Sirtuins as Therapeutic Targets for Treating Cancer, Metabolic Diseases, and Neurodegenerative Diseases
Abstract
1. Introduction
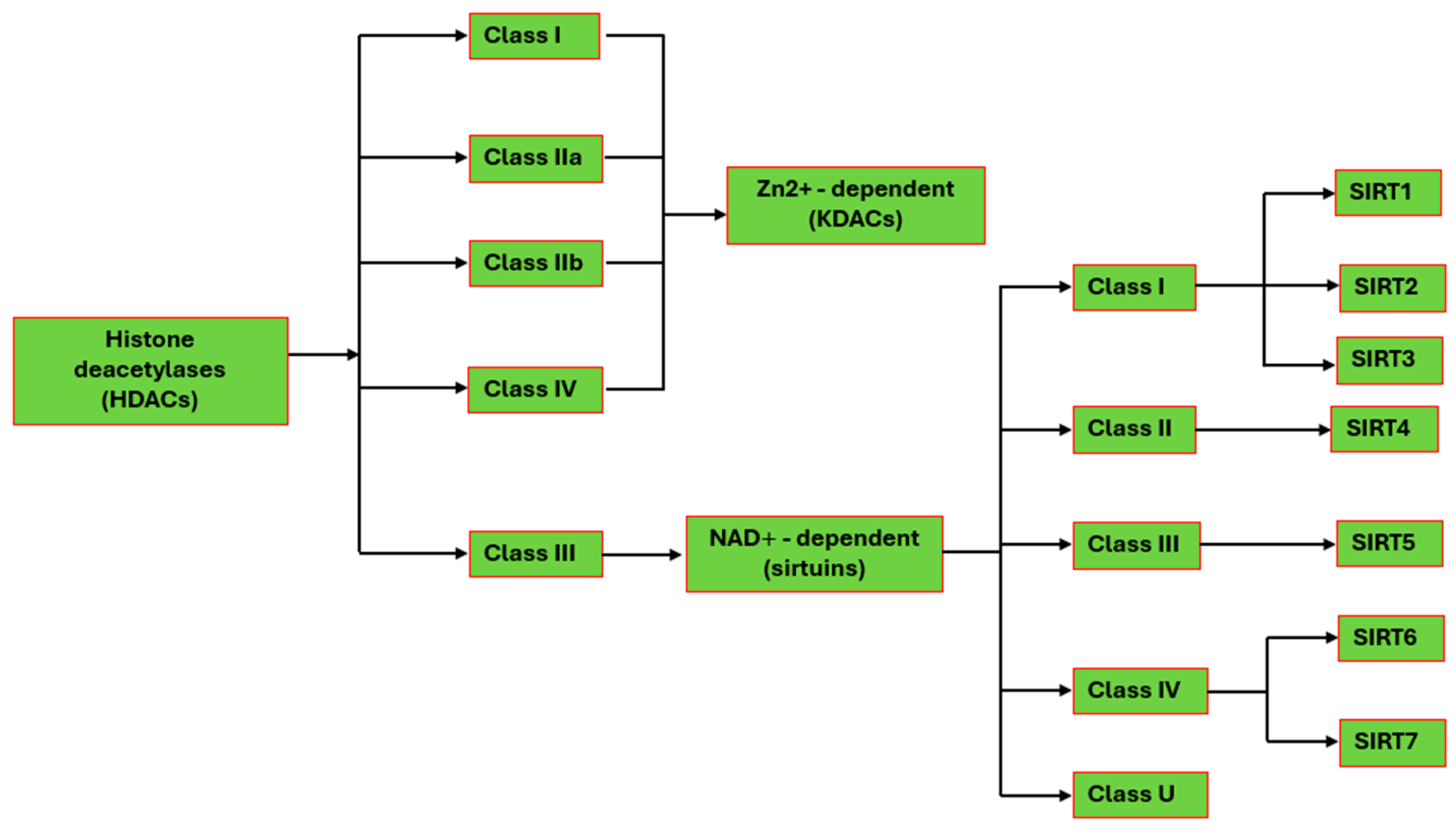
2. Protein Deacetylation in Human Cells
2.1. Zn2+-Dependent HDACs
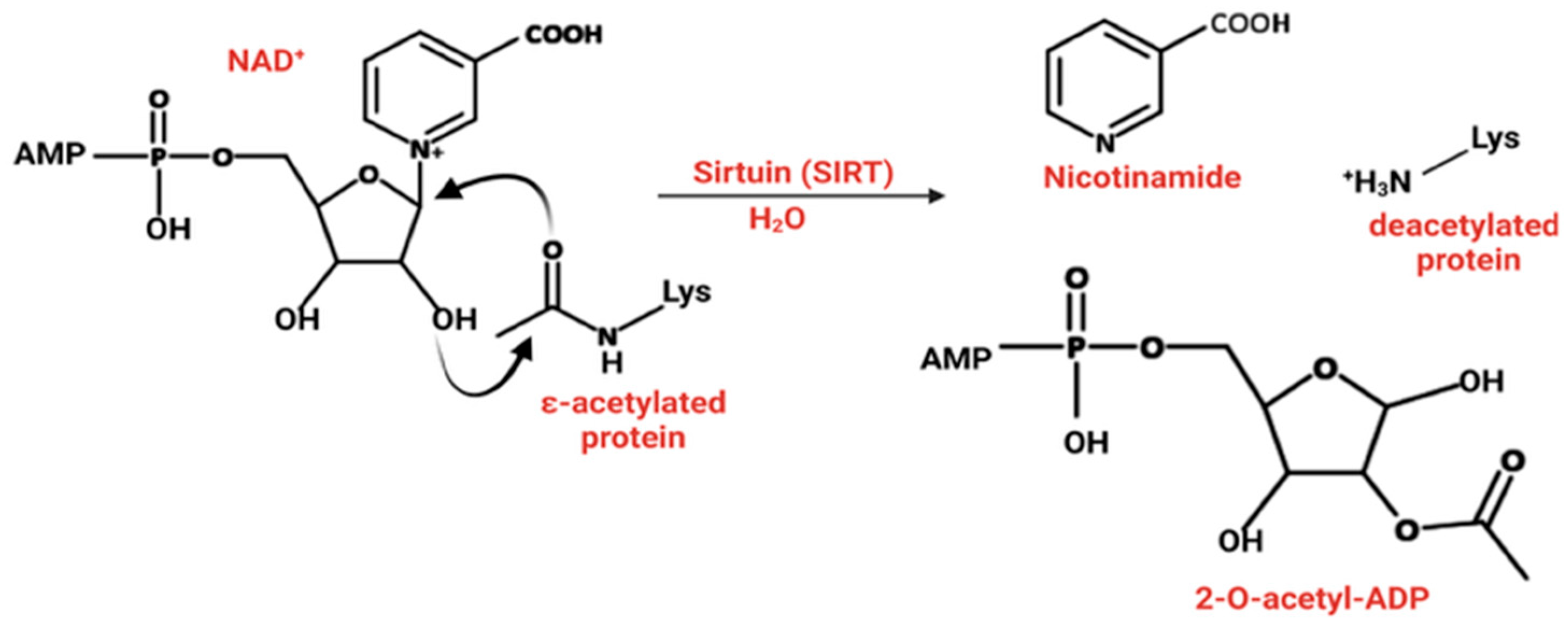
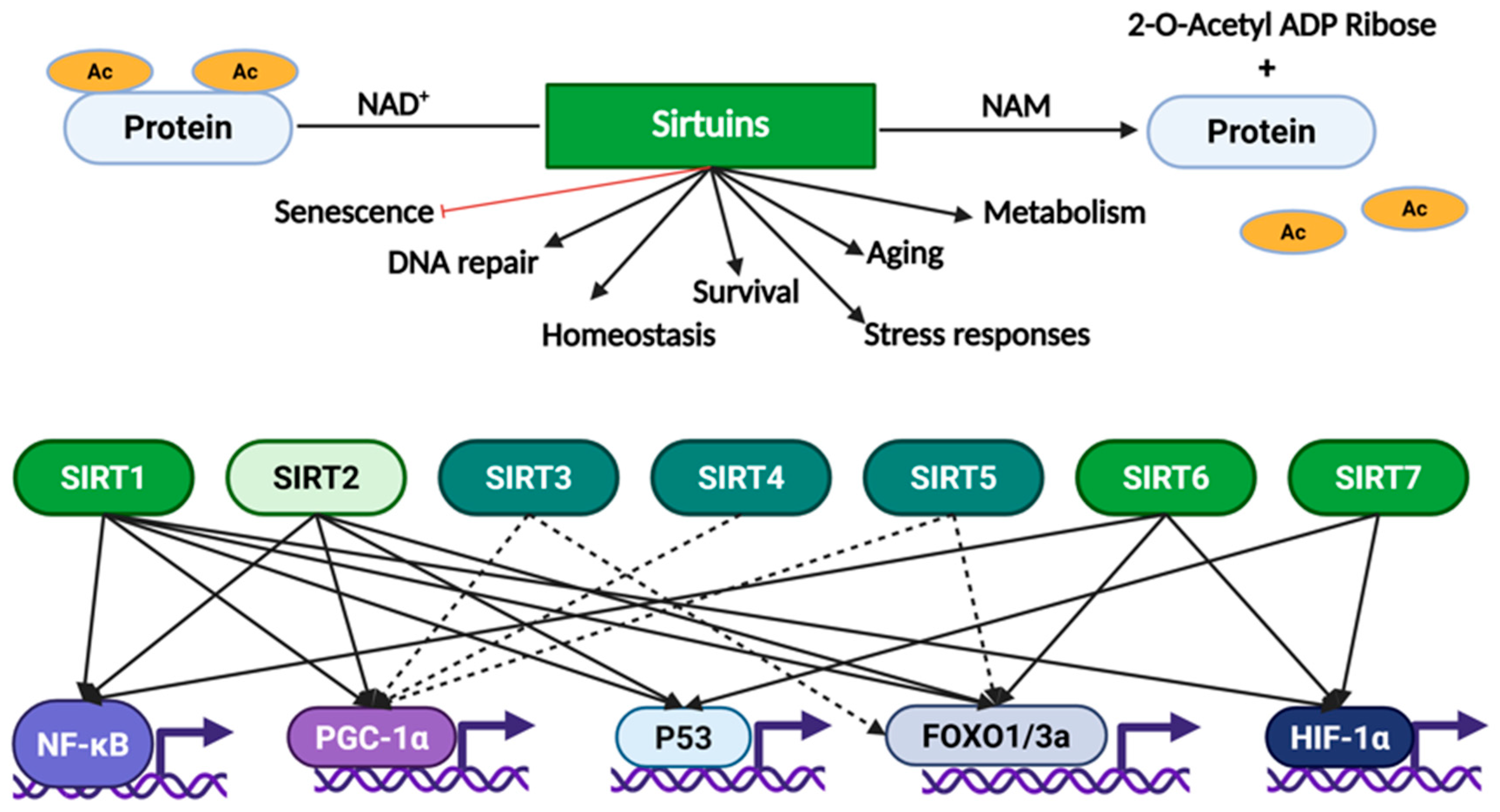
2.2. The Human Sirtuins
3. Sirtuins and Cancer
3.1. SIRT1, a Contextual Oncogene
3.2. SIRT2, a Second Context-Dependent Regulator in Cancer
3.3. The Roles of SIRT3 in Cancer Are Also Context-Dependent
3.4. The Roles of SIRT4 in Cancer
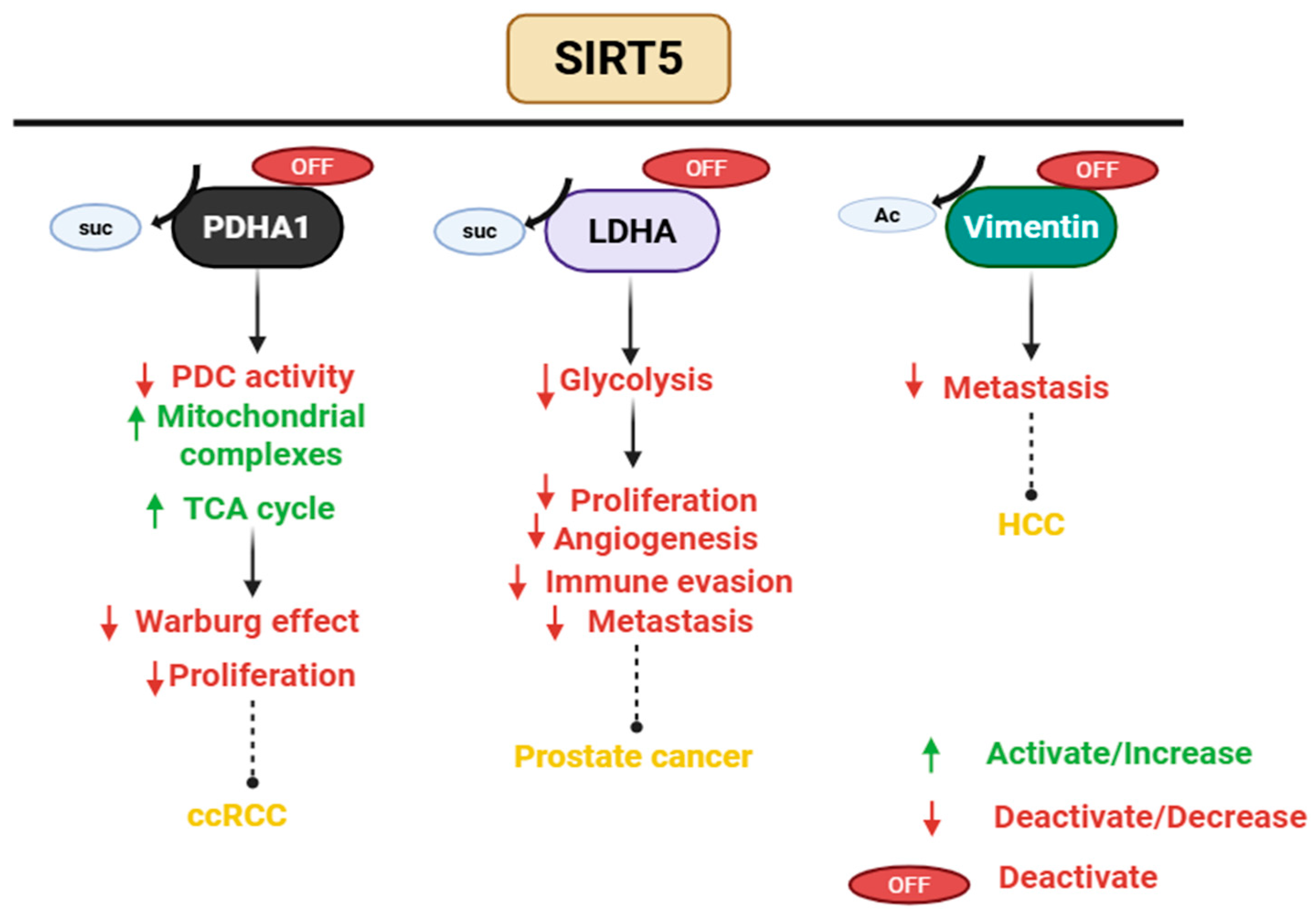
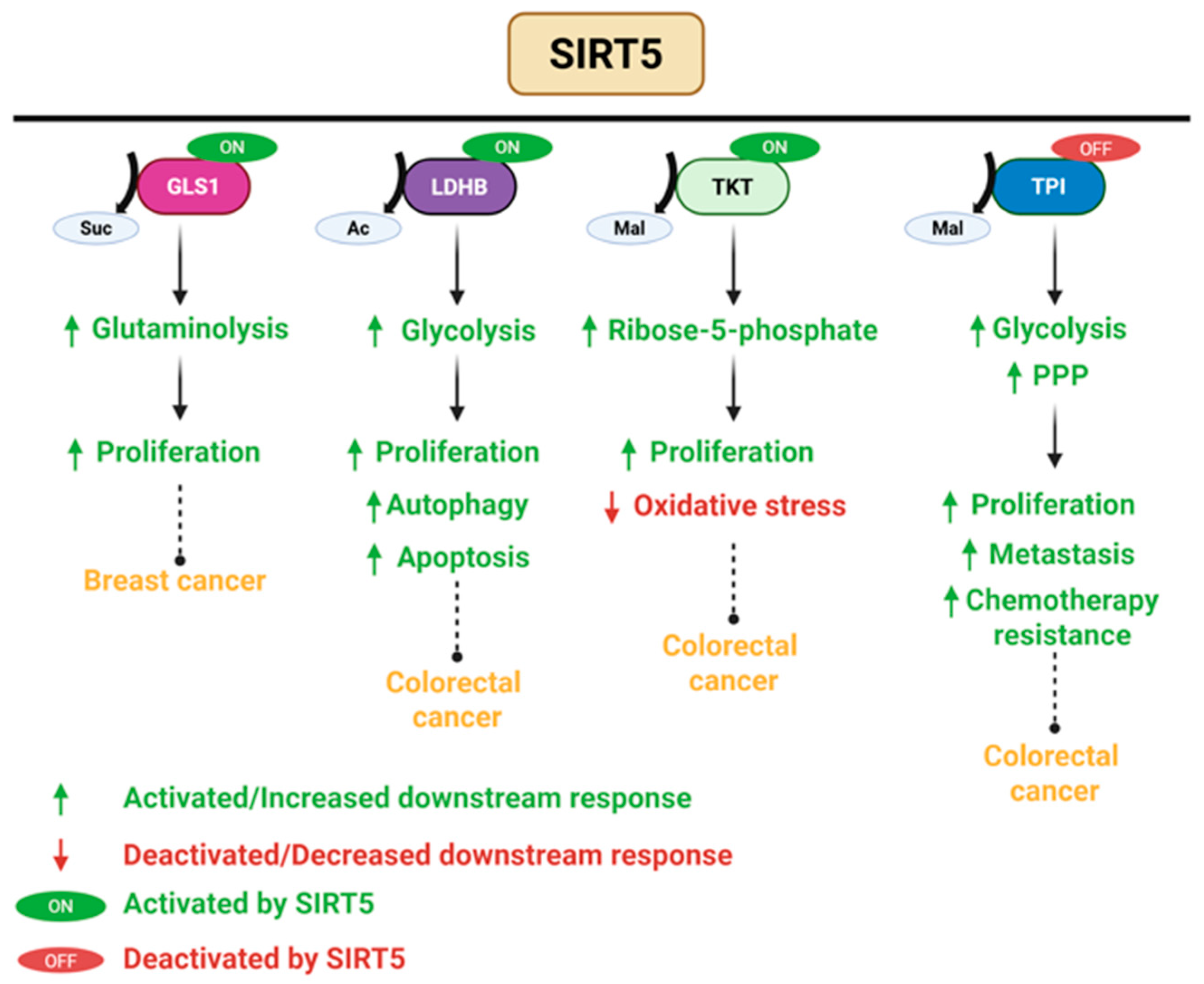
3.5. SIRT5 Plays a Dual Role in Cancer
3.6. The Context Dependent Role of SIRT6 in Cancer
3.7. The Role of SIRT7 in Cancer
3.8. Small-Molecule Modulators of Sirtuins as Cancer Therapy
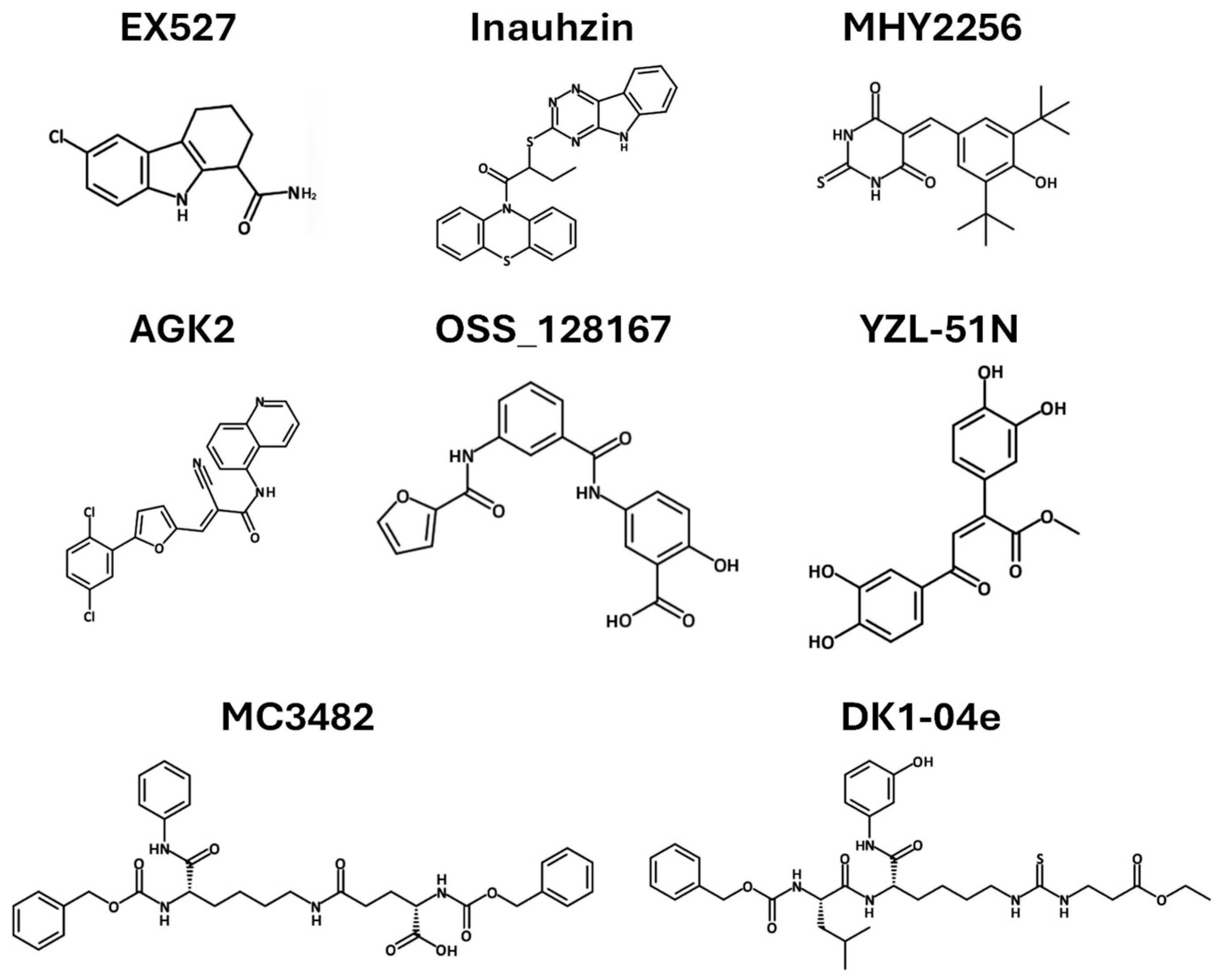
3.8.1. Sirtuin Inhibitors as Cancer Therapy
| Compound | Target | Cancer Type | Mechanism of Action | REF |
|---|---|---|---|---|
| EX527 | SIRT1 | Glioma | Upregulates p53, increases acetylated p53 and p21, induces apoptosis | [88] |
| Inauhzin | SIRT 1 | Lung Colon | Inhibits the binding of NAD+ to SIRT1, inhibits SIRT1 activity and blocks MDM2-mediated ubiquitination, triggers p53-dependent apoptosis | [90,91] |
| MHY2256 | SIRT1-3 | Breast | Reduces SIRT1-3 levels, increases p53 acetylation, induces apoptosis | [92,93] |
| AGK2 | SIRT2 | Breast | Suppresses cell proliferation and viability, and induces cell cycle arrest and apoptosis | [94] |
| DK1-04e | SIRT5 | Breast | Reduces tumor burden and total tumor weight | [95] |
| MC3482 | SIRT5 | Breast | Increases intracellular ammonia and promotes ammonia-induced autophagy | [74,96] |
| OSS_128167 | SIRT6 | Large B-cell lymphoma | Decreases cell proliferation, induces cell apoptosis, and blocks cell cycle | [97] |
| YZL-51N | SIRT7 | Colon | Suppresses DNA repair, increases chromatin instability | [98] |
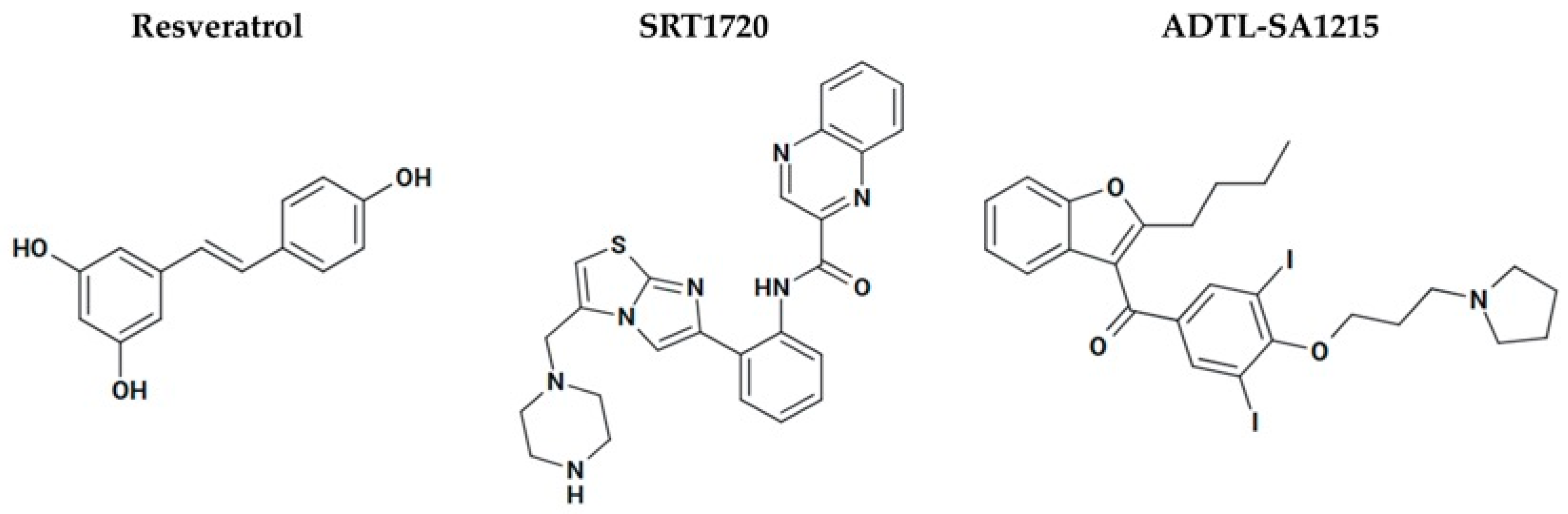
3.8.2. Sirtuin Activators as Cancer Therapy
| Compound | Models Studied | Mechanism of Action | Outcome | REF |
|---|---|---|---|---|
| Resveratrol | A431 human skin cancer cells | ↓ Cyclin D1, D2, D3, E; ↓ CDK2, CDK4, CDK6; ↑ p21 expression | Inhibited tumor growth | [101,102] |
| MCF-7 breast cancer cells DU-145 prostate cancer cells | Regulation of CDK4 and cyclin D1, ↑ p21 and p53 pathways | Antiproliferation | [102,103] | |
| MDA-MB-231 xenografts (ER-β+, ER-α−, nude mice) | ↑ apoptosis, ↓ angiogenesis | Inhibited tumor growth | [104] | |
| 4T1 breast cancer xenografts (ER-α−) | ↓ MMP-9 | Inhibited tumor growth ↓ lung metastasis | [105] | |
| PC-3 prostate cancer xenografts (AR-) | ↓ tumor volume and proliferation, ↑ apoptosis | ↓ tumor volume, ↓ proliferation, ↑ apoptosis | [106] | |
| DU-145 orthotopic prostate cancer (mice) | ↓ tumor volume, invasion, proliferation, metastases | Inhibited tumor growth, ↓invasion, ↓ metastasis | [106] | |
| Clinical (healthy and cancer patients) | PK, rapid metabolism, poor bioavailability | Safe up to 5 g/day, ↓ Ki-67 in colorectal tissue | [107,108] | |
| SRT1720 | Bladder cancer cells | Blocks late-stage autophagy (↓ fusion with lysosomes), deacetylates LAMP2 | Inhibited migration/invasion, ↑ apoptosis | [109] |
| Bladder cancer organoid cultures and mouse models | SIRT1 deacetylates HIF-1α ↓ hypoxia signaling | Suppressed tumor growth | [110] | |
| Multiple myeloma cell line | ATM-dependent apoptosis; Caspase activation; DNA damage, ER stress, ↑ ROS; inhibition of NF-κB and VEGF signaling | Selective toxicity (IC50: 3–7 µM); ↓ tumor growth; synergistic with bortezomib/dexamethasone | [111] | |
| ADTL-SA1215 | MDA-MB-231 human breast cancer cells; xenografts | ↑ SIRT3-mediated autophagy and mitophagy; suppression of proliferation and migration | Inhibited tumor growth and migration | [112,113] |
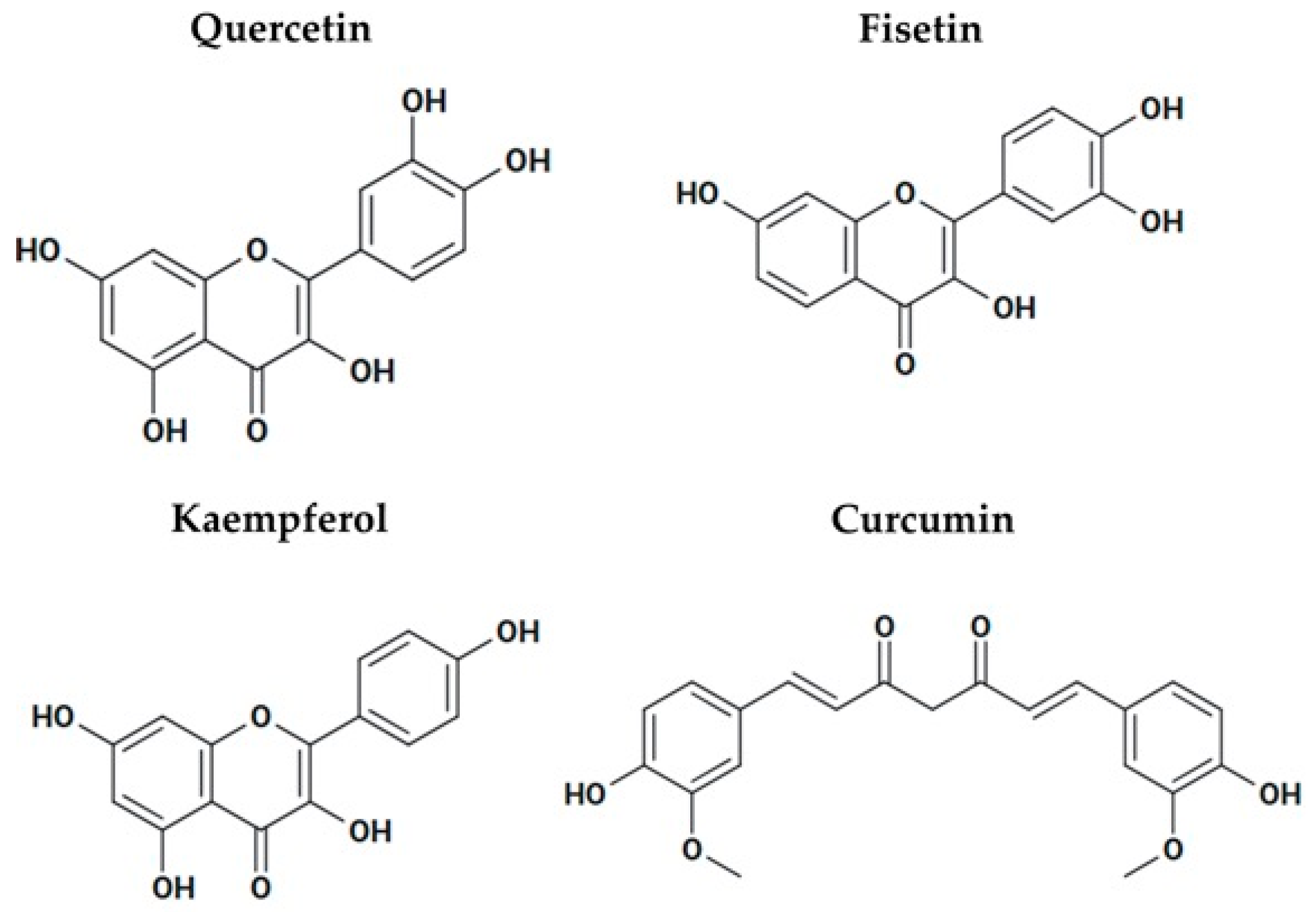
3.8.3. Polyphenols That Activate Sirtuins as Possible Anticancer Agents
4. Beyond Cancer: The Role of Sirtuins in Metabolic Diseases
4.1. The Role of Sirtuins in Diabetes
4.2. The Therapeutic Potential of Sirtuins in Obesity
4.3. Sirtuins and Osteoporosis
4.4. Sirtuin Activators for Treating Metabolic Diseases
| Compound | Mechanism of Action | Findings | REFs |
|---|---|---|---|
| Metformin | Directly activates SIRT1, promotes mitochondrial biogenesis, and improves insulin sensitivity. Indirect activation of SIRT1 via AMPK activation and ↑ NAD+ levels. | Used as a first-line therapy for type 2 diabetes | [152,153,154] |
| Resveratrol | Blocks PDE, ↑ cAMP levels ↑ calcium, ↑ NAD+ levels, thereby activating SIRT1. | In mice, decreased the risk of obesity and insulin resistance. | [157,158,159] |
| SRT2104 | ↑ SIRT1 expression, and reduces p53 acetylation, oxidative stress, and inflammation | In animal studies, SRT2104 enhanced insulin sensitivity and regulated blood glucose levels effectively. Phase II trial: 28 days improved lipid profile (↓ LDL and triglycerides), but no significant change in blood glucose or insulin sensitivity | [160,161,163] |
5. Sirtuins Role in Neurodegenerative Diseases and as Possible Therapeutic Targets
5.1. Sirtuins as Possible Contributors to PD Development
5.2. Sirtuins Role in AD Development
5.3. Sirtuins Role in HD
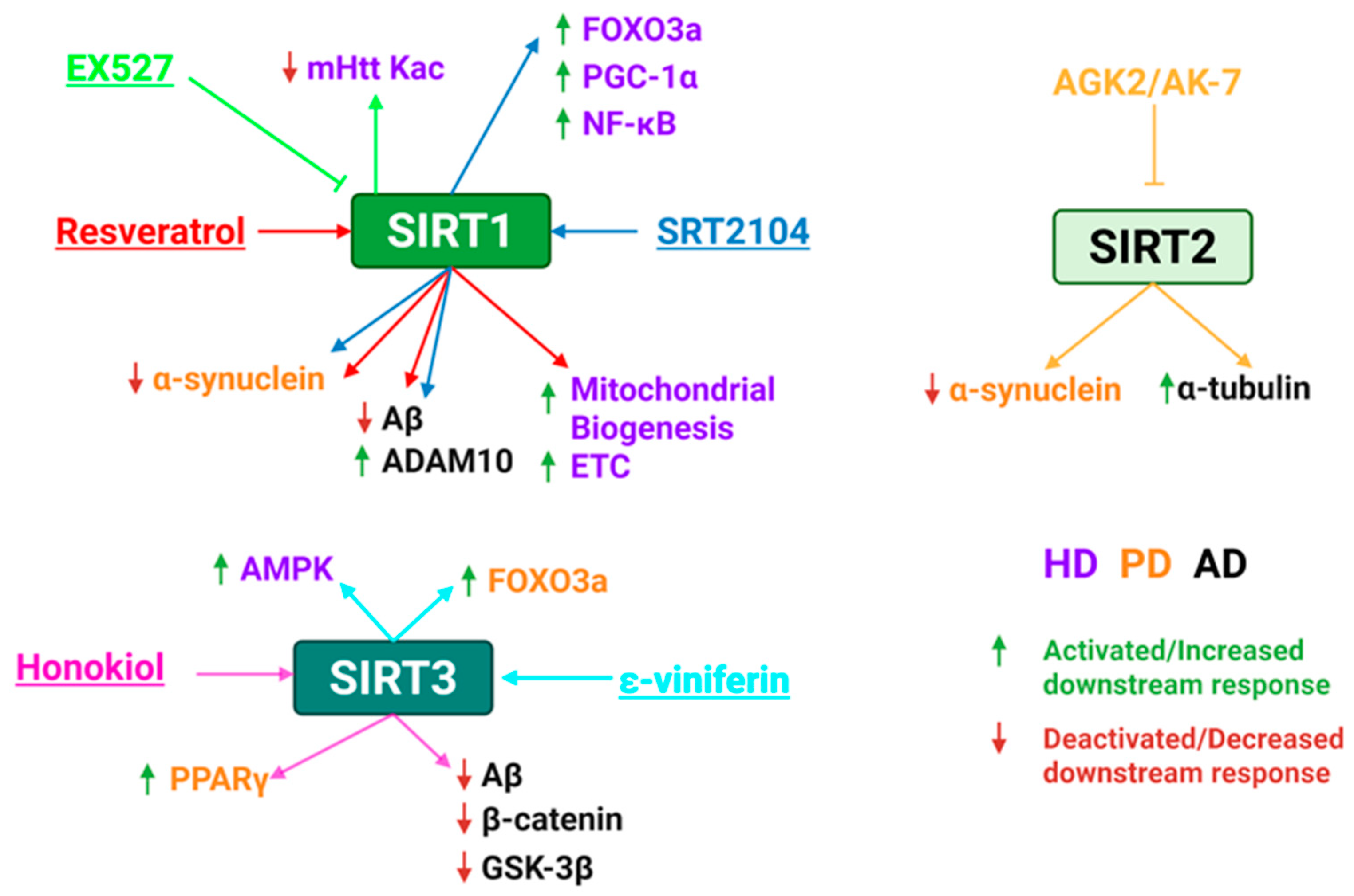
5.4. Sirtuin Activators for Treating Neurodegenerative Diseases
| Compound | Target | Disease | Mechanism of Action | Findings | REFs |
|---|---|---|---|---|---|
| Resveratrol | SIRT1 | PD | Promotes autophagic degeneration of α-synuclein, reduces neuroinflammation and oxidative damage | Improved motor and cognitive function in mice. | [211,212,213] |
| AD | Increases ADAM10, reduces Aβ levels, modulates inflammation | Neuroprotective in AD models. Phase II trials show its safe, greater brain volume loss, reduced Aβ accumulation in the brain. | [214,215] | ||
| HD | Enhances mitochondrial biogenesis and electron transport chain activity | Improved motor coordination and mitochondrial gene expression mouse models; Clinical trial completed but unpublished. | [216,217] | ||
| Honokiol | SIRT3 | PD | Restores motor function, prevents dopaminergic neuron loss, reduces oxidative stress, etc. | Neuroprotective in PD models with improved behavioral outcomes. | [218] |
| AD | Improves mitochondrial ATP production, reduces ROS, enhances mitophagy and neuronal survival | Improved memory and spatial learning in mouse models; reduced Aβ aggregation, oxidative stress, and NF-κB. | [171,218,219,220] | ||
| SRT2104 | SIRT1 | PD | Restores autophagy, reduces dopaminergic neuron loss | Improved coordination and motor function in PD mice; restored autophagy | [160] |
| AD | Protects cerebrovascular endothelial cells from Aβ-induced stress, reduces endothelial dysfunction | Improve endothelial viability; reduce detrimental cognitive effects | [160] | ||
| HD | Improves mitochondrial function and autophagy | Improve motor coordination, reduced brain atrophy, prolonged survival in HD mice | [221,222] | ||
| ɛ-Viniferin | SIRT3 | PD | Enhances FOXO3 deacetylation/nuclear translocation, boosts ATP, reduces ROS | Reduced mitochondrial depolarization and apoptosis. | [223] |
| HD | Stimulates AMPK, promotes mitochondrial biogenesis | Reduced ROS, prevented mitochondrial dysfunction in HD models | [203] |
5.5. Sirtuin Inhibitors for Treating Neurodegenerative Diseases
| Compound | Target | Disease | Mechanism of Action | Findings | REFs |
|---|---|---|---|---|---|
| EX527 | SIRT1 | HD | Increases acetylation of mHtt, enhances macroautophagic degradation of mutant protein, reduces toxicity | Phase I trials: safe and well-tolerated. Phase II HD trials: safe, but no effects on circulating levels of soluble Htt. | [228,229,230,231] |
| AGK2 | SIRT2 | HD | Protects against α-synuclein toxicity, reduces neuronal death, decreases sterol biosynthesis. | Limited BBB permeability, restricts in vivo efficacy. | [232,233] |
