Content and Availability of Minerals in Plant-Based Burgers Compared with a Meat Burger
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Sample Collection and Preparation
2.3. Moisture Content Analysis
2.4. Total Mineral Content of Samples
2.5. In Vitro Simulated Gastrointestinal Digestion of Samples
2.6. Mineral Uptake by Caco-2 Cells
2.7. Protein Analysis
2.8. Statistical Analysis
3. Results
3.1. Moisture and Mineral Composition of Burger Samples
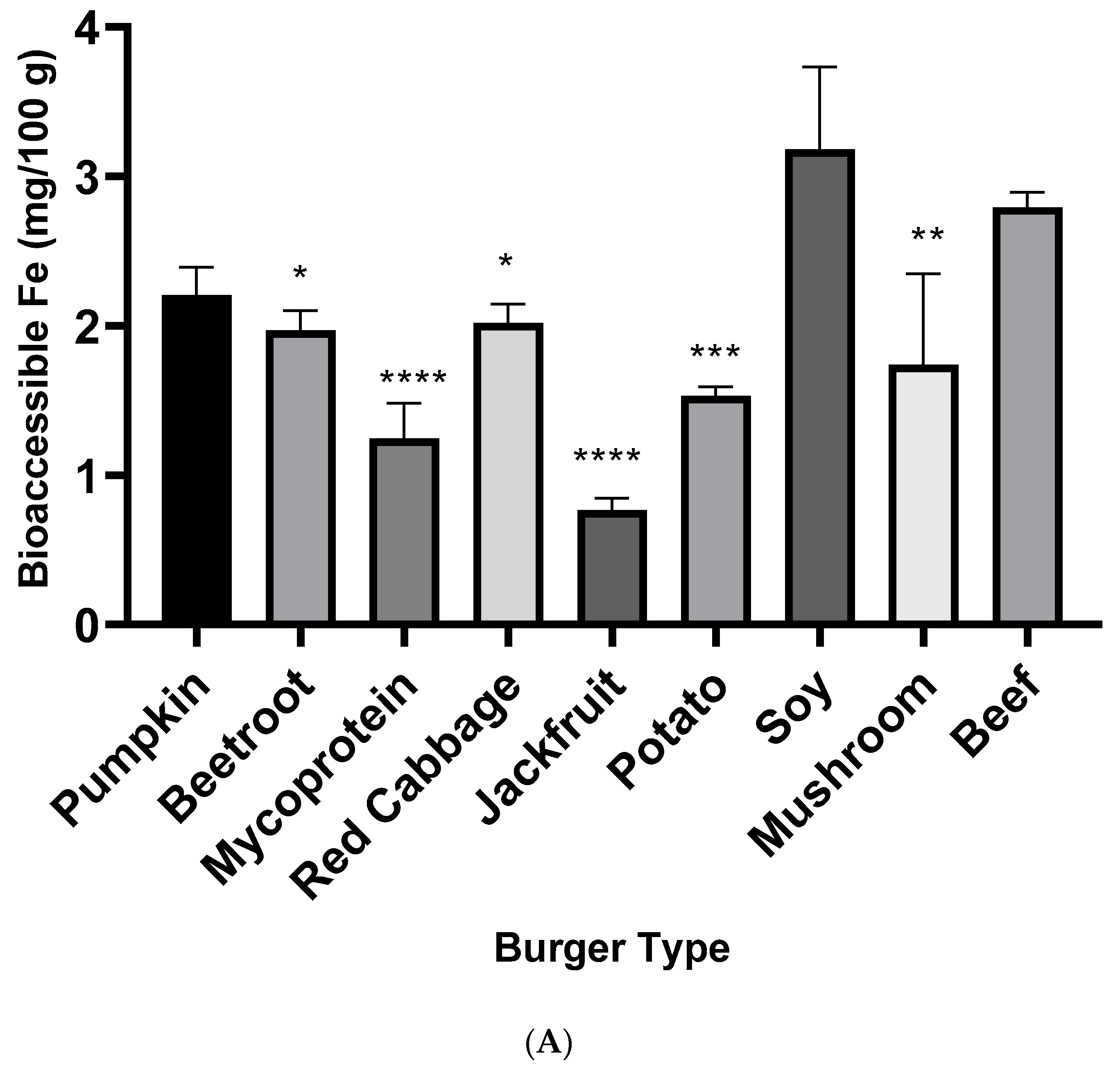
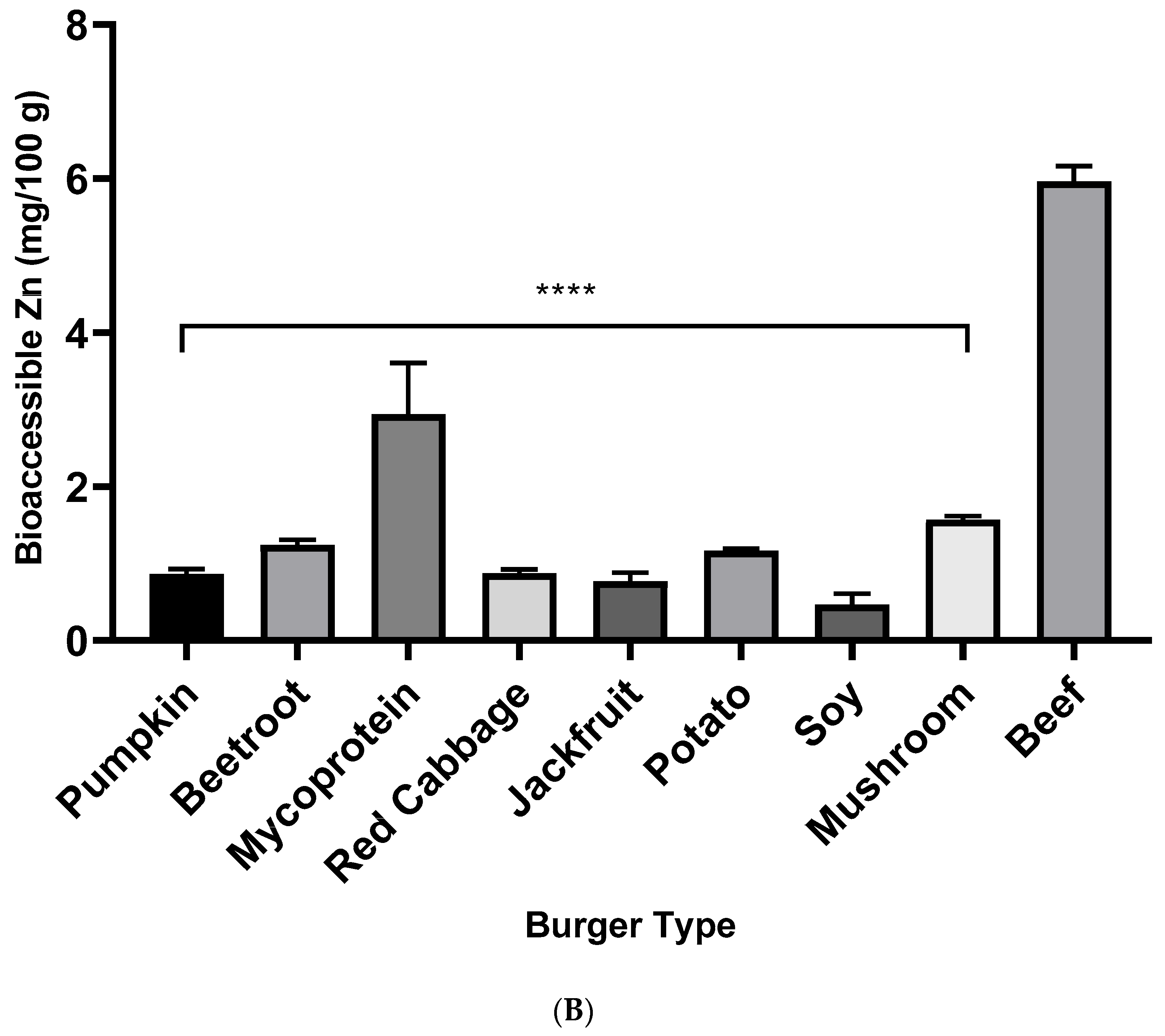
3.2. Iron and Zinc Bioaccessibility from Burger Samples
3.3. Mineral Uptake from Plant-Based Burger Digests by Caco-2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; Declerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Craig, W.J. Health effects of vegan diets. Am. J. Clin. Nutr. 2009, 89, 1627S–1633S. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Iron status of vegetarians. Am. J. Clin. Nutr. 1994, 59, 1233S–1237S. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 17, 6–47. [Google Scholar] [CrossRef] [PubMed]
- Tonheim, L.E.; Groufh-Jacobsen, S.; Stea, T.H.; Henjum, S. Consumption of meat and dairy substitute products amongst vegans, vegetarians and pescatarians. Food Nutr. Res. 2023, 67, 1–11. [Google Scholar] [CrossRef]
- Craig, W.J. Nutrition concerns and health effects of vegetarian diets. Nutr. Clin. Pract. 2010, 25, 613–620. [Google Scholar] [CrossRef]
- Mclean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; De Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444. [Google Scholar] [CrossRef]
- WHO. World Health Organisation: The Global Prevalence of Anaemia in 2011. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/177094/9789241564960_eng.pdf (accessed on 15 March 2023).
