The Neuroprotection of Verbascoside in Alzheimer’s Disease Mediated through Mitigation of Neuroinflammation via Blocking NF-κB-p65 Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Feeding and Agent Administration
2.2. Label-Free Quantification Proteomics
2.3. Cell Culture
2.4. Cell Viability and Lactate Dehydrogenase (LDH) Cytotoxicity Assay
2.5. Nitric Oxide (NO) Assay
2.6. Transmission Electron Microscopy (TEM)
2.7. Immunohistochemistry and Immunofluorescence
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Western Blot
2.10. Statistical Analysis
3. Results
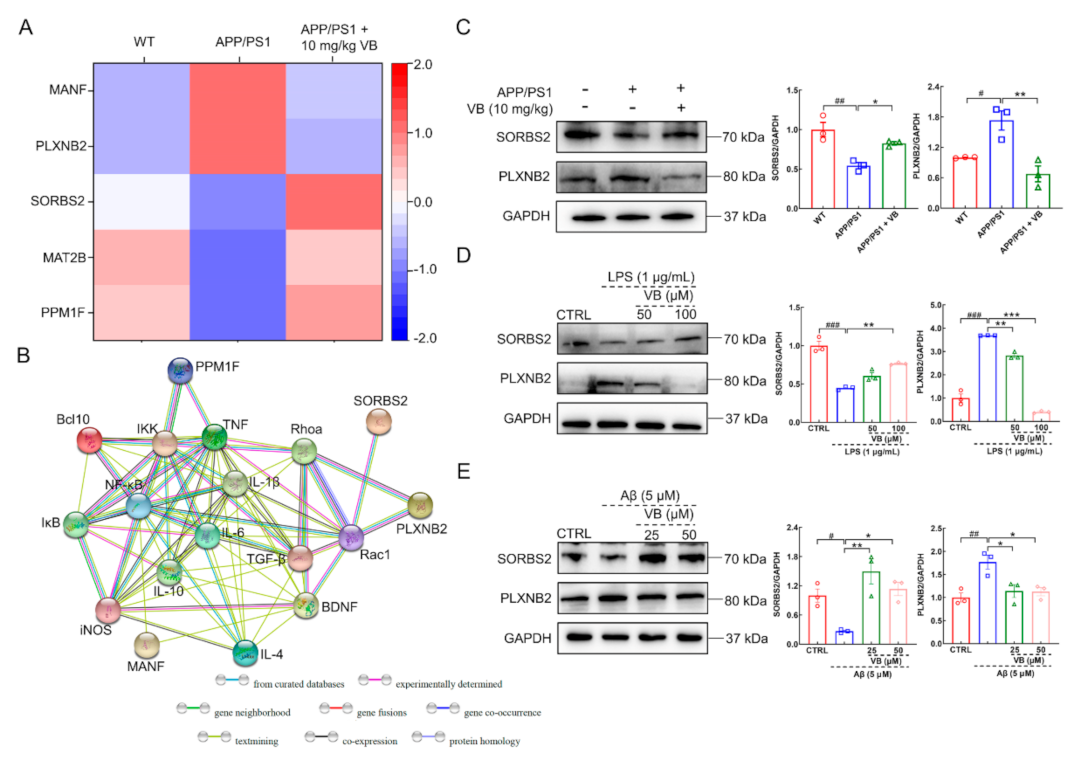
3.1. Neuroprotection of VB Links Closely with Microglia and Astrocyte Activation
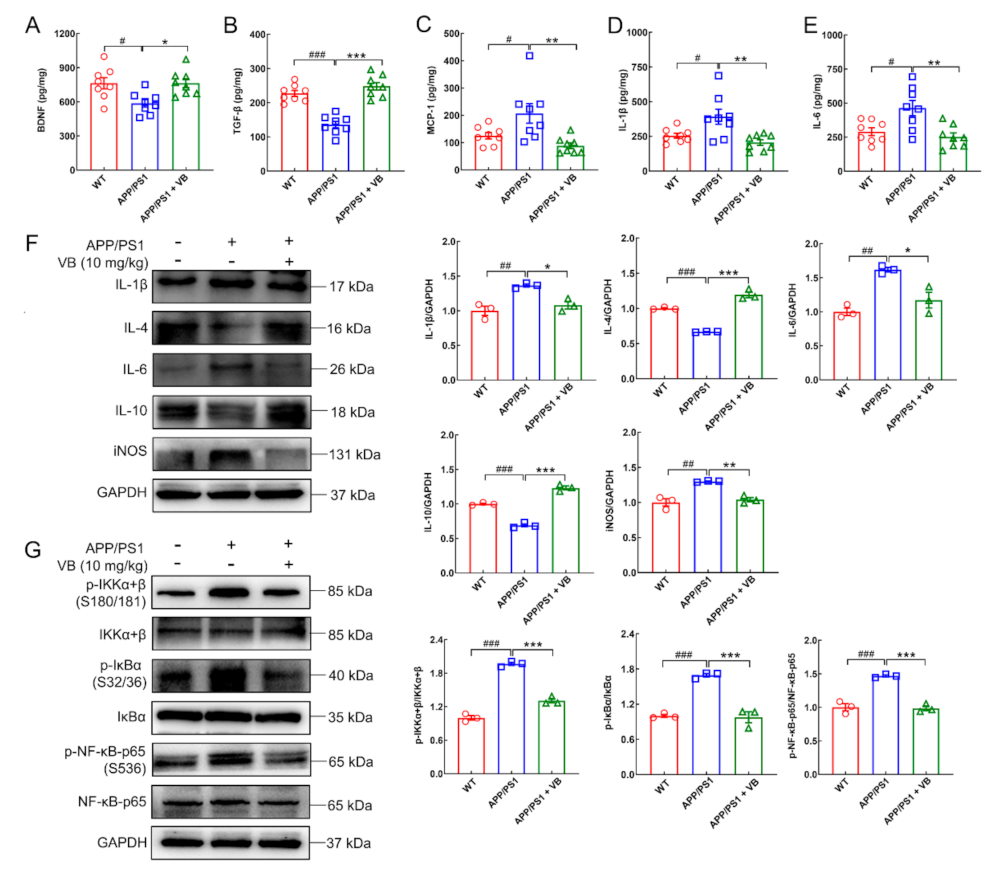
3.2. VB Mediates Anti-Neuroinflammatory Properties through Modulation of NF-κB-p65 Signaling in APP/PS1 Mice
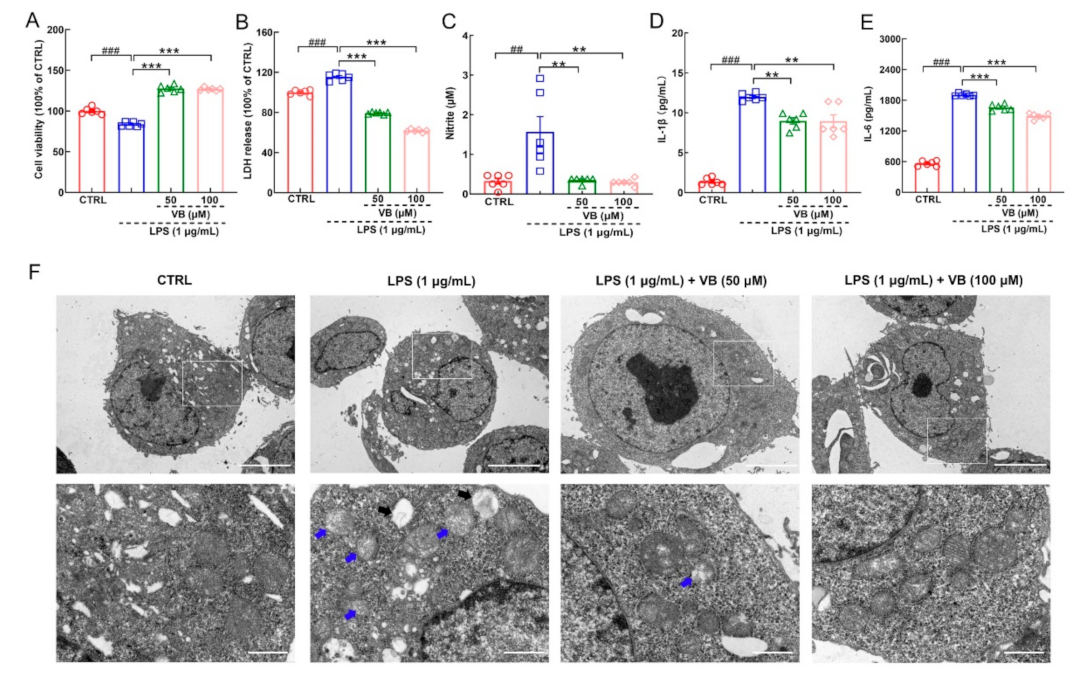
3.3. VB Exerts Anti-Inflammatory Properties in LPS-Induced BV2 Cells by Regulating NF-κB-p65 Signaling
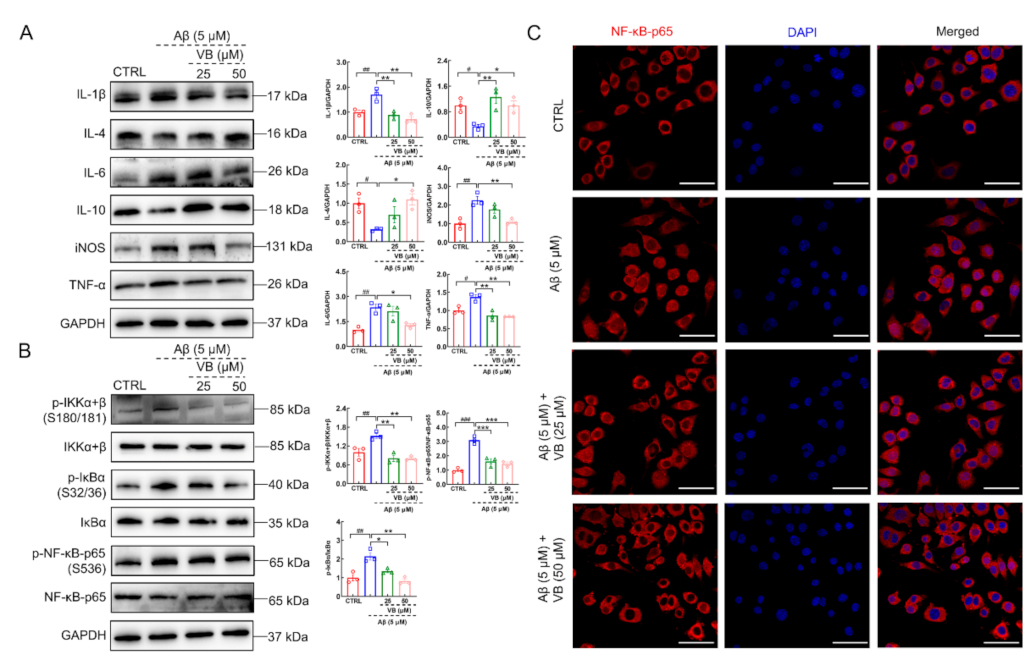
3.4. VB Exerts Neuroprotection in Aβ1-42-Stimulated N2a Cells by Inhibiting Neuroinflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daneschvar, H.L.; Aronson, M.D.; Smetana, G.W. Do statins prevent Alzheimer’s disease? A narrative review. Eur. J. Intern. Med. 2015, 26, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Darby, D.G.; Pietrzak, R.H.; Fredrickson, J.; Woodward, M.; Moore, L.; Fredrickson, A.; Sach, J.; Maruff, P. Intraindividual cognitive decline using a brief computerized cognitive screening test. Alzheimers Dement. 2012, 8, 95–104. [Google Scholar] [CrossRef]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cubinkova, V.; Valachova, B.; Uhrinova, I.; Brezovakova, V.; Smolek, T.; Jadhav, S.; Zilka, N. Alternative hypotheses related to Alzheimer’s disease. Bratisl. Lek. Listy 2018, 119, 210–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickstead, E.S.; Irving, M.A.; Getting, S.J.; McArthur, S. Exploiting formyl peptide receptor 2 to promote microglial resolution: A new approach to Alzheimer’s disease treatment. FEBS J. 2021. [Google Scholar] [CrossRef]
