The Impact of Phytochemicals in Obesity-Related Metabolic Diseases: Focus on Ceramide Metabolism
Abstract
1. Introduction
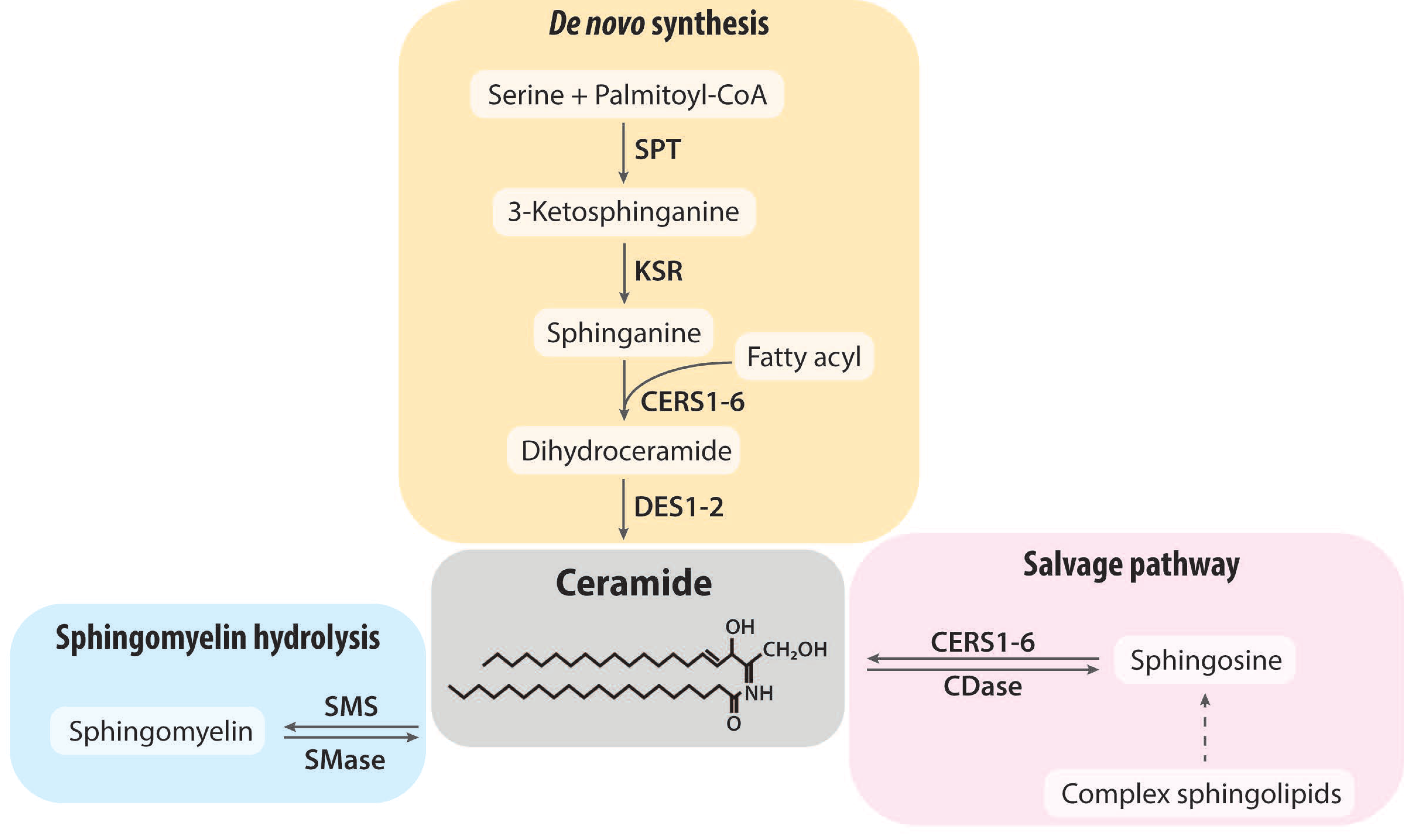
2. Ceramide Synthesis and Degradation
3. Ceramide Metabolism in Obesity-Related Metabolic Diseases
3.1. Associations between Ceramide Levels and Obesity-Related Metabolic Diseases
3.2. Accumulation of Ceramide in Obesity
3.3. Cellular Actions of Ceramide Related to Metabolic Disturbances
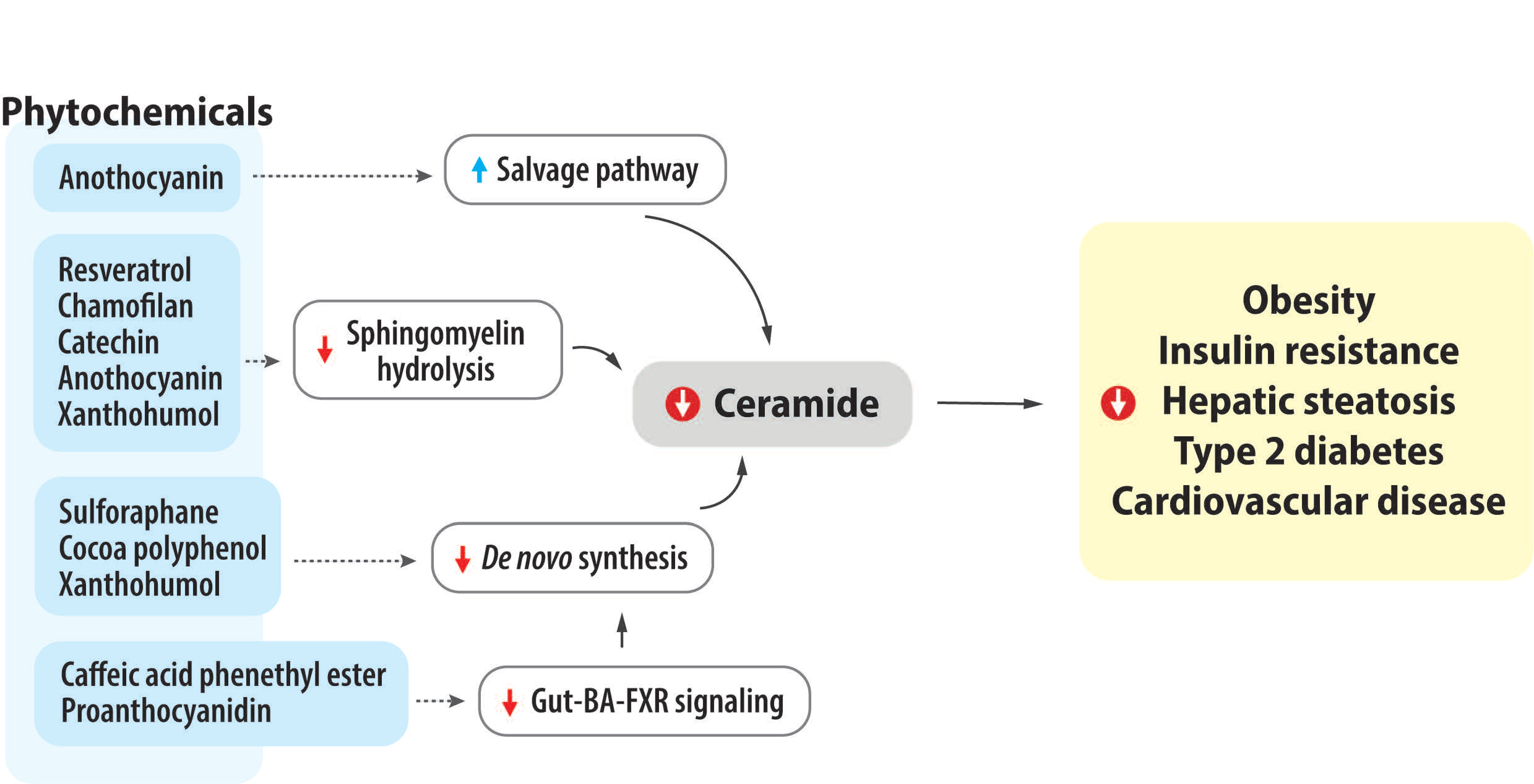
4. Impact of Bioactive Phytochemicals on Ceramide Metabolism and Obesity-Related Metabolic Diseases
4.1. Coffee, Caffeine, and Caffeine Derivative
4.2. Sulforaphane
4.3. Resveratrol
| Author | Animal Model | Treatment | Duration | Ceramide Levels | Sphingolipids Levels | Sphingolipid Metabolism-Related Expression or Activity |
|---|---|---|---|---|---|---|
| Sinha et al. [85] | C57BL/6 mice, ♂ | Caffeine (30 mg/kg BW) I.P. injection + ND vs. Untreated + ND | 3 d | ↓ Hepatic C22:0-, C25:0-Cer ∅ Hepatic C2:0-, C16:0-, C18:0-, C20:0-, C22:1-, C23:0-, C24:0-, C24:1-Cer | ↓ Hepatic C22:0-DhCer ∅ Hepatic C16:0-, C24:0-, C24:1-DhCer ↑ Hepatic Sphiganine, Sphingosine | - |
| Velázquez et al. [86] | Sprague Dawley rats, ♀ | Caffeine + HF-HFr vs. GCE + HF-HFr vs. HF-HFr | HF-HFr for 2 mo + Additional treatment for 1 mo | Caffeine: ↓ Hepatic 18:1-Cer ∅ Hepatic C14:0-, C16:0-, C18:0-, C20:0-, C22:0-, C24:0-, C24:1-Cer GCE: ↓ Hepatic 20:0-Cer ∅ Hepatic C14:0-, C16:0-, C18:0-, C22:0-, C24:0, C18:1-Cer | Caffeine: ∅ Hepatic C16:0-, C18:0-, C20:0-, C22:0-, C24:0-, C24:1-HexCer GCE: ↓ Hepatic 18:0-, 20:0-, 22:0-HexCer ∅ Hepatic C16:0-, C24:0-, C24:1-HexCer | - |
| Zhong et al. [87] | C57BL/6 mice, ♂ | CAPE (75 mg/kg/d) + HFD vs. HFD | 8 wk | - | - | ↓ Ileum Sptlc2 CerS2 CerS4 |
| FXRfl/fl FXRΔIE, ♂ | CAPE (75 mg/kg/d) vs. saline | 8 wk | CAPE: ↓ Serum total-Cer ↓ Ileum total-, C16:0-,C18:0-,C20:0-,C22:0-, C24:0-Cer | - | - | |
| Teng et al. [90] | C57BL/6J mice, ♂ | SFN (0.5 mg/kg, 5 mg/kg, 3 times/wk i.p.) + HFD vs. HFD | 10 wk | ↓ Hepatic total-Cer | - | ↓ Hepatic Sptlc3 CerS4 |
| Li et al. [91] | C57BL/6J mice, ♂ | SFN (10 mg/kg/d, i.p.) + HFD vs. HFD | 17 wk | ↓ Hepatic total-Cer | - | - |
| Alrob et al. [94] | BALB/c mice, ♂ | RES (30 mg/kg, every other day i.p.) + LFD vs. HFD | 4 wk HFD + 4 wk treatment | ↓ Muscle total-Cer | - | - |
| Babenko et al. [79] | Wistar rat, ♂ | Chamiloflan (160 mg/kg BW, daily i.p.) | 1 wk | 3-mo-old: ∅ Hepatic total-Cer 24-mo-old: ↓ Hepatic total-Cer 27–28-mo-old: ↓ Hepatic total-Cer | 3-mo-old: ∅ Hepatic SM 24-mo-old: ↑ Hepatic SM 27–28-mo-old: ↑ Hepatic SM | 24-mo-old: ↓ nSMase activity |
| Tveter et al. [95] | db/db mice | LFD (10% SPI) with 1% GP vs. LFD (10% SPI) | 28 d | - | - | ↓ Hepatic Sptlc2 CerS4 Fxr ↓ Ileum Smpd3 Fxr |
| Seo et al. [96] | C57BL/6J mice, ♂ | ChrSd (10% w/w) + HFD vs. HFD | 5 wk | - | - | ↓ Hepatic Sptlc3 (mRNA, protein) |
| Cho et al. [97] | C57BL/6J mice, ♂ | GSF (10% w/w) + HFD vs. HFD | 5 wk HFD + 9 wk treatment | - | - | ↓ Intestinal Fxr ∅ Adipose tissue |
| Huang et al. [98] | C57BL/6J mice, ♂ | EGCG (3.2 g/kg diet) + HFD vs. HFD | 17 wk | ↓ Hepatic d18:1/16:0-, d18:1/26:0- d18:1/26:1-Cer ↑ Hepatic d18:1/18:0-, d18:1/22:1-, d18:1/24:2-Cer ↓ Serum d18:1/16:0-, d18:1/22:3-Cer | ↓ Hepatic d18:1/18:3-SM ↑ Hepatic d18:1/20:0-, d18:1/22:0-, d18:1/22:1-, d18:1/24:0-, d18:1/24:1-, d18:1/24:2-, d18:1/24:3-, d18:1/26:3-, d18:1/26:4-SM ↑ Serum d18:1/16:0-, d18:1/18:1-, d18:1/18:3-, d18:1/20:0-, d18:1/22:0-, d18:1/22:1-, d18:1/24:0-, d18:1/24:3-SM | - |
| Nam et al. [99] | C57BL/6J mice, ♂ | Green tea extract (0.25% w/w) + HFD vs. HFD | 12 wk | ↑ Hepatic d18:1/22:0-Cer | - | - |
| Ali et al. [100] | Sprague–Dawley rats, ♂ | cocoa polyphenol (600 mg/kg BW/d) + HFD vs. HFD | HFD for 12 wk + Treatment for 4 wk | - | - | ↓ MES-WAT Cers5 Fa2h |
| Si et al. [101] | C57BL/6 mice, ♂ | BAE (200 mg/kg BW) + HFD vs. HFD | 8 wk | ↓ Serum Total-Cer All examined Cer | ↓ Serum SM | ↑ Serum SMS1, SMS2 ↓ Serum Spt, CerS1, CerS2, CerS4, Degs, ASMase |
