Sugar-Sweetened Beverages and Metabolic Risk in Children and Adolescents with Obesity: A Narrative Review
Abstract
1. Introduction
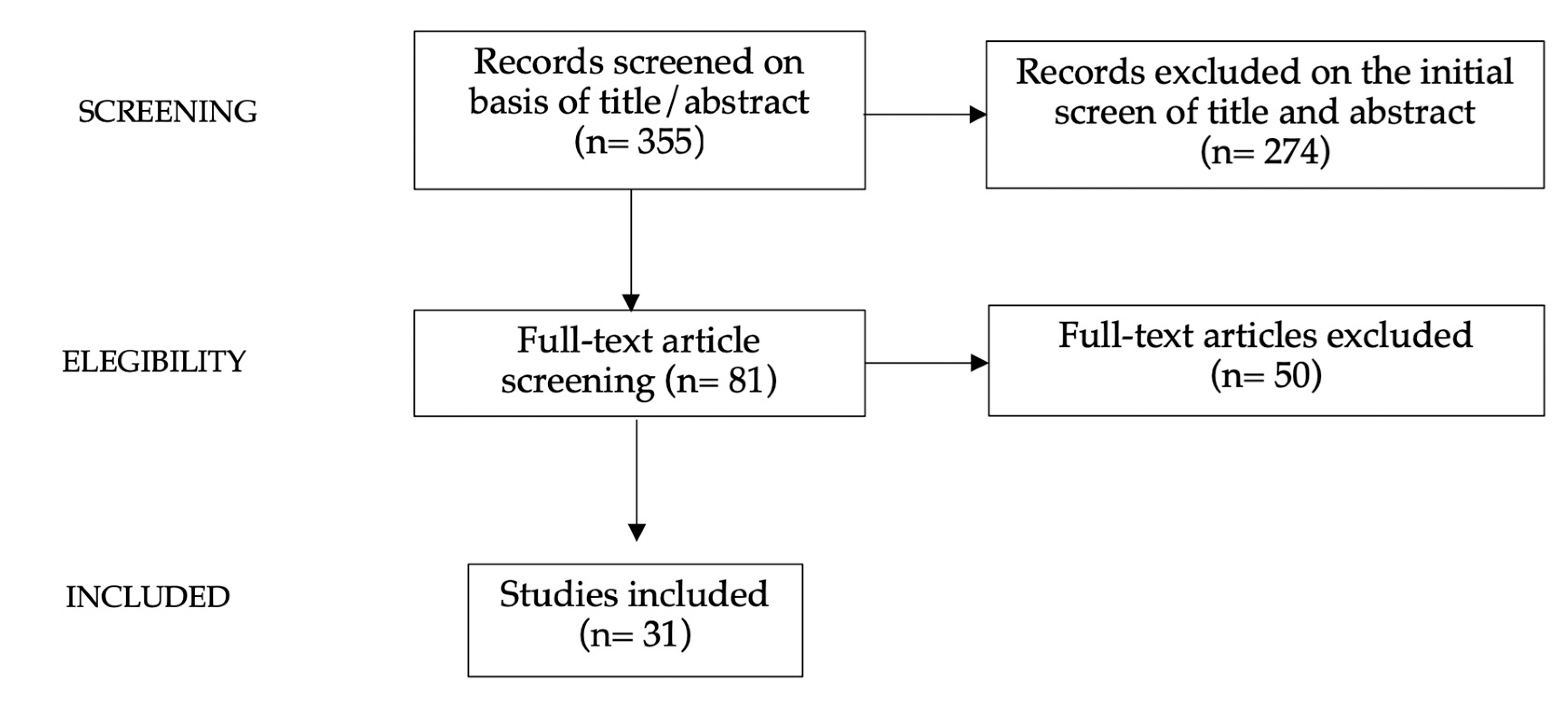
2. Methods
3. Results
4. Discussion of the Results
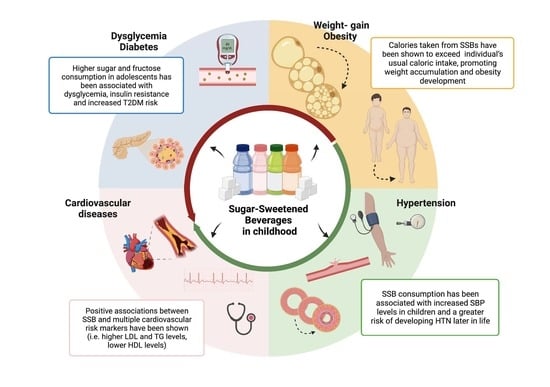
4.1. Sugar-Sweetened Beverage Consumption and Mechanism to Impact Health Outcomes
4.1.1. SSB Consumption and Associated Factors
4.1.2. SSBs and Metabolic Impact
4.1.3. SSBs and Impact on Gut Microbiota
4.1.4. SSBs and Dietary Behavior and Emotional Eating
4.2. Sugar-Sweetened Beverages, Weight Gain and Metabolic Complications
4.2.1. SSBs and Weight Gain
4.2.2. SSBs and Type 2 Diabetes Mellitus
4.2.3. SSBs, Hypertension, Cardiovascular Risk and Metabolic Syndrome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradwisch, S.A.; Smith, E.M.; Mooney, C.; Scaccia, D. Obesity in Children and Adolescents: An Overview. Nursing 2020, 50, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Wang, J.P.; Jiang, Y.Y.; Li, H.; Hu, Y.H.; Lee, K.O.; Li, G.W. Fasting Plasma Insulin at 5 Years of Age Predicted Subsequent Weight Increase in Early Childhood over a 5-Year Period—The Da Qing Children Cohort Study. PLoS ONE 2015, 10, e0127389. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. JAMA 2014, 311, 806. [Google Scholar] [CrossRef]
- Kelly, A.S.; Barlow, S.E.; Rao, G.; Inge, T.H.; Hayman, L.L.; Steinberger, J.; Urbina, E.M.; Ewing, L.J.; Daniels, S.R. Severe Obesity in Children and Adolescents: Identification, Associated Health Risks, and Treatment Approaches: A Scientific Statement From the American Heart Association. Circulation 2013, 128, 1689–1712. [Google Scholar] [CrossRef]
- Anderson, S.E.; Whitaker, R.C. Prevalence of Obesity Among US Preschool Children in Different Racial and Ethnic Groups. Arch. Pediatr. Adolesc. Med. 2009, 163, 344. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of Obesity and Trends in Body Mass Index Among US Children and Adolescents, 1999–2010. JAMA 2012, 307, 483. [Google Scholar] [CrossRef]
- Cena, H.; Fiechtner, L.; Vincenti, A.; Magenes, V.C.; De Giuseppe, R.; Manuelli, M.; Zuccotti, G.V.; Calcaterra, V. COVID-19 Pandemic as Risk Factors for Excessive Weight Gain in Pediatrics: The Role of Changes in Nutrition Behavior. A Narrative Review. Nutrients 2021, 13, 4255. [Google Scholar] [CrossRef]
- Greydanus, D.E.; Agana, M.; Kamboj, M.K.; Shebrain, S.; Soares, N.; Eke, R.; Patel, D.R. Pediatric Obesity: Current Concepts. Dis. Mon. 2018, 64, 98–156. [Google Scholar] [CrossRef]
- Stunkard, A.J. A Twin Study of Human Obesity. JAMA J. Am. Med. Assoc. 1986, 256, 51. [Google Scholar] [CrossRef]
- Cole, T.J. Establishing a Standard Definition for Child Overweight and Obesity Worldwide: International Survey. BMJ 2000, 320, 1240. [Google Scholar] [CrossRef] [PubMed]
- Neri, C.; Edlow, A.G. Effects of Maternal Obesity on Fetal Programming: Molecular Approaches. Cold Spring Harb. Perspect. Med. 2016, 6, a026591. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, F.; Montella, S.; Pietrobelli, A. Obesity and Pulmonary Disease: Unanswered Questions: Obesity-Related Pulmonary Disease. Obes. Rev. 2012, 13, 822–833. [Google Scholar] [CrossRef]
- Delgado, J.; Barranco, P.; Quirce, S. Obesity and Asthma. J. Investig. Allergol. Clin. Immunol. 2008, 18, 420–425. [Google Scholar]
- Verhulst, S.L.; Aerts, L.; Jacobs, S.; Schrauwen, N.; Haentjens, D.; Claes, R.; Vaerenberg, H.; Van Gaal, L.F.; De Backer, W.A.; Desager, K.N. Sleep-Disordered Breathing, Obesity, and Airway Inflammation in Children and Adolescents. Chest 2008, 134, 1169–1175. [Google Scholar] [CrossRef]
- Chou, C.H.; Kang, K.T.; Weng, W.C.; Lee, P.L.; Hsu, W.C. Central Sleep Apnea in Obese Children with Sleep-Disordered Breathing. Int. J. Obes. 2014, 38, 27–31. [Google Scholar] [CrossRef]
- Boxer, G.H.; Bauer, A.M.; Miller, B.D. Obesity-Hypoventilation in Childhood. J. Am. Acad. Child Adolesc. Psychiatry 1988, 27, 552–558. [Google Scholar] [CrossRef]