- Singh, S.; Sahu, K.; Singh, C.; Singh, A. Lipopolysaccharide induced altered signaling pathways in various neurological disorders. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 285–294. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Portugal, C.C.; Almeida, T.O.; Socodato, R.; Relvas, J.B. Src family kinases (SFKs): Critical regulators of microglial homeostatic functions and neurodegeneration in Parkinson’s and Alzheimer’s diseases. FEBS J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Li, L. Microglia Regulate Neuronal Circuits in Homeostatic and High-Fat Diet-Induced Inflammatory Conditions. Front. Cell. Neurosci. 2021, 15, 722028. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-Q.; Liu, J.-F.; Luo, W.; Wong, C.-H.; So, K.-F.; Hu, Y.; Chiu, K. Lycium barbarum extract promotes M2 polarization and reduces oligomeric amyloid-beta-induced inflammatory reactions in microglial cells. Neural Regen Res. 2022, 17, 203–209. [Google Scholar]
- Wang, J.; Qin, X.; Sun, H.; He, M.; Lv, Q.; Gao, C.; He, X.; Liao, H. Nogo receptor impairs the clearance of fibril amyloid-beta by microglia and accelerates Alzheimer’s-like disease progression. Aging Cell 2021, 20, e13515. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, B.; von Bernhardi, R. Transforming Growth Factor beta 1 Modulates Amyloid beta-Induced Glial Activation Through the Smad3-Dependent Induction of MAPK Phosphatase-1. J. Alzheimers Dis. 2012, 32, 417–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padmavathy, J.; Saravanan, D. Natural product as a source of prodrug. Bangl J. Pharm. 2017, 12, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Boldi, A.M. Libraries from natural product-like scaffolds. Curr. Opin. Chem. Biol. 2004, 8, 281–286. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, C.; Huang, C. Verbascoside Attenuates Acute Inflammatory Injury Caused by an Intracerebral Hemorrhage Through the Suppression of NLRP3. Neurochem. Res. 2021, 46, 770–777. [Google Scholar] [CrossRef]
- Zivkovic, J.C.; Barreira, J.C.M.; Savikin, K.P.; Alimpic, A.Z.; Stojkovic, D.S.; Dias, M.I.; Santos-Buelga, C.; Duletic-Lausevic, S.N.; Ferreira, I.C.F.R. Chemical Profiling and Assessment of Antineurodegenerative and Antioxidant Properties of Veronica teucrium L. and Veronica jacquinii Baumg. Chem. Biodivers. 2017, 14, e1700167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Biophenols: Enzymes (beta-secretase, Cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 2018, 128, 118–129. [Google Scholar] [CrossRef]
