Head and Neck Cancer Patient Population, Management, and Oncologic Outcomes from the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. End Points and Statistical Analysis
3. Results
3.1. Patient Population
3.2. Survival Analyses
3.3. Clinical Time Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID-19: Recommendations for Management of Elective Surgical Procedures. ACS. Available online: https://www.facs.org/for-medical-professionals/covid-19/clinical-guidance/elective-surgery/ (accessed on 11 November 2022).
- Centers for Medicare & Medicaid Services. CMS Adult Elective Surgery and Procedures Recommendations. 7 April 2020. p. 2. Available online: https://www.cms.gov/files/document/covid-elective-surgery-recommendations.pdf (accessed on 11 November 2022).
- Wells, C.R.; Galvani, A.P. Impact of the COVID-19 pandemic on cancer incidence and mortality. Lancet Public Health 2022, 7, 490–491. [Google Scholar] [CrossRef]
- Survey: COVID-19 Affecting Patients’ Access to Cancer Care. American Cancer Society Cancer Action Network. Available online: https://www.fightcancer.org/releases/survey-covid-19-affecting-patients%E2%80%99-access-cancer-care (accessed on 11 November 2022).
- Mehanna, H.; Hardman, J.C.; Shenson, J.A.; Abou-Foul, A.K.; Topf, M.C.; AlFalasi, M.; Chan, J.Y.K.; Chaturvedi, P.; Chow, V.L.Y.; Dietz, A.; et al. Recommendations for head and neck surgical oncology practice in a setting of acute severe resource constraint during the COVID-19 pandemic: An international consensus. Lancet Oncol. 2020, 21, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.J.; Palma, D.; Guckenberger, M.; Balermpas, P.; Beitler, J.J.; Blanchard, P.; Brizel, D.; Budach, W.; Caudell, J.; Corry, J.; et al. Practice Recommendations for Risk-Adapted Head and Neck Cancer Radiation Therapy during the COVID-19 Pandemic: An ASTRO-ESTRO Consensus Statement. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 618–627. [Google Scholar] [CrossRef] [PubMed]
- MD Anderson Head and Neck Surgery Treatment Guidelines Consortium. Head and neck surgical oncology in the time of a pandemic: Subsite-specific triage guidelines during the COVID-19 pandemic. Head Neck 2020, 42, 1194–1201. [CrossRef]
- Lee, S.S.; Ceasar, D.; Margolis, B.; Venkatesh, P.; Espino, K.; Gerber, D.; Boyd, L.R. The impact of the ban on elective surgery in New York City during the coronavirus outbreak on gynecologic oncology patient care. Gynecol. Oncol. Rep. 2022, 41, 100997. [Google Scholar] [CrossRef]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, 4087. [Google Scholar] [CrossRef] [PubMed]
- Biagi, J.J.; Raphael, M.J.; Mackillop, W.J.; Kong, W.; King, W.D.; Booth, C.M. Association between Time to Initiation of Adjuvant Chemotherapy and Survival in Colorectal Cancer: A Systematic Review and Meta-analysis. JAMA 2011, 305, 2335–2342. [Google Scholar] [CrossRef]
- Raphael, M.J.; Biagi, J.J.; Kong, W.; Mates, M.; Booth, C.M.; Mackillop, W.J. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2016, 160, 17–28. [Google Scholar] [CrossRef]
- Malagón, T.; Yong, J.H.E.; Tope, P.; Miller, W.H.; Franco, E.L. McGill Task Force on the Impact of COVID-19 on Cancer Control and Care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int. J. Cancer 2022, 150, 1244–1254. [Google Scholar] [CrossRef]
- Meyers, J. The %NEWSURV Family of Macros: An Update on the Survival Plotting Macro %NEWSURV and an Introduction to Expansion Macros. In PharmaSUG 2017-Paper DV15. 2017. Available online: https://www.pharmasug.org/proceedings/2017/DV/PharmaSUG-2017-DV15.pdf (accessed on 4 January 2024).
