The Effect of Electrolytes on Blood Pressure: A Brief Summary of Meta-Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Data Extraction
3. Results
3.1. Effect of Dietary Sodium/Salt Intake/Reduction on Blood Pressure
3.2. Effect of Potassium Supplementation on Blood Pressure
3.3. Calcium Intake in Form of Supplements or Diets and Risk for Gestational Hypertension or Blood Pressure Lowering
3.4. Effect of Magnesium on Blood Pressure or Hypertension Risk
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Rahimi, K.; Emdin, C.A.; Macmahon, S. The Epidemiology of Blood Pressure and Its Worldwide Management. Circ. Res. 2015, 116, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Qi, Y.; Zheng, Z.; Wang, Y.; Zhang, X.; Li, H.; Liu, H.; Zhang, X.-T.; Du, J.; Liu, J. Dietary factors associated with hypertension. Nat. Rev. Cardiol. 2011, 8, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Rust, P.; Ekmekcioglu, C. Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension. Adv. Exp. Med. Biol. 2017, 956, 61–84. [Google Scholar] [PubMed]
- Ekmekcioglu, C.; Blasche, G.; Dorner, T.E. Too much salt and how we can get rid of it. Forsch. Komplementmed. 2013, 20, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C.; Elmadfa, I.; Meyer, A.L.; Moeslinger, T. The role of dietary potassium in hypertension and diabetes. J. Physiol. Biochem. 2016, 72, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Flack, J.M.; Calhoun, D.; Schiffrin, E.L. The New ACC/AHA Hypertension Guidelines for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Am. J. Hypertens. 2018, 31, 133–135. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Issues New Guidance on Dietary Salt and Potassium. Available online: https://www.who.int/mediacentre/news/notes/2013/salt_potassium_20130131/en/ (accessed on 14 June 2019).
- Imdad, A.; Jabeen, A.; Bhutta, Z.A. Role of calcium supplementation during pregnancy in reducing risk of developing gestational hypertensive disorders: A meta-analysis of studies from developing countries. BMC Public Health 2011, 11, S18. [Google Scholar] [CrossRef] [PubMed]
- Khanam, F.; Hossain, B.; Mistry, S.K.; Mitra, D.K.; Raza, W.A.; Rifat, M.; Afsana, K.; Rahman, M. The association between daily 500 mg calcium supplementation and lower pregnancy-induced hypertension risk in Bangladesh. BMC Pregnancy Childbirth 2018, 18, 406. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The role of magnesium in hypertension and cardiovascular disease. J. Clin. Hypertens. 2011, 13, 843–847. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Del Gobbo, L.C.; Rosanoff, A.; Wang, J.; Zhang, W.; Song, Y. Effects of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Hypertension 2016, 68, 324–333. [Google Scholar] [CrossRef]
- McCallum, L.; Lip, S.; Padmanabhan, S. The hidden hand of chloride in hypertension. Pflugers Arch. Eur. J. Physiol. 2015, 467, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, T.W.; Morris, R.C. Dietary Chloride as a Determinant of “Sodium-Dependent” Hypertension. Science 1983, 222, 1139–1141. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, T.W.; Al-Bander, H.A.; Morris, R.C. “Salt-sensitive” essential hypertension in men. Is the sodium ion alone important? N. Engl. J. Med. 1987, 317, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Shore, A.C.; Markandu, N.D.; MacGregor, G.A. A randomized crossover study to compare the blood pressure response to sodium loading with and without chloride in patients with essential hypertension. J. Hypertens. 1988, 6, 613–617. [Google Scholar] [CrossRef] [PubMed]
- De Bacquer, D.; De Backer, G.; De Buyzere, M.; Kornitzer, M. Is low serum chloride level a risk factor for cardiovascular mortality? J. Cardiovasc. Risk 1998, 5, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, H.; Van Den Born, J.C.; Hillebrands, J.L.; Joles, J.A. Hydrogen sulfide in hypertension. Curr. Opin. Nephrol. Hypertens. 2016, 25, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ried, K. Garlic Lowers Blood Pressure in Hypertensive Individuals, Regulates Serum Cholesterol, and Stimulates Immunity: An Updated Meta-analysis and Review. J. Nutr. 2016, 146, 389S–396S. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.; Stamler, J.; Griep, L.M.O.; Daviglus, M.L.; Van Horn, L.; Elliott, P. An update on nutrients and blood pressure: Summary of INTERMAP Study findings. J. Atheroscler. Thromb. 2016, 23, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Kesteloot, H.; Appel, L.J.; Dyer, A.R.; Ueshima, H.; Chan, Q.; Brown, I.J.; Zhao, L.; Stamler, J. Dietary Phosphorus and Blood Pressure: International Study of Macro- and Micro-Nutrients and Blood Pressure. Hypertension 2008, 51, 669–675. [Google Scholar] [CrossRef]
- Alonso, A.; Nettleton, J.A.; Ix, J.H.; de Boer, I.H.; Folsom, A.R.; Bidulescu, A.; Kestenbaum, B.R.; Chambless, L.E.; Jacobs, D.R. Dietary phosphorus, blood pressure and incidence of hypertension in the Atherosclerosis Risk in Communities (ARIC) Study and the Multi-Ethnic Study of Atherosclerosis (MESA). Hypertension 2010, 55, 776–784. [Google Scholar] [CrossRef]
- The Lancet. GBD 2017: A fragile world. Lancet 2018, 392, 1683. [Google Scholar] [CrossRef]
- World Heart Foundation. Fact Sheet: Cardiovascular Disease Risk Factors—World Heart Federation. Available online: https://www.world-heart-federation.org/resources/risk-factors/?cats=29 (accessed on 14 June 2019).
