Abstract
Wearable sensors are becoming increasingly popular for complementing classical clinical assessments of gait deficits. The aim of this review is to examine the existing knowledge by systematically reviewing a large number of papers focusing on the use of wearable inertial sensors for the assessment of gait during the 6-minute walk test (6MWT), a widely recognized, simple, non-invasive, low-cost and reproducible exercise test. After a systematic search on PubMed and Scopus databases, two raters evaluated the quality of 28 full-text articles. Then, the available knowledge was summarized regarding study design, subjects enrolled (number of patients and pathological condition, if any, age, male/female ratio), sensor characteristics (type, number, sampling frequency, range) and body placement, 6MWT protocol and extracted parameters. Results were critically discussed to suggest future directions for the use of inertial sensor devices in the clinics.
1. Introduction
The 6-minute walk test (6MWT) is a simple, non-invasive, low-cost and reproducible exercise test used to evaluate endurance during self-paced, submaximal walk by measuring the distance walked within 6 minutes (6MWD) along a flat, straight course with a hard surface [1]. The 6MWT was originally developed for assessing exercise capacity in patients with cardiopulmonary diseases [2]. However, it has also been commonly used to measure functional status [3,4], as a predictor of morbidity and mortality [5,6], and for pre/post-treatment comparisons [7,8]. Besides the 6MWD, other useful outcome measures may be extracted from the 6MWT, including pulmonary function metrics [9], patient-reported fatigue at the beginning and end of the test using the Borg scale [10], and 6MWT work, calculated as the product of 6MWD and body weight [11]. However, a major limitation of the traditional 6MWT is that the only standardized outcome measure is the 6MWD, which does not take into account additional subject-specific factors such as gait length and width or body posture. Standard 6MWT performed in the clinics do not consider the granularity of overall gait patterns or body segment kinematics, and gait parameters are not commonly measured during the test. Moreover, it involves costs and some practical limitations such as the need for a dedicated space in the hospital and healthcare personnel to observe the test and to note down the measurements.
Accelerometers, gyroscopes and magnetometers are the most common wearable sensors used in human motion analysis and physical activity monitoring [12,13]. Accelerometers sense linear acceleration along one or several axis, and are the most widespread sensors used in ambulatory gait analysis, because they are miniaturized, low powered, durable, inexpensive, highly mobile, and readily available [14]. Modern gyroscopes measure angular velocity about one or several axes, with rotations around them commonly defined as Euler angles. Magnetometers sense amplitude and direction of the magnetic field exploiting the principle of the Lorentz force [15]. These sensors can be combined in devices called magneto-inertial measurement units, or MIMUs, which are gaining increasing popularity in human motion analysis. A recent review outlining the clinical impact of wearable sensors for gait analysis highlighted that MIMUs are the most widely used wearable technologies in the clinical field [16]. Readings obtained from the different sensors can be extracted and processed separately to obtain key features, or combined using fusion algorithms, such as Kalman filters, in order to obtain an estimated three-dimensional orientation [17].
Several MIMU-based methods have been proposed in the literature for the detection of gait events [18] and the computation of basic temporal parameters (stride and step duration, swing and stance phase duration). Spatial parameters (stride length, walking speed) can also be obtained by direct (i.e., integration of the accelerometer signal) or indirect (i.e., gait models) methods [19,20]. Gait dynamics, i.e., the study of stride-to-stride fluctuations in gait, includes basic variability metrics as well as advanced analytic methods, such as detrended fluctuation analysis [21], and can also be measured by means of MIMUs [22,23]. Wearable MIMU systems may also be able to estimate gait kinematics [24] and kinetics [25]. As a recent systematic review showed, new methods in artificial intelligence and machine learning are also increasingly being applied to wearable sensors data [26] because the latter exhibits many of the typical qualities of “big data” [27].
Despite the increasing body of evidence for the use of wearable sensing during gait, the use of MIMUs during standard clinical tests such as the 6MWT is not widespread. The well-defined setting of the 6MWT and its long duration compared to other walking tests represent favorable conditions to extract valuable information from both turn and straight walking, potentially increasing test efficiency. This study aims at reorganizing the existing knowledge by systematically reviewing a large number of papers focusing on the use of wearable inertial sensors for the assessment of advanced gait features during the 6MWT. It was not the interest of the authors to review papers focusing on energy expenditure, physical activity estimation or basic gait metrics extraction (i.e., step count). The objectives of this work are: (1) to select and perform a quality check on papers that assess gait during the 6MWT using wearable inertial sensors; (2) to identify the characteristics of the populations included in the studies; (3) to describe the features of the sensors used in terms of technical characteristics, number and location on the human body; (4) to indicate the main parameters extracted; (5) to suggest future directions for the use of inertial sensor devices in the clinics, especially fostering their testing and validation in the pediatric population.
2. Materials and Methods
For this review the authors followed the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [28], and the original methodology of the systematic review was submitted to PROSPERO.
2.1. Search Strategy
Pubmed and Scopus search engines were accessed in November 2019 to identify articles measuring advanced quantitative parameters associated to gait with MIMU wearable sensors. The queries used for the database search within the title and/or abstract were the following: (“6MWT” OR “six-minute walk test” OR “six-minute walking test” OR “6MWD” OR "6-min walk test" OR "6-minute walk test") AND (“IMU” OR “inertial measurement unit” OR “MIMU” OR “magneto inertial measurement unit” OR “inertial sensor” OR “accelerometer” OR “wearable sensor” OR “smartphone” OR “activity tracker”). In addition, cross-referencing was applied to all included papers, in order to identify additional relevant studies. The literature search was conducted by F.S.
2.2. Study Selection and Quality Assessment
After completion of the preliminary electronic database search, one rater (F.S.) screened titles and abstracts and evaluated the suitability of each paper for inclusion in the present review. Papers were excluded if they: (i) Did not use a wearable inertial sensor during the 6MWT; (ii) were an abstract and/or were conference proceedings; (iii) were a review article or a case study; iv) were a study protocol; (v) were not written in English; (vi) were not ranked on Thomson Reuters; or (vii) were published before 2010. In addition, papers were excluded from further quality assessment if they were out of topic with respect to the aims of the present review i.e., the study of advanced gait parameters using wearable inertial sensors during the 6MWT. For example, studies were excluded if they focused on energy expenditure and physical activity intensity or used wearable sensors only to extract basic information such as the number of steps walked during the 6MWT.
