Abstract
A gluten-free diet is the mainstay method of treatment and the prevention of celiac disease complications. However, an inadequately balanced gluten-free diet can increase the risk of obesity, negatively affect glucose and lipid metabolism, and increase the risk of the metabolic syndrome. Therefore, an adequate nutritional counselling is necessary for patients diagnosed with celiac disease in order to prevent and treat the components of the metabolic syndrome.
1. Introduction
Celiac disease (CD) is a chronic autoimmune enteropathy of the small intestine which lasts a lifetime [1,2,3]. It is characterized by digestive, as well as absorption disorders, and is caused by an abnormal immune response to prolamines (alcohol-soluble protein fractions) contained in cereals [4]. These compounds, commonly known as gluten, include wheat protein, gliadin; the protein contained in rye, secalin; and in barley, hordein [5,6]. According to the hypothesis of Kӧttgen, the occurrence of celiac disease is determined by genetic, environmental, infectious, metabolic, and immunological factors [7,8,9,10,11].
In the general population, the prevalence of celiac disease amounts to 0.5–1% [12,13]. An increased occurrence of celiac disease can be observed in individuals with autoimmune diseases, such as type 1 diabetes, autoimmune hepatitis, thyroiditis, alopecia areata, Dermatitis Herpetiformis, or psoriasis [13,14,15,16]. While the incidence of celiac disease in autoimmune hepatitis ranges between 3–6% in adults and about 20% in children, the co-occurrence of type 1 diabetes and CD can be found in 1–10% of patients in the European population [17,18]. An increased risk of developing celiac disease has been reported in patients affected by the chromosomal disorders, such as Down’s syndrome, as well as Williams’ syndrome, and Turner’s syndrome [19,20]. According to Fasano et al., the prevalence of CD amounted to 1:10 in first-degree relatives and 1:39 in second-degree relatives [21]. A systematic review conducted in the MEDLINE (1966–2003) and EMBASE (1947–2003) databases highlights the incidence of celiac disease in 20% of first-degree relatives [22]. A gluten-free diet (GFD) is the mainstay method of both treatment and the prevention of celiac disease complications. However, it should be emphasized that an inadequately balanced gluten-free diet, rather than CD diagnosis, may result in the metabolic consequences [23]. Gluten elimination is recommended only in the treatment of clinical gluten sensitivity, e.g., in celiac disease, or non-celiac gluten sensitivity. [24] A randomized, double-blind trial involving 30 volunteers who had not been diagnosed with celiac disease, did not reveal any symptomatic benefits of a GFD in individuals without gluten-dependent diseases. DRCT (double-blind randomized controlled trial) has also demonstrated that gluten consumption does not cause any symptoms in healthy volunteers [25].
A gluten-free diet requires a complete elimination of wheat, rye and barley [26,27,28]. Indeed, even as little as 50 mg of gluten, which can be found in a few breadcrumbs or a small piece of pasta, can increase enteropathy [29]. Furthermore, many products can be contaminated with gluten during harvesting, processing or packaging [27]. A good example can be gluten-free oats, which are often contaminated with wheat or barley [30]. Furthermore, wheat is often used as a thickening or filling agent for numerous meat products, soups, sauces, and as an coating for meat or eggs [31]. According to the Codex Alimentarius Commission, gluten-free products are foods which contain ≤20 ppm gluten (20 mg of gluten per 1 kg of food sold) [32]. A growing body of evidence suggests that most individuals with celiac disease will tolerate foods with low gluten content (≤20 mg/kg) [33]. Nevertheless, patients with non-Responsive Celiac Disease (NRCD) are particularly sensitive even to trace amounts of gluten [34]. In the study of Hollon JR et al., patients with suspected refractory celiac disease type 1 followed a diet eliminating gluten completely for a period of 3–6 months. This alimentation, which was solely based on fresh, unprocessed foods, was defined as the Gluten Contamination Elimination Diet (GCED). Consequently, a remission of some symptoms was observed in the patients with NRCD taking part in the study [35]. It is believed that GCED can also be used to distinguish between individuals who are “hypersensitive” to the classic gluten-free diet and patients with true Refractory Celiac Disease (RCD) [36]. According to the study conducted by Freeman which included 182 patients, a regeneration and normalization of the small intestinal mucosa could occur even within 6 months of a strict compliance to a gluten-free diet, while the best results were observed after a year or two of such a procedure. Furthermore, it was observed that after the six-month-long exclusion of gluten, the health of women improved to a greater extent than that of men. However, in individuals over 65 years of age, the improvement was slower than in younger age groups, although it was mainly observed in men [37].
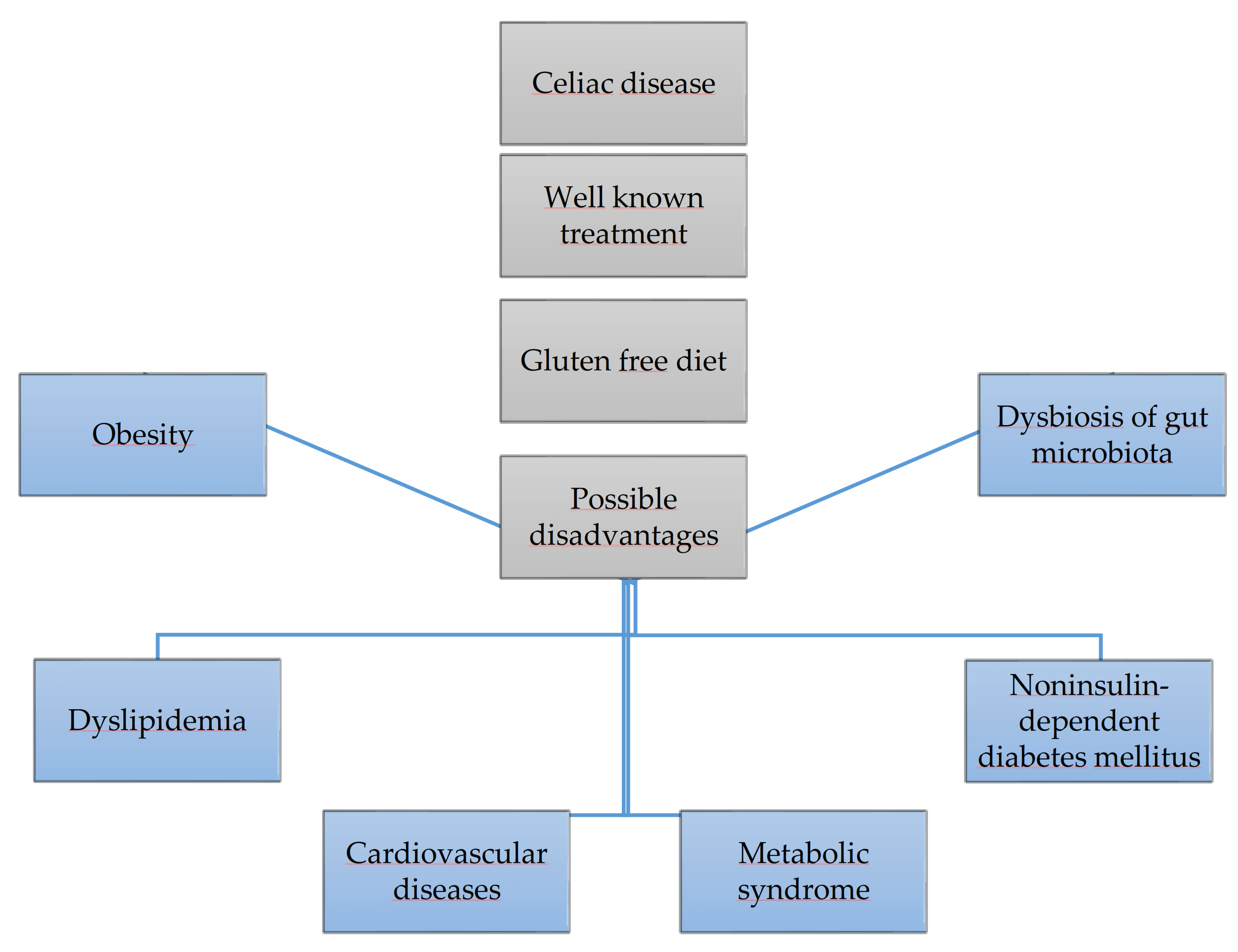
A gluten-free diet is the only effective method of treating celiac disease. However, it may increase the risk of obesity, themetabolic syndrome, as well as negatively affect glucose,lipid metabolism and the intestinal microbiota, it is shown in Figure 1. This paper reviews the available published papers regarding this topic.