- Lozoff, B.; Beard, J.; Connor, J.; Felt, B.; Georgieff, M.; Schallert, T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006, 64, 34–43. [Google Scholar] [CrossRef]
- Hercberg, S.; Preziosi, P.; Galan, P. Iron deficiency in Europe. Public Health Nutr. 2001, 4, 537–545. [Google Scholar] [CrossRef]
- Wyness, L. The role of red meat in the diet: Nutrition and health benefits. Proc. Nutr. Soc. 2016, 75, 227–232. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.; Hurrell, R.F. Bioavailability of minerals and trace elements. Nutr. Res. Rev. 1996, 9, 295–324. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Reddy, M.; Cook, J.D. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br. J. Nutr. 1999, 81, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.R.; Cook, J.D. Interaction of vitamin C and iron. Ann. N. Y. Acad. Sci. 1980, 355, 32–44. [Google Scholar] [CrossRef] [PubMed]
- National Diet and Nutrition Survey. Results from Years 1–4 (Combined) of the Rolling Programme (2008/2009–2011/12). 2014. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/594361/NDNS_Y1_to_4_UK_report_full_text_revised_February_2017.pdf (accessed on 15 March 2023).
- Hunt, J.R. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 2003, 78 (Suppl. S3), 633S–639S. [Google Scholar] [CrossRef]
- Orlich, M.J.; Jaceldo-Siegl, K.; Sabaté, J.; Fan, J.; Singh, P.N.; Fraser, G.E. Patterns of food consumption among vegetarians and non-vegetarians. Br. J. Nutr. 2014, 112, 1644–1653. [Google Scholar] [CrossRef]
- Alessandrini, R.; Brown, M.K.; Pombo-Rodrigues, S.; Bhageerutty, S.; He, F.J.; MacGregor, G.A. Nutritional Quality of Plant-Based Meat Products Available in the UK: A Cross-Sectional Survey. Nutrients 2021, 13, 4225. [Google Scholar] [CrossRef]
- Higuera, J.M.; Santos, H.M.; Oliveira, A.F.; Nogueira, A.R.A. Animal and vegetable protein burgers: Bromatological analysis, mineral composition, and bioaccessibility evaluation. ACS Food Sci. Technol. 2021, 1, 1821–1829. [Google Scholar] [CrossRef]
- Salomé, M.; Huneau, J.-F.; Le Baron, C.; Kesse-Guyot, E.; Hélène Fouillet, H.; Mariotti, F. Substituting Meat or Dairy Products with Plant-Based Substitutes Has Small and Heterogeneous Effects on Diet Quality and Nutrient Security: A Simulation Study in French Adults (INCA3). J. Nutr. 2021, 151, 2435–2445. [Google Scholar] [CrossRef]
- Melville, H.; Shahid, M.; Gaines, A.; McKenzie, B.L.; Alessandrini, R.; Trieu, K.; Wu, J.H.Y.; Rosewarne, E.; Coyle, D.H. The nutritional profile of plant-based meat analogues available for sale in Australia. Nutr. Diet. 2023, 80, 211–222. [Google Scholar] [CrossRef]
- Bryngelsson, S.; Moshtaghian, H.; Bianchi, M.; Hallström, E. Nutritional assessment of plant-based meat analogues on the Swedish market. Int. J. Food Sci. Nutr. 2022, 73, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Tso, R.; Forde, C.G. Unintended Consequences: Nutritional Impact and Potential Pitfalls of Switching from Animal- to Plant-Based Foods. Nutrients 2021, 13, 2527. [Google Scholar] [CrossRef] [PubMed]