- Rosen, C.L. Clinical Features of Obstructive Sleep Apnea Hypoventilation Syndrome in Otherwise Healthy Children. Pediatr. Pulmonol. 1999, 27, 403–409. [Google Scholar] [CrossRef]
- Størdal, K.; Johannesdottir, G.B.; Bentsen, B.S.; Carlsen, K.C.L.; Sandvik, L. Asthma and Overweight Are Associated with Symptoms of Gastro-Oesophageal Reflux. Acta Paediatr. 2006, 95, 1197–1201. [Google Scholar] [CrossRef]
- Calcaterra, V.; Regalbuto, C.; Porri, D.; Pelizzo, G.; Mazzon, E.; Vinci, F.; Zuccotti, G.; Fabiano, V.; Cena, H. Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules 2020, 10, 1324. [Google Scholar] [CrossRef]
- Stevenson, S.B. Pseudotumor Cerebri: Yet Another Reason to Fight Obesity. J. Pediatr. Health Care 2008, 22, 40–43. [Google Scholar] [CrossRef]
- Paley, G.L.; Sheldon, C.A.; Burrows, E.K.; Chilutti, M.R.; Liu, G.T.; McCormack, S.E. Overweight and Obesity in Pediatric Secondary Pseudotumor Cerebri Syndrome. Am. J. Ophthalmol. 2015, 159, 344–352.e1. [Google Scholar] [CrossRef]
- Stiebel-Kalish, H.; Serov, I.; Sella, R.; Chodick, G.; Snir, M. Childhood Overweight or Obesity Increases the Risk of IIH Recurrence Fivefold. Int. J. Obes. 2014, 38, 1475–1477. [Google Scholar] [CrossRef]
- Friedman, D.I.; Liu, G.T.; Digre, K.B. Revised Diagnostic Criteria for the Pseudotumor Cerebri Syndrome in Adults and Children. Neurology 2013, 81, 1159–1165. [Google Scholar] [CrossRef]
- Anderson, S.E.; Cohen, P.; Naumova, E.N.; Jacques, P.F.; Must, A. Adolescent Obesity and Risk for Subsequent Major Depressive Disorder and Anxiety Disorder: Prospective Evidence. Psychosom. Med. 2007, 69, 740–747. [Google Scholar] [CrossRef]
- Roth, B.; Munsch, S.; Meyer, A.; Isler, E.; Schneider, S. The Association between Mothers’ Psychopathology, Childrens’ Competences and Psychological Well-Being in Obese Children. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2008, 13, 129–136. [Google Scholar] [CrossRef]
- Vander Wal, J.S.; Mitchell, E.R. Psychological Complications of Pediatric Obesity. Pediatr. Clin. North Am. 2011, 58, 1393–1401. [Google Scholar] [CrossRef]
- Yoshida, Y.; Simoes, E.J. Sugar-Sweetened Beverage, Obesity, and Type 2 Diabetes in Children and Adolescents: Policies, Taxation, and Programs. Curr. Diab. Rep. 2018, 18, 31. [Google Scholar] [CrossRef]
- Popkin, B.M. Patterns of Beverage Use across the Lifecycle. Physiol. Behav. 2010, 100, 4–9. [Google Scholar] [CrossRef]
- Gan, Q.; Xu, P.; Yang, T.; Cao, W.; Xu, J.; Li, L.; Pan, H.; Zhao, W.; Zhang, Q. Sugar-Sweetened Beverage Consumption Status and Its Association with Childhood Obesity among Chinese Children Aged 6–17 Years. Nutrients 2021, 13, 2211. [Google Scholar] [CrossRef]
- Roesler, A.; Rojas, N.; Falbe, J. Sugar-Sweetened Beverage Consumption, Perceptions, and Disparities in Children and Adolescents. J. Nutr. Educ. Behav. 2021, 53, 553–563. [Google Scholar] [CrossRef]
- Morgan, K.; Lowthian, E.; Hawkins, J.; Hallingberg, B.; Alhumud, M.; Roberts, C.; Murphy, S.; Moore, G. Sugar-Sweetened Beverage Consumption from 1998–2017: Findings from the Health Behaviour in School-Aged Children/School Health Research Network in Wales. PLoS ONE 2021, 16, e0248847. [Google Scholar] [CrossRef]
- Nielsen, S.J.; Popkin, B.M. Changes in Beverage Intake between 1977 and 2001. Am. J. Prev. Med. 2004, 27, 205–210. [Google Scholar] [CrossRef]
- World Health Organization Home Page. Available online: https://www.who.int/childgrowth/en/ (accessed on 15 September 2020).
- Malik, V.S.; Hu, F.B. The Role of Sugar-Sweetened Beverages in the Global Epidemics of Obesity and Chronic Diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- Sousa, A.; Sych, J.; Rohrmann, S.; Faeh, D. The Importance of Sweet Beverage Definitions When Targeting Health Policies—The Case of Switzerland. Nutrients 2020, 12, 1976. [Google Scholar] [CrossRef]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef]
