Profile of Nucleotides in Chinese Mature Breast Milk from Six Regions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Selection
2.2. Data Collection
2.3. Sample Collection
2.4. Analytical Methods
2.4.1. Chemicals and Reagents
2.4.2. Sample Preparation
2.4.3. HPLC Analysis
2.5. Data Analysis
3. Results
3.1. Characteristics of the Subjects
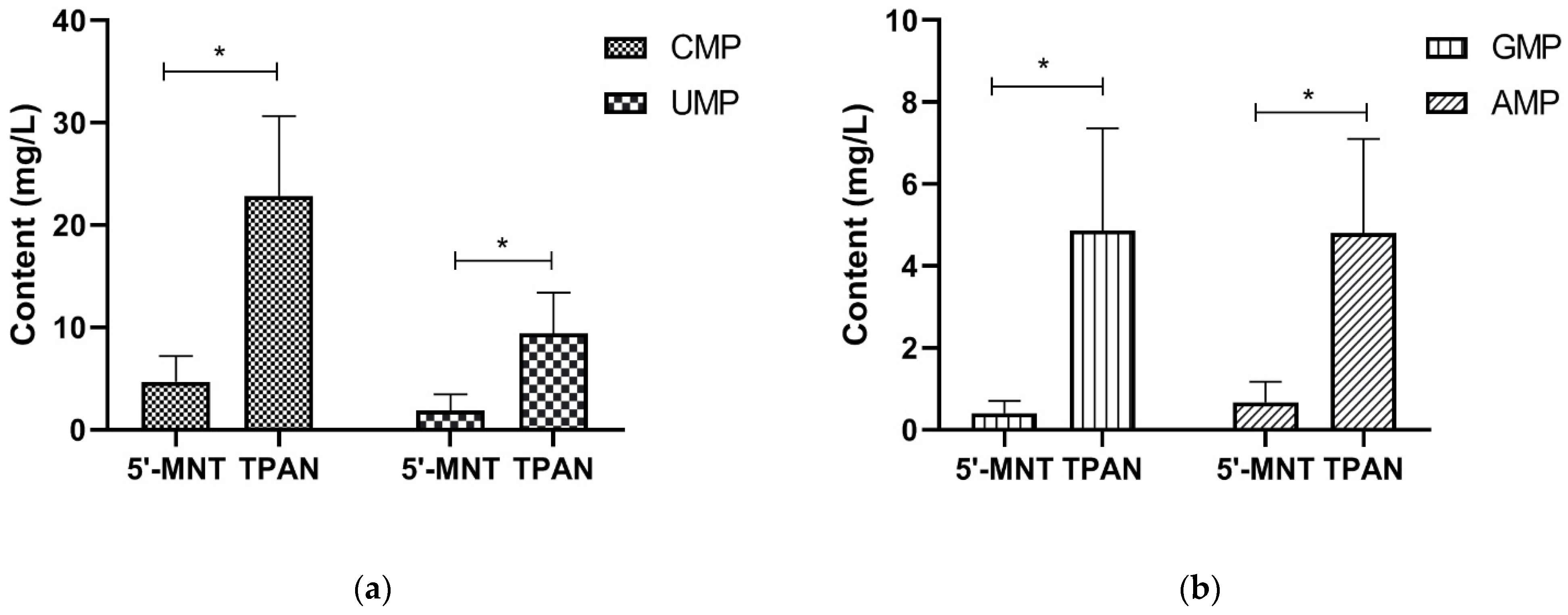
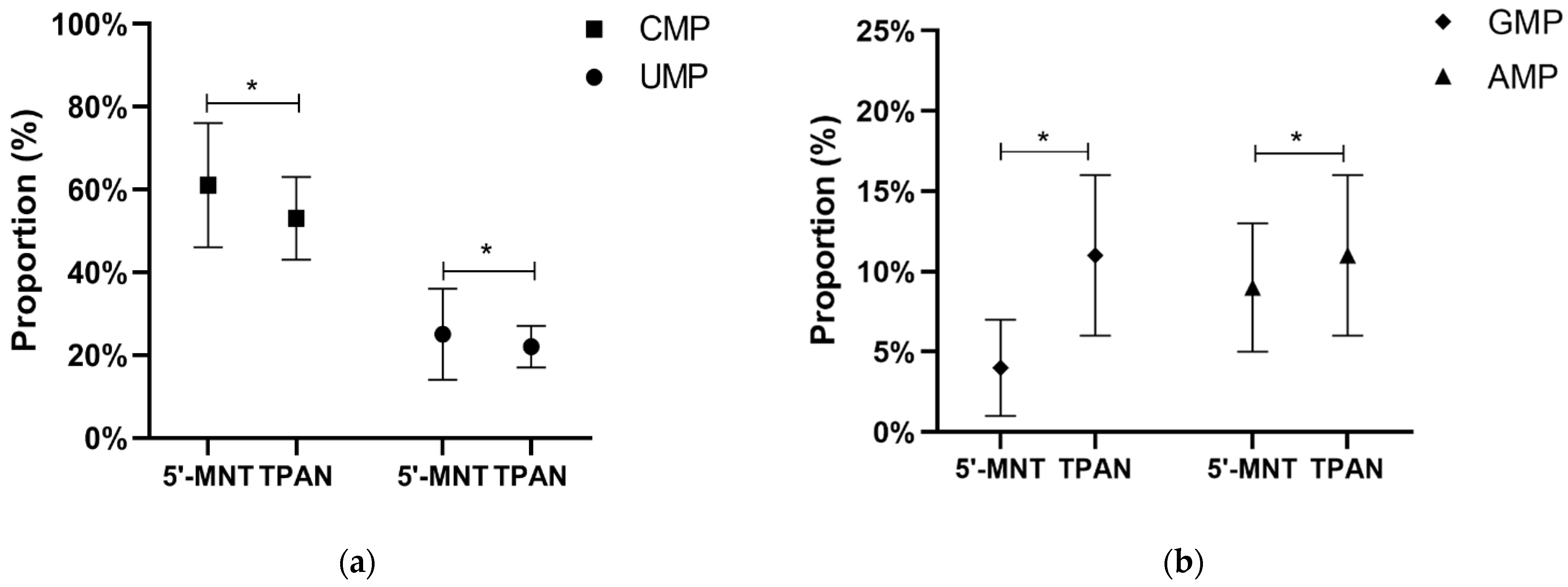
3.2. TPAN and 5′-MNT Content of Mature Milk
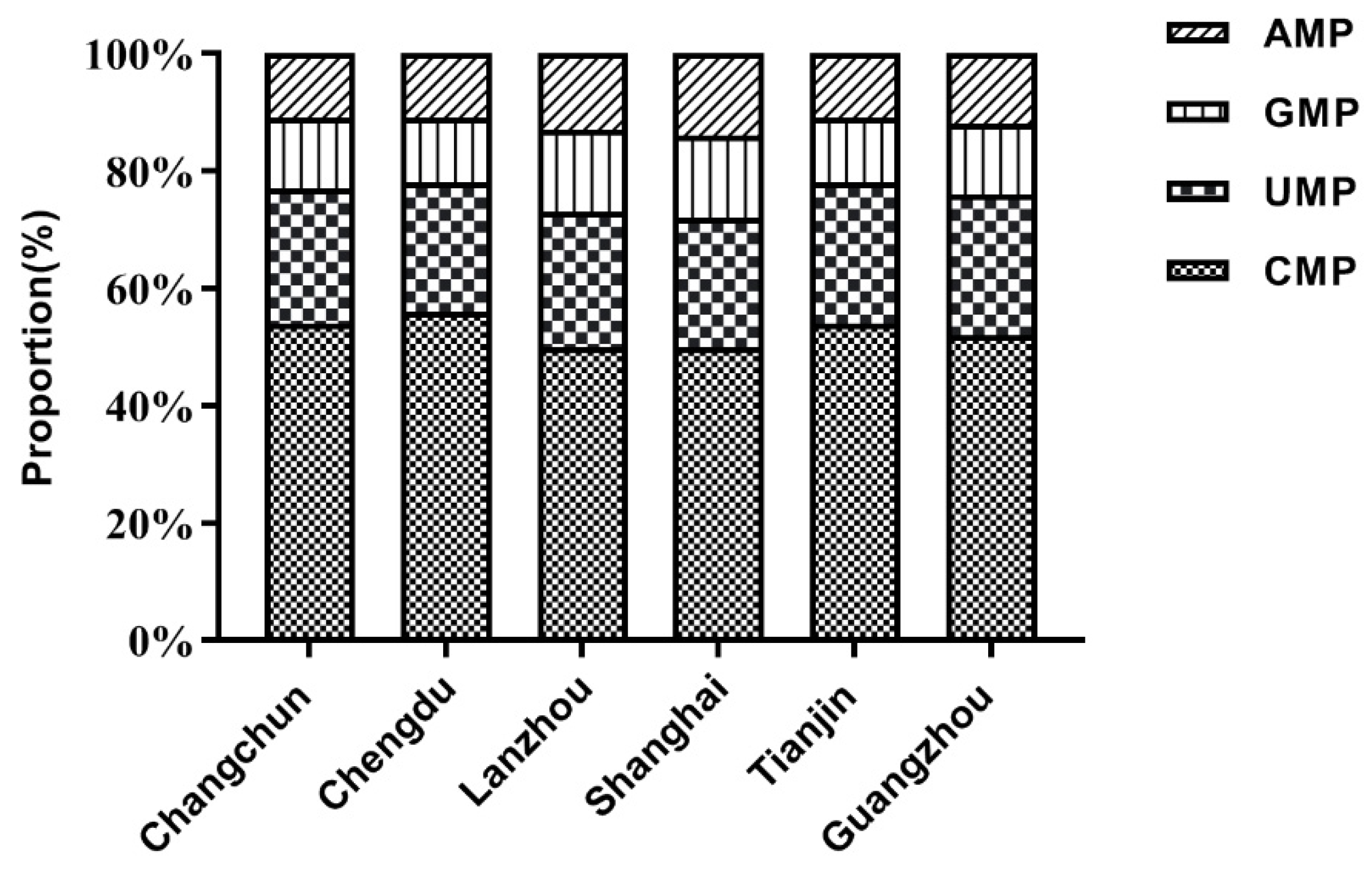
3.3. TPAN Content of Mature Milk from the Six Regions
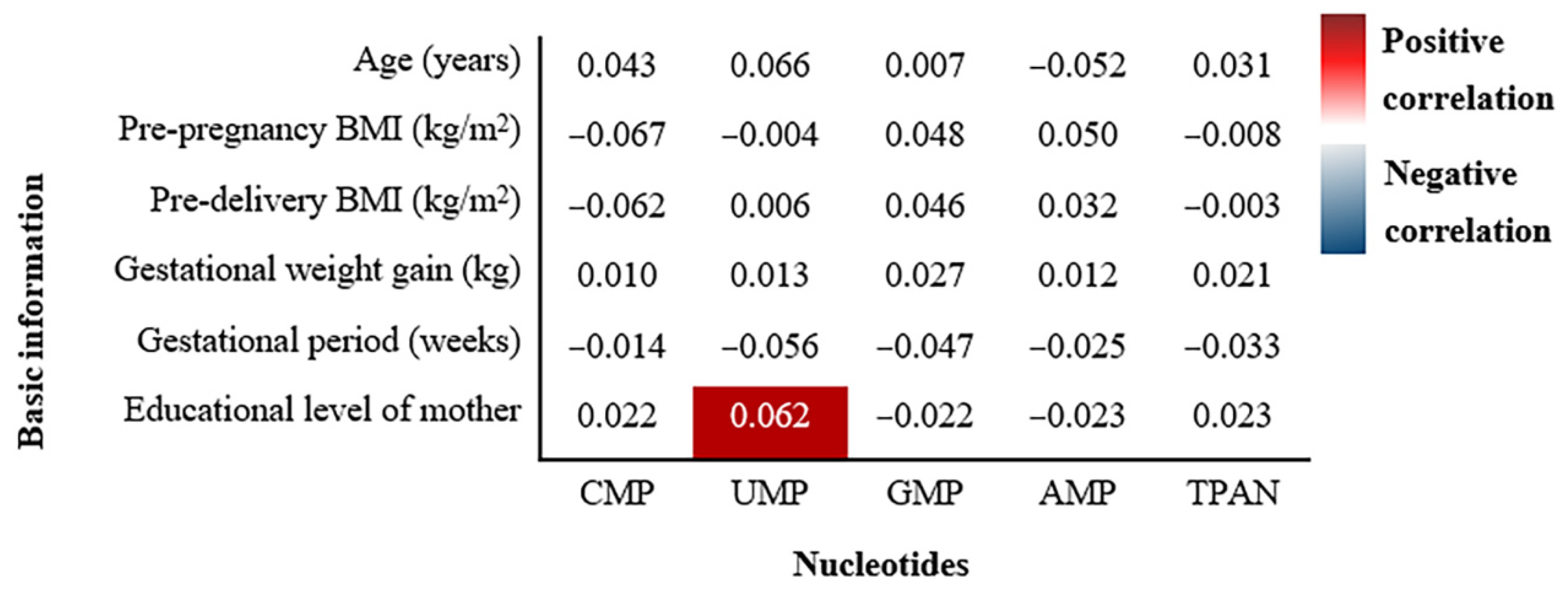
3.4. Correlations between Basic Demographic Characteristics and Nucleotides Content of Breast Milk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Grote, V.; Verduci, E.; Scaglioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C.; European Childhood Obesity Project. Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur. J. Clin. Nutr. 2016, 70, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Van de Perre, P. Transfer of antibody via mother’s milk. Vaccine 2003, 21, 3374–3376. [Google Scholar] [CrossRef]
- Donovan, S.M.; Lonnerdal, B. Non-protein nitrogen and true protein in infant formulas. Acta Paediatr. Scand. 1989, 78, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, M.L.; Santoro, J.R.; Jorge, S.M.; Goncalves, A.L. Total nitrogen and electrolyte levels in colostrum and transition human milk. J. Perinat. Med. 1986, 14, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.Y. Scientific rationale and benefits of nucleotide supplementation of infant formula. J. Paediatr. Child Health 2002, 38, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Carver, J.D. Dietary nucleotides: Effects on the immune and gastrointestinal systems. Acta Paediatr. Suppl. 