Full-text publications that met the inclusion criteria were downloaded into Mendeley Desktop 1.19.4. Then, a quality assessment was performed for each article. The quality assessment checklist was based on similar published checklists used for systematical and/or meta-analysis reviews [29,30,31] and modified according to the specific needs and the topic of the present review. Items assessing internal (1, 3, 4, 6, 7, 9, 12, 13, and 14), external (2, 3, 5, 6, 8, 10, and 11) and statistical (15, 16, 17, and 18) validity were included [32,33] and are reported in Table 1. Each item of the checklist was given a score of 1, 0.5 or 0 corresponding to “met”, “partially met” or “not met”, respectively. The total score was then computed as the sum of scores of all the items in the checklist. Two authors assessed the quality of the included papers. The papers scoring above 10/15 corresponded to a “high quality” publication, those scoring between 9.5/15 and 5/15 were regarded as “medium quality” publications and those scoring below 5/15 were considered “poor quality” articles [34]. At the end of the quality assessment, Cohen’s kappa statistic [35] was used to compute the reliability of the agreement between the two raters, and the average of the scores was taken for the final quality assessment outcome. This statistic is used to measure inter-rater reliability for categorical items [36]: values ≤ 0 indicate no agreement, 0.01–0.20 none to slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1.00 almost perfect agreement.

Table 1.
Quality assessment checklist of internal, external and statistical validity (IV, EV and SV).
2.3. Data Extraction
The following information was collected from each paper: (i) aim, (ii) study design, (iii) subjects enrolled (number of patients and pathological condition, if any, age, male/female ratio), (iv) sensor characteristics (type, number, sampling frequency, range), (v) sensor body placement, (vi) length of the 6MWT walkway, (vii) calibration and filtering of raw data, (viii) parameters extracted, and (ix) synthetic conclusions.
3. Results
A flowchart of the systematic review process is reported in Figure 1. A total of 273 articles were included in the initial screening. A total of 232 articles were excluded after applying the exclusion criteria because they: (i) did not use a wearable inertial sensor during the 6MWT (168 papers); (ii) were an abstract and/or were conference proceedings (15 papers); (iii) were a review article or a case study (12 papers); (iv) were a study protocol (10 papers); (v) were not written in English (3 papers); (vi) were not ranked on Thomson Reuters (4 papers); or (vii) were published before 2010 (20 papers). In addition, 15 papers were excluded because they were out-of-topic, and two papers were included after cross-referencing. The remaining 28 articles were reviewed in full-text form and were all included in the systematic review after quality assessment.
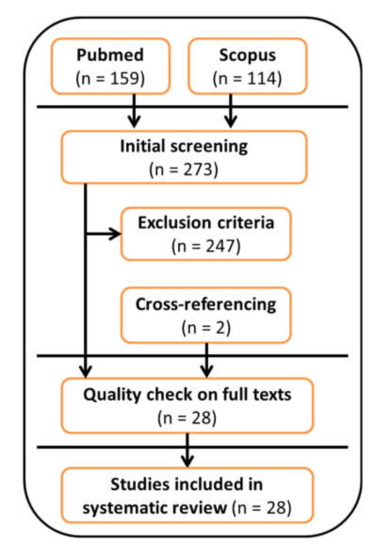
Figure 1.
Flowchart of the systematic review process.
3.1. Quality Assessment
Internal, external and statistical validity of the 28 included papers were evaluated by two authors (F.S and E.B.). The summary of the quality assessment process is shown in Table 2. Overall, 20 papers were classified as “high” quality (71.4%) and 8 papers were classified as “medium” quality (28.6%). Detailed results are provided in the Supplementary Materials (Table S1). The inter-rater agreement, computed by means of the Cohen’s kappa, was equal to 0.69, suggesting a substantial agreement between raters. A detailed summary of the papers included in this review is reported in Table 2.

Table 2.
Summary of the main characteristics of the articles included in the review.
3.2. Aims and Study Design
The aims of the studies reflected the predominant exploratory and preliminary nature of most articles. Based on the information reported in the reviewed articles, most of the papers were pilot (n = 12) or validation studies (n = 12). In addition, there were 2 feasibility studies and 2 cohort studies.
3.3. Population Characteristics
Populations with a variety of disease characteristics were investigated in the reviewed papers, the following being the most represented patient populations: people with multiple sclerosis (MS, 5 papers), chronic obstructive pulmonary disease (COPD, 5 papers), symptomatic lumbar spinal stenosis (sLSS, 2 papers) and cancer (2 papers). Healthy elderly and young healthy individuals were reported as studied populations in 2 papers each. Of the 28 papers, 12 (43%) included a healthy control group. Further details are reported in Table 3.

Table 3.
Summary of the population types reported in the included papers.
3.4. Sensor Characteristics
The authors of the papers included in the review used a variety of sensor types and supports to collect data, including research-grade MIMUs, triaxial accelerometers or gyroscopes, consumer-based sensors and smartphones with embedded sensors. All of the 28 included papers collected signals from at least one triaxial accelerometer. In general, 14 papers (50%) processed data collected through triaxial accelerometers only, 8 papers (28.6%) also collected signals from triaxial rate gyroscopes, and 6 papers (21.4%) used full MIMU data. Mostly, sensors were embedded in commercial research-grade sensors (19 papers, 67.9%). Some authors used sensors embedded in commercial smartphones or similar devices (7 papers, 25.0%) or used in-house manufactured devices (2 papers, 7.1%). The sampling frequencies (SF) at which the sensors collected data varied widely across papers. The most recurring SF was 60 Hz (6 papers, 21.4%), followed by 128 Hz and 100 Hz (3 papers each, 10.7%). SFs of 400 Hz and 50 Hz were reported by two papers each (7.1%). SFs of 1000 Hz, 200 Hz, 32 Hz, 30 Hz and 10 Hz were reported by one paper each (3.6%). Finally, 3 papers (10.7%) reported more than one SF, and 4 did not report any (14.3%). The majority of the papers did not report any sensor range (19 papers, 67.9%). The accelerometer sensor range was reported in 9/28 papers, the rate gyroscope range was reported in 5/14 papers, while the magnetometer range was reported in 1/6 studies.