- Vien, S.; Luhovyy, B.L.; Patel, B.P.; Panahi, S.; El Khoury, D.; Mollard, R.C.; Hamilton, J.K.; Anderson, G.H. Pre- and within-Meal Effects of Fluid Dairy Products on Appetite, Food Intake, Glycemia, and Regulatory Hormones in Children. Appl. Physiol. Nutr. Metab. 2017, 42, 302–310. [Google Scholar] [CrossRef]
- Maersk, M.; Belza, A.; Holst, J.J.; Fenger-Grøn, M.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Satiety Scores and Satiety Hormone Response after Sucrose-Sweetened Soft Drink Compared with Isocaloric Semi-Skimmed Milk and with Non-Caloric Soft Drink: A Controlled Trial. Eur. J. Clin. Nutr. 2012, 66, 523–529. [Google Scholar] [CrossRef]
- Ramne, S.; Brunkwall, L.; Ericson, U.; Gray, N.; Kuhnle, G.G.C.; Nilsson, P.M.; Orho-Melander, M.; Sonestedt, E. Gut Microbiota Composition in Relation to Intake of Added Sugar, Sugar-Sweetened Beverages and Artificially Sweetened Beverages in the Malmö Offspring Study. Eur. J. Nutr. 2021, 60, 2087–2097. [Google Scholar] [CrossRef]
- Yan, T.; Shi, L.; Xu, K.; Bai, J.; Wen, R.; Liao, X.; Dai, X.; Wu, Q.; Zeng, L.; Peng, W.; et al. Habitual Intakes of Sugar-Sweetened Beverages Associated with Gut Microbiota Related Metabolites and Metabolic Health Outcomes in Young Chinese Adults. Nutr. Metab. Cardiovasc. Dis. 2022, S0939475322004410. [Google Scholar] [CrossRef]
- Sweetman, C.; Wardle, J.; Cooke, L. Soft Drinks and “desire to Drink” in Preschoolers. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 60. [Google Scholar] [CrossRef]
- Elfhag, K.; Tholin, S.; Rasmussen, F. Consumption of Fruit, Vegetables, Sweets and Soft Drinks Are Associated with Psychological Dimensions of Eating Behaviour in Parents and Their 12-Year-Old Children. Public Health Nutr. 2008, 11, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, H.; Lindi, V.; Schwab, U.; Kiiskinen, S.; Venäläinen, T.; Karhunen, L.; Lakka, T.A.; Eloranta, A.M. Eating Behaviour Is Associated with Eating Frequency and Food Consumption in 6–8 Year-Old Children: The Physical Activity and Nutrition in Children (PANIC) Study. Appetite 2017, 114, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Rodenburg, G.; Kremers, S.P.J.; Oenema, A.; van de Mheen, D. Associations of Children’s Appetitive Traits with Weight and Dietary Behaviours in the Context of General Parenting. PLoS ONE 2012, 7, e50642. [Google Scholar] [CrossRef] [PubMed]
- Ariza, A.J. Risk Factors for Overweight in Five- to Six-Year-Old Hispanic-American Children: A Pilot Study. J. Urban Health Bull. N.Y. Acad. Med. 2004, 81, 150–161. [Google Scholar] [CrossRef]
- Grimes, C.A.; Riddell, L.J.; Campbell, K.J.; Nowson, C.A. Dietary Salt Intake, Sugar-Sweetened Beverage Consumption, and Obesity Risk. Pediatrics 2013, 131, 14–21. [Google Scholar] [CrossRef]
- Nicklas, T.A.; Yang, S.-J.; Baranowski, T.; Zakeri, I.; Berenson, G. Eating Patterns and Obesity in Children. Am. J. Prev. Med. 2003, 25, 9–16. [Google Scholar] [CrossRef]
- Gui, Z.-H.; Zhu, Y.-N.; Cai, L.; Sun, F.-H.; Ma, Y.-H.; Jing, J.; Chen, Y.-J. Sugar-Sweetened Beverage Consumption and Risks of Obesity and Hypertension in Chinese Children and Adolescents: A National Cross-Sectional Analysis. Nutrients 2017, 9, 1302. [Google Scholar] [CrossRef]
- Davis, J.N.; Koleilat, M.; Shearrer, G.E.; Whaley, S.E. Association of Infant Feeding and Dietary Intake on Obesity Prevalence in Low-Income Toddlers: Infant Feeding and Obesity in Hispanics. Obesity 2014, 22, 1103–1111. [Google Scholar] [CrossRef]
