n-3 PUFA-Enriched Diet Preserves Skeletal Muscle Mitochondrial Function and Redox State and Prevents Muscle Mass Loss in Mice with Chronic Heart Failure
Abstract
1. Introduction
2. Methods
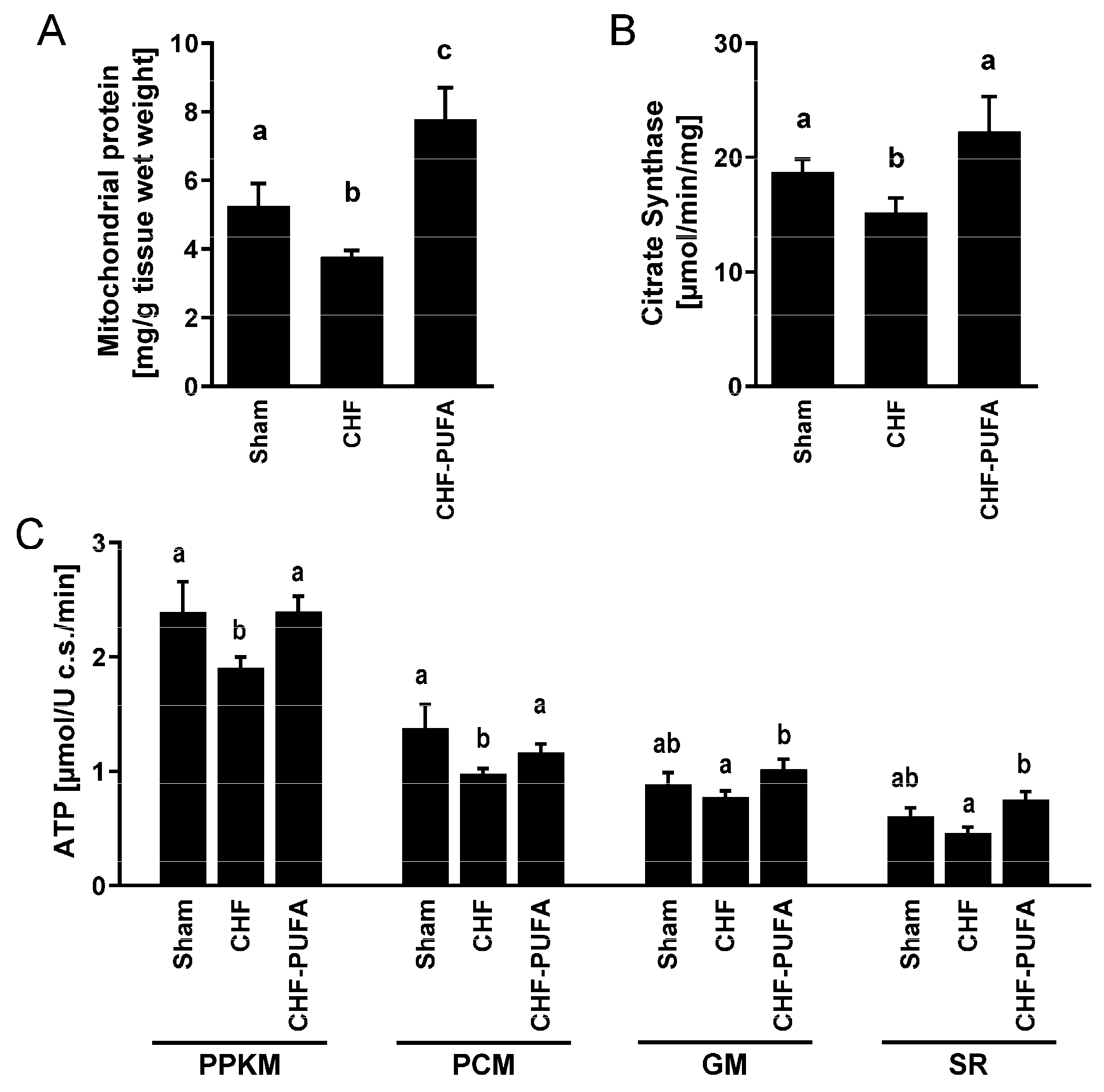
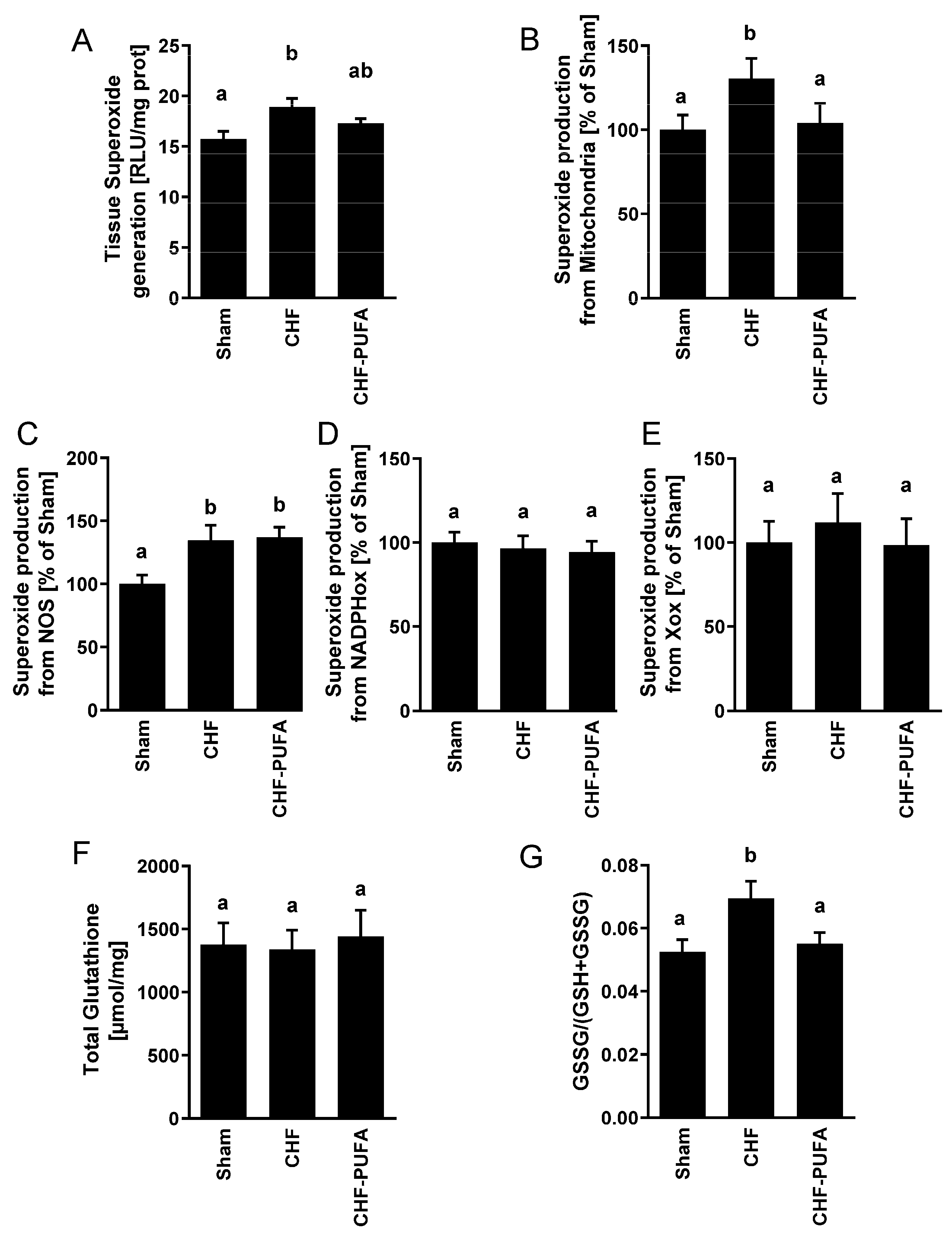
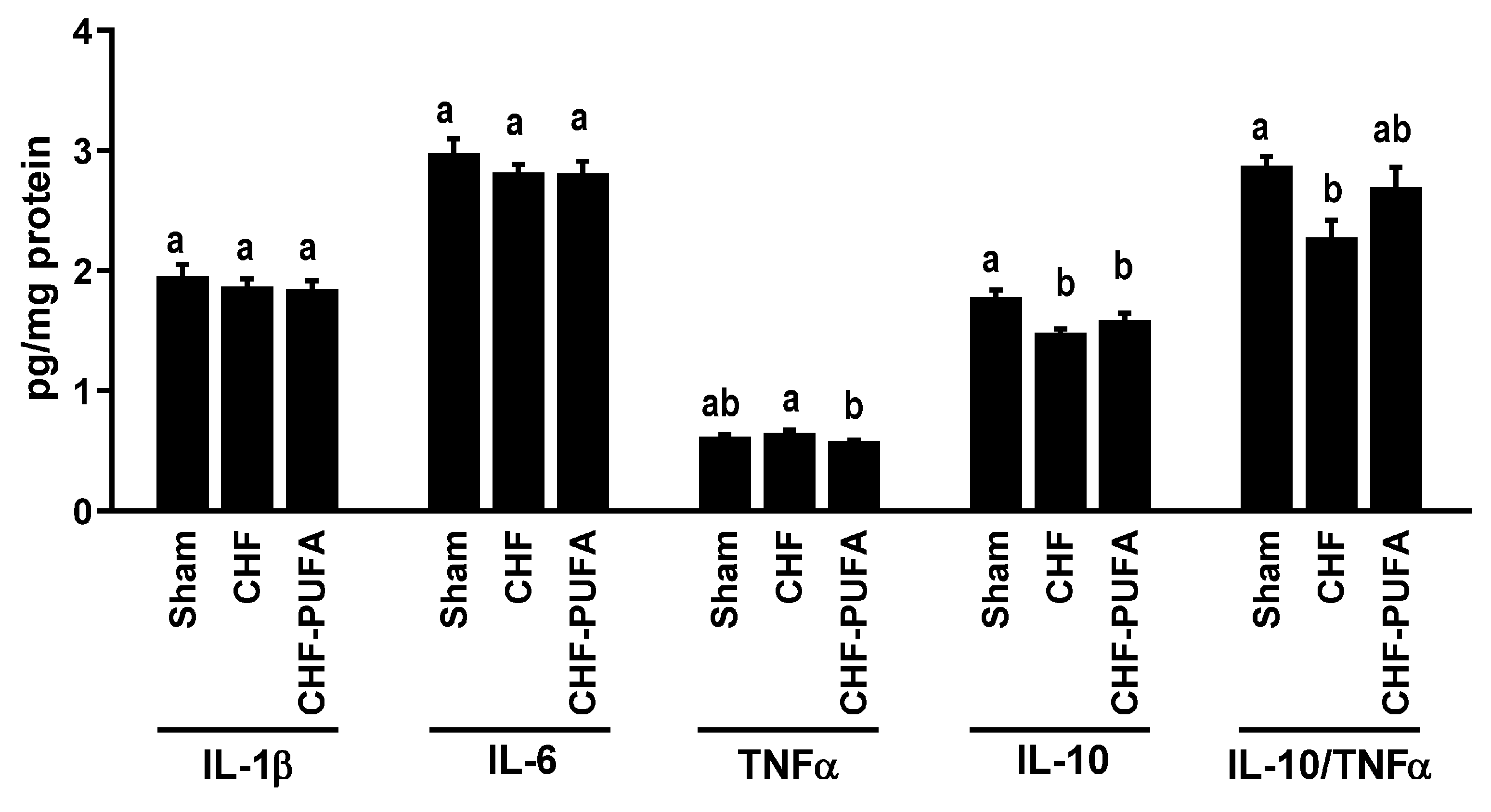
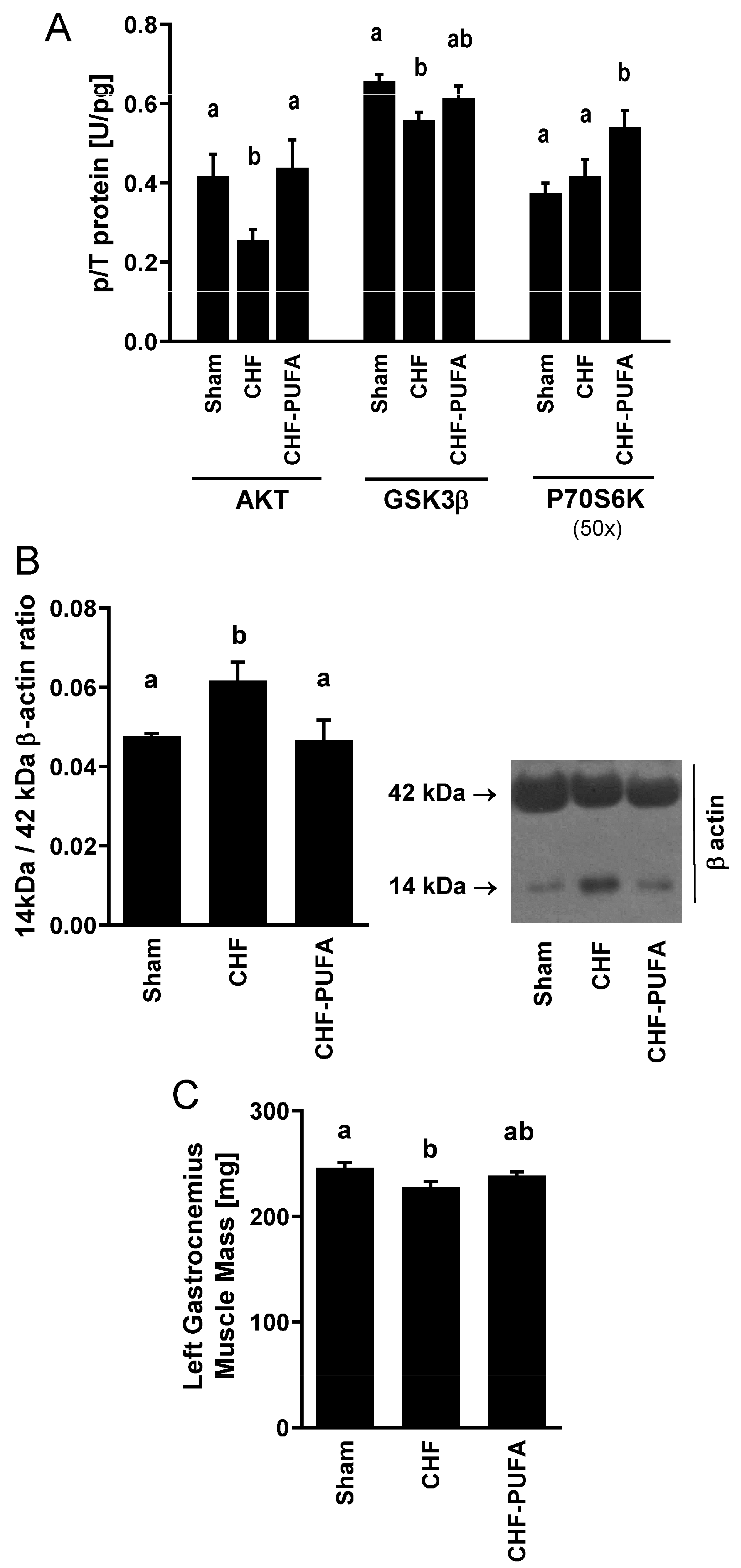
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anker, S.D.; Sharma, R. The syndrome of cardiac cachexia. Int. J. Cardiol. 2002, 85, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Lu, X.; Qian, Z.; Xu, W.; Zhou, X. New insights into the pathogenesis and treatment of sarcopenia in chronic heart failure. Theranostics 2019, 9, 4019–4029. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Billingsley, H.E.; Rodriguez-Miguelez, P.; Kirkman, D.L.; Garten, R.; Franco, R.L.; Lee, D.-C.; Lavie, C.J. Lean Mass Abnormalities in Heart Failure: The Role of Sarcopenia, Sarcopenic Obesity, and Cachexia. Curr. Probl. Cardiol. 2019, 45, 100417. [Google Scholar] [CrossRef] [PubMed]
- Fulster, S.; Tacke, M.; Sandek, A.; Ebner, N.; Tschope, C.; Doehner, W.; Anker, S.D.; von Haehling, S. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur. Heart J. 2013, 34, 512–519. [Google Scholar] [CrossRef]
- Emami, A.; Saitoh, M.; Valentova, M.; Sandek, A.; Evertz, R.; Ebner, N.; Loncar, G.; Springer, J.; Doehner, W.; Lainscak, M.; et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: Results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Eur. J. Heart Fail. 2018, 20, 1580–1587. [Google Scholar] [CrossRef]