- Therneau, T.; Crowson, C.; Atkinson, E. Adjusted Survival Curves. 2014. Available online: https://www.semanticscholar.org/paper/Adjusted-Survival-Curves-Therneau-Crowson/59353b51032c2061eb2cb050a72af52568c5a2da (accessed on 2 January 2024).
- Kind, A.J.H.; Buckingham, W.R. Making Neighborhood-Disadvantage Metrics Accessible—The Neighborhood Atlas. N. Engl. J. Med. 2018, 378, 2456–2458. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, C.; Hu, C. Effect of COVID-19 Epidemic on Delay of Diagnosis and Treatment Path for Patients with Nasopharyngeal Carcinoma. Cancer Manag. Res. 2020, 12, 3859–3864. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.G.; Pasea, L.; Banerjee, A.; Denaxas, S.; Katsoulis, M.; Chang, W.H.; Williams, B.; Pillay, D.; Noursadeghi, M.; Linch, D.; et al. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. medRxiv 2020. [Google Scholar] [CrossRef]
- Giannakoulis, V.G.; Papoutsi, E.; Siempos, I.I. Effect of Cancer on Clinical Outcomes of Patients with COVID-19: A Meta-Analysis of Patient Data. JCO Glob. Oncol. 2020, 6, 799–808. [Google Scholar] [CrossRef]
- Johansson, A.L.V.; Skog, A.; Johannesen, T.B.; Myklebust, T.A.; Skovlund, C.W.; Mørch, L.S.; Friis, S.; Gamborg, M.; Kristiansen, M.F.; Pettersson, D.; et al. Were cancer patients worse off than the general population during the COVID-19 pandemic? A population-based study from Norway, Denmark and Iceland during the pre-vaccination era. Lancet Reg. Health-Eur. 2023, 31, 100680. [Google Scholar] [CrossRef]
- Achard, V.; Aebersold, D.M.; Allal, A.S.; Andratschke, N.; Baumert, B.G.; Beer, K.T.; Betz, M.; Breuneval, T.; Bodis, S.; Bari, B.; et al. A national survey on radiation oncology patterns of practice in Switzerland during the COVID-19 pandemic: Present changes and future perspectives. Radiother. Oncol. 2020, 150, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Anacak, Y.; Onal, C.; Ozyigit, G.; Agaoglu, F.; Akboru, H.; Akyurek, S.; Gursel, B.; Igdem, S.; Yalman, D.; Yildiz, F.; et al. Changes in radiotherapy practice during COVID-19 outbreak in Turkey: A report from the Turkish Society for Radiation Oncology. Radiother. Oncol. 2020, 150, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors|JCO Clinical Cancer Informatics. Available online: https://ascopubs.org/doi/10.1200/CCI.20.00134?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 11 November 2022).
- COVIDSurg Collaboartive. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: An international, prospective, cohort study. Lancet Oncol. 2022, 22, 1507–1517. [Google Scholar] [CrossRef]
- Riera, R.; Bagattini, Â.M.; Pacheco, R.L.; Pachito, D.V.; Roitberg, F.; Ilbawi, A. Delays and Disruptions in Cancer Health Care Due to COVID-19 Pandemic: Systematic Review. JCO Glob. Oncol. 2021, 7, 311–323. [Google Scholar] [CrossRef]
- Venkatasai, J.; John, C.; Kondavetti, S.S.; Appasamy, M.; Parsuraman, L.; Amblathandi, R.; Masilamani, H. Impact of COVID-19 Pandemic on Patterns of Care and Outcome of Head and Neck Cancer: Real-World Experience from a Tertiary Care Cancer Center in India. JCO Glob. Oncol. 2022, 8, e2100339. [Google Scholar] [CrossRef]
- Marcum, M.; Kurtzweil, N.; Vollmer, C.; Schmid, L.; Vollmer, A.; Kastl, A.; Acker, K.; Gulati, S.; Grover, P.; Herzgo, T.J.; et al. COVID-19 pandemic and impact on cancer clinical trials: An academic medical center perspective. Cancer Med. 2020, 9, 6141–6146. [Google Scholar] [CrossRef]
- Lamont, E.B.; Diamond, S.S.; Katriel, R.G.; Ensign, L.L.; Liu, J.; Rusli, E.; Alexander, G.C. Trends in Oncology Clinical Trials Launched Before and During the COVID-19 Pandemic. JAMA Netw. Open 2021, 4, e2036353. [Google Scholar] [CrossRef] [PubMed]
- The Sex, Gender and COVID-19 Project|Global Health 50/50. Available online: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/ (accessed on 22 December 2022).