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ 2013, 346, f1326. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Taylor, F.; Martin, N.; Gottlieb, S.; Taylor, R.; Ebrahim, S. Reduced dietary salt for the prevention of cardiovascular disease (Review). Cochrane Database Syst. Rev. 2014, CD009217. [Google Scholar] [CrossRef]
- Graudal, N.; Hubeck-Graudal, T.; Jürgens, G.; McCarron, D.A. The Significance of Duration and Amount of Sodium Reduction Intervention in Normotensive and Hypertensive Individuals: A Meta-Analysis. Adv. Nutr. 2015, 6, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Graudal, N.; Jürgens, G. The blood pressure sensitivity to changes in sodium intake is similar in Asians, Blacks and Whites. An analysis of 92 randomized controlled trials. Front. Physiol. 2015, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Gay, H.C.; Rao, S.G.; Vaccarino, V.; Ali, M.K. Effects of Different Dietary Interventions on Blood Pressure; Systematic Review and Meta-Analysis of Randomized Controlled Trials. Hypertension 2016, 67, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Graudal, N.; Hubeck-Graudal, T.; Jurgens, G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Review). Cochrane Database Syst. Rev. 2017, 2017, CD004022. [Google Scholar]
- He, F.J.; Li, J.; MacGregor, G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013, 346, f1325. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. Salt reduction lowers cardiovascular risk: Meta-analysis of outcome trials. Lancet 2011, 378, 380–382. [Google Scholar] [CrossRef]
- Kelly, J.; Khalesi, S.; Dickinson, K.; Hines, S.; Coombes, J.S.; Todd, A.S. The effect of dietary sodium modification on blood pressure in adults with systolic blood pressure less than 140 mmHg: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2016, 14, 196–237. [Google Scholar] [CrossRef]
- Peng, Y.; Li, W.; Wen, X.; Li, Y.; Hu, J.; Zhao, L. Effects of salt substitutes on blood pressure: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Ashton, K.E.; Moxham, T.; Hooper, L.; Ebrahim, S. Reduced dietary salt for the prevention of cardiovascular disease: A meta-analysis of randomized controlled trials (cochrane review). Am. J. Hypertens. 2011, 24, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Moran, A.E.; Liu, J.; Qi, Y.; Xie, W.; Tzong, K.; Zhao, D. A Meta-Analysis of Effect of Dietary Salt Restriction on Blood Pressure in Chinese Adults. Glob. Heart 2015, 10, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.R.R.; Rutherford, S.; Huang, C.; Phung, D.; Islam, M.Z.; Chu, C. Drinking water salinity and risk of hypertension: A systematic review and meta-analysis. Arch. Environ. Occup. Heal. 2017, 72, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Subasinghe, A.K.; Arabshahi, S.; Busingye, D.; Evans, R.G.; Walker, K.Z.; Riddell, M.A.; Thrift, A.G. Association between salt and hypertension in rural and urban populations of low to middle income countries: A systematic review and meta-analysis of population based studies. Asia Pac. J. Clin. Nutr. 2016, 25, 402–413. [Google Scholar]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta-analyses. BMJ 2013, 346, f1378. [Google Scholar] [CrossRef] [PubMed]
- Binia, A.; Jaeger, J.; Hu, Y.; Singh, A.; Zimmermann, D. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: A meta-analysis of randomized controlled trials. J. Hypertens. 2015, 33, 1509–1520. [Google Scholar] [CrossRef]
- Filippini, T.; Violi, F.; D’Amico, R.; Vinceti, M. The effect of potassium supplementation on blood pressure in hypertensive subjects: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 230, 127–135. [Google Scholar] [CrossRef]
- Poorolajal, J.; Zeraati, F.; Soltanian, A.R.; Sheikh, V.; Hooshmand, E.; Maleki, A. Oral potassium supplementation for management of essential hypertension: A meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0174967. [Google Scholar] [CrossRef]
- Bommel, E.V.; Cleophas, T. Potassium treatment for hypertension in patients with high salt intake: A meta-analysis. Int. J. Clin. Pharmacol. Ther. 2012, 50, 478–482. [Google Scholar] [CrossRef]
- Hofmeyr, G.J.; Lawrie, T.A.; Atallah, Á.N.; Duley, L.; Torloni, M.R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems (Review). Cochrane Database Syst. Rev. 2014, CD001059. [Google Scholar] [CrossRef]