- von Haehling, S. Muscle wasting and sarcopenia in heart failure: A brief overview of the current literature. ESC Heart Fail. 2018, 5, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.; O’Doherty, A.F.; Taylor, C.; Clark, A.L.; Carroll, S.; Ingle, L. Low skeletal muscle mass is associated with low aerobic capacity and increased mortality risk in patients with coronary heart disease—A CARE CR study. Clin. Physiol. Funct. Imaging 2019, 39, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Amiya, E.; Hatano, M.; Nitta, D.; Maki, H.; Bujo, C.; Saito, A.; Hosoya, Y.; Minatsuki, S.; Hara, T.; et al. Abdominal skeletal muscle mass as a predictor of mortality in Japanese patients undergoing left ventricular assist device implantation. ESC Heart Fail. 2019, 6, 526–535. [Google Scholar] [CrossRef]
- Seiler, M.; Bowen, T.S.; Rolim, N.; Dieterlen, M.-T.; Werner, S.; Hoshi, T.; Fischer, T.; Mangner, N.; Linke, A.; Schuler, G.; et al. Skeletal Muscle Alterations Are Exacerbated in Heart Failure with Reduced Compared with Preserved Ejection Fraction: Mediated by Circulating Cytokines? Circ. Heart Fail. 2016, 9, e003027. [Google Scholar] [CrossRef]
- Barazzoni, R. Skeletal muscle mitochondrial protein metabolism and function in ageing and type 2 diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 97–102. [Google Scholar] [CrossRef]