3.5. Sensor Number and Locations
The relative majority of the papers (13/28, 46.4%) reported the use of a single sensor. Of the remaining 15 papers, 2 papers (7.1%) reported the use of 2 sensors, 4 papers (14.3%) reported 3 sensors, 2 papers (7.1%) reported 4 sensors, 1 paper (3.6%) reported 5 sensors, 2 papers (7.1%) reported 6 sensors, 3 papers reported 7 sensors (10.7%) and 1 paper (3.6%) reported the use of 17 sensors. Overall, the majority of the studies (64.3%, 18/28 papers) reported placing at least one wearable sensor on the lower back, with the preferred locations being at the level of the lower back (L3, L5 and the sacrum). Shanks and ankles were also frequent locations chosen by researchers, with 8 (28.6%) and 7 (25.0%) papers, respectively. Further details on the placement of the wearable sensors used in the included articles are summarized in Figure 2.
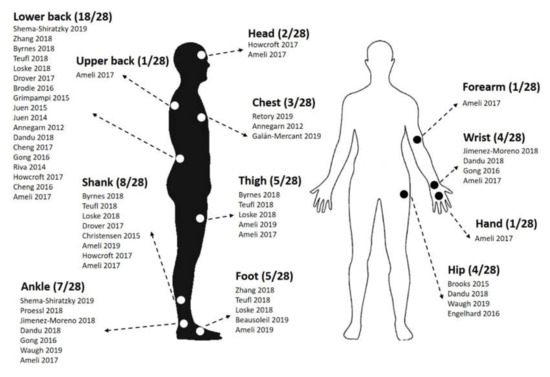
Figure 2.
Sensor placement reported in the reviewed papers.
3.6. 6MWT Characteristics
All 6MWT were performed indoors along a flat straight corridor with 180° turns, apart for one study that used a rectangular set of corridors [39]. Most studies used a 30 m long path (11 papers, 39.3%), 8 articles did not report the length of the path (28.6%), 3 papers reported 15–16 m (14.3%), 3 papers (10.7%) reported 23 m (75 feet), 2 papers reported a variable number of path lengths, and one paper reported a length of 25 m.
3.7. Parameters Extracted during the 6MWT
The assessed papers reported a wide variety of outcome parameters obtained from MIMU data. Pre-processing of the signals involved a number of different techniques. Calibration procedures were reported by only 6 papers [41,45,49,56,58,59] and raw-data filtering was reported by 10 papers. The majority used lowpass Butterworth filters, one paper used a band-pass filter and one paper explicitly processed raw data without filtering (see Table 2 for details). Data processing was performed mostly using Matlab (10 papers, 35.7%, [38,41,44,45,46,51,58,59,62,63]). Eight papers did not report the software used (28.6%, [37,39,40,48,53,56,60,64]), 4 papers used custom software (14.3%, [47,49,50,57]). The remaining 6 papers (21.4%, [42,43,52,54,55,61]) reported the use of other software.
Spatio-temporal parameters were reported by the majority of the papers (16, 57.1%), acceleration descriptive statistics were reported by 10 papers (35.7%), 5 papers reported frequency and kinematics measures (17.9%), 3 papers reported stability indexes or other type of measures (10.7%), gait events were reported by only one study. A summary of the outcome parameters with a brief description are shown in Table 4.

Table 4.
Summary and definition of the main outcome parameters of the reviewed papers.
4. Discussion
This work demonstrated that a significant number of papers (high quality = 20) have been published assessing gait using wearable inertial sensors during the 6MWT. We obtained substantial inter-rater agreement for the assessment of the quality of the papers analyzed (Cohen’s kappa = 0.69) [35]. When performing the quality assessment, the authors tuned their scores according to the available literature. For example, small sample sizes of the reviewed papers reflecting their piloting nature were not scored low a priori, but only if the conclusions of the papers were not justified or supported by the number of subjects recruited.
Technical advances in MIMU research are allowing this technology to become a valid alternative to classic laboratory-based and clinical assessments. While many methods and metrics still need to be validated, in the near future it will be possible to extend quantitative measures outside the classic clinical environment, even including free-living data to inform diagnosis and treatment of diseases [65,66].
In the authors’ opinion, the benefits of using MIMUs and wearable sensors in general during the 6MWT are evident: a thorough and comprehensive assessment of gait can be performed without adding further burden to the patient. They provide an alternative to traditional gait analysis and postural control assessments which require expensive equipment, are time consuming and can provide detailed information only for a very limited number of consecutive gait cycles [67]. Considering the hundreds of steps taken during the 6MWT, the information that can be extracted from this clinical test appears to be very valuable. Further information could be gathered from measures obtained during turning, which have shown to be more sensitive to detect impaired mobility than gait speed or clinical measures [68].
Of the reviewed articles, the majority of the pilot studies aimed at comparing gait features between a clinical population and a healthy control group. The preliminary outcomes gathered from these studies will serve to inform larger scale studies. Validation studies aimed generally at addressing accuracy, reliability and robustness of outcome measures against a clinical scale or a validated gold standard. The most represented clinical population were patients with MS, where wearable inertial sensors were used to inform on additional aspects of gait, especially walking-related fatigue. The reviewed papers showed that gait fatigability measured using both traditional and novel outcome parameters was significantly correlated with physical fatigue, gait disability, and fall history [37,56,62]. In patients with chronic obstructive pulmonary disease, most studies aimed at validating MIMU-based outcomes to predict pulmonary function and transitions in oxygen saturation [57,64]. Research on healthy elderly subjects mostly focused on the prospective fall-risk prediction and classification of fallers and non-fallers [44,63]. MIMUs were also useful in evaluating postoperative improvements in patients with symptomatic lumbar spinal stenosis and people recovering from total knee arthroplasty [43,48]. Healthy individuals were mostly included in fundamental research studies exploring newly proposed metrics or for validation studies. These results confirm the exploratory nature of most studies using MIMUs during the 6MWT, and the variety of outcomes and approaches that this technology allows. Some of the reviewed studies also used “big data” and machine learning approaches for disease classification [57] and fall risk prediction [63]. Our review confirms, as concluded by a recent paper [26], that machine learning in the field of gait analysis using inertial sensors is still in its preliminary steps. Although only a limited amount of studies used these techniques, we believe that this approach has a great potential to support the clinics that is still unexplored. Surprisingly, none of the reviewed studies included a pediatric cohort. Studies on healthy and pathologic young populations are therefore needed to evaluate the feasibility and to validate this approach also in the developmental age.
