Abstract
The Povarov reaction of p-anisidine, cinnamaldehyde and methacrolein dimethylhydrazone afforded a 1,2,3,4-tetrahydroquinoline derivative bearing 2-styryl, 4-methyl and 4-dimethylhydrazono substituents in a fully diastereoselective fashion. This is the first example of the combination of a type I aza-vinylogous Povarov reaction and a type II vinylogous Povarov reaction in the same process.
1. Introduction
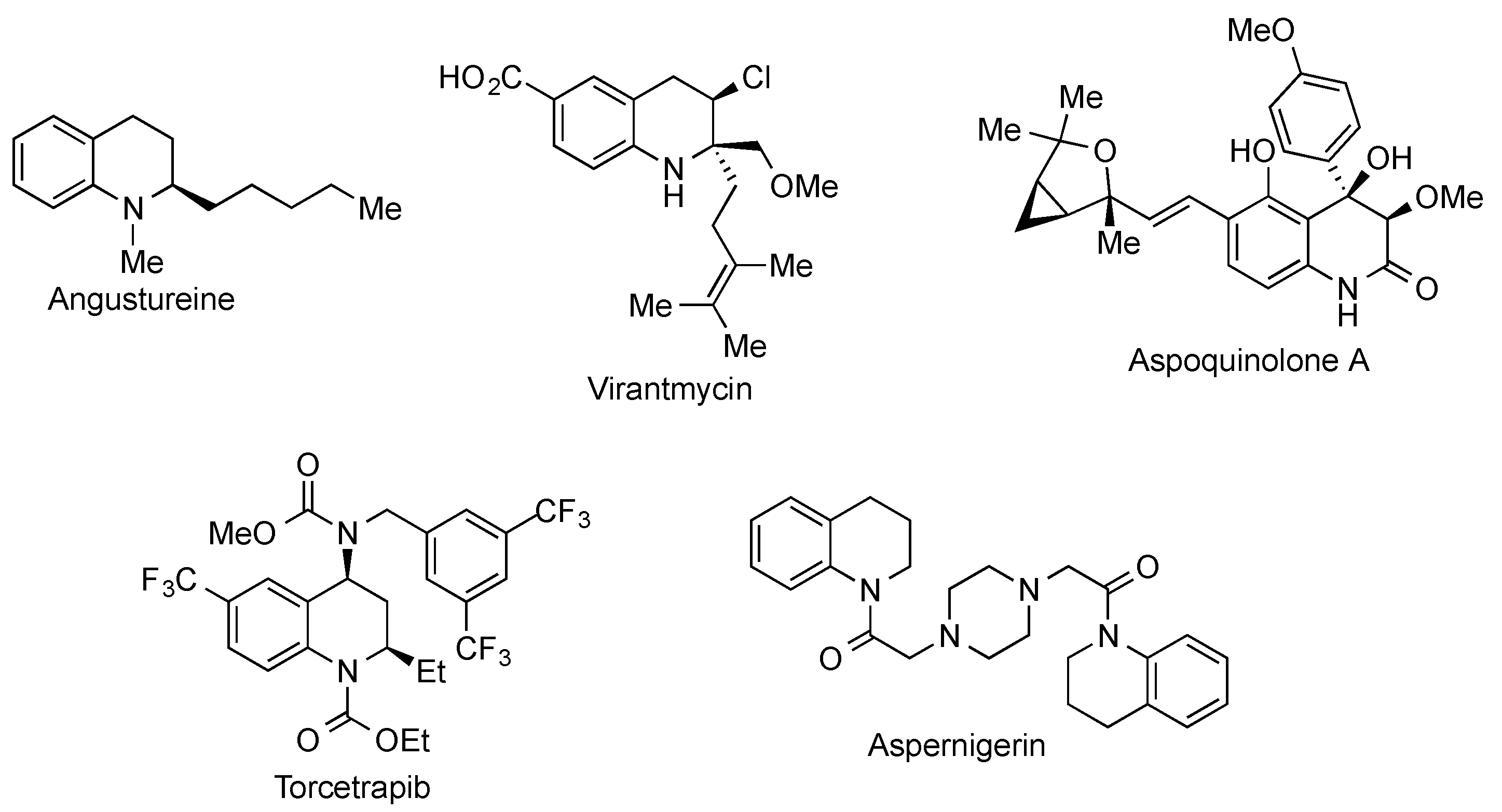
The 1,2,3,4-tetrahydroquinoline ring system is present in a broad variety of natural products and synthetic bioactive molecules [1,2] and can be considered as one of the most relevant simple heterocycles. Some important tetrahydroquinolines are summarized in Figure 1, including the alkaloids angustureine, virantmycin and aspoquinolone A, the cholesterol-lowering agent torcetrapib and the insecticidal, herbicidal and fungicidal aspernigerin (Figure 1).