- Gallagher, C.; Moschonis, G.; Lambert, K.A.; Karaglani, E.; Mavrogianni, C.; Gavrili, S.; Manios, Y.; Erbas, B. Sugar-Sweetened Beverage Consumption Is Associated with Visceral Fat in Children. Br. J. Nutr. 2021, 125, 819–827. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.M.; Yang, S.-J.; Nicklas, T.A. Beverage Intake Among Preschool Children and Its Effect on Weight Status. Pediatrics 2006, 118, e1010–e1018. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.L.; Kirzner, J.; Pietrobelli, A.; St-Onge, M.-P.; Faith, M.S. Increased Sweetened Beverage Intake Is Associated with Reduced Milk and Calcium Intake in 3- to 7-Year-Old Children at Multi-Item Laboratory Lunches. J. Am. Diet. Assoc. 2009, 109, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.; Farmer, A.; Girard, M.; Peterson, K. Regular Sugar-Sweetened Beverage Consumption between Meals Increases Risk of Overweight among Preschool-Aged Children. J. Am. Diet. Assoc. 2007, 107, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary Sugars and Body Weight: Systematic Review and Meta-Analyses of Randomised Controlled Trials and Cohort Studies. BMJ 2012, 346, e7492. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, R.; Park, S.; Galuska, D.A.; Sherry, B.; Freedman, D.S. A Longitudinal Analysis of Sugar-Sweetened Beverage Intake in Infancy and Obesity at 6 Years. Pediatrics 2014, 134, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Rangan, A.; Olsen, N.J.; Bo Andersen, L.; Wedderkopp, N.; Kristensen, P.; Grøntved, A.; Ried-Larsen, M.; Lempert, S.M.; Allman-Farinelli, M.; et al. Sugar-Sweetened Beverages Consumption in Relation to Changes in Body Fatness over 6 and 12 Years among 9-Year-Old Children: The European Youth Heart Study. Eur. J. Clin. Nutr. 2014, 68, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Laurson, K.; Eisenmann, J.C.; Moore, S. Lack of Association between Television Viewing, Soft Drinks, Physical Activity and Body Mass Index in Children. Acta Paediatr. 2008, 97, 795–800. [Google Scholar] [CrossRef]
- Forshee, R.A.; Anderson, P.A.; Storey, M.L. Sugar-Sweetened Beverages and Body Mass Index in Children and Adolescents: A Meta-Analysis. Am. J. Clin. Nutr. 2008, 87, 1662–1671. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Feldman, H.A.; Chomitz, V.R.; Antonelli, T.A.; Gortmaker, S.L.; Osganian, S.K.; Ludwig, D.S. A Randomized Trial of Sugar-Sweetened Beverages and Adolescent Body Weight. N. Engl. J. Med. 2012, 367, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- de Ruyter, J.C.; Olthof, M.R.; Seidell, J.C.; Katan, M.B. A Trial of Sugar-Free or Sugar-Sweetened Beverages and Body Weight in Children. N. Engl. J. Med. 2012, 367, 1397–1406. [Google Scholar] [CrossRef]
- Malik, A.H.; Akram, Y.; Shetty, S.; Malik, S.S.; Yanchou Njike, V. Impact of Sugar-Sweetened Beverages on Blood Pressure. Am. J. Cardiol. 2014, 113, 1574–1580. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Nikniaz, L.; Khodarahmi, M. Sugar-Sweetened Beverages Increases the Risk of Hypertension among Children and Adolescence: A Systematic Review and Dose–Response Meta-Analysis. J. Transl. Med. 2020, 18, 344. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.K.; Bundy, V.; Kanto, W.; Davis, C.L.; Bernard, P.J.; Zhu, H.; Gutin, B.; Dong, Y. Greater Fructose Consumption Is Associated with Cardiometabolic Risk Markers and Visceral Adiposity in Adolescents. J. Nutr. 2012, 142, 251–257. [Google Scholar] [CrossRef]
- Welsh, J.A.; Sharma, A.; Cunningham, S.A.; Vos, M.B. Consumption of Added Sugars and Indicators of Cardiovascular Disease Risk Among US Adolescents. Circulation 2011, 123, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; He, Y.; Wang, Z.; He, X.; Zang, J.; Guo, C.; Jia, X.; Ren, Y.; Shan, C.; Sun, J.; et al. The Associations between Sugar-sweetened Beverage Intake and Cardiometabolic Risks in Chinese Children and Adolescents. Pediatr. Obes. 2020, 15, e12634. [Google Scholar] [CrossRef]
- Mirmiran, P.; Yuzbashian, E.; Asghari, G.; Hosseinpour-Niazi, S.; Azizi, F. Consumption of Sugar Sweetened Beverage Is Associated with Incidence of Metabolic Syndrome in Tehranian Children and Adolescents. Nutr. Metab. 2015, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, M.; Yang, C.; Zheng, H.; Zhu, Y. Association of Sugar-Sweetened Beverage Intake with Risk of Metabolic Syndrome among Children and Adolescents in Urban China. Public Health Nutr. 2020, 23, 2770–2780. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20210727-1 (accessed on 10 January 2023).
- Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweianhanes-overview/ (accessed on 10 January 2023).
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf (accessed on 10 January 2023).
- Beal, T.; Morris, S.S.; Tumilowicz, A. Global Patterns of Adolescent Fruit, Vegetable, Carbonated Soft Drink, and Fast-Food Consumption: A Meta-Analysis of Global School-Based Student Health Surveys. Food Nutr. Bull. 2019, 40, 444–459. [Google Scholar] [CrossRef]
- Della Corte, K.; Fife, J.; Gardner, A.; Murphy, B.L.; Kleis, L.; Della Corte, D.; Schwingshackl, L.; LeCheminant, J.D.; Buyken, A.E. World Trends in Sugar-Sweetened Beverage and Dietary Sugar Intakes in Children and Adolescents: A Systematic Review. Nutr. Rev. 2021, 79, 274–288. [Google Scholar] [CrossRef]
- Pettigrew, S.; Jongenelis, M.; Chapman, K.; Miller, C. Factors Influencing the Frequency of Children’s Consumption of Soft Drinks. Appetite 2015, 91, 393–398. [Google Scholar] [CrossRef]
- Khan, R.; Siraj, M.A.; Kheya, H.; Khalid, S.; Tabassum, M.; Zaman, S.B. Consumption of Sugar-Sweetened Beverages and Their Health Impact on Children. Discoveries 2021, 4, e17. [Google Scholar]
- Case, A.; Paxson, C. Parental Behavior And Child Health. Health Aff. 2002, 21, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Hebestreit, A.; Intemann, T.; Siani, A.; De Henauw, S.; Eiben, G.; Kourides, Y.; Kovacs, E.; Moreno, L.; Veidebaum, T.; Krogh, V.; et al. Dietary Patterns of European Children and Their Parents in Association with Family Food Environment: Results from the I.Family Study. Nutrients 2017, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lin, M.; Onufrak, S.; Li, R. Association of Sugar-Sweetened Beverage Intake during Infancy with Dental Caries in 6-Year-Olds. Clin. Nutr. Res. 2015, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, E. Why Socioeconomic Status Affects the Health of Children: A Psychosocial Perspective. Curr. Dir. Psychol. Sci. 2004, 13, 112–115. [Google Scholar] [CrossRef]
- Pabayo, R.; Spence, J.C.; Cutumisu, N.; Casey, L.; Storey, K. Sociodemographic, Behavioural and Environmental Correlates of Sweetened Beverage Consumption among Pre-School Children. Public Health Nutr. 2012, 15, 1338–1346. [Google Scholar] [CrossRef]
- Reid, M.; Hammersley, R.; Hill, A.J.; Skidmore, P. Long-Term Dietary Compensation for Added Sugar: Effects of Supplementary Sucrose Drinks over a 4-Week Period. Br. J. Nutr. 2007, 97, 193–203. [Google Scholar] [CrossRef]
- DellaValle, D.M.; Roe, L.S.; Rolls, B.J. Does the Consumption of Caloric and Non-Caloric Beverages with a Meal Affect Energy Intake? Appetite 2005, 44, 187–193. [Google Scholar] [CrossRef]
- Solomi, L.; Rees, G.A.; Redfern, K.M. The Acute Effects of the Non-Nutritive Sweeteners Aspartame and Acesulfame-K in UK Diet Cola on Glycaemic Response. Int. J. Food Sci. Nutr. 2019, 70, 894–900. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Ludwig, D.S. The Glycemic Index: Physiological Mechanisms Relating to Obesity, Diabetes, and Cardiovascular Disease. JAMA 2002, 287, 2414. [Google Scholar] [CrossRef]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.-P.; Willett, W.C.; Hu, F.B. Sugar-Sweetened Beverages and Risk of Metabolic Syndrome and Type 2 Diabetes. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose Metabolism and Metabolic Disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. Sugar-Sweetened Beverages and Cardiometabolic Health: An Update of the Evidence. Nutrients 2019, 11, 1840. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Medici, V.; Bremer, A.A.; Lee, V.; Lam, H.D.; Nunez, M.V.; Chen, G.X.; Keim, N.L.; Havel, P.J. A Dose-Response Study of Consuming High-Fructose Corn Syrup–Sweetened Beverages on Lipid/Lipoprotein Risk Factors for Cardiovascular Disease in Young Adults. Am. J. Clin. Nutr. 2015, 101, 1144–1154. [Google Scholar] [CrossRef]
- Choi, H.K.; Curhan, G. Soft Drinks, Fructose Consumption, and the Risk of Gout in Men: Prospective Cohort Study. BMJ 2008, 336, 309–312. [Google Scholar] [CrossRef]
- Choi, H.K.; Willett, W.; Curhan, G. Fructose-Rich Beverages and Risk of Gout in Women. JAMA 2010, 304, 2270. [Google Scholar] [CrossRef]
- Richette, P.; Bardin, T. Gout. Lancet 2010, 375, 318–328. [Google Scholar] [CrossRef]
- Nakagawa, T.; Tuttle, K.R.; Short, R.A.; Johnson, R.J. Hypothesis: Fructose-Induced Hyperuricemia as a Causal Mechanism for the Epidemic of the Metabolic Syndrome. Nat. Clin. Pract. Nephrol. 2005, 1, 80–86. [Google Scholar] [CrossRef]
- Di Rienzi, S.C.; Britton, R.A. Adaptation of the Gut Microbiota to Modern Dietary Sugars and Sweeteners. Adv. Nutr. 2020, 11, 616–629. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut Microbiota-Derived Metabolites as Central Regulators in Metabolic Disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut Microbial Metabolites as Multi-Kingdom Intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Lee, C.J.; Sears, C.L.; Maruthur, N. Gut Microbiome and Its Role in Obesity and Insulin Resistance. Ann. N.Y. Acad. Sci. 2020, 1461, 37–52. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Liu, B.-N.; Liu, X.-T.; Liang, Z.-H.; Wang, J.-H. Gut Microbiota in Obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Yu, Y.C.; Han, J.M.; Kim, S. Aminoacyl-TRNA Synthetases and Amino Acid Signaling. Biochim. Biophys. Acta BBA Mol. Cell Res. 2021, 1868, 118889. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W.L. Sugar Addiction: Is It Real? A Narrative Review. Br. J. Sport. Med. 2018, 52, 910–913. [Google Scholar] [CrossRef]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Evidence for Sugar Addiction: Behavioral and Neurochemical Effects of Intermittent, Excessive Sugar Intake. Neurosci. Biobehav. Rev. 2008, 32, 20–39. [Google Scholar] [CrossRef]