- Wu, C.-R.; Lin, H.-C.; Su, M.-H. Reversal by aqueous extracts of Cistanche tubulosa from behavioral deficits in Alzheimer’s disease-like rat model: Relevance for amyloid deposition and central neurotransmitter function. BMC Complement. Altern. Med. 2014, 14, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Cai, X.; Wang, R.; Zhai, S.; Zhang, Y.; Hu, W.; Zhang, Y.; Wang, D. Neuroprotective effects of verbascoside against Alzheimer’s disease via the relief of endoplasmic reticulum stress in A beta-exposed U251 cells and APP/PS1 mice. J. Neuroinflamm. 2020, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.I.; Kim, D.K.; Kim, T.-H.; Kang, K.-R.; Seo, J.-Y.; Cho, S.S.; Yun, Y.; Choi, Y.-y.; Leem, J.; Kim, H.-W.; et al. Acteoside Counteracts Interleukin-1 beta-Induced Catabolic Processes through the Modulation of Mitogen-Activated Protein Kinases and the NF kappa B Cellular Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 8684725. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Z.; Zhou, X.; Zhao, Z.; Zhao, R.; Xu, X.; Kong, X.; Ren, J.; Yao, X.; Wen, Q.; et al. Microglia and macrophage exhibit attenuated inflammatory response and ferroptosis resistance after RSL3 stimulation via increasing Nrf2 expression. J. Neuroinflamm. 2021, 18, 249. [Google Scholar] [CrossRef] [PubMed]
- He, J.-H.; Liu, R.-P.; Peng, Y.-M.; Guo, Q.; Zhu, L.-B.; Lian, Y.-Z.; Hu, B.-L.; Fan, H.-H.; Zhang, X.; Zhu, J.-H. Differential and paradoxical roles of new-generation antidepressants in primary astrocytic inflammation. J. Neuroinflamm. 2021, 18, 47. [Google Scholar] [CrossRef]
- Vdovenko, D.; Bachmann, M.; Wijnen, W.J.; Hottiger, M.O.; Eriksson, U.; Valaperti, A. The adaptor protein c-Cbl-associated protein (CAP) limits pro-inflammatory cytokine expression by inhibiting the NF-kappa B pathway. Int. Immunopharmacol. 2020, 87, 106822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, C.; Dang, E.; Cao, J.; Zhu, Z.; Fu, M.; Yao, X.; Liu, Y.; Jin, B.; Wang, G.; et al. CD100-Plexin-B2 Promotes the Inflammation in Psoriasis by Activating NF-kappa B and the Inflammasome in Keratinocytes. J. Investig. Dermatol. 2018, 138, 375–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thadathil, N.; Nicklas, E.H.; Mohammed, S.; Lewis, T.L., Jr.; Richardson, A.; Deepa, S.S. Necroptosis increases with age in the brain and contributes to age-related neuroinflammation. Geroscience 2021, 43, 2345–2361. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-K.; Holland, C.V.; Loxton, K.; Barghouth, U. Cerebral Toxocariasis: Silent Progression to Neurodegenerative Disorders? Clin. Microbiol. Rev. 2015, 28, 663–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.-H.; Yu, J.-T.; Tan, L. Brain-Derived Neurotrophic Factor in Alzheimer’s Disease: Risk, Mechanisms, and Therapy. Mol. Neurobiol. 2015, 52, 1477–1493. [Google Scholar] [CrossRef]
- Chen, J.-j.; Wang, T.; An, C.-d.; Jiang, C.-y.; Zhao, J.; Li, S. Brain-derived neurotrophic factor: A mediator of inflammation-associated neurogenesis in Alzheimer’s disease. Rev. Neurosci. 2016, 27, 793–811. [Google Scholar] [CrossRef]
- Han, R.; Liu, Z.; Sun, N.; Liu, S.; Li, L.; Shen, Y.; Xiu, J.; Xu, Q. BDNF Alleviates Neuroinflammation in the Hippocampus of Type 1 Diabetic Mice via Blocking the Aberrant HMGB1/RAGE/NF-kappa B Pathway. Aging Dis. 2019, 10, 611–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawyer, A.J.; Tian, W.; Saucier-Sawyer, J.K.; Rizk, P.J.; Saltzman, W.M.; Bellamkonda, R.V.; Kyriakides, T.R. The effect of inflammatory cell-derived MCP-1 loss on neuronal survival during chronic neuroinflammation. Biomaterials 2014, 35, 6698–6706. [Google Scholar] [CrossRef] [Green Version]
- Olivera, G.C.; Ren, X.; Vodnala, S.K.; Lu, J.; Coppo, L.; Leepiyasakulchai, C.; Holmgren, A.; Kristensson, K.; Rottenberg, M.E. Nitric Oxide Protects against Infection-Induced Neuroinflammation by Preserving the Stability of the Blood-Brain Barrier. PLoS Path. 2016, 12, e1005442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, L.G.; Angelo, Y.d.S.; Iglesias, A.H.; Peron, J.P.S. Unraveling the Link Between Mitochondrial Dynamics and Neuroinflammation. Front. Immunol. 2021, 12, 624919. [Google Scholar] [CrossRef]
- Fan, Z.; Aman, Y.; Ahmed, I.; Chetelat, G.; Landeau, B.; Ray Chaudhuri, K.; Brooks, D.J.; Edison, P. Influence of microglial activation on neuronal function in Alzheimer’s and Parkinson’s disease dementia. Alzheimers Dement. 2015, 11, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, Y.; Di, C.; Zhao, C.; Chen, J.; Su, W.; Wu, Q.; Wu, M.; Su, X.; Xia, Z. Increased airway epithelial cell-derived exosomes activate macrophage-mediated allergic inflammation via CD100 shedding. J. Cell. Mol. Med. 2021, 25, 8850–8862. [Google Scholar] [CrossRef] [PubMed]
