Abstract
Background/Objectives: Cytarabine (Ara-C) remains central to acute myeloid leukemia therapy but is limited by unpredictable systemic toxicities. Preclinical studies have long documented multi-organ injury, yet findings remain fragmented. This systematic review synthesizes animal evidence to clarify the spectrum, dose–response patterns, and mechanisms of cytarabine-induced toxicity. Methods: Following PRISMA 2020 guidelines and PROSPERO registration (CRD420251081384), a comprehensive search of PubMed, MEDLINE, Scopus, Cochrane Library and Embase identified eligible in vivo animal studies. Data extraction covered animal models, dosing regimens, routes of administration, histopathological and biochemical endpoints and mechanistic findings. Risk of bias and study quality were assessed using SYRCLE’s tool, CAMARADES checklist and an adapted Newcastle–Ottawa Scale, with reporting benchmarked against ARRIVE 2.0. Results: Eighty-one studies (1964–2024) were included. Cytarabine produced dose- and regimen-dependent toxicities across multiple organs. Neurotoxicity was most frequently reported, followed by intestinal mucositis, ocular injury, alopecia, hepatotoxicity, nephrotoxicity, and developmental anomalies. Mechanistic analyses consistently implicated oxidative stress, inflammatory cascades, apoptosis, and epigenetic dysregulation. Study quality was moderate, with frequent deficiencies in randomization, blinding, and sample-size justification, raising concerns about reproducibility. Cardiotoxicity, despite clinical relevance, was virtually absent from preclinical evaluation. Conclusions: Preclinical evidence suggests cytarabine’s systemic toxicity as a multifactorial process extending beyond rapidly proliferating tissues. While animal studies provide mechanistic insights, methodological weaknesses and translational gaps limit predictive value. Future research must adopt rigorous design, systematically assess underexplored toxicities, and integrate molecular profiling to identify biomarkers and protective strategies.
1. Introduction
Cytarabine, also known as cytosine arabinoside or 1-β-D-arabinofuranosylcytosine, is a deoxycytidine nucleoside analog that remains a fundamental pharmacologic agent in induction and consolidation therapy for patients diagnosed with acute myeloid leukemia (AML), as well as Hodgkin and non-Hodgkin lymphomas [1,2,3,4,5]. As a pyrimidine nucleoside analog, it mimics the physiological nucleoside deoxycytidine. Its primary mechanism of action involves intracellular phosphorylation to the active metabolite cytarabine triphosphate (ara-CTP), which competes with deoxycytidine triphosphate for incorporation into DNA during the S-phase of the cell cycle, inhibiting DNA polymerase and resulting in chain termination and the subsequent induction of apoptosis in rapidly proliferating malignant cells [1,2].
Despite its proven antileukemic efficacy, clinical application is constrained by a narrow therapeutic index, with significant burdens of severe, life-threatening, or fatal adverse effects that remain largely unpredictable despite decades of use [1,6,7,8,9]. This unpredictability stems from inter-individual variability in pharmacokinetics, which is modulated by genetic polymorphisms in enzymes such as cytidine deaminase (CDA). Reduced CDA activity can lead to excessive intracellular accumulation of ara-CTP, thereby precipitating heightened systemic toxicity [6,8].
The toxicity profile of cytarabine is highly dose- and regimen-dependent, posing challenges for optimizing therapeutic efficacy while minimizing harm [1]. In standard 7-day regimens (typically 100–200 mg/m2 daily), myelosuppression is the principal dose-limiting toxicity, manifesting as profound neutropenia, thrombocytopenia, and anemia that increase infection risks [1,10]. Gastrointestinal toxicities commonly present as mild to moderate mucositis and diarrhea, though rare but severe complications like acute pancreatitis have been reported, potentially linked to direct acinar cell damage or inflammatory cascades [11,12]. Furthermore, the “cytarabine syndrome” may occur within 12 h of infusion initiation and is characterized by fever, myalgias, arthralgias, bone pain, maculopapular rash, keratoconjunctivitis, and retrosternal pain, likely driven by cytokine release or hypersensitivity reactions [13].
The advent of high-dose cytarabine (HiDAC) regimens (2–3 g/m2) for consolidation therapy has further amplified the toxicity burden. HiDAC is associated with severe adverse events including biphasic pancytopenia, neurotoxicity (cerebellar ataxia, seizures or encephalopathy due to ara-CTP accumulation in cerebrospinal fluid), dermatologic rashes, alopecia and hyperbilirubinemia affecting over 10% of patients [1,10,14]. Although less frequently discussed in the context of standard chemotherapy, cardiovascular complications, such as fatal cardiomyopathy when combined with cyclophosphamide in transplant conditioning regimens, angina, pericarditis and bradycardia (incidence up to 2.8%), further underscore the drug’s multi-organ impact and may involve endothelial damage or oxidative stress [15,16].
Critically, these toxicities extend beyond immediate physiological harm, comprising patient quality of life, increased healthcare costs and necessitating treatment interruptions or dose reductions that may affect survival outcomes [6,7]. Preclinical animal models have served as an indispensable platform for elucidating the pathophysiology of these off-target effects, demonstrating reproducible patterns of multi-organ toxicity across species. However, this vast body of evidence remains fragmented, dispersed across various species, dosing schedules and areas of research, hindering cross-study comparison and translational extrapolation to safer clinical applications in AML therapy.
Therefore, the primary objective of this systematic review is to address and bridge the gap between animal and human-based studies by comprehensively synthesizing animal evidence on cytarabine’s systemic multi-organ toxicity, with the objectives to (1) catalog the spectrum of organ toxicities reported in animal models following cytarabine administration, (2) characterize dose–response relationships and treatment schedules associated with the onset, severity and temporal progression of cytarabine-induced toxicity, (3) identify the histopathological, biochemical, and functional endpoints used to quantify organ-specific damage and (4) map proposed molecular and cellular mechanisms underlying cytarabine toxicity in preclinical studies.
2. Materials and Methods
To guarantee methodological transparency and rigor, this systematic review was conducted in accordance with the PRISMA 2020 (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [17], as detailed in Supplementary Table S1. The review was registered in PROSPERO on 15 July 2025, with ID CRD420251081384.
2.1. Identifying Research Questions
To clarify the research query, a PICO (population, intervention, comparator, outcome) framework [18] was employed, as detailed in Table 1. Accordingly, the following review question was developed: “In preclinical animal models (mice, rats, rabbits or drosophila), what are the systemic multi-organ toxicities induced by various dosing regimens of cytarabine (Ara-C) administration compared to control groups with no agent administered?”.

Table 1.
Population, Intervention, Comparator, Outcome table (PICO).
2.2. Identifying Relevant Studies
A comprehensive and systematic search was conducted on 15 July 2025 across multiple biomedical databases, including PubMed, MEDLINE, Scopus, Cochrane Library and Embase, using the following query strings: “(“Arabinofuranosylcytosine” OR “Arabinosylcytosine” OR “Aracytidine” OR “Cytosine Arabinoside” OR “Arabinoside, Cytosine” OR “Cytarabine Hydrochloride” OR “Cytosar” OR “beta-Ara C” OR “Aracytine” OR “Ara-C” “Cytonal” OR “Cytarabine”) AND (animal study OR animal model)”. References were reviewed for the identification of any additional relevant articles.
2.3. Study Selection—Eligibility and Screening
The review was restricted to full-text research studies published in peer-reviewed journals in the English language. Eligible studies had to meet the following criteria: the study must include preclinical animal models (mice, rats, rabbits or drosophila of all species and breeds); the study design must be a randomized or nonrandomized controlled study, conducted in vivo; the study should focus primarily on the systemic multi-organ toxicity of cytarabine (Ara-C) and, when available, include a control group with no agent administered. Duplicate records, review articles, systematic reviews and meta-analyses, protocols and guidelines, in vitro studies, clinical trials (all phases) on humans, conference abstracts and presentations, preprints, studies deemed irrelevant, unavailable full-text, and studies in other languages apart from English were excluded.
After the exclusion of articles based on the above criteria by both automated tools and the researchers, the final set of articles was retrieved. To ensure accuracy and objectivity, two independent reviewers (I.K. and S.T.) initially screened the titles and abstracts in a double-blinded process. For studies that passed this initial screening, the full texts were obtained and further evaluated to determine their final eligibility. Any discrepancies during the screening process were resolved by a third reviewer (T.P.).
2.4. Data Charting
Key data were independently extracted from all included studies by two reviewers (I.K. and S.T.), comprising the first author and year of publication, DOI, study design including experimental groups, control group and number of animals per group, animal model details such as species, sex, weight and age, intervention details including dose of cytarabine, timing of administration, frequency of administration, route of administration and vehicle, primary outcomes such as main toxicity, biomarker results and main histopathological findings of cytarabine’s toxicity and secondary outcomes including key methods used to assess cytarabine’s organ-specific toxicity.
2.5. Collating, Summarizing, and Reporting Results
The extracted data were synthesized into results tables. Given the systematic nature of this review, a meta-analysis was not conducted. Instead, a narrative synthesis of the principal findings was performed to explore and evaluate the systemic multi-organ toxicity of cytarabine in preclinical animal models.
2.6. Study Quality and Risk of Bias Assessment
The risk of bias and study quality of the included studies were assessed by two independent reviewers (I.K. and S.T.) using SYRCLE’s risk of bias tool for assessing risk of bias [19] and the CAMARADES checklist [20] and the Newcastle–Ottawa Scale (NOS) adapted for animal models [21] for study quality. Any discrepancies were resolved through discussion or by consulting a third reviewer (T.P.). Additionally, adherence to the ARRIVE 2.0 Essential 10 guidelines was evaluated, and the overall quality of evidence was assessed using the GRADE framework.
3. Results
3.1. Study Selection
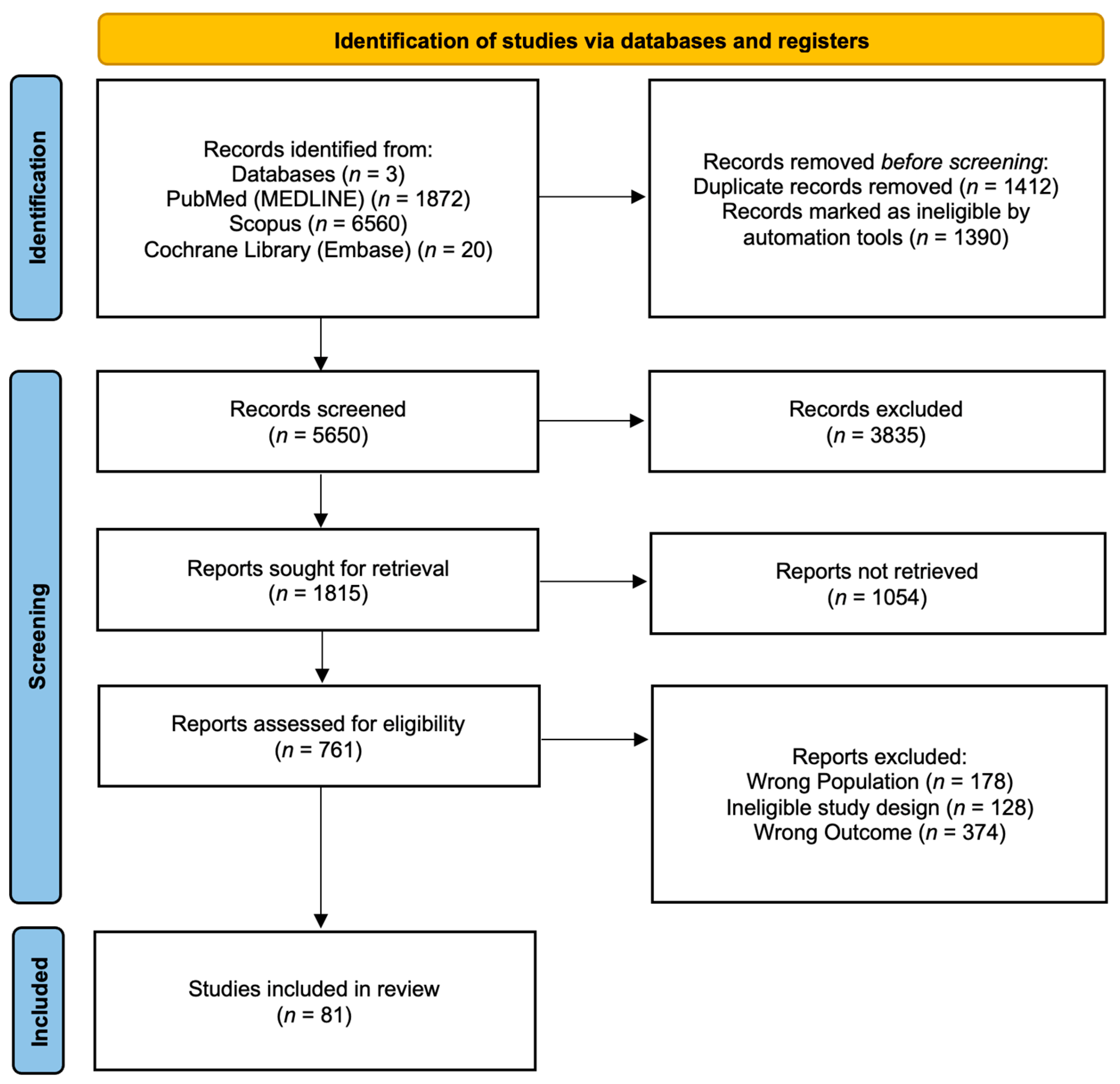
The PRISMA flow diagram (Figure 1) outlines the review selection and exclusion process. Initially, a total of 8452 records were retrieved from the aforementioned databases (PubMed and MEDLINE, n = 1872; Scopus, n = 6560; Cochrane and Embase, n = 20). Automated screening excluded 1390 records, leaving 7062 records for further consideration. Of these, 1412 duplicate records were removed manually. Subsequently, 3835 studies were excluded based on ineligible study design, as determined through title and abstract screening, and 1054 studies were not retrieved due to no full-text availability. After a full-text review of the remaining 761 articles, 81 studies met the inclusion criteria and were included in the review.
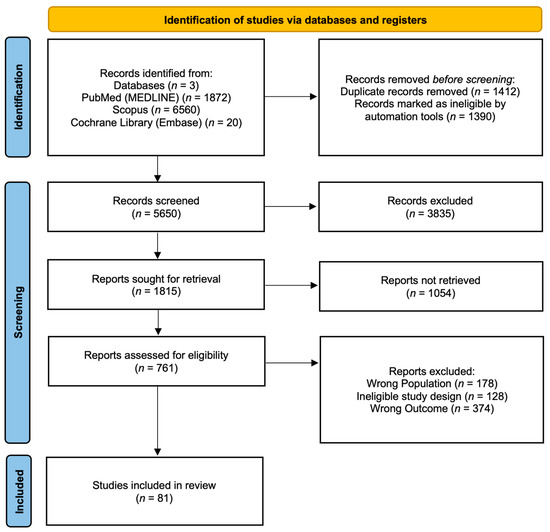
Figure 1.
PRISMA flow diagram.
3.2. Study Characteristics
The included studies spanned dates from 1964 to 2024, exclusively focusing on in vivo animal models of cytarabine-induced toxicity. They primarily employed rodent models, with mice (n = 40 studies) and rats (n = 35 studies) representing the majority, whereas rabbits (n = 6) and Drosophila (n = 2) were used less frequently. Animal ages ranged from embryonic stages (gestation days 10–12 in teratogenicity models) to adulthood (6–8 weeks old in most murine investigations), with body weights generally between 20 and 300 g, depending on species and strain. Common strains included Wistar rats (n = 18), Sprague–Dawley rats (n = 14), C57BL/6J mice (n = 11), and BALB/c mice (n = 6), which were frequently chosen for their genetic consistency and suitability for modeling human physiological responses. Cytarabine regimens exhibited considerable variability, reflecting efforts to emulate clinical dosing in the treatment of acute myeloid leukemia. Doses ranged from 1 mg/kg to 1000 mg/kg and were administered through various routes, including intraperitoneal (i.p.; n = 63 studies), intravenous (i.v.; n = 4), subcutaneous (s.c.; n = 6), oral (n = 3), intravitreal (n = 1), subconjunctival (n = 1), ophthalmic drops (n = 1), intratesticular (n = 1), and intrathecal (n = 1). Treatment regimens comprised single doses, daily administrations spanning 3 to 7 days, or intermittent protocols extending over several weeks, with vehicles such as saline or phosphate-buffered saline frequently employed. Experimental groups generally consisted of cytarabine-only cohorts (n = 6–20 animals per group) in conjunction with saline control groups, and, in certain instances, included adjunctive interventions such as antioxidants or cytoprotectants to reduce toxicity. Group sizes ranged from 5 to 30 animals, with larger cohorts used in studies evaluating functional outcomes such as behavioral assessments.
3.3. Risk of Bias and Study Quality
Using the SYRCLE’s tool, there were moderate to high risks of concern in most of the studies, with only 10% (n = 8) rated as low overall risk (Supplementary Table S2). Sequence generation (randomization) was reported in only 30% of studies, while baseline characteristics were consistently described. Allocation concealment and random housing were unclear in nearly all cases, contributing to potential selection and performance biases. Blinding of caregivers and investigators was absent in nearly all studies, though outcome assessors were blinded in only 15%. Incomplete outcome data and selective reporting were low risks overall, with most studies addressing all planned endpoints. Other biases, such as funding conflicts, were unclear in older publications.
Study quality, as assessed per the CAMARADES 10-point checklist, indicated moderate quality overall, with a median score of 4 (range 3–8) out of 10 (maximum score) and only six studies of high quality, with strengths in peer-reviewed publication, appropriate animal models and compliance with welfare regulations (Supplementary Table S3). Weaknesses encompassed absent sample-size calculations (in all studies), absent blinded model induction (in all studies) and lack of temperature control (in 62% of studies). The adaptation of the Newcastle–Ottawa Scale for cohort-like animal studies yielded a median score of 7 (range 6–9) out of 9 (maximum score), indicating high quality among the included studies, with high representativeness of exposed cohorts and ascertainment of exposure, but variable comparability regarding confounders like age or comorbidities in 93% of studies (Supplementary Table S4). Adherence to ARRIVE 2.0 Essential 10 guidelines was moderate, with a median score of 6 (range 5–9.5) out of 10 (maximum score), with robust reporting of study design, outcome measures, experimental animals and procedures, but deficiencies in sample-size justification (absent in all studies), blinding (absent in 88%) and randomization (absent in 79%) processes (Supplementary Table S5). It is worth noting that publication bias was not detected, though the predominance of positive toxicity findings suggests potential underreporting of null results.