| PD | Mitigates α-synuclein toxicity; protected dopaminergic neurons. | ||||
| AK-7 | SIRT2 | HD | Increases α-tubulin acetylation, reduces mHtt aggregates and degeneration, improves motor function. | Improved BBB penetration, no clinical trials | [234,235] |
| PD | Protects substantia nigra dopaminergic neurons, reduces oxidative stress, and mitochondrial dysfunction. |
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| HD | Huntington’s disease |
| PTMs | Post-translational modifications |
| KATs | Lysine acetyltransferases |
| KDACs | Lysine deacetylases |
| HDACs | Histone deacetylases |
| NAM | Nicotinamide |
| SIRTs | Sirtuins |
| FOXO | Forkhead box O |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| EMT | Epithelial-mesenchymal transition |
| ROS | Reactive oxygen species |
| HIF1α | Hypoxia-inducible factor 1α |
| PDHA1 | Pyruvate dehydrogenase complex E1α subunit |
| LDHA | Lactate dehydrogenase |
| TCA | Tricarboxylic acid |
| LAMP2 | Lysosomal-associated membrane protein 2 |
| MnSOD | Manganese superoxide dismutase |
| IDH2 | Isocitrate dehydrogenase 2 |
| PPAR | Proliferator-activated receptor |
| FABP4 | Fatty acid binding protein 4 |
| Aβ | Amyloid-β |
| mHtt | Mutant huntingtin |
| BDNF | Brain-derived neurotrophic factor |
| TORC1 | Transcription coactivator 1 |
| GKRP | Glucokinase regulatory protein |
| GCK | Glucokinase |
| OXPHOS | Oxidative phosphorylation |
| Drp1 | Dynamin-related protein 1 |
References
- Omenn, G.S.; Orchard, S.; Lane, L.; Lindskog, C.; Pineau, C.; Overall, C.M.; Budnik, B.; Mudge, J.M.; Packer, N.H.; Weintraub, S.T.; et al. The 2024 Report on the Human Proteome from the HUPO Human Proteome Project. J. Proteome Res. 2024, 23, 5296–5311. [Google Scholar] [CrossRef] [PubMed]
- Varabyou, A.; Sommer, M.J.; Erdogdu, B.; Shinder, I.; Minkin, I.; Chao, K.H.; Park, S.; Heinz, J.; Pockrandt, C.; Shumate, A.; et al. CHESS 3: An improved, comprehensive catalog of human genes and transcripts based on large-scale expression data, phylogenetic analysis, and protein structure. Genome Biol. 2023, 24, 249. [Google Scholar] [CrossRef] [PubMed]
- Graw, S.; Chappell, K.; Washam, C.L.; Gies, A.; Bird, J.; Robeson, M.S., 2nd; Byrum, S.D. Multi-omics data integration considerations and study design for biological systems and disease. Mol. Omics 2021, 17, 170–185. [Google Scholar] [CrossRef]
- Ramazi, S.; Zahiri, J. Posttranslational modifications in proteins: Resources, tools and prediction methods. Database 2021, 2021, baab012. [Google Scholar] [CrossRef]
- Xu, D.; Wang, X. Lysine Acetylation is an Important Post-Translational Modification that Modulates Heat Shock Response in the Sea Cucumber Apostichopus japonicus. Int. J. Mol. Sci. 2019, 20, 4423. [Google Scholar] [CrossRef]
- Popova, L.; Carr, R.A.; Carabetta, V.J. Recent Contributions of Proteomics to Our Understanding of Reversible N(ε)-Lysine Acylation in Bacteria. J. Proteome Res. 2024, 23, 2733–2749. [Google Scholar] [CrossRef]
- Leutert, M.; Entwisle, S.W.; Villén, J. Decoding Post-Translational Modification Crosstalk with Proteomics. Mol. Cell. Proteom. 2021, 20, 100129. [Google Scholar] [CrossRef]
- Allfrey, V.G.; Faulkner, R.; Mirsky, A.E. Acetylation and Methylation of Histones and Their Possible Role in the Regulation of RNA Synthesis. Proc. Natl. Acad. Sci. USA 1964, 51, 786–794. [Google Scholar] [CrossRef]
- Dutta, H.; Jain, N. Post-translational modifications and their implications in cancer. Front. Oncol. 2023, 13, 1240115. [Google Scholar] [CrossRef]
- Graf, L.G.; Vogt, R.; Blasl, A.T.; Qin, C.; Schulze, S.; Zühlke, D.; Sievers, S.; Lammers, M. Assays to Study Enzymatic and Non-Enzymatic Protein Lysine Acetylation In Vitro. Curr. Protoc. 2021, 1, e277. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J.; Encarnacion-Guevara, S. Bacterial protein acetylation: Mechanisms, functions, and methods for study. Front. Cell. Infect. Microbiol. 2024, 14, 1408947. [Google Scholar] [CrossRef]
- Teixeira, C.S.S.; Cerqueira, N.; Gomes, P.; Sousa, S.F. A Molecular Perspective on Sirtuin Activity. Int. J. Mol. Sci. 2020, 21, 8609. [Google Scholar] [CrossRef]
- Imai, S.; Guarente, L. Ten years of NAD-dependent SIR2 family deacetylases: Implications for metabolic diseases. Trends Pharmacol. Sci. 2010, 31, 212–220. [Google Scholar] [CrossRef]
- Martínez-Redondo, P.; Vaquero, A. The diversity of histone versus nonhistone sirtuin substrates. Genes. Cancer 2013, 4, 148–163. [Google Scholar] [CrossRef]
- Hirschey, M.D. Old enzymes, new tricks: Sirtuins are NAD(+)-dependent de-acylases. Cell Metab. 2011, 14, 718–719. [Google Scholar] [CrossRef]
- Milazzo, G.; Mercatelli, D.; Di Muzio, G.; Triboli, L.; De Rosa, P.; Perini, G.; Giorgi, F.M. Histone Deacetylases (HDACs): Evolution, Specificity, Role in Transcriptional Complexes, and Pharmacological Actionability. Genes 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Greco, T.M.; Guise, A.J.; Luo, Y.; Yu, F.; Nesvizhskii, A.I.; Cristea, I.M. The functional interactome landscape of the human histone deacetylase family. Mol. Syst. Biol. 2013, 9, 672. [Google Scholar] [CrossRef]
- Yang, X.J.; Seto, E. The Rpd3/Hda1 family of lysine deacetylases: From bacteria and yeast to mice and men. Nat. Rev. Mol. Cell Biol. 2008, 9, 206–218. [Google Scholar] [CrossRef]
- Delcuve, G.P.; Khan, D.H.; Davie, J.R. Roles of histone deacetylases in epigenetic regulation: Emerging paradigms from studies with inhibitors. Clin. Epigenetics 2012, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhao, D.; Wu, C.; Wu, Z.; Zhang, W.; Chen, S.; Zhao, X.; Wu, S. Role of histone deacetylase inhibitors in non-neoplastic diseases. Heliyon 2024, 10, e33997. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, M.W. Lysine deacetylase (KDAC) regulatory pathways: An alternative approach to selective modulation. ChemMedChem 2014, 9, 511–522. [Google Scholar] [CrossRef]
- Imai, S.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef]
- Smith, J.S.; Brachmann, C.B.; Celic, I.; Kenna, M.A.; Muhammad, S.; Starai, V.J.; Avalos, J.L.; Escalante-Semerena, J.C.; Grubmeyer, C.; Wolberger, C.; et al. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl. Acad. Sci. USA 2000, 97, 6658–6663. [Google Scholar] [CrossRef]
- Poltronieri, P.; Čerekovic, N. Roles of Nicotinamide Adenine Dinucleotide (NAD+) in Biological Systems. Challenges 2018, 9, 3. [Google Scholar] [CrossRef]
- Chio, U.S.; Rechiche, O.; Bryll, A.R.; Zhu, J.; Leith, E.M.; Feldman, J.L.; Peterson, C.L.; Tan, S.; Armache, J.P. Cryo-EM structure of the human Sirtuin 6-nucleosome complex. Sci. Adv. 2023, 9, eadf7586. [Google Scholar] [CrossRef]
- Hu, J.; Jing, H.; Lin, H. Sirtuin inhibitors as anticancer agents. Future Med. Chem. 2014, 6, 945–966. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mahur, P.; Muthukumaran, J.; Singh, A.K.; Jain, M. Shedding light on structure, function and regulation of human sirtuins: A comprehensive review. 3 Biotech. 2023, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Herskovits, A.Z.; Guarente, L. Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res. 2013, 23, 746–758. [Google Scholar] [CrossRef]
- Frye, R.A. Evolution of Sirtuins from Archaea to Vertebrates. In Histone Deacetylases: Transcriptional Regulation and Other Cellular Functions; Verdin, E., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 183–202. [Google Scholar]
- Betsinger, C.N.; Cristea, I.M. Mitochondrial Function, Metabolic Regulation, and Human Disease Viewed through the Prism of Sirtuin 4 (SIRT4) Functions. J. Proteome Res. 2019, 18, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.A.; Greco, T.M.; Oberstein, A.; Budayeva, H.G.; Chakrabarti, R.; Rowland, E.A.; Kang, Y.; Shenk, T.; Cristea, I.M. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell 2014, 159, 1615–1625. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef]
- Choudhary, C.; Weinert, B.T.; Nishida, Y.; Verdin, E.; Mann, M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 536–550. [Google Scholar] [CrossRef]