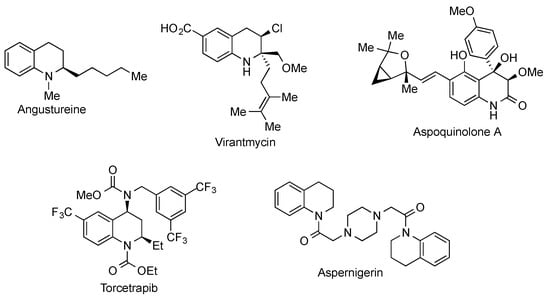
Figure 1.
Structures of selected tetrahydroquinoline derivatives.
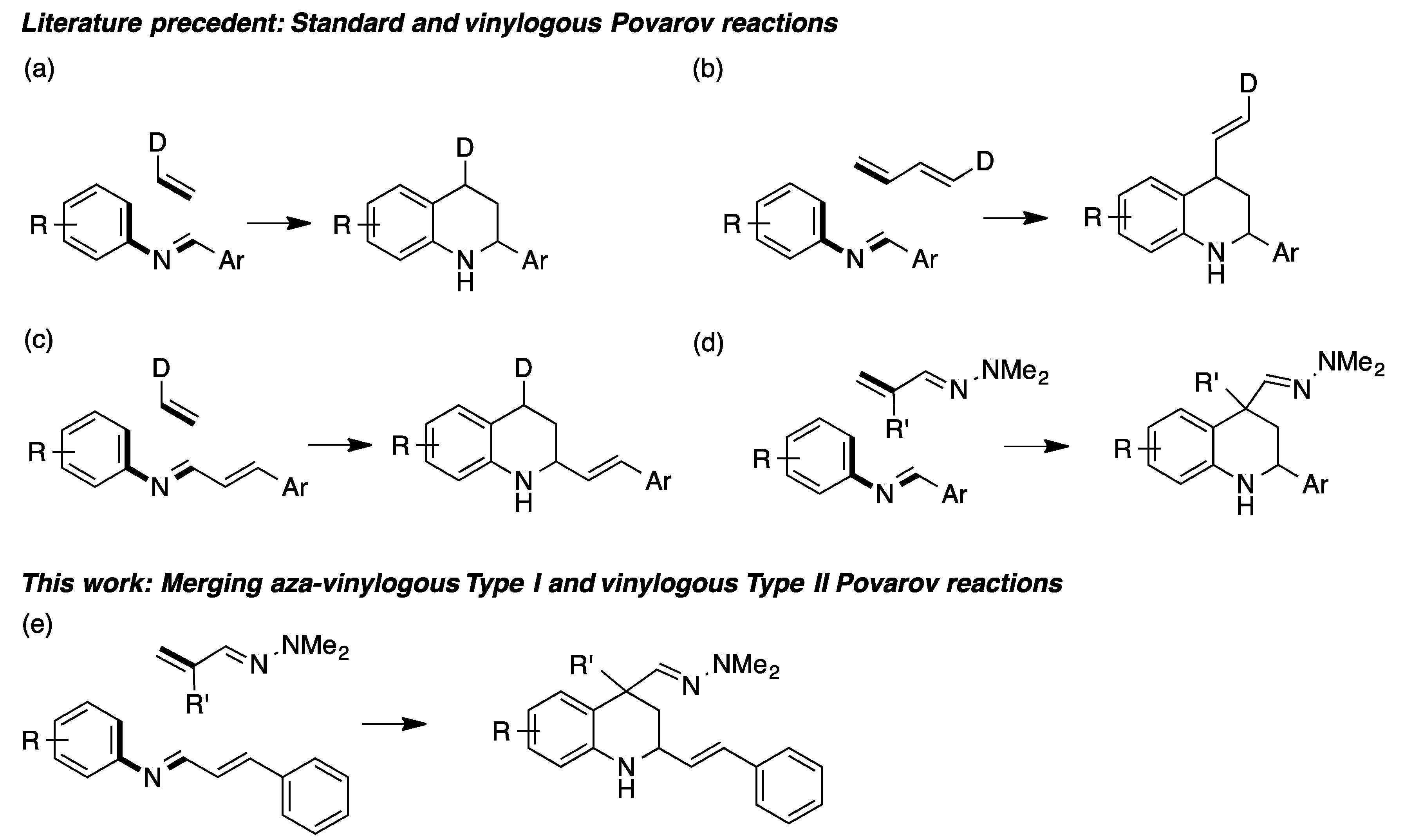
The importance of tetrahydroquinolines has prompted a great deal of research into their synthesis. Among the many known approaches to this framework [1,2,3], the Povarov reaction, i.e., the acid-catalyzed formal inverse electron demand [4+2] cycloaddition of electron-rich olefins with N-arylimines, arising from arylamines and aldehydes (Scheme 1a), stands out as one of the most studied methods [4,5]. Vinylogous Povarov reactions are also known, and have the advantage of generating tetrahydroquinolines with an olefin substituent either at C-4 (Type I, Scheme 1b) or C-2 (Type II, Scheme 1c). We have described [6,7] a type I aza-vinylogous Povarov reaction using α,β-unsaturated