3.4. Spectrum of Organ Toxicities
The included studies documented a wide range of organ toxicities induced by cytarabine, mostly impacting rapidly proliferating tissues in accordance with the drug’s antimetabolite mechanism of action. Table 2 encapsulates the results from the studies included, Supplementary Table S6 reports the results for individual studies. Neurotoxicity was the most commonly observed toxicity, with it occurring in an estimated 30% of cases in a total of 23 studies [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44], presenting as cerebellar degeneration, ataxia, and hippocampal-dependent cognitive deficits, predominantly in rat and mouse models. Intestinal toxicity, encompassing mucositis and villous atrophy, was observed in 14% of studies (n = 11) [45,46,47,48,49,50,51,52,53,54,55,56]. Ocular toxicity, including meibomian gland dysfunction, acinar atrophy, ductal hyperkeratinization, lacrimal gland hyposecretion, corneal epithelial erosions and retinal atrophy, was reported in ~10% of studies (n = 8) [35,36,57,58,59,60,61,62,63], particularly in mice exposed to high-dose i.p. regimens. Alopecia was assessed in 6 studies [64,65,66,67,68,69]. Hepatotoxicity, characterized by elevated transaminases, steatosis and fibrosis, affected ~5% of investigations (n = 4) [70,71,72,73]. Nephrotoxicity, characterized by tubular necrosis and glomerular damage, appeared in only 3 studies [35,36,70]. Less common injuries included myelotoxicity [74,75,76,77,78], pulmonary fibrosis [79] and reproductive toxicity, such as testicular atrophy with a detrimental impact on testicular Leydig cell number and consequently, reduced testosterone levels, following cytarabine exposure [80]. Multi-organ involvement was evident in 20% of studies, emphasizing cytarabine’s systemic impact, with no organ spared in high-dose models. Finally, 27 studies also assessed developmental toxicity following gestational exposure, along with other organ injuries, including craniofacial defects (cleft palate, encephalocele), limb and tail deformities (hemimelia, phocomelia, clubbing), digit anomalies (polydactyly, oligodactyly, ectrodactyly) and skeletal dysplasias (carpal bone fusions, absence, incomplete ossification, hypoplasia of skull, vertebral fusions) [33,34,35,36,39,41,42,43,55,61,71,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96]. Notably, cardiotoxicity, which cytarabine potentially induces, has not been systematically evaluated in preclinical animal models according to the published literature.

Table 2.
Summary of Findings and Certainty of Evidence Table.
3.5. Dose–Response Relationships, Treatment Schedules and Human Equivalent Dose Translation
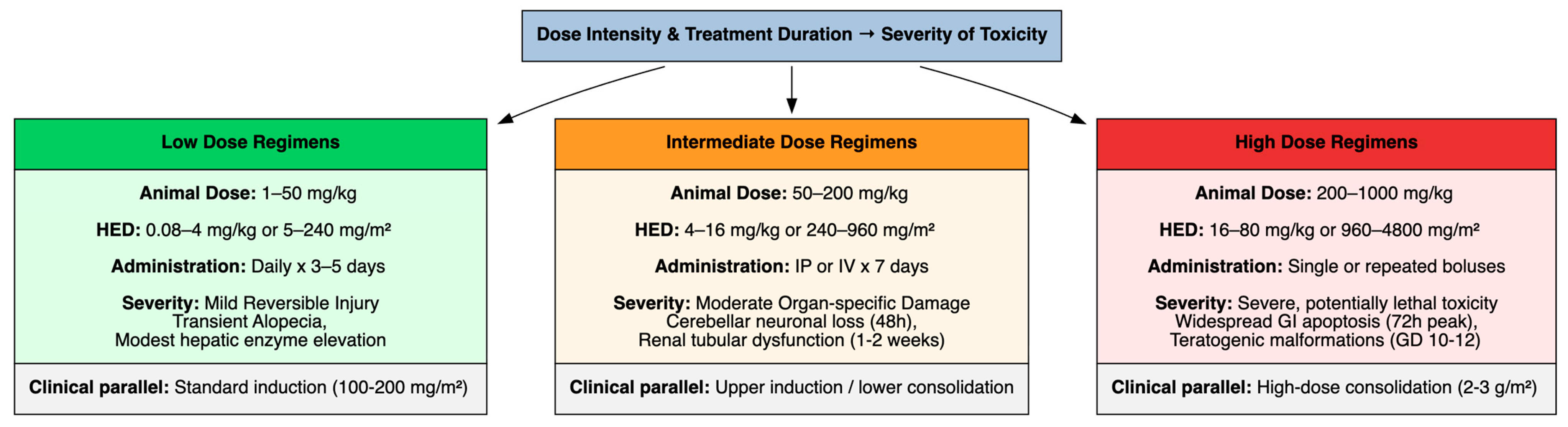
The severity of toxicity was closely linked to dose intensity and treatment duration (Figure 2). To enhance translational utility, animal doses have been converted to human equivalent doses (HEDs) using body-surface area normalization according to FDA guidance, with conversion factors of 0.08 for mouse and 0.16 for rat models [97].
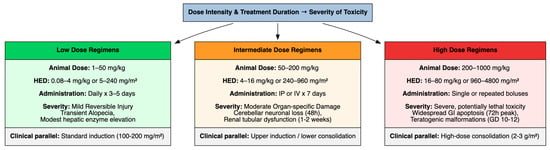
Figure 2.
Dose-Dependent Toxicity Profile and Human Equivalent Dose Translation.
Low doses, such as 1–50 mg/kg for animal models, approximately equivalent to 0.08–4 mg/kg or 5–240 mg/m2 in humans, often administered daily for 3–5 days, elicited mild, reversible injuries such as transient alopecia or hepatic enzyme elevations. These doses approximate conventional low-to-standard clinically used cytarabine regimens (100–200 mg/m2) used in induction chemotherapy for AML.
Intermediate-dose regimens, such as 50–200 mg/kg for animal models, approximately equivalent to 4–16 mg/kg or 240–960 mg/m2 in humans, administered i.p. or i.v. for 7 days, induced moderate organ-specific damage, including neuronal loss in the cerebellum (observed within 48 h post-administration) and renal tubular dysfunction progressing over 1–2 weeks. These doses approximate the upper range of standard induction and lower range of consolidation regimens, where neurotoxicity emerges as a dose-limiting toxicity.
High-dose regimens, such as 200–1000 mg/kg for animal models, approximately equivalent to 16–80 mg/kg or 960–4800 mg/m2 in humans, administered as single or repeated bolus injections, were associated with severe, potentially lethal toxicity. Widespread apoptosis in the gut mucosa, peaking at 72 h, and teratogenic malformations when administered during critical gestational windows, such as gestational days 10–12 in rats, corresponding to early organogenesis, were induced. These high-dose regimens are consistent with intensive consolidation protocols (2–3 g/m2 per dose) employed in AML therapy, where neurotoxicity, gastrointestinal injury and ocular complications become clinically prominent.
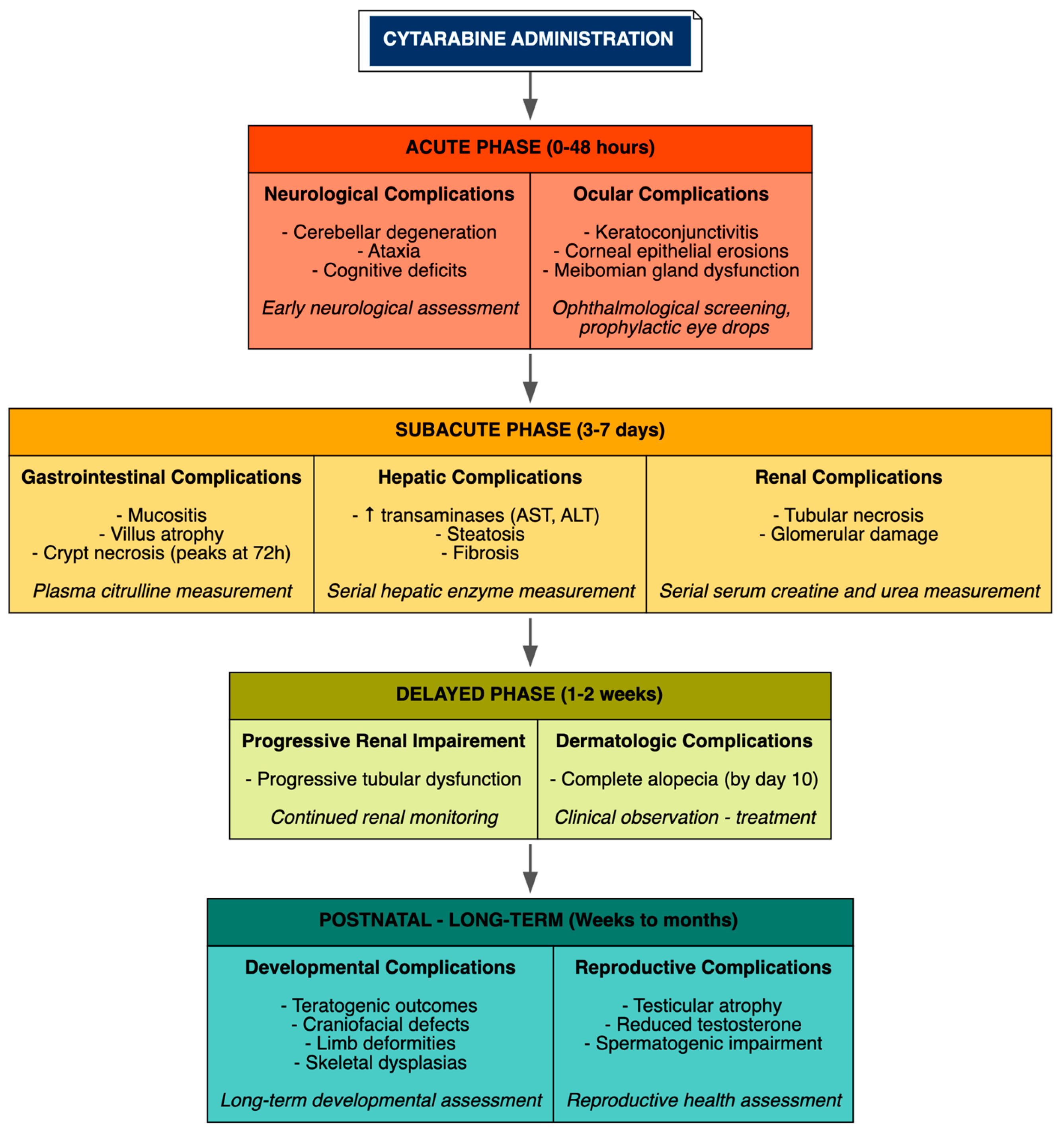
Temporal progression varied by organ, reflecting distinct vulnerabilities of proliferating tissues and organ-specific metabolic demands. Ocular and neurological effects appeared acutely within 24–48 h post-administration, with neuronal loss in the cerebellum observed within 48 h and corneal epithelial erosions and keratoconjunctivitis manifesting early in high-dose models, necessitating early neurological and ophthalmological assessment. Hepatic and renal injuries developed subacutely over 3–7 days, with progressive renal tubular dysfunction extending over 1–2 weeks, emphasizing the need for serial measurement of hepatic transaminases and renal function markers starting from day 3. Gastrointestinal toxicity, characterized by widespread apoptosis in the gut mucosa, peaked at 72 h, with catastrophic villus and crypt destruction observed by day 3 in high-dose exposures. Dermatologic toxicity followed a delayed course, with complete alopecia typically evident by day 10 following treatment initiation. Teratogenic outcomes manifested postnatally when cytarabine was administered during critical gestational windows, such as gestational days 10–12 in rats corresponding to early organogenesis. Routes of administration influenced severity, with intrathecal administration exacerbating neurotoxicity, while treatment schedules mimicked clinical consolidation, such as 100 mg/kg daily for 5 consecutive days, reproducibly induced multi-organ patterns across preclinical models (Figure 3).
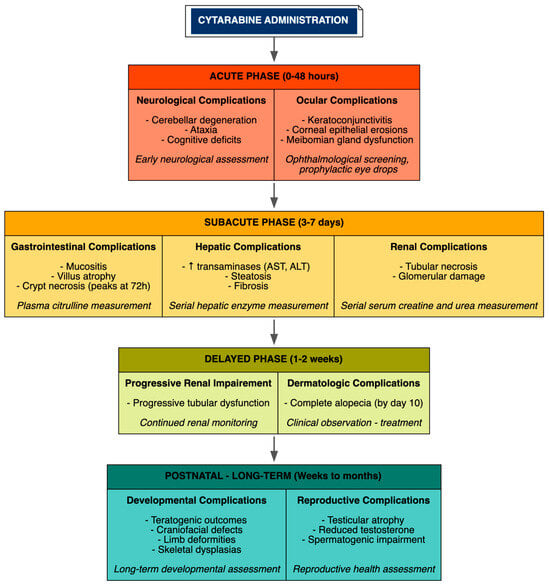
Figure 3.
Temporal Progression of Organ-Specific Toxicities.
3.6. Histopathological, Biochemical and Functional Endpoints
Across studies, a wide range of histopathological, biochemical, and functional endpoints were employed. Histological analyses revealed acinar dropout in meibomian glands, villus shortening and crypt necrosis in the intestine, dendritic retraction in cortical neurons and mitochondrial disruption in Drosophila midgut epithelia. Biochemical assays consistently suggested oxidative stress, with increased lipid peroxidation markers (MDA, 4-HNE, 8-OHdG) and reduced antioxidant enzyme activity (SOD, CAT, GSH-Px). Functional outcomes included impaired tear production, reduced locomotor activity, cognitive deficits in Morris water maze testing and decreased fertility indices.
3.7. Molecular and Cellular Mechanisms
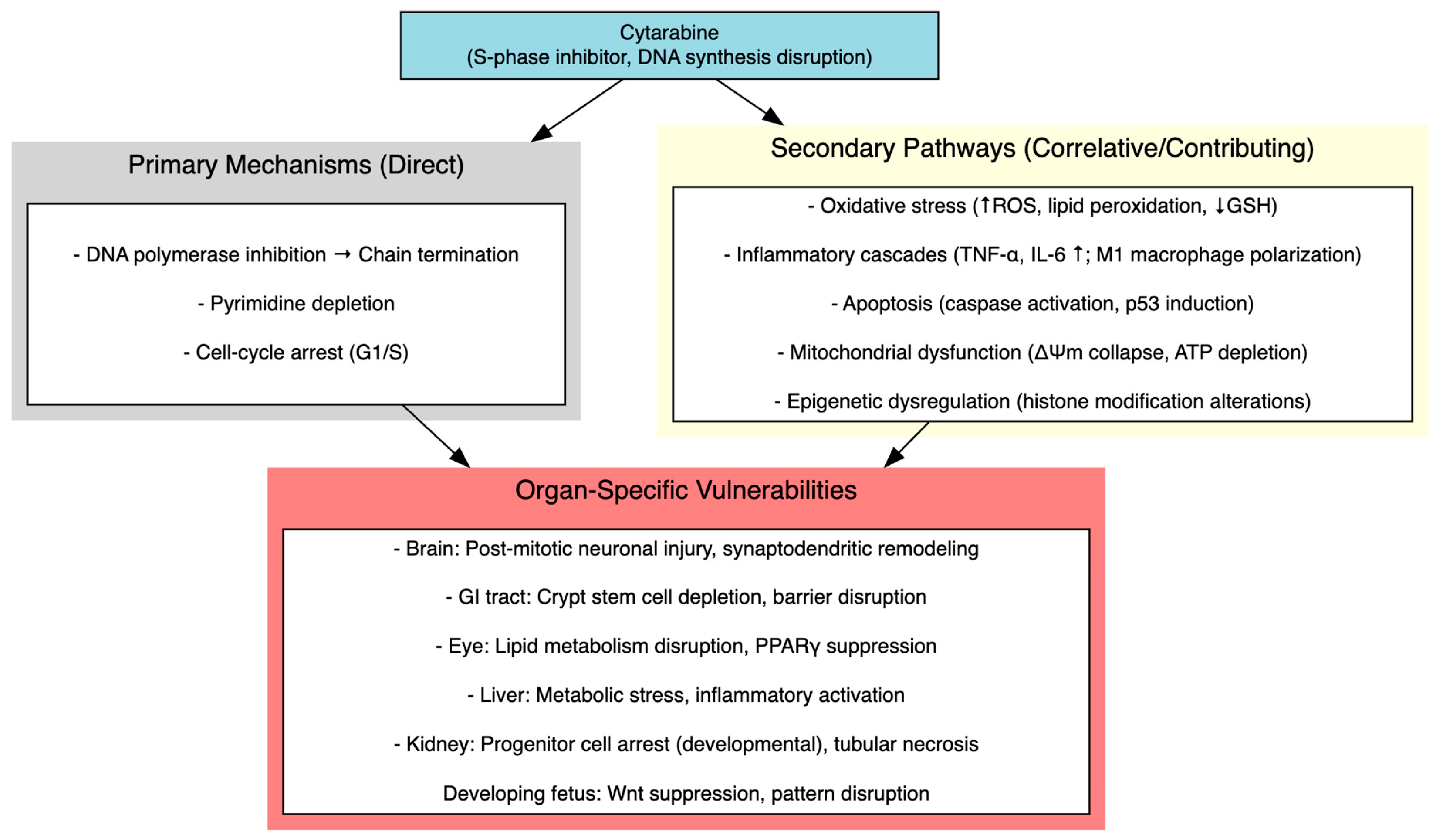
Multiple, interrelated mechanistic pathways were consistently implicated across organ systems, highlighting that cytarabine toxicity cannot be reduced to a single mode of injury. Oxidative stress and the collapse of endogenous antioxidant defenses emerged as central drivers, particularly in ocular, intestinal, hepatic and developmental models. Studies consistently documented accumulation of lipid peroxidation products and depletion of glutathione and enzymatic scavengers that temporally preceded structural injury. Inflammatory cascades, typified by TNF-α and IL-6 upregulation and skewed macrophage polarization toward pro-inflammatory M1 phenotypes, were robustly documented in intestinal injury models and correlated with amplified mucosal damage. Interventional studies showing that macrophage depletion or anti-inflammatory agents attenuate injury provide strong preliminary evidence for a causal role of inflammation. Apoptotic signaling, evidenced by caspase activation, p53 induction and DNA fragmentation, was a ubiquitous feature across intestinal, developmental and neural tissues.
