Abstract
(1) Background: Aggressive angiomyxoma is a mesenchymal cancer that is rare during pregnancy. It is a neoplasm that relapses and infiltrates the nearest structures. Our aim is to evaluate the management and outcomes of an observed case, in light of the current literature. (2) Methods: We observed this condition at the “Maggiore della Carità” Hospital in Novara (Italy) in a patient with an initial twin pregnancy and a suspected pelvic mass. The words “angiomyxoma” and “pregnancy” were searched on the main online scientific search sources (PubMed, Google Scholar, Scopus, WES, and Embase, etc.). (3) Results: The patient underwent surgery with a complicated follow-up, but recent negative controls. We analyzed the literature about the topic and found only 24 similar clinical cases. (4) Conclusions: Considering the current literature, it is useful to assess an aggressive angiomyxoma in the differential diagnosis of soft masses in pregnant women. The treatment of choice is surgical excision, and vaginal delivery is feasible. The therapeutic decision depends on each case.
1. Introduction
Angiomyxoma is a rare mesenchymal tumor characterized by a possible infiltration of the nearest tissues: it is more frequent in women, and in the third and fourth decade of life [1,2]. The most interested anatomical sites are: vulva, vagina, pelvis, and perineum. It is a neoplasm that is prone to misdiagnosis and relapse (30% of cases) [3]. In general, its clinical presentation is asymptomatic and, initially, the neoformation is painless at the level of the vulva or vagina, with a variable size observed at the beginning of gestation, which then increases during the following months. The main differential diagnoss are: Bartholin gland cyst, lipoma, vaginal wall cyst, spindle cell lipoma, myxoid neurofibroma, intrauterine myxoma, and myxoid liposarcoma [2,4,5,6].
Our aim is to describe the clinical management of this rare cancer in a patient with a twin pregnancy who underwent surgical excision during gestation, considering the current literature.
2. Materials and Methods
We observed this condition at the “Maggiore della Carità” Hospital in Novara (Italy) in a pregnant patient with a suspected pelvic mass.
The words “angiomyxoma” and “pregnancy” on the main online scientific search sources (PubMed, Google Scholar, Scopus, WES, and Embase, etc.) highlighted 128 results. Including case reports, case series, cohort studies, and meta-analyses in English, we reviewed 24 cases about this topic. We evaluated the published research, from the first study resulted in 1995 until today, with our described clinical case (Table 1).

Table 1.
Review of the literature.
The study conformed to the Ethical Guidelines of the Helsinki Declaration. The patient gave informed consent for the use of her clinical data for research purposes. The review follows PRISMA Checklist 2020 indications.
3. Results
Our Clinical Experience: The Case Report
Firstly, we described our clinical experience about this condition, and after, we reviewed the current literature, including our case (Table 1).
A 30-year-old patient with a silent history, except subclinical hypothyroidism, came to the attention of the gynecologist for her first obstetric control. The pregnancy was a spontaneous dichorionic diamniotic gestation, without significant clinical events.