- CDC. COVID Data Tracker. Centers for Disease Control and Prevention. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 22 December 2022).
- Jin, J.; Bai, P.; He, W.; Wu, F.; Liu, X.; Han, D.; Liu, S.; Yang, J. Gender Differences in Patients with COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Penfold, R.; Magee, L. Gender balance in an unprecedented time. Future Healthc. J. 2020, 7, 212–213. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Impact on Women and Gender Equality|McKinsey. Available online: https://www.mckinsey.com/featured-insights/future-of-work/covid-19-and-gender-equality-countering-the-regressive-effects (accessed on 22 December 2022).
- Guerrina, R.; Borisch, B.; Callahan, L.F.; Howick, J.; Reginster, J.; Mobasheri, A. Health and Gender Inequalities of the COVID-19 Pandemic: Adverse Impacts on Women’s Health, Wealth and Social Welfare. Front. Glob. Womens Health 2021, 2, 670310. Available online: https://www.frontiersin.org/articles/10.3389/fgwh.2021.670310 (accessed on 22 December 2022). [CrossRef]
- Connor, J.; Madhavan, S.; Mokashi, M.; Amanuel, H.; Johnson, N.R.; Pace, L.E.; Bartz, D. Health risks and outcomes that disproportionately affect women during the COVID-19 pandemic: A review. Soc. Sci. Med. 2020, 266, 113364. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Young, S.; Williams, R.; Liang, P.S. Impact of the COVID-19 pandemic on colorectal cancer screening in New York City. J. Med. Screen. 2022, 30, 81–86. [Google Scholar] [CrossRef]
- Henderson, L.M.; Benefield, T.; Bosemani, T.; Long, J.M.; Rivera, M.P. Impact of the COVID-19 Pandemic on Volumes and Disparities in Lung Cancer Screening. Chest 2021, 160, 379–382. [Google Scholar] [CrossRef]
