Beneficial Effects of Probiotics on Benign Gynaecological Disorders: A Review
Abstract
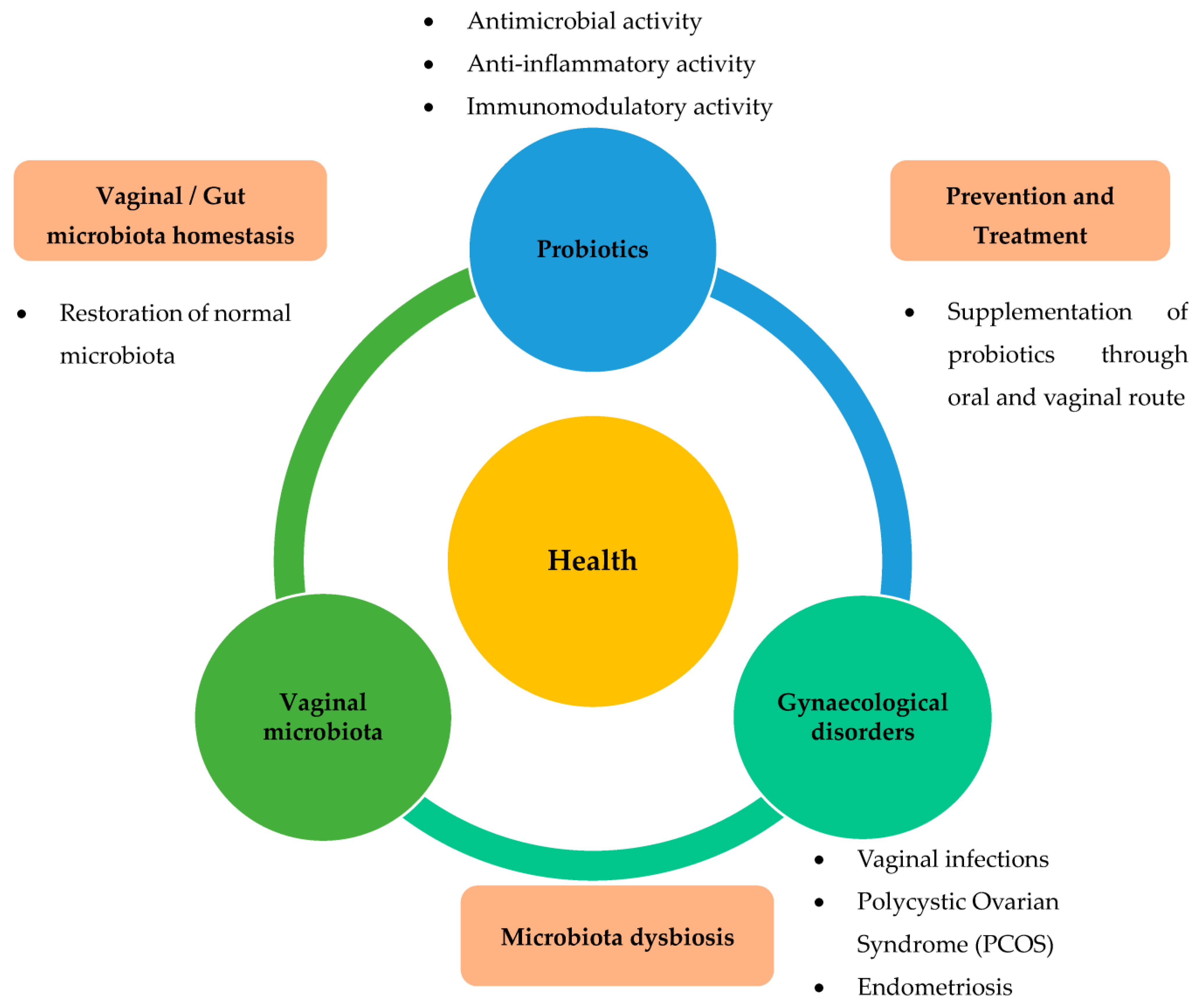
1. Introduction
2. Methodology
3. Beneficial Effects of Probiotics on Benign Gynaecological Disorders
3.1. Effects of Probiotics on Vaginal Infections
3.2. Effects of Probiotics on PCOS
3.3. Probiotics Effects on Endometriosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Hotel, A.C.P.; Cordoba, A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 2001, 5, 1–10. [Google Scholar]
- Holzapfel, W.H.; Haberer, P.; Geisen, R.; Björkroth, J.; Schillinger, U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am. J. Clin. Nutr. 2001, 73, 365s–373s. [Google Scholar] [CrossRef]
- Fuller, R.; Tannock, G.W. Probiotics a Critical Review; Horizon Scientific: Wymondham, UK, 1999; pp. 15–22. [Google Scholar]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Markowiak, P.; Slizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Homayouni, A.; Bastani, P.; Ziyadi, S.; Mohammad-Alizadeh-Charandabi, S.; Ghalibaf, M.; Mortazavian, A.M.; Mehrabany, E.V. Effects of Probiotics on the Recurrence of Bacterial Vaginosis: A Review. J. Low. Genit. Tract Dis. 2014, 18, 79–86. [Google Scholar] [CrossRef]
- Buggio, L.; Somigliana, E.; Borghi, A.; Vercellini, P. Probiotics and vaginal microecology: Fact or fancy? BMC Womens Health 2019, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Ya, W.; Reifer, C.; Miller, L.E. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: A double-blind, randomized, placebo-controlled study. Am. J. Obstet. Gynecol. 2010, 203, 120.e1–120.e6. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Pirotta, M.; De Guingand, D.; Hocking, J.S.; Morton, A.N.; Garland, S.M.; Fehler, G.; Morrow, A.; Walker, S.; Vodstrcil, L.A.; et al. Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: Randomised placebo-controlled double-blind trial. PLoS ONE 2012, 7, e34540. [Google Scholar] [CrossRef]
- Mastromarino, P.; Vitali, B.; Mosca, L. Bacterial vaginosis: A review on clinical trials with probiotics. New Microbiol. 2013, 36, 229–238. [Google Scholar]
- Ozkinay, E.; Terek, M.C.; Yayci, M.; Kaiser, R.; Grob, P.; Tuncay, G. The effectiveness of live lactobacilli in combination with low dose oestriol (Gynoflor) to restore the vaginal flora after treatment of vaginal infections. BJOG 2005, 112, 234–240. [Google Scholar] [CrossRef]
- Vujic, G.; Jajac Knez, A.; Despot Stefanovic, V.; Kuzmic Vrbanovic, V. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: A double-blind, randomized, placebo-controlled study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 75–79. [Google Scholar] [CrossRef]
- Ehrstrom, S.; Daroczy, K.; Rylander, E.; Samuelsson, C.; Johannesson, U.; Anzen, B.; Pahlson, C. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 2010, 12, 691–699. [Google Scholar] [CrossRef]
