2-((4-((E)-1-(Hydroxyimino)ethyl)phenyl)amino)-2-oxoethyl Cinnamate
Abstract
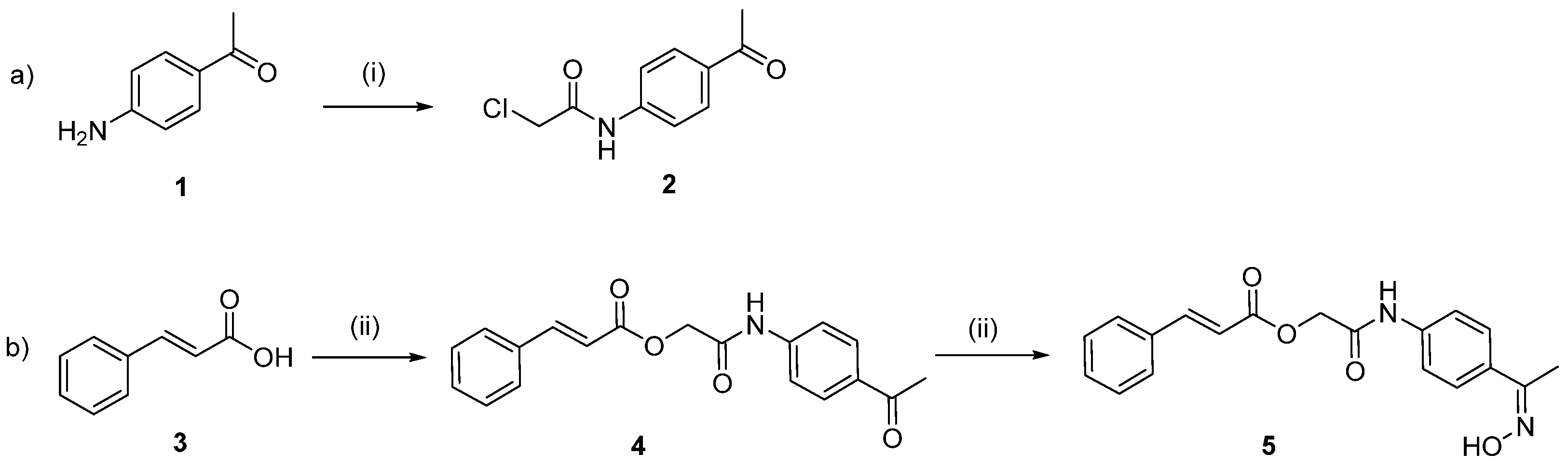
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Physicochemical Studies
2.3. Biological Evaluation
3. Materials and Methods
3.1. General Information
3.2. Chemistry General Procedure
3.2.1. N-(4-Acetylphenyl)-2-Chloroacetamide (2)
3.2.2. 2-((4-Acetylphenyl)amino)-2-oxoethyl Cinnamate (4)
3.2.3. Synthesis of 2-((4-((E)-1-(Hydroxyimino)ethyl)phenyl)amino)-2-oxoethyl Cinnamate (5)
3.3. Biological In Vitro Assays
3.3.1. Inhibition of Linoleic Acid Lipid Peroxidation
3.3.2. Inhibition of Soybean Lipoxygenase In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and Cancer: How Hot is the Link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.; Lipsky, P.E. Cyclooxygenase in Biology and Disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rådmark, O.; Samuelsson, B. 5-Lipoxygenase: Mechanisms of Regulation. J. Lipid Res. 2009, 50, S40–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontiki, E.; Hadjipavlou-Litina, D. Synthesis and Pharmacochemical Evaluation of Novel Aryl-Acetic Acid Inhibitors of Lipoxygenase, Antioxidants, and Anti-Inflammatory Agents. Bioorganic Med. Chem. 2007, 15, 5819–5827. [Google Scholar] [CrossRef] [PubMed]
- Peperidou, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Voulgari, E.; Avgoustakis, K. Multifunctional Cinnamic Acid Derivatives. Molecules 2017, 22, 1247. [Google Scholar] [CrossRef]
- De Cássia, R.; Andrade, L.N.; Barreto, R.; De Sousa, D.P. A Review on Anti-Inflammatory Activity of Phenylpro-Panoids Found in Essential Oils. Molecules 2014, 19, 1459–1480. [Google Scholar] [CrossRef] [Green Version]
- Sova, M. Antioxidant and Antimicrobial Activities of Cinnamic Acid Derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Baltas, M.; Bedos-Belval, F. Cinnamic Acid Derivatives as Anticancer Agents-A Review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef]
- Fotopoulos, I.; Pontiki, E.; Hadjipavlou-Litina, D. Targeting Inflammation with Conjugated Cinnamic Amides, Ethers and Esters. Lett. Drug Des. Discov. 2019, 17, 3–11. [Google Scholar] [CrossRef]
- Strijdom, H.; Chamane, N.; Lochner, A. Nitric Oxide in the Cardiovascular System: A Simple Molecule with Complex Actions. Cardiovasc. J. Afr. 2009, 20, 303–310. [Google Scholar] [PubMed]
- Esplugues, J.V. NO as a Signalling Molecule in the Nervous System. Br. J. Pharmacol. 2002, 135, 1079–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdan, C. Nitric Oxide and the Immune Response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Anthwal, A.; Thakur, B.K.; Rawat, M.S.M.; Rawat, D.S.; Tyagi, A.K.; Aggarwal, B.B. Synthesis, Characterization and In Vitro Anticancer Activity of C-5 Curcumin Analogues with Potential to Inhibit TNF-α -Induced NF-B Activation. Biomed. Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiq, M.; Nazir, Y.; Ashraf, Z.; Rafique, H.; Afzal, S.; Mumtaz, A.; Hassan, M.; Ali, A.; Afzal, K.; Yousuf, M.R.; et al. Synthesis, Computational Studies, Tyrosinase Inhibitory Kinetics and Antimelanogenic Activity of Hydroxy Substituted 2-[(4-acetylphenyl)amino]-2-oxoethyl derivatives. J. Enzym. Inhib. Med. Chem. 2019, 34, 1562–1572. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Gupta, M.; Lee, H.J.; Barden, C.J.; Weaver, D.F. The Blood-Brain Barrier (BBB) Score. J. Med. Chem. 2019, 62, 9824–9836. [Google Scholar] [CrossRef] [PubMed]
- Betigeri, S.; Thakur, A.; Raghavan, K. Use of 2,2′-Azobis(2-Amidinopropane) Dihydrochloride as a Reagent Tool for Evaluation of Oxidative Stability of Drugs. Pharm. Res. 2005, 22, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Peperidou, A.; Kapoukranidou, D.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Multitarget Molecular Hybrids of Cinnamic Acids. Molecules 2014, 19, 20197–20226. [Google Scholar] [CrossRef] [PubMed]
- Minor, W.; Steczko, J.; Bolin, J.T.; Otwinowski, Z.; Axelrod, B. Crystallographic Determination of the Active Site Iron and Its Ligands in Soybean Lipoxygenase L-1. Biochemistry 1993, 32, 6320–6323. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolotti, O.; Carotti, A. Design, Synthesis and Pharmacobiological Evaluation of Novel Acrylic Acid Derivatives Acting as Lipoxygenase and Cyclooxygenase-1 Inhibitors with Antioxi-dant and Anti-Inflammatory Activities. Eur. J. Med. Chem. 2011, 46, 191–200. [Google Scholar] [CrossRef] [PubMed]
| milogP a | TPSA b | No. of Atoms | No of O and N c | No of OH and NH d | No of Violations | No of Rotational Bonds e | Volume f | MW g | logBB h [16] |
|---|---|---|---|---|---|---|---|---|---|
| 3.29 | 88.00 | 25 | 6 | 2 | 0 | 7 | 306.86 | 338.36 | 2.90 |
| A/A | Compound | AAPH at 100 μM | LOX Inhibition (IC50) μM |
|---|---|---|---|
| 4 | 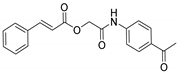 | 32% | no |
| 5 | 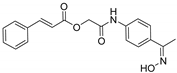 | 50.2% | 50 μM |
| Nordihydroguaretic acid (NDGA) | nt | 0.45 μM | |
| Trolox | 93% | nt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsopka, I.-C.; Hadjipavlou-Litina, D. 2-((4-((E)-1-(Hydroxyimino)ethyl)phenyl)amino)-2-oxoethyl Cinnamate. Molbank 2021, 2021, M1239. https://doi.org/10.3390/M1239
Tsopka I-C, Hadjipavlou-Litina D. 2-((4-((E)-1-(Hydroxyimino)ethyl)phenyl)amino)-2-oxoethyl Cinnamate. Molbank. 2021; 2021(3):M1239. https://doi.org/10.3390/M1239
Chicago/Turabian StyleTsopka, Ioanna-Chrysoula, and Dimitra Hadjipavlou-Litina. 2021. "2-((4-((E)-1-(Hydroxyimino)ethyl)phenyl)amino)-2-oxoethyl Cinnamate" Molbank 2021, no. 3: M1239. https://doi.org/10.3390/M1239
APA StyleTsopka, I.-C., & Hadjipavlou-Litina, D. (2021). 2-((4-((E)-1-(Hydroxyimino)ethyl)phenyl)amino)-2-oxoethyl Cinnamate. Molbank, 2021(3), M1239. https://doi.org/10.3390/M1239