- Chen, R.C.; Haynes, K.; Du, S.; Barron, J.; Katz, A.J. Association of Cancer Screening Deficit in the United States with the COVID-19 Pandemic. JAMA Oncol. 2021, 7, 878–884. [Google Scholar] [CrossRef]
- COVID-19: A Gender Lens. United Nations Population Fund. Available online: https://www.unfpa.org/resources/covid-19-gender-lens (accessed on 22 December 2022).
- John, N.; Casey, S.E.; Carino, G.; McGovern, T. Lessons Never Learned: Crisis and gender-based violence. Dev. World Bioeth. 2020, 20, 65–68. [Google Scholar] [CrossRef]
- Magesh, S.; John, D.; Li, W.T.; Li, Y.; Mattingly-app, A.; Jain, S.; Chang, E.Y.; Ongkeko, W.M. Disparities in COVID-19 Outcomes by Race, Ethnicity, and Socioeconomic Status: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2134147. [Google Scholar] [CrossRef]
- Nordhues, H.C.; Bhagra, A.; Stroud, N.N.; Vencill, J.A.; Kuhle, C.L. COVID-19 Gender Disparities and Mitigation Recommendations: A Narrative Review. Mayo Clin. Proc. 2021, 96, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hu, X.; Zhao, J.; Jemal, A.; Yabroff, K.R. Identification of Deaths Caused by Cancer and COVID-19 in the US during March to December 2020. JAMA Oncol. 2022, 8, 1696–1698. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | 2019 (N = 74) | 2020 (N = 54) | p Value |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 61.6 (13.6) | 62.5 (14.6) | 0.6916 |
| Median (Min, Q1, Q3, Max) | 61.5 (21, 56, 71, 87) | 62.5 (26, 55, 75, 87) | 0.5938 |
| Gender, N (%) | |||
| Female | 31 (41.9) | 13 (24.1) | 0.0361 * |
| Male | 43 (58.1) | 41 (75.9) | |
| Stage, N (%) | |||
| Benign | 6 (8.1) | 1 (1.9) | 0.0726 |
| 1 | 15 (20.3) | 9 (16.7) | |
| 2 | 10 (13.5) | 9 (16.7) | |
| 3 | 12 (16.2) | 19 (35.2) | |
| 4 | 31 (41.9) | 16 (29.6) | |
| Area Deprivation Index (ADI) | |||
