Abstract
A new azo compound was prepared via the azo coupling reaction between 4-(ethoxycarbonyl)-3-methyl-1H-pyrazole-5-diazonium chloride and 8-hydroxyquinoline (oxine). The ester functional group of the obtained compound was hydrolyzed and thus a new chemical structure with a carboxylic functional group resulted. The structures of the new compounds were fully characterized by: UV–Vis, FT-IR, 1D and 2D NMR spectroscopy, and HRMS spectrometry.
1. Introduction
Azo compounds contain the chromophoric azo (diazene, according to IUPAC nomenclature) functional group (–N=N–). The substituents attached to the diazene group can be aromatic or heterocyclic. An extended conjugation occurs, thus giving the compounds the property either to absorb or to emit light [1]. Azo colorants form the biggest and most well-known group of colorants, and account for approximately 60–70% of them [2].
Pyrazole containing azo compounds have a variety of uses, which include food coloring [3], corrosion inhibition for soft-cast steel [4], polypropylene dyeing [5], Electro Fluidic Displays (EFDs) [6], antimicrobial agents [7], and colorimetric detection of metal ions [8].
8-Hydroxyquinoline and its derivatives belong to the most well-known ligands for metal complexes used for example in Organic Light Emitting Diodes (OLEDs) [9], chemosensors for metal ions, antibacterial, anti-HIV agents, and gravimetric metal analysis [10].
Given the above, we decided to synthesize azo compounds which would carry two important moieties: pyrazole and 8-hydroxyquinoline. The properties of the new compounds such as dyeing capability, maximum emission wavelength, metal chelating capability, and thermal stability are subject of further studies.
2. Results and Discussion
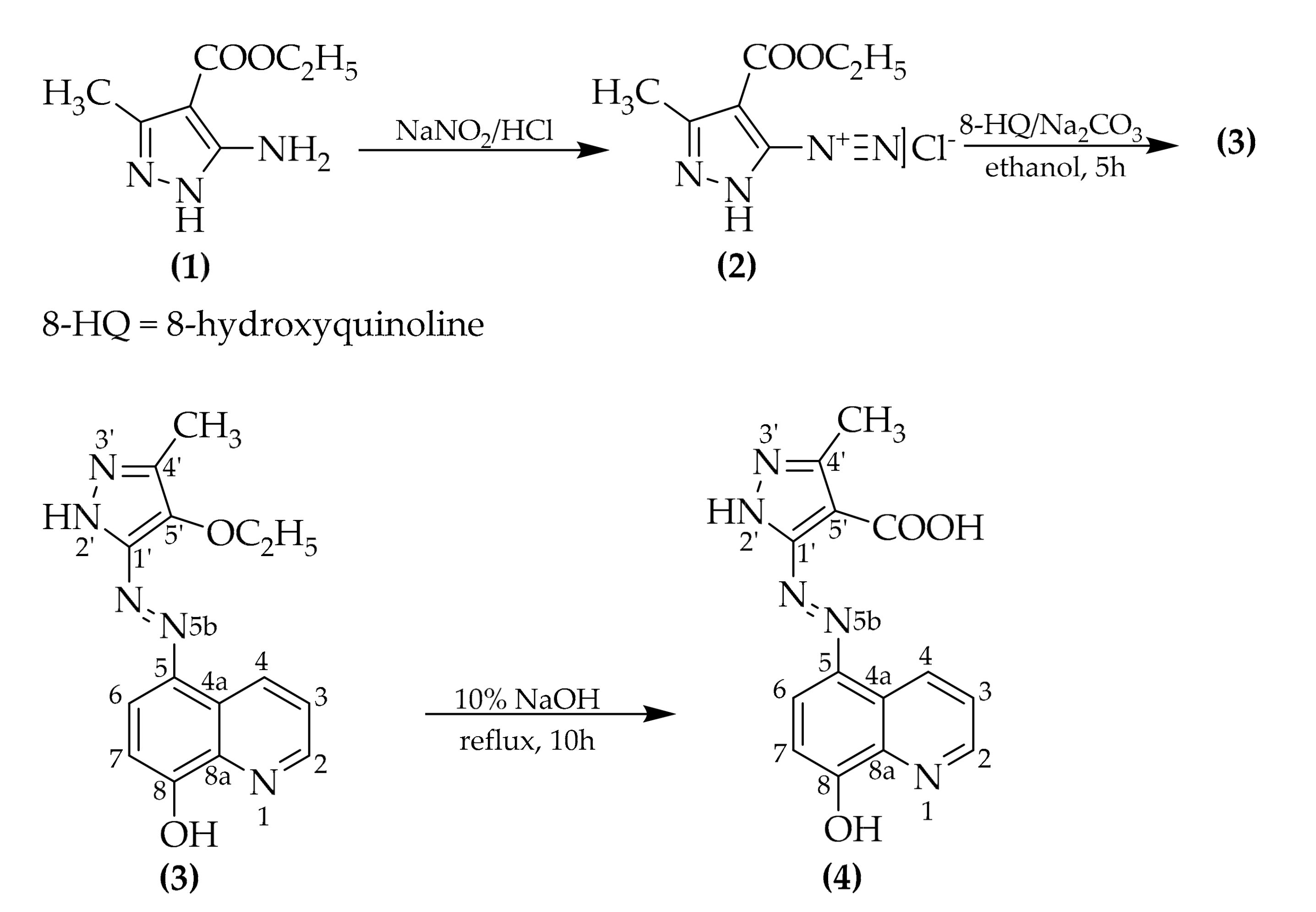
Ethyl 5-((8-hydroxyquinolin-5-yl)diazenyl)-3-methyl-1H-pyrazole-4-carboxylate was synthesized by a classic azo coupling reaction between 4-(ethoxycarbonyl)-3-methyl-1H-pyrazole-5-diazonium chloride and 8-hydroxyquinoline. The diazotization and coupling were conducted following procedures from the literature [11,12,13,14], and the conditions were adapted in order to obtain the desired product. The synthesis followed the below Scheme 1:
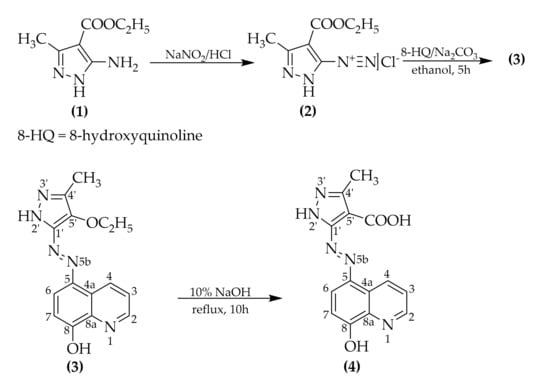
Scheme 1.
Synthetic route of the novel azo compounds (3) and (4).
The compound (3) obtained after azo coupling carries an ester functional group, so the hydrolysis was conducted in alkaline medium, as by this means it is irreversible [15].