- Garnham, J.O.; Roberts, L.D.; Espino-Gonzalez, E.; Whitehead, A.; Swoboda, P.P.; Koshy, A.; Gierula, J.; Paton, M.F.; Cubbon, R.M.; Kearney, M.T.; et al. Chronic heart failure with diabetes mellitus is characterized by a severe skeletal muscle pathology. J. Cachexia Sarcopenia Muscle 2019, 11, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, G.G.; Semolic, A.; Ruozi, G.; Vinci, P.; Guarnieri, G.; Bortolotti, F.; Barbetta, D.; Zanetti, M.; Giacca, M.; Barazzoni, R. Unacylated ghrelin normalizes skeletal muscle oxidative stress and prevents muscle catabolism by enhancing tissue mitophagy in experimental chronic kidney disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 5159–5171. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Kinugawa, S.; Matsushima, S.; Ono, T.; Sobirin, M.A.; Inoue, N.; Yokota, T.; Hirabayashi, K.; Tsutsui, H. Oxidative stress impairs insulin signal in skeletal muscle and causes insulin resistance in postinfarct heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1637–H1644. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, G.G.; Aleksova, A.; Ferro, M.D.; Cannatà, A.; Semolic, A.; Zanetti, M.; Springer, J.; Anker, S.D.; Giacca, M.; Sinagra, G.; et al. Preserved Skeletal Muscle Mitochondrial Function, Redox State, Inflammation and Mass in Obese Mice with Chronic Heart Failure. Nutrients 2020, 12, 3393. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Cappellari, G.G.; Palus, S.; Vinci, P.; Ruozi, G.; Zanetti, M.; Semolic, A.; Ebner, N.; Von Haehling, S.; Sinagra, G.; et al. Acylated ghrelin treatment normalizes skeletal muscle mitochondrial oxidative capacity and AKT phosphorylation in rat chronic heart failure. J. Cachexia Sarcopenia Muscle 2017, 8, 991–998. [Google Scholar] [CrossRef]