- An, L.; Li, W.; Xie, T.; Peng, X.; Li, B.; Xie, S.; Xu, J.; Zhou, X.; Guo, S. Calcium supplementation reducing the risk of hypertensive disorders of pregnancy and related problems: A meta-analysis of multicentre randomized controlled trials. Int. J. Nurs. Pract. 2015, 21, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.J.M.; Soedamah-muthu, S.S.; Mishra, G.D. The association between dietary factors and gestational hypertension and pre-eclampsia: A systematic review and meta-analysis of observational studies. BMC Med. 2014, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, D. Effects of calcium plus Vitamin D supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. J. Hum. Hypertens. 2017, 31, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G.; Ciapponi, A.; Cafferata, M.L.; Belizán, J.M. Calcium supplementation for prevention of primary hypertension (Review). Cochrane Database Syst. Rev. 2015, CD010037. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; Song, Y.; Rosanoff, A.; Shechter, M.; He, K. The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 106, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Garg, R. Effect of magnesium supplementation on type 2 diabetes associated cardiovascular risk factors: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2017, 30, 621–633. [Google Scholar] [CrossRef]
- Kass, L.; Weekes, J.; Carpenter, L. Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef]
- Rosanoff, A.; Plesset, M.R. Oral magnesium supplements decrease high blood pressure (SBP > 155mmHg) in hypertensive subjects on anti-hypertensive medications: A targeted meta-analysis. Magnes. Res. 2013, 26, 93–99. [Google Scholar]
- Han, H.; Fang, X.; Wei, X.; Liu, Y.; Jin, Z.; Chen, Q.; Fan, Z.; Aaseth, J.; Hiyoshi, A.; He, J.; et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: A systematic review and meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 26. [Google Scholar] [CrossRef]
- Wu, J.; Xun, P.; Tang, Q.; Cai, W.; He, K. Circulating magnesium levels and incidence of coronary heart diseases, hypertension, and type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Adrogué, H.J.; Madias, N.E. Sodium and Potassium in the Pathogenesis of Hypertension. N. Engl. J. Med. 2007, 356, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yoshika, M.; Komiyama, Y.; Nishimura, M. The central mechanism underlying hypertension: A review of the roles of sodium ions, epithelial sodium channels, the renin-angiotensin-aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertens. Res. 2011, 34, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.C.; Schmidlin, O.; Sebastian, A.; Tanaka, M.; Kurtz, T.W. Vasodysfunction That Involves Renal Vasodysfunction, Not Abnormally Increased Renal Retention of Sodium, Accounts for the Initiation of Salt-Induced Hypertension. Circulation 2016, 133, 881–893. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Galletti, F.; La Fata, E.; Sabino, P.; Strazzullo, P. Effect of dietary sodium restriction on arterial stiffness: Systematic review and meta-analysis of the randomized controlled trials. J. Hypertens. 2018, 36, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. The Importance of potassium in managing hypertension. Curr. Hypertens. Rep. 2011, 13, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Murao, S.; Takata, Y.; Yasuda, M.; Osawa, H.; Kohi, F. The Influence of Sodium and Potassium Intake and Insulin Resistance on Blood Pressure in Normotensive Individuals Is More Evident in Women. Am. J. Hypertens. 2018, 31, 876–885. [Google Scholar] [CrossRef]
- Feyh, A.; Bracero, L.; Lakhani, H.V.; Santhanam, P.; Shapiro, J.; Khitan, Z.; Sodhi, K. Role of Dietary Components in Modulating Hypertension. J. Clin. Exp. Cardiol. 2016, 7, 433. [Google Scholar] [CrossRef]
- Sontia, B.; Touyz, R.M. Role of magnesium in hypertension. Arch. Biochem. Biophys. 2007, 458, 33–39. [Google Scholar] [CrossRef]
- Egeland, G.M.; Skurtveit, S.; Sakshaug, S.; Daltveit, A.K.; Vikse, B.E.; Haugen, M. Low Calcium Intake in Midpregnancy Is Associated with Hypertension Development within 10 Years after Pregnancy: The Norwegian Mother and Child Cohort Study. J. Nutr. 2017, 147, 1757–1763. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendation: Calcium Supplementation during Pregnancy for the Prevention of Pre-eclampsia and Its Complications. Available online: https://apps.who.int/iris/bitstream/handle/10665/277235/9789241550451-eng.pdf?ua=1 (accessed on 14 June 2019).