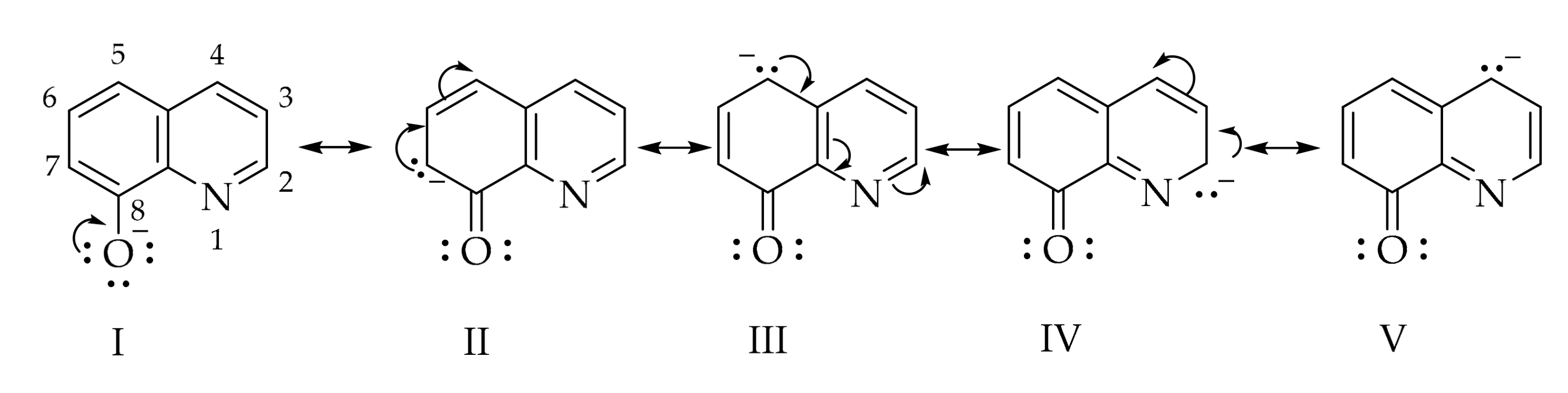
The azo coupling reaction was carried out in alkaline medium because in this medium, 8-hydroxyquinoline exists as the 8-quinolinate anion which has a higher electron density in the positions 2, 4, 5, and 7, as seen in the below resonance structures (Scheme 2).
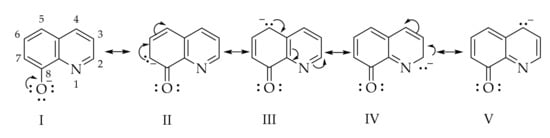
Scheme 2.
Resonance structures of the 8-hydroxyquinolinate anion.
The so-called pyridine ring of the 8-quinolinate anion is not very active towards electrophilic substitution reactions [16], the azo coupling reaction occurs only in the C-5 and C-7 positions due to increased electron density in these positions. However, the experimental NMR data from our study (available in the Supplementary Materials Section) shows that coupling took place regioselectively in the C-5 position. From the literature [17] we found that at a molar ratio of 1:1 (diazonium salt:8-hydroxyquinoline) the coupling took place only in the C-5 position, but on increasing the diazonium salt ratio the C-5 and C-7 disubstituted compound could be obtained. If the C-5 position is occupied, e.g., as in the case of 5-chloro-8-hydroxyquinoline or 8-hydroxyquinoline-5-sulfonic acid the coupling takes place in the C-7 position [18].
Experimental NMR Data for Compound (3) Explanation
From the long-range coupling spectrum 1H-15N HMBC it can be seen that the nitrogen atom, whose signal is found at 298.8 ppm couples with the proton whose signal is found at 8.97 ppm, with the proton at 7.52 ppm, and even with the proton at 7.47 ppm.
From the 1H-13C COSY spectrum it can be noted that the proton at 7.47 ppm couples with the protons at 9.45 ppm and 8.97 ppm, also the proton at 7.52 ppm couples with the proton at 8.56 ppm. We can conclude that the proton at 7.47 ppm is the 3-H proton, because it couples with two other protons, and the protons with signals at 8.56 ppm and 7.52 ppm are the 6-H and 7-H protons.
Due to the fact that the coupling between the nitrogen atom 1-N and the proton from 7.52 ppm takes place and this coupling is a long-range coupling (over four bonds), we can conclude that the signal at 7.52 ppm corresponds to the 7-H proton, thus the signal from the 8.56 ppm corresponds to the 6-H proton.
From the coupling of the 1-N nitrogen atom and the proton at 8.97 ppm we can conclude that this signal corresponds to the 2-H proton, and the remaining signal at 9.45 ppm can be attributed to the 4-H proton. Using the long-range coupling information we can conclude that the 7-H position remained unoccupied and the azo-coupling took place only in the position 5 of 8-hydroxyquinoline.