- Liu, S.Z.; Marcinek, D.J. Skeletal muscle bioenergetics in aging and heart failure. Heart Fail. Rev. 2017, 22, 167–178. [Google Scholar] [CrossRef]
- Bowen, T.S.; Rolim, N.P.L.; Fischer, T.; Baekkerud, F.H.; Medeiros, A.; Werner, S.; Bronstad, E.; Rognmo, O.; Mangner, N.; Linke, A.; et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur. J. Heart Fail. 2015, 17, 263–272. [Google Scholar] [CrossRef]
- Lena, A.; Coats, A.J.S.; Anker, M.S. Metabolic disorders in heart failure and cancer. ESC Heart Fail. 2018, 5, 1092–1098. [Google Scholar] [CrossRef]
- Cappellari, G.G.; Semolic, A.; Ruozi, G.; Barbetta, D.; Bortolotti, F.; Vinci, P.; Zanetti, M.; Mak, R.H.; Garibotto, G.; Giacca, M.; et al. n-3 PUFA dietary lipid replacement normalizes muscle mitochondrial function and oxidative stress through enhanced tissue mitophagy and protects from muscle wasting in experimental kidney disease. Metab. Clin. Exp. 2022, 133, 155242. [Google Scholar] [CrossRef]
- Gamboa, J.L.; Roshanravan, B.; Towse, T.; Keller, C.A.; Falck, A.M.; Yu, C.; Frontera, W.R.; Brown, N.J.; Ikizler, T.A. Skeletal Muscle Mitochondrial Dysfunction Is Present in Patients with CKD before Initiation of Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. CJASN 2020, 15, 926–936. [Google Scholar] [CrossRef]
- Lv, J.; Li, Y.; Shi, S.; Xu, X.; Wu, H.; Zhang, B.; Song, Q. Skeletal muscle mitochondrial remodeling in heart failure: An update on mechanisms and therapeutic opportunities. Biomed. Pharmacother.=Biomed. Pharmacother. 2022, 155, 113833. [Google Scholar] [CrossRef] [PubMed]