- Leung, A.A.; Nerenberg, K.; Daskalopoulou, S.S.; McBrien, K.; Zarnke, K.B.; Dasgupta, K.; Cloutier, L.; Gelfer, M.; Lamarre-Cliche, M.; Milot, A.; et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can. J. Cardiol. 2016, 32, 569–588. [Google Scholar] [CrossRef] [PubMed]
- Ndanuko, R.N.; Tapsell, L.C.; Charlton, K.E.; Neale, E.P.; Batterham, M.J. Dietary Patterns and Blood Pressure in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2016, 7, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Agnoletti, D.; Safar, M.E.; Blacher, J. Effect of antihypertensive agents on blood pressure variability: The natrilix SR versus candesartan and amlodipine in the reduction of systolic blood pressure in hypertensive patients (X-CELLENT) study. Hypertension 2011, 58, 155–160. [Google Scholar] [CrossRef] [PubMed]
- McClure, S.T.; Rebholz, C.M.; Medabalimi, S.; Hu, E.A.; Xu, Z.; Selvin, E.; Appel, L.J. Dietary phosphorus intake and blood pressure in adults: A systematic review of randomized trials and prospective observational studies. Am. J. Clin. Nutr. 2019, 109, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J.; Frassetto, L.A.; Katzinger, J. Diet-induced acidosis: Is it real and clinically relevant? Br. J. Nutr. 2010, 103, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Mazoteras-Pardo, V.; Becerro-De-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; López-López, D.; Palomo-López, P.; Rodríguez-Sanz, D.; Calvo-Lobo, C. The QardioArm Blood Pressure App for Self-Measurement in an Obese Population: Validation Study Using the European Society of Hypertension International Protocol Revision 2010. JMIR Mhealth Uhealth 2018, 6, e11632. [Google Scholar] [CrossRef] [PubMed]
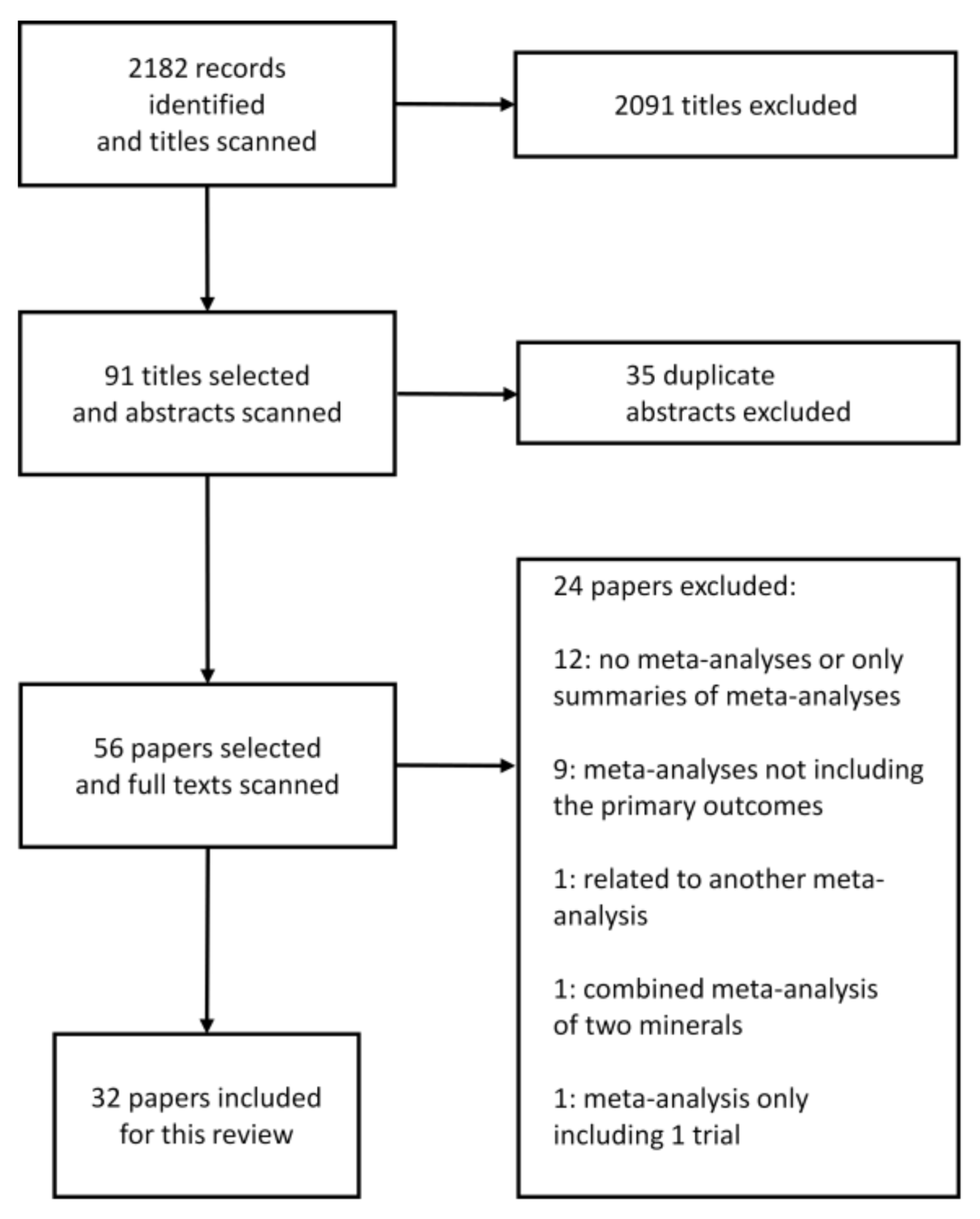
| Author/Year | No. of Trials | Study Characteristics | No. of Participants | Patient Characteristics | Duration of Trials | Sodium/Salt Intake or Reduction | Blood Pressure Lowering in mmHg (95% CI) | Further Remarks/Summary |
|---|---|---|---|---|---|---|---|---|
| Aburto et al., 2013a [24] | 36 | Randomized controlled trials | 6736 | 2273 (hypertensive) | Most studies (n = 31) <3 months | Different reductions in sodium intake Relative sodium reduction in the intervention group: ≥1/3 of control | SBP: −3.4 (−4.3 to −2.5) DBP: −1.5 (−2.1 to −1.0) | Reduced sodium intake decreases blood pressure in people both with and without hypertension. The reduction in blood pressure was greater in those with hypertension. |
| Adler et al., 2014 [25] | 6 (SBP) 5 (DBP) | Randomized controlled trials | 3362 (SBP) 2754 (DBP) | SBP (end of trial): (normotensive) 2079 (hypertensive) 1283 DBP (end of trial): (normotensive) 2079 (hypertensive) 675 | 7–36 months | Sodium intake: 70 to >100 mmol/day | Normotensive: SBP: −1.2 (−2.3 to 0.02) DBP: −0.8 (−1.4 to −0.2) Hypertensive: SBP: −4.1 (−5.8 to −2.4) DBP: −3.7 (−8.4 to 0.9) | Normotensive persons: small blood pressure reduction. Hypertensive patients: greater reduction in SBP, no difference in DBP. |
| Graudal et al., 2015 [26] | 15 | Randomized controlled trials | 12–114 | “time to maximal efficacy” analysis 7 studies with hypertensive patients 7 studies with normotensive persons 1 study hypertensive + normotensive | 1 to 6 weeks | Sodium reduction range: 55–118 mmol/day | No significant differences in SBP or DBP after initiation of salt reduction between week 1 and subsequent weeks. | Time dependent effects of salt reduction on blood pressure. The effect of salt reduction on blood pressure appears to reach maximal efficacy at 1 week and remain stable over subsequent time intervals. |
| Graudal and Jürgens 2015 [27] | 92 | Randomized controlled trials | 661 Asians 561 Blacks 3782 Whites | 9 Asian/9 Black/74 White population | 7–365 days | Sodium reduction: 63–103 mmol | SBP: −3.2 (−4.0 to −2.5) (in Whites) −4.7 (−7.1 to −2.3) (in Blacks) −3.8 (−6.4 to −1.3) (in Asians) DBP:−1.5 (−2.1 to −1.0) (in Whites) −3.0 (−4.0 to −2.0) (in Blacks) −2.0 (−3.0 to −0.9) (in Asians) | SBP: no differences in ethnic groups. DBP: small differences between black and white people. |
