Elevation of Intracellular Alpha-Ketoglutarate Levels Inhibits Osteoclastogenesis by Suppressing the NF-κB Signaling Pathway in a PHD1-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Regents
2.2. Animals
2.3. Cell Culture
2.4. Cell Viability Assays
2.5. Osteoclastogenesis from RAW264.7 Cells
2.6. Osteoclastogenesis from Bone Marrow-Derived Macrophages (BMMs)
2.7. Mitochondrial Oxidative Phosphorylation Assays
2.8. Cell Transfection
2.9. Double Nicking CRISPR-Cas9
2.10. Real-Time Quantitative PCR (RT-qPCR)
2.11. Western Blot Analysis
2.12. Immunofluorescence
2.13. Statistical Analyses
3. Results
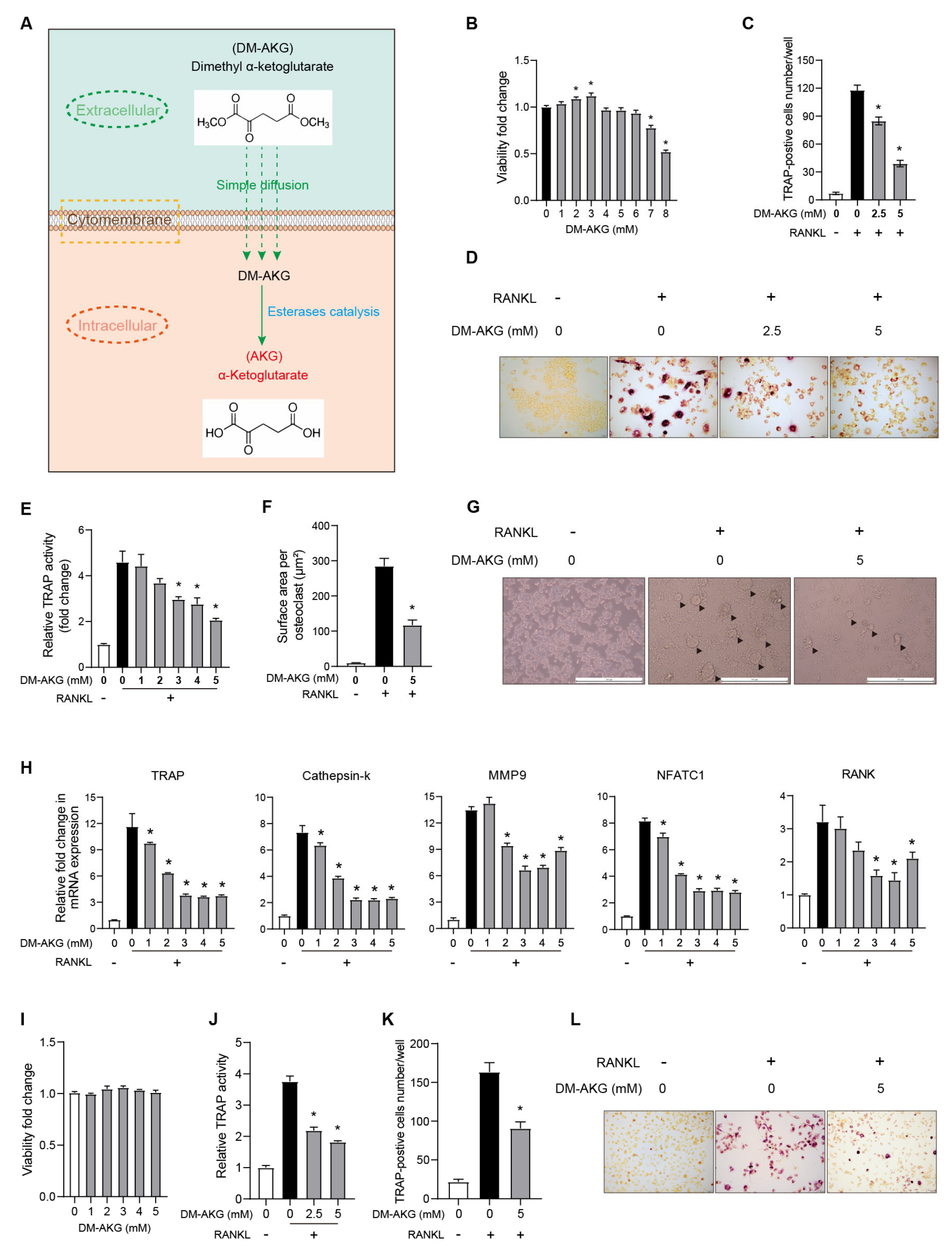
3.1. The Elevation of Intracellular AKG Levels Inhibits Osteoclastogenesis
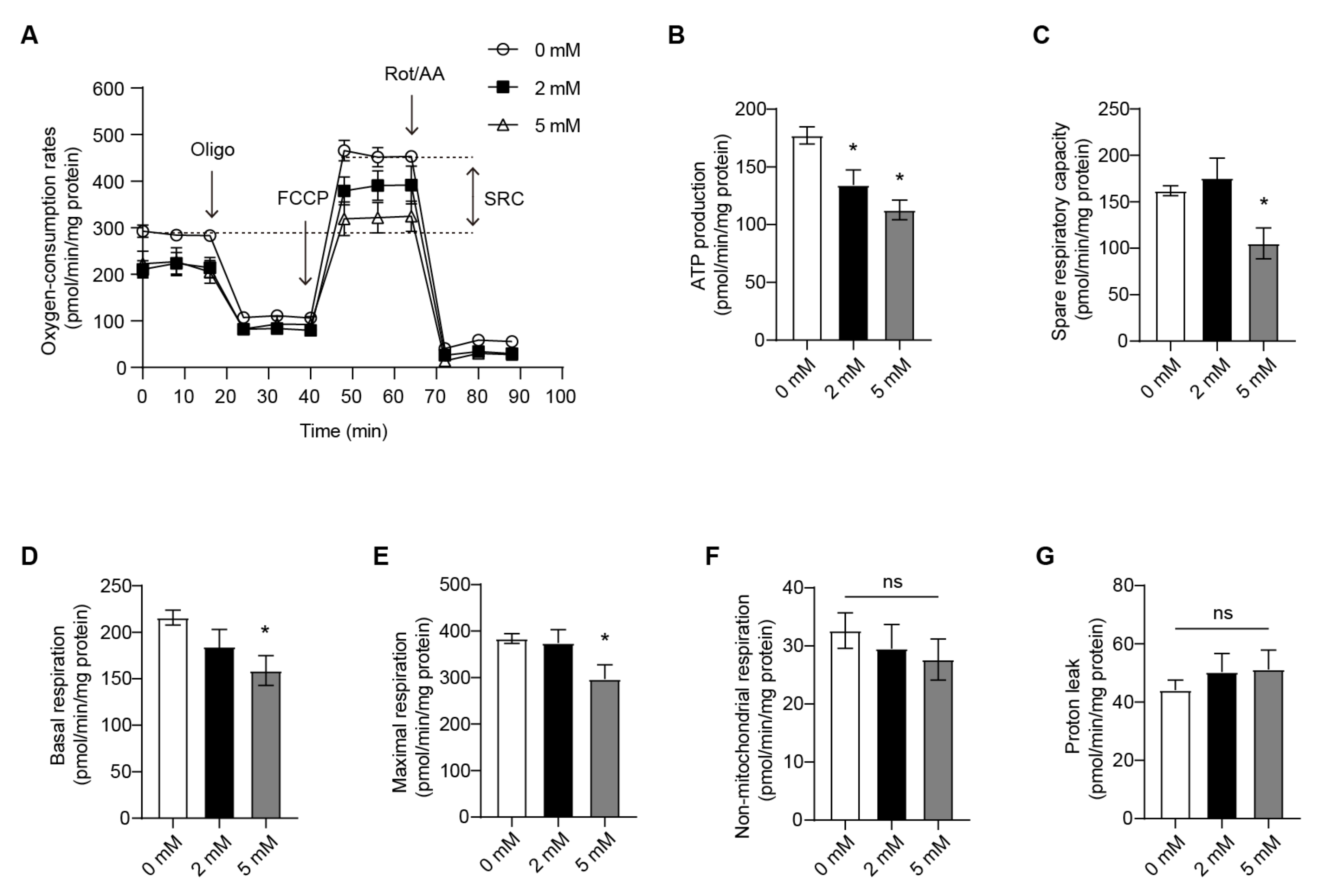
3.2. OXPHOS of the RANKL-Stimulated RAW264.7 Cells Is Attenuated by DM-AKG Treatment
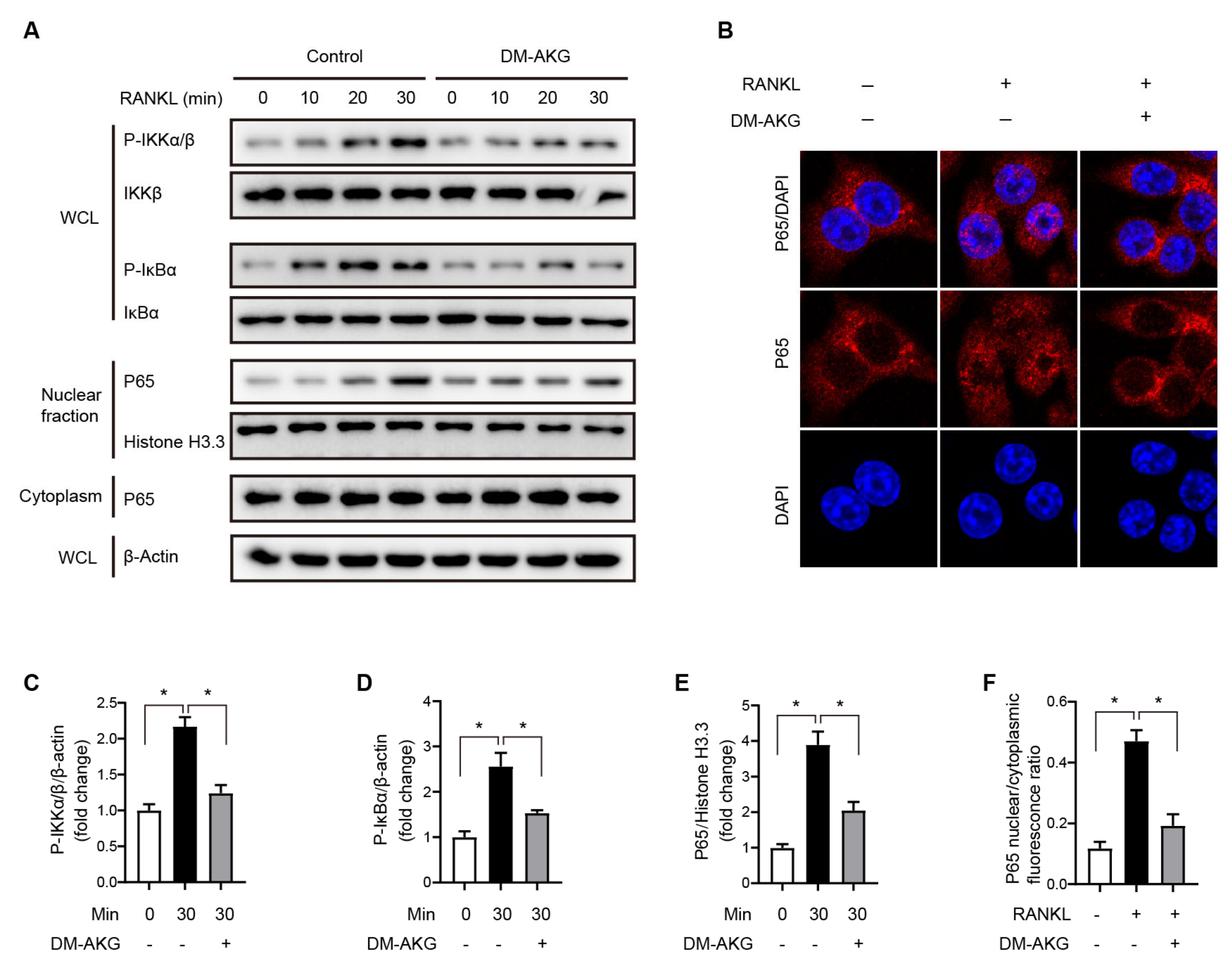
3.3. The Elevation of Intracellular AKG Levels Suppresses NF-κB Signaling during RANKL-Induced Osteoclast Differentiation
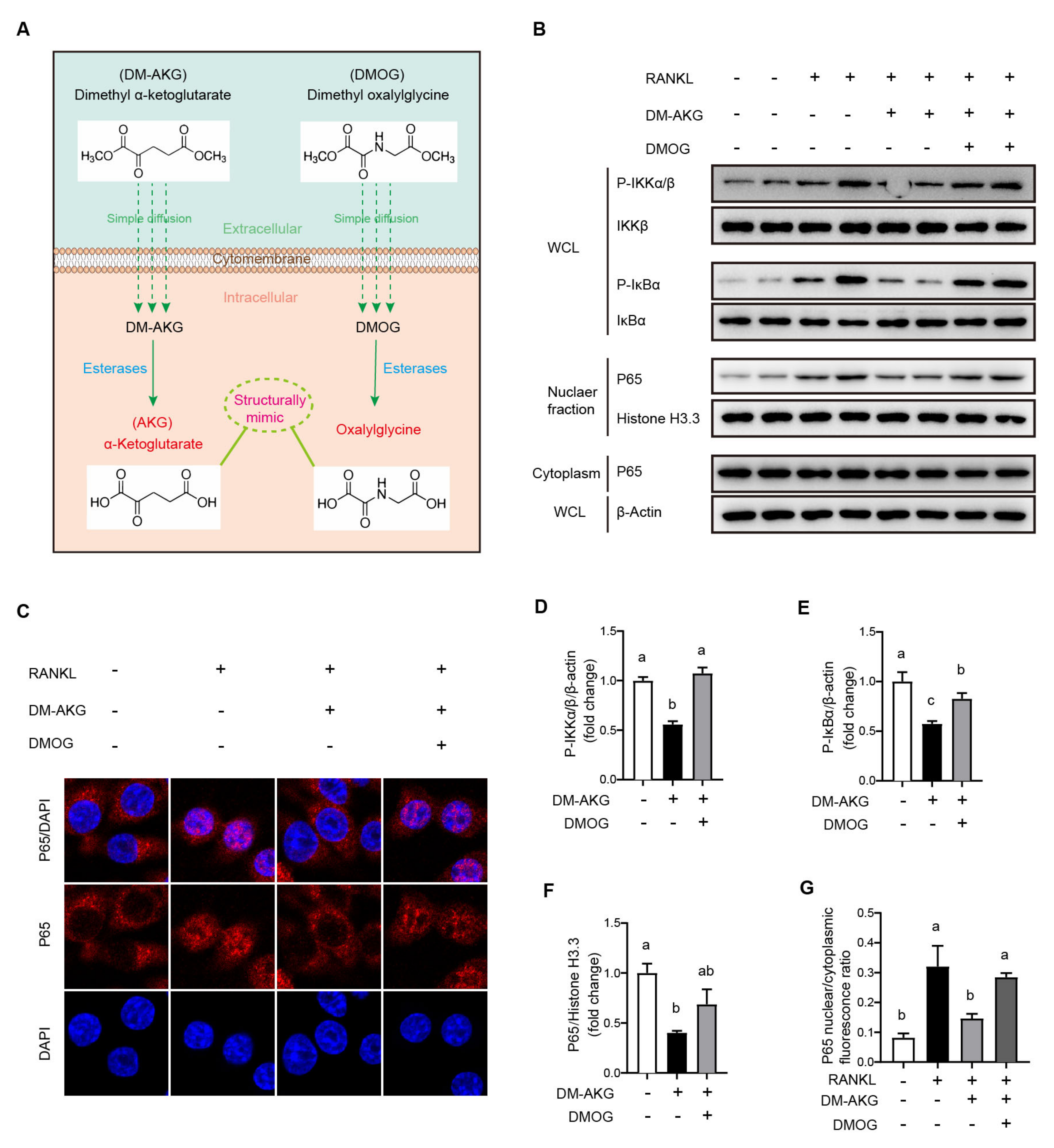
3.4. DMOG Antagonizes the Inhibition Effects of DM-AKG on NF-κB Signaling Activation during Osteoclast Differentiation
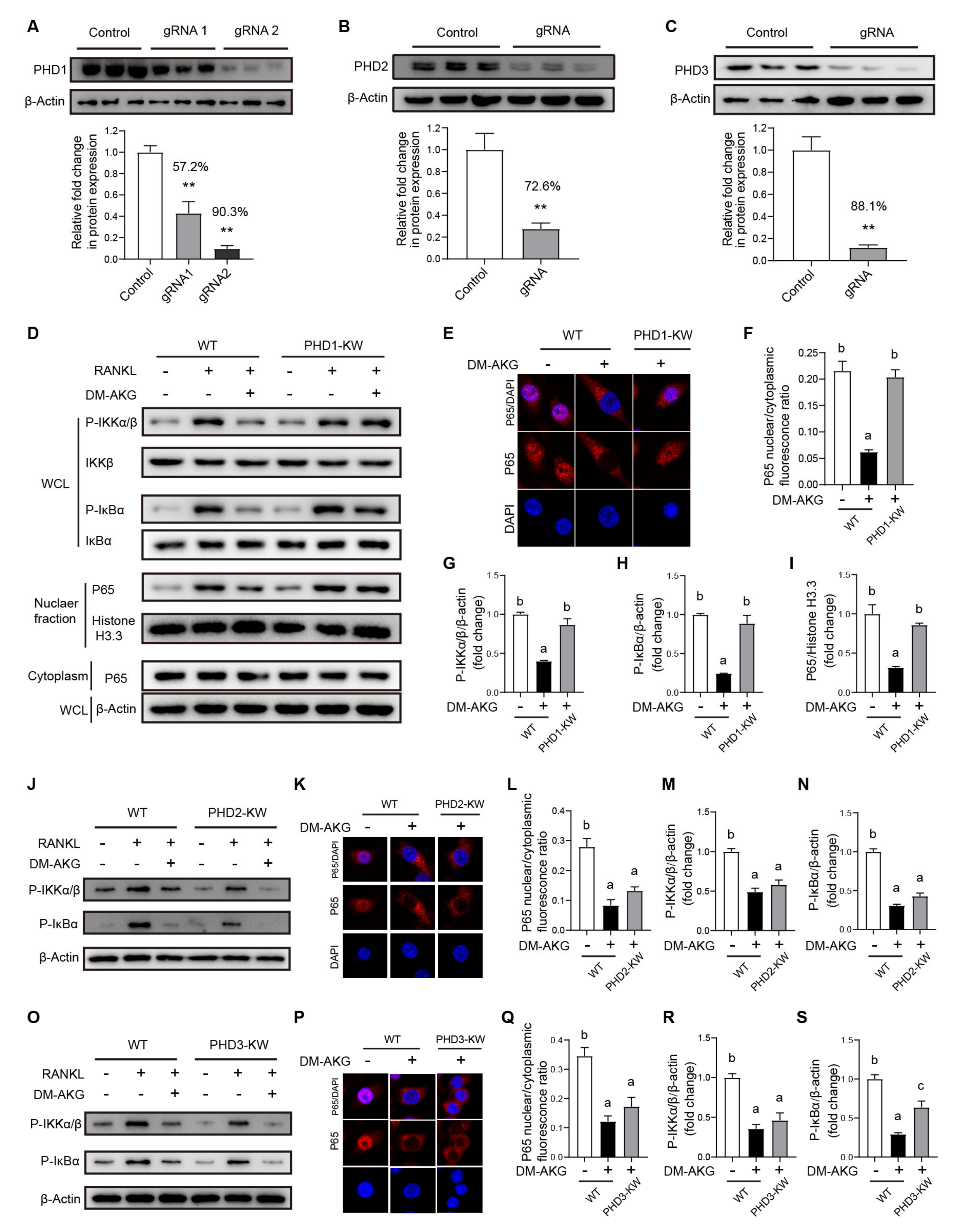
3.5. PHD1 Deficiency Reverses the Inhibitory Effects of DM-AKG Treatment on NF-κB Signaling during Osteoclast Differentiation
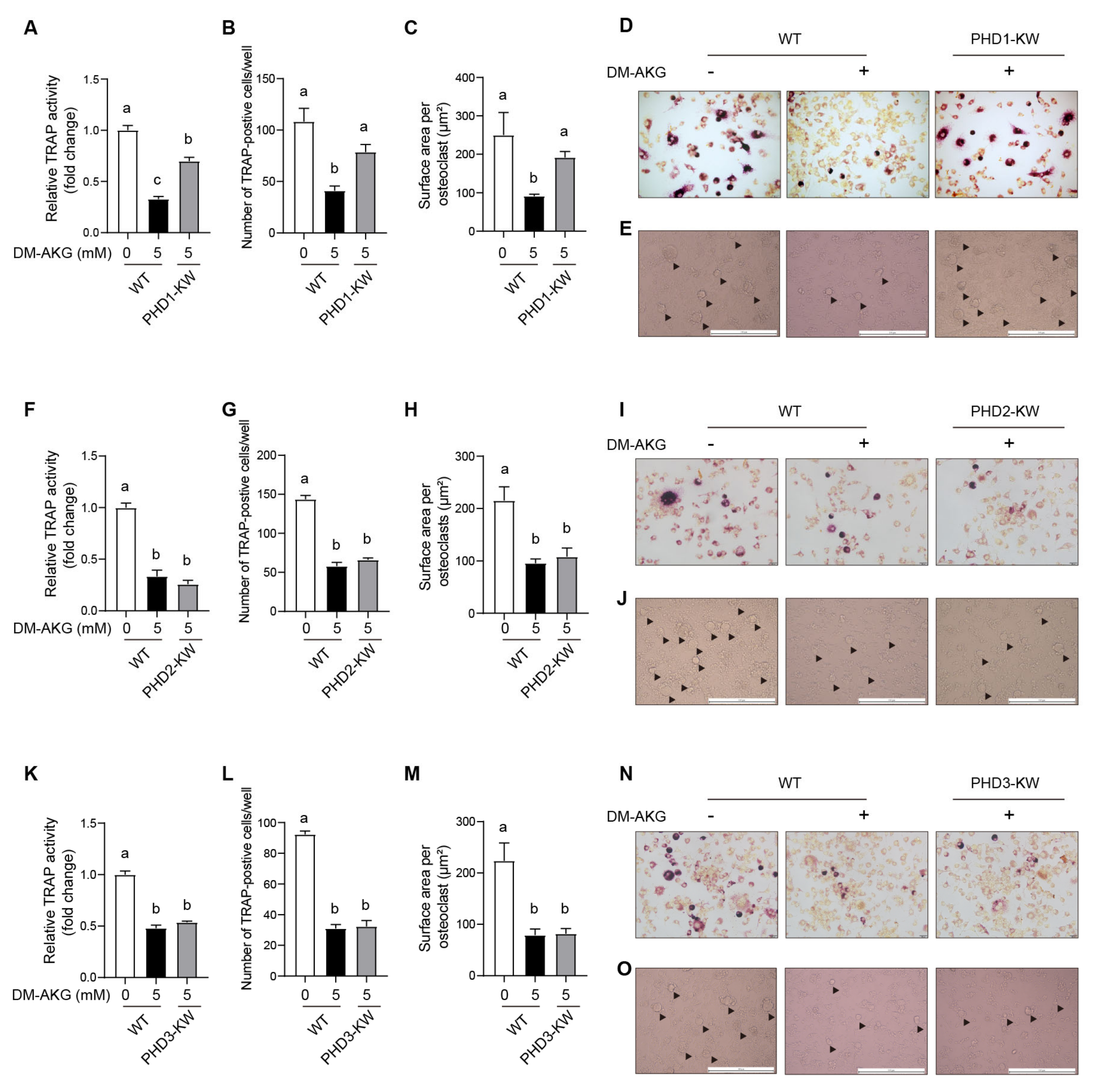