- Verdin, E. NAD⁺ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef]
- Weng, H.; Ma, Y.; Chen, L.; Cai, G.; Chen, Z.; Zhang, S.; Ye, Q. A New Vision of Mitochondrial Unfolded Protein Response to the Sirtuin Family. Curr. Neuropharmacol. 2020, 18, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Kunadis, E.; Piperi, C. Exploring the Multi-Faceted Role of Sirtuins in Glioblastoma Pathogenesis and Targeting Options. Int. J. Mol. Sci. 2022, 23, 12889. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, X. The role of acetylation and deacetylation in cancer metabolism. Clin. Transl. Med. 2025, 15, e70145. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, S. Specific regulation of epigenome landscape by metabolic enzymes and metabolites. Biol. Rev. Camb. Philos. Soc. 2024, 99, 878–900. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Zhao, E.; Hou, J.; Ke, X.; Abbas, M.N.; Kausar, S.; Zhang, L.; Cui, H. The Roles of Sirtuin Family Proteins in Cancer Progression. Cancers 2019, 11, 1949. [Google Scholar] [CrossRef]
- Tang, Y.; Ju, W.; Liu, Y.; Deng, Q. The role of SIRT1 in autophagy and drug resistance: Unveiling new targets and potential biomarkers in cancer therapy. Front. Pharmacol. 2024, 15, 1469830. [Google Scholar] [CrossRef]
- Carafa, V.; Altucci, L.; Nebbioso, A. Dual Tumor Suppressor and Tumor Promoter Action of Sirtuins in Determining Malignant Phenotype. Front. Pharmacol. 2019, 10, 38. [Google Scholar] [CrossRef]
- Yousafzai, N.A.; Jin, H.; Ullah, M.; Wang, X. Recent advances of SIRT1 and implications in chemotherapeutics resistance in cancer. Am. J. Cancer Res. 2021, 11, 5233–5248. [Google Scholar]
- Chen, G.; Huang, P.; Hu, C. The role of SIRT2 in cancer: A novel therapeutic target. Int. J. Cancer 2020, 147, 3297–3304. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, X.; Wang, X.; Zheng, Y.; Qiu, J.; Peng, Y.; Xu, J.; Chai, Z.; Liu, C. Multiple Roles of SIRT2 in Regulating Physiological and Pathological Signal Transduction. Genet. Res. 2022, 2022, 9282484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Y.; Li, S.; Qin, Y.; Zhu, G.; Zhang, Q.; Zhang, Y.; Guan, F.; Fan, T.; Liu, H. SIRT2-mediated deacetylation of ACLY promotes the progression of oesophageal squamous cell carcinoma. J. Cell. Mol. Med. 2024, 28, e18129. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, S.; Ren, X. The Clinical Significance of SIRT2 in Malignancies: A Tumor Suppressor or an Oncogene? Front. Oncol. 2020, 10, 1721. [Google Scholar] [CrossRef] [PubMed]
- Fiskus, W.; Coothankandaswamy, V.; Chen, J.; Ma, H.; Ha, K.; Saenz, D.T.; Krieger, S.S.; Mill, C.P.; Sun, B.; Huang, P.; et al. SIRT2 Deacetylates and Inhibits the Peroxidase Activity of Peroxiredoxin-1 to Sensitize Breast Cancer Cells to Oxidant Stress-Inducing Agents. Cancer Res. 2016, 76, 5467–5478. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Qi, X.; Hu, Y.; Wang, Y.; Zhang, J.; Liu, Z.; Qin, Z. Targeting sirtuins for cancer therapy: Epigenetics modifications and beyond. Theranostics 2024, 14, 6726–6767. [Google Scholar] [CrossRef]
- Zahra, K.; Dey, T.; Ashish; Mishra, S.P.; Pandey, U. Pyruvate Kinase M2 and Cancer: The Role of PKM2 in Promoting Tumorigenesis. Front. Oncol. 2020, 10, 159. [Google Scholar] [CrossRef]
- Park, S.H.; Ozden, O.; Liu, G.; Song, H.Y.; Zhu, Y.; Yan, Y.; Zou, X.; Kang, H.J.; Jiang, H.; Principe, D.R.; et al. SIRT2-Mediated Deacetylation and Tetramerization of Pyruvate Kinase Directs Glycolysis and Tumor Growth. Cancer Res. 2016, 76, 3802–3812. [Google Scholar] [CrossRef]
- Ouyang, S.; Zhang, Q.; Lou, L.; Zhu, K.; Li, Z.; Liu, P.; Zhang, X. The Double-Edged Sword of SIRT3 in Cancer and Its Therapeutic Applications. Front. Pharmacol. 2022, 13, 871560. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.R.; Liu, B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef]
- Finley, L.W.; Carracedo, A.; Lee, J.; Souza, A.; Egia, A.; Zhang, J.; Teruya-Feldstein, J.; Moreira, P.I.; Cardoso, S.M.; Clish, C.B.; et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell 2011, 19, 416–428. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Z.; Jiang, H.; Shi, F. The sirtuin 3 expression profile is associated with pathological and clinical outcomes in colon cancer patients. Biomed. Res. Int. 2014, 2014, 871263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, J.; Zhu, S.; Han, B.; Liu, B. Context-dependent role of SIRT3 in cancer. Trends Pharmacol. Sci. 2024, 45, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Trinh, D.; Al Halabi, L.; Brar, H.; Kametani, M.; Nash, J.E. The role of SIRT3 in homeostasis and cellular health. Front. Cell. Neurosci. 2024, 18, 1434459. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cai, Z. SIRT3 regulates mitochondrial biogenesis in aging-related diseases. J. Biomed. Res. 2022, 37, 77–88. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Zhu, Y.; Kong, C. Functions of mammalian SIRT4 in cellular metabolism and research progress in human cancer. Oncol. Lett. 2020, 20, 11. [Google Scholar] [CrossRef]
- Onyiba, C.I.; Scarlett, C.J.; Weidenhofer, J. The Mechanistic Roles of Sirtuins in Breast and Prostate Cancer. Cancers 2022, 14, 5118. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Liu, T.; Hu, B.; Li, J.; Liu, C.; Liu, T.; Li, F. Mitochondrial PAK6 inhibits prostate cancer cell apoptosis via the PAK6-SIRT4-ANT2 complex. Theranostics 2020, 10, 2571–2586. [Google Scholar] [CrossRef]
- Yue, X.; Shi, Y.; Luo, Q. Advances of SIRT4 in cancer metabolism and therapy. Pediatr. Discov. 2023, 1, e17. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. Role of SIRT5 in cancer. Friend or Foe? Biochimie 2023, 209, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.K.; Bang, I.H.; Choi, S.Y.; Jeon, J.M.; Na, A.Y.; Gao, Y.; Cho, S.S.; Ki, S.H.; Choe, Y.; Lee, J.N.; et al. LDHA Desuccinylase Sirtuin 5 as A Novel Cancer Metastatic Stimulator in Aggressive Prostate Cancer. Genom. Proteom. Bioinform. 2023, 21, 177–189. [Google Scholar] [CrossRef]
- Lu, X.; Yang, P.; Zhao, X.; Jiang, M.; Hu, S.; Ouyang, Y.; Zeng, L.; Wu, J. OGDH mediates the inhibition of SIRT5 on cell proliferation and migration of gastric cancer. Exp. Cell Res. 2019, 382, 111483. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Song, X.; Guo, T.; Gu, S.; Chang, X.; Su, T.; Yang, X.; Liang, B.; Huang, D. Vimentin acetylation is involved in SIRT5-mediated hepatocellular carcinoma migration. Am. J. Cancer Res. 2018, 8, 2453–2466. [Google Scholar]
- He, S.; Jia, Q.; Zhou, L.; Wang, Z.; Li, M. SIRT5 is involved in the proliferation and metastasis of breast cancer by promoting aerobic glycolysis. Pathol. Res. Pract. 2022, 235, 153943. [Google Scholar] [CrossRef]
- Greene, K.S.; Lukey, M.J.; Wang, X.; Blank, B.; Druso, J.E.; Lin, M.J.; Stalnecker, C.A.; Zhang, C.; Negrón Abril, Y.; Erickson, J.W.; et al. SIRT5 stabilizes mitochondrial glutaminase and supports breast cancer tumorigenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 26625–26632. [Google Scholar] [CrossRef] [PubMed]
- Tharayil, J.S.; Kandettu, A.; Chakrabarty, S. The curious case of mitochondrial sirtuin in rewiring breast cancer metabolism: Mr Hyde or Dr Jekyll? Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167691. [Google Scholar] [CrossRef]
- Fabbrizi, E.; Fiorentino, F.; Carafa, V.; Altucci, L.; Mai, A.; Rotili, D. Emerging Roles of SIRT5 in Metabolism, Cancer, and SARS-CoV-2 Infection. Cells 2023, 12, 852. [Google Scholar] [CrossRef]