- Ahmadi, S.; Jamilian, M.; Karamali, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Probiotic supplementation and the effects on weight loss, glycaemia and lipid profiles in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Hum. Fertil. 2017, 20, 254–261. [Google Scholar] [CrossRef]
- Tabrizi, R.; Ostadmohammadi, V.; Akbari, M.; Lankarani, K.B.; Vakili, S.; Peymani, P.; Karamali, M.; Kolahdooz, F.; Asemi, Z. The Effects of Probiotic Supplementation on Clinical Symptom, Weight Loss, Glycemic Control, Lipid and Hormonal Profiles, Biomarkers of Inflammation, and Oxidative Stress in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Probiotics Antimicrob. Proteins 2022, 14, 1–14. [Google Scholar] [CrossRef]
- Nasri, K.; Jamilian, M.; Rahmani, E.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Asemi, Z. The effects of synbiotic supplementation on hormonal status, biomarkers of inflammation and oxidative stress in subjects with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. BMC Endocr. Disord. 2018, 18, 21. [Google Scholar] [CrossRef]
- Jamilian, M.; Mansury, S.; Bahmani, F.; Heidar, Z.; Amirani, E.; Asemi, Z. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J. Ovarian Res. 2018, 11, 80. [Google Scholar] [CrossRef]
- Rashad, N.M.; El-Shal, A.S.; Amin, A.I.; Soliman, M.H. Effects of probiotics supplementation on macrophage migration inhibitory factor and clinical laboratory feature of polycystic ovary syndrome. J. Funct. Foods 2017, 36, 317–324. [Google Scholar] [CrossRef]
- Ghanei, N.; Rezaei, N.; Amiri, G.A.; Zayeri, F.; Makki, G.; Nasseri, E. The probiotic supplementation reduced inflammation in polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. J. Funct. Foods 2018, 42, 306–311. [Google Scholar] [CrossRef]
- Shoaei, T.; Heidari-Beni, M.; Tehrani, H.G.; Feizi, A.; Esmaillzadeh, A.; Askari, G. Effects of Probiotic Supplementation on Pancreatic beta-cell Function and C-reactive Protein in Women with Polycystic Ovary Syndrome: A Randomized Double-blind Placebo-controlled Clinical Trial. Int. J. Prev. Med. 2015, 6, 27. [Google Scholar] [CrossRef]
- Khodaverdi, S.; Mohammadbeigi, R.; Khaledi, M.; Mesdaghinia, L.; Sharifzadeh, F.; Nasiripour, S.; Gorginzadeh, M. Beneficial Effects of Oral Lactobacillus on Pain Severity in Women Suffering from Endometriosis: A Pilot Placebo-Controlled Randomized Clinical Trial. Int. J. Fertil. Steril. 2019, 13, 178–183. [Google Scholar] [CrossRef]
- Itoh, H.; Sashihara, T.; Hosono, A.; Kaminogawa, S.; Uchida, M. Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology 2011, 63, 205–210. [Google Scholar] [CrossRef]
- Uchida, M.; Kobayashi, O. Effects of Lactobacillus gasseri OLL2809 on the induced endometriosis in rats. Biosci. Biotechnol. Biochem. 2013, 77, 1879–1881. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A. The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function. Nutrients 2022, 14, 902. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, Y.J. Probiotics in the Prevention and Treatment of Postmenopausal Vaginal Infections: Review Article. J. Menopausal Med. 2017, 23, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Maretti, C.; Cavallini, G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: A pilot study. Andrology 2017, 5, 439–444. [Google Scholar] [CrossRef]