1999, 88, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Janas, L.M.; Picciano, M.F. The nucleotide profile of human milk. Pediatr. Res. 1982, 16, 659–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, J.R.; Greenberg, N.A. The role of nucleotides in the immune and gastrointestinal systems: Potential clinical applications. Nutr. Clin. Pract. 2012, 27, 281–294. [Google Scholar] [CrossRef]
- Moffatt, B.A.; Ashihara, H. Purine and pyrimidine nucleotide synthesis and metabolism. Arab. Book 2002, 1, e0018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boza, J.J.; Moennoz, D.; Bournot, C.E.; Blum, S.; Zbinden, I.; Finot, P.A.; Ballevre, O. Role of glutamine on the de novo purine nucleotide synthesis in Caco-2 cells. Eur. J. Nutr. 2000, 39, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Aggett, P.; Leach, J.L.; Rueda, R.; MacLean, W.C., Jr. Innovation in infant formula development: A reassessment of ribonucleotides in 2002. Nutrition 2003, 19, 375–384. [Google Scholar] [CrossRef]
- Mateos-Vivas, M.; Rodriguez-Gonzalo, E.; Dominguez-Alvarez, J.; Garcia-Gomez, D.; Ramirez-Bernabe, R.; Carabias-Martinez, R. Analysis of free nucleotide monophosphates in human milk and effect of pasteurisation or high-pressure processing on their contents by capillary electrophoresis coupled to mass spectrometry. Food Chem. 2015, 174, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Gomez-Leon, C.; Rueda, R. Exogenous nucleic acids and nucleotides are efficiently hydrolysed and taken up as nucleosides by intestinal explants from suckling piglets. Br. J. Nutr. 2007, 98, 285–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, N.; Mosenthin, R.; Bauer, E. The role of dietary nucleotides in single-stomached animals. Nutr. Res. Rev. 2011, 24, 46–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manley-Harris, I.M. Determination of total potentially available nucleosides in bovine, caprine, and ovine milk. Int. Dairy J. 2012, 24, 40–43. [Google Scholar]