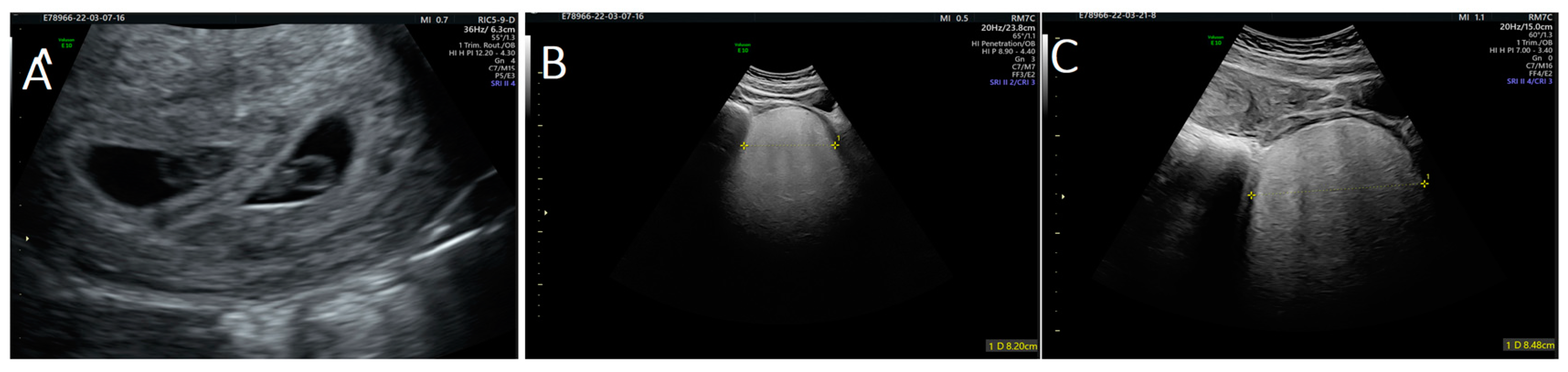
During the first visit at 10 weeks of gestation, a transvaginal ultrasound revealed a hypervascularized and inhomogeneous pelvic formation of about 10 cm in maximum diameter (Figure 1), which was not present at the last gynecological visit performed the previous year. Our patient had no symptoms until this ultrasound check.
Figure 1.
Ultrasound images of: (A) twin pregnancy; and (B,C) angiomyxoma.
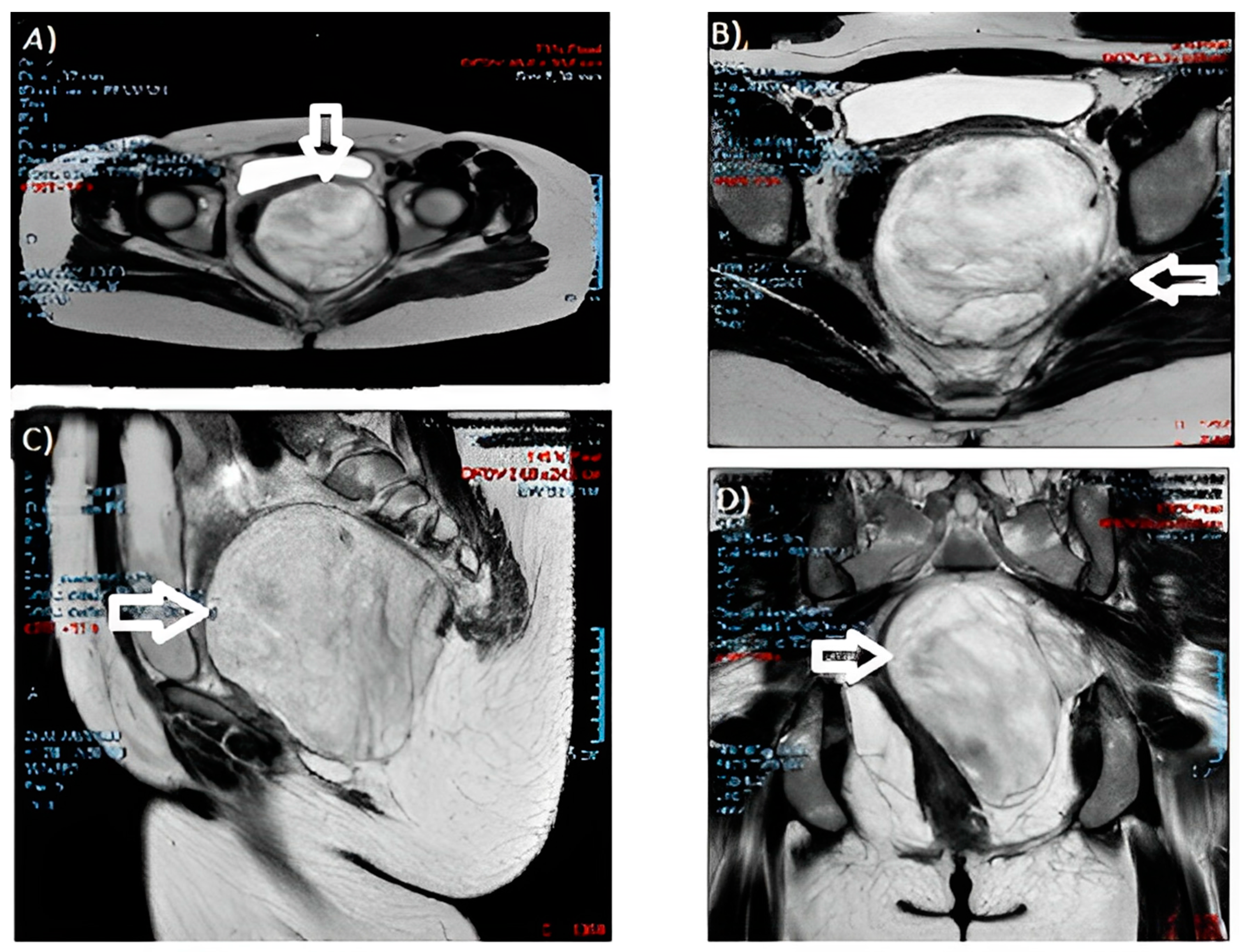
During the gynecological examination, a swelling of the left posterolateral vaginal wall with an antero-deviated portio was noted. The gynecologist performed further checks. Blood markers resulted negative. A protruding pelvic mass was described from the left perineal plane to the ischiorectal fossa on abdominal magnetic resonance imaging (MRI) (Figure 2), with