- Diao, R.Y.; Gustafsson, A.B. Mitochondrial quality surveillance: Mitophagy in cardiovascular health and disease. Am. J. Physiol. Cell Physiol. 2022, 322, C218–C230. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, Y.; Kanki, T.; Aoki, Y.; Hirota, Y.; Saigusa, T.; Uchiumi, T.; Kang, D. Mitophagy plays an essential role in reducing mitochondrial production of reactive oxygen species and mutation of mitochondrial DNA by maintaining mitochondrial quantity and quality in yeast. J. Biol. Chem. 2012, 287, 3265–3272. [Google Scholar] [CrossRef] [PubMed]
- Lanza, I.R.; Blachnio-Zabielska, A.; Johnson, M.L.; Schimke, J.M.; Jakaitis, D.R.; Lebrasseur, N.K.; Jensen, M.D.; Sreekumaran Nair, K.; Zabielski, P. Influence of fish oil on skeletal muscle mitochondrial energetics and lipid metabolites during high-fat diet. Am. J. Physiology. Endocrinol. Metab. 2013, 304, E1391–E1403. [Google Scholar] [CrossRef] [PubMed]
- Lipina, C.; Hundal, H.S. Lipid modulation of skeletal muscle mass and function. J. Cachexia Sarcopenia Muscle 2017, 8, 190–201. [Google Scholar] [CrossRef]
- Nisr, R.B.; Shah, D.S.; Hundal, H.S. Mono- and Polyunsaturated Fatty Acids Counter Palmitate-Induced Mitochondrial Dysfunction in Rat Skeletal Muscle Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2020, 54, 975–993. [Google Scholar] [CrossRef]
- Garnier, A.; Fortin, D.; Zoll, J.; N’Guessan, B.; Mettauer, B.; Lampert, E.; Veksler, V.; Ventura-Clapier, R. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 43–52. [Google Scholar] [CrossRef]