| Gay et al., 2016 [28] | 24 | Randomized controlled trials | 23,858 | 11 to 2570 participants (median: 129) participants >19 years old | Trial durations ranged from 6 to 48 months of follow−up (median: 12 months) | Dietary interventions (including low sodium diets) | Overall pooled net effect of diets: SBP: −3.1 (−3.9 to −2.3) DBP: −1.8 (−2.2 to −1.4) | This meta-analysis shows that dietary interventions (including low sodium diets) provide clinically significant net blood pressure reductions, and that some dietary patterns may be more effective than others. |
| Graudal et al., 2017 [29] | 177 | Randomized controlled trials | 12,210 | White people with hypertension. (84 studies; 5925 participants in SBP; 85 studies; 6001 participants in DBP) Black people with hypertension. (8 studies; 619 participants in SBP and DBP) Asian people with hypertension. (8 studies; 501 participants in SBP and DBP) White people with normotension. (89 studies, 8569 participants in SBP; 90 studies, 8833 participants in DBP) Black people with normotension. (7 studies, 506 participants in SBP and DBP) Asian people with normotension. (3 studies, 393 participants in SBP and DBP) | 4–1100 days | Mean sodium reduction: 135 mmol/day range; <100 to ≥250 mmol/day | Hypertensive (White): SBP: −5.5 (−6.5 to −4.6) DBP: −2.9 (−3.4 to −2.3) Hypertensive (Black): SBP: −6.6 (−9.0 to −4.2) DBP: −2.9 (−4.5 to −1.30) Hypertensive (Asian): SBP: −7.8 (−11.4 to −4.1) DBP: −2.7 (−4.2 to −1.2) Normotensive (White): SBP: −1.1 (−1.6 to −0.6) DBP: 0.03 (−0.4 to 0.4) Normotensive (Black) SBP: −4.0 (−7.4 to −0.7) DBP: −2.0 (−4.4 to 0.4) Normotensive (Asian): SBP: −0.7 (−3.9 to 2.4) DBP: −1.6 (−3.4 to 0.1) | High-quality evidence for White people; moderate-quality evidence for Black/Asian people |
| He et al., 2013 [30] | 34 | Randomized trials | 3230 | 990 (of 22 trials) hypertensive 2240 (of 12 trials) normotensive | Median duration: 5 weeks in hypertensive people, 4 weeks in normotensive people | Salt reduction: 75 mmol/day (4.4 g/day). Reduction of urinary sodium: 40–120 mmol/day (2.3–7.0 g/day). | Total SBP: −4.2 (−5.2 to −3.2) DBP: −2.1 (−2.7 to −1.5) Hypertensive: SBP: −5.4 (−6.6 to −4.2) DBP: −2.8 (−3.5 to −2.1) Normotensive: SBP: −2.4 (−3.6 to −1.3) DBP: −1.0 (−1.9 to −0.2) | Reduction in SBP was significant in both black and white people and in women and men. Significant effects on blood pressure were seen in hypertensives and normotensives. Dose-response relation: the greater the reduction in salt intake, the greater the fall in blood pressure. |
| He and MacGregor 2011 [31] | 6 | Outcome trials | 6250 | 3 trials in normotensive participants 3 trials in hypertensive patients | 6–36 months | Salt reduction: 2–2.3 g/day | Normotensive: SBP: −1.1 (−0.1 to 2.3) DBP: −0.8 (0.2 to 1.4) Hypertensive: SBP: −4.1 (2.4 to 5.8) DBP: −3.7 (−0.9 to 8.4) | Significant reduction in cardiovascular events |
| Kelly et al., 2016 [32] | 5 | Randomized and non-randomized controlled trials | 1214 | Normotensive participants (≥18 years) with SBP ≤140 mmHg | 4 weeks to 48 months | Salt reduction: −75 mmol/day (range; −37 to −136 mmol). | SBP: −0.7 (−2.6 to 1.2) DBP: −0.6 (−1.3 to 0.1) | No significant change in SBP or DBP following reduction of dietary sodium over the period of 4 weeks to 36 months |
| Peng et al., 2014 [33] | 5 | Randomized controlled trials | 1974 | Hypertensive and normotensive participants | 6 months to 2 years | Different salt substitutes vs. common salt (NaCl). | SBP: −4.9 (−7.3 to −2.5) DBP: −1.5 (−2.7 to −0.3) | Salt substitutes significantly reduced both SBP and DBP |
| Taylor et al., 2011 [34] | 7 | Randomized controlled trials | 3 trials normotensive (3518), 2 trials hypertensive (758), 1 trial mixed pop. (1981), 1 trial with heart failure (234) | Adults ≥18 years, irrespective of gender/ethnicity. Studies of children/pregnant women were excluded. | Trials follow-up ranged 6 to 71 months | Salt reduction; <70–100 mmol/ day. Urinary 24-h sodium excretion: Normotensive (mean diff.): 34.2 mmol/24 h (18.8–49.6), Hypertensive (mean diff.): 39.1 mmol/24 h (31.1–47.1) | Normotensives (mean difference) SBP:−1.1 (−2.3 to 0.1) DBP:−0.8 (−1.4 to −0.2) Hypertensives SBP: −4.1 (−5.8 to −2.4) DBP: −3.7 (−8.4 to 0.9) | Significant reduction of SBP in hypertensive patients |