The assignment of the signals of the carbon atoms was made easier by the 1H-13C HSQC direct coupling spectrum between carbon atoms and the proton directly bonded to them.
From the 2D NOESY spectrum through-space correlations can be observed between the 3-H proton (at 7.47 ppm) and 4-H (at 9.45 ppm) and 2-H (8.97 ppm). In addition through-space correlation between the 7-H proton (at 7.52 ppm) and the 6-H proton (at 8.49 ppm can be observed).
From the HMBC spectrum the coupling can be observed between the carbon atom from 140.8 ppm (8a-C) and the 7-H proton from 7.52 ppm).
In the table below (Table 1) correlations are presented between direct bonding protons to the carbon atoms (1H-13C HSQC experiment), long-range correlations between protons, and carbon atoms (1H-13C HMBC experiment) long-range correlation between protons and nitrogen atom (1H-15N HMBC), long-range correlations between protons (1H-1H COSY) and couplings through-space between protons (2D-NOESY):

Table 1.
Experimental NMR data * for the compound (3).
In conclusion, it was shown (mainly by 1D and 2D NMR data) that the azo coupling reaction between the diazonium salt (2) and 8-hydroxyquinoline takes place regioselectively in the C-5 position of 8-hydroxyquinoline. The hydrolysis of the azo compound (3) yielded the corresponding carboxylic acid (4) (confirmed by NMR and IR data). Both compounds (3) and (4) were obtained in a high yield and purity and can be used in further studies.
3. Materials and Methods
The reagents were purchased from commercial sources and were used as received. The 5-amino-3-methyl-1H-pyrazole-4-carboxylate was synthesized earlier in our laboratory following a modified procedures from the literature [19].
The 1D and 2D NMR spectra were recorded on a Bruker Avance III 500 MHz spectrometer. Chemical shifts (δ) were measured in ppm and the coupling constants (J) in Hz. Samples were dissolved in Py-d5 or DMSO-d6.
IR spectra were recorded on a Bruker Vertex 70 (Bruker Optics GmbH, Ettlingen, Germany) equipped with a Platinum ATR spectrometer, Bruker Diamond Type A225/Q.I.
UV–Vis spectra were recorded on a Jasco V-530 spectrometer and the samples were dissolved in 96% ethanol. The concentration for compound (3) solution was 5.56·10−5 M and the concentration for compound (4) solution was 6.73·10−5 M.
Melting points were measured on a Böetius PHMK (Veb Analytik, Dresden, Germany) apparatus and were uncorrected.
High resolution MS (HRMS) spectra were recorded on a Bruker Maxis II QTOF spectrometer (Bruker Daltonics, Bremen, Germany) with electrospray ionization (ESI) either in positive or negative mode. The compounds were initially dissolved in DMSO and further diluted 1:100 with acetonitrile. MS spectra processing and isotope pattern simulations were performed with Compass Data Analysis V.4.4 (Bruker Daltonics, Bremen, Germany).
4. Experimental
4.1. Synthesis of Ethyl 5-((8-Hydroxyquinolin-5-Yl)Diazenyl)-3-Methyl-1h-Pyrazole-4-Carboxylate (3)
In a 100 mL round bottom flask equipped with a magnetic stirrer, 8-hydroxyquinoline (0.363 g, 2.5 mmol) was added together with 11 mL (22.930 mmol) of 20% Na2CO3 (aq.) and 50 mL of ethanol.
After cooling down the 8-hydroxyquinoline solution to a temperature below 5 °C, the solution of 4-(ethoxycarbonyl)-3-methyl-1H-pyrazole-5-diazonium chloride (2), which was obtained by diazotization of 0.444 g (2.625 mmol) of ethyl 5-amino-3-methyl-1H-pyrazole-4-carboxylate (1) using 0.192 g (2.756 mmol) NaNO2 and 0.68 mL (8.125 mmol) concentrated HCl, was added dropwise. An intense red coloring of the reaction mass was observed. After addition of the diazonium chloride solution, the pH of the reaction mass was checked and adjusted to pH 7–8. Besides the pH checking, the rate of the azo compound formation was monitored by reacting a few drops of reaction mass with an alkaline H acid (4-amino-5-hydroxynaphthalene-2,7-disulfonic acid) solution on a filter paper. By this method it was determined whether the diazonium salt was present or not (if present a dark violet spot appears on the filter paper). The reaction mass was left stirring for about 5 h.
