3) | 17 (37.8) | 14 (29.2) | 22 (44.0) | ||
| 1 portion per day | 3 (21.4) | 6 (40.0) | 4 (25.0) | 13 (28.9) | 18 (37.5) | 13 (26.0) | ||
| several portions daily | 0 (0.0) | 1 (6.7) | 1 (6.3) | 2 (4.4) | 1 (2.1) | 3 (6.0) | ||
| Consumption of salty snacks, N (%) | never/rarely | 6 (40.0) | 12 (80.0) | 9 (56.3) | 27 (58.7) | 45 (88.2) | 26 (51.0) | p = 0.001 * |
| 1–6 portions per week | 8 (53.3) | 3 (20.0) | 6 (37.5) | 17 (37.0) | 4 (7.8) | 23 (45.1) | ||
| 1 portion per day / several portions daily | 1 (6.7) | 0 (0.0) | 1 (6.3) | 2 (4.3) | 2 (3.9) | 2 (3.9) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraus, D.A.; Medibach, A.; Behanova, M.; Kocijan, A.; Haschka, J.; Zwerina, J.; Kocijan, R. Nutritional Behavior of Patients with Bone Diseases: A Cross-Sectional Study from Austria. Nutrients 2024, 16, 1920. https://doi.org/10.3390/nu16121920
Kraus DA, Medibach A, Behanova M, Kocijan A, Haschka J, Zwerina J, Kocijan R. Nutritional Behavior of Patients with Bone Diseases: A Cross-Sectional Study from Austria. Nutrients. 2024; 16(12):1920. https://doi.org/10.3390/nu16121920
Chicago/Turabian StyleKraus, Daniel A., Amadea Medibach, Martina Behanova, Annemarie Kocijan, Judith Haschka, Jochen Zwerina, and Roland Kocijan. 2024. "Nutritional Behavior of Patients with Bone Diseases: A Cross-Sectional Study from Austria" Nutrients 16, no. 12: 1920. https://doi.org/10.3390/nu16121920
APA StyleKraus, D. A., Medibach, A., Behanova, M., Kocijan, A., Haschka, J., Zwerina, J., & Kocijan, R. (2024). Nutritional Behavior of Patients with Bone Diseases: A Cross-Sectional Study from Austria. Nutrients, 16(12), 1920. https://doi.org/10.3390/nu16121920