hydrazones as the dienophile component that has the advantage of simultaneously installing a quaternary stereocenter and functional group at C-4 (Scheme 1d).
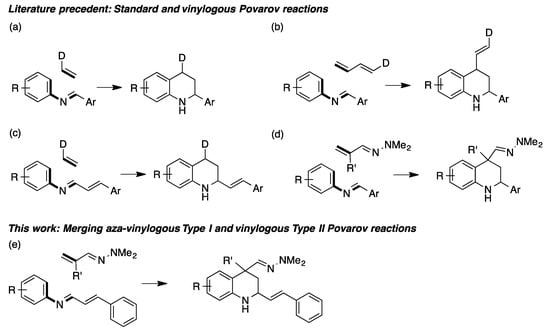
Scheme 1.
(a) The standard Povarov reaction; (b) type I vinylogous Povarov reaction; (c) type II vinylogous Povarov reaction; (d) type I aza-vinylogous Povarov reaction; (e) the reaction described in this work, which combines the features of the type I aza-vinylogous and type II vinylogous Povarov reactions.
In this context, we describe here the first example of a Povarov reaction that combines the features of a type I aza-vinylogous and a type II vinylogous Povarov reaction in a single transformation and allows the preparation of 2,4-difunctionalized 2-styryltetrahydroquinolines bearing a quaternary stereocenter at C-4 (Scheme 1e).