- Shi, L.; Yan, H.; An, S.; Shen, M.; Jia, W.; Zhang, R.; Zhao, L.; Huang, G.; Liu, J. SIRT5-mediated deacetylation of LDHB promotes autophagy and tumorigenesis in colorectal cancer. Mol. Oncol. 2019, 13, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Ma, W.; Hu, Y.; Liu, Y.; Song, Y.; Fu, L.; Qin, Z. Mitochondrial Sirtuins in Cancer: A Revisited Review from Molecular Mechanisms to Therapeutic Strategies. Theranostics 2024, 14, 2993–3013. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Chen, Y.; Wang, Y.Q.; Tao, E.W.; Tan, J.; Liu, Q.Q.; Li, C.M.; Tong, X.M.; Gao, Q.Y.; Hong, J.; et al. Sirtuin5 protects colorectal cancer from DNA damage by keeping nucleotide availability. Nat. Commun. 2022, 13, 6121. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Liu, M.; Luo, J. Protein post-translational modifications in the regulation of cancer hallmarks. Cancer Gene Ther. 2023, 30, 529–547. [Google Scholar] [CrossRef]
- Desantis, V.; Lamanuzzi, A.; Vacca, A. The role of SIRT6 in tumors. Haematologica 2018, 103, 1–4. [Google Scholar] [CrossRef]
- Al-Azzam, N. Sirtuin 6 and metabolic genes interplay in Warburg effect in cancers. J. Clin. Biochem. Nutr. 2020, 66, 169–175. [Google Scholar] [CrossRef]
- Garcia-Peterson, L.M.; Guzmán-Pérez, G.; Krier, C.R.; Ahmad, N. The sirtuin 6: An overture in skin cancer. Exp. Dermatol. 2020, 29, 124–135. [Google Scholar] [CrossRef]
- Blank, M.F.; Grummt, I. The seven faces of SIRT7. Transcription 2017, 8, 67–74. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Zhu, K.S.; Wang, H.; Zhu, W.-G. Advances in Cellular Characterization of the Sirtuin Isoform, SIRT7. Front. Endocrinol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jing, Z.X.; Ke, Z.; Yi, P. Sirtuin 7 plays an oncogenic role in human osteosarcoma via downregulating CDC4 expression. Am. J. Cancer Res. 2017, 7, 1788–1803. [Google Scholar] [PubMed]
- Ianni, A.; Kumari, P.; Tarighi, S.; Braun, T.; Vaquero, A. SIRT7: A novel molecular target for personalized cancer treatment? Oncogene 2024, 43, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, B.N.; Fernández-Duran, I.; Hernandez, Y.; Tarighi, S.; Thackray, J.K.; Espinosa-Alcantud, M.; Kumari, P.; Ianni, A.; Cesaire, L.; Braun, T.; et al. SIRT7 and p53 interaction in embryonic development and tumorigenesis. Front. Cell Dev. Biol. 2023, 11, 1281730. [Google Scholar] [CrossRef]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: The p53 family and its regulators provide endless cancer therapy opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Ianni, A.; Kumari, P.; Tarighi, S.; Simonet, N.G.; Popescu, D.; Guenther, S.; Hölper, S.; Schmidt, A.; Smolka, C.; Yue, S.; et al. SIRT7-dependent deacetylation of NPM promotes p53 stabilization following UV-induced genotoxic stress. Proc. Natl. Acad. Sci. USA 2021, 118, e2015339118. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Tarighi, S.; Braun, T.; Ianni, A. SIRT7 Acts as a Guardian of Cellular Integrity by Controlling Nucleolar and Extra-Nucleolar Functions. Genes 2021, 12, 1361. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Sun, S.L. EX527, a Sirt-1 inhibitor, induces apoptosis in glioma via activating the p53 signaling pathway. Anticancer Drugs 2020, 31, 19–26. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Song, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Z.; Wang, Y. The dual role of sirtuins in cancer: Biological functions and implications. Front. Oncol. 2024, 14, 1384928. [Google Scholar] [CrossRef]
- Zhang, Q.; Zeng, S.X.; Lu, H. Determination of Maximum Tolerated Dose and Toxicity of Inauhzin in Mice. Toxicol. Rep. 2015, 2, 546–554. [Google Scholar] [CrossRef]
- Yin, J.Y.; Lu, X.T.; Hou, M.L.; Cao, T.; Tian, Z. Sirtuin1-p53: A potential axis for cancer therapy. Biochem. Pharmacol. 2023, 212, 115543. [Google Scholar] [CrossRef]
- Park, E.Y.; Woo, Y.; Kim, S.J.; Kim, D.H.; Lee, E.K.; De, U.; Kim, K.S.; Lee, J.; Jung, J.H.; Ha, K.T.; et al. Anticancer Effects of a New SIRT Inhibitor, MHY2256, against Human Breast Cancer MCF-7 Cells via Regulation of MDM2-p53 Binding. Int. J. Biol. Sci. 2016, 12, 1555–1567. [Google Scholar] [CrossRef]
- De, U.; Son, J.Y.; Sachan, R.; Park, Y.J.; Kang, D.; Yoon, K.; Lee, B.M.; Kim, I.S.; Moon, H.R.; Kim, H.S. A New Synthetic Histone Deacetylase Inhibitor, MHY2256, Induces Apoptosis and Autophagy Cell Death in Endometrial Cancer Cells via p53 Acetylation. Int. J. Mol. Sci. 2018, 19, 2743. [Google Scholar] [CrossRef]
- Wawruszak, A.; Luszczki, J.; Czerwonka, A.; Okon, E.; Stepulak, A. Assessment of Pharmacological Interactions between SIRT2 Inhibitor AGK2 and Paclitaxel in Different Molecular Subtypes of Breast Cancer Cells. Cells 2022, 11, 1211. [Google Scholar] [CrossRef]
- Abril, Y.L.N.; Fernandez, I.R.; Hong, J.Y.; Chiang, Y.L.; Kutateladze, D.A.; Zhao, Q.; Yang, M.; Hu, J.; Sadhukhan, S.; Li, B.; et al. Pharmacological and genetic perturbation establish SIRT5 as a promising target in breast cancer. Oncogene 2021, 40, 1644–1658. [Google Scholar] [CrossRef]
- Polletta, L.; Vernucci, E.; Carnevale, I.; Arcangeli, T.; Rotili, D.; Palmerio, S.; Steegborn, C.; Nowak, T.; Schutkowski, M.; Pellegrini, L.; et al. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy 2015, 11, 253–270. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Li, Y.; Zhang, Y.; Fang, X.; Chen, N.; Wang, X. Targeting Sirt6 with OSS_128167 Displays Anti-Tumor Activities in Diffuse Large B-Cell Lymphoma through Down-Regulation of PI3K Signaling. Blood 2019, 134, 5065. [Google Scholar] [CrossRef]
- Kang, T.S.; Yan, Y.M.; Tian, Y.; Zhang, J.; Zhang, M.; Shu, Y.; Huang, J.; He, J.; Tao, C.T.; Zhu, Q.; et al. YZL-51N functions as a selective inhibitor of SIRT7 by NAD(+) competition to impede DNA damage repair. iScience 2024, 27, 110014. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, S.A.; Ahn, S.G. Sirtuin inhibitors, EX527 and AGK2, suppress cell migration by inhibiting HSF1 protein stability. Oncol. Rep. 2016, 35, 235–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, N.; Guan, X.; Zhang, S.; Wang, Y.; Wang, X.; Lu, Z.; Chong, D.; Wang, J.Y.; Yu, R.; Yu, W.; et al. Discovery of a pyrrole-pyridinimidazole derivative as novel SIRT6 inhibitor for sensitizing pancreatic cancer to gemcitabine. Cell Death Dis. 2023, 14, 499. [Google Scholar] [CrossRef]
- Ahmad, N.; Adhami, V.M.; Afaq, F.; Feyes, D.K.; Mukhtar, H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin. Cancer Res. 2001, 7, 1466–1473. [Google Scholar] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Kim, Y.A.; Rhee, S.H.; Park, K.Y.; Choi, Y.H. Antiproliferative effect of resveratrol in human prostate carcinoma cells. J. Med. Food 2003, 6, 273–280. [Google Scholar] [CrossRef]
- Garvin, S.; Ollinger, K.; Dabrosin, C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006, 231, 113–122. [Google Scholar] [CrossRef]
- Lee, H.S.; Ha, A.W.; Kim, W.K. Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nutr. Res. Pract. 2012, 6, 294–300. [Google Scholar] [CrossRef]
- Li, K.; Dias, S.J.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Lewin, J.R.; Levenson, A.S. Pterostilbene acts through metastasis-associated protein 1 to inhibit tumor growth, progression and metastasis in prostate cancer. PLoS ONE 2013, 8, e57542. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Alsayed, A.R.; Farhan, F.; Al Kury, L.T. Resveratrol and Tumor Microenvironment: Mechanistic Basis and Therapeutic Targets. Molecules 2020, 25, 4282. [Google Scholar] [CrossRef]
- Li, L.; Fu, S.; Wang, J.; Lu, J.; Tao, Y.; Zhao, L.; Fu, B.; Lu, L.; Xiang, C.; Sun, X.; et al. SRT1720 inhibits bladder cancer cell progression by impairing autophagic flux. Biochem. Pharmacol. 2024, 222, 116111. [Google Scholar] [CrossRef]
- Tan, P.; Wang, M.; Zhong, A.; Wang, Y.; Du, J.; Wang, J.; Qi, L.; Bi, Z.; Zhang, P.; Lin, T. SRT1720 inhibits the growth of bladder cancer in organoids and murine models through the SIRT1-HIF axis. Oncogene 2021, 40, 6081–6092. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Bandi, M.; Singh, A.V.; Ray, A.; Raje, N.; Richardson, P.; Anderson, K.C. Preclinical evaluation of a novel SIRT1 modulator SRT1720 in multiple myeloma cells. Br. J. Haematol. 2011, 155, 588–598. [Google Scholar] [CrossRef]