| Paraiso et al. [102] | C57Bl/6J WT, FXRLiver−/− mice, ♂ ♀ | XN (60 mg/kg BW/d) + HFD vs. HFD | 12 wk | XN (WT, ♀): ↓ Hepatic total-Cer XN (WT, ♂): ∅ Hepatic total-Cer XN (FXRLiver−/−, ♀): ∅ Hepatic total-Cer XN (FXRLiver−/−, ♂): ↓ Hepatic total-Cer | XN (WT, ♀): ↓ Hepatic SM XN (WT, ♂): ↑ Hepatic SM XN (FXRLiver−/−, ♀): ∅ Hepatic SM XN (FXRLiver−/−, ♂): ∅ Hepatic SM | - |
| Paraiso et al. [103] | C57Bl/6J mice, ♂ | XN +HFD DXN HFD TXN + HFD (each flavonoid dose, 30 mg/kg BW per day) vs. HFD | 13 wk | XN, DXN, TXN: ↓ Hepatic total-Cer ↓ Hippocampal total- Cer | XN, DXN, TXN: ∅ Hepatic total SM | XN: ↑ Hepatic Degs2, Cers2,4,5,6, Smpd1,3,4, Sgms1,2 DXN, TXN: ↑ Hepatic Sptlc1, Smpd4 |
4.4. Tea Flavonoids and Chamiloflan
4.5. Grape Seed and Proanthocyanidin
4.6. Green Tea, Cocoa, and Catechin
4.7. Anthocyanins
4.8. Xanthohumol
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Keramat, S.A.; Alam, K.; Rana, R.H.; Chowdhury, R.; Farjana, F.; Hashmi, R.; Gow, J.; Biddle, S.J.H. Obesity and the risk of developing chronic diseases in middle-aged and older adults: Findings from an Australian longitudinal population survey, 2009–2017. PLoS ONE 2021, 16, e0260158. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E. The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev. Endocr. Metab. Disord. 2020, 21, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H. Protective mechanisms of the Mediterranean diet in obesity and type 2 diabetes. J. Nutr. Biochem. 2007, 18, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Chavez, J.A.; Summers, S.A. A Ceramide-Centric View of Insulin Resistance. Cell Metab. 2012, 15, 585–594. [Google Scholar] [CrossRef]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef]
- Chaurasia, B.; Holland, W.L.; Summers, S.A. Does This Schlank Make Me Look Fat? Trends Endocrinol. Metab. 2018, 29, 597–599. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Luberto, C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000, 10, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. The Ceramide-centric Universe of Lipid-mediated Cell Regulation: Stress Encounters of the Lipid Kind *. J. Biol. Chem. 2002, 277, 25847–25850. [Google Scholar] [CrossRef] [PubMed]
- Kien, C.L.; Bunn, J.Y.; Poynter, M.E.; Stevens, R.; Bain, J.; Ikayeva, O.; Fukagawa, N.K.; Champagne, C.M.; Crain, K.I.; Koves, T.R.; et al. A Lipidomics Analysis of the Relationship Between Dietary Fatty Acid Composition and Insulin Sensitivity in Young Adults. Diabetes 2013, 62, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Coster, A.C.F.; Campbell, L.V.; Greenfield, J.R.; Lange, K.; Christopher, M.J.; Meikle, P.J.; Samocha-Bonet, D. The effect of short-term overfeeding on serum lipids in healthy humans. Obesity 2013, 21, E649–E659. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Kullberg, J.; Ståhlman, M.; Cedernaes, J.; Heurling, K.; Johansson, H.-E.; Iggman, D.; Wilking, H.; Larsson, A.; Eriksson, O.; et al. Overeating Saturated Fat Promotes Fatty Liver and Ceramides Compared With Polyunsaturated Fat: A Randomized Trial. J. Clin. Endocrinol. Metab. 2019, 104, 6207–6219. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr. De Novo Sphingolipid Biosynthesis: A Necessary, but Dangerous, Pathway *. J. Biol. Chem. 2002, 277, 25843–25846. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Gupta, S.D.; Gable, K.; Niranjanakumari, S.; Moitra, P.; Eichler, F.; Brown, R.H., Jr.; Harmon, J.M.; Dunn, T.M. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc. Natl. Acad. Sci. USA 2009, 106, 8186–8191. [Google Scholar] [CrossRef] [PubMed]
- Hornemann, T.; Penno, A.; Rütti, M.F.; Ernst, D.; Kivrak-Pfiffner, F.; Rohrer, L.; von Eckardstein, A. The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. J. Biol. Chem. 2009, 284, 26322–26330. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Futerman, A.H. Mammalian ceramide synthases. IUBMB Life 2010, 62, 347–356. [Google Scholar] [CrossRef]