After completion, the reaction mass was acidified down to a pH of 5–6 and then was precipitated in distilled water. A dark red precipitate was formed. The precipitate was vacuum filtered and dried. The final product (3) had an appearance of a dark brown-red solid.
An amount of 728 mg (2.221 mmol) of compound (3) was obtained, the isolation yield was 89%. Melting point: 218–219 °C;
1H-NMR (Py-d5, 500 MHz, 25 °C) δ (ppm): 9.45 (d, 1H, J = 8.4 Hz, 4-H); 8.97 (dd, 1H, J = 4.0 Hz, 1H, 2-H); 8.49 (d, 1H, J = 8.6 Hz, 6-H); 7.52 (d, 1H, J = 8.6 Hz, 7-H); 7.47 (dd, 1H, J = 8.6 Hz, J = 4.1 Hz, 3-H); 4.47 (q, 2H, J = 7.1 Hz, -O-CH2-CH3); 2.73 (s, 3H, -CH3); 1.32 (t, 3H, J = 7.1 Hz, -O-CH2-CH3); 13C-NMR (Py-d5, 125 MHz, 25 °C) δ (ppm): 164.8 (-C=O); 160.6 (8-C); 159.4 (4′-C); 150.7 (1′-C); 149.6 (2-C); 140.8 (8a-C); 139.7 (4a-C); 133.3 (4-C); 128.6 (5-C); 123.6 (3-C); 118.9 (6-C); 113.0 (7-C); 108.1 (5′-C); 60.8 (-O-CH2-CH3); 15.1 (-O-CH2-CH3); 13.6 (-CH3); 15N-NMR (Py-d5, 50 MHz, 25 °C) δ (ppm): 298.8 (1-N).
FT-IR (cm−1): 3201 (νNH), 2981 (νas.CH3); 2939 (νas.CH2); 1704 (νC = O); 1569, 1504 (νquinoline skeleton); 1473 (νpyrazole skeleton); 1209 (νas.C-O). The IR bands indicate the presence of the quinoline and pyrazole rings, the ester functional group, and the alkyl fragment.
UV–Vis (EtOH 96%): λ max = 393 nm (n → π* transition), ε(λ max) = 11,502.230 M−1 cm−1; HRMS: calculated for C16H15N5O3: 325.1175; found: 325.1124.
4.2. Synthesis of 5-((8-Hydroxyquinolin-5-Yl)Diazenyl)-3-Methyl-1h-Pyrazole-4-Carboxylic Acid (4)
An amount of 350 mg (1.078 mmol) of compound (3) and 15 mL of 10% NaOH (aq.) were added to a round bottom flask equipped with a condenser column and magnetic stirrer. Then the reaction mass was refluxed for about 10 h to ensure full conversion of ester to carboxylic acid (reaction monitoring by means of TLC was not possible due to the low solubility of the compounds in the usual organic solvents).
After 10 h the reaction mass was cooled down and acidified to pH 5–6. The precipitate was vacuum filtered and allowed to dry.
Then 218 mg (0.726 mmol) of a brown compound (4) were obtained, the isolation yield was 70%.