- Reid, J.N.; Bisanz, J.E.; Monachese, M.; Burton, J.P.; Reid, G. The rationale for probiotics improving reproductive health and pregnancy outcome. Am. J. Reprod. Immunol. 2013, 69, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, R.; Qing, W.; Zhang, Y.; Zhou, Z.; Hou, Y.; Shi, Y.; Zhou, H.; Chen, M. Probiotics are a good choice for the treatment of bacterial vaginosis: A meta-analysis of randomized controlled trial. Reprod. Health 2022, 19, 137. [Google Scholar] [CrossRef]
- Wang, Z.; He, Y.; Zheng, Y. Probiotics for the Treatment of Bacterial Vaginosis: A Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3859. [Google Scholar] [CrossRef]
- Reid, G.; Younes, J.A.; Van der Mei, H.C.; Gloor, G.B.; Knight, R.; Busscher, H.J. Microbiota restoration: Natural and supplemented recovery of human microbial communities. Nat. Rev. Microbiol. 2011, 9, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Jefferson, K.K.; Cerca, N. Interactions between Lactobacillus crispatus and bacterial vaginosis (BV)-associated bacterial species in initial attachment and biofilm formation. Int. J. Mol. Sci. 2013, 14, 12004–12012. [Google Scholar] [CrossRef]
- Iqbal, Z.; Ahmed, S.; Tabassum, N.; Bhattacharya, R.; Bose, D. Role of probiotics in prevention and treatment of enteric infections: A comprehensive review. 3 Biotech 2021, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Cerca, N. Influence of Biofilm Formation by Gardnerella vaginalis and Other Anaerobes on Bacterial Vaginosis. J. Infect. Dis. 2015, 212, 1856–1861. [Google Scholar] [CrossRef]
- Teede, H.; Deeks, A.; Moran, L. Review Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41–50. [Google Scholar] [CrossRef]
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, A.; Roychoudhury, S.; Paul Choudhury, A.; Ahmed, A.B.F.; Dutta, S.; Mottola, F.; Verma, V.; Kalita, J.C.; Kumar, D.; Sengupta, P.; et al. Polycystic Ovary Syndrome: An Updated Overview Foregrounding Impacts of Ethnicities and Geographic Variations. Life 2022, 12, 1974. [Google Scholar] [CrossRef]
- Committee on Practice Bulletins—Gynecology. Polycystic ovary syndrome. Obstet. Gynecol. 2018, 131, e157–e171. [Google Scholar] [CrossRef]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K.; Endocrine, S. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef]
- Barber, T.M.; Hanson, P.; Weickert, M.O.; Franks, S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119874042. [Google Scholar] [CrossRef]
- Barber, T.M. Why are women with polycystic ovary syndrome obese? Br. Med. Bull. 2022, 143, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2014, 111, 1507–1519. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; Li, X.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota. Food Funct. 2020, 11, 5192–5204. [Google Scholar] [CrossRef]