In the nervous system, toxicity extended beyond antimetabolite-mediated mitotic arrest of progenitors to encompass dendritic atrophy, selective loss of mature spines, mitochondrial dysfunction, neurofilament degradation and epigenetic dysregulation, collectively disrupting synaptic plasticity and circuit integrity rather than producing uniform neuronal loss. Ocular injury involved dysregulated lipid metabolism, including loss of PPARγ nuclear localization, impaired lipogenic enzyme expression (AWAT2, SOAT1, ELOVL4), compensatory upregulation of cholesterologenesis and suppression of the AKT/FoxO survival signaling axis. These findings suggest that metabolic disruption, particularly in lipid-rich tissues such as meibomian glands, may represent a tissue-specific vulnerability. Oxidative disequilibrium, with accumulation of lipid peroxidation markers and blunted Nrf2/HO-1 antioxidant defenses, likely compounds these metabolic deficits. Developmental anomalies reflected a convergence of proliferation arrest, Wnt suppression, p53-dependent apoptosis and premature gliogenic shifts, consistent with disrupted embryonic patterning. Finally, in Drosophila, activation of JAK-STAT, JNK and Toll/IMD pathways suggested that cytarabine engages conserved innate immune and apoptotic programs, reinforcing the view that its systemic toxicity arises from a nexus of oxidative, inflammatory, apoptotic and epigenetic disturbances rather than a single linear pathway. Figure 4 summarizes the proposed molecular and cellular mechanisms of cytarabine- induced multi-organ toxicity.
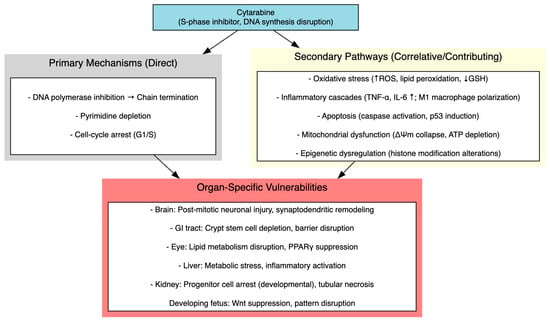
Figure 4.
Molecular and Cellular Mechanisms of Cytarabine-Induced Multi-Organ Toxicity.
4. Discussion
This systematic review of preclinical animal research provides preliminary evidence for cytarabine’s S-phase-specific antimetabolite activity that can lead to dose- and regimen-dependent systemic multi-organ toxicities, mostly in rapidly proliferating tissues. Neurotoxicity, gastrointestinal mucositis, and ophthalmic impairment were the most common side effects, but hepatic, renal, reproductive, and developmental toxicities suggest cytarabine’s systemic influence. These findings support clinical observations of chemotherapy-related complications in acute myeloid leukemia patients and provide translational insights into underlying mechanisms like oxidative stress, inflammation, apoptosis, and cell-cycle disruption, enabling organ-specific protective interventions and dosing optimization to improve therapeutic indices in humans.
4.1. Cytarabine-Induced Neurotoxicity
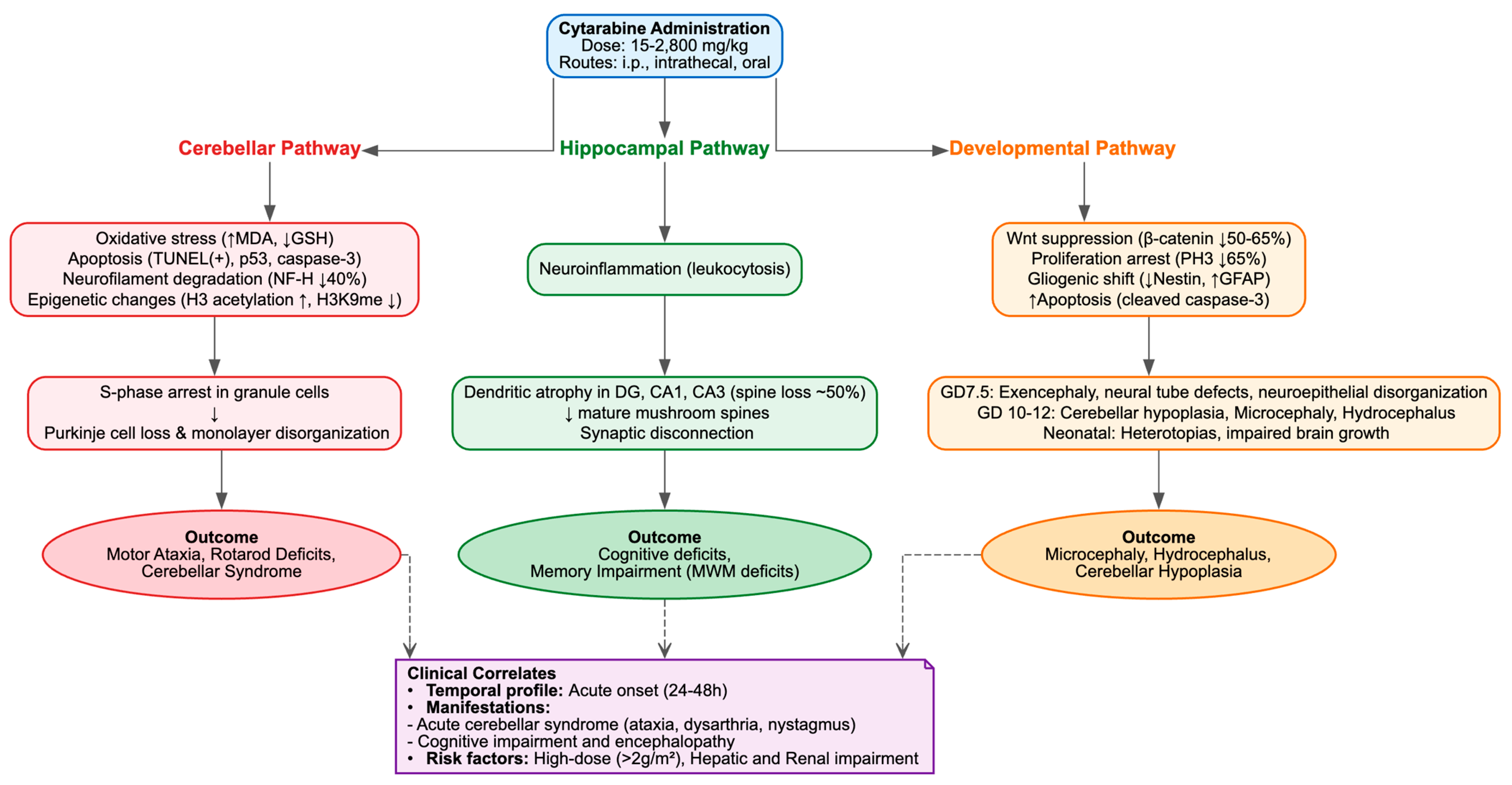
Neurotoxicity was identified as the predominant adverse effect of cytarabine in 23 preclinical studies, corresponding to clinical observations where it represents a significant dose-limiting toxicity, impacting up to 12% of patients undergoing high-dose intravenous treatments and as many as 55% of those with renal impairment [1,98]. It presented as cerebellar degeneration, disruption of hippocampal circuits, cognitive and behavioral deficits and developmental anomalies, predominantly in juvenile rodents and, in one instance, a Drosophila model, with cumulative doses varying from 15 to 2800 mg/kg delivered via intraperitoneal, intrathecal, or oral routes.
Cerebellar pathology was characterized by the loss of Purkinje cells, disorganization of the monolayer, depletion of granule cells, and cytotoxicity, alongside molecular changes such as hyporeactivity of axonal neurofilaments (NFs), selective degradation of the NF-H isoform (approximately 40% reduction), dysregulation of calbindin D-28K, oxidative stress (elevated MDA, reduced GSH), DNA strand breaks, apoptosis (TUNEL+, p53+, caspase-3+), and epigenetic modifications (increased H3 acetylation and K4 methylation, decreased H3K9 methylation) [27,28,29]. The lesions were associated with motor ataxia, as suggested by an expanded footprint base, increased step width, deficits in rotarod latency, and ambulatory hypokinesia [29], mirroring the clinically observed acute cerebellar syndrome, which is characterized by dysarthria, nystagmus, ataxia, and, in severe instances, irreversible disability, typically occurring 3–8 days following the initiation of high-dose treatment [1,99].
Hippocampal vulnerability was marked by dendritic atrophy, arbor simplification and selective loss of mature mushroom spines (~50% reduction in DG, CA1 and CA3), without overt necrosis or cell loss, but accompanied by leukocytosis suggestive of neuroinflammation. These alterations likely underline the cognitive deficits, memory impairments and encephalopathy reported in long-term survivors, particularly pediatric patients [100]. Juvenile intrathecal administration of Ara-C plus methotrexate impaired Morris Water Maze (MWM) probe trial performance, reducing quadrant preference and platform crossings while sparing acquisition and swim speed [22]. In the anterior cingulate cortex (ACC), layer II/III pyramidal neurons exhibited apical dendritic retraction (~15% reduced length, ~36% fewer branch points, ~51% reduced spine density), impairing remote but not recent memory, consistent with delayed circuit remodeling [24] and potentially explaining the delayed neurocognitive sequelae observed in leukemia survivors [100]. By contrast, some studies reported no MWM deficits despite ~9% weight loss [23].
Supratentorial pathology included neuronal pyknosis, cytoplasmic depletion, nuclear vacuolization and granule layer fragmentation across multiple regions, mediated by monoamine depletion, cholinesterase inhibition, oxidative stress (increased TBARS, MDA and H2O2; decreased GSH) and mitochondrial dysfunction (decreased succinate dehydrogenase, increased ROS, swelling and ΔΨm collapse) [25,26,31,37]. Such widespread injury plausibly accounts for clinical manifestations including seizures, somnolence, confusion and, rarely, fatal outcomes, with heightened risk in elderly patients or those with hepatic or renal dysfunction [1,98].
Developmental neurotoxicity encompassed neural tube defects (exencephaly ~30% at GD7.5), neuroepithelial disorganization, proliferation arrest (~65% reduction in PH3+ cells), increased apoptosis (2–3-fold rise in cleaved caspase-3), gliogenic shift (reduced Nestin, increased GFAP) and Wnt suppression (~50–65% reduction in β-catenin), recapitulating embryonic stem cell G1 arrest and differentiation skew [30]. Neonatal exposure induced cerebellar hypoplasia, Purkinje degeneration, heterotopic gray matter, microcephaly, hydrocephalus and impaired brain growth [32,34,35,36,40,41,42,44], alongside p53-dependent fetal lesions [101], sensorimotor gating deficits [43] and neurochemical disturbances [39]. In a rabbit herpes simplex encephalitis model, Ara-C evoked pathological brain changes [38]. In Drosophila, chronic exposure produced dose-dependent locomotor impairment, reduced lifespan and upregulation of the ROS, JNK, and JAK-STAT pathways [55].
Concluding, these findings emphasize that Ara-C neurotoxicity is not limited to antimetabolite-mediated mitotic arrest in progenitors, but also extends to oxidative and mitochondrial injury, epigenetic disruption, apoptosis and protracted synaptodendritic remodeling in post-mitotic neurons (Table 3, Figure 5). This pattern is region-specific (cerebellum > hippocampus > supratentorial) and translates into enduring behavioral and cognitive consequences.

Table 3.
Neurotoxicity reported from individual studies.
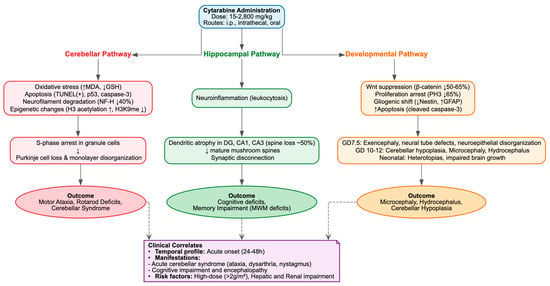
Figure 5.
Cytarabine-Induced Neurotoxicity.
4.2. Cytarabine-Induced Gastrointestinal Toxicity
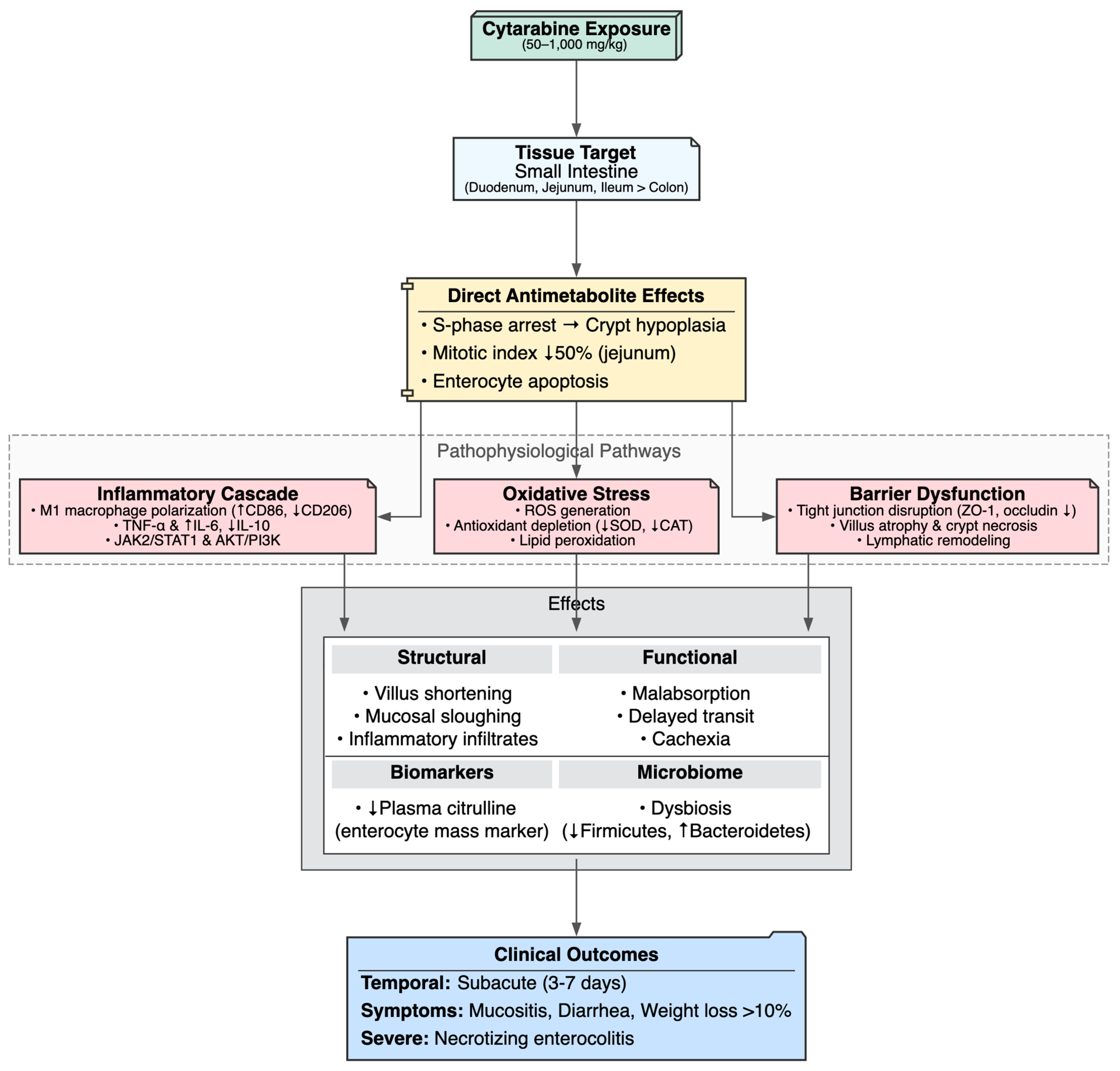
Cytarabine induced marked dose- and regimen-dependent gastrointestinal injury in 11 preclinical studies, predominantly targeting the small intestine (duodenum, jejunum, ileum) and, to a lesser extent, the colon. The dominant pathological picture was mucositis, characterized by villus atrophy (shortening, blunting, flattening), crypt hypoplasia or necrosis, enterocyte vacuolization and sloughing, dense lamina propria and submucosal inflammatory infiltrates, muscularis edema, cryptal abscesses and, in some cases, colonic polyps or xanthomatized enterocytes [46,47,49,51,52]. These findings mirror clinical observations in AML patients, where cytarabine frequently causes dose-dependent mucositis and diarrhea, often accompanied by abdominal pain and, in severe cases, necrotizing enterocolitis [1,102]. Additional complications such as ileus, hematemesis, melena, and vascular lesions including telangiectasia or intramural hematomas have also been reported [103].
The ileum appeared particularly vulnerable, showing flattened villi, crypt epithelial necrosis, vacuolated enterocytes, inflammatory infiltrates and tight-junction disruption with reduced ZO-1/occludin expression [46]. In contrast, jejunal crypts showed ~50% reduction in mitotic index without overt necrosis, suggesting impaired regenerative capacity [45,52]. In germ-free and conventional mice, short-chain fatty acids partially mitigated villus blunting, necrosis and lamina propria inflammation, though these lesions worsened under elemental diets [47]. Ultrastructural studies in Drosophila revealed epithelial edema, apical nuclear displacement, sparse microvilli and mitochondrial membrane rupture with matrix rarefaction, producing shortened and thinned guts but preserved permeability [55]. Notably, the study by Park M-R et al. (2023) reported preserved villus and crypt architecture with intact tight junctions, yet profound functional impairment was evident, including remodeling of lacteal lymphatic endothelium from permeable “button-like” to impermeable “zipper-like” VE-cadherin junctions, VEGFR2/AKT hyperphosphorylation, sequestration of oversized chylomicrons (>600 nm) in trans-Golgi cisternae, impaired lipid absorption, delayed intestinal transit, and cachexia independent of anorexia [104]. This functional malabsorption correlates with clinical reports of cytarabine-induced diarrhea, weight loss and metabolic derangements, particularly under high-dose regimens where complications such as pancreatitis, electrolyte disturbances, and protein-losing enteropathy have been documented [1,103].
The study by Minden MD et al. (2024) showed the lethality of high-dose exposure, with 100% mortality by day 3 following 300 mg/kg Ara-C, accompanied by catastrophic villus and crypt destruction, inflammatory infiltrates, reduced plasma citrulline (a surrogate for enterocyte mass) and dysbiosis marked by decreased Firmicutes and increased Bacteroidetes species [51]. Notably, reduced citrulline is already recognized clinically as a biomarker of enterocyte loss and a predictor of mucositis severity [105].