Melting point: >300 °C;
1H-NMR (DMSO-d6, 500 MHz, 25 °C) δ (ppm): 12.79 (s, 1H, -COOH); 9.15 (d, 1H, J = 8.2 Hz, 2-H); 8.69 (s, 1H, 4-H); 7.95 (d, 1H, J = 8.9 Hz, 7-H); 7.55 − 7.53 (m, 1H, 3-H); 6.74 − 6.73 (m, 1H, 6-H); 6.32 (s, 1H, -OH); 2.26 (s, 3H, -CH3); 13C-NMR (DMSO-d6, 125 MHz, 25 °C) δ (ppm): 163.9 (-COOH); 146.5 (4-C); 144.7 (1′-C); 143.4 (4′-C); 141.0 (8-C); 132.9 (8a-C); 131.3 (2-C); 129.2 (4a-C); 124.01 (5-C); 122.3 (3-C); 117.3 (7-C); 113.9 (6-C); 83.0 (5′-C); 11.5 (-CH3);
FT-IR (cm−1): 3000 (νassociated COOH); 1600, 1500 (νquinoline skeleton); 1550 (νC = O); 1463 (νpyrazole skeleton); 1321 (δC-OH). The IR bands indicate the presence of the carboxylic functional group (wide band at 3000 cm−1) and the absence of ethyl from the ester group.
UV–Vis (EtOH 96%): λ max = 385.5 nm (n → π* transition), ε(λ max) = 15,468.593 M−1 cm−1;
HRMS: calculated for C14H11N5O3: 297.0862; found: 297.0781.
Supplementary Materials
The following materials are available online: Figure S1. 1H-NMR spectrum of the compound (3), Figure S2. 13C-NMR spectrum of the compound (3), Figure S3. DEPT135 spectrum of the compound (3), Figure S4. COSY 1H-1H spectrum of the compound (3) Figure S5. HSQCED 1H-13C spectrum of the compound (3), Figure S6. HMBC 1H-15N spectrum of the compound (3), Figure S7. HMBC 1H-13C spectrum of the compound (3), Figure S8. 2D NOESY 1H-1H spectrum of the compound (3), Figure S9. 1H NMR spectrum of the compound (4), Figure S10. 13C NMR spectrum of the compound (4), Figure S11. DEPT135 spectrum of the compound (4), Figure S12. COSY 1H-1H spectrum of the compound (4), Figure S13. HSQCED 1H-13C spectrum of the compound (4), Figure S14. HMBC 1H-13C spectrum of the compound (4), Figure S15. 2D NOESY 1H- 1H spectrum of the compound (4), Figure S16. FT-IR spectrum of the compound (3), Figure S17. FT-IR spectrum of the compound (4), Figure S18. UV-Vis spectrum of the compound (3), Figure S19. UV–Vis spectrum of the compound (4), Figure S20. HRMS spectrum of the compound (3), Figure S21. HRMS spectrum of the compound (4).
Author Contributions
Designed the experiments, V.B.; performed the experiments, I.B. and V.-N.B.; analyzed the spectral data, V.B. and C.D.; wrote the manuscript, V.B. and I.B.; supervision and funding acquisitions, V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI—UEFISCDI, project number PN-III-P2-2.1-PED-2019-3414, within PNCDI III.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hunger, K.; Mischke, P.; Rieper, W.; Raue, R.; Kunde, K.; Engel, A. Azo Dyes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar] [CrossRef]
- Clark, M. Azoic dyeing. In Handbook of Textile and Industrial Dyeing; Woodhead Publishing Limited: Cambridge, UK, 2011; Volume 1, pp. 604–605. [Google Scholar]
- Leulescu, M.; Rotaru, A.; Pălărie, I.; Moanţă, A.; Cioateră, N.; Popescu, M.; Morîntale, E.; Bubulică, M.V.; Florian, G.; Hărăbor, A.; et al. Tartrazine: Physical, thermal and biophysical properties of the most widely employed synthetic yellow food-colouring azo dye. J. Therm. Anal. Calorim. 2018, 134, 209–231. [Google Scholar] [CrossRef]
- Devika, B.G.; Doreswamy, B.H.; Tandon, H.C. Corrosion behaviour of metal complexes of antipyrine based azo dye ligand for soft-cast steel in 1 M hydrochloric acid. J. King Saud Univ. Sci. 2020, 32, 881–890. [Google Scholar] [CrossRef]