- Taniguchi, M.; Okazaki, T. Role of ceramide/sphingomyelin (SM) balance regulated through “SM cycle” in cancer. Cell. Signal. 2021, 87, 110119. [Google Scholar] [CrossRef]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 2008, 20, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M., II.; Pratipanawatr, T.; Berria, R.; Wang, E.; DeFronzo, R.A.; Sullards, M.C.; Mandarino, L.J. Ceramide Content Is Increased in Skeletal Muscle From Obese Insulin-Resistant Humans. Diabetes 2004, 53, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Brönneke, H.S.; et al. Obesity-Induced CerS6-Dependent C16:0 Ceramide Production Promotes Weight Gain and Glucose Intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Brozinick, J.T.; Hawkins, E.; Hoang Bui, H.; Kuo, M.S.; Tan, B.; Kievit, P.; Grove, K. Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. Int. J. Obes. 2013, 37, 1064–1070. [Google Scholar] [CrossRef]
- Samad, F.; Hester, K.D.; Yang, G.; Hannun, Y.A.; Bielawski, J. Altered Adipose and Plasma Sphingolipid Metabolism in Obesity: A Potential Mechanism for Cardiovascular and Metabolic Risk. Diabetes 2006, 55, 2579–2587. [Google Scholar] [CrossRef]
- Shah, C.; Yang, G.; Lee, I.; Bielawski, J.; Hannun, Y.A.; Samad, F. Protection from High Fat Diet-induced Increase in Ceramide in Mice Lacking Plasminogen Activator Inhibitor 1 *. J. Biol. Chem. 2008, 283, 13538–13548. [Google Scholar] [CrossRef]
- Dubé, J.J.; Amati, F.; Toledo, F.G.S.; Stefanovic-Racic, M.; Rossi, A.; Coen, P.; Goodpaster, B.H. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 2011, 54, 1147–1156. [Google Scholar] [CrossRef]
- Huang, H.; Kasumov, T.; Gatmaitan, P.; Heneghan, H.M.; Kashyap, S.R.; Schauer, P.R.; Brethauer, S.A.; Kirwan, J.P. Gastric Bypass Surgery Reduces Plasma Ceramide Subspecies and Improves Insulin Sensitivity in Severely Obese Patients. Obesity 2011, 19, 2235–2240. [Google Scholar] [CrossRef]
- Hammerschmidt, P.; Ostkotte, D.; Nolte, H.; Gerl, M.J.; Jais, A.; Brunner, H.L.; Sprenger, H.-G.; Awazawa, M.; Nicholls, H.T.; Turpin-Nolan, S.M.; et al. CerS6-Derived Sphingolipids Interact with Mff and Promote Mitochondrial Fragmentation in Obesity. Cell 2019, 177, 1536–1552.e1523. [Google Scholar] [CrossRef]
- Gosejacob, D.; Jäger, P.S.; vom Dorp, K.; Frejno, M.; Carstensen, A.C.; Köhnke, M.; Degen, J.; Dörmann, P.; Hoch, M. Ceramide Synthase 5 Is Essential to Maintain C16:0-Ceramide Pools and Contributes to the Development of Diet-induced Obesity *. J. Biol. Chem. 2016, 291, 6989–7003. [Google Scholar] [CrossRef]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating Ceramides Predict Cardiovascular Outcomes in the Population-Based FINRISK 2002 Cohort. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Anroedh, S.; Hilvo, M.; Akkerhuis, K.M.; Kauhanen, D.; Koistinen, K.; Oemrawsingh, R.; Serruys, P.; van Geuns, R.J.; Boersma, E.; Laaksonen, R.; et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J. Lipid Res. 2018, 59, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Akashi, H.; Drosatos, K.; Liao, X.; Jiang, H.; Kennel, P.J.; Brunjes, D.L.; Castillero, E.; Zhang, X.; Deng, L.Y.; et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2017, 2, e82922. [Google Scholar] [CrossRef]
- Hojjati, M.R.; Li, Z.; Zhou, H.; Tang, S.; Huan, C.; Ooi, E.; Lu, S.; Jiang, X.-C. Effect of Myriocin on Plasma Sphingolipid Metabolism and Atherosclerosis in apoE-deficient Mice *. J. Biol. Chem. 2005, 280, 10284–10289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-J.; Holland, W.L.; Wilson, L.; Tanner, J.M.; Kearns, D.; Cahoon, J.M.; Pettey, D.; Losee, J.; Duncan, B.; Gale, D.; et al. Ceramide Mediates Vascular Dysfunction in Diet-Induced Obesity by PP2A-Mediated Dephosphorylation of the eNOS-Akt Complex. Diabetes 2012, 61, 1848–1859. [Google Scholar] [CrossRef]
- Bharath, L.P.; Ruan, T.; Li, Y.; Ravindran, A.; Wan, X.; Nhan, J.K.; Walker, M.L.; Deeter, L.; Goodrich, R.; Johnson, E.; et al. Ceramide-Initiated Protein Phosphatase 2A Activation Contributes to Arterial Dysfunction In Vivo. Diabetes 2015, 64, 3914–3926. [Google Scholar] [CrossRef]