At the mechanistic level, cytarabine-induced gastrointestinal toxicity converged on dysregulated inflammation, oxidative stress, and impaired repair responses. Key features included pro-inflammatory M1 macrophage polarization (increased CD86+, iNOS+, CD11b+F4/80+ with reduced CD206+ M2), upregulation of TNF-α and IL-6 with IL-10 suppression via AKT/PI3K [46] and JAK2/STAT1 signaling [49], increased ROS production with reduced antioxidant defenses (SOD, CAT), activation of innate immune pathways (Toll/IMD, JAK-STAT, JNK) and apoptosis [55].
Together, these processes culminated in epithelial barrier breakdown, regenerative failure, nutrient malabsorption, systemic inflammation, progressive weight loss exceeding 10% and, ultimately, mortality. The evidence highlights cytarabine’s profound disruption of intestinal homeostasis in rapidly renewing mucosa, raising critical translational questions about how such injury can be predicted, mitigated, or reversed in clinical practice (Table 4, Figure 6).

Table 4.
Gastrointestinal toxicity reported from individual studies.
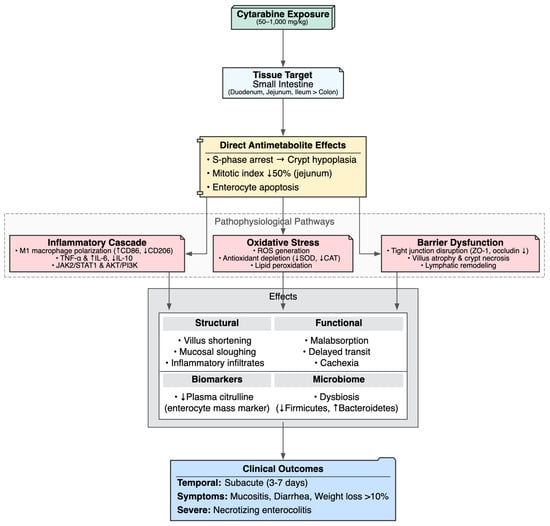
Figure 6.
Cytarabine-Induced Gastrointestinal Toxicity.
4.3. Cytarabine-Induced Ocular Toxicity
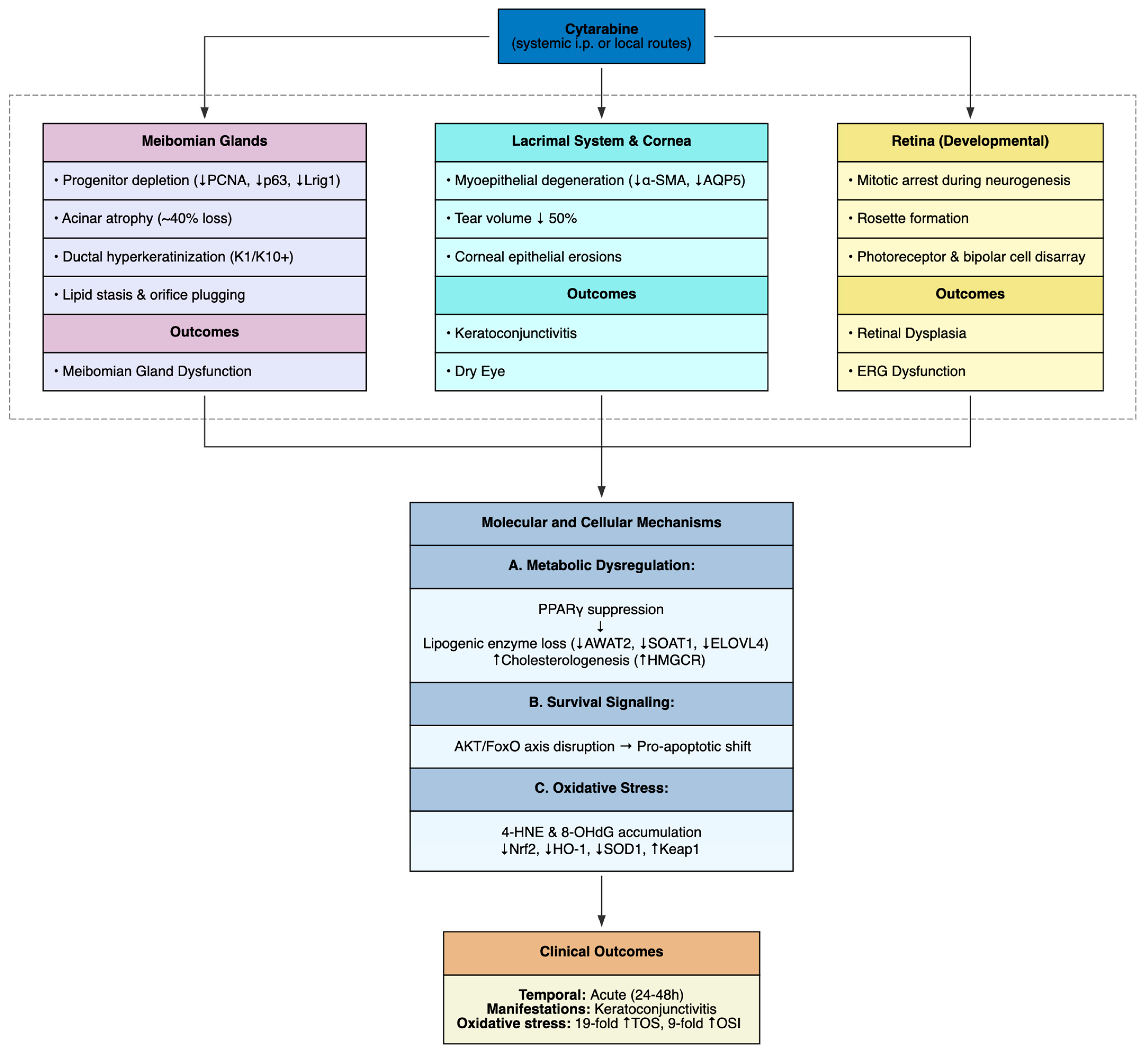
Ocular toxicity, observed predominantly under high-dose intraperitoneal or locally administered regimens, was reported across eight preclinical investigations in murine, rabbit and rat models. The study by Liu et al. (2024) showed that systemic cytarabine (50 mg/kg i.p. daily for 7 days) induces meibomian gland dysfunction and ocular surface injury in C57BL/6J mice with punctate corneal epithelial erosions (elevated corneal fluorescein staining), a ~50% reduction in tear volume due to lacrimal myoepithelial degeneration (reduced α-SMA and AQP5) and meibomian gland anomalies including orifice plugging and a 40% loss of acinar area [57]. Histopathology revealed acinar atrophy, ductal dilation with lipid stasis, full-thickness ductal hyperkeratinization (K1+/K10+) and depletion of progenitor basal cells (reduced PCNA+, p63+, Lrig1+) [57].
The study by Balci YI et al. (2017) corroborated these findings in Wistar rats (400 mg/kg i.p. for 5 days), reporting keratoconjunctivitis with a 19-fold rise in total oxidant status and a 9-fold increase in oxidative stress index [58]. These results implicate reactive oxygen species as central mediators of ocular surface injury, compounding cytarabine’s antimitotic effects.
Other studies highlighted route-dependent differences. The study by Rootman et al. (1983) compared subconjunctival and intravenous administration (37.5 mg/kg) in New Zealand white rabbits, where subconjunctival injection induced transient conjunctival inflammatory infiltrates and focal superficial erosions resolving within one week, while deeper ocular structures remained unaffected [59]. The study by Diets-Ouwehand et al. (1992) examined intravitreal cytarabine (600–2700 μg) in chinchilla rabbits, where doses ≥1500 μg caused transient blood–retina barrier disruption and irreversible b-wave attenuation on electroretinography, consistent with photoreceptor or bipolar cell dysfunction [60]. Although light microscopy showed no overt pathology, electron microscopy revealed synaptic pedicle abnormalities in photoreceptors [60]. The study by Percy and Danylchuk (1977) described severe retinal dysplasia in postnatal rats with rosette formations, photoreceptor and bipolar cell disarray, nuclear displacement, degeneration and retinal thinning, attributed to mitotic arrest during gliogenesis and neurogenesis [61]. Similarly, the study by Shimada et al. (1973) reported retinal rosettes in mice, suggesting cytarabine’s disruption of retinal progenitor proliferation [62]. The study by Kaufman et al. (1964) documented corneal epithelial toxicity in rabbits, characterized by edema, opacity and sloughing [63]. Developmental ocular defects were also evident in the research by Percy and Albert (1974), who suggested postnatal proliferative blockade in rodents [35], while the study by Percy (1975) proposed teratogenic retinal anomalies in late fetal models [36].
At the mechanistic level, cytarabine-induced ocular toxicity appears to converge on a cascade of disrupted lipid homeostasis, impaired survival signaling, and unchecked oxidative stress. Loss of PPARγ nuclear localization undermines lipogenic programs (AWAT2, SOAT1, ELOVL4), while simultaneously driving a compensatory but maladaptive upregulation of cholesterologenesis (increased HMGCR and cholesterol accumulation) [57]. This metabolic imbalance is compounded by suppression of the AKT/FoxO axis: reduced phosphorylation of AKT and FoxO1/3a permits their nuclear retention, shifting transcriptional control toward pro-apoptotic and stress-responsive pathways [57]. In parallel, oxidative disequilibrium becomes entrenched, with accumulation of lipid peroxidation and DNA damage markers (4-HNE, 8-OHdG), alongside increased Keap1 and blunted Nrf2/HO-1/SOD1 antioxidant defenses [57].
Taken together, these studies reveal cytarabine’s consistent targeting of proliferative ocular compartments through S-phase inhibition, oxidative stress, and developmental interference, culminating in epithelial attrition, glandular atrophy, dyskeratosis, and neuroretinal maldevelopment (Table 5, Figure 7). These preclinical findings mirror clinical experience, where high-dose cytarabine frequently induces keratoconjunctivitis, manifesting as conjunctivitis or keratitis in a substantial proportion of patients [106,107].

Table 5.
Ocular toxicity reported from individual studies.
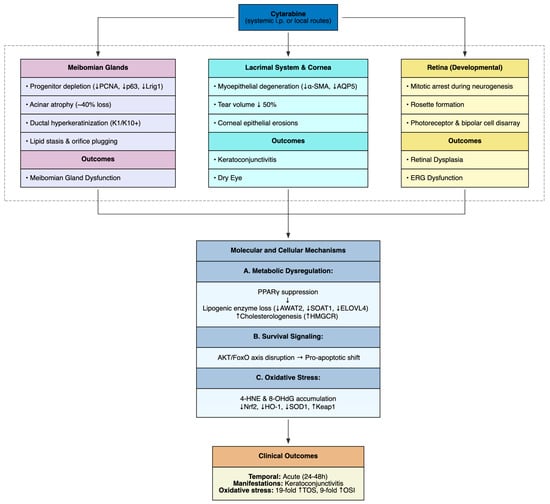
Figure 7.
Cytarabine-Induced Ocular Toxicity.
4.4. Cytarabine-Induced Alopecia
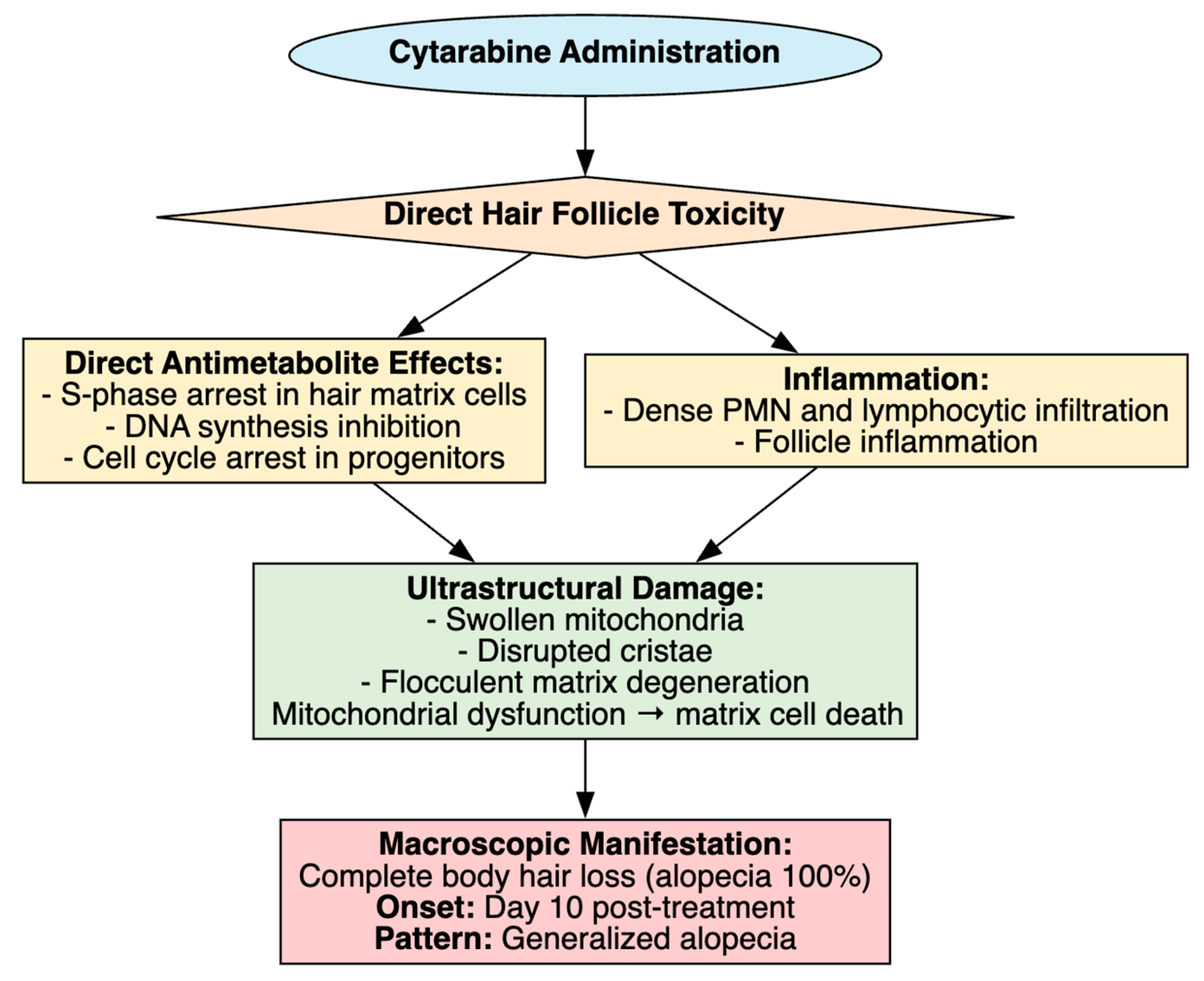
Several studies have investigated cytarabine-induced alopecia in rodent models, highlighting its macroscopic manifestation as complete body hair loss. The study by Jimenez et al. (1992) showed that intraperitoneal Ara-C at 20 mg/kg daily for 7 days in 7-day-old Fisher rats with C51 chloroleukemia resulted in complete alopecia by day 10, whereas co-administration of recombinant human interleukin-1β not only prevented hair loss but also enhanced leukemia suppression, plausibly through IL-1–mediated cell-cycle arrest of follicular matrix cells [64]. In a parallel study by Jimenez et al. (1992) reported that combined cyclophosphamide (50 mg/kg) and Ara-C (50 mg/kg) for 4–5 days induced total alopecia in all Sprague–Dawley rats, while topical or subcutaneous liposomal ImuVert plus N-acetylcysteine conferred 70–100% protection, likely by attenuating oxidative stress and modulating immune responses within follicles [65]. Extending these findings, Jimenez et al. (1992) suggested that IL-1 protected follicles both in vivo and in vitro, suggesting that cytokine-induced quiescence of proliferating matrix cells may allow them to evade Ara-C’s antimitotic effects [66].
The study by Sun et al. (2009) further suggested that Ara-C induced alopecia in rodents, with severity reduced by active hexose correlated compound (AHCC), an immunostimulatory extract thought to act through antioxidant and anti-inflammatory pathways [67]. The study by Hussein et al. (1995) reported that topical minoxidil completely prevented alopecia in Ara-C-treated rats, most likely by enhancing follicular perfusion and counteracting Ara-C-induced anagen disruption [68]. Clinically, minoxidil has also been trialed in chemotherapy-induced alopecia, with low-dose oral formulations accelerating regrowth in some patients [108,109]. Finally, the study by Hagiwara et al. (2011) observed that Ara-C (20 mg/kg i.p. daily for 7 days) induced complete alopecia in 8-day-old Wistar rats, accompanied by dense polymorphonuclear and lymphocytic infiltration of hair follicles, suggesting an inflammatory component, while electron microscopy (EM) showed swollen mitochondria, disrupted cristae and flocculent matrix in hair-matrix cells [69].
Collectively, these studies highlight Ara-C’s direct cytotoxicity to rapidly dividing hair matrix cells, with alopecia serving as a visible marker of its broader antimetabolite effects (Table 6, Figure 8). Clinically, alopecia is most pronounced at higher Ara-C doses, where complete hair loss is more frequent than with standard regimens [110] and it is often distressing, with over 50% of affected patients considering it the worst aspect of chemotherapy [111]. Advances in formulation, such as liposomal CPX-351, which has shown markedly lower alopecia rates (11%) compared with conventional cytarabine plus daunorubicin, suggest that drug delivery innovations may offer the most realistic path toward mitigating this psychologically burdensome toxicity [112].

Table 6.
Alopecia reported from individual studies.
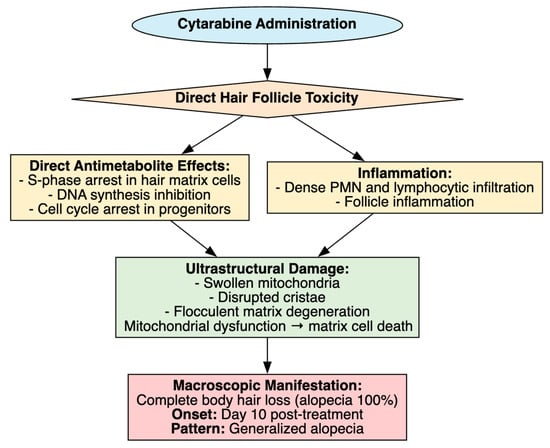
Figure 8.
Cytarabine-Induced Alopecia.