3.6. PHD1 Deficiency Antagonizes the Inhibitory Effects of DM-AKG on Osteoclast Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilaca, T.; Eastell, R.; Schini, M. Osteoporosis in men. Lancet Diabetes Endocrinol. 2022, 10, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Demidenko, O.; Barardo, D.; Budovskii, V.; Finnemore, R.; Palmer, F.R.; Kennedy, B.K.; Budovskaya, Y.V. Rejuvant®, a potential life-extending compound formulation with alpha-ketoglutarate and vitamins, conferred an average 8 year reduction in biological aging, after an average of 7 months of use, in the TruAge DNA methylation test. Aging 2021, 13, 24485–24499. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhou, Z.; Guo, M.; Zhou, Z. The study of skin hydration, anti-wrinkles function improvement of anti-aging cream with alpha-ketoglutarate. J. Cosmet. Dermatol. 2022, 21, 1736–1743. [Google Scholar] [CrossRef]
- Shahmirzadi, A.A.; Edgar, D.; Liao, C.-Y.; Hsu, Y.-M.; Lucanic, M.; Shahmirzadi, A.A.; Wiley, C.D.; Gan, G.; Kim, D.E.; Kasler, H.G.; et al. Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab. 2020, 32, 447–456. [Google Scholar] [CrossRef]
- Chin, R.M.; Fu, X.; Pai, M.Y.; Vergnes, L.; Hwang, H.; Deng, G.; Diep, S.; Lomenick, B.; Meli, V.S.; Monsalve, G.C.; et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 2014, 510, 397–401. [Google Scholar] [CrossRef]
- Su, Y.; Wang, T.; Wu, N.; Li, D.; Fan, X.; Xu, Z.; Mishra, S.K.; Yang, M. Alpha-ketoglutarate extends Drosophila lifespan by inhibiting mTOR and activating AMPK. Aging 2019, 11, 4183–4197. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, P.; Liu, Y.; Wu, Y.; Chen, Y.; Guo, Y.; Zhang, S.; Zheng, X.; Zhou, L.; Liu, W.; et al. Alpha-ketoglutarate ameliorates age-related osteoporosis via regulating histone methylations. Nat. Commun. 2020, 11, 5596. [Google Scholar] [CrossRef]