2. Results and Discussion
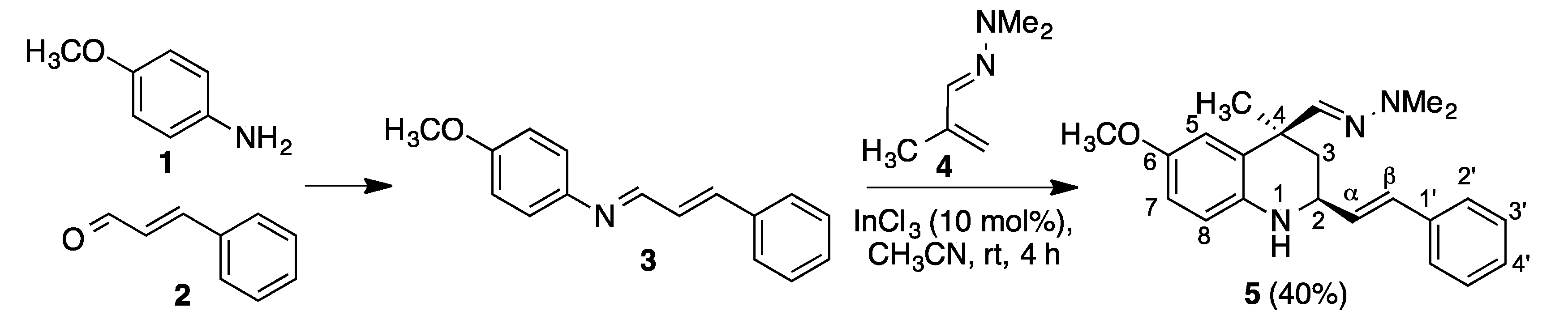
The doubly vinylogous Povarov reaction that we describe here is summarized in Scheme 2. The reaction between p-anisidine 1 and cinnamaldehyde 2 afforded the corresponding imine 3, which was treated in crude state with methacrolein dimethylhydrazone 4 in acetonitrile containing 10% indium trichloride as a Lewis acid catalyst, at room temperature, affording compound 5 as a single diastereomer in 40% overall yield (Scheme 2).
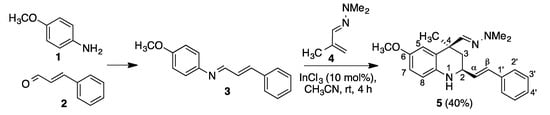
Scheme 2.
Synthesis of compound 5.
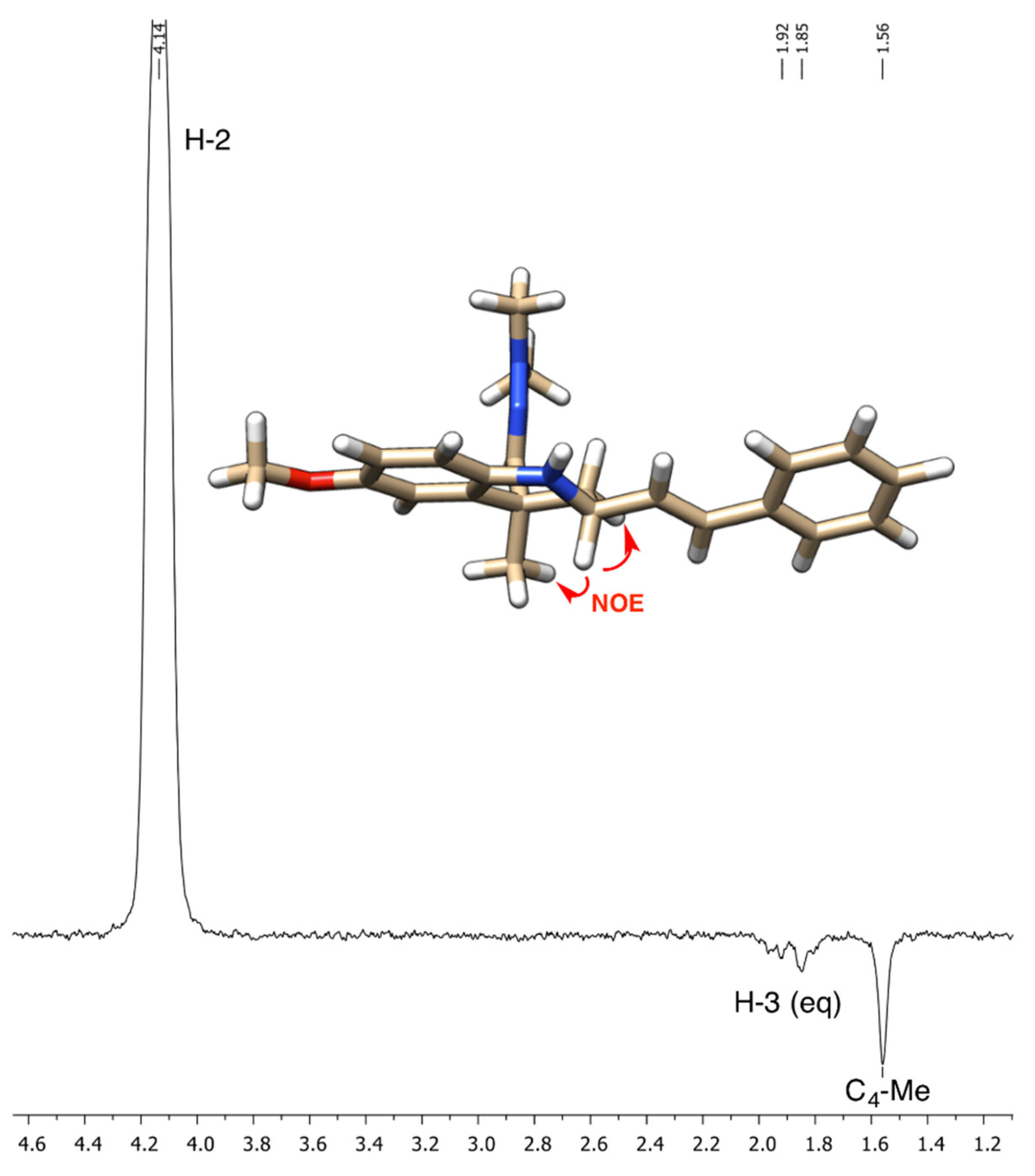
The structure of compound 5 is consistent with a high-resolution mass measurement and with its IR, 1H-NMR and 13C-NMR spectral data, which were assigned with the aid of 2D-NMR experiments. Thus, the hydrazone group gave a CH=N stretching vibration at 1600 cm−1 in the IR spectrum, a 1H-NMR signal in the 6.74–6.64 interval, which overlapped with other signals