- Olszewski, P.K.; Wood, E.L.; Klockars, A.; Levine, A.S. Excessive Consumption of Sugar: An Insatiable Drive for Reward. Curr. Nutr. Rep. 2019, 8, 120–128. [Google Scholar] [CrossRef]
- Wang, Y.C.; Bleich, S.N.; Gortmaker, S.L. Increasing Caloric Contribution From Sugar-Sweetened Beverages and 100% Fruit Juices Among US Children and Adolescents, 1988–2004. Pediatrics 2008, 121, e1604–e1614. [Google Scholar] [CrossRef]
- Bleich, S.N.; Wolfson, J.A. Trends in SSBs and Snack Consumption among Children by Age, Body Weight, and Race/Ethnicity: Snacking Among SSB and Non-SSB Drinkers. Obesity 2015, 23, 1039–1046. [Google Scholar] [CrossRef]
- Beck, A.L.; Martinez, S.; Patel, A.I.; Fernandez, A. Trends in Sugar-Sweetened Beverage Consumption among California Children. Public Health Nutr. 2020, 23, 2864–2869. [Google Scholar] [CrossRef]
- Dai, J.; Soto, M.J.; Dunn, C.G.; Bleich, S.N. Trends and Patterns in Sugar-Sweetened Beverage Consumption among Children and Adults by Race and/or Ethnicity, 2003–2018. Public Health Nutr. 2021, 24, 2405–2410. [Google Scholar] [CrossRef]
- Tasevska, N.; DeLia, D.; Lorts, C.; Yedidia, M.; Ohri-Vachaspati, P. Determinants of Sugar-Sweetened Beverage Consumption among Low-Income Children: Are There Differences by Race/Ethnicity, Age, and Sex? J. Acad. Nutr. Diet. 2017, 117, 1900–1920. [Google Scholar] [CrossRef]
- Scharf, R.J.; DeBoer, M.D. Sugar-Sweetened Beverages and Children’s Health. Annu. Rev. Public Health 2016, 37, 273–293. [Google Scholar] [CrossRef]
- Schulze, M.B.; Liu, S.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic Index, Glycemic Load, and Dietary Fiber Intake and Incidence of Type 2 Diabetes in Younger and Middle-Aged Women. Am. J. Clin. Nutr. 2004, 80, 348–356. [Google Scholar] [CrossRef]
- Abbasi, A.; Juszczyk, D.; van Jaarsveld, C.H.M.; Gulliford, M.C. Body Mass Index and Incident Type 1 and Type 2 Diabetes in Children and Young Adults: A Retrospective Cohort Study. J. Endocr. Soc. 2017, 1, 524–537. [Google Scholar] [CrossRef]
- SACN, Scientific Advisory Committee on Nutrition Carbohydrates and Health. 2015. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/445503/SACN_Carbohydrates_and_Health.pdf (accessed on 6 January 2023).
- Fidler Mis, N.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; et al. Sugar in Infants, Children and Adolescents: A Position Paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 681–696. [Google Scholar] [CrossRef]
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.E.L.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Association between Sugar-Sweetened and Artificially Sweetened Soft Drinks and Type 2 Diabetes: Systematic Review and Dose–Response Meta-Analysis of Prospective Studies. Br. J. Nutr. 2014, 112, 725–734. [Google Scholar] [CrossRef]
- Ambrosini, G.L.; Oddy, W.H.; Huang, R.C.; Mori, T.A.; Beilin, L.J.; Jebb, S.A. Prospective Associations between Sugar-Sweetened Beverage Intakes and Cardiometabolic Risk Factors in Adolescents. Am. J. Clin. Nutr. 2013, 98, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Xu, F.; Ye, Q.; Zhou, H.; Li, C.; He, J.; Wang, Z.; Hong, X.; Hou, X. Sugar-Sweetened Beverages and School Students’ Hypertension in Urban Areas of Nanjing, China. J. Hum. Hypertens. 2018, 32, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.d.S.N.; Cunha, D.B.; Pereira, R.A.; Sichieri, R. Soft Drink Consumption, Mainly Diet Ones, Is Associated with Increased Blood Pressure in Adolescents. J. Hypertens. 2016, 34, 221–225. [Google Scholar] [CrossRef]
- Nguyen, S.; Choi, H.K.; Lustig, R.H.; Hsu, C. Sugar-Sweetened Beverages, Serum Uric Acid, and Blood Pressure in Adolescents. J. Pediatr. 2009, 154, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Sun, W.; Chen, Y.; Jiang, Y.; Song, Y.; Lin, Q.; Zhu, L.; Zhu, Q.; Wang, X.; et al. Investigating the Relationship between Precocious Puberty and Obesity: A Cross-Sectional Study in Shanghai, China. BMJ Open 2017, 7, e014004. [Google Scholar] [CrossRef]
- Rupérez, A.I.; Mesana, M.I.; Moreno, L.A. Dietary Sugars, Metabolic Effects and Child Health. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 206–216. [Google Scholar] [CrossRef]
- Cook, S.; Weitzman, M.; Auinger, P.; Nguyen, M.; Dietz, W.H. Prevalence of a Metabolic Syndrome Phenotype in Adolescents: Findings From the Third National Health and Nutrition Examination Survey, 1988–1994. Arch. Pediatr. Adolesc. Med. 2003, 157, 821. [Google Scholar] [CrossRef]
| Authors | Journal/Year | Study Design | Population Involved (Sample Size and Age) | Type of SSBs Studied | Main Results |
|---|---|---|---|---|---|
| Vien et al. [38] | Appl. Physiol. Nutr. Metab. 2017 | Interventional randomized controlled trial (two experiments) | 32 children, 6–14 years old (experiment 1) 20 children, 6–14 years old (experiment 2) | Fruit punch | SSB consumption has negative effects on food intake, appetite and satiety hormones compared to other beverages tested (dairy products) |