4.5. Cytarabine-Induced Hepatotoxicity
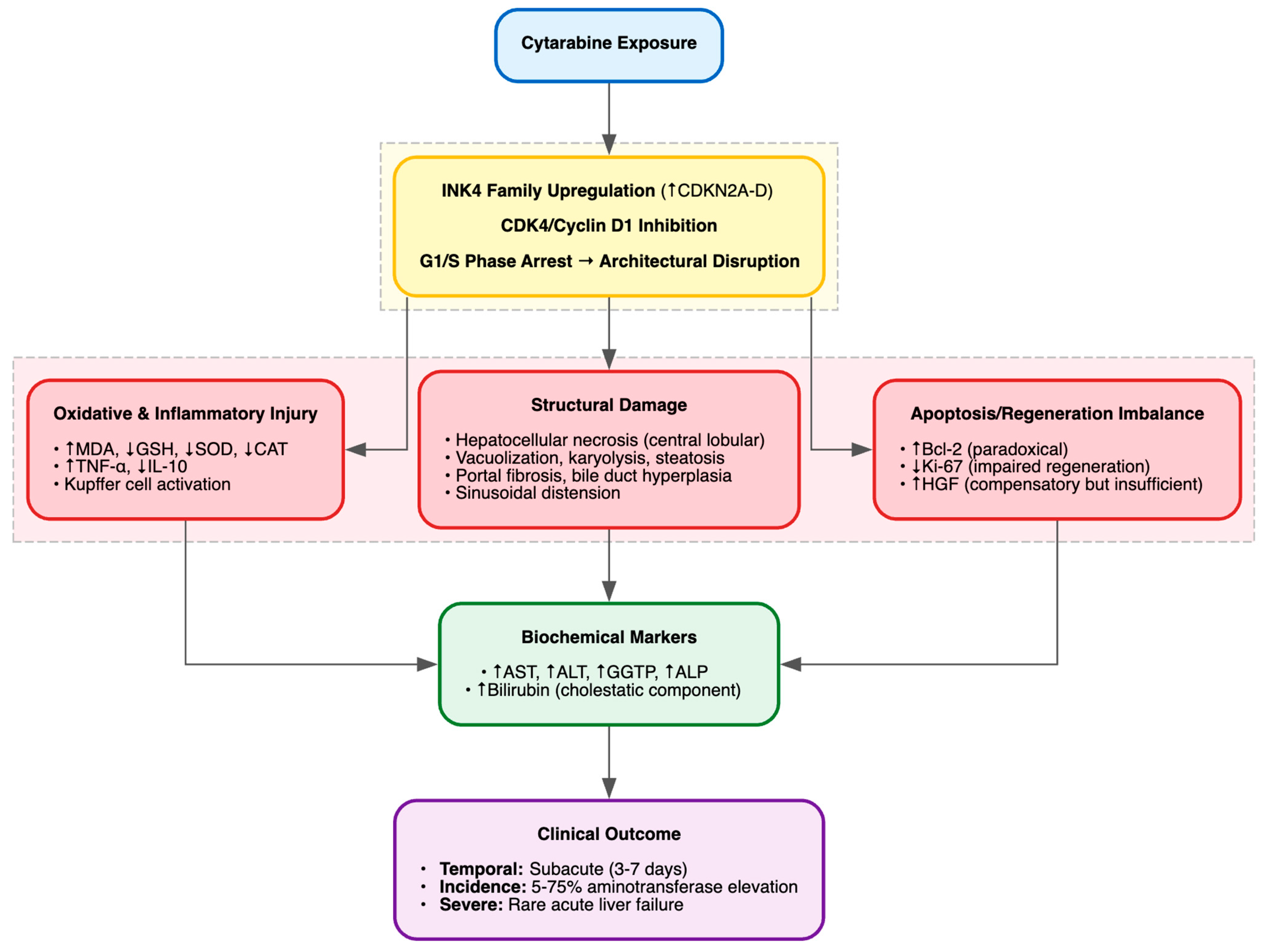
Cytarabine-induced hepatotoxicity has been consistently suggested in preclinical models, revealing patterns of oxidative stress, inflammatory activation and structural injury. In BALB/c nude mice given i.p. cytarabine at 2.5 mg/kg daily for up to 20 days, the study by Sun et al. (2019) reported progressive liver atrophy with concomitant weight loss, mechanistically linked to G1/S cell-cycle arrest via upregulation of the INK4 family genes (CDKN2A–D) and inhibition of the CDK4/cyclin D1 complex [70]. Histopathology showed growth-restrictive architectural disruption [70]. The study by Kolure et al. (2023) extended these findings in pregnant Sprague–Dawley rats exposed orally to 25 mg/kg daily from gestational days 8–20, documenting maternal hepatotoxicity with marked elevations in AST, ALT, urea, and creatinine, increased hepatic MDA, and suppression of antioxidant defenses (CAT, SOD, GSH, GSH-Px) [71]. H&E sections revealed lobular disorganization, vacuolization, pycnotic nuclei, and sinusoidal dilation. In rabbits treated intraperitoneally with 50 mg/kg daily for 7 days, the study by Al-Jammas et al. (2020) observed mononuclear and Kupffer cell infiltrates, portal fibrosis, bile-duct hyperplasia, vascular congestion, coagulative necrosis and sinusoidal distension [72]. Finally, the study by Dudina et al. (2018) reported acute cytotoxic liver injury in Wistar rats administered intravenous cytarabine at 2 g/m2 daily for 5 days, with elevated AST, ALT, GGTP and ALP, central-lobular necrosis, karyolysis, vacuolar degeneration, steatosis, portal fibrosis and bile-duct hyperplasia [73]. Mechanistically, ELISA and immunohistochemistry revealed increased TNF-α and HGF, reduced IL-10, upregulated Bcl-2 and diminished Ki-67, indicating a dysregulated balance of inflammation, apoptosis and regeneration [73].
Summarizing, these studies underscore a hepatotoxic profile driven by cell-cycle arrest, oxidative imbalance, and pro-inflammatory signaling, yet the evidence base remains narrow and methodologically limited (Table 7, Figure 9). Clinically, cytarabine, especially at high doses, has been associated with hepatotoxicity ranging from transient aminotransferase elevations in 5–75% of patients to rare but severe cases of acute liver failure [113]. Cases reports describe acute hepatic failure with elevated transaminases, mild hyperbilirubinemia and preserved ALP [114], as well as isolated direct hyperbilirubinemia suggestive of cholestatic injury [115]. These clinical observations echo preclinical findings of hepatocellular necrosis, cholestasis and inflammatory injury, underscoring the translational relevance but also exposing the lack of systematic evaluation.

Table 7.
Hepatotoxicity reported from individual studies.
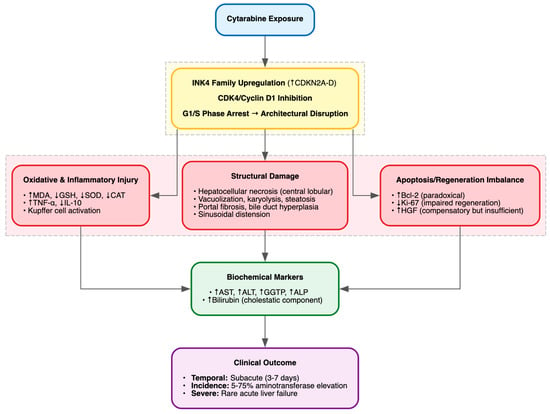
Figure 9.
Cytarabine-Induced Hepatotoxicity.
4.6. Cytarabine-Induced Renal Toxicity
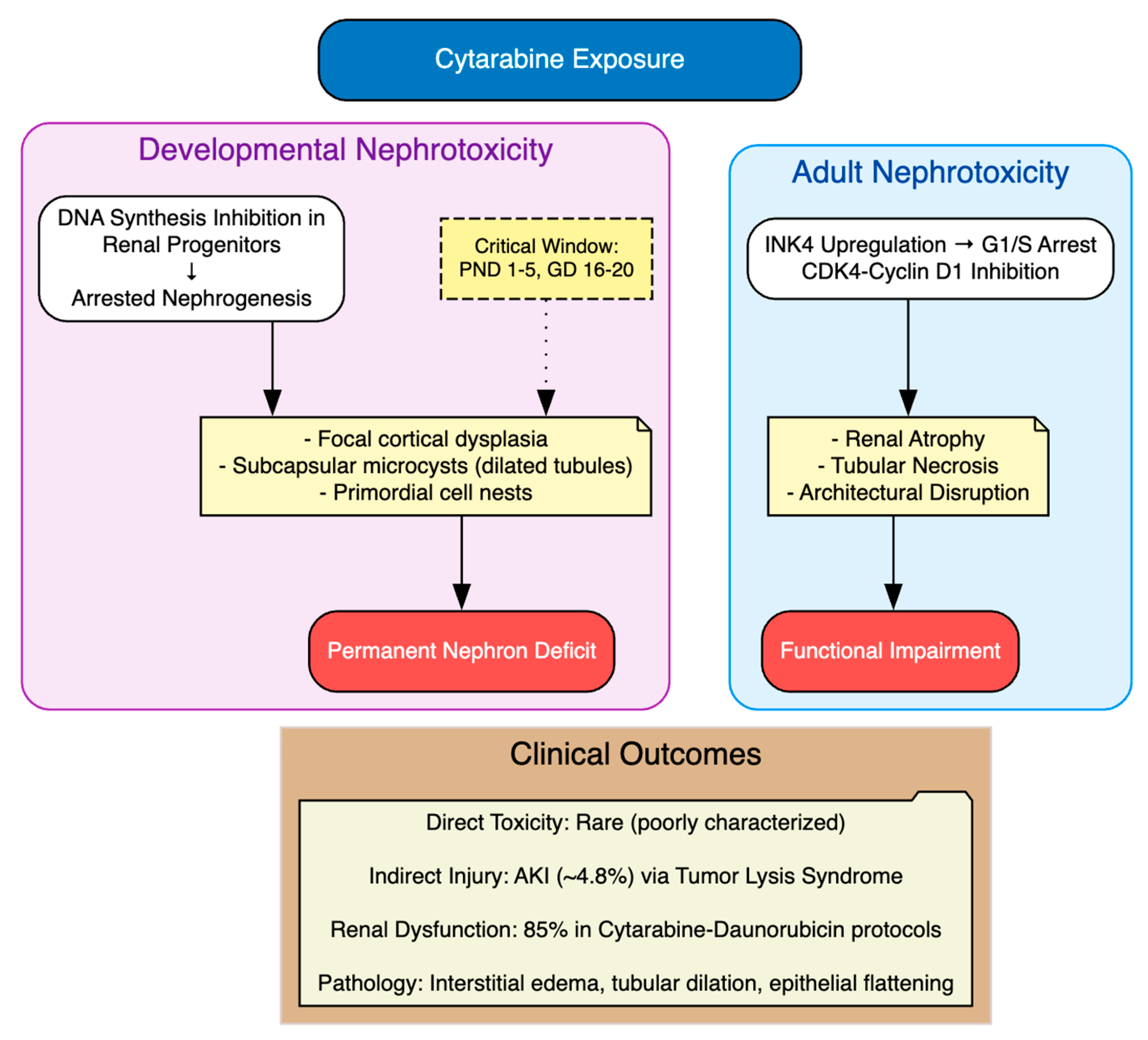
In preclinical models, cytarabine has been shown to induce nephrotoxicity primarily through disruption of DNA synthesis and inhibition of proliferative capacity, as shown in three key studies. The study by Percy et al. (1974) administered cytarabine at 3.125–50 mg/kg daily for five consecutive days beginning on postnatal day 1, 5 or 10 in ICR Swiss albino mice and Sprague–Dawley rats [35]. They reported developmental defects, including kidney lesions characterized by focal cortical dysplasia, subcapsular nests of undifferentiated primordial renal cells and arrested glomerular and tubular development, with severity greatest when dosing began on postnatal day 1 at doses ≥25 mg/kg/day. In a subsequent study, the study by Percy (1975) examined late fetal exposure in ICR Swiss albino mice (gestational days 16–18) and Sprague–Dawley rats (gestational days 18–20) using subcutaneous injections of 12.5–50 mg/kg/day for three days [36]. Cytarabine produced dose-dependent renal malformations, most pronounced at 50 mg/kg/day, including focal subcapsular microcysts composed of dilated tubules lined by vacuolated epithelium and nests of primordial cells, consistent with arrested nephrogenesis [36]. Mechanistically, these effects reflected impaired pyrimidine incorporation into DNA, leading to defective progenitor cell division and disrupted organogenesis [36]. More recently, the study by Sun et al. (2019) showed cytarabine-induced renal atrophy in 5–8-week-old BALB/c nude mice treated intraperitoneally with 2.5 mg/kg daily for up to 20 days [70]. Findings showed dose-dependent body-weight loss, gross kidney atrophy and histological evidence of inflammation and architectural disruption, mediated by upregulation of the INK4 family genes (CDKN2A–D) and inhibition of the CDK4/cyclin D1 complex, resulting in G1/S cell cycle arrest and impaired tissue growth [70].
To sum up, these studies establish cytarabine’s nephrotoxic potential via proliferative inhibition, with consequences for both developmental and adult renal integrity. Yet, translation to the clinical setting remains problematic: direct nephrotoxicity in humans is poorly characterized and often inferred indirectly (Table 8, Figure 10). Cytarabine has been associated with acute kidney injury (AKI) in approximately 4.8% of patients, most often secondary to tumor lysis syndrome (TLS) with urate and calcium phosphate deposition in renal tubules [116]. Clinical regimens incorporating cytarabine have also been linked to high rates of renal impairment. For example, 85% of patients receiving a cytarabine–daunorubicin protocol developed renal dysfunction, with pathology showing interstitial edema, tubular dilatation, epithelial flattening, focal atypia and mitotic figures in tubular epithelium [103]. Rare but notable cases of direct renal dysfunction have been described, such as a 49-year-old woman with myelodysplastic syndrome who developed transient acute renal failure and hepatic dysfunction during low-dose cytarabine therapy, possibly reflecting an allergic mechanism [117].

Table 8.
Renal toxicity reported from individual studies.
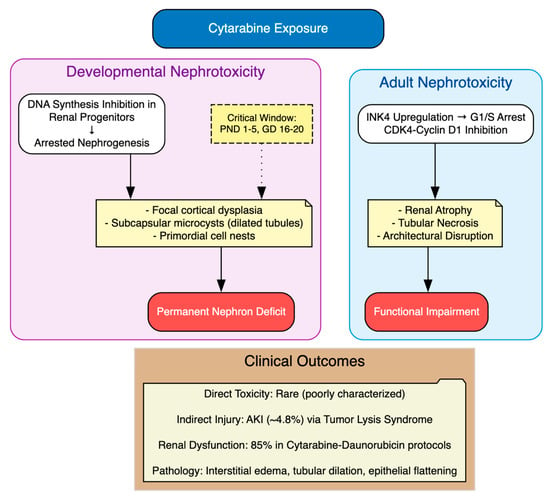
Figure 10.
Cytarabine-Induced Nephrotoxicity.
4.7. Cytarabine-Induced Developmental Toxicity
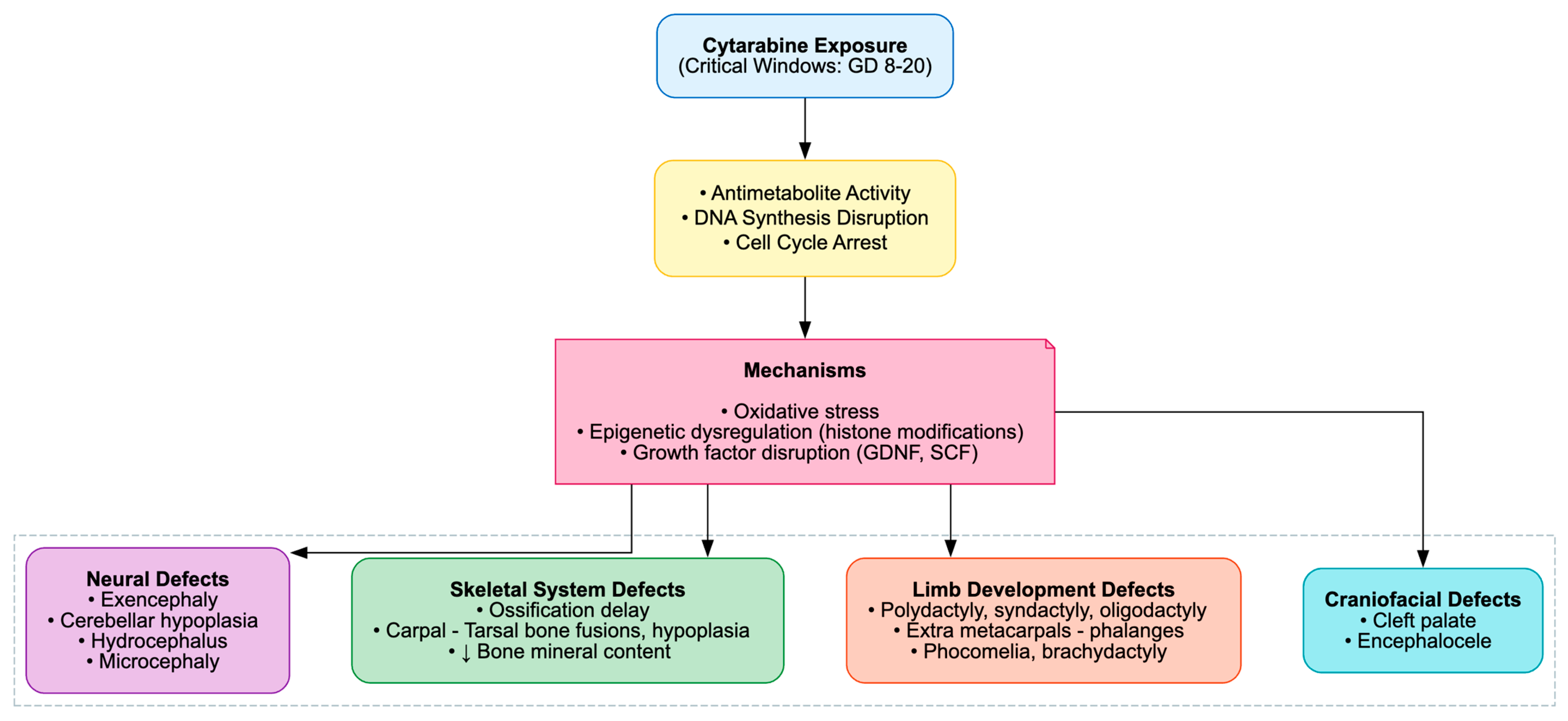
The developmental toxicity of cytarabine is well-established across rodent and non-mammalian models, manifesting as fetal growth restriction, congenital malformations, skeletal anomalies and neurodevelopmental impairments. These outcomes are consistently attributed to its antimetabolite activity, which disrupts DNA synthesis and triggers apoptosis.