| Mean (SD) | 3.4 (2.1) | 3.4 (2.1) | 0.8953 |
| Median (Min, Q1, Q3, Max) | 3 (1, 2, 5, 8) | 3 (1, 2, 5, 9) | 0.9536 |
| Treatment Intent, N (%) | |||
| Adjuvant | 32 (43.2) | 20 (37.0) | 0.7113 |
| Definitive | 40 (54.1) | 32 (59.3) | |
| Palliative | 2 (2.7) | 2 (3.7) | |
| Induction Chemotherapy, N (%) | |||
| No | 70 (94.6) | 42 (79.3) | 0.0114 * |
| Yes | 4 (5.4) | 11 (20.7) | |
| Trial, N (%) | |||
| No | 66 (89.2) | 48 (88.9) | 0.9571 |
| Yes | 8 (10.8) | 6 (11.1) | |
| Dose Received (cGy) | |||
| Mean (SD) | 5995.9 (1551.7) | 5953.8 (1512.0) | 0.8804 |
| Median (Min, Q1, Q3, Max) | 6600 (1480, 5900, 7000, 10,000) | 6600 (848, 5700, 7000, 7000) | 0.9173 |
| Fraction Received | |||
| Mean (SD) | 28.8 (8.5) | 28.8 (8.2) | 0.9860 |
| Median (Min, Q1, Q3, Max) | 30 (1, 28, 35, 35) | 33 (4, 27.5, 35, 35) | 0.9689 |
| Treatment Receipt, N (%) | |||
| No | 2 (2.7) | 2 (3.7) | 1.0000 |
| Yes | 72 (97.3) | 52 (96.3) | |
| Telemedicine visit, N (%) | |||
| No | 74 (100) | 15 (27.8) | <0.0001 * |
| Yes | 0 (0) | 39 (72.2) | |
| Treatment discontinued, N (%) | |||
| No | 74 (100) | 50 (92.6) | 0.02964 * |
| Yes | 0 (0) | 4 (7.4) | |
| Total treatment duration (in days) | |||
| Mean (SD) | 42.8 (14.3) | 41.4 (16.0) | 0.6098 |
| Median (Min, Q1, Q3, Max) | 44 (5, 39, 49, 103) | 44.5 (11, 36.5, 49, 120) | 0.5244 |
| Median follow-up (in months) | |||
| Median (95% CI) | 31.0 (27.9–32.1) | 19.4 (19.0–22.1) | <0.0001 * |
| Treatment altered, N (%) | |||
| No | 74 (100) | 49 (90.7) | 0.0119 * |
| Yes | 0 (0) | 5 (9.3) | |
| Race, N (%) | |||
| African American/Black | 6 (8.1) | 3 (5.6) | 0.0560 |
| Arab | 0 (0) | 1 (1.8) | |
| Asian | 9 (12.2) | 11 (20.4) | |
| Caucasian/White | 33 (44.6) | 22 (40.7) | |
| Hispanic/Latino | 6 (8.1) | 0 (0) | |