- Huynh, K.; Barlow, C.K.; Jayawardana, K.S.; Weir, J.M.; Mellett, N.A.; Cinel, M.; Magliano, D.J.; Shaw, J.E.; Drew, B.G.; Meikle, P.J. High-Throughput Plasma Lipidomics: Detailed Mapping of the Associations with Cardiometabolic Risk Factors. Cell Chem. Biol. 2019, 26, 71–84.e74. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Yu, C.; Hoofnagle, A.; Hari, N.; Jensen, P.N.; Fretts, A.M.; Umans, J.G.; Howard, B.V.; Sitlani, C.M.; Siscovick, D.S.; et al. Circulating Sphingolipids, Insulin, HOMA-IR, and HOMA-B: The Strong Heart Family Study. Diabetes 2018, 67, 1663–1672. [Google Scholar] [CrossRef]
- Fretts, A.M.; Jensen, P.N.; Hoofnagle, A.N.; McKnight, B.; Howard, B.V.; Umans, J.; Sitlani, C.M.; Siscovick, D.S.; King, I.B.; Djousse, L.; et al. Plasma ceramides containing saturated fatty acids are associated with risk of type 2 diabetes. J. Lipid Res. 2021, 62, 100119. [Google Scholar] [CrossRef]
- Wigger, L.; Cruciani-Guglielmacci, C.; Nicolas, A.; Denom, J.; Fernandez, N.; Fumeron, F.; Marques-Vidal, P.; Ktorza, A.; Kramer, W.; Schulte, A.; et al. Plasma Dihydroceramides Are Diabetes Susceptibility Biomarker Candidates in Mice and Humans. Cell Rep. 2017, 18, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, P.K.; Zhou, Y.; Sädevirta, S.; Leivonen, M.; Arola, J.; Orešič, M.; Hyötyläinen, T.; Yki-Järvinen, H. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Kolak, M.; Westerbacka, J.; Velagapudi, V.R.; Wågsäter, D.; Yetukuri, L.; Makkonen, J.; Rissanen, A.; Häkkinen, A.-M.; Lindell, M.; Bergholm, R.; et al. Adipose Tissue Inflammation and Increased Ceramide Content Characterize Subjects With High Liver Fat Content Independent of Obesity. Diabetes 2007, 56, 1960–1968. [Google Scholar] [CrossRef]
- Yang, G.; Badeanlou, L.; Bielawski, J.; Roberts, A.J.; Hannun, Y.A.; Samad, F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E211–E224. [Google Scholar] [CrossRef]
- Holland, W.L.; Brozinick, J.T.; Wang, L.-P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of Ceramide Synthesis Ameliorates Glucocorticoid-, Saturated-Fat-, and Obesity-Induced Insulin Resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef]
- Lopez, X.; Goldfine, A.B.; Holland, W.L.; Gordillo, R.; Scherer, P.E. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J. Pediatr. Endocrinol. Metab. 2013, 26, 995–998. [Google Scholar] [CrossRef]
- Wasilewska, N.; Bobrus-Chociej, A.; Harasim-Symbor, E.; Tarasów, E.; Wojtkowska, M.; Chabowski, A.; Lebensztejn, D.M. Increased serum concentration of ceramides in obese children with nonalcoholic fatty liver disease. Lipids Health Dis. 2018, 17, 216. [Google Scholar] [CrossRef]
- Yetukuri, L.; Katajamaa, M.; Medina-Gomez, G.; Seppänen-Laakso, T.; Vidal-Puig, A.; Orešič, M. Bioinformatics strategies for lipidomics analysis: Characterization of obesity related hepatic steatosis. BMC Syst. Biol. 2007, 1, 12. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Sädevirta, S.; Zhou, Y.; Kayser, B.; Ali, A.; Ahonen, L.; Lallukka, S.; Pelloux, V.; Gaggini, M.; Jian, C.; et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care 2018, 41, 1732–1739. [Google Scholar] [CrossRef]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Holland, W.L.; Bikman, B.T.; Wang, L.-P.; Yuguang, G.; Sargent, K.M.; Bulchand, S.; Knotts, T.A.; Shui, G.; Clegg, D.J.; Wenk, M.R.; et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid–induced ceramide biosynthesis in mice. J. Clin. Investig. 2011, 121, 1858–1870. [Google Scholar] [CrossRef]
- Schilling, J.D.; Machkovech, H.M.; He, L.; Sidhu, R.; Fujiwara, H.; Weber, K.; Ory, D.S.; Schaffer, J.E. Palmitate and Lipopolysaccharide Trigger Synergistic Ceramide Production in Primary Macrophages *. J. Biol. Chem. 2013, 288, 2923–2932. [Google Scholar] [CrossRef]
- Xu, J.; Yeh, C.H.; Chen, S.; He, L.; Sensi, S.L.; Canzoniero, L.M.; Choi, D.W.; Hsu, C.Y. Involvement of de novo ceramide biosynthesis in tumor necrosis factor-alpha/cycloheximide-induced cerebral endothelial cell death. J. Biol. Chem. 1998, 273, 16521–16526. [Google Scholar] [CrossRef] [PubMed]