but is clearly visible in the HMQC experiment, and a 13C-NMR signal at 145.3 ppm. One of the olefinic protons was clearly observed at 6.27 ppm as a doublet of doublets with J = 15.8 and 7.3 Hz, which allowed its assignment as H-α. The other proton corresponding to the olefin part of the styryl group (H-β) is part of the multiplet at 6.74–6.64, as revealed by the COSY experiment, and the H-C correlation experiments allowed the assignment of the olefin carbons to the CH signals at 132.4 (C-α) and 131.0 (C-β). The cis arrangement of the styryl and dimethylhydrazono substituents agrees with the literature precedents [6,7] and was unequivocally established by the observation of a NOE enhancement of the axial C-4 methyl substituent upon irradiation of the H-2 proton (Figure 2). The alternative trans isomer was not observed in the crude reaction product.
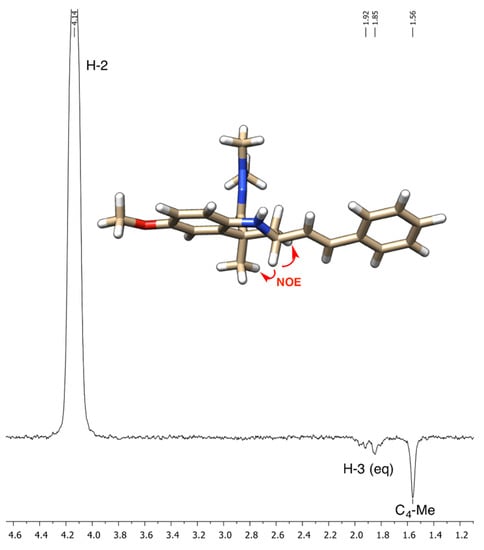
Figure 2.
NOE assignment of the relative configuration of compound 5.