| Not reported | 8 (10.8) | 2 (3.7) | |
| Other | 12 (16.2) | 15 (27.8) | |
| Language, N (%) | |||
| English | 63 (85.1) | 43 (79.6) | 0.5626 |
| Spanish | 7 (9.5) | 5 (9.3) | |
| Other | 4 (5.4) | 6 (11.1) | |
| Morphology, N (%) | |||
| Squamous cell carcinoma, NOS | 57 (77.0) | 42 (79.2) | 0.5956 |
| Adenoid cystic carcinoma | 2 (2.7) | 3 (5.7) | |
| Others | 15 (20.3) | 8 (15.1) | |
| 2019 (N = 74) | 2020 (N = 54) | Log-Rank p Value | |
|---|---|---|---|
| Relapse-Free Survival (RFS) | |||
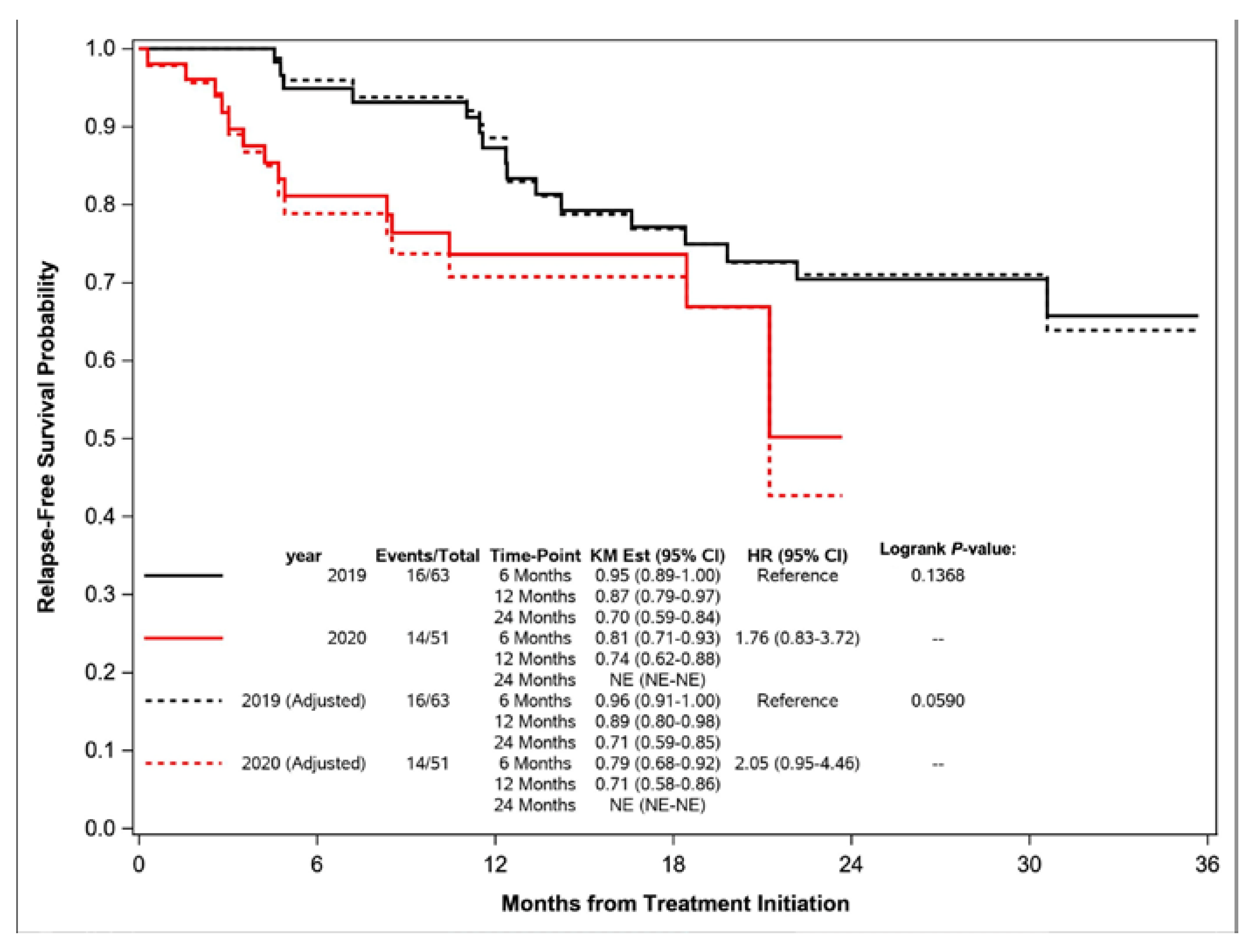
| Recurred, N (%) | 10 (15.9) | 11 (21.6) | 0.1368 |
| Local Relapse | 7 (11.1) | 9 (17.6) | |
| Distant Metastasis | 3 (4.8) | 2 (3.9) | |
| Deceased, N (%) | 6 (9.5) | 3 (5.9) | |
| Alive and Relapse-Free, N (%) | 47 (74.6) | 37 (72.5) | |
| Excluded, N (%) | 11 (14.9) | 3 (5.6) | |
| 6-Month RFS [95% CI] | 0.95 (0.89–1.00) | 0.81 (0.71–0.93) | |
| 12-Month RFS [95% CI] | 0.87 (0.79–0.97) | 0.74 (0.62–0.88) | |
| 24-Month RFS [95% CI] | 0.70 (0.59–0.84) | Not Reached | |
| Median [95% CI] | Not Reached | Not Reached | |
| Overall Survival (OS) | |||
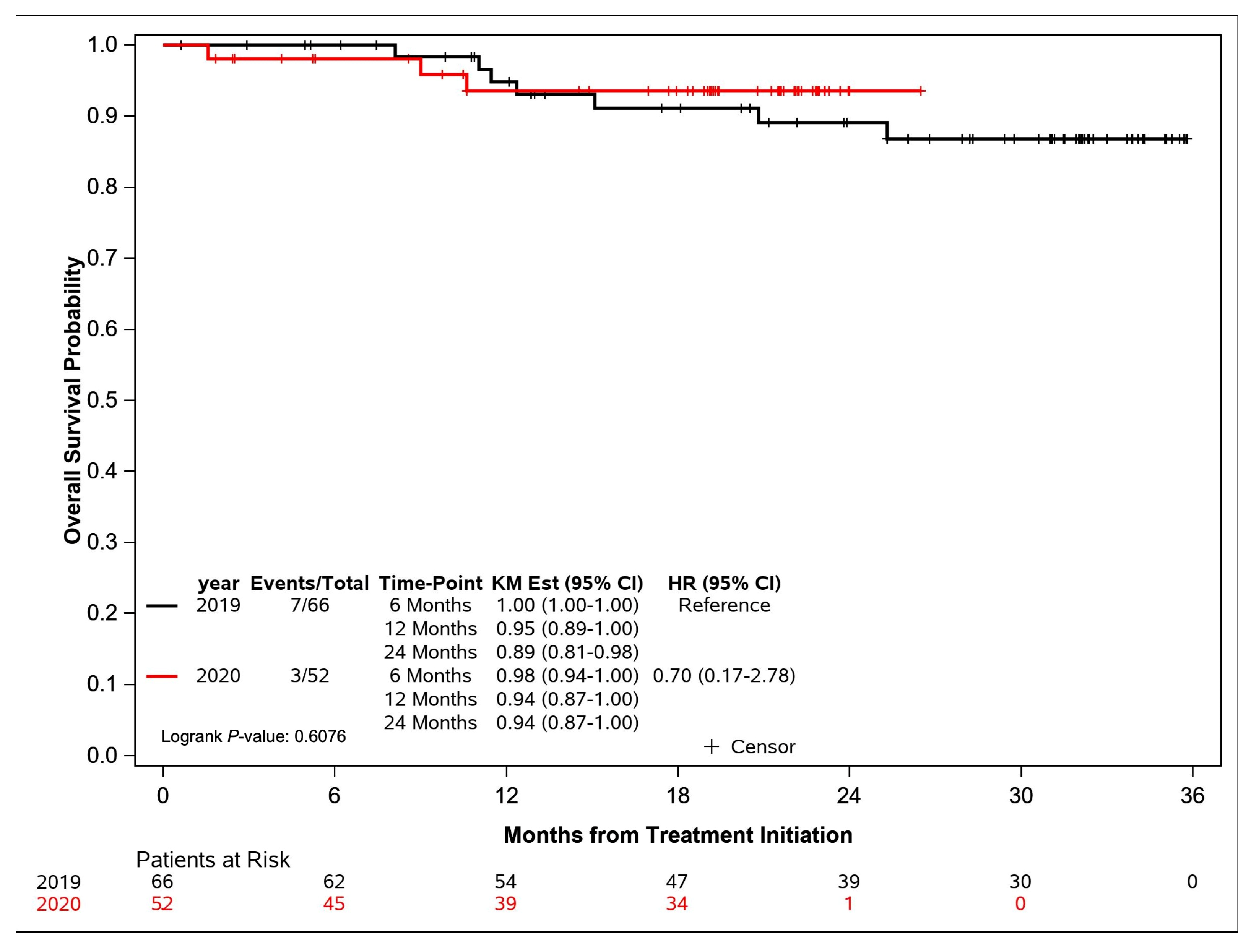
| Deceased, N (%) | 7 (10.6) | 3 (5.8) | 0.6076 |
| Alive, N (%) | 59 (89.4) | 49 (94.2) | |
| Excluded, N (%) | 8 (10.8) | 2 (3.7) | |
| 6-Month OS [95% CI] | 1.00 (1.00–1.00) | 0.98 (0.94–1.00) | |
| 12-Month OS [95% CI] | 0.95 (0.89–1.00) | 0.94 (0.87–1.00) | |
| 24-Month OS [95% CI] | 0.89 (0.81–0.98) | 0.94 (0.87–1.00) | |
| Median [95% CI] | Not Reached | Not Reached | |
| Median Time Parameters [95% CI] | 2019 (N = 74) | 2020 (N = 54) | Log-Rank p Value |
|---|---|---|---|
| Weeks from Biopsy to Consult | 4.0 (3.3–5.4) | 3.4 (2.4–3.7) | 0.0839 |
| Weeks from Biopsy to Treatment | 9.9 (8.0–12.0) | 7.6 (6.7–10.0) | 0.2984 |