- He, F.F.; Li, Y.M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.J.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology Bethesda 2016, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Lolou, V. The Role of Probiotics and Synbiotics on Hirsutism. Fermentation 2021, 7, 10. [Google Scholar] [CrossRef]
- Ju, H.; Jones, M.; Mishra, G. The prevalence and risk factors of dysmenorrhea. Epidemiol. Rev. 2014, 36, 104–113. [Google Scholar] [CrossRef]
- Morrow, C.; Naumburg, E.H. Dysmenorrhea. Prim. Care 2009, 36, 19–32. [Google Scholar] [CrossRef]
- Itani, R.; Soubra, L.; Karout, S.; Rahme, D.; Karout, L.; Khojah, H.M.J. Primary Dysmenorrhea: Pathophysiology, Diagnosis, and Treatment Updates. Korean J. Fam. Med. 2022, 43, 101–108. [Google Scholar] [CrossRef]
- Nur Azurah, A.G.; Sanci, L.; Moore, E.; Grover, S. The quality of life of adolescents with menstrual problems. J. Pediatr. Adolesc. Gynecol. 2013, 26, 102–108. [Google Scholar] [CrossRef]
- Guimaraes, I.; Povoa, A.M. Primary Dysmenorrhea: Assessment and Treatment. Rev. Bras. Ginecol. Obstet. 2020, 42, 501–507. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Salliss, M.E.; Farland, L.V.; Mahnert, N.D.; Herbst-Kralovetz, M.M. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum. Reprod Update 2021, 28, 92–131. [Google Scholar] [CrossRef]
- Leonardi, M.; Hicks, C.; El-Assaad, F.; El-Omar, E.; Condous, G. Endometriosis and the microbiome: A systematic review. BJOG 2020, 127, 239–249. [Google Scholar] [CrossRef]
- Zhang, S.; Lai, X.; Wang, X.; Liu, G.; Wang, Z.; Cao, L.; Zhang, X.; Xiao, W.; Li, S. Deciphering the Pharmacological Mechanisms of Guizhi-Fuling Capsule on Primary Dysmenorrhea Through Network Pharmacology. Front. Pharmacol. 2021, 12, 613104. [Google Scholar] [CrossRef]
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Snydman, D.R. The safety of probiotics. Clin. Infect. Dis. 2008, 46 (Suppl. 2), S104–S111, discussion S144–S151. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.; Hormannsperger, G.; Huys, G.; Levy, D.D.; Lutgendorff, F.; Mack, D.; et al. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef] [PubMed]

| Microorganisms Considered Probiotics | |||
|---|---|---|---|
| Lactobacillus Species | Bifidobacterium Species | Other Lactic Acid Bacteria | Non-Lactic Acid Bacteria |
| L. acidophilus | B. adolescentis | Enterococcus faecalis | Bacillus cereus var. toyoi |
| L. amylovorus | B. animalis | Enterococcus faecium | Escherichia coli strain nissle |
| L. casei | B. bifidum | Lactococcus lactis | Propionibacterium freudenreichii |
| L. crispatus | B. breve | Leuconstoc mesenteroides | Saccharomyces cerevisiae |
| L. delbrueckii | B. infantis | Pedioccocus acidilactic | Saccharomyces boulardii |
| L. gallinarum | B. lactis | Sprolactobacillus inulinus | |
| L. gasseri | B. longum | Streprococcus thermophilus | |
| L. johnsonii | |||
| L. paracasei | |||
| L. reuteri | |||
| L. rhamnosus | |||