In prenatal exposure studies, Ara-C produced clear, dose-dependent fetotoxicity, including resorptions, reduced fetal weight and length and external malformations such as phocomelia, oligodactyly, brachydactyly and hematomas. Skeletal ossification was markedly delayed, with diminished ossification centers, reduced bone mineral content and histopathological evidence of impaired ossification in the skull, vertebrae, sternum and limbs [36,80,82]. Limb malformations, particularly polydactyly and syndactyly, were notable, with histopathology revealing extra metacarpals and phalanges. Mechanistically, these defects were linked to expanded FGF4 expression in limb buds and disrupted Wnt/β-catenin signaling, driving premature differentiation and reduced neuroepithelial proliferation [30,81,84,85,86,87,88,89,90,91,92,93,94,95,96].
Neurodevelopmental effects included neural tube defects like exencephaly with disorganized neuroepithelium, cerebellar hypoplasia with Purkinje and granule cell loss, misalignment and heterotopic granule cells, as well as hydrocephalus and microcephaly with gray matter heterotopia. These were mediated by p53-dependent apoptosis, cell-cycle arrest, oxidative stress, and cytoskeletal disruption, highlighting the vulnerability of the developing nervous system to Ara-C [32,33,34,35,38,39,40,41,42,43,44,83]. In Drosophila, Ara-C induced developmental delay, reduced pupal size and eclosion rates and midgut epithelial injury characterized by edema, truncated microvilli, and mitochondrial rupture. These effects were mediated by oxidative stress, apoptosis and upregulation of the JAK-STAT and JNK signaling pathways [55].
Postnatal and juvenile exposures further exacerbated cerebellar neuronal injury, dendritic retraction and long-term spermatogenic impairment, with seminiferous tubule distortion, vacuolization, and reduced germ-cell layers. These outcomes were associated with oxidative stress, histone modifications, and dysregulation of growth factors such as GDNF and SCF (Table 9, Figure 11) [22,23,24,25,26,27,28,29,31].

Table 9.
Developmental toxicity reported from individual studies.
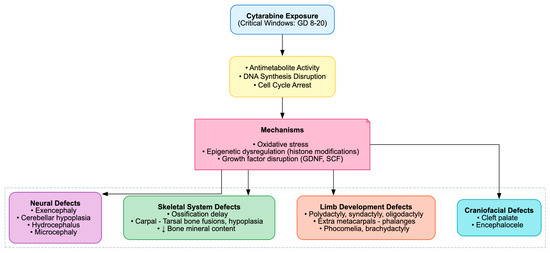
Figure 11.
Cytarabine-Induced Developmental Toxicity.
4.8. Other Cytarabine-Induced Toxicities
Pulmonary toxicity, though rarely investigated, was exemplified by Bilgin et al. [79] (2020). They reported non-cardiogenic diffuse alveolar edema and hemorrhage, and inflammatory infiltration in rats, with increased oxidative stress markers (MDA and GSH depletion) and NF-κB activation with TNF-α upregulation, after i.p. cytarabine 200 mg/kg for 14 days. These findings raise the possibility that cytarabine may contribute to respiratory compromise in susceptible patients.
Salivary gland toxicity, while less immediately life-threatening, illuminates another troubling blind spot. Saif A-J et al. (2024) [50] documented parotid gland acinar necrosis, ductal degeneration and stromal thickening and fibrosis in rabbits receiving 60 mg/kg i.p. for 10 days, with TNF-α elevation and Bcl-2 modulation indicating apoptotic injury. This mirrors clinical observations of xerostomia and sialadenitis that impair quality of life [1].
Reproductive toxicity remains strikingly underexplored. The study by Khaleel et al. (2022) [118] addressed the critical question of pediatric exposure and reported that juvenile exposure led to irreversible azoospermia, persistent testicular atrophy and a complete loss of spermatogonia that stemmed from dysregulation of GDNF, SCF and IL-10. The study by Orth et al. (1988) [119] focused primarily on the cytarabine-induced reduction in the Sertoli cells, confirmed by the reduced level of Androgen-Binding Protein, that can lead to permanent oligospermia after exposure during the perinatal or neonatal period. Moreover, Watanabe et al. (1992) [120] observed that cytarabine causes significant atrophy and weight loss in the prostate and seminal vesicles, possibly by disrupting androgen signaling or protein synthesis. Also, they identified a specific metabolic signature, an increased ratio of Putrescine to Spermidine, which suggests that cytarabine’s metabolic toxicity extends to the biochemical pathways supporting glandular secretion. Finally, Palo AK et al. (2009) [74] showed clastogenicity and a dose-dependent increase in chromosomal aberrations and micronuclei in both bone marrow and spermatogonial cells, raising concerns about genotoxic transmission through the male germline. These findings question the long-term reproductive safety of cytarabine, particularly in younger populations, and underscore the necessity of genetic counseling and potentially delaying conception for survivors of chemotherapy to allow for the clearance of damaged germline cohorts.
Hematopoietic suppression, consistently observed in studies confirms myelotoxicity as a central, dose-limiting adverse effect, yet the mechanistic nuances remain superficially studied. Lee JY et al. (2018) [75] investigated the integrity of the vascular niche in mice treated with 100 mg/kg cytarabine and they observed a structural collapse of the sinusoids, leading to a fragmented and leaky vascular network, and a concurrent depletion of megakaryocytes, likely secondary to the loss of their vascular scaffold. The study also explored the CXCL12/CXCR4 axis and found that a CXCR4 antagonist promoted the restoration of the endothelium and, by mobilizing cells or altering the local signaling dynamics, facilitated the physical reconstruction of the vascular niche, thereby accelerating hematopoietic recovery. Furthermore, Zhu RJ et al. (2013) [77] found that cytarabine triggered massive adipogenesis, with the bone marrow being crowded with adipocytes, physically occupying the space needed for hematopoietic regeneration and secreting adipokines that negatively regulate hematopoiesis, through PPAR-γ activation. Crucially, they reported that BADGE-induced adipogenesis inhibition improved hematopoietic recovery. Wang J et al. (2018) [78] documented a severe reduction in both T and B lymphocyte populations and NK cell activity depression and described lienal peptide’s dual regulatory effects on immune reconstitution. Finally, Castañeda-Yslas IY et al. (2024) [76] assessed genotoxicity in the erythroid lineage and observed a spike in micronucleated erythrocytes, a definitive marker of chromosomal breakage during the erythroblast stage, while, also, evaluating silver nanoparticle protection. Yet, these studies, taken collectively, fail to coalesce into a unified model of cytarabine myelotoxicity.
Taken together, these diverse toxicities reveal cytarabine’s capacity to perturb multiple organ systems in ways that are neither incidental nor fully anticipated by current clinical monitoring (Table 10). The absence of systematic evaluation in domains such as cardiopulmonary and reproductive health reflects a persistent blind spot in translational research.

Table 10.
Other reported toxicities from individual studies.
4.9. Limitations
Despite the comprehensive synthesis provided in this systematic review, several limitations warrant consideration. First, the methodological quality of the included animal studies was moderate at best, with consistent deficiencies in randomization, blinding and sample-size justification. Only a minority of studies reported random sequence generation, allocation concealment, or blinded outcome assessment, while none provided formal sample-size calculations. These omissions raise substantial concerns about internal validity, reproducibility and the risk of bias. Without adequate randomization and blinding, observed toxicities may reflect uncontrolled confounders or observer bias rather than true drug effects. Similarly, the absence of sample-size justification increases the likelihood of underpowered experiments, selective outcome reporting and exaggerated effect sizes. Second, although formal publication bias was not detected, the predominance of positive toxicity findings across studies strongly suggests the presence of reporting bias. Negative or null results may be underrepresented in the published literature, either due to selective reporting within studies or non-publication of inconclusive experiments. This skew toward positive outcomes inflates the apparent consistency and severity of cytarabine-induced toxicities, potentially overstating their translational relevance. The lack of transparency regarding unpublished data further limits the ability to assess the true spectrum of cytarabine’s systemic effects. Finally, heterogeneity in animal species, dosing regimens, routes of administration and outcome measures complicates cross-study comparison and limits extrapolation to human contexts. While body-surface area conversions provide approximate human equivalent doses, differences in metabolism, immune responses and developmental biology between species reduce the predictive value of these models.
4.10. Translational Implications and Future Perspectives
This systematic synthesis of preclinical evidence underscores several translational priorities that warrant consideration for clinical practice. Several biochemical and histopathological endpoints demonstrated consistent utility across studies and warrant clinical validation. Plasma citrulline, a potential marker of enterocyte mass, exhibited a dose-dependent reduction in animal models of cytarabine-induced mucositis and is already recognized clinically as a predictor of mucositis severity. Similarly, oxidative stress markers, such as MDA 4-HNE and 8-OHdG, were consistently elevated across multiple organ systems prior to overt histopathological damage, suggesting potential as early-warning indicators. Validation of these biomarkers in clinical cohorts receiving cytarabine therapy could enable timely intervention and personalized dose adjustment strategies.
Furthermore, preclinical evidence suggests that organ-specific toxicities exhibit distinct temporal profiles. Neurotoxicity and ocular injury manifested acutely (24–48 h post-administration), whereas hepatic and renal injuries developed subacutely (3–7 days). These temporal patterns imply that monitoring protocols should be tailored accordingly. For patients receiving high-dose cytarabine (≥2 g/m2), early neurological assessment (within 48 h), ophthalmological screening and, potentially, prophylactic corticosteroid eye drops and serial measurement of hepatic transaminases and renal function markers (starting day 3) may facilitate early detection and prompt intervention. Also, baseline assessment of cytidine deaminase (CDA) polymorphisms, known to influence ara-CTP accumulation, could inform individualized dosing strategies to mitigate toxicity risk.
Moreover, future clinical trials evaluating cytarabine regimens should incorporate prespecified toxicity endpoints aligned with preclinical observations. Standardized grading systems for cytarabine-specific toxicities, particularly neurotoxicity (cerebellar syndrome, cognitive impairment), gastrointestinal injury (mucositis severity, plasma citrulline) and ocular dysfunction (tear production, corneal fluorescein staining), should be implemented. It is also crucial that future research should prioritize underexplored domains such as cardiotoxicity, pulmonary injury and long-term reproductive outcomes, which remain insufficiently characterized in the current literature. Additionally, trials should stratify patients by known risk factors, including renal function, age and CDA polymorphism status, to enable subgroup analyses and identification of high-risk populations. Finally, mechanistic substudies incorporating molecular profiling, such as circulating microRNAs, proteomic and metabolomic signatures may elucidate early predictors of toxicity onset and inform adaptive dosing algorithms.
5. Conclusions
Preclinical data from 81 studies indicate that cytarabine induces dose- and regimen-dependent multi-organ toxicities in animal models, primarily affecting rapidly proliferating tissues through mechanisms such as oxidative stress, inflammation, apoptosis and cell-cycle disruption. Neurotoxicity was the most frequently observed adverse effect, followed by gastrointestinal mucositis, ocular injuries, alopecia, hepatotoxicity, nephrotoxicity and developmental anomalies. Pulmonary and reproductive toxicities were less studied and cardiotoxicity remains unassessed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cimb48010004/s1.
Author Contributions
Conceptualization, I.K. and S.T.; methodology, I.K.; software, A.K.; validation, E.G., G.D. and T.P.; formal analysis, I.K. and S.T.; investigation, I.K. and S.T.; resources, I.K.; data curation, I.K. and S.T.; writing—original draft preparation, I.K., S.T. and A.K.; writing—review and editing, K.M., E.G., G.D. and T.P.; visualization, I.K.; supervision, T.P.; project administration, T.P.; funding acquisition, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ↓ | Decreased |
| ↑ | Increased |
| AML | Acute Myeloid Leukemia |
| Ara-C | Cytarabine (Cytosine Arabinoside) |
| ara-CTP | Cytarabine Triphosphate |
| CDA | Cytidine Deaminase |
| i.p. | Intraperitoneal |
| i.v. | Intravenous |
| s.c. | Subcutaneous |
| i.t. | Intrathecal |
| GD | Gestational Day |
| MWM | Morris Water Maze |
| ACC | Anterior Cingulate Cortex |
| DG | Dentate Gyrus |
| CA1/CA3 | Cornu Ammonis regions of hippocampus |
| NF | Neurofilament |
| NF-H/NF-M/NF-L | Neurofilament Heavy/Medium/Light isoforms |
| MDA | Malondialdehyde |
| GSH | Glutathione (reduced) |
| GSSG | Glutathione disulfide (oxidized) |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| GSH-Px | Glutathione Peroxidase |
| TBARS | Thiobarbituric Acid Reactive Substances |
| ROS | Reactive Oxygen Species |
| ΔΨm | Mitochondrial Membrane Potential |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP Nick End Labeling |
| H3K9me | Histone H3 Lysine 9 Methylation |
| H3K4me | Histone H3 Lysine 4 Methylation |
| H3 acetylation | Histone H3 Acetylation |
| GFAP | Glial Fibrillary Acidic Protein |
| PH3 | Phospho-Histone H3 |
| Wnt | Wingless/Integrated signaling pathway |
| JAK-STAT | Janus Kinase—Signal Transducer and Activator of Transcription |
| JNK | c-Jun N-terminal Kinase |
| IMD | Immune Deficiency pathway (Drosophila) |
| TNF-α | Tumor Necrosis Factor alpha |
| IL-6/IL-10 | Interleukin-6/Interleukin-10 |
| PPARγ | Peroxisome Proliferator-Activated Receptor gamma |
| AQP5 | Aquaporin 5 |
| α-SMA | Alpha-Smooth Muscle Actin |
| PCNA | Proliferating Cell Nuclear Antigen |
| ZO-1 | Zonula Occludens-1 |
| VE-cadherin | Vascular Endothelial Cadherin |
| VEGFR2 | Vascular Endothelial Growth Factor Receptor 2 |
| AKT | Protein Kinase B |
| FoxO1/FoxO3a | Forkhead Box O1/O3a |
| Keap1 | Kelch-like ECH-associated protein 1 |
| Nrf2 | Nuclear Factor Erythroid 2–Related Factor 2 |
| HO-1 | Heme Oxygenase-1 |
| OSI | Oxidative Stress Index |
| TOS | Total Oxidant Status |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| ALP | Alkaline Phosphatase |
| GGTP | Gamma-Glutamyl Transpeptidase |
| HGF | Hepatocyte Growth Factor |
| Bcl-2 | B-cell lymphoma 2 (anti-apoptotic protein) |
| NOS | Newcastle–Ottawa Scale |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| SYRCLE | Systematic Review Centre for Laboratory Animal Experimentation |
| CAMARADES | Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| PICO | Population, Intervention, Comparator, Outcome |
| EM | Electron Microscopy |
| IHC | Immunohistochemistry |
| H&E | Hematoxylin and Eosin |
| SCF | Stem Cell Factor |
| GDNF | Glial cell line-Derived Neurotrophic Factor |
| MCSF | Macrophage Colony-Stimulating Factor |
| FSH | Follicle-Stimulating Hormone |
| LH | Luteinizing Hormone |
| 3β-HSD/17β-HSD | 3β-/17β-Hydroxysteroid Dehydrogenase |
| ABP | Androgen-Binding Protein |
| NAC | N-Acetylcysteine |
| AHCC | Active Hexose Correlated Compound |
| ALA | Alpha-Lipoic Acid |
| AS-IV | Astragaloside IV |
| GQBZP | Guiqi Baizhu Prescription |
| LP | Lienal Peptide |
| AgNPs | Silver Nanoparticles |
| BADGE | Bisphenol A Diglycidyl Ether (PPARγ antagonist) |
| CPX-351 | Liposomal Cytarabine + Daunorubicin formulation |
| HU | Hydroxyurea |
| Ara-CP | Cytarabine Palmitate (long-acting formulation) |
| Ara-A | Adenine Arabinoside |
| Ara-U | Uracil Arabinoside |
| CdR | Deoxycytidine |
| dCMP/CMP/CDP/CR/TdR | Deoxycytidine Monophosphate/Cytidine Monophosphate/Cytidine Diphosphate/Cytidine/Thymidine |
| 5-aza-C | 5-Azacytidine |
| 3MG | 3-O-Methylglucose |
| NE | Norepinephrine |
| DA | Dopamine |
| 5-HT | Serotonin |
| 5-HIAA | 5-Hydroxyindoleacetic Acid |
| CNPase | 2′,3′-Cyclic-Nucleotide 3′-Phosphodiesterase |
| MAP-2 | Microtubule-Associated Protein 2 |
| VZ | Ventricular Zone |
| IZ | Intermediate Zone |
| GE | Ganglionic Eminence |
| EGL | External Granular Layer |
| IGL | Internal Granular Layer |
| ML | Molecular Layer |
| BM | Bone Marrow |
| HSC | Hematopoietic Stem Cell |
| HPC | Hematopoietic Progenitor Cell |
| CFU | Colony-Forming Unit |
| WBC | White Blood Cell |
| Hb | Hemoglobin |
| TAS | Total Antioxidant Status |
| tGSH | Total Glutathione |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| SDS | Sodium Dodecyl Sulfate |
| TEM | Transmission Electron Microscopy |
| EB | Embryoid Body |
| mESC | Mouse Embryonic Stem Cell |
| CFS | Corneal Fluorescein Staining |
| AWAT2 | Acyl-CoA:Wax Alcohol Acyltransferase 2 |
| SOAT1 | Sterol O-Acyltransferase 1 |
| ELOVL4 | Elongation of Very Long Chain Fatty Acids Protein 4 |
| HMGCR | 3-Hydroxy-3-Methylglutaryl-CoA Reductase |
| SDH | Succinate Dehydrogenase |
| AER | Apical Ectodermal Ridge |
| SCFAs | Short-Chain Fatty Acids |
References
- Di Francia, R.; Crisci, S.; De Monaco, A.; Cafiero, C.; Re, A.; Iaccarino, G.; De Filippi, R.; Frigeri, F.; Corazzelli, G.; Micera, A.; et al. Response and Toxicity to Cytarabine Therapy in Leukemia and Lymphoma: From Dose Puzzle to Pharmacogenomic Biomarkers. Cancers 2021, 13, 966. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Fridley, B.; Kalari, K.; Jenkins, G.; Batzler, A.; Safgren, S.; Hildebrandt, M.; Ames, M.; Schaid, D.; Wang, L. Gemcitabine and Cytosine Arabinoside Cytotoxicity: Association with Lymphoblastoid Cell Expression. Cancer Res. 2008, 68, 7050–7058. [Google Scholar] [CrossRef] [PubMed]
- Gökbuget, N.; Hoelzer, D. Treatment of Adult Acute Lymphoblastic Leukemia. Semin. Hematol. 2009, 46, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.W. Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Hematology/the Education Program of the American Society of Hematology. Am. Soc. Hematol. Educ. Program 2011, 2011, 498–505. [Google Scholar] [CrossRef]
- Burnett, A.; Wetzler, M.; Löwenberg, B. Therapeutic Advances in Acute Myeloid Leukemia. J. Clin. Oncol. 2011, 29, 487–494. [Google Scholar] [CrossRef]
- Evrard, A.; Lacarelle, B.; Ciccolini, J. Severe or Lethal Toxicities with Nucleosidic Analogs: Time for Action. Pharmacogenomics 2013, 14, 227–230. [Google Scholar] [CrossRef]
- Chagnon, K.; Boissel, N.; Raffoux, E.; Dombret, H.; Tazi, A.; Bergeron, A. A New Pattern of Cytosine-Arabinoside-Induced Lung Toxicity. Br. J. Haematol. 2009, 147, 771–774. [Google Scholar] [CrossRef]
- Ciccolini, J.; Evrard, A.; M’Batchi, L.; Pourroy, B.; Mercier, C.; Iliadis, A.; Lacarelle, B.; Verschuur, A.; Ouafik, L.; André, N. CDA Deficiency as a Possible Culprit for Life-Threatening Toxicities after Cytarabine plus 6-Mercaptopurine Therapy: Pharmacogenetic Investigations. Pharmacogenomics 2012, 13, 393–397. [Google Scholar] [CrossRef]
- Baker, W.J.; Royer, G.L.; Weiss, R.B. Cytarabine and Neurologic Toxicity. J. Clin. Oncol. 1991, 9, 679–693. [Google Scholar] [CrossRef]
- Mayer, R.J.; Davis, R.B.; Schiffer, C.A.; Berg, D.T.; Powell, B.L.; Schulman, P.; Omura, G.A.; Moore, J.O.; McIntyre, O.R.; Frei, E. Intensive Postremission Chemotherapy in Adults with Acute Myeloid Leukemia. N. Engl. J. Med. 1994, 331, 896–903. [Google Scholar] [CrossRef]
- McGrail, L.H.; Sehn, L.H.; Weiss, R.B.; Robson, M.R.; Antin, J.H.; Byrd, J.C. Pancreatitis during Therapy of Acute Myeloid Leukemia: Cytarabine Related? Ann. Oncol. 1999, 10, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- McBride, C.E.; Yavorski, R.T.; Moses, F.M.; Robson, M.E.; Solimando, D.A.; Byrd, J.C. Acute Pancreatitis Associated with Continuous Infusion Cytarabine Therapy: A Case Report. Cancer 1996, 77, 2588–2591. [Google Scholar] [CrossRef]
- Powell, B.L.; Zekan, P.J.; Muss, H.B.; Richards, F.; Lyerly, E.S.; Capizzi, R.L. Ara-C Syndrome during Low-dose Continuous Infusion Therapy. Med. Pediatr. Oncol. 1986, 14, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.O.; George, S.L.; Dodge, R.K.; Amrein, P.C.; Powell, B.L.; Kolitz, J.E.; Baer, M.R.; Davey, F.R.; Bloomfield, C.D.; Larson, R.A.; et al. Sequential Multiagent Chemotherapy Is Not Superior to High-Dose Cytarabine Alone as Postremission Intensification Therapy for Acute Myeloid Leukemia in Adults under 60 Years of Age: Cancer and Leukemia Group B Study 9222. Blood 2005, 105, 3420–3427. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Tsokkou, S.; Grigoriadis, S.; Chrysavgi, L.; Gavriilaki, E. Cardiotoxicity in Acute Myeloid Leukemia in Adults: A Scoping Study. Cancers 2024, 16, 2474. [Google Scholar] [CrossRef]
- Nagahata, Y.; Kondo, T.; Ono, Y.; Hiramoto, N.; Kitano, T.; Hishizawa, M.; Yamashita, K.; Hashimoto, H.; Ishikawa, T.; Takaori-Kondo, A. High-Dose Cytarabine Chemotherapy (≥4 g/M2/Day) before Allogeneic Hematopoietic Stem Cell Transplantation for Non-Core-Binding-Factor AML in the First Complete Remission. Leuk. Lymphoma 2020, 61, 3128–3136. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The Well-Built Clinical Question: A Key to Evidence-Based Decisions. ACP J. Club 1995, 123, A12-3. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Bahor, Z.; Liao, J.; Currie, G.; Ayder, C.; MacLeod, M.; McCann, S.K.; Bannach-Brown, A.; Wever, K.; Soliman, N.; Wang, Q.; et al. Development and Uptake of an Online Systematic Review Platform: The Early Years of the CAMARADES Systematic Review Facility (SyRF). BMJ Open Sci. 2021, 5, e100103. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://ohri.ca/en/who-we-are/core-facilities-and-platforms/ottawa-methods-centre/newcastle-ottawa-scale (accessed on 19 November 2025).