dimensions of 10 × 10 × 11 cm and cottony content, hyperintense in T2, without a restriction of diffusivity. The neoformation compressed and contralaterally displaced the levator ani muscle and the wall of the rectum, anteriorly the bladder and the vaginal canal, with a cranial dislocation of the uterus. There were no appreciable signs of the infiltration of contiguous structures and no fluid flaps or lymphadenopathies. The growth and description were suggestive of an angiomyxoma.
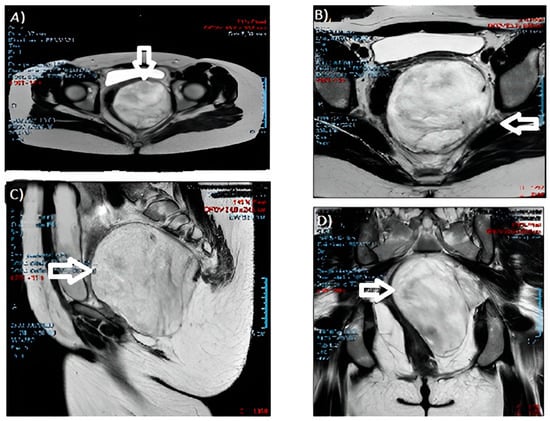
Figure 2.
MRI images of the angiomyxoma: (A,B) cross-sections; (C) sagittal section; and (D) coronal section. (The arrow indicates the neoformation).
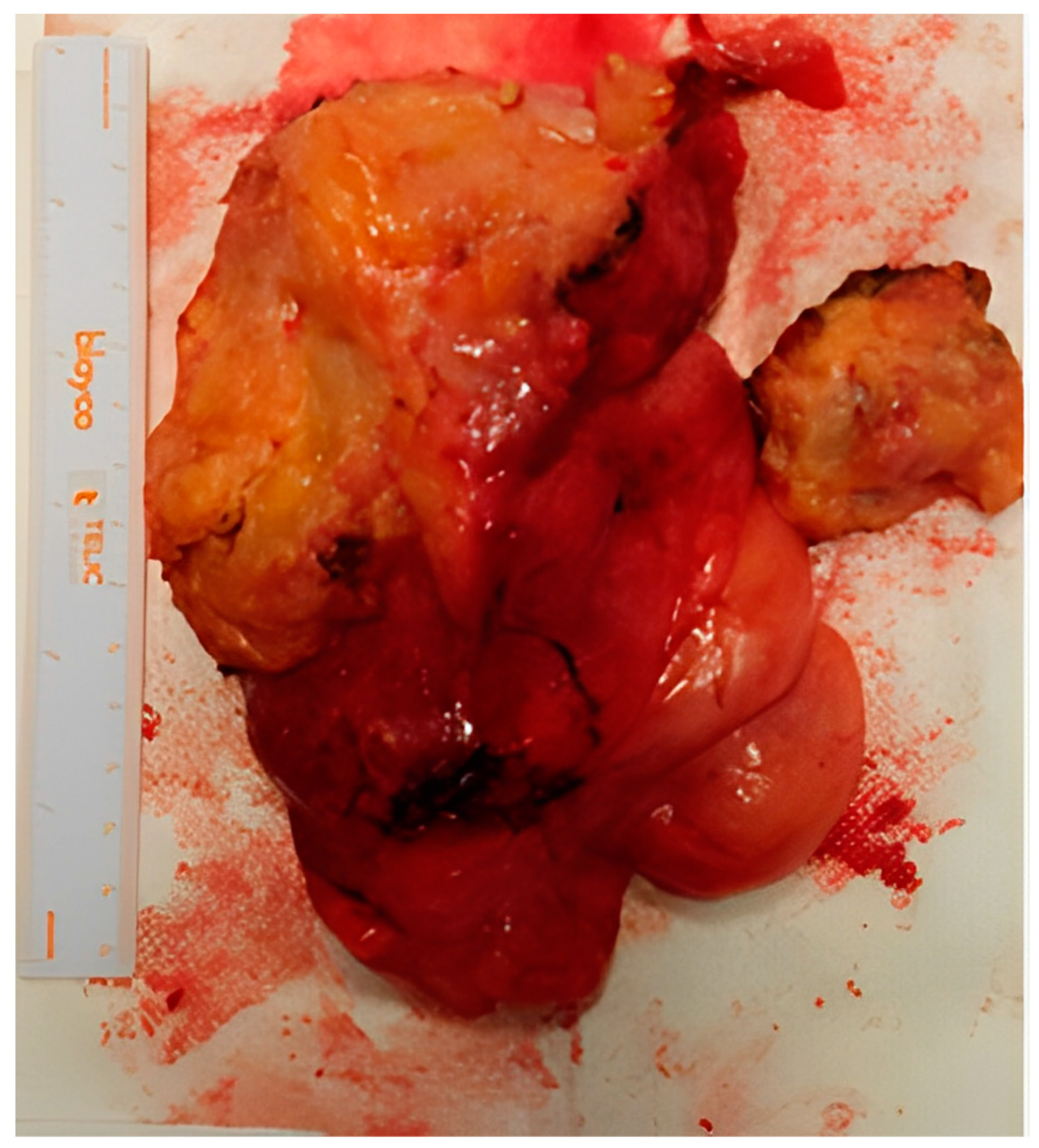
The clinical case was examined in a multidisciplinary setting with indication for surgery, due to the symptomatic increase and the