| Population Sample | Health Condition | Probiotic Strain Administered | Dosage | Duration of Treatment | Clinical Effects/Health Parameter Modifications | References |
|---|---|---|---|---|---|---|
| 120 | History of BV | Probaclac Vaginal capsule (108 CFU of Lactobacillus rhamnosus, L. acidophilus, and Streptococcus thermophilus) | 1 vaginal capsule per treatment day | 21 days (7 days on, 7 days off, 7 days on) | Probiotic prophylaxis resulted in lower recurrence rates for women with BV. | [9] |
| 268 | BV | 107 CFU of live L. acidophilus KS400 with combined use of oral metronidazole 400 mg. | Vaginal probiotic cream | 12 days | Improved restoration of normal vaginal flora in BV-positive women. | [10] |
| 125 | History of BV | 109 L. rhamnosus GR-1 and L. reuteri RC-14 (105 CFU) with combined use of oral metronidazole 500 mg. | 1 oral capsule twice daily | 30 days | BV cure rate is 88% compared to 40% control. | [11] |
| 19 | Vaginal infections | Vaginal tablet containing >107 CFU of L. acidophilus, 0.03 mg of estriol and 600 mg of lactose | 1 tablet daily | 6 days for pre-menopausal women, 12 days for post-menopausal women | Vaginal flora was enhanced significantly by the probiotic administration in combination with low-dose estriol. | [12] |
| 544 | Vaginal infection | Oral capsules containing >109 CFU Lactobacillus rhamnosus GR-1 and L-reuteri RC-14 | 2 capsules daily | 6 weeks | Restitution to balance vaginal microbiota is reported in 243 subjects (61.52%) in the probiotic group. High counts (>105 CFU/mL) of lactobacilli were recovered from 81.5% of subjects who received probiotics. | [13] |
| 360 | BV, vulvovaginal candidiasis | Vaginal capsules containing between 108 and 1010 CFU of L. gasseri LN40, L. fermentum LN99, L. casei subsp. Rhamnosus LN113, and P. acidilactici LN23, after treatment | 1 vaginal tablet daily | 5 days | LN had a good colonization rate in The vagina of patients with BV, and women receiving LN were cured after administration. | [14] |
| Population Sample/Sample Size | Health Condition | Probiotic Strain Administered | Dosage | Duration of Treatment | Clinical Effects/Health Parameter Modifications | References |
|---|---|---|---|---|---|---|
| 60 | PCOS patients | Lactobacillus acidophilus (2 × 109 CFU/g), Lactobacillus casei (2 × 109 CFU/g) and Bifidobacterium bifidum (2 × 109 CFU/g). | 1 capsule daily | 12 weeks | Reduction in weight and BMI. Significant reduction in FPG, serum insulin levels, serum triglycerides and VLDL–cholesterol. | [15] |
| 60 | PCOS patients | Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum (2 × 109 CFU/g each) | 1 capsule daily | 12 weeks | Significantly increased serum HGB, plasma TAC and decreased serum total testosterone, CRP | [16] |
| 60 | PCOS patients | Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum (2 × 109 CFU/g each) and 0.8 g inulin | 1 capsule daily | 12 weeks | Increased SHBG, plasma NO, and serum CRP. Reduction in serum insulin and total testosterone levels. | [17] |