- Filip, R.S.; Pierzynowski, S.G.; Lindegard, B.; Wernerman, J.; Haratym-Maj, A.; Podgurniak, M. Alpha-Ketoglutarate Decreases Serum Levels of C-terminal Cross-Linking Telopeptide of Type I Collagen (CTX) in Postmenopausal Women with Osteopenia: Six-Month Study. Int. J. Vitam. Nutr. Res. 2007, 77, 89–97. [Google Scholar] [CrossRef]
- Boskey, A.; Coleman, R. Aging and Bone. J. Dent. Res. 2010, 89, 1333–1348. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of Bone Remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Cui, J.; Shibata, Y.; Zhu, T.; Zhou, J.; Zhang, J. Osteocytes in bone aging: Advances, challenges, and future perspectives. Ageing Res. Rev. 2022, 77, 101608. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Cedeno-Veloz, B.A.; Lopez, J.E.; Gutiérrez-Valencia, M.; Alegría, L.L.; Saiz, L.C.; García, A.M.R.; Latorre, M.S.; Vélez, R.R.; Izquierdo, M.; Martínez-Velilla, N. Efficacy of Antiresorptive Treatment in Osteoporotic Older Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Nutr. Health Aging 2022, 26, 778–785. [Google Scholar] [CrossRef]
- Zdzisińska, B.; Żurek, A.; Kandefer-Szerszeń, M. Alpha-Ketoglutarate as a Molecule with Pleiotropic Activity: Well-Known and Novel Possibilities of Therapeutic Use. Arch. Immunol. Ther. Exp. 2017, 65, 21–36. [Google Scholar] [CrossRef]