patient’s severe sexual discomfort. Considering the growth of the neoformation and the patient’s will to be surgically treated, despite the possible risks and complications, the excision was vaginally performed at 13 weeks and 6 days of gestation (Figure 3). Under spinal anesthesia, an incision was made in the middle–lower third of the left vaginal wall for about 4 cm, in correspondence with the known swelling. The capsule appeared to be lardaceous and yellowish-white in color. At the end of the procedure, the formation was completely removed and sent for an extemporaneous histological examination: “myxo-lipomatous mesenchymal neoplasia without evident mitoses”. A rectal test with methylene blue was negative, the blood loss was <50 mL, and the fetal heartbeats were regular for both fetuses.
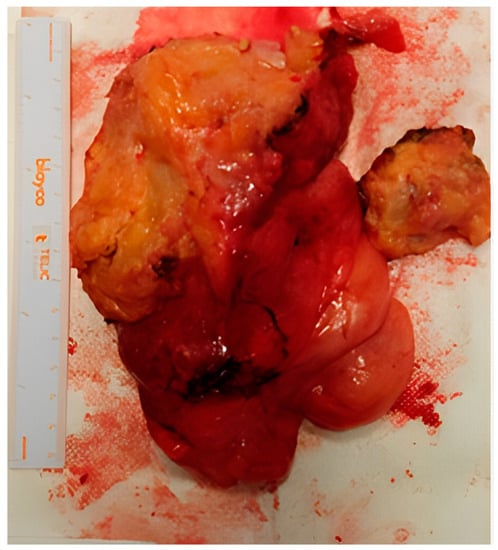
Figure 3.