| 60 | PCOS patients | 8 × 109 CFU probiotic containing Lactobacillus acidophilus, Lactobacillus reuteri, Lactobacillus fermentum and Bifdobacterium bifidum and 200 µg selenium | 1 capsule daily | 12 weeks | Reduced serum total testosterone, hs-CRP, hirsutism, GSH. | [18] |
| 100 | PCOS patients | 10 billions probiotic capsules (Lactobacillus delbruekii and Lactobacillus fermentum) | Twice daily | 12 weeks | Significant improvement to metabolic parameters: glucose, serum lipid, significant decrease in inflammatory biomarker upon supplementation with probiotics. | [19] |
| 60 | PCOS patients | 1 × 109 CFU/g L. acidophilus, Lactobacillus plantarum, Lactobacillus fermentum, and Lactobacillus gasseri | Two capsules daily | 12 weeks | Positive effects that reduce inflammation: considerable reduction in hs-CRP, IL-6 and increased IL-10. | [20] |
| 72 | PCOS patients | Lactobacillus casei 7 × 109 CFU/g, Lactobacillus acidophilus 2 × 109 CFU/g, Lactobacillus rhamnosus 1.5 × 109 CFU/g, Lactobacillus bulgaricus 2 × 108 CFU/g, Bifdo-bacterium breve 2 × 1010 CFU/g, Bifdobacterium longum 7 × 109 CFU/g, Streptococcus thermophiles 1.5 × 109 CFU/g | 1 capsule daily | 8 weeks | Reduced glycemic index and hs-CRP, reduced serum insulin. | [21] |
| Population Sample/Sample Size | Health Condition | Probiotic Strain Administered | Dosage | Duration of Treatment | Clinical Effects/Health Parameter Modifications | References |
|---|---|---|---|---|---|---|
| 37 | Endometriosis patients | 109 Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus fermentum and Lactobacillus gasseri | Once daily | 8 weeks | Significant improvement regarding endometriosis-associated pain. | [22] |
| 27 | Endometriosis—induced Female Wistarr-Imamichi rats | Lactobacillus gasseri OLL2809 | 40 mg | 4 weeks | OLL2809 significantly enhanced the regression of endometriosis, and 2 out of 9 rats were completely healed. | [24] |
| 62 | Endometriosis patients | 100 mg of Lactobacillus gasseri OLL2809 | Two tablets daily | 3 months | Decrease in VAS of pain intensity in intervention group was significantly greater than placebo. | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norfuad, F.A.; Mokhtar, M.H.; Nur Azurah, A.G. Beneficial Effects of Probiotics on Benign Gynaecological Disorders: A Review. Nutrients 2023, 15, 2733. https://doi.org/10.3390/nu15122733
Norfuad FA, Mokhtar MH, Nur Azurah AG. Beneficial Effects of Probiotics on Benign Gynaecological Disorders: A Review. Nutrients. 2023; 15(12):2733. https://doi.org/10.3390/nu15122733
Chicago/Turabian StyleNorfuad, Farisha Alia, Mohd Helmy Mokhtar, and Abdul Ghani Nur Azurah. 2023. "Beneficial Effects of Probiotics on Benign Gynaecological Disorders: A Review" Nutrients 15, no. 12: 2733. https://doi.org/10.3390/nu15122733
APA StyleNorfuad, F. A., Mokhtar, M. H., & Nur Azurah, A. G. (2023). Beneficial Effects of Probiotics on Benign Gynaecological Disorders: A Review. Nutrients, 15(12), 2733. https://doi.org/10.3390/nu15122733