- De Mello, V.D.F.; Lankinen, M.; Schwab, U.; Kolehmainen, M.; Lehto, S.; Seppänen-Laakso, T.; Orešič, M.; Pulkkinen, L.; Uusitupa, M.; Erkkilä, A.T. Link between plasma ceramides, inflammation and insulin resistance: Association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia 2009, 52, 2612–2615. [Google Scholar] [CrossRef]
- Majumdar, I.; Mastrandrea, L.D. Serum sphingolipids and inflammatory mediators in adolescents at risk for metabolic syndrome. Endocrine 2012, 41, 442–449. [Google Scholar] [CrossRef]
- Ahima, R.S. Metabolic Actions of Adipocyte Hormones: Focus on Adiponectin. Obesity 2006, 14, 9S–15S. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef]
- Vasiliauskaité-Brooks, I.; Sounier, R.; Rochaix, P.; Bellot, G.; Fortier, M.; Hoh, F.; De Colibus, L.; Bechara, C.; Saied, E.M.; Arenz, C.; et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 2017, 544, 120–123. [Google Scholar] [CrossRef]
- Holland, W.L.; Xia, J.Y.; Johnson, J.A.; Sun, K.; Pearson, M.J.; Sharma, A.X.; Quittner-Strom, E.; Tippetts, T.S.; Gordillo, R.; Scherer, P.E. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol. Metab. 2017, 6, 267–275. [Google Scholar] [CrossRef]
- Choi, S.R.; Lim, J.H.; Kim, M.Y.; Kim, E.N.; Kim, Y.; Choi, B.S.; Kim, Y.-S.; Kim, H.W.; Lim, K.-M.; Kim, M.J.; et al. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism 2018, 85, 348–360. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Koutsari, C.; Tchkonia, T.; Jensen, M.D. Sphingolipid Content of Human Adipose Tissue: Relationship to Adiponectin and Insulin Resistance. Obesity 2012, 20, 2341–2347. [Google Scholar] [CrossRef]
- Johnson, E.L.; Heaver, S.L.; Waters, J.L.; Kim, B.I.; Bretin, A.; Goodman, A.L.; Gewirtz, A.T.; Worgall, T.S.; Ley, R.E. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat. Commun. 2020, 11, 2471. [Google Scholar] [CrossRef]
- Heaver, S.L.; Johnson, E.L.; Ley, R.E. Sphingolipids in host–microbial interactions. Curr. Opin. Microbiol. 2018, 43, 92–99. [Google Scholar] [CrossRef]
- Jiang, C.; Xie, C.; Lv, Y.; Li, J.; Krausz, K.W.; Shi, J.; Brocker, C.N.; Desai, D.; Amin, S.G.; Bisson, W.H.; et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 2015, 6, 10166. [Google Scholar] [CrossRef]
- Jiang, C.; Xie, C.; Li, F.; Zhang, L.; Nichols, R.G.; Krausz, K.W.; Cai, J.; Qi, Y.; Fang, Z.Z.; Takahashi, S.; et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Investig. 2015, 125, 386–402. [Google Scholar] [CrossRef]
- Salinas, M.; López-Valdaliso, R.; Martín, D.; Alvarez, A.; Cuadrado, A. Inhibition of PKB/Akt1 by C2-Ceramide Involves Activation of Ceramide-Activated Protein Phosphatase in PC12 Cells. Mol. Cell. Neurosci. 2000, 15, 156–169. [Google Scholar] [CrossRef]
- Zinda, M.J.; Vlahos, C.J.; Lai, M.T. Ceramide Induces the Dephosphorylation and Inhibition of Constitutively Activated Akt in PTEN Negative U87MG Cells. Biochem. Biophys. Res. Commun. 2001, 280, 1107–1115. [Google Scholar] [CrossRef]
- Stratford, S.; Hoehn, K.L.; Liu, F.; Summers, S.A. Regulation of Insulin Action by Ceramide: Dual Mechanisms Linking Ceramide Accumulation to the Inhibition of Akt/Protein Kinase B *. J. Biol. Chem. 2004, 279, 36608–36615. [Google Scholar] [CrossRef]
- Raichur, S.; Wang, S.T.; Chan, P.W.; Li, Y.; Ching, J.; Chaurasia, B.; Dogra, S.; Öhman, M.K.; Takeda, K.; Sugii, S.; et al. CerS2 Haploinsufficiency Inhibits β-Oxidation and Confers Susceptibility to Diet-Induced Steatohepatitis and Insulin Resistance. Cell Metab. 2014, 20, 687–695. [Google Scholar] [CrossRef]
- Dekker, M.J.; Baker, C.; Naples, M.; Samsoondar, J.; Zhang, R.; Qiu, W.; Sacco, J.; Adeli, K. Inhibition of sphingolipid synthesis improves dyslipidemia in the diet-induced hamster model of insulin resistance: Evidence for the role of sphingosine and sphinganine in hepatic VLDL-apoB100 overproduction. Atherosclerosis 2013, 228, 98–109. [Google Scholar] [CrossRef]
- Chaurasia, B.; Tippetts, T.S.; Mayoral Monibas, R.; Liu, J.; Li, Y.; Wang, L.; Wilkerson, J.L.; Sweeney, C.R.; Pereira, R.F.; Sumida, D.H.; et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 2019, 365, 386–392. [Google Scholar] [CrossRef]