- Gill, B.D.; Indyk, H.E.; Manley-Harris, M. Determination of total potentially available nucleosides in bovine milk. Int. Dairy J. 2011, 21, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef] [Green Version]
- Klein, C.J. Nutrient requirements for preterm infant formulas. J. Nutr. 2002, 132, 1395S–1577S. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Sanchez-Medina, F. Acid-soluble nucleotides of human milk at different stages of lactation. J. Dairy Res. 1982, 49, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Sato, N.; Nakano, T.; Idota, T.; Nakajima, I. Profile of nucleotides and nucleosides of human milk. J. Nutr. Sci. Vitaminol. 1995, 41, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leach, J.L.; Baxter, J.H.; Molitor, B.E.; Ramstack, M.B.; Masor, M.L. Total potentially available nucleosides of human milk by stage of lactation. Am. J. Clin. Nutr. 1995, 61, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Tressler, R.L.; Ramstack, M.B.; White, N.R.; Molitor, B.E.; Chen, N.R.; Alarcon, P.; Masor, M.L. Determination of total potentially available nucleosides in human milk from Asian women. Nutrition 2003, 19, 16–20. [Google Scholar] [CrossRef]
- Carver, J.D. Dietary nucleotides: Cellular immune, intestinal and hepatic system effects. J. Nutr. 1994, 124, 144S–148S. [Google Scholar] [CrossRef] [PubMed]
- Barness, L.A. Dietary sources of nucleotides--from breast milk to weaning. J. Nutr. 1994, 124, 128S–130S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosgrove, M. Perinatal and infant nutrition. Nucleotides. Nutrition 1998, 14, 748–751. [Google Scholar] [CrossRef]