- Fraisl, P.; Aragonés, J.; Carmeliet, P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat. Rev. Drug Discov. 2009, 8, 139–152. [Google Scholar] [CrossRef]
- Chisolm, D.A.; Weinmann, A.S. Metabolites, genome organization, and cellular differentiation gene programs. Curr. Opin. Immunol. 2018, 51, 62–67. [Google Scholar] [CrossRef]
- Carey, B.W.; Finley, L.W.S.; Cross, J.; Allis, C.D.; Thompson, C.B. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 2015, 518, 413–416. [Google Scholar] [CrossRef]
- TeSlaa, T.; Chaikovsky, A.C.; Lipchina, I.; Escobar, S.L.; Hochedlinger, K.; Huang, J.; Graeber, T.; Braas, D.; Teitell, M.A. α-Ketoglutarate Accelerates the Initial Differentiation of Primed Human Pluripotent Stem Cells. Cell Metab. 2016, 24, 485–493. [Google Scholar] [CrossRef]
- Liu, P.-S.; Wang, H.; Li, X.; Chao, T.; Teav, T.; Christen, S.; Di Conza, G.; Cheng, W.-C.; Chou, C.-H.; Vavakova, M.; et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017, 18, 985–994. [Google Scholar] [CrossRef]
- Hsu, H.; Lacey, D.L.; Dunstan, C.R.; Solovyev, I.; Colombero, A.; Timms, E.; Tan, H.-L.; Elliott, G.; Kelley, M.J.; Sarosi, I.; et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 1999, 96, 3540–3545. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Soysa, N.S.; Alles, N.; Shimokawa, H.; Jimi, E.; Aoki, K.; Ohya, K. Inhibition of the classical NF-κB pathway prevents osteoclast bone-resorbing activity. J. Bone Miner. Metab. 2009, 27, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L. Heterogeneous cellular effects of α-ketoglutarate esters. Aging 2019, 11, 3412–3413. [Google Scholar] [CrossRef]