- Xia, J.Y.; Holland, W.L.; Kusminski, C.M.; Sun, K.; Sharma, A.X.; Pearson, M.J.; Sifuentes, A.J.; McDonald, J.G.; Gordillo, R.; Scherer, P.E. Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell Metab. 2015, 22, 266–278. [Google Scholar] [CrossRef]
- Zigdon, H.; Kogot-Levin, A.; Park, J.-W.; Goldschmidt, R.; Kelly, S.; Merrill, A.H., Jr.; Scherz, A.; Pewzner-Jung, Y.; Saada, A.; Futerman, A.H. Ablation of Ceramide Synthase 2 Causes Chronic Oxidative Stress Due to Disruption of the Mitochondrial Respiratory Chain *. J. Biol. Chem. 2013, 288, 4947–4956. [Google Scholar] [CrossRef]
- Park, M.; Kaddai, V.; Ching, J.; Fridianto, K.T.; Sieli, R.J.; Sugii, S.; Summers, S.A. A Role for Ceramides, but Not Sphingomyelins, as Antagonists of Insulin Signaling and Mitochondrial Metabolism in C2C12 Myotubes *. J. Biol. Chem. 2016, 291, 23978–23988. [Google Scholar] [CrossRef]
- Sentelle, R.D.; Senkal, C.E.; Jiang, W.; Ponnusamy, S.; Gencer, S.; Panneer Selvam, S.; Ramshesh, V.K.; Peterson, Y.K.; Lemasters, J.J.; Szulc, Z.M.; et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 2012, 8, 831–838. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Cancer Prevention and Therapy with Polyphenols: Sphingolipid-Mediated Mechanisms. Nutrients 2018, 10, 940. [Google Scholar] [CrossRef]
- Bikman, B.T.; Guan, Y.; Shui, G.; Siddique, M.M.; Holland, W.L.; Kim, J.Y.; Fabriàs, G.; Wenk, M.R.; Summers, S.A. Fenretinide Prevents Lipid-induced Insulin Resistance by Blocking Ceramide Biosynthesis *. J. Biol. Chem. 2012, 287, 17426–17437. [Google Scholar] [CrossRef]
- Momchilova, A.; Petkova, D.; Staneva, G.; Markovska, T.; Pankov, R.; Skrobanska, R.; Nikolova-Karakashian, M.; Koumanov, K. Resveratrol alters the lipid composition, metabolism and peroxide level in senescent rat hepatocytes. Chem. Biol. Interact. 2014, 207, 74–80. [Google Scholar] [CrossRef]
- Babenko, N.A.; Shakhova, E.G. Effects of Chamomilla recutita flavonoids on age-related liver sphingolipid turnover in rats. Exp. Gerontol. 2006, 41, 32–39. [Google Scholar] [CrossRef]
- Babenko, N.A.; Shakhova, E.G. Effects of flavonoids on sphingolipid turnover in the toxin-damaged liver and liver cells. Lipids Health Dis. 2008, 7, 1. [Google Scholar] [CrossRef]
- Van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Signori, C.; Meessen, J.M.T.A.; Laaksonen, R.; Maggioni, A.P.; Novelli, D.; Blanda, A.; Jylhä, A.; Nicolis, E.; Targher, G.; Tavazzi, L.; et al. Coffee, Atrial Fibrillation, and Circulating Ceramides in Patients with Chronic Heart Failure. J. Agric. Food Chem. 2021, 69, 11236–11245. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.J.; Low, D.Y.; Pan, W.-C.; Gunther, S.H.; Sim, X.; Torta, F.; Herr, D.R.; Kovalik, J.-P.; Ching, J.; Khoo, C.M.; et al. Coffee, Black Tea, and Green Tea Consumption in Relation to Plasma Metabolites in an Asian Population. Mol. Nutr. Food Res. 2020, 64, 2000527. [Google Scholar] [CrossRef] [PubMed]
- Wittenbecher, C.; Cuadrat, R.; Johnston, L.; Eichelmann, F.; Jäger, S.; Kuxhaus, O.; Prada, M.; Del Greco, M.F.; Hicks, A.A.; Hoffman, P.; et al. Dihydroceramide- and ceramide-profiling provides insights into human cardiometabolic disease etiology. Nat. Commun. 2022, 13, 936. [Google Scholar] [CrossRef]
- Sinha, R.A.; Farah, B.L.; Singh, B.K.; Siddique, M.M.; Li, Y.; Wu, Y.; Ilkayeva, O.R.; Gooding, J.; Ching, J.; Zhou, J.; et al. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology 2014, 59, 1366–1380. [Google Scholar] [CrossRef]
- Velázquez, A.M.; Roglans, N.; Bentanachs, R.; Gené, M.; Sala-Vila, A.; Lázaro, I.; Rodríguez-Morató, J.; Sánchez, R.M.; Laguna, J.C.; Alegret, M. Effects of a Low Dose of Caffeine Alone or as Part of a Green Coffee Extract, in a Rat Dietary Model of Lean Non-Alcoholic Fatty Liver Disease without Inflammation. Nutrients 2020, 12, 3240. [Google Scholar] [CrossRef]
- Zhong, X.-c.; Liu, Y.-m.; Gao, X.-x.; Krausz, K.W.; Niu, B.; Gonzalez, F.J.; Xie, C. Caffeic acid phenethyl ester suppresses intestinal FXR signaling and ameliorates nonalcoholic fatty liver disease by inhibiting bacterial bile salt hydrolase activity. Acta Pharmacol. Sin. 2023, 44, 145–156. [Google Scholar] [CrossRef]