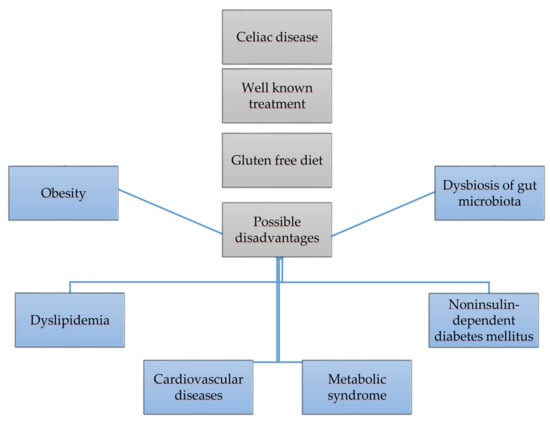
Figure 1.
Gluten free diet and its risks.
2. Material and Methods
In order to collect the literature data related to the presented topic, the PubMed database (www.pubmed.ncbi.nlm.nih.gov, accessed on 22 April 2020) was explored using the terms: “celiac disease”, “gluten-free diet”, “dyslipidemia”, “obesity”, “diabetes mellitus t.2”, “metabolic syndrome”, “cardiovascular diseases”, “gut microbiota”, and “microbiome gut dysbiosis”.
3. GFD and Its Risks
3.1. Gluten-Free Diet and Obesity
Although one of the classic manifestations of celiac disease is body weight deficiency, the number of patients suffering from overweight or obesity at the time of diagnosis is on the rise [38,39,40,41,42,43]. Research conducted over the past decade suggested that following a gluten-free diet may result in weight gain [44,45,46]. In fact, Valletta et al. demonstrated a significant increase in body weight following the implementation of a gluten-free diet [47]. Additionally, the researchers hypothesized that the reduction in dietary fiber, excess fats and the supply of hypercaloric drinks and high-glycemic grain products resulted in an elevated body weight [47,48,49,50,51,52]. Moreover, the authors emphasized that the improper eating habits of these patients may be due to the reduced taste values of gluten-free products, which in turn translated into an increased consumption of sweet and salty snacks [44]. This hypothesis is supported in the study conducted by Dall’Asta C. et al., which indicated a preference in high-fat meals and a high consumption of sweets as well as sweetened, colorful drinks, and a low consumption of fruit or vegetables among the 118 study participants [53]. Furthermore, two meta-analyses from 2018 and 2019 demonstrated that both adults and children on a gluten-free diet consumed too much energy, fats, cereals with a high glycemic index and a low content of dietary fiber and nutrients [54,55].
In 1986, Semeraro L.A. suggested that an increase in the absorption in the distal sections of the intestine might compensate the jejunal atrophy in patients with celiac disease [56]. He assumed that this process might be similar to structural changes in the residual intestine occurring after a partial surgical resection of the intestine. This adaptation includes morphological changes, such as an increase in villi height, as well as an increase of the depth of the intestinal crypts, and the number of intestinal epithelial cells. Furthermore, in patients with CD, atrophy causes loss of normal bowel function which may induce an increased absorption in the functionally preserved segments of the gastrointestinal tract. Thus, if this process leads to an overcompensation, it may result in the child’s energy requirements being exceeded, thus increasing the risk of overweight or obesity [56]. This compensatory hypothesis seems to be supported by some of the first published cases of adolescents with CD who were still overweight or obese despite villi atrophy in the jejunal biopsy [57,58]. The compensatory surface area of the small intestine appears to increase with age. Therefore, the intestines can develop the ability to absorb the adequate amount of energy [56]. This is confirmed by the broad spectrum of symptoms following the diagnosis of CD, which appears to be age-related [59,60,61]. Moreover, while children under 2 years of age often present with the classic form of CD, including malabsorption, older children, adolescents, and adults report unusual symptoms [3,62] whichseems to be consistent with the compensation hypothesis. In fact, the classic symptoms may be due to a lack of intestinal adaptation, which is less developed in the young individuals. Additionally, a lack of intestinal adaptation causes severe and classic symptoms, including malabsorption and visceral crisis, which occur in very young children recently diagnosed with CD. Intestinal adaptation is a time-dependent phenomenon, and the likelihood of modifying a person’s small intestine mucosa increases with age; hence, it is possible to relieve the symptoms of CD in older children and adolescents [63]. According to this hypothesis, there is no correlation between CD presentation and the degree of villous atrophy or the degree of the intestinal involvement visualized by endoscopic procedures and video-capsules [64,65]. Moreover, the nutritional status of the general population is of utmost importance for the correct interpretation of BMI in children at the time of the diagnosis of CD. It is vital to bear in mind that celiac disease can develop in overweight/obese patients, reflecting the individual predispositions (i.e., genetic, nutritional and environmental factors) [66,67]. Furthermore, the worldwide prevalence of overweight and obesity in children has increased over the past two decades; in fact, it is estimated that 60 million children will have been overweight or obese by 2020 [68]. In a 10-year study by Dickey W. involving 371 patients, the mean BMI amounted to 24.6 kg/m2 (range 16.3–43.5). 17 patients (5%) were underweight (BMI <18.5), 211 (57%) had a normal body weight and 143 (39%) were overweight (BMI ≥ 25), of whom 48 (13% of all patients) were obese (BMI ≥ 30.0). In the groups of patients following a gluten-free diet, 81% gained weight after 2 years, with 82% of subjects initially being overweight [69]. On the other hand, Murray et al. demonstrated that 6% of 215 adult CD patients who were obese at the time of the diagnosis, presented with a decrease in BMI following 6 months of a GFD [70]. According to West et al., 3.9% of 3590 CD patients were obese, and 17% were overweight [71]. Furthermore, Viljamaa et al., in their 14-year study involving 50 patients with CD, found that 15% of them were categorized as obese [72]. Conversely, in the prospective study involving 698 subjects with newly diagnosed celiac disease, 4% of the participants were underweight, 57% presented a normal body weight, 28% were overweight, and 11% were obese. Following the implementation of a gluten-free diet, 69% of the weight-deficient patients gained weight, and 18% of those who were overweight and 42% of the obese patients experienced weight loss [73]. A comparison of mean BMI values at the time of the diagnosis and after following a gluten-free diet is shown in Table 1.