- Mayer Labba, I.C.; Hoppe, M.; Gramatkovski, E.; Hjellström, M.; Abdollahi, M.; Undeland, I.; Hulthén, L.; Sandberg, A.S. Lower Non-Heme Iron Absorption in Healthy Females from Single Meals with Texturized Fava Bean Protein Compared to Beef and Cod Protein Meals: Two Single-Blinded Randomized Trials. Nutrients 2022, 14, 3162. [Google Scholar] [CrossRef] [PubMed]
- Khoja, K.; Buckley, A.; FAslam, M.; ASharp, P.; Latunde-Dada, G.O. In vitro bioaccessibility and bioavailability of iron from mature and microgreen fenugreek, rocket and broccoli. Nutrients 2020, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Glahn, R.P.; Lee, O.A.; Yeung, A.; Goldman, M.I.; Miller, D.D. Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/caco-2 cell culture model. Nutr. J. 1998, 128, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Latunde-Dada, G.O.; Yang, W.; Vera Aviles, M. In vitro iron availability from insects and sirloin beef. J. Agric. Food Chem. 2016, 64, 8420–8424. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: Health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef]
- PHE. Public Health England—McCance and Widdowson’s the Composition of Foods Integrated Dataset. 2019. Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid (accessed on 4 April 2020).
- Curtain, F.; Grafenauer, S. Plant-based meat substitutes in the flexitarian age: An audit of products on supermarket shelves. Nutrients 2019, 11, 2603. [Google Scholar] [CrossRef]
- Field, M.S.; Mithra, P.; Estevez, D.; Peña-Rosas, J.P. Wheat flour fortification with iron for reducing anaemia and improving iron status in populations. Cochrane Database Syst. Rev. 2020, 7, CD011302. [Google Scholar]
- Harnack, L.; Mork, S.; Valluri, S.; Weber, C.; Schmitz, K.; Stevenson, J.; Pettit, J. Nutrient composition of a selection of plant-based ground beef alternative products available in the United States. J. Acad. Nutr. Diet. 2021, 121, 2401–2408.e12. [Google Scholar] [CrossRef] [PubMed]
- Swing, C.J.; Thompson, T.W.; Guimaraes, O.; Geornaras, I.; Engle, T.E.; Belk, K.E.; Gifford, C.L.; Narayanan, M.N. Nutritional composition of novel plant-based meat alternatives and traditional animal-based meats. J. Food Sci. Nutr. 2021, 7, 109. [Google Scholar]
- Souza Filho, P.F.; Andersson, D.; Ferreira, J.A.; Taherzadeh, M.J. Mycoprotein: Environmental impact and health aspects. World J. Microbiol. Biotechnol. 2019, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hashempour-Baltork, F.; Khosravi-Darani, K.; Hosseini, H.; Farshi, P.; Reihani, S. Mycoproteins as safe meat substitutes. J. Clean. Prod. 2020, 253, 119958. [Google Scholar] [CrossRef]
- Erdman, J.W.; Fordyce, E.J. Soy products and the human diet. Am. J. Clin. Nutr. 1989, 49, 725–737. [Google Scholar] [CrossRef]
- Gillooly, M.J.; Torrance, J.D.; Bothwell, T.H.; MacPhail, A.P.; Derman, D.; Mills, W.; Mayet, F. The relative effect of ascorbic acid on iron absorption from soy-based and milk-based infant formulas. Am. J. Clin. Nutr. 1984, 40, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Rizk, S.W.; Clydesdale, F.M. Effectiveness of organic acids to solubilize iron from a wheat-soy drink. J. Food Prot. 1985, 48, 648–652. [Google Scholar] [CrossRef]
- Gibson, R.S. Content and bioavailability of trace elements in vegetarian diets. Am. J. Clin. Nutr. 1994, 59 (Suppl. S5), 1223S–1232S. [Google Scholar] [CrossRef]