- Conzatti, A.; Fróes, F.C.; Schweigert Perry, I.D.; Souza, C.G. Clinical and molecular evidence of the consumption of broccoli, glucoraphanin and sulforaphane in humans. Nutr. Hosp. 2014, 31, 559–569. [Google Scholar] [CrossRef]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 2017, 9, eaah4477. [Google Scholar] [CrossRef]
- Teng, W.; Li, Y.; Du, M.; Lei, X.; Xie, S.; Ren, F. Sulforaphane Prevents Hepatic Insulin Resistance by Blocking Serine Palmitoyltransferase 3-Mediated Ceramide Biosynthesis. Nutrients 2019, 11, 1185. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.; Teng, W. Sulforaphane Attenuates Nonalcoholic Fatty Liver Disease by Inhibiting Hepatic Steatosis and Apoptosis. Nutrients 2022, 14, 76. [Google Scholar] [CrossRef]
- Kalantari, H.; Das, D.K. Physiological effects of resveratrol. BioFactors 2010, 36, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2015, 1852, 1114–1123. [Google Scholar] [CrossRef]
- Abo Alrob, O.; Al-Horani, R.A.; Altaany, Z.; Nusair, M.B. Synergistic Beneficial Effects of Resveratrol and Diet on High-Fat Diet-Induced Obesity. Medicina 2022, 58, 1301. [Google Scholar] [CrossRef]
- Tveter, K.M.; Villa-Rodriguez, J.A.; Cabales, A.J.; Zhang, L.; Bawagan, F.G.; Duran, R.M.; Roopchand, D.E. Polyphenol-induced improvements in glucose metabolism are associated with bile acid signaling to intestinal farnesoid X receptor. BMJ Open Diabetes Res. Care 2020, 8, e001386. [Google Scholar] [CrossRef]
- Seo, K.-H.; Bartley, G.E.; Tam, C.; Kim, H.-S.; Kim, D.-H.; Chon, J.-W.; Kim, H.; Yokoyama, W. Chardonnay Grape Seed Flour Ameliorates Hepatic Steatosis and Insulin Resistance via Altered Hepatic Gene Expression for Oxidative Stress, Inflammation, and Lipid and Ceramide Synthesis in Diet-Induced Obese Mice. PLoS ONE 2016, 11, e0167680. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Lee, H.G.; Seo, K.-H.; Yokoyama, W.; Kim, H. Antiobesity Effect of Prebiotic Polyphenol-Rich Grape Seed Flour Supplemented with Probiotic Kefir-Derived Lactic Acid Bacteria. J. Agric. Food Chem. 2018, 66, 12498–12511. [Google Scholar] [CrossRef]
- Huang, J.; Feng, S.; Liu, A.; Dai, Z.; Wang, H.; Reuhl, K.; Lu, W.; Yang, C.S. Green Tea Polyphenol EGCG Alleviates Metabolic Abnormality and Fatty Liver by Decreasing Bile Acid and Lipid Absorption in Mice. Mol. Nutr. Food Res. 2018, 62, 1700696. [Google Scholar] [CrossRef]
- Nam, M.; Choi, M.-S.; Choi, J.-Y.; Kim, N.; Kim, M.-S.; Jung, S.; Kim, J.; Ryu, D.H.; Hwang, G.-S. Effect of green tea on hepatic lipid metabolism in mice fed a high-fat diet. J. Nutr. Biochem. 2018, 51, 1–7. [Google Scholar] [CrossRef]
- Ali, F.; Ismail, A.; Esa, N.M.; Pei, C.P. Transcriptomics expression analysis to unveil the molecular mechanisms underlying the cocoa polyphenol treatment in diet-induced obesity rats. Genomics 2015, 105, 23–30. [Google Scholar] [CrossRef]
- Si, X.; Tian, J.; Shu, C.; Wang, Y.; Gong, E.; Zhang, Y.; Zhang, W.; Cui, H.; Li, B. Serum Ceramide Reduction by Blueberry Anthocyanin-Rich Extract Alleviates Insulin Resistance in Hyperlipidemia Mice. J. Agric. Food Chem. 2020, 68, 8185–8194. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Tran, T.Q.; Magana, A.A.; Kundu, P.; Choi, J.; Maier, C.S.; Bobe, G.; Raber, J.; Kioussi, C.; Stevens, J.F. Xanthohumol ameliorates Diet-Induced Liver Dysfunction via Farnesoid X Receptor-Dependent and Independent Signaling. Front. Pharmacol. 2021, 12, 643857. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Revel, J.S.; Choi, J.; Miranda, C.L.; Lak, P.; Kioussi, C.; Bobe, G.; Gombart, A.F.; Raber, J.; Maier, C.S.; et al. Targeting the Liver-Brain Axis with Hop-Derived Flavonoids Improves Lipid Metabolism and Cognitive Performance in Mice. Mol. Nutr. Food Res. 2020, 64, 2000341. [Google Scholar] [CrossRef]
- Yi, M.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Zhao, R.; Wan, Q.; Du, L.; Zhou, Y. Tea Consumption and Health Outcomes: Umbrella Review of Meta-Analyses of Observational Studies in Humans. Mol. Nutr. Food Res. 2019, 63, 1900389. [Google Scholar] [CrossRef]