Table 1.
Comparison of mean BMI values at the time of the diagnosis and after following a gluten-free diet.
3.2. Gluten-Free Diet and the Metabolic Syndrome
A gluten-free diet is based on the products which have a high glycemic index and are devoid of dietary fiber. These foods contain many simple carbohydrates and fats. These factors can give rise to nutritional deficiencies, constipation, and the development of the metabolic syndrome [82]. The malabsorption syndrome observed in celiac disease, as well as an improperly balanced gluten-free diet lead to nutritional deficiencies including iron, folic acid, calcium, and vitamin D, as well as B vitamins and zinc [83].
Numerous publications emphasize that an improperly balanced gluten-free diet may entail the development of the metabolic syndrome due to the consumption of high-glycemic cereal products and foods with a high content of fatty acids [74,79,84]. The metabolic syndrome is referred to as a set of interrelated factors which significantly increase the risk of developing atherosclerosis, type 2 diabetes, and their cardiovascular complications. The consensus of the International Diabetes Federation (IDF), National Heart, Lung, and Blood Institute (NHLBI), American Heart Association (AHA), World Heart Federation (WHF), International Atherosclerosis Society (IAS), and the International Association for the Study of Obesity (IASO) dating back to 2009 states that for the diagnosis of the metabolic syndrome (MetS) any three of the following five criteria should be fulfilled:
- Waist circumference (depending on the country of origin and ethnic group—in the European population ≥80 cm in women and ≥94 cm in men);
- Triglyceride concentration>1.7 mmol/L (150 mg/dL) or the treatment of hypertriglyceridemia;
- HDL-C (high-density lipoprotein) concentration <1.0 mmol/L (40 mg/dL) in men and <1.3 mmol/L (50 mg/dL) in women or the treatment of low HDL-C;
- Systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg, or the treatment of previously diagnosed arterial hypertension;
- Fasting glucose ≥ 5.6 mmol/L (100 mg/dL) or a drug treatment of type 2 diabetes [85].
In the study of Tortora R involving 98 newly diagnosed patients, it was observed that the number of individuals diagnosed with the metabolic syndrome 6 vs. 29 (p < 0.01; OR: 20) was increased following one year of a GFD. Pertaining to the diagnosis of the metabolic syndrome, a rise was observed in the number of participants presenting with the following: an increased waist circumference 48 vs. 72 (p < 0.01; OR: 2.8), high blood pressure 4 vs. 18 (p < 0.01; OR: 5.2), exceeded glucose threshold 7 vs. 25 (p = 0.01; OR: 4.4) and an elevated triglycerides concentration level 7 vs. 16 (p = 0.05) [74]. Moreover, similar conclusions were reached by Italian researchers who enrolled 185 adults with celiac disease in their study. At the time of the diagnosis, the metabolic syndrome was diagnosed in 3.24% (n = 6) of the participants, and after the introduction of a gluten-free diet, the number of patients with MetS increased to 14.59% (n = 26; p < 0.0001) [79]. A prospective study, including 301 patients with celiac disease, evaluated the predictors of the metabolic syndrome at the time of the diagnosis and after one year of gluten-free dieting. While upon the diagnosis 4.3% of the participants met the criteria for MetS, 23.9% developed the metabolic syndrome (4.3% vs. 23.9%; p < 0.001; OR 6.9) following 1 year of a GFD [84]. In the retrospective study conducted by Kabbani et al. 840 patients with celiac disease were compared with 840 healthy individuals in terms of age, gender, and ethnicity. The incidence of the metabolic syndrome was significantly lower in patients with celiac disease, as compared to the control group (3.5% vs. 12.7%; p < 0.0001). In fact, the mean BMI of patients with CD was significantly lower than in the control group (24.7 vs. 27.5; p < 0.0001) [86].
3.3. Gluten-Free Diet and Dyslipidemia
An inadequately balanced gluten-free diet may be associated with an increase in the total cholesterol, LDL, triglycerides, and a decreased concentration of HDL fraction [79,81,87]. In the following research studies, lipids concentrations were evaluated before and after the introduction of a gluten-free diet. Tortora R et al. compared the concentration of triglycerides at the time of the diagnosis and after 4 years of a gluten-free diet compliance. However, in another prospective study, the same authors did not demonstrate statistically significant changes in the triglyceride levels following a year of a gluten-free diet [74,75]. A cohort retrospective study involving 185 celiac disease patients revealed an increase in cholesterol and triglycerides, as well as a reduction in high-density lipoproteins after complying to a gluten-free diet [79]. On the other hand, in one cross-sectional study, the incidence of dyslipidemia was significantly lower in comparison to the control group (18.3% vs. 34.9%; p < 0.0001) [86]. The lipid profile of patients at the time of the CD diagnosis and after the introduction of a gluten-free diet is shown in Table 2.

Table 2.
The lipid profile ofpatients at the time of the CD diagnosis and after the introduction of a gluten-free diet.
3.4. Gluten-Free Diet and Cardiovascular Diseases (CVD)
The impact of a gluten-free diet on the development of cardiovascular disease remains unclear. There are studies which confirm the atherogenic effects of a gluten-free diet, whereas others report that this diet may have antiatherosclerosis effects. In the study by Brar P et al. involving 132 patients with celiac disease, the concentration of total cholesterol, LDL, and HDL fractions was assessed after six months of adherence to a strict gluten-free diet. The exclusion criteria comprised an increased concentration of total cholesterol, LDL fraction, triglycerides, diabetes, thyroid, liver, and pancreatic diseases at the time of the diagnosis of CD. An increase in the total cholesterol and high-density lipoprotein (p < 0.0001), but not the low-density lipoprotein (p = 0.06) was observed. The study indicated that the LDL/HDL ratio in the study group decreased by 0.36 ± 0.7 (p < 0.0001) [64]. In contrast, Zanini B. et al. in the retrospective analysis of the effect of a 1–5 years of a gluten-free diet with715 celiac patients, demonstrated a significant increase in BMI (21.4 ± 3.4 vs. 21.4 ± 3.4 vs. 22.5 ± 3.5; p < 0.0001), in the total cholesterol (171.2 ± 37.4 mg/dL vs. 181.4 ± 35.1 mg/dL; p < 0.0001), and in γ-Glutamyl transpeptidase (16.5 ± 14.9 vs. 19.5 ± 19.2 U/L; p < 0.0001), with a simultaneous reduction in the concentration of triglycerides (87.9 ± 49.5 vs. 80.2 ± 42.8 mg/dL; p < 0.0001) and homocysteine (16.9 ± 9, 6 vs. 13.3 ± 8.0 μmol/L; p = 0.018). Nevertheless, in this case, due to the unavailability of comprehensive information, it was not possible to estimate the CVD risk. Therefore, an alternative concept of patients with “a low risk of CVD” was applied which included a combination of factors, such as BMI> 25, blood glucose <100 mg/dL, total cholesterol <200 mg/dL, triglycerides <150 mg/dL and γ -GT 14–27 U/L, which decreased from 58% to 47% [80]. A cross-sectional study conducted at the Israel Schneider Medical Center for Children (Petach Tiqva, Israel) and San Paolo Hospital (Milan, Italy) enrolled 114 children with CD in a serologic remission who had been on a gluten-free for at least one year. This study evaluated BMI, waist circumference, LDL, triglycerides, blood pressure, and insulin resistance. The authors found three or more coexisting CVD factors in 14.4% of the subjects, where the most common ones included a high level of triglycerides (34.8%), increased blood pressure (29.4%), and a high LDL cholesterol (24.1%) [88].