| Wang et al., 2015 [35] | 6 | Interventional studies | 3153 | Chinese adults aged ≥35 years | At most 1 week | Salt level reduced in hypertensive patients: 9.6 g/day (163.0 mmol/day sodium). | Normotensive + hypertensive: SBP: −6.3 (−7.2 to −5.4) DBP: −3.2 (−3.7 to −2.7) Hypertensive: SBP: −8.9 (−14.1 to −3.7) DBP: −5.9 (−9.7 to −2.1) | Salt restriction lowers mean BP in Chinese adults, with the strongest effect among hypertensive participants. |
| Observational Studies | ||||||||
| Talukder et al., 2017 [36] | 10 | Observational studies | 8093 | 7 studies (12 datasets) with 3747 participants with low/high water sodium exposure groups | 4–405 mg/L water sodium level | Standardized mean difference: SBP: 0.1 (−0.2 to 0.3) DBP: 0.2 (0.1 to 0.4) | An (inconclusive) association between water sodium and human blood pressure is suggested, more consistently for DBP. | |
| Subasinghe et al., 2016 [37] | 18 | Observational studies | 134,916 | Participants in urban and rural areas in low-and-middle income countries (LMICs). Age: 24–65. | Daily salt intake range: 6.9 to 42.3 g/day | Effect size (ES) of hypertension ES 1.36 (1.24 to 1.48) ES 1.28 (1.13 to 1.45) | Excessive salt intake has a greater impact on the prevalence of hypertension in urban than rural regions. | |
| Author/Year | No. of trials | Study Characteristic | No. of Participants | Patient Characteristics | Duration of Trials | Potassium Dosage (Supplements) | Blood Pressure Lowering in mmHg (95%CI) | Further Remarks/Summary |
|---|---|---|---|---|---|---|---|---|
| Aburto et al., 2013b [38] | 21 | Randomized controlled trials | 1892/1857 | Hypertensive 818(SBP)/828(DBP) | <2 to >4 months | <90 mmol/day to >155 mmol/day in the intervention group | SBP: −3.5 (−5.2 to −1.8) DBP: −2.0 (−3.1 to −0.9) | Effect seen in people with hypertension but not in those without hypertension. Intake above 120 mmol/day did not seem to have any additional benefit. Potassium may be more effective in reducing blood pressure at higher levels of sodium consumption. |
| Binia et al., 2015 [39] | 15 | Randomized controlled trials | 917 | 400 hypertensives 329 normotensives 188 hypertensive or normotensive persons (mixed population) | 4–24 weeks | <40–120 mmol/day | All: SBP: −4.7 (−7.0 to −2.4) DBP: −3.5 (−5.7 to −1.3) Hypertensive patients: SBP: −6.8 (−9.3 to −4.3) DBP: −4.7 (−7.5 to −1.8) | Potassium supplementation is associated with reduction of blood pressure in patients who are not on antihypertensive medication, and the effect is significant in hypertensive patients. |
| Filippini et al., 2017 [40] | 33 | Randomized controlled trials | 1829 | 1163 (studies ≥4 weeks overall) | <4 to ≥12 weeks | 25–250 mmol/day | SBP: −4.5 (−5.9 to −3.1) DBP: −3.0 (−4.8 to −1.1) | Potassium supplementation in hypertensives was generally associated with decreased blood pressure, particularly in high sodium consumers. |
| Poorolajal et al., 2017 [41] | 23 | Randomized controlled trials | 1213 | Primary hypertension: 732 (SBP) 695 (DBP) | 4–52 weeks | 6–200 mmol/day | SBP: −4.3 (−6.0 to −2.5) DBP: −2.5 (−4.1 to −1.0) | Potassium supplementation has a modest but significant impact on blood pressure. |
| Bommel and Cleophas 2012 [42] | 10 | Crossover and parallel design studies | 556 | High salt intake, >170 mmol/24h | Follow up 8–16 weeks | Not available | SBP: −9.5 (−10.8 to −8.1) DBP: −6.4 (−7.3 to −5.6) | Potassium treatment reduces considerably the blood pressure of hypertensive patients on salt rich diets. |
| Author/Year | No. of Trials | Study Characteristic | No. of Participants | Study Aims | Duration of Trials | Calcium Dosage (Diet or Supplement) | Blood Pressure Lowering in mmHg or RR/OR for Gestational Hypertension (95% CI) | Further Remarks/Summary |
|---|---|---|---|---|---|---|---|---|
| Imdad et al., 2011 [8] | 6 | Randomized controlled trials | Calcium-group: 4919 Control group: 4942 | Effect of calcium supplementation on gestational hypertensive disorders in studies from developing countries | Calcium supplements in all the included studies were before 20–32 weeks of gestation and continued till delivery. | 0.5–2 g/day | RR: 0.55 (0.36 to 0.85) | Calcium supplementation during pregnancy was associated with a significant reduced risk of acquiring gestational hypertension. |