Macroscopic appearance of the aggressive angiomyxoma after surgery.
After the definitive histological examination, the description was of an aggressive angiomyxoma, with fibroadipose tissue weighing 362 g and measuring 15 × 11 × 4.5 cm, yellowish with whitish streaks composed of adipose tissue, a myxoid appearance, and seeded by bands of loose collagen, including a modest cellularity, partly fused and partly stellate elements with a focal perivascular distribution, and a low mitotic index (<1/10 HPF). The immunophenotype was: actin +, desmin +, estrogen +/−, progesterone +/−, CD34+, S100−, MDM2−.
The patient was discharged home on the fourth postoperative day, with a regular course. The pregnancy was ongoing without complications. The recommendation was to perform a pelvic MRI and abdomen/chest computer-assisted tomography (CT) with contrast agent after delivery.
On the 10th postoperative day (at 14 weeks and 1 day of gestation), the patient came to the emergency room due to the appearance of hyperpyrexia and abdominal pain. Upon investigation, blood tests showed raised inflammation indexes. A vaginal ultrasound described a left pararectal dysomogeneous area of 8 × 5.7 × 4.1 cm. In a speculum examination, no abnormal genital discharge or signs of dehiscence were noted. There were no obstetric complications. She was therefore hospitalized with the diagnosis of a pelvic hematoma, and subjected to intravenous antibiotic therapy with piperacillin/tazobactam (4.5 g for three times/day). During her hospitalization, she was subjected to urine tests, urine cultures, and vaginal swabs with negative results. The progressive reduction in inflammation indexes, the resolution of her pelvic-abdominal pain, and the regression in temperature were observed. Daily fetal monitoring was regular. At the discharge visit, there was a dimensional reduction in the left pararectal suspected area (4 × 2.5 cm), with the presence of an inflammatory vaginal polyp of 1.5 × 0.5 cm between the middle and the distal third of the vaginal wound. She was discharged home on the 6th day of hospitalization, on the 16th post-operative day, with oral antibiotic therapy (amoxicillin/clavulanic acid 1 g for three times/day for another six days) and a scheduled follow-up visit for two months after the operation. Therefore, an elective cesarean section was planned at 38 gestational weeks of gestation, with a contextual excision of the suspected inflammatory vaginal polyp. The second and third trimester ultrasounds resulted in regular biometrics and anatomy for both fetuses.
At 36 weeks and 4 days of gestation, she arrived in the obstetric emergency department for the discharge of amniotic fluid. Upon examination, the PROM (premature rupture of membrane) test resulted positive, and at the obstetrical visit, the cervix was posterior, closed, with 20 percent effacement, and resistant. The twins were both cephalic and had regular amniotic fluid (the amniotic fluid deepest pockets were 4 and 5 cm, respectively, for the two fetuses). The CTG was normally reactive for both fetuses, with no perceived contractile activity. The vagino-rectal swabs were negative.
The patient tested positive at the routine SarsCOVID2 swab. She was therefore hospitalized for pPROM (preterm premature rupture of membrane) and subjected to short-cycle corticosteroid prophylaxis and antibiotic therapy, as per a protocol with Cefazolin 2 g and Azithromycin 500 mg. After two days, she was then subjected to a cesarean section and the contextual excision of the vaginal polyp, with a final blood loss of 400 mL. At the delivery, we reported the birth of a male newborn weighing 2740 g with Apgar score 8–9, and a male newborn weighing 2620 g with Apgar score 8–9.
The patient was discharged on the fifth post-operative day, with a regular course and two healthy children. Follow-up of the previous diagnosis of aggressive angiomyxoma demonstrated a normal CT and MRI.