Not surprisingly, accelerometers were the most common source of raw data, due to their low power consumption and widespread use in comparison to rate gyroscopes and magnetometers [14]. Researchers used a variety of commercial sensors. Research-grade sensors were the most frequent choice in hospital settings, while sensors embedded in smartphones were favored when data were collected at home or the aim of the study was to validate these methodologies for future at-home use. Sampling frequencies varied between 10 Hz and 1000 Hz. This large spread may be partially justified by the variety of outcome measures proposed in the reviewed articles. For example, studies on physiological tremors and impacts may require sensing accelerations at up to 25–60 Hz [69,70]. However, it is worth noting that although accelerations at the foot that occur during initial contact can reach up to 60 Hz [71], 99% of the acceleration power during walking is concentrated below 15 Hz [72], suggesting that collecting data at very high sampling frequencies may be unnecessary. Inertial sensing measurement range is generally not critical for gait measurement applications, since some accelerometers may reach a range of up to 100 g and rate gyroscopes reach ranges of up to 2000°/s. However, saturation may occur when the full scale is set to ±2 g [73]. Most studies did not report this information in the methodology sections. As already highlighted by a previous systematic review focusing on balance assessment using wearable sensors [29], there was also a general lack of information related to sensor calibration in the papers reviewed in the present work, with only six reporting a calibration procedure, and only one acknowledging the lack of calibration as a potential limit of the work [39]. Most sensor axes were manually aligned to the anteroposterior, mediolateral and vertical axes without additional procedures for the minimization of sensor misalignment. A few static and dynamic calibration techniques to reduce the risk of cross-talk have been proposed in the literature [20,74]. In fact, one of the reviewed articles proposes a novel approach to improve the quality of accelerometer data during a 6MWT [45]. Information on pre-processing of raw data was also generally scarce, with only a minority of papers reporting the use of digital filters. Among them, there was a general uniformity in using low-pass Butterworth filters, with cut-off frequencies ranging between 2.5 and 40 Hz. The high variability of cut-off frequencies is justified by the variety of parameters extracted by the authors of the papers, and the locations of the sensors on the human body. It has been shown that low cut-off frequencies attenuate inertial sensor signals, while less restrictive filtering may provide more movement-related signals, but with the risk of higher noise [75]. Future studies should include more technical details regarding pre-processing of raw data used to calculate gait metrics, justifying their choices in light of existing literature. The research protocols of the reviewed articles included data collected from sensors positioned at 12 different body locations. Waist-placement was often preferred for single sensor configurations, because the sensor is positioned close to the center of mass of the human body, and hence thought to better represent human motions [76]. While the reviewed articles mostly focused on lower limbs (thighs, shanks, ankles and feet), there is also increasing awareness that upper body movements play a crucial role during locomotion and may be impaired for people with a variety of pathologies [77]. Future research should also focus on upper body variables, proposed as potential markers of disease progression [78], and on investigating the correlation between sensor location and outcome parameters.
Overall, the reviewed articles followed the technical guidelines for the 6MWT [2]. Regarding the 6MWT path length, a multicenter study found no significant effect of the length of straight courses ranging from 15 to 50 m, but patients walked further on continuous tracks [79]. While all papers reporting the path length were within this range, for a number of papers the length was not mentioned. For this reason, the authors believe that studies reporting 6MWT evaluation should carefully describe details about test administration.
The most frequently used outcome measures were those in the spatio-temporal domain, especially cadence. This is not surprising since a large amount of literature concerning the development of algorithms for step detection and step counting has been published in the last decades [80,81,82,83]. Other common spatio-temporal measures were variability and symmetry metrics, both being associated to gait impairment and balance disorders. Quantification of specific gait phases was also performed. The correct discrimination of gait phases can be important to distinguish between normal and pathologic gait, and for the evaluation of recovery after interventions or rehabilitation [18].
In the reviewed articles, the first quartile of Fourier transform and the ratio of even/odd harmonics were the most frequent outcome measures in the frequency domain. Both metrics have been linked to instability and higher fall risk [84,85]. The use of frequency domain measures obtained from accelerometer signals is increasing especially in the field of balance assessments. These metrics may provide better insights into balance also during gait, particularly for neurological disorders such as ataxia and Parkinson’s Disease [86].
Among acceleration descriptive statistics, root mean square (RMS) was one of the most reported outcome measures. RMS is a statistical measure of the magnitude of acceleration, it is simple to compute and of clear clinical meaning; however, its high correlation with walking speed needs to be taken into account when experiments are performed at self-selected pace. To overcome this limit, Sekine and colleagues recently proposed the RMS ratio, which represents the ratio between RMS in each direction and the RMS vector magnitude [87].
Non-linear analysis of inertial sensor data has also been proposed in recent years. Local dynamic stability of the gait pattern measured by means of accelerometers was assessed by some of the reviewed articles using short-term Lyapunov exponents [60,63], which quantify stride-to-stride local stability, taking also into account how the locomotor control system responds to perturbations [88]. The reviewed studies showed that these outcome measures could be used during the 6MWT in a clinical setting to obtain valuable information, especially during the turning phases of the test.
The most common kinematic indexes reported in the reviewed articles were angles computed at the lower limb joints. These metrics were generally compared against a gold standard, such as optical motion capture system [41,45]. Measurement of 3D joint angles using MIMUs is still a developing field and often lacks reliability and validity. However, a full kinematic analysis during the 6MWT using wearable MIMUs has already been attempted and proved to be technically feasible [24,89]; this kind of analysis would add significant value to the assessment.