- Buddington, R.K.; Pajor, A.; Buddington, K.K.; Pierzynowski, S. Absorption of α-ketoglutarate by the gastrointestinal tract of pigs. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 138, 215–220. [Google Scholar] [CrossRef]
- Wolffram, S.; Hagemann, C.; Grenacher, B.; Scharrer, E. Characterization of the transport of tri- and dicarboxylates by pig intestinal brush-border membrane vesicles. Comp. Biochem. Physiol. Part A Physiol. 1992, 101, 759–767. [Google Scholar] [CrossRef]
- Qi, M.; Liao, S.; Wang, J.; Deng, Y.; Zha, A.; Shao, Y.; Cui, Z.; Song, T.; Tang, Y.; Tan, B.; et al. MyD88 deficiency ameliorates weight loss caused by intestinal oxidative injury in an autophagy-dependent mechanism. J. Cachexia Sarcopenia Muscle 2022, 13, 677–695. [Google Scholar] [CrossRef]
- Tang, Y.; Li, J.; Li, F.; Hu, C.-A.A.; Liao, P.; Tan, K.; Tan, B.; Xiong, X.; Liu, G.; Li, T.; et al. Autophagy protects intestinal epithelial Cells against Deoxynivalenol toxicity by alleviating oxidative stress via IKK signaling pathway. Free. Radic. Biol. Med. 2015, 89, 944–951. [Google Scholar] [CrossRef]
- Jiang, Q.; Tian, J.; Liu, G.; Yin, Y.; Yao, K. Endoplasmic Reticulum Stress and Unfolded Protein Response Pathways Involved in the Health-Promoting Effects of Allicin on the Jejunum. J. Agric. Food Chem. 2019, 67, 6019–6031. [Google Scholar] [CrossRef]