- Liao, K.Y.; Wu, T.C.; Huang, C.F.; Lin, C.C.; Huang, I.F.; Wu, L. Profile of nucleotides and nucleosides in Taiwanese human milk. Pediatr. Neonatol. 2011, 52, 93–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueda, R.; Gomez-Leon, C.; Gil, A. Ribonucleic acid hydrolysis by intestinal explants of neonatal piglets. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Jardi, C.; Aparicio, E.; Bedmar, C.; Aranda, N.; Abajo, S.; March, G.; Basora, J.; Arija, V.; Study Group, T.E. Food Consumption during Pregnancy and Post-Partum. ECLIPSES Study. Nutrients 2019, 11, 2447. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Campos-Gimenez, E.; Redeuil, K.M.; Leveques, A.; Actis-Goretta, L.; Vinyes-Pares, G.; Zhang, Y.; Wang, P.; Thakkar, S.K. Concentrations of Carotenoids and Tocopherols in Breast Milk from Urban Chinese Mothers and Their Associations with Maternal Characteristics: A Cross-Sectional Study. Nutrients 2017, 9, 1229. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Qi, C.; Yu, R.; Wang, X.; Zhou, Q.; Sun, J.; Jin, Q.; Wang, X. Total and sn-2 fatty acid profile of breast milk from women delivering preterm infants under the influence of maternal characteristics. Food Funct. 2018, 9, 5750–5758. [Google Scholar] [CrossRef] [PubMed]
- Beser Ozmen, E.; Okman, E.; Sari, F.N.; Kucukoglu Keser, M.; Dizdar, E.A.; Tayman, C.; Oguz, S.S. Comparison of the Macronutrient Content of Colostrum: Locals with Refugees. Breastfeed. Med. 2020, 15, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Hu, L.; Liu, Y.; Yan, C.; Peng, X.; Xu, Q.; Wang, R.; Cheng, Y.; Chen, H.; Fang, Z.; et al. Dietary Nucleotides Supplementation Improves the Intestinal Development and Immune Function of Neonates with Intra-Uterine Growth Restriction in a Pig Model. PLoS ONE 2016, 11, e0157314. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, M.; Davies, D.P.; Jenkins, H.R. Nucleotide supplementation and the growth of term small for gestational age infants. Arch. Dis. Child. Fetal Neonatal Ed. 1996, 74, F122–F125. [Google Scholar] [CrossRef] [Green Version]