Other measures have been proposed that do not fit into the previous categories, for example Dandu and colleagues propose dynamic time warping (DTW) and warp scores, outcome measures specifically designed and fitted to the 6MWT to quantify progressive changes to walking patterns [56]. Byrnes and colleagues describe outcome measures starting from the definition of an attractor, which represents the mean cycle of all strides and may reflect specific differences in pathologic gait [40].
Clinical Implications
Gait has been acknowledged as a biomarker in many pathological conditions and the variety of gait parameters provided by inertial sensors can help clinicians to enhance assessments of interventions, disease progression and rehabilitation programs.
This review showed that wearable sensors are a technical solution that can be adopted in clinical settings to complement traditional assessments of gait deficits. The 6MWT is a well-suited test for this purpose because of its relatively long duration. Reporting additional insights contributes to inform clinical decisions and benefits the health care system by increasing test efficiency.
For example, parameters obtained using the methods reviewed in the present study were sensitive to disability in post-stroke patients [39], were suggested as outcome parameters for surgical procedures in lumbar spinal stenosis [40], and were useful in assessing the evolution of lower limb amputees during rehabilitation [52]. This technology also provided objective markers of gait fatigability in patients with multiple sclerosis [37,62]. Deterioration of gait features during the 6MWT using MIMUs appears to be a particularly promising direction of research for many neurological disorders where motor fatigue is an important symptom.
None of the reviewed papers included a pediatric population. This may be surprising because the 6MWT is a common functional outcome measure for chronic pediatric conditions [90]. However, it is likely that majority of the research involves adult populations and this is reflected proportionally in the papers included in the present review. Future studies may investigate how analytics from MIMUs during this standard test may improve our understanding of disability and disease progression in neurodegenerative disorders such as ataxia and muscular dystrophies. This technology also paves the way for remote monitoring in more ecologically valid environments.
5. Conclusions
After a systematic literature search and a quality assessment, we reviewed the current knowledge on the assessment of gait during the 6MWT using wearable MIMUs to extract advanced gait parameters, highlighting the main objectives of the studies, describing the preferred sensors and body location, and the outcome measures used in the studies. The results suggest that MIMUs could be successfully used to obtain additional information of clinical significance and increase the meaningfulness of the 6MWT evaluation. Future works should focus on extending the clinical populations studied, with particular attention to the pediatric population, on the validation of additional outcome measures and on extending the use of machine learning approaches.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-8220/20/9/2660/s1, Table S1: Detailed results of the quality assessment conducted by each rater on the articles included in the systematic review.
Author Contributions
Conceptualization, F.A.S., A.C.; G.R. and E.B.; methodology, F.A.S.; formal analysis, F.A.S.; data curation, F.A.S., A.C. and E.B.; writing—original draft preparation, F.A.S.; writing—review and editing, F.A.S., A.C., G.R. and E.B.; supervision, E.B.; funding acquisition, G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Italian Ministry of Health (Ricerca Corrente 2019 to Eng. G. Reni, Ricerca Corrente 2020 to E. Biffi). The funding body did not have any role in the collection, analysis, and interpretation of data nor in the writing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European respiratory society/American thoracic society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Crapo, R.O.; Casaburi, R.; Coates, A.L.; Enright, P.L.; MacIntyre, N.R.; McKay, R.T.; Johnson, D.; Wanger, J.S.; Zeballos, R.J.; Bittner, V.; et al. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar]
- Enright, P.L.; McBurnie, M.A.; Bittner, V.; Tracy, R.P.; McNamara, R.; Arnold, A.; Newman, A.B. The 6-min walk test: A quick measure of functional status in elderly adults. Chest 2003, 123, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Kotsia, A.; Michalis, L.K.; Naka, K.K. 6-min walking test: A useful tool in the management of heart failure patients. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719870084. [Google Scholar] [CrossRef]
- Karanth, M.P.S.; Awad, N.T. Six minute walk test: A tool for predicting mortality in chronic pulmonary diseases. J. Clin. Diagn. Res. 2017, 11, OC34. [Google Scholar] [CrossRef]
- Du Bois, R.M.; Albera, C.; Bradford, W.Z.; Costabel, U.; Leff, J.A.; Noble, P.W.; Sahn, S.A.; Valeyre, D.; Weycker, D.; King, T.E. 6-min walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2014, 43, 1421–1429. [Google Scholar] [CrossRef]
- Beretta, E.; Storm, F.A.; Strazzer, S.; Frascarelli, F.; Petrarca, M.; Colazza, A.; Cordone, G.; Biffi, E.; Morganti, R.; Maghini, C.; et al. Effect of robotic-assisted gait training in a large population of children with motor impairment due to cerebral palsy or acquired brain injury. Arch. Phys. Med. Rehabil. 2020, 101, 112. [Google Scholar] [CrossRef]
- Beretta, E.; Molteni, E.; Biffi, E.; Morganti, R.; Avantaggiato, P.; Strazzer, S. Robotically-driven orthoses exert proximal-to-distal differential recovery on the lower limbs in children with hemiplegia, early after acquired brain injury. Eur. J. Paediatr. Neurol. 2018, 22, 652–661. [Google Scholar] [CrossRef]