- Bursch, K.L.; Goetz, C.J.; Smith, B.C. Current Trends in Sirtuin Activator and Inhibitor Development. Molecules 2024, 29, 1185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, L.; Shi, D.; Liu, J.; Zhang, J.; Zhao, R.; Wang, G.; Zhang, L.; Ouyang, L.; Liu, B. Structure-Guided Design of a Small-Molecule Activator of Sirtuin-3 that Modulates Autophagy in Triple Negative Breast Cancer. J. Med. Chem. 2021, 64, 14192–14216. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Q.; Jia, R.; Xiang, S.; Xu, L. A comprehensive review of the relationship between autophagy and sorafenib-resistance in hepatocellular carcinoma: Ferroptosis is noteworthy. Front. Cell Dev. Biol. 2023, 11, 1156383. [Google Scholar] [CrossRef]
- Jo, H.; Park, Y.; Kim, T.; Kim, J.; Lee, J.S.; Kim, S.Y.; Chung, J.I.; Ko, H.Y.; Pyun, J.C.; Kim, K.S.; et al. Modulation of SIRT3 expression through CDK4/6 enhances the anti-cancer effect of sorafenib in hepatocellular carcinoma cells. BMC Cancer 2020, 20, 332. [Google Scholar] [CrossRef]
- Liu, T.; Li, Z.; Tian, F. Quercetin inhibited the proliferation and invasion of hepatoblastoma cells through facilitating SIRT6-medicated FZD4 silence. Hum. Exp. Toxicol. 2021, 40, S96–S107. [Google Scholar] [CrossRef]
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac. Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, P.; Terada, T.; Sui, M.; Furuta, H.; Iida, K.; Katayama, Y.; Lu, Y.; Okamoto, K.; Suzuki, M.; et al. Quercetin 3,5,7,3′,4′-pentamethyl ether from Kaempferia parviflora directly and effectively activates human SIRT1. Commun. Biol. 2021, 4, 209. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, Y.H.; Son, S.W.; Moon, E.Y.; Pyo, S.; Um, S.H. Fisetin induces Sirt1 expression while inhibiting early adipogenesis in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2015, 467, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, Y.; Gao, Z.; Li, X.; Weng, M.; Shi, C.; Wang, C.; Sun, L. Fisetin inhibits the proliferation, migration and invasion of pancreatic cancer by targeting PI3K/AKT/mTOR signaling. Aging 2021, 13, 24753–24767. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Biswas, S.; Al-Dayan, N.; Alhegaili, A.S.; Sarwat, M. Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma. Antioxidants 2021, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Anto, R.J.; M, S.; Nath, L.R. Kaempferol-Mediated Sensitization Enhances Chemotherapeutic Efficacy of Sorafenib Against Hepatocellular Carcinoma: An In Silico and In Vitro Approach. Adv. Pharm. Bull. 2020, 10, 472–476. [Google Scholar] [CrossRef]
- Li, Q.; Wei, L.; Lin, S.; Chen, Y.; Lin, J.; Peng, J. Synergistic effect of kaempferol and 5-fluorouracil on the growth of colorectal cancer cells by regulating the PI3K/Akt signaling pathway. Mol. Med. Rep. 2019, 20, 728–734. [Google Scholar] [CrossRef]
- Zendedel, E.; Butler, A.E.; Atkin, S.L.; Sahebkar, A. Impact of curcumin on sirtuins: A review. J. Cell. Biochem. 2018, 119, 10291–10300. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Song, N.Y.; Suh, J.; Kim, D.H.; Kim, W.; Ann, J.; Lee, J.; Baek, J.H.; Na, H.K.; Surh, Y.J. Curcumin suppresses oncogenicity of human colon cancer cells by covalently modifying the cysteine 67 residue of SIRT1. Cancer Lett. 2018, 431, 219–229. [Google Scholar] [CrossRef]
- Chen, H.H.; Zhang, Y.X.; Lv, J.L.; Liu, Y.Y.; Guo, J.Y.; Zhao, L.; Nan, Y.X.; Wu, Q.J.; Zhao, Y.H. Role of sirtuins in metabolic disease-related renal injury. Biomed. Pharmacother. 2023, 161, 114417. [Google Scholar] [CrossRef]
- Zhang, E.; Wu, Y. Metabolic memory: Mechanisms and implications for diabetic vasculopathies. Sci. China Life Sci. 2014, 57, 845–851. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, H.; Li, J.; Li, T.; Zheng, B.; Zheng, Y.; Jin, H.; He, Y.; Gu, Q.; Xu, X. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes 2012, 61, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhao, H.; Liu, Y.; Yang, Z.; Yao, H.; Liu, T.; Gou, T.; Wang, L.; Zhang, J.; Tian, Y.; et al. Novel Role of the SIRT1 in Endocrine and Metabolic Diseases. Int. J. Biol. Sci. 2023, 19, 484–501. [Google Scholar] [CrossRef] [PubMed]
- Lemos, V.; de Oliveira, R.M.; Naia, L.; Szegö, É.; Ramos, E.; Pinho, S.; Magro, F.; Cavadas, C.; Rego, A.C.; Costa, V.; et al. The NAD+-dependent deacetylase SIRT2 attenuates oxidative stress and mitochondrial dysfunction and improves insulin sensitivity in hepatocytes. Hum. Mol. Genet. 2017, 26, 4105–4117. [Google Scholar] [CrossRef]
- Watanabe, H.; Inaba, Y.; Kimura, K.; Matsumoto, M.; Kaneko, S.; Kasuga, M.; Inoue, H. Sirt2 facilitates hepatic glucose uptake by deacetylating glucokinase regulatory protein. Nat. Commun. 2018, 9, 30. [Google Scholar] [CrossRef]
- Zheng, X.; Li, J.; Sheng, J.; Dai, Y.; Wang, Y.; Liu, J.; Xu, Y. Haplotypes of the Mutated SIRT2 Promoter Contributing to Transcription Factor Binding and Type 2 Diabetes Susceptibility. Genes 2020, 11, 569. [Google Scholar] [CrossRef]
- Kanwal, A.; Dsouza, L.A. Sirtuins and diabetes: Optimizing the sweetness in the blood. Transl. Med. Commun. 2019, 4, 3. [Google Scholar] [CrossRef]
- Xian, Y.; Liu, B.; Shen, T.; Yang, L.; Peng, R.; Shen, H.; An, X.; Wang, Y.; Ben, Y.; Jiang, Q. Enhanced SIRT3 expression restores mitochondrial quality control mechanism to reverse osteogenic impairment in type 2 diabetes mellitus. Bone Res. 2025, 13, 30. [Google Scholar] [CrossRef]
- Song, M.Y.; Wang, J.; Ka, S.O.; Bae, E.J.; Park, B.H. Insulin secretion impairment in Sirt6 knockout pancreatic β cells is mediated by suppression of the FoxO1-Pdx1-Glut2 pathway. Sci. Rep. 2016, 6, 30321. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.G.; Ramadori, G.; Ioris, R.M.; Galiè, M.; Berglund, E.D.; Coate, K.C.; Fujikawa, T.; Pucciarelli, S.; Moreschini, B.; Amici, A.; et al. Enhanced insulin sensitivity in skeletal muscle and liver by physiological overexpression of SIRT6. Mol. Metab. 2015, 4, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Yao, L.; Yang, X.; Gao, Y.; Fang, F.; Zhang, J.; Wang, Q.; Chang, Y. SIRT6 regulates metabolic homeostasis in skeletal muscle through activation of AMPK. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E493–E505. [Google Scholar] [CrossRef]
- Sociali, G.; Magnone, M.; Ravera, S.; Damonte, P.; Vigliarolo, T.; Von Holtey, M.; Vellone, V.G.; Millo, E.; Caffa, I.; Cea, M.; et al. Pharmacological Sirt6 inhibition improves glucose tolerance in a type 2 diabetes mouse model. FASEB J. 2017, 31, 3138–3149. [Google Scholar] [CrossRef]
- Du, Y.; Huo, Y.; Yang, Y.; Lin, P.; Liu, W.; Wang, Z.; Zeng, W.; Li, J.; Liang, Z.; Yuan, C.; et al. Role of sirtuins in obesity and osteoporosis: Molecular mechanisms and therapeutic targets. Cell Commun. Signal. 2025, 23, 20. [Google Scholar] [CrossRef]
- Hirschey, M.D.; Shimazu, T.; Jing, E.; Grueter, C.A.; Collins, A.M.; Aouizerat, B.; Stančáková, A.; Goetzman, E.; Lam, M.M.; Schwer, B.; et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell 2011, 44, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, F.; Zhang, L.; Lou, R.; Zhang, C.; Wan, J.; Ma, X.; Lin, L. Adipocyte-expressed SIRT3 manipulates carnitine pool to orchestrate metabolic reprogramming and polarization of macrophages. Cell Death Dis. 2025, 16, 381. [Google Scholar] [CrossRef]
- Vargas-Ortiz, K.; Perez-Vazquez, V.; Diaz-Cisneros, F.J.; Figueroa, A.; Jiménez-Flores, L.M.; Rodriguez-DelaRosa, G.; Macias, M.H. Aerobic Training Increases Expression Levels of SIRT3 and PGC-1α in Skeletal Muscle of Overweight Adolescents Without Change in Caloric Intake. Pediatr. Exerc. Sci. 2015, 27, 177–184. [Google Scholar] [CrossRef]
- Kanfi, Y.; Peshti, V.; Gil, R.; Naiman, S.; Nahum, L.; Levin, E.; Kronfeld-Schor, N.; Cohen, H.Y. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell 2010, 9, 162–173. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, H.; Zhong, Y.; Chen, Y.; Tang, K.; Pan, Z.; Huang, J.; Yang, X.; Wang, Q.; Gao, Y. Microglia Sirt6 modulates the transcriptional activity of NRF2 to ameliorate high-fat diet-induced obesity. Mol. Med. 2023, 29, 108. [Google Scholar] [CrossRef]