- Mayyas, F.; Jaradat, R.; Alzoubi, K.H. Cardiac effects of fish oil in a rat model of streptozotocin-induced diabetes. Nutr. Metab. Cardiovasc. Dis. NMCD 2018, 28, 592–599. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Nabavi, S.F.; Nabavi, S.M.; Jardim, F.R. Omega-3 polyunsaturated fatty acids and mitochondria, back to the future. Trends Food Sci. Technol. 2017, 67, 76–92. [Google Scholar] [CrossRef]
- Martins, A.R.; Crisma, A.R.; Masi, L.N.; Amaral, C.L.; Marzuca-Nassr, G.N.; Bomfim, L.H.M.; Teodoro, B.G.; Queiroz, A.L.; Serdan, T.D.A.; Torres, R.P.; et al. Attenuation of obesity and insulin resistance by fish oil supplementation is associated with improved skeletal muscle mitochondrial function in mice fed a high-fat diet. J. Nutr. Biochem. 2018, 55, 76–88. [Google Scholar] [CrossRef]
- Miller, C.J.; Gounder, S.S.; Kannan, S.; Goutam, K.; Muthusamy, V.R.; Firpo, M.A.; Symons, J.D.; Paine, R., 3rd; Hoidal, J.R.; Rajasekaran, N.S. Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle. Biochim. Biophys. Acta 2012, 1822, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Putti, R.; Migliaccio, V.; Sica, R.; Lionetti, L. Skeletal Muscle Mitochondrial Bioenergetics and Morphology in High Fat Diet Induced Obesity and Insulin Resistance: Focus on Dietary Fat Source. Front. Physiol. 2015, 6, 426. [Google Scholar] [CrossRef]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G.; et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 1999, 354, 447–455.