- Alhamad, E.H.; Shaik, S.A.; Idrees, M.M.; Alanezi, M.O.; Isnani, A.C. Outcome measures of the 6 min walk test: Relationships with physiologic and computed tomography findings in patients with sarcoidosis. BMC Pulm. Med. 2010, 10, 42. [Google Scholar] [CrossRef]
- Borg, G.A.V. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Okuro, R.T.; de Oliveira Ribeiro, M.A.G.; Ribeiro, J.D.; Minsky, R.C.; Schivinski, C.I.S. Alternative indexes to estimate the functional capacity from the 6-min walk test in children and adolescents with Cystic Fibrosis. Respir. Care 2017, 62, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Ben Mansour, K.; Rezzoug, N.; Gorce, P. Analysis of several methods and inertial sensors locations to assess gait parameters in able-bodied subjects. Gait Posture 2015, 42, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Godinho, C.; Domingos, J.; Cunha, G.; Santos, A.T.; Fernandes, R.M.; Abreu, D.; Gonçalves, N.; Matthews, H.; Isaacs, T.; Duffen, J.; et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson’s disease. J. Neuroeng. Rehabil. 2016, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, J.J.; Menz, H.B. Accelerometry: A technique for quantifying movement patterns during walking. Gait Posture 2008, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Herrera-May, A.L.; Aguilera-Cortés, L.A.; García-Ramírez, P.J.; Manjarrez, E. Resonant magnetic field sensors based on MEMS technology. Sensors 2009, 9, 7785–7813. [Google Scholar] [CrossRef] [PubMed]
- Shull, P.B.; Jirattigalachote, W.; Hunt, M.A.; Cutkosky, M.R.; Delp, S.L. Quantified self and human movement: A review on the clinical impact of wearable sensing and feedback for gait analysis and intervention. Gait Posture 2014, 40, 11–19. [Google Scholar] [CrossRef]
- Sabatini, A.M. Quaternion-based extended Kalman filter for determining orientation by inertial and magnetic sensing. IEEE Trans. Biomed. Eng. 2006, 53, 1346–1356. [Google Scholar] [CrossRef]
- Taborri, J.; Palermo, E.; Rossi, S.; Cappa, P. Gait Partitioning Methods: A Systematic Review. Sensors 2016, 16, 66. [Google Scholar] [CrossRef]
- Zijlstra, W.; Hof, A. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture 2003, 18, 1–10. [Google Scholar] [CrossRef]
- Moe-Nilssen, R. A new method for evaluating motor control in gait under real-life environmental conditions. Part 1: The Instrument. Clin. Biomech. 1998, 13, 328–335. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Peng, C.K.; Ladin, Z.; Wei, J.Y.; Goldberger, A.L. Is walking a random walk? Evidence for long-range correlations in stride interval of human gait. J. Appl. Physiol. 1995, 78, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M. Gait dynamics, fractals and falls: Finding meaning in the stride-to-stride fluctuations of human walking. Hum. Mov. Sci. 2007, 26, 555–589. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.G.; Dingwell, J.B. Effects of walking speed, strength and range of motion on gait stability in healthy older adults. J. Biomech. 2008, 41, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Picerno, P. 25 years of lower limb joint kinematics by using inertial and magnetic sensors: A review of methodological approaches. Gait Posture 2017, 51, 239–246. [Google Scholar] [CrossRef]
- Liu, T.; Inoue, Y.; Shibata, K. Development of a wearable sensor system for quantitative gait analysis. Meas. J. Int. Meas. Confed. 2009, 42, 978–988. [Google Scholar] [CrossRef]
- Caldas, R.; Mundt, M.; Potthast, W.; Buarque de Lima Neto, F.; Markert, B. A systematic review of gait analysis methods based on inertial sensors and adaptive algorithms. Gait Posture 2017, 57, 204–210. [Google Scholar] [CrossRef]
- Demchenko, Y.; Grosso, P.; De Laat, C.; Membrey, P. Addressing big data issues in Scientific Data Infrastructure. In Proceedings of the International Conference on Collaboration Technologies and Systems, CTS 2013, San Diego, CA, USA, 20–24 May 2013. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Ghislieri, M.; Gastaldi, L.; Pastorelli, S.; Tadano, S.; Agostini, V. Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review. Sensors 2019, 19, 4075. [Google Scholar] [CrossRef]
- Taborri, J.; Agostini, V.; Artemiadis, P.K.; Ghislieri, M.; Jacobs, D.A.; Roh, J.; Rossi, S. Feasibility of Muscle Synergy Outcomes in Clinics, Robotics, and Sports: A Systematic Review. Appl. Bionics Biomech. 2018. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, Obesity, and Depression. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Cooper, H.M. Research Synthesis and Meta-Analysis: A Step-by-Step Approach; SAGE Publications: London, UK, 2016. [Google Scholar]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, T.; Van Der Windt, D.A.W.M.; Van Der Heijden, G.J.M.G.; Bouter, L.M. Systematic review of prognostic cohort studies on shoulder disorders. Pain 2004, 109, 420–431. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Earlbam Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Shema-Shiratzky, S.; Gazit, E.; Sun, R.; Regev, K.; Karni, A.; Sosnoff, J.J.; Herman, T.; Mirelman, A.; Hausdorff, J.M. Deterioration of specific aspects of gait during the instrumented 6-min walk test among people with multiple sclerosis. J. Neurol. 2019, 266, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Retory, Y.; David, P.; Niedzialkowski, P.; de Picciotto, C.; Bonay, M.; Petitjean, M. Gait Monitoring and Walk Distance Estimation with an Accelerometer During 6-Minute Walk Test. Respir. Care 2019, 64, 923–930. [Google Scholar] [CrossRef]