- Hemida, A.S.; Mareae, A.H.; Elbasiony, A.S.A.; Shehata, W.A. Plexin-B2 in psoriasis; a clinical and immunohistochemical study. J. Immunoass. Immunochem. 2020, 41, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.C.; Gutierrez-Vazquez, C.; Wheeler, M.A.; Li, Z.; Rothhammer, V.; Linnerbauer, M.; Sanmarco, L.M.; Guo, L.; Blain, M.; Zandee, S.E.J.; et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 2021, 372, eabf1230. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bei, Y.; Shen, S.; Huang, P.; Shi, J.; Zhang, J.; Sun, Q.; Chen, Y.; Yang, Y.; Xu, T.; et al. miR-21-3p controls sepsis-associated cardiac dysfunction via regulating SORBS2. J. Mol. Cell. Cardiol. 2016, 94, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, Z.; Hu, H.; Zhao, M.; Sun, L. Microglia in Alzheimer’s Disease: A Target for Therapeutic Intervention. Front. Cell. Neurosci. 2021, 15, 749587. [Google Scholar] [CrossRef]
- Liu, B.; Huang, B.; Hu, G.; He, D.; Li, Y.; Ran, X.; Du, J.; Fu, S.; Liu, D. Isovitexin-Mediated Regulation of Microglial Polarization in Lipopolysaccharide-Induced Neuroinflammation via Activation of the CaMKKbeta/AMPK-PGC-1alpha Signaling Axis. Front. Immunol. 2019, 10, 2650. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.Y.; Lim, S.M.; Oh, K.-W.; Cho, K.-A.; Park, J.; Kim, K.-S.; Lee, S.-J.; Kwon, M.-S.; Kim, S.H. Mesenchymal Stem Cells Modulate the Functional Properties of Microglia via TGF-beta Secretion. Stem Cells Transl. Med. 2016, 5, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.-Y.; Cho, G.-S.; Piao, H.Z.; Kim, W.-K. Role of TGF-beta in Survival of Phagocytizing Microglia: Autocrine Suppression of TNF-alpha Production and Oxidative Stress. Exp. Neurobiol. 2012, 21, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Ye, R.D. Microglial A beta Receptors in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2015, 35, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhu, Y.; Lin, N.; Zhang, J.; Ye, Q.; Huang, H.; Chen, X. Microglial phagocytosis induced by fibrillar beta-amyloid is attenuated by oligomeric beta-amyloid: Implications for Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Diniz, L.P.; Matias, I.; Siqueira, M.; Stipursky, J.; Gomes, F.C.A. Astrocytes and the TGF-β1 Pathway in the Healthy and Diseased Brain: A Double-Edged Sword. Mol. Neurobiol. 2018, 56, 4653–4679. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Feng, Z.; Zhang, Q.Z.; Zhang, J.T. Beneficial effects of melatonin in experimental models of Alzheimer disease. Acta Pharmacol. Sin. 2006, 27, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Maudsley, S.; Martin, B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004, 27, 589–594. [Google Scholar] [CrossRef]