4. Discussion
As emerged from our experience, and confirmed in the literature, the aspect of an aggressive angiomyxoma is often like a benign neoformation, such as lipomas or cysts, with a sort of soft, compact texture [2,4,5,6] and lobulated or spherical form, with an undefined capsule. The aggressivity of this type of tumor is usually limited to the nearest tissues, but some rare cases of pulmonary and lymph nodal metastasis have been reported [24,25,26,27].
Our patient had no symptoms until her first obstetric ultrasound check: despite the precious information of this technique, the gold standard remains a pelvic RMI and CT scan as an extension study. The images were similar to multiple twisted strip shadows, with “whirlpool” or “stratified” changes [28].
At the pathological level, the section could appear translucent, gray, white, or brown, with a homogeneous jelly-like consistency, cystic, and bleeding areas. Hypocellular tissues with myxoid stroma, edematous mucoid, collagen matrix, and stellate or spindle cells plus an elongated cytoplasm and abnormal vascularization are pathognomonic. The immunohistochemical examination is based on positivity for vimentin, desmin, CD34, hormone receptors (estrogens and progesterone, up to 80% of cases), S100, and a low Ki67 (<1% tumor cells) [2].
During pregnancy, the literature reports an important growth action of hormones, which should not be underrated [2]. As in our case, the suggested treatment is a surgical excision of the neoformation, trying to obtain radicality with free margins. If a neoplasm occurs during pregnancy, it could be possible to decide to treat it after delivery if there are no symptoms or discomfort [2].
In our case, the goal of surgery was reached, but a pelvic hematoma occurred as a complication and an inflammatory polyp was observed at the surgical site. The patient was then treated, with the resolution of these conditions. Vaginal delivery was not contraindicated [2], but a cesarean section was successfully performed after preterm premature membrane rupture, which was preferred due to the risk of bleeding and vulnerability of the treated perineal area.
In the literature, there are suggestions for treatment, with hormonal therapy as agonists of gonadotropin-releasing hormone (GnRh) also as an adjuvant drug, but there are no clear data on its use [29,30].
Despite radicality, it is very important to adhere to a regular imaging follow-up to diagnose eventual recurrences. It was suggested to our patient to perform a pelvic MRI and an abdomen/chest CT with contrast agent after delivery and 6 months later.
5. Conclusions
The uniqueness of our work is not only for the analysis of the pathological management, but also for the presence of a complex, ongoing twin pregnancy and the observed sequelae of the treatment. To our knowledge, this is the most updated review on the topic [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. The variety of the possible body locations, the association with pregnancy, and the few cases known in the literature make it difficult to formulate unique guidelines on this subject.
This article could be helpful for all health practitioners that have to manage pelvic neoformations and make complete diagnostic hypotheses.
In fact, aggressive angiomyxoma is a very rare disease, but it is necessary to include it in differential diagnoses in the case of pelvic neoformations, also during pregnancy. Surgical excision is its key therapy. Follow-ups through imaging (preferable MRI) are highly suggested to recognize recurrence and treat it promptly, with an attention comparable to other oncological conditions subjected to recurrence [31].
Usually, its obstetric outcomes are very encouraging: the delivery route could be differently decided for each gestation.
In the future, imaging techniques and therapeutic strategies could be implemented and diversify the treatment choice.