- Zhang, W.; Smuck, M.; Legault, C.; Ith, M.A.; Muaremi, A.; Aminian, K. Gait symmetry assessment with a low back 3d accelerometer in post-stroke patients. Sensors 2018, 18, 3322. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, S.K.; Nüesch, C.; Loske, S.; Leuenberger, A.; Schären, S.; Netzer, C.; Mündermann, A. Inertial sensor-based gait and attractor analysis as clinical measurement tool: Functionality and sensitivity in healthy subjects and patients with symptomatic lumbar spinal stenosis. Front. Physiol. 2018, 9, 1095. [Google Scholar] [CrossRef]
- Teufl, W.; Miezal, M.; Taetz, B.; Fröhlich, M.; Bleser, G. Validity, test-retest reliability and long-term stability of magnetometer free inertial sensor based 3D joint kinematics. Sensors 2018, 18, 1980. [Google Scholar] [CrossRef]
- Proessl, F.; Swanson, C.W.; Rudroff, T.; Fling, B.W.; Tracy, B.L. Good agreement between smart device and inertial sensor-based gait parameters during a 6-min walk. Gait Posture 2018, 64, 63–67. [Google Scholar] [CrossRef]
- Loske, S.; Nüesch, C.; Byrnes, K.S.; Fiebig, O.; Schären, S.; Mündermann, A.; Netzer, C. Decompression surgery improves gait quality in patients with symptomatic lumbar spinal stenosis. Spine J. 2018, 18, 2195–2204. [Google Scholar] [CrossRef]
- Drover, D.; Howcroft, J.; Kofman, J.; Lemaire, E.D. Faller classification in older adults using wearable sensors based on turn and straight-walking accelerometer-based features. Sensors 2017, 17, 1321. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.A.D.; Psarakis, M.; Hoang, P. Gyroscopic corrections improve wearable sensor data prior to measuring dynamic sway in the gait of people with Multiple Sclerosis. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Grimpampi, E.; Oesen, S.; Halper, B.; Hofmann, M.; Wessner, B.; Mazzà, C. Reliability of gait variability assessment in older individuals during a six-minute walk test. J. Biomech. 2015, 48, 4185–4189. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.C.; Vittinghoff, E.; Iyer, S.; Tandon, D.; Kuhar, P.; Madsen, K.A.; Marcus, G.M.; Pletcher, M.J.; Olgin, J.E. Accuracy and Usability of a Self-Administered Six-Minute Walk Test Smartphone Application. Circ. Heart Fail. 2015, 8, 905–913. [Google Scholar] [CrossRef]
- Christiansen, C.L.; Bade, M.J.; Paxton, R.J.; Stevens-Lapsley, J.E. Measuring movement symmetry using tibial-mounted accelerometers for people recovering from total knee arthroplasty. Clin. Biomech. 2015, 30, 732–737. [Google Scholar] [CrossRef]
- Juen, J.; Cheng, Q.; Schatz, B. A Natural Walking Monitor for Pulmonary Patients Using Mobile Phones. IEEE J. Biomed. Health Inform. 2015, 19, 1399–1405. [Google Scholar] [CrossRef]
- Juen, J.; Cheng, Q.; Prieto-Centurion, V.; Krishnan, J.A.; Schatz, B. Health monitors for chronic disease by gait analysis with mobile phones. Telemed. J. e-Health 2014, 20, 1035–1041. [Google Scholar] [CrossRef]
- Annegarn, J.; Spruit, M.A.; Savelberg, H.H.C.M.; Willems, P.J.B.; van de Bool, C.; Schols, A.M.W.J.; Wouters, E.F.M.; Meijer, K. Differences in walking pattern during 6-min walk test between patients with COPD and healthy subjects. PLoS ONE 2012, 7, e37329. [Google Scholar] [CrossRef]
- Beausoleil, S.; Miramand, L.; Turcot, K. Evolution of gait parameters in individuals with a lower-limb amputation during a six-minute walk test. Gait Posture 2019, 72, 40–45. [Google Scholar] [CrossRef]
- Galán-Mercant, A.; Ortiz, A.; Herrera-Viedma, E.; Tomas, M.T.; Fernandes, B.; Moral-Munoz, J.A. Assessing physical activity and functional fitness level using convolutional neural networks. Knowl.-Based Syst. 2019, 185, 104939. [Google Scholar] [CrossRef]
- Ameli, S.; Naghdy, F.; Stirling, D.; Naghdy, G.; Aghmesheh, M. Chemotherapy-induced fatigue estimation using hidden Markov model. Biocybern. Biomed. Eng. 2019, 39, 176–187. [Google Scholar] [CrossRef]
- Jimenez-Moreno, A.C.; Charman, S.J.; Nikolenko, N.; Larweh, M.; Turner, C.; Gorman, G.; Lochmüller, H.; Catt, M. Analyzing walking speeds with ankle and wrist worn accelerometers in a cohort with myotonic dystrophy. Disabil. Rehabil. 2018, 41, 2972–2978. [Google Scholar] [CrossRef] [PubMed]
- Dandu, S.R.; Engelhard, M.M.; Qureshi, A.; Gong, J.; Lach, J.C.; Brandt-Pearce, M.; Goldman, M.D. Understanding the physiological significance of four inertial gait features in multiple sclerosis. IEEE J. Biomed. Health Inform. 2018, 22, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Juen, J.; Bellam, S.; Fulara, N.; Close, D.; Silverstein, J.C.; Schatz, B. Predicting Pulmonary Function from Phone Sensors. Telemed. e-Health 2017, 23, 913–919. [Google Scholar] [CrossRef]
- Ameli, S.; Naghdy, F.; Stirling, D.; Naghdy, G.; Aghmesheh, M. Objective clinical gait analysis using inertial sensors and six minute walking test. Pattern Recognit. 2017, 63, 246–257. [Google Scholar] [CrossRef]
- Gong, J.; Qi, Y.; Goldman, M.D.; Lach, J. Causality Analysis of Inertial Body Sensors for Multiple Sclerosis Diagnostic Enhancement. IEEE J. Biomed. Health Inform. 2016, 20, 1273–1280. [Google Scholar] [CrossRef]
- Riva, F.; Grimpampi, E.; Mazzà, C.; Stagni, R. Are gait variability and stability measures influenced by directional changes? Biomed. Eng. Online 2014, 13, 56. [Google Scholar] [CrossRef]
- Waugh, J.L.S.; Huang, E.; Fraser, J.E.; Beyer, K.B.; Trinh, A.; McIlroy, W.E.; Kulić, D. Online Learning of Gait Models from Older Adult Data. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 733–742. [Google Scholar] [CrossRef]
- Engelhard, M.M.; Dandu, S.R.; Patek, S.D.; Lach, J.C.; Goldman, M.D. Quantifying Six-Minute Walk Induced Gait Deterioration with Inertial Sensors in Multiple Sclerosis Subjects. Gait Posture 2016, 49, 340–345. [Google Scholar] [CrossRef]
- Howcroft, J.; Kofman, J.; Lemaire, E.D. Prospective Fall-Risk Prediction Models for Older Adults Based on Wearable Sensors. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1812–1820. [Google Scholar] [CrossRef]