- Luo, J.; Li, L.; Shi, W.; Xu, K.; Shen, Y.; Dai, B. Oxidative stress and inflammation: Roles in osteoporosis. Front. Immunol. 2025, 16, 1611932. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, J.C.; Jiang, Q.; Lee, W.Y. Role of sirtuins in bone biology: Potential implications for novel therapeutic strategies for osteoporosis. Aging Cell 2021, 20, e13301. [Google Scholar] [CrossRef]
- Lin, L.; Guo, Z.; He, E.; Long, X.; Wang, D.; Zhang, Y.; Guo, W.; Wei, Q.; He, W.; Wu, W.; et al. SIRT2 regulates extracellular vesicle-mediated liver-bone communication. Nat. Metab. 2023, 5, 821–841. [Google Scholar] [CrossRef]
- Jing, Y.; Zhou, Y.; Zhou, F.; Wang, X.; Tao, B.; Sun, L.; Liu, J.; Zhao, H. SIRT2 deficiency prevents age-related bone loss in rats by inhibiting osteoclastogenesis. Cell. Mol. Biol. 2019, 65, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jia, X.; Cui, Y.; Song, Y.; Wang, S.; Geng, Y.; Li, R.; Gao, W.; Fu, D. Sirt3-mediated mitophagy regulates AGEs-induced BMSCs senescence and senile osteoporosis. Redox Biol. 2021, 41, 101915. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Krager, K.; Richardson, K.K.; Warren, A.D.; Ponte, F.; Aykin-Burns, N.; Manolagas, S.C.; Almeida, M.; Kim, H.N. Mitochondrial Sirt3 contributes to the bone loss caused by aging or estrogen deficiency. JCI Insight 2021, 6, e146728. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Cuyàs, E.; Verdura, S.; Llorach-Parés, L.; Fernández-Arroyo, S.; Joven, J.; Martin-Castillo, B.; Bosch-Barrera, J.; Brunet, J.; Nonell-Canals, A.; Sanchez-Martinez, M.; et al. Metformin is a direct SIRT1-activating compound: Computational modeling and experimental validation. Front. Endocrinol. 2018, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, H.; Liu, T.; Qin, C.; Sesaki, H.; Guo, S.; Radovick, S.; Hussain, M.; Maheshwari, A.; Wondisford, F.E.; et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019, 29, 1511–1523.e1515. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Carling, D. AMP-activated protein kinase: The current landscape for drug development. Nat. Rev. Drug Discov. 2019, 18, 527–551. [Google Scholar] [CrossRef]
- Moin, T.; Schmittdiel, J.A.; Flory, J.H.; Yeh, J.; Karter, A.J.; Kruge, L.E.; Schillinger, D.; Mangione, C.M.; Herman, W.H.; Walker, E.A. Review of Metformin Use for Type 2 Diabetes Prevention. Am. J. Prev. Med. 2018, 55, 565–574. [Google Scholar] [CrossRef]
- Xu, K.; Li, J.; Wen, R.; Chang, B.; Cheng, Y.; Yi, X. Role of SIRT3 in bone homeostasis and its application in preventing and treating bone diseases. Front. Pharmacol. 2023, 14, 1248507. [Google Scholar] [CrossRef]
- Wu, S.K.; Wang, L.; Wang, F.; Zhang, J. Resveratrol improved mitochondrial biogenesis by activating SIRT1/PGC-1α signal pathway in SAP. Sci. Rep. 2024, 14, 26216. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Chang, N.; Li, J.; Lin, S.; Zhang, J.; Zeng, W.; Ma, G.; Wang, Y. Emerging roles of SIRT1 activator, SRT2104, in disease treatment. Sci. Rep. 2024, 14, 5521. [Google Scholar] [CrossRef]
- Wu, H.; Wu, J.; Zhou, S.; Huang, W.; Li, Y.; Zhang, H.; Wang, J.; Jia, Y. SRT2104 attenuates diabetes-induced aortic endothelial dysfunction via inhibition of P53. J. Endocrinol. 2018, 237, 1–14. [Google Scholar] [CrossRef]
- Ma, F.; Wu, J.; Jiang, Z.; Huang, W.; Jia, Y.; Sun, W.; Wu, H. P53/NRF2 mediates SIRT1’s protective effect on diabetic nephropathy. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1272–1281. [Google Scholar] [CrossRef]
- Baksi, A.; Kraydashenko, O.; Zalevkaya, A.; Stets, R.; Elliott, P.; Haddad, J.; Hoffmann, E.; Vlasuk, G.P.; Jacobson, E.W. A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes. Br. J. Clin. Pharmacol. 2014, 78, 69–77. [Google Scholar] [CrossRef]
- Adam, H.; Gopinath, S.C.B.; Md Arshad, M.K.; Adam, T.; Parmin, N.A.; Husein, I.; Hashim, U. An update on pathogenesis and clinical scenario for Parkinson’s disease: Diagnosis and treatment. 3 Biotech. 2023, 13, 142. [Google Scholar] [CrossRef]
- Haque, M.E.; Akther, M.; Azam, S.; Kim, I.S.; Lin, Y.; Lee, Y.H.; Choi, D.K. Targeting α-synuclein aggregation and its role in mitochondrial dysfunction in Parkinson’s disease. Br. J. Pharmacol. 2022, 179, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Lu, Y.; Liu, Y.; Feng, H.; Li, C.; Sheng, W.; Cui, Z.; Zhu, S.; Gu, X.; Yang, Z.; et al. General Control of Amino Acid Synthesis 5-Like 1-Mediated Acetylation of Manganese Superoxide Dismutase Regulates Oxidative Stress in Diabetic Kidney Disease. Oxid. Med. Cell. Longev. 2021, 2021, 6691226. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, Q.; Shi, J.; Zhou, S. Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson’s disease. Biomed. Pharmacother. 2020, 132, 110928. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Chen, B.; Dai, R.; Xi, Z.; Xu, H. Insights into Manganese Superoxide Dismutase and Human Diseases. Int. J. Mol. Sci. 2022, 23, 15893. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.A.; Scherzer, C.R.; Potashkin, J.A. Network analysis identifies SOD2 mRNA as a potential biomarker for Parkinson’s disease. PLoS ONE 2014, 9, e109042. [Google Scholar] [CrossRef]
- Chen, H.H.; Chang, P.C.; Chen, C.; Chan, M.H. Protective and therapeutic activity of honokiol in reversing motor deficits and neuronal degeneration in the mouse model of Parkinson’s disease. Pharmacol. Rep. 2018, 70, 668–676. [Google Scholar] [CrossRef]
- Leite, J.A.; Ghirotto, B.; Targhetta, V.P.; de Lima, J.; Câmara, N.O.S. Sirtuins as pharmacological targets in neurodegenerative and neuropsychiatric disorders. Br. J. Pharmacol. 2022, 179, 1496–1511. [Google Scholar] [CrossRef]
- Chen, H.H.; Chang, P.C.; Wey, S.P.; Chen, P.M.; Chen, C.; Chan, M.H. Therapeutic effects of honokiol on motor impairment in hemiparkinsonian mice are associated with reversing neurodegeneration and targeting PPARγ regulation. Biomed. Pharmacother. 2018, 108, 254–262. [Google Scholar] [CrossRef]
- Weng, H.; Song, W.; Fu, K.; Guan, Y.; Cai, G.; Huang, E.; Chen, X.; Zou, H.; Ye, Q. Proteomic profiling reveals the potential mechanisms and regulatory targets of sirtuin 4 in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s mouse model. Front. Neurosci. 2022, 16, 1035444. [Google Scholar] [CrossRef]
- Laurent, G.; de Boer, V.C.; Finley, L.W.; Sweeney, M.; Lu, H.; Schug, T.T.; Cen, Y.; Jeong, S.M.; Li, X.; Sauve, A.A.; et al. SIRT4 represses peroxisome proliferator-activated receptor α activity to suppress hepatic fat oxidation. Mol. Cell. Biol. 2013, 33, 4552–4561. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, G.D.; Colak, M. SIRT4 prevents excitotoxicity via modulating glutamate metabolism in glioma cells. Hum. Exp. Toxicol. 2020, 39, 938–947. [Google Scholar] [CrossRef]
- Wang, X.W.; Sun, Y.J.; Chen, X.; Zhang, W.Z. Interleukin-4-induced FABP4 promotes lipogenesis in human skeletal muscle cells by activating the PPAR γ signaling pathway. Cell Biochem. Biophys. 2022, 80, 355–366. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Wang, X.X.; Truong, D.; Wu, Y.C. The Critical Role of SIRT1 in Parkinson’s Disease: Mechanism and Therapeutic Considerations. Aging Dis. 2020, 11, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Hanson, P.S.; Morris, C.M. SIRT1 ameliorates oxidative stress induced neural cell death and is down-regulated in Parkinson’s disease. BMC Neurosci. 2017, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.F.; Martins, A.; Majolo, F.; Contini, V.; Laufer, S.; Goettert, M.I. Neural regeneration research model to be explored: SH-SY5Y human neuroblastoma cells. Neural Regen. Res. 2023, 18, 1265–1266. [Google Scholar] [CrossRef]
- Singh, A.S.; Naqvi, A.R.; Chanu, M.T. Neuroinflammation and progress in clinical trials for the treatment of Alzheimer’s disease and related dementias: An update. Innov. Med. Omics 2025, 2, 36–50. [Google Scholar] [CrossRef]