- Cherif, H.; Duhamel, F.; Cecyre, B.; Bouchard, A.; Quintal, A.; Chemtob, S.; Bouchard, J.-F. Receptors of intermediates of carbohydrate metabolism, GPR91 and GPR99, mediate axon growth. PLoS Biol. 2018, 16, e2003619. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, P.; Jiang, Q.; Cai, X.; Wang, T.; Peng, W.; Sun, J.; Zhu, C.; Zhang, C.; Yue, D.; et al. Exercise-induced α-ketoglutaric acid stimulates muscle hypertrophy and fat loss through OXGR1-dependent adrenal activation. EMBO J. 2020, 39, e103304. [Google Scholar] [CrossRef]
- Xu, C.; Yuan, Y.; Zhang, C.; Zhou, Y.; Yang, J.; Yi, H.; Gyawali, I.; Lu, J.; Guo, S.; Ji, Y.; et al. Smooth muscle AKG/OXGR1 signaling regulates epididymal fluid acid-base balance and sperm maturation. Life Metab. 2022, 1, 67–80. [Google Scholar] [CrossRef]
- Indo, Y.; Takeshita, S.; Ishii, K.-A.; Hoshii, T.; Aburatani, H.; Hirao, A.; Ikeda, K. Metabolic regulation of osteoclast differentiation and function. J. Bone Miner. Res. 2013, 28, 2392–2399. [Google Scholar] [CrossRef]
- Fan, J.; Jahed, V.; Klavins, K. Metabolomics in Bone Research. Metabolites 2021, 11, 434. [Google Scholar] [CrossRef]
- Dobrowolski, P.J.; Piersiak, T.; Surve, V.V.; Kruszewska, D.; Gawron, A.; Pacuska, P.; Håkanson, R.; Pierzynowski, S.G. Dietary α-ketoglutarate reduces gastrectomy-evoked loss of calvaria and trabecular bone in female rats. Scand. J. Gastroenterol. 2008, 43, 551–558. [Google Scholar] [CrossRef]
- Radzki, R.P.; Bienko, M.; Pierzynowski, S.G. Anti-osteopenic effect of alpha-ketoglutarate sodium salt in ovariectomized rats. J. Bone Miner. Metab. 2012, 30, 651–659. [Google Scholar] [CrossRef]