3.5. Celiac Disease, a Gluten-Free Diet, and Type 2 Diabetes
The data regarding the association of celiac disease, a gluten-free diet, and the development of type 2 diabetes is inconsistent. Despite the fact that extensive evidence reports that CD patients are less likely to develop carbohydrate disorders, numerous studies correlate the use of a highly processed gluten-free diet with an increased risk of elevated glucose levels [74,80,86]. A cross-sectional study involving 840 patients with celiac disease and 840 controls matched by age, gender and ethnicity, revealed that subjects with CD presented a three times lower incidence of DM2 compared to healthy controls (26 vs. 81). However, it should be noted that one of the alternative possibilities is the overlapping of genes which predispose to celiac disease and protect against type 2 diabetes [86]. Tissue transglutaminase, in celiac disease, increases inflammation resulting in a reduction in the expression of the γ receptor (PPARG) activated by the proliferator peroxisomes, which may correlate with a reduced risk of developing type 2 diabetes [89,90]. According to the study by Tortora involving 98 patients with a newly diagnosed celiac disease, sevenpatients presented glucose levels indicative of hyperglycemia, whereas following 12 months of a gluten-free diet, hyperglycemia was reported in 25 patients (p < 0.01). The mean blood glucose value in the study group after a GFD increased from 86 mg/dL to 92 mg/dL [74]. In another study by Tortora, an increase in glucose levels was observed in patients with CD following a gluten-free diet in comparison with the baseline (mean ± SD: 88.7 ± 13.4 mg/dL vs. 84.1 ± 19.8 mg/dL) [75]. Moreover, as indicated by Zanini B et al., a statistically significant increase in fasting glucose after a gluten-free diet (12–48 months of observation) in 497 patients was observed—87.9 ± 10.8 vs. 89.7 ± 12.2 (p < 0.0001) [80].
3.6. Gluten-Free Diet and the Intestinal Microbiota
The gut microbiota affects the metabolic processes and immunity, consequently influencing the pathophysiological mechanisms of numerous diseases [91,92,93,94,95]. There is an increasing recognition supporting the hypothesis that alterations in both the composition and function of the intestinal microbiome are associated with many chronic inflammatory diseases, including celiac disease [96,97,98]. Nevertheless, research regarding the gut microbiome has certain limitations. Indeed, it usually involves small trials and the use of low-throughput techniques, such as culture techniques and simple molecular techniques which do not involve sequencing, thus they do not account for the entire gut microbiome [99]. In the study by Nistal E et al., the composition of the microbiome in healthy subjects (n = 11) was compared with the composition of the microbiome in patients with CD on a gluten-free diet (n = 11) and with patients suffering from CD who did not follow a GFD (n = 10). Differences in the composition of the microbiota involving Lactobacillus and Bifidobacterium strains (p < 0.05) between the healthy individuals and patients with CD were observed. Moreover, it was also reported that CD patients following a GFD presented a much higher concentration of short chain fatty acids (SCFA) in stools [94]. Additionally, in a study comparing the composition of microbiota in 24 CD patients (aged 2–12) not adhering to a GFD, 18 CD patients on a GFD (aged 1–12) for at least 2 years, and 20 healthy children not on a GFD (at the age of 2–11), it was found that Bifidobacterium, Clostridium histolyticum, Clostridium lituseburense and Faecalibacterium prausnitzii were less abundant (p < 0.050) in CD patients not on a GFD as compared to the control group. However, the ratio of the Bacteroides-Prevotella genera was more abundant (p < 0.050) in CD patients without a GFD than in the control group [100]. Another study involving children with celiac disease on a GFD (n = 19) and the healthy children (n = 15) found that the levels of Lactobacillus, Enterococcus, and Bifidobacteria were significantly higher (respectively: p = 0.028; p = 0.019; p = 0.023) in stool samples of the healthy individuals than in children with CD. In contrast, Bacteroides, Staphylococcus, Salmonella, Shigella, and Klebsiella were observed in significantly higher amounts (p = 0.014) in children with celiac disease than in the healthy subjects [101]. Furthermore, in the study involving 19 children (6–12 years old) complying with a GFD for at least 2 years and 15 healthy children, it was noted that differences in the biodiversity of the intestinal microbiota were found between the groups. Additionally, among the patients with CD, the differences were related to the celiac disease activity, and Bacteroides vulgatus and Escherichia coli were detected more frequently in this patient group than in the controls (p < 0.0001) [102]. Spanish research compared the microbiota composition of 20 children with a symptomatic CD who did not follow a GFD,10 children with asymptomatic CD who complied with a GFD for 1–2 years, and 8 healthy children who served as controls. The Bacteroides and Escherichia genera were more numerous in CD patients with active disease than in the control group, whereas the ratio of Lactobacillus—Bifidobacterium to Bacteroides—Escherichia was significantly reduced in patients with the active disease or the disease in remission compared to the control group [93]. According to the Italian study involving asymptomatic patients with CD (n = 7) on a GFD for a minimum of 2 years,patients with symptomatic CD (n = 7) who did not follow a GFD, and healthy controls (n = 7). It was observed that Lactobacillus abundance was lower in children with the active form of the disease compared to the healthy individuals and the patients on a GFD. Additionally, the characterization and differentiation of Lactobacillus strains in children with CD following a GFD was similar to that in healthy children. Lactobacillus brevis, Lactobacillus rossiae and Lactobacillus pentosus were identified only in faecal samples of healthy and asymptomatic patients with CD. In addition, Lactobacillus fermentum, Lactobacillus delbrueckii subsp. bulgaricus and Lactobacillus gasseri were identified in only a few stool samples of the healthy subjects. The ratio of Lactobacillus and Bifidobacterium to Bacteroides and Enterobacteria was lower in the group of children with CD on a GFD in comparison with the healthy children [103].
4. Conclusions
The research in the last decade has highlighted the fact that that an improperly balanced gluten-free diet might result in weight gain, thus, leading to obesity and the development of the metabolic disorders, such as type 2 diabetes, the metabolic syndrome and dyslipidemia. Therefore, a particular attention should be paid in order to maintain a balanced gluten-free diet in both newly diagnosed and ongoing celiac patients. In fact, there is little evidence to support the hypothesis that alterations in the gut microbiota leading to gut dysbiosis can be observed in patients with celiac disease treated with GFD.
However, it should be emphasized that GFD is the only effective method of treating celiac disease and should not be used without medical indications by healthy individuals, due to the risk of possible complications.