| Hofmeyr et al., 2014 [43] | 12 trials | Randomized controlled trials | 15,470 women | Assessing the effects of calcium supplementation during pregnancy on hypertensive disorders of pregnancy and related maternal and child outcomes | Calcium supplementation started at the latest from 34 weeks of pregnancy. | High-dose calcium supplementation (≥1 g/day) | RR: 0.65 (0.53 to 0.81) | Average risk of high blood pressure was reduced with calcium supplementation compared with placebo. There was also a reduction in hypertension with low-dose calcium supplementation (<1 g/day). |
| An et al., 2015 [44] | 4 | Randomized controlled trials | Gestational hypertension: 7252 Control group: 7272 Severe gestational hypertension: 6673 Control group: 6684 | Assessing the effectiveness of calcium supplementation during pregnancy on reducing the risk of hypertensive disorders of pregnancy and related problems. | From ~11–24 weeks of pregnancy to delivery | Supplementation with calcium (at least >1 g/day) | Gestational hypertension: RR: 0.91 (0.84 to 0.99) Severe gestational hypertension: RR: 0.81 (0.60 to 1.09) | Calcium supplementation appears to reduce the risk of hypertension in pregnancy. No significant reduction in the risk of severe gestational hypertension. |
| Wu and Sun 2017 [46] | 8 | Randomized controlled trials | 36,806 | Evaluation the effect of calcium plus vitamin-D (CaD) supplements on the changes in BP from baseline to the longest follow-up time point in male and female participants. | 8 weeks to 7 years | Intervention dose of calcium (≤1000 mg/day, 5 trials or >1000 mg/day, 3 trials) | Mean differences in SBP: 0.6 (−1 to 2.20) Mean differences in DBP: −0.2 (−0.9 to 0.5) | Calcium plus vitamin D supplementation slightly increased SBP, but the difference was not statistically significant. Calcium plus vitamin D supplementation did not significantly affected DBP reduction. |
| Cormick et al., 2015 [47] | 16 | Randomized controlled trials | SBP: 3048 (16 studies) DBP: 2947 (15 studies) | Assessing the efficacy and safety of calcium supplementation versus placebo or control for reducing blood pressure in normotensive people | Median follow up intervention period of 3.5 months | For most studies the intervention was 1000 mg to 2000 mg of elemental calcium per day | Mean difference: SBP: −1.4 (−2.2 to −0.7) DBP: −1 (−1.5 to −0.5) | The quality of evidence was high for doses of calcium of 1000 to 1500 mg/day and was moderate for lower or higher doses. Calcium intake slightly reduced both SBP and DBP in normotensive people. |
| Observational studies | ||||||||
| Schoenaker et al., 2014 [45] | 16 | Observational studies | Case-control studies: 757 pregnant women Cohort studies: 41,214 pregnant women, 908 gestational hypertension | Assessing the effect of dietary factors on hypertensive disorders during pregnancy (gestational hypertension and pre-eclampsia) | Highest group >1600 mg/day versus lowest group <1000 mg/day | Gestational hypertension (comparing highest to lowest): OR: 0.63 (0.41 to 0.97) | Results from case–control studies consistently showed lower reported calcium intake for pregnant women with hypertensive disorders (gestational hypertension and preeclampsia) | |
| Author-Year | No. of Trials | Study Characteristics | No. Participants | Study Aims | Duration of Trials | Magnesium Dosage (Diet or Supplement) | Blood Pressure Lowering in mmHg or RR (95% CI) | Further Remarks/Summary |
|---|---|---|---|---|---|---|---|---|
| Zhang et al., 2016 [11] | 27 | Randomized controlled trials | Magnesium group: 822 Placebo group: 800 | Effect of magnesium supplementation in normotensive and hypertensive adults (age 18–84 years). | 3 weeks–6 months | Median dose of 368 mg/day (range: 238–960 mg/day) | SBP: −2 (−0.4 to −3.6) DBP: −1.8 (−0.7 to –2.8) | Magnesium supplementation at a median dose of 368 mg/day for a median duration of 3 months significantly reduced SBP and DBP. Magnesium supplementation at a dose of 300 mg/day or duration of 1 month is enough to elevate serum magnesium and reduce blood pressure. Serum magnesium was negatively associated with DBP but not SBP. |