| Maersk et al. [39] | Eur. J. Clin. Nutr. 2012 | Cross-over | 24 subjects, 20–50 years old | Sucrose-sweetened regular cola | Total energy intake (ad libitum energy intake plus energy intake from the drink) was higher after the energy-containing drinks compared with diet cola and water (p < 0.01). SSBs induced lower subjective fullness and higher hunger than other beverages tested (still water and diet cola). |
| Ramne et al. [40] | Eur. J. Nutr. 2021 | Cross-sectional | 1371 Swedish participants, 18–70 years old | Any type | Negative association between SSB intake and Lachnobacterium. Positive association between SSB intake and Firmicutes/Bacteroidetes ratio after adjusting for both BMI and fiber intake. |
| Yan et al. [41] | Nutr. Metab. Cardiovasc. Dis. 2022 | Cross-sectional | 86 young Chinese adults, age = 23 ± 4 for men and age = 22 ± 3 for women | Carbonated beverages, fruit juice, lactobacillus drinks, energy drinks, tea drinks, bubble tea, pre-sweetened coffee, milk, yogurt and other dairy drinks. | Carbonated beverages, fruit juice, energy drinks, and bubble tea exhibited positive associations with obesity-related markers and blood lipids. |
| Sweetman et al. [42] | Int. J. Behav. Nutr. Phys. Act. 2008 | Cross-sectional | 346 same-sex twin English preschoolers, 9–12 years old | Sweetened soft drinks, fruit juice, fruit squash | Higher scores on the Desire to Drink subscale, measured through the Children’s Eating Behaviour Questionnaire, were associated with higher preferences and greater frequency of SSB consumption. |
| Elfhag et al. [43] | Public Health Nutr. 2008 | Cross-sectional | 1441 children, approximately 12 years old | Soft drinks | Lower SSB consumption among children with higher response to internal satiety cues. |
| Jalkanen et al. [44] | Appetite 2017 | Cross-sectional | 406 children, 6–8 years old | Carbonated and non-carbonated beverages | No association between higher score in Desire to Drink subscale and higher consumption of SSBs. |
| Rodenburg et al. [45] | PLoS ONE 2012 | Cross-sectional | 1275 children from the IMPACT study in 2009–2010, 9 years in 2009 | Any type | No consistent associations were found between children’s appetitive traits and SSB intake. |
| Ariza et al. [46] | J. Urban Health Bull. N.Y. Acad. Med. 2004 | Cross-sectional | 250 kindergarten students, 5–6 years old | Any type (excluding fruit juice) | Children who regularly consumed SSBs had an odds ratio (OR) of obesity of 3.7. |
| Grimes et al. [47] | Pediatrics 2013 | Cross-sectional | 4283 Australian children and adolescents, 5–6 years old | Any type | Children drinking SSBs 26% more likely to be overweight/obese (OR 1.26, 95% CI: 1.03–1.53). |
| Nicklas et al. [48] | Am. J. Prev. Med. 2003 | Cross-sectional | 562 children aged 10 years (Bogalusa Heart Study) | Soft drinks, fruit-flavored drinks, tea and coffee | OR for overweight of 1.33 for SSB consumers compared to non-SSB-drinkers. |
| Gui et al. [49] | Nutrients 2017 | Cross-sectional | 53,151 children and adolescents aged 6–17 years | Coca-Cola, Sprite, orange juice, Nutrition Express, and Red Bull | SSB consumers among the participants had a higher OR (1.133, 95%) than non-consumers for abdominal obesity. |
| Davis et al. [50] | Obesity 2014 | Cross-sectional | 2295 low-income children 2–4 years old | Soda and fruit drinks | High intake of SSBs was linked to increases in obesity prevalence; on the other hand, no SSB intake was associated with a 28% reduction in prevalence of obesity. |
| Gallagher et al. [51] | Br. J. Nutr. 2021 | Cross-sectional | 2665 Greek school children aged 9–13 years | Any type | Positive association between high SSB consumption and visceral adipose tissue (p = 0,01). |
| O’Connor et al. [52] | Pediatrics 2006 | Cross-sectional | 1160 children aged 2–5 years from the 1999–2003 NHANES cohort | Any type (including 100% fruit juice) | No higher BMI in children drinking >12 ounces daily of SSBs compared to non-drinkers. |
| Keller et al. [53] | Am. Diet. Assoc. 2009 | Cross-sectional | 126 children, 3–7 years old | Any type | No difference in BMI according to SSB intake. |
| Dubois et al. [54] | J. Am. Diet. Assoc. 2007 | Longitudinal | 1944 children, born in 1998 | Carbonated drinks and fruit flavored drinks (excluding 100% fruit juice) | Children who drank SSBs between the ages of 2.5–4.5 years had an OR of 2.4 for being overweight at age 4.5 years compared to non-drinkers. |
| Te Morenga et al. [55] | BMJ 2012 | Meta-analysis | 22 randomized controlled trials and cohort studies among children and adults | Any type (including fruit juices) | OR of 1.55 for higher levels of BMI among SSB drinkers over time. |
| Pan et al. [56] | Pediatrics 2014 | Longitudinal | 1189 children involved in the Infant Feeding Practices Study II and followed up at 6 years | Juice drinks, soft drinks, soda, sweet tea, Kool-Aid etc. | Odds for obesity detected were 71% higher for any SSB intake and 92% higher for SSB introduction before age 6 months. |