Author Contributions
Conceptualization, M.M., I.K.-K.; writing—original draft preparation, M.M.; D.M.; critical revision of the manuscript, I.K.-K., A.S.-T.; P.E.; A.D.; supervision, I.K.-K.; acceptance of the final version: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AHA | American Heart Association |
| CD | Celiac Disease |
| BMI | body mass index |
| DRCT | double-blind randomized controlled trial |
| GFD | Gluten Free Diet |
| GCED | Gluten Contamination Elimination Diet |
| HDL | high-density lipoprotein |
| IAS | International Atherosclerosis Society |
| IASO | International Association for the Study of Obesity. |
| IDF | International Diabetes Federation |
| LDL | low-density lipoprotein |
| NHLBI | National Heart, Lung, and Blood Institute |
| NRCD | Non-Responsive Celiac Disease |
| RCD | Refractory Celiac Disease |
| SCFA | Short chain fatty acids |
| WHF | World Heart Federation |
References
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo Definitions for Coeliac Disease and Related Terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Kaswala, D.H.; Veeraraghavan, G.; Kelly, C.P.; Leffler, D.A. Celiac Disease: Diagnostic Standards and Dilemmas. Diseases 2015, 3, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Brusca, I. Overview of Biomarkers for Diagnosis and Monitoring of Celiac Disease. Adv. Clin. Chem. 2015, 68, 1–55. [Google Scholar] [CrossRef]
- Catassi, C.; Yachha, S.K. The Global Village of Celiac Disease. Front. Celiac Dis. 2008, 12, 23–31. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The Gluten-Free Diet: Safety and Nutritional Quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef]
- Gujral, N.; Freeman, H.J.; Thomson, A.B. Celiac Disease: Prevalence, Diagnosis, Pathogenesis and Treatment. World J. Gastroenterol. 2012, 18, 6036–6059. [Google Scholar] [CrossRef]
- Kuja-Halkola, R.; Lebwohl, B.; Halfvarson, J.; Wijmenga, C.; Magnusson, P.K.E.; Ludvigsson, J.F. Heritability of Non-HLA Genetics in Coeliac Disease: A Population-Based Study in 107000 Twins. Gut 2016, 65, 1793–1798. [Google Scholar] [CrossRef]
- Lionetti, E.; Castellaneta, S.; Francavilla, R.; Pulvirenti, A.; Tonutti, E.; Amarri, S.; Barbato, M.; Barbera, C.; Barera, G.; Bellantoni, A.; et al. Introduction of Gluten, HLA Status, and the Risk of Celiac Disease in Children. N. Engl. J. Med. 2014, 371, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Lee, H.-S.; Aronsson, C.A.; Hagopian, W.A.; Koletzko, S.; Rewers, M.J.; Eisenbarth, G.S.; Bingley, P.J.; Bonifacio, E.; Simell, V.; et al. Risk of Pediatric Celiac Disease According to HLA Haplotype and Country. N. Engl. J. Med. 2014, 371, 42–49. [Google Scholar] [CrossRef]
- Vriezinga, S.L.; Auricchio, R.; Bravi, E.; Castillejo, G.; Chmielewska, A.; Escobar, P.C.; Kolaček, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mummert, E.; et al. Randomized Feeding Intervention in Infants at High Risk for Celiac Disease. N. Engl. J. Med. 2014, 371, 1304–1315. [Google Scholar] [CrossRef]
- Oxentenko, A.S.; Rubio-Tapia, A. Celiac Disease. Mayo Clin. Proc. 2019, 94, 2556–2571. [Google Scholar] [CrossRef]
- Metso, S.; Hyytiä-Ilmonen, H.; Kaukinen, K.; Huhtala, H.; Jaatinen, P.; Salmi, J.; Taurio, J.; Collin, P. Gluten-Free Diet and Autoimmune Thyroiditis in Patients with Celiac Disease. A Prospective Controlled Study. Scand. J. Gastroenterol. 2012, 47, 43–48. [Google Scholar] [CrossRef]
- Naveh, Y.; Rosenthal, E.; Ben-Arieh, Y.; Etzioni, A. Celiac Disease-Associated Alopecia in Childhood. J. Pediatr. 1999, 134, 362–364. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; van Heel, D.A.; et al. Diagnosis and Management of Adult Coeliac Disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef]
- Abenavoli, L.; Dastoli, S.; Bennardo, L.; Boccuto, L.; Passante, M.; Silvestri, M.; Proietti, I.; Potenza, C.; Luzza, F.; Nisticò, S.P. The Skin in Celiac Disease Patients: The Other Side of the Coin. Medicina 2019, 55, 578. [Google Scholar] [CrossRef] [PubMed]
- Panetta, F.; Nobili, V.; Sartorelli, M.R.; Papa, R.E.; Ferretti, F.; Alterio, A.; Diamanti, A. Celiac Disease in Pediatric Patients with Autoimmune Hepatitis: Etiology, Diagnosis, and Management. Paediatr. Drugs 2012, 14, 35–41. [Google Scholar] [CrossRef]
- Volta, U.; Tovoli, F.; Caio, G. Clinical and Immunological Features of Celiac Disease in Patients with Type 1 Diabetes Mellitus. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Frost, A.R.; Band, M.M.; Conway, G.S. Serological Screening for Coeliac Disease in Adults with Turner’s Syndrome: Prevalence and Clinical Significance of Endomysium Antibody Positivity. Eur. J. Endocrinol. 2009, 160, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Wouters, J.; Weijerman, M.E.; van Furth, A.M.; Schreurs, M.W.J.; Crusius, J.B.A.; von Blomberg, B.M.E.; de Baaij, L.R.; Broers, C.J.M.; Gemke, R.J.B.J. Prospective Human Leukocyte Antigen, Endomysium Immunoglobulin A Antibodies, and Transglutaminase Antibodies Testing for Celiac Disease in Children with Down Syndrome. J. Pediatr. 2009, 154, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Berti, I.; Gerarduzzi, T.; Not, T.; Colletti, R.B.; Drago, S.; Elitsur, Y.; Green, P.H.R.; Guandalini, S.; Hill, I.D.; et al. Prevalence of Celiac Disease in at-Risk and Not-at-Risk Groups in the United States: A Large Multicenter Study. Arch. Intern. Med. 2003, 163, 286–292. [Google Scholar] [CrossRef]
- Dubé, C.; Rostom, A.; Sy, R.; Cranney, A.; Saloojee, N.; Garritty, C.; Sampson, M.; Zhang, L.; Yazdi, F.; Mamaladze, V.; et al. The Prevalence of Celiac Disease in Average-Risk and at-Risk Western European Populations: A Systematic Review. Gastroenterology 2005, 128, S57–S67. [Google Scholar] [CrossRef]
- Remes-Troche, J.M.; Cobos-Quevedo, O.D.J.; Rivera-Gutiérrez, X.; Hernández, G.; de la Cruz-Patiño, E.; Uscanga-Domínquez, L.F. Metabolic Effects in Patients with Celiac Disease, Patients with Nonceliac Gluten Sensitivity, and Asymptomatic Controls, after Six Months of a Gluten-Free Diet. Rev. Gastroenterol. Mex. 2020, 85, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Itzlinger, A.; Branchi, F.; Elli, L.; Schumann, M. Gluten-Free Diet in Celiac Disease-Forever and for All? Nutrients 2018, 10, 1796. [Google Scholar] [CrossRef]
- Croall, I.D.; Aziz, I.; Trott, N.; Tosi, P.; Hoggard, N.; Sanders, D.S. Gluten Does Not Induce Gastrointestinal Symptoms in Healthy Volunteers: A Double-Blind Randomized Placebo Trial. Gastroenterology 2019, 157, 881–883. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac Disease: A Comprehensive Current Review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef]
- Kupper, C. Dietary Guidelines and Implementation for Celiac Disease. Gastroenterology 2005, 128, S121–S127. [Google Scholar] [CrossRef]
- Cichewicz, A.B.; Mearns, E.S.; Taylor, A.; Boulanger, T.; Gerber, M.; Leffler, D.A.; Drahos, J.; Sanders, D.S.; Craig, K.J.T.; Lebwohl, B. Diagnosis and Treatment Patterns in Celiac Disease. Dig. Dis. Sci. 2019, 64, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Fabiani, E.; Iacono, G.; D’Agate, C.; Francavilla, R.; Biagi, F.; Volta, U.; Accomando, S.; Picarelli, A.; De Vitis, I.; et al. A Prospective, Double-Blind, Placebo-Controlled Trial to Establish a Safe Gluten Threshold for Patients with Celiac Disease. Am. J. Clin. Nutr. 2007, 85, 160–166. [Google Scholar] [CrossRef]
- Koerner, T.B.; Cléroux, C.; Poirier, C.; Cantin, I.; Alimkulov, A.; Elamparo, H. Gluten Contamination in the Canadian Commercial Oat Supply. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2011, 28, 705–710. [Google Scholar] [CrossRef]
- Zarkadas, M.; Cranney, A.; Case, S.; Molloy, M.; Switzer, C.; Graham, I.D.; Butzner, J.D.; Rashid, M.; Warren, R.E.; Burrows, V. The Impact of a Gluten-Free Diet on Adults with Coeliac Disease: Results of a National Survey. J. Hum. Nutr. Diet. 2006, 19, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Standards|CODEXALIMENTARIUS FAO-WHO. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/jp/ (accessed on 12 July 2020).