- Carver, J.D.; Pimentel, B.; Cox, W.I.; Barness, L.A. Dietary nucleotide effects upon immune function in infants. Pediatrics 1991, 88, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Kennedy, K.; Lanigan, J.; Clough, H.; Jenkins, W.; Elias-Jones, A.; Stephenson, T.; Dudek, P.; Lucas, A. Dietary nucleotides and early growth in formula-fed infants: A randomized controlled trial. Pediatrics 2010, 126, e946–e953. [Google Scholar] [CrossRef] [PubMed]
- Yau, K.I.; Huang, C.B.; Chen, W.; Chen, S.J.; Chou, Y.H.; Huang, F.Y.; Kua, K.E.; Chen, N.; McCue, M.; Alarcon, P.A.; et al. Effect of nucleotides on diarrhea and immune responses in healthy term infants in Taiwan. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 37–43. [Google Scholar] [CrossRef]
| Characteristic | Changchun | Chengdu | Lanzhou | Shanghai | Tianjin | Guangzhou |
|---|---|---|---|---|---|---|
| N | 110 | 104 | 92 | 100 | 111 | 114 |
| Age (years) | 30.62 ± 3.21 | 30.09 ± 3.53 | 29.00 ± 4.34 | 29.58 ± 3.53 | 29.95 ± 3.19 | 28.91 ± 2.99 |
| BMI (kg/m2), Pre-gestation | 21.7 ± 3.2 | 21.0 ± 2.7 | 21.6 ± 3.0 | 21.3 ± 3.0 | 22.2 ± 4.0 | 20.5 ± 3.5 |
| BMI (kg/m2), Pre-delivery | 28.0 ± 4.9 | 26.4 ± 2.9 | 27.2 ± 3.2 | 26.8 ± 3.1 | 27.6 ± 3.5 | 25.9 ± 3.7 |
| Gestational weight gain (kg) | 17.4 ± 5.7 | 13.8 ± 4.2 | 14.7 ± 4.4 | 14.5 ± 5.1 | 14.6 ± 4.9 | 13.9 ± 5.2 |
| Gestational period (weeks) | 38.9 ± 1.2 | 39.0 ± 1.1 | 38.8 ± 2.0 | 39.5 ± 1.0 | 39.3 ± 1.1 | 38.9 ± 3.8 |
| First pregnancy | 59 (53.6) | 35 (34.2) | 50 (54.4) | 53 (53.0) | 68 (61.3) | 70 (61.4) |
| Parity | 73 (66.4) | 37 (35.6) | 50 (54.4) | 54 (54.0) | 70 (63.1) | 70 (61.4) |
| Vaginal delivery | 35 (31.8) | 31 (29.8) | 46 (50.0) | 70 (70.0) | 79 (71.2) | 90 (79.0) |
| Educational level | ||||||
| High school or below | 7 (6.4) | 22 (21.2) | 31 (33.7) | 15 (15.0) | 28 (25.2) | 19 (16.7) |
| College | 71 (64.6) | 64 (61.5) | 57 (62.0) | 72 (72.0) | 64 (57.7) | 84 (73.7) |