- Radzki, R.P.; Bieńko, M.; Filip, R.; Pierzynowski, S.G. The protective and therapeutic effect of exclusive and combined treatment with alpha-ketoglutarate sodium salt and ipriflavone on bone loss in orchidectomized rats. J. Nutr. Health Aging 2016, 20, 628–636. [Google Scholar] [CrossRef]
- Tian, Q.; Zhao, J.; Yang, Q.; Wang, B.; DeAvila, J.M.; Zhu, M.-J.; Du, M. Dietary alpha-ketoglutarate promotes beige adipogenesis and prevents obesity in middle-aged mice. Aging Cell 2020, 19, e13059. [Google Scholar] [CrossRef]
- He, W.; Miao, F.J.-P.; Lin, D.C.-H.; Schwandner, R.T.; Wang, Z.; Gao, J.; Chen, J.-L.; Tian, H.; Ling, L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 2004, 429, 188–193. [Google Scholar] [CrossRef]
- Wright, S.H.; Hirayama, B.; Kaunitz, J.D.; Results, T.; Support, T.F.; Wright, E.M. NaDC-1 (A-20): Sc-23539; Santa Cruz Biotechnoly, Inc.: Santa Cruz, CA, USA, 2015. [Google Scholar]
- Cummins, E.P.; Berra, E.; Comerford, K.M.; Ginouves, A.; Fitzgerald, K.T.; Seeballuck, F.; Godson, C.; Nielsen, J.E.; Moynagh, P.; Pouyssegur, J.; et al. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc. Natl. Acad. Sci. USA 2006, 103, 18154–18159. [Google Scholar] [CrossRef]
- Takeda, Y.; Costa, S.; Delamarre, E.; Roncal, C.; De Oliveira, R.L.; Squadrito, M.L.; Finisguerra, V.; Deschoemaeker, S.; Bruyère, F.; Wenes, M.; et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature 2011, 479, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.P.; Pierzynowski, S.G. Biological effects of 2-oxoglutarate with particular emphasis on the regulation of protein, mineral and lipid absorption/metabolism, muscle performance, kidney function, bone formation and cancerogenesis, all viewed from a healthy ageing perspective state of the art-review article. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008, 59, 91–106. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Bao, X.; Yang, F.; Tang, X.; Jiang, Q.; Li, Y.; Yao, K.; Yin, Y. Elevation of Intracellular Alpha-Ketoglutarate Levels Inhibits Osteoclastogenesis by Suppressing the NF-κB Signaling Pathway in a PHD1-Dependent Manner. Nutrients 2023, 15, 701. https://doi.org/10.3390/nu15030701
Tian J, Bao X, Yang F, Tang X, Jiang Q, Li Y, Yao K, Yin Y. Elevation of Intracellular Alpha-Ketoglutarate Levels Inhibits Osteoclastogenesis by Suppressing the NF-κB Signaling Pathway in a PHD1-Dependent Manner. Nutrients. 2023; 15(3):701. https://doi.org/10.3390/nu15030701
Chicago/Turabian StyleTian, Junquan, Xuetai Bao, Fan Yang, Xiongzhuo Tang, Qian Jiang, Yuying Li, Kang Yao, and Yulong Yin. 2023. "Elevation of Intracellular Alpha-Ketoglutarate Levels Inhibits Osteoclastogenesis by Suppressing the NF-κB Signaling Pathway in a PHD1-Dependent Manner" Nutrients 15, no. 3: 701. https://doi.org/10.3390/nu15030701
APA StyleTian, J., Bao, X., Yang, F., Tang, X., Jiang, Q., Li, Y., Yao, K., & Yin, Y. (2023). Elevation of Intracellular Alpha-Ketoglutarate Levels Inhibits Osteoclastogenesis by Suppressing the NF-κB Signaling Pathway in a PHD1-Dependent Manner. Nutrients, 15(3), 701. https://doi.org/10.3390/nu15030701