- Makovicky, P.; Makovicky, P.; Caja, F.; Rimarova, K.; Samasca, G.; Vannucci, L. Celiac Disease and Gluten-Free Diet: Past, Present, and Future. Gastroenterol. Hepatol. Bed. Bench. 2020, 13, 1–7. [Google Scholar]
- Mooney, P.D.; Evans, K.E.; Singh, S.; Sanders, D.S. Treatment Failure in Coeliac Disease: A Practical Guide to Investigation and Treatment of Non-Responsive and Refractory Coeliac Disease. J. Gastrointestin Liver Dis. 2012, 21, 197–203. [Google Scholar] [PubMed]
- Hollon, J.R.; Cureton, P.A.; Martin, M.L.; Puppa, E.L.L.; Fasano, A. Trace Gluten Contamination May Play a Role in Mucosal and Clinical Recovery in a Subgroup of Diet-Adherent Non-Responsive Celiac Disease Patients. BMC Gastroenterol. 2013, 13, 40. [Google Scholar] [CrossRef]
- Leonard, M.M.; Cureton, P.; Fasano, A. Indications and Use of the Gluten Contamination Elimination Diet for Patients with Non-Responsive Celiac Disease. Nutrients 2017, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Mucosal Recovery and Mucosal Healing in Biopsy-Defined Adult Celiac Disease. Int. J. Celiac Dis. 2017, 5, 14–18. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac Disease: Understanding the Gluten-Free Diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef]
- Singh, I.; Agnihotri, A.; Sharma, A.; Verma, A.K.; Das, P.; Thakur, B.; Sreenivas, V.; Gupta, S.D.; Ahuja, V.; Makharia, G.K. Patients with Celiac Disease May Have Normal Weight or May Even Be Overweight. Indian J. Gastroenterol. 2016, 35, 20–24. [Google Scholar] [CrossRef]
- Olén, O.; Montgomery, S.M.; Marcus, C.; Ekbom, A.; Ludvigsson, J.F. Coeliac Disease and Body Mass Index: A Study of Two Swedish General Population-Based Registers. Scand. J. Gastroenterol. 2009, 44, 1198–1206. [Google Scholar] [CrossRef]
- Venkatasubramani, N.; Telega, G.; Werlin, S.L. Obesity in Pediatric Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 295–297. [Google Scholar] [CrossRef]
- Furse, R.M.; Mee, A.S. Atypical Presentation of Coeliac Disease. BMJ 2005, 330, 773–774. [Google Scholar] [CrossRef] [PubMed]
- Tucker, E.; Rostami, K.; Prabhakaran, S.; Al Dulaimi, D. Patients with Coeliac Disease Are Increasingly Overweight or Obese on Presentation. J. Gastrointestin Liver Dis. 2012, 21, 11–15. [Google Scholar]
- Mariani, P.; Viti, M.G.; Montuori, M.; La Vecchia, A.; Cipolletta, E.; Calvani, L.; Bonamico, M. The Gluten-Free Diet: A Nutritional Risk Factor for Adolescents with Celiac Disease? J. Pediatr. Gastroenterol. Nutr. 1998, 27, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Farnetti, S.; Zocco, M.A.; Garcovich, M.; Gasbarrini, A.; Capristo, E. Functional and Metabolic Disorders in Celiac Disease: New Implications for Nutritional Treatment. J. Med. Food 2014, 17, 1159–1164. [Google Scholar] [CrossRef]
- Lojou, M.; Sahakian, N.; Dutour, A.; Vanbiervliet, G.; Bege, T.; Gaborit, B. Celiac Disease and Obesity: Is Bariatric Surgery an Option? Obes. Surg. 2020, 30, 2791–2799. [Google Scholar] [CrossRef]
- Valletta, E.; Fornaro, M.; Cipolli, M.; Conte, S.; Bissolo, F.; Danchielli, C. Celiac Disease and Obesity: Need for Nutritional Follow-Up after Diagnosis. Eur. J. Clin. Nutr. 2010, 64, 1371–1372. [Google Scholar] [CrossRef]
- Miranda, J.; Lasa, A.; Bustamante, M.A.; Churruca, I.; Simon, E. Nutritional Differences between a Gluten-Free Diet and a Diet Containing Equivalent Products with Gluten. Plant. Foods Hum. Nutr. 2014, 69, 182–187. [Google Scholar] [CrossRef]
- Ferrara, P.; Cicala, M.; Tiberi, E.; Spadaccio, C.; Marcella, L.; Gatto, A.; Calzolari, P.; Castellucci, G. High Fat Consumption in Children with Celiac Disease. Acta Gastroenterol. Belg. 2009, 72, 296–300. [Google Scholar]
- Jenkins, D.J.; Thorne, M.J.; Wolever, T.M.; Jenkins, A.L.; Rao, A.V.; Thompson, L.U. The Effect of Starch-Protein Interaction in Wheat on the Glycemic Response and Rate of In Vitro Digestion. Am. J. Clin. Nutr. 1987, 45, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Robins, G.G.; Burley, V.J.; Howdle, P.D. Evidence of High Sugar Intake, and Low Fibre and Mineral Intake, in the Gluten-Free Diet. Aliment. Pharmacol. Ther. 2010, 32, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.; Fabiano, V.; Dilillo, D.; Picca, M.; Cravidi, C.; Brambilla, P. Intakes of Nutrients in Italian Children with Celiac Disease and the Role of Commercially Available Gluten-Free Products. J. Hum. Nutr Diet. 2013, 26, 436–444. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Scarlato, A.P.; Galaverna, G.; Brighenti, F.; Pellegrini, N. Dietary Exposure to Fumonisins and Evaluation of Nutrient Intake in a Group of Adult Celiac Patients on a Gluten-Free Diet. Mol. Nutr. Food Res. 2012, 56, 632–640. [Google Scholar] [CrossRef]
- Taetzsch, A.; Das, S.K.; Brown, C.; Krauss, A.; Silver, R.E.; Roberts, S.B. Are Gluten-Free Diets More Nutritious? An Evaluation of Self-Selected and Recommended Gluten-Free and Gluten-Containing Dietary Patterns. Nutrients 2018, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Villa, M.P.; Conti, L.; Ranucci, G.; Pacchiarotti, C.; Principessa, L.; Raucci, U.; Parisi, P. Nutritional Deficiencies in Children with Celiac Disease Resulting from a Gluten-Free Diet: A Systematic Review. Nutrients 2019, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, L.A.; Barwick, K.W.; Gryboski, J.D. Obesity in Celiac Sprue. J. Clin. Gastroenterol. 1986, 8, 177–180. [Google Scholar] [CrossRef]
- Nibali, S.C.; Magazzù, G.; De Luca, F. Obesity in a Child with Untreated Coeliac Disease. Helv. Paediatr. Acta 1987, 42, 45–48. [Google Scholar]
- Czaja-Bulsa, G.; Garanty-Bogacka, B.; Syrenicz, M.; Gebala, A. Obesity in an 18-Year-Old Boy with Untreated Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 226. [Google Scholar] [CrossRef]