| Weeks from Surgery to Treatment | 6.2 (6.0–7.6) | 6.6 (5.7–7.9) | 0.8263 |
| Weeks from Consult to Simulation | 1.8 (1.6–2.6) | 1.8 (1.6–2.6) | 0.9272 |
| Weeks from Simulation to Treatment | 1.7 (1.6–1.7) | 1.6 (1.4–1.7) | 0.2399 |
| Weeks from Consult to Treatment | 3.9 (3.4–5.0) | 3.6 (3.0–5.1) | 0.8787 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloom, J.R.; Rodriguez-Russo, C.; Hsieh, K.; Dickstein, D.R.; Sheu, R.-D.; Jain, M.; Moshier, E.; Liu, J.; Gupta, V.; Kirke, D.N.; et al. Head and Neck Cancer Patient Population, Management, and Oncologic Outcomes from the COVID-19 Pandemic. Curr. Oncol. 2024, 31, 436-446. https://doi.org/10.3390/curroncol31010029
Bloom JR, Rodriguez-Russo C, Hsieh K, Dickstein DR, Sheu R-D, Jain M, Moshier E, Liu J, Gupta V, Kirke DN, et al. Head and Neck Cancer Patient Population, Management, and Oncologic Outcomes from the COVID-19 Pandemic. Current Oncology. 2024; 31(1):436-446. https://doi.org/10.3390/curroncol31010029
Chicago/Turabian StyleBloom, Julie R., Carlos Rodriguez-Russo, Kristin Hsieh, Daniel R. Dickstein, Ren-Dih Sheu, Mayuri Jain, Erin Moshier, Jerry Liu, Vishal Gupta, Diana N. Kirke, and et al. 2024. "Head and Neck Cancer Patient Population, Management, and Oncologic Outcomes from the COVID-19 Pandemic" Current Oncology 31, no. 1: 436-446. https://doi.org/10.3390/curroncol31010029
APA StyleBloom, J. R., Rodriguez-Russo, C., Hsieh, K., Dickstein, D. R., Sheu, R.-D., Jain, M., Moshier, E., Liu, J., Gupta, V., Kirke, D. N., Roof, S., Misiukiewicz, K., Posner, M., Bakst, R., Sindhu, K. K., & Sharma, S. (2024). Head and Neck Cancer Patient Population, Management, and Oncologic Outcomes from the COVID-19 Pandemic. Current Oncology, 31(1), 436-446. https://doi.org/10.3390/curroncol31010029