3. Materials and Methods
General experimental information. All reagents (Sigma-Aldrich, Madrid, Spain; Fischer Chemical, Madrid, Spain; Alpha Aesar, Kändel, Germany) and solvents (Scharlau, Barcelona, Spain; Fischer Chemical, Madrid, Spain) were of commercial quality and were used as received. Reactions were monitored by thin layer chromatography on aluminum plates coated with silica gel and fluorescent indicator (Merck, Madrid, Spain). Infrared spectra were recorded with an Agilent Cary630 FTIR spectrophotometer (Madrid, Spain) working by attenuated total reflection (ATR), with a diamond accessory for solid and liquid samples. NMR spectroscopic data were recorded using a Bruker Avance 250 spectrometer (Bruker, Rivas-Vaciamadrid, Spain) operating at 250 MHz for 1H-NMR and 63 MHz for 13C-NMR maintained by the NMR facility of Universidad Complutense (CAI de Resonancia Magnética Nuclear, Madrid, Spain); chemical shifts are given in ppm and coupling constants in Hertz. 1H- and 13C-NMR assignments were supported by 2D-NMR experiments and were aided by simulations performed with MestreNova and ChemDraw Pro. Copies of spectra and 2D-NMR experiments are provided in the Supporting Information. Time-of-flight mass spectrometric measurements were performed using a MALDI- TOF/TOF Bruker ULTRAFLEX (mass range: 300–150,000 u) at the CAI of Espectrometría de Masas, Universidad Complutense.
(E)-4-methoxy-N-((E)-3-phenylallylidene)aniline (3). A solution of p-anisidine (1 mmol, 123 mg) and cinnamaldehyde (1 eq, 148 mg) in CH2Cl2 (5 mL) was stirred vigorously in the presence of anhydrous Na2SO4 (5 g) for 30 min and then the reaction mixture was filtered and the solvent evaporated under vacuum to afford 3 as a brown foam in quantitative yield. The crude was used in the following reaction without further purification. IR (neat): 2955.4 (C-H), 1626.0 (C=N), 1601.3 (C=C), 1498.8 (Csp2-Csp2), 1242.3 (Csp2-O) cm−1. 1H NMR (250 MHz, CDCl3) δ: 8.31 (dd, J = 5.9, 2.4 Hz, 1H), 7.62–7.52 (m, 2H), 7.47–7.32 (m, 3H), 7.32–7.21 (m, 2H), 7.20–7.11 (m, 2H), 7.01–6.91 (m, 2H), 3.84 (s, 3H) ppm. 13C{1H} NMR (63 MHz, CDCl3) δ: 159.8, 158.7, 144.8, 143.4, 136.1, 129.7, 129.2, 129.1, 127.7, 122.6, 114.7, 55.8.
(±)-(2S*,4S*)-4-((E)-(2,2-dimethylhydrazono)methyl)-6-methoxy-4-methyl-2-((E)- styryl)-1,2,3,4-tetrahydroquinoline (5). To a stirred solution of compound 3 (1.0 mmol, 237 mg) and InCl3 (0.1 mmol, 22 mg) in acetonitrile (20 mL) was added dropwise methacrolein dimethylhydrazone (1.1 mmol, 123 mg). Stirring at room temperature was continued until completion of the reaction, as indicated by TLC (Rf 0.29, eluting with 8:2 hexane/ethyl acetate), and the mixture was then diluted with water (10 mL), extracted with CH2Cl2 (4 × 10 mL), dried with anhydrous Na2SO4 and evaporated under reduced pressure. The resulting crude was purified by silica gel column chromatography using hexane/ethyl acetate (92.5:7.5) as the mobile phase to obtain 140 mg (40%) of compound 5 as a yellow oil. IR (neat): 3369.8 (N-H), 2951.1 (C-H), 2826.0 (C-H), 1600.2 (C=N), 1501.6 (C=C), 1235.3 (Csp2-O) cm−1. 1H NMR (250 MHz, CDCl3) δ: 7.45–7.27 (m, 5H, Ph), 6.74–6.64 (m, 4H, H-5, H-7, CH=N, H-β), 6.57 (d, J = 9.1 Hz, 1H, H-8), 6.27 (dd, J = 15.8, 7.3 Hz, 1H, H-α), 4.19–4.08 (m, 1H, H-2), 3.76 (s, 3H, OCH3), 2.79 (s, 6H, N(CH3)2), 2.05–1.90 (m, 1H, H-3ax), 1.82 (dd, J = 13.1, 2.9 Hz, 1H, H-3eq), 1.56 (s, 3H, C4-CH3) ppm; NH is absent. 13C{1H} NMR (63 MHz, CDCl3) δ: 152.6 (Cq, C-6), 145.3 (CH=N), 138.2 (Cq, C-8a), 137.2 (Cq, C-1’), 132.4 (C-α), 131.0 (C-β), 129.1 (C-3’, C-5’), 128.7 (Cq, C-4a), 128.1 (C-4’), 126.8 (C-2’, C-6’), 116.1 (C-8), 114.7 (C-7), 113.9 (C-5), 56.3 (OCH3), 51.6 (C-2), 43.9 (N(CH3)2), 41.9 (C-3), 41.0 (Cq, C-4), 28.5 (C4-CH3) ppm. HRMS (MALDI-TOF): calculated for C22H27N3O, 349.2154. Found, 349.2161.