- Moloney, C.M.; Labuzan, S.A.; Crook, J.E.; Siddiqui, H.; Castanedes-Casey, M.; Lachner, C.; Petersen, R.C.; Duara, R.; Graff-Radford, N.R.; Dickson, D.W.; et al. Phosphorylated tau sites that are elevated in Alzheimer’s disease fluid biomarkers are visualized in early neurofibrillary tangle maturity levels in the post mortem brain. Alzheimers Dement. 2023, 19, 1029–1040. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, Z. Therapeutic potential of natural molecules against Alzheimer’s disease via SIRT1 modulation. Biomed. Pharmacother. 2023, 161, 114474. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef] [PubMed]
- Fagerli, E.; Escobar, I.; Ferrier, F.J.; Jackson, C.W.; Perez-Lao, E.J.; Perez-Pinzon, M.A. Sirtuins and cognition: Implications for learning and memory in neurological disorders. Front. Physiol. 2022, 13, 908689. [Google Scholar] [CrossRef]
- Mehramiz, M.; Porter, T.; O’Brien, E.K.; Rainey-Smith, S.R.; Laws, S.M. A Potential Role for Sirtuin-1 in Alzheimer’s Disease: Reviewing the Biological and Environmental Evidence. J. Alzheimers Dis. Rep. 2023, 7, 823–843. [Google Scholar] [CrossRef]
- Aroor, A.; Brewster, A.L. Seizing the Alzheimer’s Brain: A Role for Sirtuin 3 in Hyperexcitability. Epilepsy Curr. 2020, 20, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Li, B.; Jia, Z.; Guo, L. Sirtuin 3 mRNA Expression is Downregulated in the Brain Tissues of Alzheimer’s Disease Patients: A Bioinformatic and Data Mining Approach. Med. Sci. Monit. 2020, 26, e923547. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Wu, G.; Yang, H.; Yang, X.; Wang, D.; He, Y. Role of SIRT3 in neurological diseases and rehabilitation training. Metab. Brain Dis. 2023, 38, 69–89. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Liu, T.; Hwang, Y.J.; Hyeon, S.J.; Im, H.; Lee, K.; Alvarez, V.E.; McKee, A.C.; Um, S.J.; et al. SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer’s disease. Aging Cell 2018, 17, e12679. [Google Scholar] [CrossRef]
- Li, S.; Yin, J.; Nielsen, M.; Beach, T.G.; Guo, L.; Shi, J. Sirtuin 3 Mediates Tau Deacetylation. J. Alzheimers Dis. 2019, 69, 355–362. [Google Scholar] [CrossRef]
- Foret, M.K.; Orciani, C.; Welikovitch, L.A.; Huang, C.; Cuello, A.C.; Do Carmo, S. Early oxidative stress and DNA damage in Aβ-burdened hippocampal neurons in an Alzheimer’s-like transgenic rat model. Commun. Biol. 2024, 7, 861. [Google Scholar] [CrossRef]
- Fan, Y.; Cheng, J.; Yang, Q.; Feng, J.; Hu, J.; Ren, Z.; Yang, H.; Yang, D.; Ding, G. Sirt6-mediated Nrf2/HO-1 activation alleviates angiotensin II-induced DNA DSBs and apoptosis in podocytes. Food Funct. 2021, 12, 7867–7882. [Google Scholar] [CrossRef]
- Jung, E.S.; Choi, H.; Song, H.; Hwang, Y.J.; Kim, A.; Ryu, H.; Mook-Jung, I. p53-dependent SIRT6 expression protects Aβ42-induced DNA damage. Sci. Rep. 2016, 6, 25628. [Google Scholar] [CrossRef]
- Welty, S.; Thathiah, A.; Levine, A.S. DNA Damage Increases Secreted Aβ40 and Aβ42 in Neuronal Progenitor Cells: Relevance to Alzheimer’s Disease. J. Alzheimers Dis. 2022, 88, 177–190. [Google Scholar] [CrossRef]
- Reddy, P.H.; Beal, M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 2008, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, P.; Ge, J.; Li, H. SIRT6 in Aging, Metabolism, Inflammation and Cardiovascular Diseases. Aging Dis. 2022, 13, 1787–1822. [Google Scholar] [CrossRef]
- Gatto, E.M.; Rojas, N.G.; Persi, G.; Etcheverry, J.L.; Cesarini, M.E.; Perandones, C. Huntington disease: Advances in the understanding of its mechanisms. Clin. Park. Relat. Disord. 2020, 3, 100056. [Google Scholar] [CrossRef]
- Tong, H.; Yang, T.; Xu, S.; Li, X.; Liu, L.; Zhou, G.; Yang, S.; Yin, S.; Li, X.J.; Li, S. Huntington’s Disease: Complex Pathogenesis and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 3845. [Google Scholar] [CrossRef] [PubMed]
- Carmo, C.; Naia, L.; Lopes, C.; Rego, A.C. Mitochondrial Dysfunction in Huntington’s Disease. Adv. Exp. Med. Biol. 2018, 1049, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Naia, L.; Carmo, C.; Campesan, S.; Fão, L.; Cotton, V.E.; Valero, J.; Lopes, C.; Rosenstock, T.R.; Giorgini, F.; Rego, A.C. Mitochondrial SIRT3 confers neuroprotection in Huntington’s disease by regulation of oxidative challenges and mitochondrial dynamics. Free Radic. Biol. Med. 2021, 163, 163–179. [Google Scholar] [CrossRef]
- Razick, D.I.; Akhtar, M.; Wen, J.; Alam, M.; Dean, N.; Karabala, M.; Ansari, U.; Ansari, Z.; Tabaie, E.; Siddiqui, S. The Role of Sirtuin 1 (SIRT1) in Neurodegeneration. Cureus 2023, 15, e40463. [Google Scholar] [CrossRef]
- Johri, A.; Chandra, A.; Flint Beal, M. PGC-1α, mitochondrial dysfunction, and Huntington’s disease. Free Radic. Biol. Med. 2013, 62, 37–46. [Google Scholar] [CrossRef]
- Fu, J.; Jin, J.; Cichewicz, R.H.; Hageman, S.A.; Ellis, T.K.; Xiang, L.; Peng, Q.; Jiang, M.; Arbez, N.; Hotaling, K.; et al. trans-(-)-ε-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington Disease. J. Biol. Chem. 2012, 287, 24460–24472. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Wijaya, L.; Tang, B.L. SIRT1 in the brain-connections with aging-associated disorders and lifespan. Front. Cell. Neurosci. 2015, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, J.; Fu, J.; Du, L.; Jeong, H.; West, T.; Xiang, L.; Peng, Q.; Hou, Z.; Cai, H.; et al. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat. Med. 2011, 18, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Naia, L.; Rego, A.C. Sirtuins: Double players in Huntington’s disease. Biochim. Biophys. Acta 2015, 1852, 2183–2194. [Google Scholar] [CrossRef]
- Lloret, A.; Beal, M.F. PGC-1α, sirtuins and PARPs in Huntington’s disease and other neurodegenerative conditions: NAD+ to rule them all. Neurochem. Res. 2019, 44, 2423–2434. [Google Scholar] [CrossRef]
- Xu, J.; Jackson, C.W.; Khoury, N.; Escobar, I.; Perez-Pinzon, M.A. Brain SIRT1 Mediates Metabolic Homeostasis and Neuroprotection. Front. Endocrinol. 2018, 9, 702. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Duan, W. Targeting sirtuin-1 in Huntington’s disease: Rationale and current status. CNS Drugs 2013, 27, 345–352. [Google Scholar] [CrossRef]
- Zhang, L.F.; Yu, X.L.; Ji, M.; Liu, S.Y.; Wu, X.L.; Wang, Y.J.; Liu, R.T. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food Funct. 2018, 9, 6414–6426. [Google Scholar] [CrossRef]
- Jin, F.; Wu, Q.; Lu, Y.F.; Gong, Q.H.; Shi, J.S. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur. J. Pharmacol. 2008, 600, 78–82. [Google Scholar] [CrossRef]
- Gahtani, R.M.; Shoaib, S.; Hani, U.; Jayachithra, R.; Alomary, M.N.; Chauhan, W.; Jahan, R.; Tufail, S.; Ansari, M.A. Combating Parkinson’s disease with plant-derived polyphenols: Targeting oxidative stress and neuroinflammation. Neurochem. Int. 2024, 178, 105798. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Chen, H.; Xu, X.; Li, Y.; Gui, Y. Neuroprotective Effect of Trans-Resveratrol in Mild to Moderate Alzheimer Disease: A Randomized, Double-Blind Trial. Neurol. Ther. 2021, 10, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Naia, L.; Rosenstock, T.R.; Oliveira, A.M.; Oliveira-Sousa, S.I.; Caldeira, G.L.; Carmo, C.; Laço, M.N.; Hayden, M.R.; Oliveira, C.R.; Rego, A.C. Comparative Mitochondrial-Based Protective Effects of Resveratrol and Nicotinamide in Huntington’s Disease Models. Mol. Neurobiol. 2017, 54, 5385–5399. [Google Scholar] [CrossRef]
- Ho, D.J.; Calingasan, N.Y.; Wille, E.; Dumont, M.; Beal, M.F. Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Exp. Neurol. 2010, 225, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Kumari, A.; Verma, A.; Chaudhary, V.; Pathak, P.; Yadav, H.N. Nature’s Neuroprotector: Honokiol and Its Promise for Alzheimer’s and Parkinson’s. Brain Disord. 2025, 17, 100208. [Google Scholar] [CrossRef]
- Faysal, M.; Khan, J.; Zehravi, M.; Nath, N.; Singh, L.P.; Kakkar, S.; Perusomula, R.; Khan, P.A.; Nainu, F.; Asiri, M.; et al. Neuropharmacological potential of honokiol and its derivatives from Chinese herb Magnolia species: Understandings from therapeutic viewpoint. Chin. Med. 2023, 18, 154. [Google Scholar] [CrossRef]