- Meraz-Rios, M.A.; Toral-Rios, D.; Franco-Bocanegra, D.; Villeda-Hernandez, J.; Campos-Pena, V. Inflammatory process in Alzheimer’s Disease. Front. Integr. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, K.; Wei, X.; Xie, T.; Lv, B.; Zhou, Q.; Wang, X. Interaction of NF-kappa B and Wnt/beta-catenin Signaling Pathways in Alzheimer’s Disease and Potential Active Drug Treatments. Neurochem. Res. 2021, 46, 711–731. [Google Scholar] [CrossRef] [PubMed]
- Doens, D.; Fernandez, P.L. Microglia receptors and their implications in the response to amyloid beta for Alzheimer’s disease pathogenesis. J. Neuroinflamm. 2014, 11, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.M.; Han, Y.W.; Han, X.H.; Zhang, K.; Chang, Y.N.; Hu, Z.M.; Qi, H.X.; Ting, C.; Zhen, Z.; Hong, W. Upstream regulators and downstream effectors of NF-kappaB in Alzheimer’s disease. J. Neurol. Sci. 2016, 366, 127–134. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Liu, H.; Wang, S.; Jiang, H.; Gao, L.; Wang, L.; Teng, L.; Wang, C.; Wang, D. The Neuroprotection of Verbascoside in Alzheimer’s Disease Mediated through Mitigation of Neuroinflammation via Blocking NF-κB-p65 Signaling. Nutrients 2022, 14, 1417. https://doi.org/10.3390/nu14071417
Chen S, Liu H, Wang S, Jiang H, Gao L, Wang L, Teng L, Wang C, Wang D. The Neuroprotection of Verbascoside in Alzheimer’s Disease Mediated through Mitigation of Neuroinflammation via Blocking NF-κB-p65 Signaling. Nutrients. 2022; 14(7):1417. https://doi.org/10.3390/nu14071417
Chicago/Turabian StyleChen, Shanshan, Honghan Liu, Shimiao Wang, Hongbo Jiang, Le Gao, Lu Wang, Lesheng Teng, Chunyue Wang, and Di Wang. 2022. "The Neuroprotection of Verbascoside in Alzheimer’s Disease Mediated through Mitigation of Neuroinflammation via Blocking NF-κB-p65 Signaling" Nutrients 14, no. 7: 1417. https://doi.org/10.3390/nu14071417
APA StyleChen, S., Liu, H., Wang, S., Jiang, H., Gao, L., Wang, L., Teng, L., Wang, C., & Wang, D. (2022). The Neuroprotection of Verbascoside in Alzheimer’s Disease Mediated through Mitigation of Neuroinflammation via Blocking NF-κB-p65 Signaling. Nutrients, 14(7), 1417. https://doi.org/10.3390/nu14071417