4. Conclusions
We describe the first example of a doubly vinylogous Povarov reaction involving an α,β-unsaturated aromatic imine as diene and an α,β-unsaturated dimethylhydrazone as the dienophile. This reaction proceeded with complete diastereoselectivity and afforded a 1,2,3,4-tetrahydroquinoline derivative bearing a C-4 quaternary stereocenter and cis-arranged functional groups at C-4 (dimethylhydrazono) and C-2 (styryl).
Supplementary Materials
The following are available online. Copies of spectra of compounds 3 and 5.
Author Contributions
Conceptualization, J.C., M.T.R. and J.C.M.; methodology, J.C.; writing—original draft preparation, J.C.M.; writing—review and editing, J.C., M.T.R. and J.C.M.; supervision, M.T.R. and J.C.M.; funding acquisition, J.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia, Innovación y Universidades (grant RTI2018-097662-B-I00 to J.C.M.) and Universidad Complutense (predoctoral contract to J.C.). The APC was not funded.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sridharan, V.; Suryavanshi, P.; Menéndez, J.C. Advances in the chemistry of tetrahydroquinolines. Chem. Rev. 2011, 111, 7157–7259. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, I.; Sridharan, V.; Menéndez, J.C. Progress in the chemistry of tetrahydroquinolines. Chem. Rev. 2019, 119, 5057–5191. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Rachwal, S.; Rachwal, B. Recent progress in the synthesis of 1,2,3,4-tetrahydroquinolines. Tetrahedron 1996, 52, 15031–15070. [Google Scholar] [CrossRef]
- Kouznetsov, V.V. Recent synthetic developments in a powerful imino Diels–Alder reaction (Povarov reaction): Application to the synthesis of N-polyheterocycles and related alkaloids. Tetrahedron 2009, 65, 2721–2750. [Google Scholar] [CrossRef]
- Ghashghaei, O.; Masdeu, C.; Alonso, C.; Palacios, F.; Lavilla, R. Recent advances of the Povarov reaction in medicinal chemistry. Drug Discov. Today Technol. 2018, 29, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Perumal, P.T.; Avendaño, C.; Menéndez, J.C. The first aza Diels–Alder reaction involving an α,β-unsaturated hydrazone as the dienophile: Stereoselective synthesis of C-4 functionalized 1,2,3,4-tetrahydroquinolines containing a quaternary stereocenter. Org. Biomol. Chem. 2007, 5, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Ribelles, P.; Estévez, V.; Villacampa, M.; Ramos, M.T.; Perumal, P.T.; Menéndez, J.C. New types of reactivity of α,β-unsaturated N,N-dimethylhydrazones: Chemodivergent, diastereoselective synthesis of functionalized tetrahydroquinolines and hexahydropyrrolo[3,2-b]indoles. Chem. Eur. J. 2012, 18, 5056–5063. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).