- Telega, G.; Bennet, T.R.; Werlin, S. Emerging New Clinical Patterns in the Presentation of Celiac Disease. Arch. Pediatr. Adolesc. Med. 2008, 162, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Alonso, M.; Fernández-Aceñero, M.; Sebastián, M. Gluten Intolerance: Sex-and Age-Related Features. Can. J. Gastroenterol. 2006, 20, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Vivas, S.; Vaquero, L.; Rodríguez-Martín, L.; Caminero, A. Age-Related Differences in Celiac Disease: Specific Characteristics of Adult Presentation. World J. Gastrointest. Pharmacol. Ther. 2015, 6, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Zanini, B.; Lanzarotto, F.; Villanacci, V.; Ricci, C.; Lanzini, A. Celiac Disease in Elderly Adults: Clinical, Serological, and Histological Characteristics and the Effect of a Gluten-Free Diet. J. Am. Geriatr. Soc. 2012, 60, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, A.; Capriati, T.; Basso, M.S.; Panetta, F.; Di Ciommo Laurora, V.M.; Bellucci, F.; Cristofori, F.; Francavilla, R. Celiac Disease and Overweight in Children: An Update. Nutrients 2014, 6, 207–220. [Google Scholar] [CrossRef]
- Brar, P.; Kwon, G.Y.; Holleran, S.; Bai, D.; Tall, A.R.; Ramakrishnan, R.; Green, P.H.R. Change in Lipid Profile in Celiac Disease: Beneficial Effect of Gluten-Free Diet. Am. J. Med. 2006, 119, 786–790. [Google Scholar] [CrossRef]
- Murray, J.A.; Rubio-Tapia, A.; Van Dyke, C.T.; Brogan, D.L.; Knipschield, M.A.; Lahr, B.; Rumalla, A.; Zinsmeister, A.R.; Gostout, C.J. Mucosal Atrophy in Celiac Disease: Extent of Involvement, Correlation with Clinical Presentation, and Response to Treatment. Clin. Gastroenterol. Hepatol. 2008, 6, 186–193, quiz 125. [Google Scholar] [CrossRef]
- Valvano, M.; Longo, S.; Stefanelli, G.; Frieri, G.; Viscido, A.; Latella, G. Celiac Disease, Gluten-Free Diet, and Metabolic and Liver Disorders. Nutrients 2020, 12, 940. [Google Scholar] [CrossRef]
- Villanueva, M.; Oyarzún, A.; Leyton, B.; González, M.; Navarro, E.; Canales, P.; Ossa, C.; Muñoz, M.P.; Bascuñán, K.A.; Araya, M. Changes in Age at Diagnosis and Nutritional Course of Celiac Disease in the Last Two Decades. Nutrients 2020, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- de Onis, M.; Blössner, M.; Borghi, E. Global Prevalence and Trends of Overweight and Obesity among Preschool Children. Am. J. Clin. Nutr. 2010, 92, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Dickey, W.; Kearney, N. Overweight in Celiac Disease: Prevalence, Clinical Characteristics, and Effect of a Gluten-Free Diet. Am. J. Gastroenterol. 2006, 101, 2356–2359. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.A.; Watson, T.; Clearman, B.; Mitros, F. Effect of a Gluten-Free Diet on Gastrointestinal Symptoms in Celiac Disease. Am. J. Clin. Nutr. 2004, 79, 669–673. [Google Scholar] [CrossRef]
- West, J.; Logan, R.F.A.; Card, T.R.; Smith, C.; Hubbard, R. Risk of Vascular Disease in Adults with Diagnosed Coeliac Disease: A Population-Based Study. Aliment. Pharmacol. Ther. 2004, 20, 73–79. [Google Scholar] [CrossRef]
- Viljamaa, M.; Collin, P.; Huhtala, H.; Sievänen, H.; Mäki, M.; Kaukinen, K. Is Coeliac Disease Screening in Risk Groups Justified? A Fourteen-Year Follow-Up with Special Focus on Compliance and Quality of Life. Aliment. Pharmacol. Ther. 2005, 22, 317–324. [Google Scholar] [CrossRef]
- Ukkola, A.; Mäki, M.; Kurppa, K.; Collin, P.; Huhtala, H.; Kekkonen, L.; Kaukinen, K. Changes in Body Mass Index on a Gluten-Free Diet in Coeliac Disease: A Nationwide Study. Eur. J. Intern. Med. 2012, 23, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Tortora, R.; Capone, P.; De Stefano, G.; Imperatore, N.; Gerbino, N.; Donetto, S.; Monaco, V.; Caporaso, N.; Rispo, A. Metabolic Syndrome in Patients with Coeliac Disease on a Gluten-Free Diet. Aliment. Pharmacol. Ther. 2015, 41, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Tortora, R.; Rispo, A.; Alisi, A.; Imperatore, N.; Crudele, A.; Ferretti, F.; Nobili, V.; Miele, L.; Gerbino, N.; Caporaso, N.; et al. PNPLA3 Rs738409 Polymorphism Predicts Development and Severity of Hepatic Steatosis but Not Metabolic Syndrome in Celiac Disease. Nutrients 2018, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, T.A.; Goldberg, A.; Kelly, C.P.; Pallav, K.; Tariq, S.; Peer, A.; Hansen, J.; Dennis, M.; Leffler, D.A. Body Mass Index and the Risk of Obesity in Coeliac Disease Treated with the Gluten-Free Diet. Aliment. Pharmacol. Ther. 2012, 35, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.C.; Liao, C.; Paski, S.; Polonsky, T.; Semrad, C.E.; Kupfer, S.S. Obesity and Cardiovascular Risk in Adults with Celiac Disease. J. Clin. Gastroenterol. 2016, 50, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Valle, N.D.; Rosania, R.; Facciorusso, A.; Trotta, A.; Cantatore, F.P.; Falco, S.; Pignatiello, S.; Viggiani, M.T.; Amoruso, A.; et al. A Comparison of the Nutritional Status between Adult Celiac Patients on a Long-Term, Strictly Gluten-Free Diet and Healthy Subjects. Eur. J. Clin. Nutr. 2016, 70, 23–27. [Google Scholar] [CrossRef]
- Ciccone, A.; Gabrieli, D.; Cardinale, R.; Di Ruscio, M.; Vernia, F.; Stefanelli, G.; Necozione, S.; Melideo, D.; Viscido, A.; Frieri, G.; et al. Metabolic Alterations in Celiac Disease Occurring after Following a Gluten-Free Diet. Digestion 2019, 100, 262–268. [Google Scholar] [CrossRef]
- Zanini, B.; Mazzoncini, E.; Lanzarotto, F.; Ricci, C.; Cesana, B.M.; Villanacci, V.; Lanzini, A. Impact of Gluten-Free Diet on Cardiovascular Risk Factors. A Retrospective Analysis in a Large Cohort of Coeliac Patients. Dig. Liver Dis. 2013, 45, 810–815. [Google Scholar] [CrossRef]