- Bladé, C.; Aragonès, G.; Arola-Arnal, A.; Muguerza, B.; Bravo, F.I.; Salvadó, M.J.; Arola, L.; Suárez, M. Proanthocyanidins in health and disease. BioFactors 2016, 42, 5–12. [Google Scholar] [CrossRef]
- Prasain, J.K.; Barnes, S. Cranberry polyphenols-gut microbiota interactions and potential health benefits: An updated review. Food Front. 2020, 1, 459–464. [Google Scholar] [CrossRef]
- Crespy, V.; Williamson, G. A Review of the Health Effects of Green Tea Catechins in In Vivo Animal Models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef]
- Khan, N.; Khymenets, O.; Urpí-Sardà, M.; Tulipani, S.; Garcia-Aloy, M.; Monagas, M.; Mora-Cubillos, X.; Llorach, R.; Andres-Lacueva, C. Cocoa Polyphenols and Inflammatory Markers of Cardiovascular Disease. Nutrients 2014, 6, 844–880. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ishizaki, Y.; Kojo, S.; Kikuzaki, H. Strong Inhibition of Secretory Sphingomyelinase by Catechins, Particularly by (−)-Epicatechin 3-O-Gallate and (−)-3′-O-Methylepigallocatechin 3-O-Gallate. J. Nutr. Sci. Vitaminol. 2016, 62, 123–129. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, H.; Tian, Z.; Wang, X.; Xu, L.; Li, K.; Gao, X.; Fan, D.; Ma, X.; Ling, W.; et al. Dose-dependent reductions in plasma ceramides after anthocyanin supplementation are associated with improvements in plasma lipids and cholesterol efflux capacity in dyslipidemia: A randomized controlled trial. Clin. Nutr. 2021, 40, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Legette, L.L.; Moreno Luna, A.Y.; Reed, R.L.; Miranda, C.L.; Bobe, G.; Proteau, R.R.; Stevens, J.F. Xanthohumol lowers body weight and fasting plasma glucose in obese male Zucker fa/fa rats. Phytochemistry 2013, 91, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.L.; Elias, V.D.; Hay, J.J.; Choi, J.; Reed, R.L.; Stevens, J.F. Xanthohumol improves dysfunctional glucose and lipid metabolism in diet-induced obese C57BL/6J mice. Arch. Biochem. Biophys. 2016, 599, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.L.; Johnson, L.A.; de Montgolfier, O.; Elias, V.D.; Ullrich, L.S.; Hay, J.J.; Paraiso, I.L.; Choi, J.; Reed, R.L.; Revel, J.S.; et al. Non-estrogenic Xanthohumol Derivatives Mitigate Insulin Resistance and Cognitive Impairment in High-Fat Diet-induced Obese Mice. Sci. Rep. 2018, 8, 613. [Google Scholar] [CrossRef]
| Author | Cells | Treatment | Ceramide/Sphingolipid Levels | Sphingolipid Metabolism-Related Expression or Activity |
|---|---|---|---|---|
| Bikman et al. [77] | C2C12 (myoblasts) | 20 μM RES + 0.75 mM PA vs. 0.75 mM PA | ↓ d18:1/16:0-, Total-Cer ↑ d18:1/16:0-, Total-DhCer | - |
| Momchilova et al. [78] | Hepatocytes from 20-mo-old Wistar rats (♂) | 50 μM RES vs. CON | ↑ SM | ↓ nSMase activity |
| Babenko et al. [79] | Hepatocytes from 24-mo-old Wistar rats (♂) | 500 μg/mL Chamiloflan 30 μM AP7Glu 30 μM LU7Glu vs. CON | Chamiloflan: ∅ Total-Cer AP7Glu: ↓ Total-Cer LU7Glu: ∅ Total-Cer | ↓ nSMase activity |
| Babenko et al. [80] | Hepatocytes from 90-d-old Wistar rats (♂) | Pretrt 40 mM CCl4 + 500 μg/mL Chamiloflan Pretrt CCl4 + 30 μM AP7Glu vs. CCl4 | Chamiloflan: ↓ Total-Cer, ↑ SM AP7Glu: ↓ Total-Cer, ∅ SM | - |
| Pretrt 70 mM EtOH + 500 μg/mL Chamiloflan vs. EtOH | Chamiloflan: ∅ Total-Cer, ∅ SM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Jeon, S. The Impact of Phytochemicals in Obesity-Related Metabolic Diseases: Focus on Ceramide Metabolism. Nutrients 2023, 15, 703. https://doi.org/10.3390/nu15030703
Kim E, Jeon S. The Impact of Phytochemicals in Obesity-Related Metabolic Diseases: Focus on Ceramide Metabolism. Nutrients. 2023; 15(3):703. https://doi.org/10.3390/nu15030703
Chicago/Turabian StyleKim, Eunkyeong, and Sookyoung Jeon. 2023. "The Impact of Phytochemicals in Obesity-Related Metabolic Diseases: Focus on Ceramide Metabolism" Nutrients 15, no. 3: 703. https://doi.org/10.3390/nu15030703
APA StyleKim, E., & Jeon, S. (2023). The Impact of Phytochemicals in Obesity-Related Metabolic Diseases: Focus on Ceramide Metabolism. Nutrients, 15(3), 703. https://doi.org/10.3390/nu15030703