Author Contributions
C.I.A., A.L. and R.B. drafted the manuscript and performed the literature review; D.S., R.T., V.R., F.S. and L.N. did the editing; D.S. and M.G. performed the surgical operation and the medical follow-up; R.L.B. performed the histological examination. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steeper, T.A.; Rosai, J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am. J. Surg. Pathol. 1983, 7, 463–475. [Google Scholar] [CrossRef]
- Xu, H.; Sun, P.; Xu, R.; Wang, L.; Shi, Y. Aggressive angiomyxoma in pregnancy: A case report and literature review. J. Int. Med. Res. 2020, 48, 0300060520936414. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.A.; Kurzeja, R.; Fietze, E.; Buscher, U. Aggressive angiomyxoma of the female perineum in pregnancy. Acta Obstet. Gynecol. Scand. 2003, 82, 484–485. [Google Scholar] [CrossRef]
- Malukani, K.; Varma, A.V.; Choudhary, D.; Dosi, S. Aggressive angiomyxoma in pregnancy: A rare and commonly misdiagnosed entity. J. Lab. Physicians 2018, 10, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, J.; Sarmento-Gonçalves, I.; Ramada, D.; Amaro, T.; Tiago-Silva, P. Aggressive Angiomyxoma in Pregnancy: A Rare Condition, a Common Misdiagnosis. Case Rep. Obstet. Gynecol. 2016, 2016, 8539704. [Google Scholar] [CrossRef]
- Güngör, T.; Zengeroglu, S.; Kaleli, A.; Kuzey, G.M. Aggressive angiomyxoma of the vulva and vagina. A common problem: Misdiagnosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 112, 114–116. [Google Scholar] [CrossRef]
- Espejo-Reina, M.P.; Prieto-Moreno, M.; De-Miguel-Blanc, M.; Pérez-Martínez, D.M.; Jiménez-López, J.S.; Monís-Rodríguez, S. Genital Prolapse in Pregnant Woman as a Presentation of Aggressive Angiomyxoma: Case Report and Literature Review. Medicina 2022, 58, 107. [Google Scholar] [CrossRef] [PubMed]
- Obi-Njoku, O.; Alberto, C.; Patel, H. Aggressive Angiomyxoid Tumour—A Very Rare Patho-logic Finding in the Urinary Bladder Co-existing with Pregnancy. Int. Arch. Urol. Complic. 2017, 3, 032. [Google Scholar] [CrossRef][Green Version]
- Orfanelli, T.; Kim, C.S.; Vitez, S.F.; Van Gurp, J.; Misra, N. A case report of aggressive angiomyxoma in pregnancy: Do hormones play a role? Case Rep. Obstet. Gynecol. 2016, 2016, 6810368. [Google Scholar] [CrossRef]
- Zangmo, R.; Kumar, S.; Singh, N.; Meena, J. Aggressive angiomyxoma of vulva in pregnancy: A case report. J. Obstet. Gynaecol. India 2016, 66, 610–612. [Google Scholar] [CrossRef][Green Version]
- Ashraf, T.; Haroon, S. Aggressive angiomyxoma in pregnancy. J. Coll. Physicians Surg. Pak. 2014, 24, S24–S26. [Google Scholar] [PubMed]
- Goyal, P.; Agrawal, D.; Sehgal, S.; Ghosh, S.; Kumar, A.; Singh, S. Aggressive angiomyxoma in pregnancy. Rare Tumors 2014, 6, 5362. [Google Scholar] [CrossRef]
- Sinha, V.; Dave, K.S.; Bhansali, R.P.; Arora, R.S. Aggressive angiomyxoma of vulva which grew with pregnancy and attained a huge size rarely seen in literature. J. Obstet. Gynaecol. India. 2014, 64 (Suppl. 1), 90–91. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, K.; Jain, S.; Duhan, N.; Nanda, S.; Kundu, P. Aggressive angiomyxoma of vulva and vagina: A series of three cases and review of literature. Arch. Gynecol. Obstet. 2011, 283, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Aye, C.; Jefferis, H.; Chung, D.Y.; Manek, S.; Kehoe, S. A case of multi-modal managed vulval aggressive angiomyxoma diagnosed before conception and monitored during pregnancy. Gynecol. Oncol. 2009, 115, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Dhingra, K.; Roy, S.; Khurana, N. Aggressive angiomyxoma of the vulva presenting as a pedunculated swelling. Indian. J. Pathol. Microbiol. 2008, 51, 259–260. [Google Scholar]