- Cheng, Q.; Juen, J.; Hsu-Lumetta, J.; Schatz, B. Predicting Transitions in Oxygen Saturation Using Phone Sensors. Telemed. e-Health 2016, 22, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, P.; Storm, F.; Buckley, C.; Bisi, M.C.; Stagni, R.; Mazzà, C. Moving from laboratory to real life conditions: Influence on the assessment of variability and stability of gait. Gait Posture 2018, 59, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Storm, F.A.; Nair, K.P.S.; Clarke, A.J.; Van der Meulen, J.M.; Mazzà, C. Free-living and laboratory gait characteristics in patients with multiple sclerosis. PLoS ONE 2018, 13, e0196463. [Google Scholar] [CrossRef]
- Muro-de-la-Herran, A.; García-Zapirain, B.; Méndez-Zorrilla, A. Gait analysis methods: An overview of wearable and non-wearable systems, highlighting clinical applications. Sensors 2014, 14, 3362–3394. [Google Scholar] [CrossRef]
- El-Gohary, M.; Pearson, S.; McNames, J.; Mancini, M.; Horak, F.; Mellone, S.; Chiari, L. Continuous monitoring of turning in patients with movement disability. Sensors 2014, 14, 356–369. [Google Scholar] [CrossRef]
- Mizrahi, J.; Verbitsky, O.; Isakov, E. Shock accelerations and attenuation in downhill and level running. Clin. Biomech. 2000, 15, 15–20. [Google Scholar] [CrossRef]
- Morrison, S.; Newell, K.M. Bilateral organization of physiological tremor in the upper limb. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Cappozzo, A. Low frequency self-generated vibration during ambulation in normal men. J. Biomech. 1982, 15, 599–609. [Google Scholar] [CrossRef]
- Antonsson, E.K.; Mann, R.W. The frequency content of gait. J. Biomech. 1985, 18, 39–47. [Google Scholar] [CrossRef]
- Mitschke, C.; Kiesewetter, P.; Milani, T.L. The effect of the accelerometer operating range on biomechanical parameters: Stride length, velocity, and peak tibial acceleration during running. Sensors 2018, 18, 130. [Google Scholar] [CrossRef]
- Brodie, M.; Walmsley, A.; Page, W. The static accuracy and calibration of inertial measurement units for 3D orientation. Comput. Methods Biomech. Biomed. Eng. 2008, 11, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, D.; Fridolfsson, J.; Börjesson, M.; Andersen, L.B.; Ekblom, Ö.; Dencker, M.; Brønd, J.C. Re-examination of accelerometer data processing and calibration for the assessment of physical activity intensity. Scand. J. Med. Sci. Sports 2019, 29, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Hsu, Y.-L. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors 2010, 10, 7772–7788. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.; Galna, B.; Rochester, L.; Mazzà, C. Quantification of upper body movements during gait in older adults and in those with Parkinson’s disease: Impact of acceleration realignment methodologies. Gait Posture 2016, 52, 265–271. [Google Scholar] [CrossRef]
- Hubble, R.P.; Naughton, G.A.; Silburn, P.A.; Cole, M.H. Wearable sensor use for assessing standing balance and walking stability in people with Parkinson’s disease: A systematic review. PLoS ONE 2015, 10, e0123705. [Google Scholar] [CrossRef]
- Sciurba, F.; Criner, G.J.; Lee, S.M.; Mohsenifar, Z.; Shade, D.; Slivka, W.; Wise, R.A. Six-Minute Walk Distance in Chronic Obstructive Pulmonary Disease: Reproducibility and Effect of Walking Course Layout and Length. Am. J. Respir. Crit. Care Med. 2003, 167, 1522–1527. [Google Scholar] [CrossRef]
- Aminian, S.; Hinckson, E.A. Examining the validity of the ActivPAL monitor in measuring posture and ambulatory movement in children. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 119. [Google Scholar] [CrossRef]
- Esliger, D.W.; Rowlands, A.V.; Hurst, T.L.; Catt, M.; Murray, P.; Eston, R.G. Validation of the GENEA accelerometer. Med. Sci. Sports Exerc. 2011, 43, 1085–1093. [Google Scholar] [CrossRef]
- Dijkstra, B.; Zijlstra, W.; Scherder, E.; Kamsma, Y. Detection of walking periods and number of steps in older adults and patients with Parkinson’s disease: Accuracy of a pedometer and an accelerometry-based method. Age Ageing 2008, 37, 436–441. [Google Scholar] [CrossRef]
- Fortune, E.; Lugade, V.; Morrow, M.; Kaufman, K. Validity of using tri-axial accelerometers to measure human movement—Part II: Step counts at a wide range of gait velocities. Med. Eng. Phys. 2014, 36, 659–669. [Google Scholar] [CrossRef]
- Doheny, E.P.; Walsh, C.; Foran, T.; Greene, B.R.; Fan, C.W.; Cunningham, C.; Kenny, R.A. Falls classification using tri-axial accelerometers during the five-times-sit-to-stand test. Gait Posture 2013, 38, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Bellanca, J.L.; Lowry, K.A.; VanSwearingen, J.M.; Brach, J.S.; Redfern, M.S. Harmonic ratios: A quantification of step to step symmetry. J. Biomech. 2013, 46, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Kusmirek, S.; Hana, K.; Socha, V.; Prucha, J.; Kutilek, P.; Svoboda, Z. Postural instability assessment using trunk acceleration frequency analysis. Eur. J. Physiother. 2016, 18, 237–244. [Google Scholar] [CrossRef]
- Sekine, M.; Tamura, T.; Yoshida, M.; Suda, Y.; Kimura, Y.; Miyoshi, H.; Kijima, Y.; Higashi, Y.; Fujimoto, T. A gait abnormality measure based on root mean square of trunk acceleration. J. Neuroeng. Rehabil. 2013, 10, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Dingwell, J.B.; Cusumano, J.P.; Cavanagh, P.R.; Sternad, D. Local Dynamic Stability Versus Kinematic Variability of Continuous Overground and Treadmill Walking. J. Biomech. Eng. 2001, 123, 27. [Google Scholar] [CrossRef]
- Sabatini, A.M. Estimating three-dimensional orientation of human body parts by inertial/magnetic sensing. Sensors 2011, 11, 1489–1525. [Google Scholar] [CrossRef]
- Bartels, B.; de Groot, J.F.; Terwee, C.B. The Six-Minute Walk Test in Chronic Pediatric Conditions: A Systematic Review of Measurement Properties. Phys. Ther. 2013, 93, 529–541. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).