- Zanetti, M.; Cappellari, G.G.; Barbetta, D.; Semolic, A.; Barazzoni, R. Omega 3 Polyunsaturated Fatty Acids Improve Endothelial Dysfunction in Chronic Renal Failure: Role of eNOS Activation and of Oxidative Stress. Nutrients 2017, 9, 895. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, G.G.; Zanetti, M.; Semolic, A.; Vinci, P.; Ruozi, G.; Falcione, A.; Filigheddu, N.; Guarnieri, G.; Graziani, A.; Giacca, M.; et al. Unacylated Ghrelin Reduces Skeletal Muscle Reactive Oxygen Species Generation and Inflammation and Prevents High-Fat Diet-Induced Hyperglycemia and Whole-Body Insulin Resistance in Rodents. Diabetes 2016, 65, 874–886. [Google Scholar] [CrossRef]
- Barazzoni, R.; Zanetti, M.; Cappellari, G.G.; Semolic, A.; Boschelle, M.; Codarin, E.; Pirulli, A.; Cattin, L.; Guarnieri, G. Fatty acids acutely enhance insulin-induced oxidative stress and cause insulin resistance by increasing mitochondrial reactive oxygen species (ROS) generation and nuclear factor-kappaB inhibitor (IkappaB)-nuclear factor-kappaB (NFkappaB) activation in rat muscle, in the absence of mitochondrial dysfunction. Diabetologia 2012, 55, 773–782. [Google Scholar] [CrossRef]
- Cappellari, G.G.; Barazzoni, R.; Cattin, L.; Muro, A.F.; Zanetti, M. Lack of Fibronectin Extra Domain A Alternative Splicing Exacerbates Endothelial Dysfunction in Diabetes. Sci. Rep. 2016, 6, 37965. [Google Scholar] [CrossRef]
- Workeneh, B.T.; Rondon-Berrios, H.; Zhang, L.; Hu, Z.; Ayehu, G.; Ferrando, A.; Kopple, J.D.; Wang, H.; Storer, T.; Fournier, M.; et al. Development of a diagnostic method for detecting increased muscle protein degradation in patients with catabolic conditions. J. Am. Soc. Nephrol. 2006, 17, 3233–3239. [Google Scholar] [CrossRef]
- Haehling, S.; Macedo, T.G.; Valentova, M.; Anker, M.S.; Ebner, N.; Bekfani, T.; Haarmann, H.; Schefold, J.C.; Lainscak, M.; Cleland, J.G.F.; et al. Muscle wasting as an independent predictor of survival in patients with chronic heart failure. J. Cachexia Sarcopenia Muscle 2020, 11, 1242–1249. [Google Scholar] [CrossRef]
- Iorio, A.; Senni, M.; Barbati, G.; Greene, S.J.; Poli, S.; Zambon, E.; Di Nora, C.; Cioffi, G.; Tarantini, L.; Gavazzi, A.; et al. Prevalence and prognostic impact of non-cardiac co-morbidities in heart failure outpatients with preserved and reduced ejection fraction: A community-based study. Eur. J. Heart Fail. 2018, 20, 1257–1266. [Google Scholar] [CrossRef]
- Bekfani, T.; Pellicori, P.; Morris, D.A.; Ebner, N.; Valentova, M.; Steinbeck, L.; Wachter, R.; Elsner, S.; Sliziuk, V.; Schefold, J.C.; et al. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int. J. Cardiol. 2016, 222, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Scandalis, L.; Kitzman, D.W.; Nicklas, B.J.; Lyles, M.; Brubaker, P.; Nelson, M.B.; Gordon, M.; Stone, J.; Bergstrom, J.; Neufer, P.D.; et al. Skeletal Muscle Mitochondrial Respiration and Exercise Intolerance in Patients With Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2023, 8, 575. [Google Scholar] [CrossRef]
- Powers, S.K.; Morton, A.B.; Ahn, B.; Smuder, A.J. Redox control of skeletal muscle atrophy. Free. Radic. Biol. Med. 2016, 98, 208–217. [Google Scholar] [CrossRef]