- Hortin, A.E.; Oduho, G.; Han, Y.; Bechtel, J.; Baker, D.H. Bioavailability of zinc in ground beef. J. Anim. Sci. 1993, 71, 119–123. [Google Scholar] [CrossRef]
- Hallberg, L.; Hulthén, L. Prediction of dietary iron absorption: An algorithm for calculating absorption and bioavailability of dietary iron. Am. J. Clin. Nutr. 2000, 71, 1147–1160. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O.; Neale, R.J. Effect of soya-bean protein on meat iron solubility and absorption in rats. Br. J. Nutr. 1986, 55, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Cofnas, N. Is vegetarianism healthy for children? Crit. Rev. Food Sci. Nutr. 2019, 59, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Soybean ferritin: Implications for iron status of vegetarians. Am. J. Clin. Nutr. 2009, 89 (Suppl. S5), 1680S–1685S. [Google Scholar] [CrossRef] [PubMed]
- Sandström, B.; Andersson, H.; Kivistö, B.; Sandberg, A.S. Apparent small intestinal absorption of nitrogen and minerals from soy and meat-protein-based diets. A study on human ileostomy subjects. J. Nutr. 1986, 116, 2209–2218. [Google Scholar] [CrossRef]
- Mayer Labba, I.C.; Steinhausen, H.; Almius, L.; Bach Knudsen, K.E.; Sandberg, A.S. Nutritional Composition and Estimated Iron and Zinc Bioavailability of Meat Substitutes Available on the Swedish Market. Nutrients 2022, 14, 3903. [Google Scholar] [CrossRef]
- Sharp, P. Methods and options for estimating iron and zinc bioavailability using Caco-2 cell models: Benefits and limitations. Int. J. Vitam. Nutr. Res. 2005, 75, 413–421. [Google Scholar] [CrossRef]
- Miller, D.D.; Berner, L.A. Is solubility in vitro a reliable predictor of iron bioavailability? Biol. Trace Elem. Res. 1989, 19, 11–24. [Google Scholar] [CrossRef]

| Sample | Ingredient List |
|---|---|
| A | Pumpkin (50%), sweet potato, soya protein, sunflower oil, potato starch, methyl cellulose, coriander, turmeric, white pepper, cumin, fenugreek, wheat fibre, salt, paprika powder, onion powder, garlic powder, red paprika |
| B | Beetroot (80%), potato starch, onion, soya protein, wheat fibres, methyl cellulose, salt, potato protein, garlic purée |
| C | MycoproteinTM (38%), textured wheat protein (wheat flour, sodium alginate, plain caramel), rehydrated free range egg white, vegetable oil, onion, smoked yeast, potassium chloride, milk proteins, roasted barley, calcium chloride, calcium acetate |
| D | Red cabbage, black turtle beans, breadcrumbs (wheat flour, calcium carbonate, iron, niacin, thiamin), sunflower oil, salt, yeast, rosemary, soya protein concentrate, sushi rice, carrot, rice vinegar, water, onion, dark soya sauce, beetroot, rapeseed oil, red chilli purée, red miso, maple syrup, garlic, black pepper |
| E | Jackfruit (45%), water, rice flour, gram flour, maize flour, maize starch, yeast extract, brown sugar, tomato paste, cornflour, potato starch, salt, sunflower oil, red pepper, spirit vinegar, muscovado sugar, cider vinegar, parsley, onion powder, garlic powder, lemon powder, sodium bicarbonate, disodium diphosphate, paprika, cane molasses, grape vinegar, fennel seed, cayenne pepper, cumin, coriander, rosemary, dextrose monohydrate, pepper, xanthan gum, black treacle, tamarind paste, pimento, ginger, cloves |