- Elmaaty, T.A.; Sofan, M.; Elsisi, H.; Kosbar, T.; Negm, E.; Hirogaki, K.; Tabata, I.; Hori, T. Optimization of an eco-friendly dyeing process in both laboratory scale and pilot scale supercritical carbon dioxide unit for polypropylene fabrics with special new disperse dyes. J. CO2 Util. 2019, 33, 365–371. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, H.; Ye, D.; Zhou, R.; Li, H.; Tang, B.; Jin, M.; Li, N.; Guo, Y.; Zhou, G. Synthesis and application of an alkylated pyrazole-based azo dye for electrofluidic display. J. Soc. Inf. Disp. 2018, 26, 369–375. [Google Scholar] [CrossRef]
- Adiguzel, R.; Turan, N.; Buldurun, K.; Korkoca, H. Spectral, Thermal and Antimicrobial Properties of Novel Mixed Ligand-Metal Complexes Derived from Saccharinate Complexes and Azo Dye Ligand. Int. J. Pharmacol. 2018, 14, 9–19. [Google Scholar] [CrossRef]
- Bartwal, G.; Aggarwala, K.; Khurana, J.M. An Ampyrone based azo dye as pH-responsive and chemo-reversible colorimetric fluorescent probe for Al3+ in semi-aqueous medium: Implication towards logic gate analysis. New J. Chem. 2018, 42, 2224–2231. [Google Scholar] [CrossRef]
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Al-Busafi, S.N.; O Suliman, F.E.; Al-Alawi, Z.R. 8-Hydroxyquinoline and its Derivatives. Res. Rev. J. Chem. 2014, 3, 1–10. [Google Scholar]
- Yet, L. 4.01—Pyrazoles. In Comprehensive Heterocyclic Chemistry III; Elsevier Science: Amsterdam, The Netherlands, 2008; Volume 4, pp. 1–141. [Google Scholar] [CrossRef]
- Roos, G.; Roos, C. Chapter 7—Functional Classes II, Reactions. In Organic Chemistry Concepts: An EFL Approach; Academic Press: Cambridge, MA, USA, 2015; pp. 103–149. [Google Scholar] [CrossRef]
- Godovikova, T.I.; Rakitin, O.A.; Khmel’nitskii, L.I. Diazotisation of Weakly Basic Aromatic and Heterocyclic Amines in Strongly Acid Media. Russ. Chem. Rev. 1983, 52, 440–445. [Google Scholar] [CrossRef]
- Butler, R.N. Diazotization of heterocyclic primary amines. Chem. Rev. 1975, 75, 241–257. [Google Scholar] [CrossRef]
- Parekh, V.J.; Rathod, V.K.; Pandit, A.B. 2.10—Substrate Hydrolysis: Methods, Mechanism, and Industrial Applications of Substrate Hydrolysis. In Comprehensive Biotechnology, 2nd ed.; Pergamon: Oxford, UK, 2011; pp. 103–118. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Sweidan, K.A.; Mubarak, M.S. Recent Advances in the Synthesis and Biological Activity of 8-Hydroxyquinolines. Molecules 2020, 25, 4321. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.P. The Reactions Of 8-Quinolinol. Chem. Rev. 1956, 56, 271–297. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.R.; Mahmoodi, N.O.; Dabiry, S. Preparation and characterization of diazenyl quinolin-8-ol with trifluoromethyl substituents. Mendeleev Commun. 2006, 16, 192–194. [Google Scholar] [CrossRef]
- Beyer, H.; Wolter, G. Über Thiazole, XXIX. Mitteil: Über die Kondensationsprodukte von Thiosemicarbazid mit α-Chloracetessigester und eine neuartige Ringverengung des 2-Amino-5- methyl-6-carbäthoxy − 1.3.4-thiodiazins zum 3-Methyl-4- carbäthoxy-5-amino-pyrazol. Chem. Ber. 1956, 89, 1652–1658. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).