- Herbst, E.A.; Paglialunga, S.; Gerling, C.; Whitfield, J.; Mukai, K.; Chabowski, A.; Heigenhauser, G.J.; Spriet, L.L.; Holloway, G.P. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J. Physiol. 2014, 592, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Kalantar-Zadeh, K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin. Nephrol. 2009, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Dick, M.F.; Guglielmo, C.G. Dietary polyunsaturated fatty acids influence flight muscle oxidative capacity but not endurance flight performance in a migratory songbird. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R362–R375. [Google Scholar] [CrossRef]
- Matravadia, S.; Herbst, E.A.; Jain, S.S.; Mutch, D.M.; Holloway, G.P. Both linoleic and alpha-linolenic acid prevent insulin resistance but have divergent impacts on skeletal muscle mitochondrial bioenergetics in obese Zucker rats. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E102–E114. [Google Scholar] [CrossRef]
- Tachtsis, B.; Camera, D.; Lacham-Kaplan, O. Potential Roles of n-3 PUFAs during Skeletal Muscle Growth and Regeneration. Nutrients 2018, 10, 309. [Google Scholar] [CrossRef]
- Slee, E.L.; McLennan, P.L.; Owen, A.J.; Theiss, M.L. Low dietary fish-oil threshold for myocardial membrane n-3 PUFA enrichment independent of n-6 PUFA intake in rats. J. Lipid Res. 2010, 51, 1841–1848. [Google Scholar] [CrossRef] [PubMed]

| Sham | CHF | CHF-PUFA | ||
|---|---|---|---|---|
| Body Weight [g] | T0 | 42.0 ± 1.5 a | 42.4 ± 0.9 a | 42.7 ± 1.2 a |
| Body Weight [g] | T84 | 44.1 ± 1.2 a | 43.1 ± 1.3 a | 45.3 ± 1.6 a |
| Body Weight [g] | T140 | 46.9 ± 1.3 a | 43.9 ± 1.1 b | 46.8 ± 2.5 ab |
| Average daily caloric intake [kcal/d] | T0–T84 | 7.7 ± 0.6 a | 7.8 ± 0.3 a | 7.4 ± 0.8 a |
| Average daily caloric intake [kcal/d] | T84–T140 | 7.7 ± 0.9 a | 8.1 ± 0.4 a | 8.8 ± 0.8 a |
| Plasma Glucose [mg/dL] | T140 | 131 ± 4 a | 140 ± 3 a | 134 ± 5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gortan Cappellari, G.; Aleksova, A.; Dal Ferro, M.; Cannatà, A.; Semolic, A.; Guarnaccia, A.; Zanetti, M.; Giacca, M.; Sinagra, G.; Barazzoni, R. n-3 PUFA-Enriched Diet Preserves Skeletal Muscle Mitochondrial Function and Redox State and Prevents Muscle Mass Loss in Mice with Chronic Heart Failure. Nutrients 2023, 15, 3108. https://doi.org/10.3390/nu15143108
Gortan Cappellari G, Aleksova A, Dal Ferro M, Cannatà A, Semolic A, Guarnaccia A, Zanetti M, Giacca M, Sinagra G, Barazzoni R. n-3 PUFA-Enriched Diet Preserves Skeletal Muscle Mitochondrial Function and Redox State and Prevents Muscle Mass Loss in Mice with Chronic Heart Failure. Nutrients. 2023; 15(14):3108. https://doi.org/10.3390/nu15143108
Chicago/Turabian StyleGortan Cappellari, Gianluca, Aneta Aleksova, Matteo Dal Ferro, Antonio Cannatà, Annamaria Semolic, Alberto Guarnaccia, Michela Zanetti, Mauro Giacca, Gianfranco Sinagra, and Rocco Barazzoni. 2023. "n-3 PUFA-Enriched Diet Preserves Skeletal Muscle Mitochondrial Function and Redox State and Prevents Muscle Mass Loss in Mice with Chronic Heart Failure" Nutrients 15, no. 14: 3108. https://doi.org/10.3390/nu15143108
APA StyleGortan Cappellari, G., Aleksova, A., Dal Ferro, M., Cannatà, A., Semolic, A., Guarnaccia, A., Zanetti, M., Giacca, M., Sinagra, G., & Barazzoni, R. (2023). n-3 PUFA-Enriched Diet Preserves Skeletal Muscle Mitochondrial Function and Redox State and Prevents Muscle Mass Loss in Mice with Chronic Heart Failure. Nutrients, 15(14), 3108. https://doi.org/10.3390/nu15143108