| Zheng et al. [57] | Eur. J. Clin. Nutr. 2014 | Longitudinal | 283 Danish children aged 9 years followed up at 6 years and 12 years | Soft drinks, fruit drinks and cordials sweetened with caloric sweeteners (excluding 100% fruit juice, flavored milk, coffee and tea) | Subjects who consumed more than 1 serving of an SSB per day at age 15 years had higher BMI and waist circumference than non-drinkers in the subsequent 6 years. Moreover, children who increased SSB intake from age 9 to 15 years had larger increases in BMI and waist circumference from 15–21 years compared to those with no increase in SSB intake. |
| Laurson et al. [58] | Acta Paediatr. 2008 | Longitudinal | 268 children (age at entry 10 years) studied over an 18-month period | Soft drinks | No association between SSB consumption and weight gain. |
| Forshee et al. [59] | Am. J. Clin. Nutr. 2008 | Meta-analysis | 12 longitudinal studies among children ages 2–19 years old | Any type | No association between SSB consumption and weight gain. |
| Ebbeling et al. [60] | N. Engl. J. Med. 2012 | Interventional randomized controlled trial | 224 adolescents followed up at 1 year | Any type (including 100% fruit juices) | Adolescents who consumed non-caloric beverages gained less weight (−1.9 kg, p = 0.04) and had lower BMI (−0.57, p = 0.045) compared to those drinking conventional SSBs. |
| De Ruyter et al. [61] | N. Engl. J. Med. 2012 | Interventional randomized controlled trial | 641 children, 4–11 years old | Any type | Children randomized to have non-caloric drinks (versus similar SSBs in a blinded fashion) by 18 months gained less weight, had lower BMI z-scores, smaller waist circumferences and lower fat percentages. |
| Malik et al. [62] | Am. J. Cardiol. 2014 | Meta-analysis | 12 studies with 409,707 participants (children >12 years and adults) | Any beverage with a caloric sweetener added | Subjects in the highest quantile of SSB intake (1–2 servings/day) had a 26% greater risk of developing T2DM with respect to individuals in the lowest quantile (none or <1 serving/month). |
| Farhangi et al. [63] | J. Transl. Med. 2020 | Systematic review and meta-analysis | 14 studies with 93,873 participants (children and adolescents <19 years) | Any type | High SSB consumption was associated with a 1.67 mmHg increase in systolic BP in children and adolescents but not with a significant rise in diastolic BP. High SSB consumers were moreover 1.36 times more likely to develop HTN compared with low SSB consumers. |
| Pollock et al. [64] | J. Nutr. 2012 | Cross-sectional | 559 US adolescents, 14–18 years old | Any type (including 100% fruit juices) | Higher total fructose consumption was positively associated with risk for cardiovascular disease and T2DM. |
| Welsh et al. [65] | Circulation 2011 | Cross-sectional | 2157 US adolescents | Any type | Positive association between consumption of added sugars with multiple cardiovascular risk markers (high LDL and TG and low HDL levels, (p trend = 0.001–0.01). |
| Zhu et al. [66] | Pediatr. Obes. 2020 | Cross-sectional | 3958 children and adolescents, 6–17 years old | Any type (excluding fruit juices) | SSB intake was positively correlated with serum total cholesterol and LDL and participants in the highest intake category of SSB consumption had higher total cholesterol and higher LDL levels than the non-consumption group. Moreover, a positive association between consumption of added sugars and BMI (p = 0.04) was shown. |
| Mirmiran et al. [67] | Nutr. Metab. 2015 | Longitudinal study | 424 children and adolescents, 6–18 years old | Sweetened carbonated soft drinks and fruit juice drinks | Compared to the first quartile of SSB intake, the OR of incident MetS in the highest quartile was 3.20; the OR for abdominal obesity was 2.49 and 2.79 for HTN. |
| Li et al. [68] | Public Health Nutr. 2020 | Cross-sectional | 7143 children and adolescents, 7–18 years old | Energy drinks, sports drinks, soda drinks, fruit drinks with added sugar, sweetened tea and coffee drinks | Subjects with high SSB intake were at higher risk of MetS (OR = 1.60) and abdominal obesity (OR = 1.55) compared with their participants with no SSB intake. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Cena, H.; Magenes, V.C.; Vincenti, A.; Comola, G.; Beretta, A.; Di Napoli, I.; Zuccotti, G. Sugar-Sweetened Beverages and Metabolic Risk in Children and Adolescents with Obesity: A Narrative Review. Nutrients 2023, 15, 702. https://doi.org/10.3390/nu15030702
Calcaterra V, Cena H, Magenes VC, Vincenti A, Comola G, Beretta A, Di Napoli I, Zuccotti G. Sugar-Sweetened Beverages and Metabolic Risk in Children and Adolescents with Obesity: A Narrative Review. Nutrients. 2023; 15(3):702. https://doi.org/10.3390/nu15030702
Chicago/Turabian StyleCalcaterra, Valeria, Hellas Cena, Vittoria Carlotta Magenes, Alessandra Vincenti, Giulia Comola, Alice Beretta, Ilaria Di Napoli, and Gianvincenzo Zuccotti. 2023. "Sugar-Sweetened Beverages and Metabolic Risk in Children and Adolescents with Obesity: A Narrative Review" Nutrients 15, no. 3: 702. https://doi.org/10.3390/nu15030702
APA StyleCalcaterra, V., Cena, H., Magenes, V. C., Vincenti, A., Comola, G., Beretta, A., Di Napoli, I., & Zuccotti, G. (2023). Sugar-Sweetened Beverages and Metabolic Risk in Children and Adolescents with Obesity: A Narrative Review. Nutrients, 15(3), 702. https://doi.org/10.3390/nu15030702