- Alexander, T.C.; Simecka, C.M.; Kiffer, F.; Groves, T.; Anderson, J.; Carr, H.; Wang, J.; Carter, G.; Allen, A.R. Changes in Cognition and Dendritic Complexity Following Intrathecal Methotrexate and Cytarabine Treatment in a Juvenile Murine Model. Behav. Brain Res. 2018, 346, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Fremouw, T.; Fessler, C.L.; Ferguson, R.J.; Burguete, Y. Recent and Remote Spatial Memory in Mice Treated with Cytosine Arabinoside. Pharmacol. Biochem. Behav. 2012, 100, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Liu, D.; Huang, L.; Wang, H.; Zhang, J.Y.; Luo, X.G. Cytosine Arabinoside Treatment Impairs the Remote Spatial Memory Function and Induces Dendritic Retraction in the Anterior Cingulate Cortex of Rats. Brain Res. Bull. 2008, 77, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, D.C.; Herrera, M.O.; Peraza, A.V.; Brizuela, N.O.; García, E.H.; Olguín, H.J.; Mejía, G.B.; del Ángel, D.S.; Ochoa, A.R. Cambios Bioquímicos e Histológicos Producidos Por Edulcorantes y Citarabina En El Cerebro de Ratas Jóvenes. Nutr. Hosp. 2018, 35, 194–200. [Google Scholar] [CrossRef]
- Calderón Guzmán, D.; Osnaya Brizuela, N.; Ortíz Herrera, M.; Juárez Olguín, H.; Hernández García, E.; Valenzuela Peraza, A.; Barragán Mejía, G. Oleic Acid Protects Against Oxidative Stress Exacerbated by Cytarabine and Doxorubicin in Rat Brain. Anticancer Agents Med. Chem. 2016, 16, 1491–1495. [Google Scholar] [CrossRef]
- Patel, R.S.; Rachamalla, M.; Chary, N.R.; Shera, F.Y.; Tikoo, K.; Jena, G. Cytarabine Induced Cerebellar Neuronal Damage in Juvenile Rat: Correlating Neurobehavioral Performance with Cellular and Genetic Alterations. Toxicology 2012, 293, 41–52. [Google Scholar] [CrossRef]
- Koros, C.; Kitraki, E. Neurofilament Isoform Alterations in the Rat Cerebellum Following Cytosine Arabinoside Administration. Toxicol. Lett. 2009, 189, 215–218. [Google Scholar] [CrossRef]
- Koros, C.; Papalexi, E.; Anastasopoulos, D.; Kittas, C.; Kitraki, E. Effects of AraC Treatment on Motor Coordination and Cerebellar Cytoarchitecture in the Adult Rat: A Possible Protective Role of NAC. Neurotoxicology 2007, 28, 83–92. [Google Scholar] [CrossRef]
- Guan, Z.; Liang, Y.; Zhu, Z.; Yang, A.; Li, S.; Guo, J.; Wang, F.; Yang, H.; Zhang, N.; Wang, X.; et al. Cytosine Arabinoside Exposure Induced Cytotoxic Effects and Neural Tube Defects in Mice and Embryo Stem Cells. Ecotoxicol. Environ. Saf. 2023, 262, 115141. [Google Scholar] [CrossRef]
- Salimi, A.; Shabani, M.; Nikjou, A.; Choupani, M.; Biniyaz, M. Exploring the Possible Mitoprotective and Neuroprotective Potency of Thymoquinone, Betanin, and Vitamin D against Cytarabine-Induced Mitochondrial Impairment and Neurotoxicity in Rats’ Brain. J. Biochem. Mol. Toxicol. 2023, 37, e23256. [Google Scholar] [CrossRef]
- Takano, T.; Akahori, S.; Takeuchi, Y.; Ohno, M. Neuronal Apoptosis and Gray Matter Heterotopia in Microcephaly Produced by Cytosine Arabinoside in Mice. Brain Res. 2006, 1089, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, H.; Katayama, K.I.; Ueno, M.; Uetsuka, K.; Nakayama, H.; Doi, K. Involvement of P53 in 1-β-d-Arabinofuranosylcytosine-Induced Rat Fetal Brain Lesions. Neurotoxicol. Teratol. 2004, 26, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Wakaizumi, S.; Kasubuchi, Y.; Kusonoki, T. Cytarabine and Its Effect on Cerebellum of Suckling Mouse. Arch. Neurol. 1975, 32, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Percy, D.H.; Albert, D.M. Developmental Defects in Mice and Rats Treated Postnatally with Cytosine Arabinoside. Exp. Mol. Pathol. 1974, 21, 381–397. [Google Scholar] [CrossRef]
- Percy, D.H. Teratogenic Effects of the Pyrimidine Analogues 5-lododeoxyuridine and Cytosine Arabinoside in Late Fetal Mice and Rats. Teratology 1975, 11, 103–117. [Google Scholar] [CrossRef]
- Guzmán, D.C.; Brizuela, N.O.; Herrera, M.O.; Olguín, H.J.; Peraza, A.V.; Ruíz, N.L.; Mejía, G.B. Intake of Oligoelements with Cytarabine or Etoposide Alters Dopamine Levels and Oxidative Damage in Rat Brain. Sci. Rep. 2024, 14, 10835. [Google Scholar] [CrossRef]
- Narang, H.K. Pathological Findings of Adenine Arabinoside (Ara-A) and Cytarabine (Ara-C) in the Treatment of Herpes Simplex Encephalitis in Rabbit Model. Antivir. Res. 1982, 2, 53–67. [Google Scholar] [CrossRef]
- Matsutani, T.; Tamaru, M.; Hayakawa, Y.; Nagayoshi, M.; Nakahara, T.; Tsukada, Y. A Neurochemical Study of Developmental Impairment of the Brain Caused by the Administration of Cytosine Arabinoside during the Fetal or Neonatal Period of Rats. Neurochem. Res. 1983, 8, 1295–1306. [Google Scholar] [CrossRef]
- Yamano, T.; Shimada, M.; Ohota, S.; Abe, Y.; Nakao, K.; Ohoya, N. Formation of Heterotopic Granule Cell in Mouse Cerebellum after Neonatal Administration of Cytosine Arabinoside. Acta Neuropathol. 1980, 49, 29–34. [Google Scholar] [CrossRef]
- Kasubuchi, Y.; Wakaizumi, S.; Shimada, M.; Kusunoki, T. Cytosine Arabinoside-induced Transplacental Dysgenetic Hydrocephalus in Mice. Teratology 1977, 16, 63–70. [Google Scholar] [CrossRef]
- ADLARD, B.P.F.; DOBBING, J.; SANDS, J. A Comparison of the Effects of Cytosine Arabinoside and Adenine Arabinoside on Some Aspects of Brain Growth and Development in the Rat. Br. J. Pharmacol. 1975, 54, 33. [Google Scholar] [CrossRef]
- Elmer, G.I.; Sydnor, J.; Guard, H.; Hercher, E.; Vogel, M.W. Altered Prepulse Inhibition in Rats Treated Prenatally with the Antimitotic Ara-C: An Animal Model for Sensorimotor Gating Deficits in Schizophrenia. Psychopharmacology 2004, 174, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Yamano, T.; Shimada, M.; Abe, Y.; Ohta, S.; Ohno, M. Destruction of External Granular Layer and Subsequent Cerebellar Abnormalities. Acta Neuropathol. 1983, 59, 41–47. [Google Scholar] [CrossRef] [PubMed]
- de Souza Silva, P.M.; de Sousa, R.V.; Simão, A.A.; Cesar, P.H.S.; Trento, M.V.C.; Marcussi, S. Protective Effect of β-D-Glucan and Glutamine on the Genomic Instability Induced by Cytarabine/Ara-C in BALB/c Mice. Int. J. Biol. Macromol. 2018, 117, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Li, Y.L.; Chu, W.; Li, G.Q.; Zhang, M.; Dong, J.J.; Li, L.; Li, C.H.; Zhang, J.B.; Li, J.W.; et al. Astragaloside IV Alleviates Cytarabine-Induced Intestinal Mucositis by Remodeling Macrophage Polarization through AKT Signaling. Phytomedicine 2023, 109, 154605. [Google Scholar] [CrossRef]
- Ramos, M.G.; Bambirra, E.A.; Cara, D.C.; Vieira, E.C.; Alvarez-Leite, J.I. Oral Administration of Short-Chain Fatty Acids Reduces the Intestinal Mucositis Caused by Treatment with Ara-C in Mice Fed Commercial or Elemental Diets. Nutr. Cancer 1997, 28, 212–217. [Google Scholar] [CrossRef]
- Ramos, M.G.; Bambirra, E.A.; Nicoli, J.R.; Cara, D.C.; Vieira, E.C.; Alvarez-Leite, J. Protection by Short-Chain Fatty Acids against 1-β-D-Arabinofuranosylcytosine-Induced Intestinal Lesions in Germfree Mice. Antimicrob. Agents Chemother. 1999, 43, 950–953. [Google Scholar] [CrossRef]
- Chu, W.; Li, Y.-L.; Li, J.-J.; Lin, J.; Li, M.; Wang, J.; He, J.-Z.; Zhang, Y.-M.; Yao, J.; Jin, X.-J.; et al. Guiqi Baizhu Prescription Ameliorates Cytarabine-Induced Intestinal Mucositis by Targeting JAK2 to Inhibit M1 Macrophage Polarization. Biomed. Pharmacother. 2023, 164, 114902. [Google Scholar] [CrossRef]
- Al-Jammas, S.; Al-Allaf, L.I.K.; Saeed, M.G. Attenuating Effects of α-Tocopherol on Cytarabine-Induced Toxicity in Parotid Salivary Gland of Rabbits: A Histological and Immunohistochemical Study. Iran. J. Vet. Med. 2024, 18, 33–42. [Google Scholar] [CrossRef]
- Minden, M.D.; Audiger, C.; Chabot-Roy, G.; Lesage, S.; Delisle, J.S.; Biemans, B.; Dimitriadou, V. The Long-Acting Glucagon-Like Peptide-2 Analog Apraglutide Enhances Intestinal Protection and Survival After Chemotherapy and Allogeneic Transplantation in Mice. Ann. Transpl. 2024, 29, e945249. [Google Scholar] [CrossRef]
- Porsani, M.Y.H.; Paludetti, M.; Orlando, D.R.; Peconick, A.P.; Costa, R.C.; Oliveira, L.E.D.; Zangeronimo, M.G.; Sousa, R.V. Protective Effect of β-Glucan and Glutamine on Intestinal and Immunological Damage in Mice Induced by Cytarabine (Ara-C). Pesqui. Veterinária Bras. 2017, 37, 977–983. [Google Scholar] [CrossRef][Green Version]
- Elli, M.; Aydin, O.; Bilge, S.; Bozkurt, A.; Dagdemir, A.; Pinarli, F.G.; Acar, S. Protective Effect of Vitamin A on ARA-C Induced Intestinal Damage in Mice. Tumori 2009, 95, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Chwalinski, S.; Potten, C.S. Crypt Base Columnar Cells in Ileum of BDF1 Male Mice—Their Numbers and Some Features of Their Proliferation. Am. J. Anat. 1989, 186, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Xiu, M.; Li, S.; Shi, Y.; Wang, X.; Lin, X.; Cai, H.; Liu, Y.; He, J. Exposure to Cytarabine Causes Side Effects on Adult Development and Physiology and Induces Intestinal Damage via Apoptosis in Drosophila. Biomed. Pharmacother. 2023, 159, 114265. [Google Scholar] [CrossRef]
- Chen, T.S. Effects of Arabinosyl Cytosine and 5-Azacytidine on the Intestinal Absorption of Nutrients. Toxicol. Appl. Pharmacol. 1982, 62, 73–80. [Google Scholar] [CrossRef]
- Liu, R.; Xue, J.; Han, J.; Tu, M.; Wang, W.; Chen, Z.; Qian, X.; Xiao, B.; Liang, L. Cytarabine Chemotherapy Induces Meibomian Gland Dysfunction. Ocul. Surf. 2024, 34, 444–458. [Google Scholar] [CrossRef]
- Balci, Y.I.; Acer, S.; Yagci, R.; Kucukatay, V.; Sarbay, H.; Bozkurt, K.; Polat, A. N-Acetylcysteine Supplementation Reduces Oxidative Stress for Cytosine Arabinoside in Rat Model. Int. Ophthalmol. 2017, 37, 209–214. [Google Scholar] [CrossRef]
- Rootman, J.; Gudauskas, G.; Kumi, C. Subconjunctival versus Intravenous Cytosine Arabinoside: Effect of Route of Administration and Ocular Toxicity. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1607–1611. [Google Scholar]
- Diets-Ouwehand, J.J.A.T.; de Keizer, R.J.W.; Vrensen, G.F.J.M.; Groen-Jansen, S.; van Best, J.A. Toxicity of 1-(β-d-Arabinofuranosyl)Cytosine after Intravitreal Injection in the Rabbit Eye. Graefe’s Arch. Clin. Exp. Ophthalmol. 1992, 230, 488–495. [Google Scholar] [CrossRef]
- Percy, D.H.; Danylchuk, K.D. Experimental Retinal Dysplasia Due to Cytosine Arabinoside. Investig. Ophthalmol. Vis. Sci. 1977, 16, 353–364. [Google Scholar]
- Shimada, M.; Wakaizumi, S.; Kasubuchi, Y.; Kusunoki, T.; Nakamura, T. Cytosine Arabinoside and Rosette Formation in Mouse Retina. Nature 1973, 246, 151–152. [Google Scholar] [CrossRef]
- Kaufman, H.E.; Capella, J.A.; Maloney, E.D.; Robbins, J.E.; Cooper, G.M.; Uotila, M.H. Corneal Toxicity of Cytosine Arabinoside. Arch. Ophthalmol. 1964, 72, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.J.; Huang, H.S. IL-1 Synergizes with ARA-C in Aborting the Development of Chloroleukemia While Protecting from ARA-C-Induced Alopecia in the Rat Model. Cytokine 1992, 4, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.J.; Huang, H.S.; Yunis, A.A. Treatment with Imuvert/JV-Acetylcysteine Protects Rats from Cyclophosphamide/Cytarabine-Induced Alopecia: Original Article. Cancer Investig. 1992, 10, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.J.; Sawaya, M.E.; Yunis, A.A. Interleukin 1 Protects Hair Follicles from Cytarabine (ARA-C)-Induced Toxicity in Vivo and in Vitro. FASEB J. 1992, 6, 911–913. [Google Scholar] [CrossRef]
- Sun, B.; Wakame, K.; Sato, E.; Nishioka, H.; Aruoma, O.I.; Fujii, H. The Effect of Active Hexose Correlated Compound in Modulating Cytosine Arabinoside-Induced Hair Loss, and 6-Mercaptopurine- and Methotrexate-Induced Liver Injury in Rodents. Cancer Epidemiol. 2009, 33, 293–299. [Google Scholar] [CrossRef]
- Hussein, A.M. Protection Against Cytosine Arabinoside-Induced Alopecia By Minoxidil in a Rat Animal Model. Int. J. Dermatol. 1995, 34, 470–473. [Google Scholar] [CrossRef]
- Hagiwara, S.; Uchida, T.; Koga, H.; Inomata, M.; Yoshizumi, F.; Moriyama, M.; Kitano, S.; Noguchi, T. The α-Lipoic Acid Derivative Sodium Zinc Dihydrolipoylhistidinate Reduces Chemotherapy-Induced Alopecia in a Rat Model: A Pilot Study. Surg. Today 2011, 41, 693–697. [Google Scholar] [CrossRef]
- Sun, F.; Li, N.; Tong, X.; Zeng, J.; He, S.; Gai, T.; Bai, Y.; Liu, L.; Lu, K.; Shen, J.; et al. Ara-c Induces Cell Cycle G1/S Arrest by Inducing Upregulation of the INK4 Family Gene or Directly Inhibiting the Formation of the Cell Cycle-Dependent Complex CDK4/Cyclin D1. Cell Cycle 2019, 18, 2293. [Google Scholar] [CrossRef]