| Master or above | 32 (29.1) | 18 (17.3) | 4 (4.4) | 13 (13.0) | 19 (17.1) | 11 (9.7) |
| Offspring gender | ||||||
| Male | 53 (48.2) | 47 (45.2) | 41 (44.6) | 60 (60.0) | 53 (47.8) | 56 (49.1) |
| Female | 57 (51.8) | 57 (54.8) | 59 (55.4) | 40 (40.0) | 58 (52.3) | 58 (50.9) |
| The occupation of mother | ||||||
| Housework | 7 (6.4) | 16 (15.4) | 24 (26.1) | 13 (13.0) | 19 (17.3) | 9 (7.9) |
| Civil servant | 23 (21.1) | 23 (22.1) | 35 (38.0) | 17 (17.0) | 27 (24.6) | 7 (6.1) |
| Company employee | 21 (19.3) | 40 (38.5) | 14 (15.2) | 60 (60.0) | 47 (42.7) | 55 (48.3) |
| Worker or other occupation | 58 (53.2) | 25 (24.0) | 19 (20.7) | 10 (10.0) | 17 (15.5) | 43 (37.7) |
| TPAN (n = 631) | 5′-MNT (n = 283) | |||||||
|---|---|---|---|---|---|---|---|---|
| Median (P25, P75) (mg/L) | Min (mg/L) | Max (mg/L) | Percentage (%) | Median (P25, P75) (mg/L) | Min (mg/L) | Max (mg/L) | Percentage (%) | |
| CMP | 22.84 (17.93, 27.95) | 2.35 | 63.88 | 52.9 | 4.67 (3.33, 6.07) | 0.51 | 14.78 | 61.2 |
| UMP | 9.37 (7.33, 12.50) | 2.69 | 32.00 | 21.9 | 1.86 (0.96, 3.23) | 0.12 | 14.73 | 25.3 |
| GMP | 4.86 (3.40, 6.68) | 1.23 | 32.28 | 11.7 | 0.40 (0.28, 0.64) | 0.06 | 6.74 | 4.0 |
| AMP | 4.80 (3.54, 6.62) | 1.24 | 28.62 | 11.6 | 0.67 (0.33, 1.09) | 0.03 | 6.54 | 8.4 |
| Total | 43.26 (35.28, 52.15) | 11.61 | 111.09 | 100 | 7.95 (5.03,11.06) | 0.90 | 25.35 | 100 |
| Changchun | Chengdu | Lanzhou | Shanghai | Tianjin | Guangzhou | p | |
|---|---|---|---|---|---|---|---|
| N | 110 | 104 | 92 | 100 | 111 | 114 | — |
| CMP | 24.20 (19.54, 31.73) a | 24.51 (20.16, 29.02) a | 20.10 (14.93,25.98) b | 21.82 (16.4, 27.09) ab | 22.83 (18.73,27.18) ab | 22.53 (17.05, 27.15) ab | <0.001 |
| UMP | 9.55 (7.54, 13.43) | 8.56 (7.06, 10.84) | 9.57 (7.72, 12.87) | 9.12 (7.10, 12.64) | 9.24 (7.62, 12.22) | 9.94 (7.40, 12.74) | 0.051 |