| Dibaba et al., 2017 [48] | 11 | Randomized controlled trials | 543 | Assessing the pooled effect of magnesium supplementation on blood pressure in participants with preclinical or non−communicable diseases. | 1 to 6 months (mean: 3.6 months) | 365–450 mg/day | Standardized mean difference: SBP: −0.2 (−0.4 to −0.03) DBP: −0.3 (−0.5 to −0.03) | Magnesium supplementation lowers blood pressure in individuals with insulin resistance, prediabetes, or other noncommunicable chronic diseases. |
| Verma and Garg 2017 [49] | 28 | Randomized controlled trials | 1694 (834 treatment arm, 860 placebo arm) | Evaluation the effect of magnesium supplementation on type 2 diabetes associated cardiovascular risk factors in both diabetic and nondiabetic individuals. Only four studies were carried out in hypertensive subjects. | 4−24 weeks | Elemental magnesium: 300–1006 mg/day | Weighted mean difference: SBP: −3.06 (−5.51 to −0.60) DBP: −1.37 (−3.02 to 0.29) | A significant improvement was observed in SBP. Insignificant improvement or no improvement was observed in DBP |
| Kass et al., 2012 [50] | 22 | Interventional studies | 1173 | Assessing the effect of magnesium supplementation on blood pressure. Adults from 12 different countries were included. | 3 to 24 weeks of follow-up | Elemental magnesium dosage: 120–973 mg/day | Overall effect size: SBP: 0.3 (0.2 to 0.4) DBP: 0.4 (0.3 to 0.4) | Summary of all trials show a decrease in SBP of 3–4 mmHg and DBP of 2–3 mmHg. Magnesium supplementation appears to achieve a small but clinically significant reduction in blood pressure. |
| Rosanoff and Plesset 2013 [51] | 7 | Interventional studies | 135 treated hypertensive subjects | Evaluation of magnesium supplementation in hypertension. Initial SBP of the patients was >155 mmHg | 6 to 17 weeks | 10.5–18.5 mmol magnesium-salt/day | Mean change: SBP: −18.7 (−22.5 to −15.0) DBP: −10.9 (−13.1 to −8.7) | This uniform subset of seven studies showed a strong effect of magnesium in treated hypertensive patients. |
| Observational studies | ||||||||
| Schoenaker et al., 2014 [45] | 3 | Observational studies | 6616 pregnant women, age range 20–40 years | Assessing the effect of dietary factors, including magnesium, on hypertensive disorders of pregnant women. | NA | Not indicated | Significantly lower mean magnesium intake of mean 7.69 mg/day for women with hypertensive disorders of pregnancy (gestational hypertension and pre-eclampsia) | Pooled results revealed statistically significantly lower mean magnesium intake for women with hypertensive disorders of pregnancy. |
| Han et al., 2017 [52] | 10 | Prospective cohort studies | 180,566 participates | Assessing the relationship between dietary magnesium intake and serum magnesium concentrations on the risk of hypertension in adults. Adult population >18 years was included. | 4–15 years | 96–425 mg/day | RR: 0.95 (0.90 to 1.00) for a 100 mg/increment in magnesium intake. Comparing highest to lowest: RR: 0.91 (0.80 to 1.02) | Increase in magnesium intake was associated with a lower risk of hypertension in a linear dose-response pattern. |
| Wu J et al., 2017 [53] | 3 | Prospective cohort studies with four cohorts | 14,876 participants (3149 cases) | Evaluation of circulating magnesium levels and incidence of coronary heart diseases, hypertension, and type 2 diabetes mellitus | Average of 6.7 years of follow-up | NA | Per 0.1 mmol/L increment in serum magnesium levels: RR: 0.96 (0.93 to 0.99) | A significant inverse linear association was observed between circulating magnesium levels and incidence of hypertension. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, S.; Klammer, N.; Ekmekcioglu, C. The Effect of Electrolytes on Blood Pressure: A Brief Summary of Meta-Analyses. Nutrients 2019, 11, 1362. https://doi.org/10.3390/nu11061362
Iqbal S, Klammer N, Ekmekcioglu C. The Effect of Electrolytes on Blood Pressure: A Brief Summary of Meta-Analyses. Nutrients. 2019; 11(6):1362. https://doi.org/10.3390/nu11061362
Chicago/Turabian StyleIqbal, Sehar, Norbert Klammer, and Cem Ekmekcioglu. 2019. "The Effect of Electrolytes on Blood Pressure: A Brief Summary of Meta-Analyses" Nutrients 11, no. 6: 1362. https://doi.org/10.3390/nu11061362
APA StyleIqbal, S., Klammer, N., & Ekmekcioglu, C. (2019). The Effect of Electrolytes on Blood Pressure: A Brief Summary of Meta-Analyses. Nutrients, 11(6), 1362. https://doi.org/10.3390/nu11061362