- Agarwal, A.; Singh, A.; Mehtab, W.; Gupta, V.; Chauhan, A.; Rajput, M.S.; Singh, N.; Ahuja, V.; Makharia, G.K. Patients with Celiac Disease Are at High Risk of Developing Metabolic Syndrome and Fatty Liver. Intest. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.; Dennis, M.; Higgins, L.A.; Lee, A.R.; Sharrett, M.K. Gluten-Free Diet Survey: Are Americans with Coeliac Disease Consuming Recommended Amounts of Fibre, Iron, Calcium and Grain Foods? J. Hum. Nutr. Diet. 2005, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Theethira, T.G.; Dennis, M. Celiac Disease and the Gluten-Free Diet: Consequences and Recommendations for Improvement. Dig. Dis. 2015, 33, 175–182. [Google Scholar] [CrossRef]
- Imperatore, N.; Tortora, R.; Testa, A.; Gerbino, N.; Caporaso, N.; Rispo, A. Proton Pump Inhibitors as Risk Factor for Metabolic Syndrome and Hepatic Steatosis in Coeliac Disease Patients on Gluten-Free Diet. J. Gastroenterol. 2018, 53, 507–516. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, T.A.; Kelly, C.P.; Betensky, R.A.; Hansen, J.; Pallav, K.; Villafuerte-Gálvez, J.A.; Vanga, R.; Mukherjee, R.; Novero, A.; Dennis, M.; et al. Patients with Celiac Disease Have a Lower Prevalence of Non-Insulin-Dependent Diabetes Mellitus and Metabolic Syndrome. Gastroenterology 2013, 144, 912–917e.1. [Google Scholar] [CrossRef]
- Caliskan, Z.; Demircioglu, K.; Sayar, S.; Kahraman, R.; Caklili, O.; Ozcan, F.B.; Kostek, O.; Baycan, O.F.; Doganay, H.L.; Caliskan, M. Lipid Profile, Atherogenic Indices, and Their Relationship with Epicardial Fat Thickness and Carotid Intima-Media Thickness in Celiac Disease. North. Clin. Istanb. 2019, 6, 242–247. [Google Scholar] [CrossRef]
- Norsa, L.; Shamir, R.; Zevit, N.; Verduci, E.; Hartman, C.; Ghisleni, D.; Riva, E.; Giovannini, M. Cardiovascular Disease Risk Factor Profiles in Children with Celiac Disease on Gluten-Free Diets. World J. Gastroenterol. 2013, 19, 5658–5664. [Google Scholar] [CrossRef] [PubMed]
- Luciani, A.; Villella, V.R.; Vasaturo, A.; Giardino, I.; Pettoello-Mantovani, M.; Guido, S.; Cexus, O.N.; Peake, N.; Londei, M.; Quaratino, S.; et al. Lysosomal Accumulation of Gliadin P31-43 Peptide Induces Oxidative Stress and Tissue Transglutaminase-Mediated PPARgamma Downregulation in Intestinal Epithelial Cells and Coeliac Mucosa. Gut 2010, 59, 311–319. [Google Scholar] [CrossRef]
- Wheeler, E.; Barroso, I. Genome-Wide Association Studies and Type 2 Diabetes. Brief. Funct. Genom. 2011, 10, 52–60. [Google Scholar] [CrossRef]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Specific Duodenal and Faecal Bacterial Groups Associated with Paediatric Coeliac Disease. J. Clin. Pathol. 2009, 62, 264–269. [Google Scholar] [CrossRef]
- de Meij, T.G.J.; Budding, A.E.; Grasman, M.E.; Kneepkens, C.M.F.; Savelkoul, P.H.M.; Mearin, M.L. Composition and Diversity of the Duodenal Mucosa-Associated Microbiome in Children with Untreated Coeliac Disease. Scand. J. Gastroenterol. 2013, 48, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Nadal, I.; Donant, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Imbalance in the Composition of the Duodenal Microbiota of Children with Coeliac Disease. J. Med. Microbiol. 2007, 56, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Nistal, E.; Caminero, A.; Vivas, S.; de Morales, J.M.R.; de Miera, L.E.S.; Rodríguez-Aparicio, L.B.; Casqueiro, J. Differences in Faecal Bacteria Populations and Faecal Bacteria Metabolism in Healthy Adults and Celiac Disease Patients. Biochimie 2012, 94, 1724–1729. [Google Scholar] [CrossRef]
- Nistal, E.; Caminero, A.; Herrán, A.R.; Pérez-Andres, J.; Vivas, S.; de Morales, J.M.R.; de Miera, L.E.S.; Casqueiro, J. Study of Duodenal Bacterial Communities by 16S RRNA Gene Analysis in Adults with Active Celiac Disease vs Non-Celiac Disease Controls. J. Appl. Microbiol. 2016, 120, 1691–1700. [Google Scholar] [CrossRef]
- Valitutti, F.; Cucchiara, S.; Fasano, A. Celiac Disease and the Microbiome. Nutrients 2019, 11, 2403. [Google Scholar] [CrossRef] [PubMed]
- Chibbar, R.; Dieleman, L.A. The Gut Microbiota in Celiac Disease and Probiotics. Nutrients 2019, 11, 2375. [Google Scholar] [CrossRef]
- Girbovan, A.; Sur, G.; Samasca, G.; Lupan, I. Dysbiosis a Risk Factor for Celiac Disease. Med. Microbiol. Immunol. 2017, 206, 83–91. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Noratto, G.; Remes-Troche, J.M. The Effect of Gluten-Free Diet on Health and the Gut Microbiota Cannot Be Extrapolated from One Population to Others. Nutrients 2018, 10, 1421. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Nadal, I.; Medina, M.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Intestinal Dysbiosis and Reduced Immunoglobulin-Coated Bacteria Associated with Coeliac Disease in Children. BMC Microbiol. 2010, 10, 63. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, M.E.; et al. Duodenal and Faecal Microbiota of Celiac Children: Molecular, Phenotype and Metabolome Characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef]
- Schippa, S.; Iebba, V.; Barbato, M.; Di Nardo, G.; Totino, V.; Checchi, M.P.; Longhi, C.; Maiella, G.; Cucchiara, S.; Conte, M.P. A Distinctive “microbial Signature” in Celiac Pediatric Patients. BMC Microbiol. 2010, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Rizzello, C.G.; Gagliardi, F.; Ricciuti, P.; Ndagijimana, M.; Francavilla, R.; Guerzoni, M.E.; Crecchio, C.; Gobbetti, M.; De Angelis, M. Different Fecal Microbiotas and Volatile Organic Compounds in Treated and Untreated Children with Celiac Disease. Appl. Environ. Microbiol. 2009, 75, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).