| GMP | 5.16 (3.47, 7.00) ab | 4.32 (3.38, 6.01) b | 6.36 (3.99, 10.47) a | 5.25 (3.68, 7.60) ab | 4.20 (2.90, 6.36) b | 4.46 (3.23, 6.57) b | <0.001 |
| AMP | 4.82 (3.29, 6.28) a | 3.97 (3.15, 5.19) b | 7.40 (4.63, 10.75) c | 6.01 (4.80, 7.25) c | 4.38 (3.19, 5.84) ab | 4.38 (3.32, 5.95) ab | <0.001 |
| TPAN | 47.01 (35.10, 54.61) | 42.58 (36.67, 48.82) | 45.36 (34.28, 57.08) | 43.83 (35.75, 50.54) | 41.02 (33.14, 51.10) | 42.12 (34.91, 51.94) | 0.218 |
| Changchun | Chengdu | Lanzhou | Shanghai | Tianjin | Guangzhou | p | |
|---|---|---|---|---|---|---|---|
| CMP | 54.7 a | 58.4 b | 46.3 c | 50.0 c | 53.6 a | 52.9 a | <0.001 |
| UMP | 21.6 ab | 20.6 a | 21.5ab | 21.1ab | 22.5 b | 23.5 b | <0.001 |
| GMP | 11.6 ab | 10.4 b | 14.8 c | 12.6 ac | 10.1 b | 11.1 ab | <0.001 |
| AMP | 10.7 a | 9.7 a | 16.2 b | 15.0 b | 10.7 a | 10.6 a | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Guo, Z.; Yu, M.; Cai, X.; Mao, Y.; Tian, F.; Xu, W.; Liu, G.; Li, X.; Zhao, Y.; et al. Profile of Nucleotides in Chinese Mature Breast Milk from Six Regions. Nutrients 2022, 14, 1418. https://doi.org/10.3390/nu14071418
Yang L, Guo Z, Yu M, Cai X, Mao Y, Tian F, Xu W, Liu G, Li X, Zhao Y, et al. Profile of Nucleotides in Chinese Mature Breast Milk from Six Regions. Nutrients. 2022; 14(7):1418. https://doi.org/10.3390/nu14071418
Chicago/Turabian StyleYang, Lutong, Zhiheng Guo, Miao Yu, Xiaokun Cai, Yingyi Mao, Fang Tian, Wenhui Xu, Guoliang Liu, Xiang Li, Yanrong Zhao, and et al. 2022. "Profile of Nucleotides in Chinese Mature Breast Milk from Six Regions" Nutrients 14, no. 7: 1418. https://doi.org/10.3390/nu14071418
APA StyleYang, L., Guo, Z., Yu, M., Cai, X., Mao, Y., Tian, F., Xu, W., Liu, G., Li, X., Zhao, Y., & Xie, L. (2022). Profile of Nucleotides in Chinese Mature Breast Milk from Six Regions. Nutrients, 14(7), 1418. https://doi.org/10.3390/nu14071418