- Xian, Y.F.; Ip, S.P.; Mao, Q.Q.; Lin, Z.X. Neuroprotective effects of honokiol against beta-amyloid-induced neurotoxicity via GSK-3β and β-catenin signaling pathway in PC12 cells. Neurochem. Int. 2016, 97, 8–14. [Google Scholar] [CrossRef]
- Jiang, M.; Zheng, J.; Peng, Q.; Hou, Z.; Zhang, J.; Mori, S.; Ellis, J.L.; Vlasuk, G.P.; Fries, H.; Suri, V.; et al. Sirtuin 1 activator SRT2104 protects Huntington’s disease mice. Ann. Clin. Transl. Neurol. 2014, 1, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.G.; Suárez-Fariñas, M.; Cueto, I.; Khacherian, A.; Matheson, R.; Parish, L.C.; Leonardi, C.; Shortino, D.; Gupta, A.; Haddad, J.; et al. A Randomized, Placebo-Controlled Study of SRT2104, a SIRT1 Activator, in Patients with Moderate to Severe Psoriasis. PLoS ONE 2015, 10, e0142081. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Y.; Feng, J. Neuroprotective mechanisms of ε-viniferin in a rotenone-induced cell model of Parkinson’s disease: Significance of SIRT3-mediated FOXO3 deacetylation. Neural Regen. Res. 2020, 15, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, C.; Wang, X.; Chen, X.; Wang, Y.; Le, W. Oxygen metabolism abnormality and Alzheimer’s disease: An update. Redox Biol. 2023, 68, 102955. [Google Scholar] [CrossRef]
- Kantham, S.; Chan, S.; McColl, G.; Miles, J.A.; Veliyath, S.K.; Deora, G.S.; Dighe, S.N.; Khabbazi, S.; Parat, M.O.; Ross, B.P. Effect of the Biphenyl Neolignan Honokiol on Aβ(42)-Induced Toxicity in Caenorhabditis elegans, Aβ(42) Fibrillation, Cholinesterase Activity, DPPH Radicals, and Iron(II) Chelation. ACS Chem. Neurosci. 2017, 8, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, J.; Wu, Y.; Yang, Y.; Zhang, W.; Tian, Y. Honokiol relieves hippocampal neuronal damage in Alzheimer’s disease by activating the SIRT3-mediated mitochondrial autophagy. CNS Neurosci. Ther. 2024, 30, e14878. [Google Scholar] [CrossRef]
- Singh, L.; Singh, S. Neuroprotective potential of Honokiol in ICV-STZ induced neuroinflammation, Aβ ((1-42)) and NF-kB expression in experimental model of rats. Neurosci. Lett. 2023, 799, 137090. [Google Scholar] [CrossRef]
- Westerberg, G.; Chiesa, J.A.; Andersen, C.A.; Diamanti, D.; Magnoni, L.; Pollio, G.; Darpo, B.; Zhou, M. Safety, pharmacokinetics, pharmacogenomics and QT concentration-effect modelling of the SirT1 inhibitor selisistat in healthy volunteers. Br. J. Clin. Pharmacol. 2015, 79, 477–491. [Google Scholar] [CrossRef]
- Smith, M.R.; Syed, A.; Lukacsovich, T.; Purcell, J.; Barbaro, B.A.; Worthge, S.A.; Wei, S.R.; Pollio, G.; Magnoni, L.; Scali, C.; et al. A potent and selective Sirtuin 1 inhibitor alleviates pathology in multiple animal and cell models of Huntington’s disease. Hum. Mol. Genet. 2014, 23, 2995–3007. [Google Scholar] [CrossRef]
- Süssmuth, S.D.; Haider, S.; Landwehrmeyer, G.B.; Farmer, R.; Frost, C.; Tripepi, G.; Andersen, C.A.; Di Bacco, M.; Lamanna, C.; Diodato, E.; et al. An exploratory double-blind, randomized clinical trial with selisistat, a SirT1 inhibitor, in patients with Huntington’s disease. Br. J. Clin. Pharmacol. 2015, 79, 465–476. [Google Scholar] [CrossRef]
- Broussy, S.; Laaroussi, H.; Vidal, M. Biochemical mechanism and biological effects of the inhibition of silent information regulator 1 (SIRT1) by EX-527 (SEN0014196 or selisistat). J. Enzyme Inhib. Med. Chem. 2020, 35, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Luthi-Carter, R.; Taylor, D.M.; Pallos, J.; Lambert, E.; Amore, A.; Parker, A.; Moffitt, H.; Smith, D.L.; Runne, H.; Gokce, O.; et al. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 7927–7932. [Google Scholar] [CrossRef]
- Outeiro, T.F.; Kontopoulos, E.; Altmann, S.M.; Kufareva, I.; Strathearn, K.E.; Amore, A.M.; Volk, C.B.; Maxwell, M.M.; Rochet, J.C.; McLean, P.J.; et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 2007, 317, 516–519. [Google Scholar] [CrossRef]
- Chopra, V.; Quinti, L.; Kim, J.; Vollor, L.; Narayanan, K.L.; Edgerly, C.; Cipicchio, P.M.; Lauver, M.A.; Choi, S.H.; Silverman, R.B.; et al. The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington’s disease mouse models. Cell Rep. 2012, 2, 1492–1497. [Google Scholar] [CrossRef]
- Chen, X.; Wales, P.; Quinti, L.; Zuo, F.; Moniot, S.; Herisson, F.; Rauf, N.A.; Wang, H.; Silverman, R.B.; Ayata, C.; et al. The sirtuin-2 inhibitor AK7 is neuroprotective in models of Parkinson’s disease but not amyotrophic lateral sclerosis and cerebral ischemia. PLoS ONE 2015, 10, e0116919. [Google Scholar] [CrossRef] [PubMed]
- Paresishvili, T.; Kakabadze, Z. Challenges and Opportunities Associated with Drug Delivery for the Treatment of Solid Tumors. Oncol. Rev. 2023, 17, 10577. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Eroglu, E.; Harmanci, N. Emerging Molecular Targets in Neurodegenerative Disorders: New Avenues for Therapeutic Intervention. Basic Clin. Pharmacol. Toxicol. 2025, 137, e70107. [Google Scholar] [CrossRef] [PubMed]
- Sundriyal, S.; Moniot, S.; Mahmud, Z.; Yao, S.; Di Fruscia, P.; Reynolds, C.R.; Dexter, D.T.; Sternberg, M.J.; Lam, E.W.; Steegborn, C.; et al. Thienopyrimidinone Based Sirtuin-2 (SIRT2)-Selective Inhibitors Bind in the Ligand Induced Selectivity Pocket. J. Med. Chem. 2017, 60, 1928–1945. [Google Scholar] [CrossRef] [PubMed]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD(+) in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019, 30, 630–655. [Google Scholar] [CrossRef]
- Yusri, K.; Jose, S.; Vermeulen, K.S.; Tan, T.C.M.; Sorrentino, V. The role of NAD(+) metabolism and its modulation of mitochondria in aging and disease. npj Metab. Health Dis. 2025, 3, 26. [Google Scholar] [CrossRef]
- Arvidsson, E.; Lobo, D.D.; Sabarese, E.; Duarte, F.; Nobre, R.J.; Quintino, L.; Lundberg, C. A systematic screening assay identifies efficient small guide RNAs for CRISPR activation. Front. Bioeng. Biotechnol. 2025, 13, 1336313. [Google Scholar] [CrossRef] [PubMed]
| Isoforms | Localization | Enzymatic Activity | Functional Roles |
|---|---|---|---|
| SIRT1 | Nucleus | Strong deacetylase activity; regulates transcription factors | Aging, metabolism, inflammation |
| SIRT2 | Cytoplasm | Strong deacetylase activity; targets, histones, cell-cycle regulators | Cell cycle control |
| SIRT3 | Mitochondria, nucleus | Strong deacetylase activity; regulates metabolic enzymes | Mitochondrial homeostasis |
| SIRT4 | Mitochondria | Weak deacetylase activity, ADP-ribosyltransferase, lipoamidase, and deacylase activities | Regulates metabolism, insulin secretion |
| SIRT5 | Mitochondria | Low deacetylase activity; potent desuccinylase, demalonylase, and deglutarylase activity | Fatty acid oxidation, regulation of glycolysis, amino acid breakdown, and cellular respiration |
| SIRT6 | Nucleus | Moderate deacetylases activity, strong ADP-ribosyltransferase, defatty-acylase activity | DNA repair, genomic stability, metabolism, inflammation, aging |
| SIRT7 | Nucleus | Moderate deacetylase activity; regulates Pol I transcription and rRNA synthesis | Ribosome biogenesis, stress resistance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akantibila, M.; Carabetta, V.J. Sirtuins as Therapeutic Targets for Treating Cancer, Metabolic Diseases, and Neurodegenerative Diseases. Pharmaceuticals 2025, 18, 1723. https://doi.org/10.3390/ph18111723
Akantibila M, Carabetta VJ. Sirtuins as Therapeutic Targets for Treating Cancer, Metabolic Diseases, and Neurodegenerative Diseases. Pharmaceuticals. 2025; 18(11):1723. https://doi.org/10.3390/ph18111723
Chicago/Turabian StyleAkantibila, Maxwell, and Valerie J. Carabetta. 2025. "Sirtuins as Therapeutic Targets for Treating Cancer, Metabolic Diseases, and Neurodegenerative Diseases" Pharmaceuticals 18, no. 11: 1723. https://doi.org/10.3390/ph18111723
APA StyleAkantibila, M., & Carabetta, V. J. (2025). Sirtuins as Therapeutic Targets for Treating Cancer, Metabolic Diseases, and Neurodegenerative Diseases. Pharmaceuticals, 18(11), 1723. https://doi.org/10.3390/ph18111723