- Kolure, R.; Nachammai, V.; Manjula, S.N.; Godela, R.; Bhavani, D.S.; Rajendra, Y. Hepatoprotective Effect of Swertiamarin from Cytarabine Induced Hepatotoxicity in Pregnant Rats. Res. J. Pharm. Technol. 2023, 16, 5707–5712. [Google Scholar] [CrossRef]
- Al-Jammas, S.; Al-Saraj, A. The Histological Changes Induced by Cytarabine on Rabbits Livers (with and without Vitamin E Administration). Iraqi J. Vet. Sci. 2020, 34, 9–13. [Google Scholar] [CrossRef]
- Dudina, M.O.; Suslova, I.R.; Khalzova, M.S.; Dergunova, J.V.; Kogan, E.A.; Roshchin, D.A.; Samyshina, E.A.; Morozov, M.A.; Dydykin, S.S. Molecular and Cellular Mechanisms of Acute Cytotoxic Liver Damage as Potential Biological Targets for Magnesium-Containing Cell-Protective Drug. Res. Results Pharmacol. 2018, 4, 9–15. [Google Scholar] [CrossRef][Green Version]
- Palo, A.K.; Sahoo, D.; Choudhury, R.C. Cytosine Arabinoside-Induced Cytogenotoxicity in Bone Marrow and Spermatogonial Cells of Mice and Its Potential Transmission through the Male Germline. Mutat. Res. 2009, 673, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Han, A.R.; Hwang, H.-S.; Kim, D.C.; Min, W.S.; Kim, H.J. Role of CXCR4 Antagonist in Megakaryocyte Reinstatement with Increased Sinusoidal Vessel Density. In Oxygen Transport to Tissue XL; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; Volume 1072, pp. 419–422. [Google Scholar] [CrossRef]
- Castañeda-Yslas, I.Y.; Torres-Bugarín, O.; Arellano-García, M.E.; Ruiz-Ruiz, B.; García-Ramos, J.C.; Toledano-Magaña, Y.; Pestryakov, A.; Bogdanchikova, N. Protective Effect of Silver Nanoparticles Against Cytosine Arabinoside Genotoxicity: An In Vivo Micronucleus Assay. Int. J. Environ. Res. Public Health 2024, 21, 1689. [Google Scholar] [CrossRef]
- Zhu, R.J.; Wu, M.Q.; Li, Z.J.; Zhang, Y.; Liu, K.Y. Hematopoietic Recovery Following Chemotherapy Is Improved by BADGE-Induced Inhibition of Adipogenesis. Int. J. Hematol. 2013, 97, 58–72. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, M.; Min, Q.; Gao, Y.; Sun, W. The Dual Regulatory Function of Lienal Peptide on Immune System. Int. Immunopharmacol. 2018, 55, 245–253. [Google Scholar] [CrossRef]
- Bilgin, A.O.; Mammadov, R.; Suleyman, B.; Unver, E.; Ozcicek, F.; Soyturk, M.; Cimen, F.K.; Kurt, N.; Suleyman, H. Effect of Rutin on Cytarabine-Associated Pulmonary Oedema and Oxidative Stress in Rats. Acad. Bras. Cienc. 2020, 92, e20190261. [Google Scholar] [CrossRef]
- Namoju, R.; Chilaka, N.K. Alpha-Lipoic Acid Ameliorates Cytarabine-Induced Developmental Anomalies in Rat Fetus. Hum. Exp. Toxicol. 2021, 40, 851–868. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, W.; Li, G.; Dong, H.; Hou, J.; Cao, Z.; Guan, D. Expression of Fibroblast Growth Factor 4 in a Rat Model of Polydactyly of the Thumb Induced by Cytarabine. Med. Sci. Monit. 2020, 26, e926953. [Google Scholar] [CrossRef]
- Chilaka, K.N.; Namoju, R. Maternal Supplementation of Alpha-Lipoic Acid Ameliorates Prenatal Cytarabine-Induced Mutilation in Reproductive Development and Function in F1 Male Adult Rats. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 4035–4053. [Google Scholar] [CrossRef]
- Yamauchi, H.; Katayama, K.; Yasoshima, A.; Uetsuka, K.; Nakayama, H.; Doi, K. 1-β-D-Arabinofuranosylcytosine (Ara-C)-Induced Apoptosis in the Rat Fetal Tissues and Placenta. J. Toxicol. Pathol. 2003, 16, 223–229. [Google Scholar] [CrossRef][Green Version]
- Kochhar, D.M.; Penner, J.D.; McDay, J.A. Limb Development in Mouse Embryos. II. Reduction Defects, Cytotoxicity and Inhibition of DNA Synthesis Produced by Cytosine Arabinoside. Teratology 1978, 18, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.M.; Dourson, M.L.; Smith, C.C. Effects of Cytosine Arabinoside on in Vivo and in Vitro Mouse Limb Development. Vitr. J. Tissue Cult. Assoc. 1977, 13, 434–442. [Google Scholar] [CrossRef]
- Rahman, M.E.; Ishikawa, H.; Watanabe, Y.; Endo, A. Carpal and Tarsal Bone Anomalies in Mice Induced by Maternal Treatment of Ara-C. Reprod. Toxicol. 1994, 8, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.E.; Ishikawa, H.; Watanabe, Y.; Endo, A. Carpal and Tarsal Bone Development Is Highly Sensitive to Three Antiproliferative Teratogens in Mice. Reprod. Toxicol. 1996, 10, 485–489. [Google Scholar] [CrossRef]
- Chiang, H.; Wu, R.Y.; Shao, B.J.; Fu, Y.D.; Yao, G.D.; Lu, D.J. Pulsed Magnetic Field from Video Display Terminals Enhances Teratogenic Effects of Cytosine Arabinoside in Mice. Bioelectromagnetics 1995, 16, 70–74. [Google Scholar] [CrossRef]
- Chiba, K.; Ishikawa, H.; Rahman, M.E.; Endo, A. Neonatal Mouse Hip Joint and Hindlimb Anomalies Induced by Prenatal Exposure to Ara-C. Reprod. Toxicol. 1996, 10, 215–219. [Google Scholar] [CrossRef]
- Ritter, E.J.; Scott, W.J.; Wilson, J.G. Teratogenesis and Inhibition of DNA Synthesis Induced in Rat Embryos by Cytosine Arabinoside. Teratology 1971, 4, 7–13. [Google Scholar] [CrossRef]
- Ritter, E.J.; Scott, W.J.; Wilson, J.G. Relationship of Temporal Patterns of Cell Death and Development to Malformations in the Rat Limb. Possible Mechanisms of Teratogenesis with Inhibitors of DNA Synthesis. Teratology 1973, 7, 219–225. [Google Scholar] [CrossRef]
- Scott, W.J.; Ritter, E.J.; Wilson, J.G. Studies on Induction of Polydactyly in Rats with Cytosine Arabinoside. Dev. Biol. 1975, 45, 103–111. [Google Scholar] [CrossRef]
- Goto, T.; Endo, A. Dose- and Stage-related Sex Difference in the Incidence of Cytosine Arabinoside Induced Digit Anomalies in the Mouse Fetus. Teratology 1987, 35, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Sakai, N.; Ohwada, K. Analysis of Diurnal Difference in Teratogen (Ara-C) Susceptibility in Mouse Embryos by a Progressive Phase-shift Method. Teratog. Carcinog. Mutagen. 1987, 7, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.E.; Ishikawa, H.; Watanabe, Y.; Endo, A. Stage Specificity of Ara-C Induced Carpal and Tarsal Bone Anomalies in Mice. Reprod. Toxicol. 1995, 9, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Chaube, S.; Kreis, W.; Uchida, K.; Murphy, M.L. The Teratogenic Effect of 1-β-d-Arabinofuranosylcytosine in the Rat: Protection by Deoxycytidine. Biochem. Pharmacol. 1968, 17, 1213–1226. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Damon, L.E.; Mass, R.; Linker, C.A. The Association between High-Dose Cytarabine Neurotoxicity and Renal Insufficiency. J. Clin. Oncol. 1989, 7, 1563–1568. [Google Scholar] [CrossRef]
- Salinsky, M.C.; Levine, R.L.; Aubuchon, J.P.; Schutta, H.S. Acute Cerebellar Dysfunction with High-Dose ARA-C Therapy. Cancer 1983, 51, 426–429. [Google Scholar] [CrossRef]
- Phillips, N.S.; Stratton, K.L.; Williams, A.L.M.; Ahles, T.; Ness, K.K.; Cohen, H.J.; Edelstein, K.; Yasui, Y.; Oeffinger, K.; Chow, E.J.; et al. Late-Onset Cognitive Impairment and Modifiable Risk Factors in Adult Childhood Cancer Survivors. JAMA Netw. Open 2023, 6, E2316077. [Google Scholar] [CrossRef]
- Yamauchi, H.; Katayama, K.I.; Ueno, M.; Uetsuka, K.; Nakayama, H.; Doi, K. Involvement of P53 in 1-β-d-Arabinofuranosylcytosine-Induced Trophoblastic Cell Apoptosis and Impaired Proliferation in Rat Placenta. Biol. Reprod. 2004, 70, 1762–1767. [Google Scholar] [CrossRef]
- Camera, A.; Andretta, C.; Villa, M.R.; Volpicelli, M.; Picardi, M.; Rossi, M.; Rinaldi, C.R.; Cioppa, P.D.; Ciancia, R.; Selleri, C.; et al. Intestinal Toxicity during Induction Chemotherapy with Cytarabine-Based Regimens in Adult Acute Myeloid Leukemia. Hematol. J. 2003, 4, 346–350. [Google Scholar] [CrossRef]
- Slavin, R.E.; Dias, M.A.; Saral, R. Cytosine Arabinoside Induced Gastrointestinal Toxic Alterations in Sequential Chemotherapeutic Protocols.A Clinical-Pathologic Study of 33 Patients. Cancer 1978, 42, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Park, M.R.; Lee, H.J.; Jang, H.M.; Kim, N.H.; Lee, J.S.; Jeong, Y.T.; Kim, I.; Choi, S.H.; Seo, K.S.; Kim, D.H. Cytarabine Induces Cachexia with Lipid Malabsorption via Zippering the Junctions of Lacteal in Murine Small Intestine. J. Lipid Res. 2023, 64, 100387. [Google Scholar] [CrossRef] [PubMed]
- Dekker, I.M.; Bruggink, H.; van der Meij, B.S.; Wierdsma, N.J. State of the Art: The Role of Citrulline as Biomarker in Patients with Chemotherapy- or Graft-versus-Host-Disease-Induced Mucositis. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Higa, G.M.; Gockerman, J.P.; Hunt, A.L.; Jones, M.R.; Horne, B.J. The Use of Prophylactic Eye Drops during High-Dose Cytosine Arabinoside Therapy. Cancer 1991, 68, 1691–1693. [Google Scholar] [CrossRef]
- Guthoff, T.; Tietze, B.; Meinhardt, B.; Becher, J.; Guthoff, R. Cytosine-Arabinoside-Induced Keratopathy: A Model of Corneal Proliferation Kinetics. Ophthalmologica 2010, 224, 308–311. [Google Scholar] [CrossRef]
- Jimenez-Cauhe, J.; Lo Sicco, K.I.; Shapiro, J.; Hermosa-Gelbard, A.; Burgos-Blasco, P.; Melian-Olivera, A.; Ortega-Quijano, D.; Pindado-Ortega, C.; Buendia-Castaño, D.; Asz-Sigall, D.; et al. Characterization and Management of Adverse Events of Low-Dose Oral Minoxidil Treatment for Alopecia: A Narrative Review. J. Clin. Med. 2025, 14, 1805. [Google Scholar] [CrossRef]
- Kearney, C.A.; Gordon, A.; Markova, A.; Freites-Martinez, A.; Tattersall, I.W.; Shapiro, J.; Lacouture, M.E.; Lo Sicco, K.I. Low-Dose Oral Minoxidil during Chemotherapy: A Review of the Mechanism and Current Evidence. Ski. Appendage Disord. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Hospira, Inc. Cytarabine Injection, Solution for Injection, 20 Mg/ML; Prescribing In-Formation; Revised December 2022; Hospira, Inc.: Lake Forest, IL, USA, 2022. [Google Scholar]
- Choi, E.K.; Kim, I.-R.; Chang, O.; Kang, D.; Nam, S.-J.; Lee, J.E.; Lee, S.K.; Im, Y.-H.; Park, Y.H.; Yang, J.-H.; et al. Impact of Chemotherapy-Induced Alopecia Distress on Body Image, Psychosocial Well-Being, and Depression in Breast Cancer Patients. Psychooncology 2014, 23, 1103–1110. [Google Scholar] [CrossRef]
- Chiche, E.; Rahmé, R.; Bertoli, S.; Dumas, P.-Y.; Micol, J.-B.; Hicheri, Y.; Pasquier, F.; Peterlin, P.; Chevallier, P.; Thomas, X.; et al. Real-Life Experience with CPX-351 and Impact on the Outcome of High-Risk AML Patients: A Multicentric French Cohort. Blood Adv. 2021, 5, 176–184. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. Cytarabine. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2017. [Google Scholar]
- Fu, S.H.; Flannery, A.H.; Thompson Bastin, M.L. Acute Hepatotoxicity After High-Dose Cytarabine for the Treatment of Relapsed Acute Myeloid Leukemia: A Case Report. Hosp. Pharm. 2019, 54, 160–164. [Google Scholar] [CrossRef]
- Laharwal, M.M.; Law, C.S.; Orosz, E.; Patel, A.V. S2680 Isolated Direct Hyperbilirubinemia Due to Cytarabine Administration. Am. J. Gastroenterol. 2020, 115, S1404. [Google Scholar] [CrossRef]
- da Lyrio, R.M.C.; Rocha, B.R.A.; Corrêa, A.L.R.M.; Mascarenhas, M.G.S.; Santos, F.L.; da Maia, R.H.; Segundo, L.B.; de Almeida, P.A.A.; Moreira, C.M.O.; Sassi, R.H. Chemotherapy-Induced Acute Kidney Injury: Epidemiology, Pathophysiology, and Therapeutic Approaches. Front. Nephrol. 2024, 4, 1436896. [Google Scholar] [CrossRef]
- Tanaka, M.; Kanamori, H.; Yamaji, S.; Mishima, A.; Fujita, H.; Fujisawa, S.; Murata, T.; Koharazawa, H.; Matsuzaki, M.; Mohri, H. Low-Dose Cytarabine-Induced Hepatic and Renal Dysfunction in a Patient with Myelodysplastic Syndrome. Anticancer Drugs 1999, 10, 289–291. [Google Scholar] [CrossRef]
- Khaleel, B.; Lunenfeld, E.; Kapelushnik, J.; Huleihel, M. Effect of Granulocyte Colony-Stimulating Factor on the Development of Spermatogenesis in the Adulthood of Juvenile AML Mice Model Treated with Cytarabine. Int. J. Mol. Sci. 2023, 24, 12229. [Google Scholar] [CrossRef]
- Orth, J.M.; Gunsalus, G.L.; Lamperti, A.A. Evidence from Sertoli Cell-Depleted Rats Indicates That Spermatid Number in Adults Depends on Numbers of Sertoli Cells Produced during Perinatal Development. Endocrinology 1988, 122, 787–794. [Google Scholar] [CrossRef]
- Watanabe, S.; Sato, S.; Nagase, S.; Saito, T. A Novel Note on the Effect of Ara-C on the Polyamine Content of the Male Accessory Organs of the Rat. Biochem. Pharmacol. 1992, 44, 593–596. [Google Scholar] [CrossRef]
- Cano, F.; Pannel, R.; Follows, G.A.; Rabbitts, T.H. Preclinical Modeling of Cytosine Arabinoside Response in Mll-Enl Translocator Mouse Leukemias. Mol. Cancer Ther. 2008, 7, 730–735. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.