| F | Potato (15%), aubergine, onion, lentils, rapeseed oil, feta, spring onion, chickpeas, sweet potato, egg, cornflour, potato starch, wheat flour, calcium carbonate, iron, niacin, thiamin, garlic purée, red chilli purée, parsley, mint, salt, cumin, extra virgin olive oil, tomato purée, pepper, coriander, paprika, yeast |
| G | SOY structure (Water, soya protein, wheat starch, wheat protein) (64%), broad beans, sunflower oil, palm oil, onion powder, pepper, garlic, ginger, mace, onion, clove, coriander, oregano, methyl cellulose, bamboo fibre, tapioca starch, seaweed, salt, iron, vitamin B12 |
| H | Water, mushrooms (32%), wheat gluten, pea flour, wheat flour, vegetable suet, pea fibre, methylcellulose, pea starch, salt, onion, yeast, pepper, sodium metabisulphite |
| I | Beef (95%), onion, salt, butter, pepper, sodium sulphite, sodium ascorbate |
| Sample | Moisture (%) | Fe | Ca | Cu | Mg | Mn | Zn |
|---|---|---|---|---|---|---|---|
| A | 67.9 | 3.36 ± 0.03 | 134 ± 0.93 *** | 0.43 ± 0.01 *** | 85.9 ± 0.34 *** | 1.20 ± 0.12 *** | 1.10 ± 0.02 *** |
| B | 75.2 | 3.39 ± 0.06 | 156 ± 1.38 *** | 0.42 ± 0.01 *** | 94.1 ± 0.70 *** | 1.88 ± 0.01 *** | 1.67 ± 0.03 *** |
| C | 62.2 | 1.13 ± 0.04 *** | 260 ± 7.01 *** | 0.56 ± 0.01 *** | 50.5 ± 0.54 *** | 3.25 ± 0.04 *** | 7.15 ± 0.08 * |
| D | 54.3 | 2.70 ± 0.05 *** | 93.4 ± 1.39 *** | 0.33 ± 0.00 *** | 70.4 ± 0.96 *** | 0.97 ± 0.01 *** | 1.14 ± 0.04 *** |
| E | 57.1 | 1.27 ± 0.03 *** | 31.7 ± 0.10 *** | 0.24 ± 0.00 *** | 40.1 ± 0.20 *** | 0.63 ± 0.00 *** | 0.89 ± 0.02 *** |
| F | 47.8 | 1.99 ± 0.08 *** | 57.9 ± 0.92 *** | 0.23 ± 0.00 *** | 39.5 ± 0.34 *** | 0.47 ± 0.01 *** | 0.91 ± 0.03 *** |
| G | 53.6 | 14.5 ± 0.03 *** | 150 ± 1.32 *** | 0.28 ± 0.00 *** | 62.5 ± 0.46 *** | 0.87 ± 0.01 *** | 1.14 ± 0.01 *** |
| H | 61.2 | 2.90 ± 0.02 *** | 46.2 ± 0.28 *** | 0.43 ± 0.00 *** | 44.5 ± 0.15 *** | 0.83 ± 0.01 *** | 2.24 ± 0.01 *** |
| I | 54.1 | 3.42 ± 0.01 | 9.59 ± 0.21 | 0.14 ± 0.00 | 37.2 ± 0.12 | 0.07 ± 0.00 | 7.41 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latunde-Dada, G.O.; Kajarabille, N.; Rose, S.; Arafsha, S.M.; Kose, T.; Aslam, M.F.; Hall, W.L.; Sharp, P.A. Content and Availability of Minerals in Plant-Based Burgers Compared with a Meat Burger. Nutrients 2023, 15, 2732. https://doi.org/10.3390/nu15122732
Latunde-Dada GO, Kajarabille N, Rose S, Arafsha SM, Kose T, Aslam MF, Hall WL, Sharp PA. Content and Availability of Minerals in Plant-Based Burgers Compared with a Meat Burger. Nutrients. 2023; 15(12):2732. https://doi.org/10.3390/nu15122732
Chicago/Turabian StyleLatunde-Dada, Gladys O., Naroa Kajarabille, Sophie Rose, Sarah M. Arafsha, Tugba Kose, Mohamad F. Aslam, Wendy L. Hall, and Paul A. Sharp. 2023. "Content and Availability of Minerals in Plant-Based Burgers Compared with a Meat Burger" Nutrients 15, no. 12: 2732. https://doi.org/10.3390/nu15122732
APA StyleLatunde-Dada, G. O., Kajarabille, N., Rose, S., Arafsha, S. M., Kose, T., Aslam, M. F., Hall, W. L., & Sharp, P. A. (2023). Content and Availability of Minerals in Plant-Based Burgers Compared with a Meat Burger. Nutrients, 15(12), 2732. https://doi.org/10.3390/nu15122732