- Bagga, R.; Keepanasseril, A.; Suri, V.; Nijhawan, R. Aggressive angiomyxoma of the vulva in pregnancy: A case report and review of management options. Medscape Gen. Med. 2007, 9, 16. [Google Scholar]
- Lepistö, A.; Heiskanen, I.; Böhling, T.; Raade, M.; Stefanovic, V.; Järvinen, H. Aggressive angiomyxoma—Report of three cases. Int. J. Colorectal Dis. 2007, 22, 1545–1546. [Google Scholar] [CrossRef]
- Han-Geurts, I.J.; Van Geel, A.N.; van Doorn, L.; den Bakker, M.D.; Eggermont, A.M.; Verhoef, C. Aggressive angiomyxoma: Multimodality treatments can avoid mutilating surgery. Eur. J. Surg. Oncol. 2006, 32, 1217–1221. [Google Scholar] [CrossRef]
- Ribaldone, R.; Piantanida, P.; Surico, D.; Boldorini, R.; Colombo, N.; Surico, N. Aggressive angiomyxoma of the vulva. Gynecol. Oncol. 2004, 95, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Smirniotis, V.; Kondi-Pafiti, A.; Theodoraki, K.; Kostopanagiotou, G.; Liapis, A.; Kourias, E. Aggressive angiomyxoma of the pelvis: A clinicopathologic study of a case. Clin. Exp. Obstet. Gynecol. 1997, 24, 209–211. [Google Scholar] [PubMed]
- Htwe, M.; Deppisch, L.M.; Saint-Julien, J.S. Hormone-dependent, aggressive angiomyxomaof the vulva. Obstet. Gynecol. 1995, 86, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Fishman, A.; Otey, L.P.; Poindexter, A.N., 3rd; Shannon, R.L.; Girtanner, R.E.; Kaplan, A.L. Aggressive angiomyxoma of the pelvis and perineum. A case report. J. Reprod. Med. 1995, 40, 665–669. [Google Scholar] [PubMed]
- Blandamura, S.; Cruz, J.; Faure Vergara, L.; Machado Puerto, I.; Ninfo, V. Aggressive angiomyxoma: A second case of metastasis with patient’s death. Hum. Pathol. 2003, 34, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Faraj, W.; Houjeij, M.; Haydar, A.; Nassar, H.; Nounou, G.; Khalife, M. Aggressive angiomyxoma presenting with back and perineal bulge; a complex surgical approach: A case report. Int. J. Surg. Case Rep. 2016, 24, 211–214. [Google Scholar] [CrossRef]
- Geng, J.; Cao, B.; Wang, L. Aggressive angiomyxoma: An unusual presentation. Korean J. Radiol. 2012, 13, 90–93. [Google Scholar] [CrossRef]
- Siassi, R.M.; Papadopoulos, T.; Matzel, K.E. Metastasizing aggressive angiomyxoma. N. Engl. J. Med. 1999, 341, 1772. [Google Scholar] [CrossRef]
- Giraudmaillet, T.; Mokrane, F.Z.; Delchier-Bellec, M.C.; Motton, S.; Cron, C.; Rousseau, H. Aggressive angiomyxoma of the pelvis with inferior vena cava involvement: MR imaging features. Diagn. Interv. Imaging 2015, 96, 111–114. [Google Scholar] [CrossRef][Green Version]
- Fucà, G.; Hindi, N.; Ray-Coquard, I.; Colia, V.; Dei Tos, A.P.; Martin-Broto, J.; Brahmi, M.; Collini, P.; Lorusso, D.; Raspagliesi, F.; et al. Treatment Outcomes and Sensitivity to Hormone Therapy of Aggressive Angiomyxoma: A Multicenter, International, Retrospective Study. Oncologist 2019, 24, e536–e541. [Google Scholar] [CrossRef]
- Shinohara, N.; Nonomura, K.S.; Ishikawa, S.; Seki, H.; Koyanagi, T. Medical management of recurrent aggressive angiomyxoma with gonadotropin-releasing hormone agonist. Int. J. Urol. 2004, 11, 432–435. [Google Scholar] [CrossRef]
- Tinelli, R.; Dellino, M.; Nappi, L.; Sorrentino, F.; D’Alterio, M.N.; Angioni, S.; Bogani, G.; Pisconti, S.; Silvestris, E. Eradication of Isolated Para-Aortic Nodal Recurrence in a Patient with an Advanced High Grade Serous Ovarian Carcinoma: Our Experience and Review of Literature. Medicina 2022, 58, 244. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).