Abstract
As part of our ongoing scaffold hopping work on antimalarial 2-aminothieno[3,2-d]pyrimidin-4-one scaffold, we explored the dihydrothieno[3,2-d][1,3,2]diazaborinin-4(1H)-one as a potential new antimalarial series. Using conditions found in the literature, we obtained 2-cyclopropyl-6-phenyl-2,3-dihydrothieno[3,2-d][1,3,2]diazaborinin-4(1H)-one with 93% yield through a simple treatment. It was then characterized by NMR (1H and 13C) and HRMS. Given the structure of this molecule, its aqueous stability was assessed to determine its suitability for biological tests. To our knowledge, this is the first dihydrothieno[3,2-d][1,3,2]diazaborinin-4(1H)-one described.
1. Introduction
Malaria is the world’s most prevalent parasitic disease in terms of deaths, with an estimated 229 million cases and 409,000 victims according to the World Health Organization (WHO) in 2019 [1]. It is caused by parasites of the Plasmodium genus (mainly P. falciparum) and transmitted by the infectious bite of Anopheles mosquitoes. The first line treatment, artemisinin-based combination therapies (ACTs), combines an artemisinin derivative with another antimalarial compound. However, the reduction of both deaths and infections is threatened by multiple factors including the spread of parasite resistance to artemisinin and its derivatives [1]. This resistance is caused by mutations of the kelch13 protein [2] and is characterized clinically by delayed parasitic clearance potentially leading to treatment failure of ACTs [3]. Initially discovered in South-East Asia, where they have become widespread [3], kelch13 mutations are now found in Africa, where they have recently been linked to in vitro resistances against artemisinin derivatives [4,5]. Thus, discovery of new antimalarial compounds with novel mechanisms of action is a priority in the battle against malaria.
In 2015, Cohen et al. described compound M1 (Figure 1) [6], a 2-aminothieno[3,2-d]pyrimidin-4-one hydrochloride salt displaying an all-stage activity against P. falciparum associated with low toxicity, no mutagenicity and possessing an unknown mechanism of action. Many thienopyrimidine compounds are described as anticancerous compounds, mainly kinase inhibitors [7] and one thienopyrimidinone compound, TAK-931 (simurosertib, Cdc7 inhibitor, Figure 1) is currently in phase II clinical trial for the treatment of metastasic cancers [8].
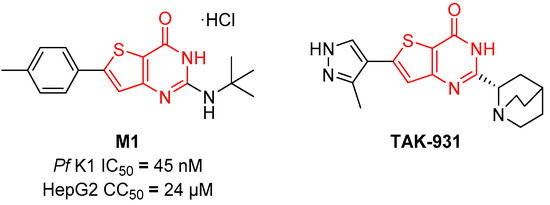
Figure 1.
Chemical structures of compounds M1 and TAK-931.
As part of our ongoing medicinal chemistry work to improve M1 properties, a scaffold hopping strategy on the thienopyrimidine core was implemented for structure-activity relationships purposes. This led to the idea of replacing the pyrimidinone moiety by a [1,3,2]diazaborinin-4-one moiety, seeking to synthesize compounds in thieno[3,2-d][1,3,2]diazaborinin-4-one series and assess their activity in vitro against multi-resistant P. falciparum K1 strain (Scheme 1).
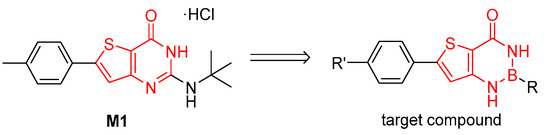
Scheme 1.
Scaffold hopping strategy for compound M1.
Boron-containing compounds possess various properties such as anti-cancerous and anti-infectious activities [9]. Boron in approved drugs (Figure 2) is found either as boronic acid (in bortezomib or ixazomib) or incorporated into a cycle, such as oxaborole or oxaborinane (like tavaborole or vaborbactam respectively).
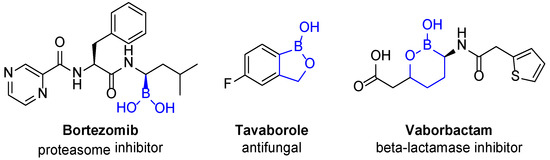
Figure 2.
Examples of marketed boron-containing drugs.
2. Results
We used reaction conditions described by Davies et al., starting from anthranilamide and various potassium trifluoroborates to afford benzo[d][1,3,2]diazaborin-4-ones in the presence of the boron trifluoride-ethylamine complex [10]. Since the tert-butylamine moiety could not be transposed onto a [1,3,2]diazaborin-4-one cycle, a cyclopropyl moiety was chosen as replacement. We decided to begin by synthesizing compound 2 (Scheme 2), since the starting materials are commercially available. Starting from 3-amino-5-phenylthiophene-2-carboxamide 1 and potassium cyclopropyltrifluoroborate with 3 equivalents of boron trifluoride ethylamine complex, we synthesized compound 2 in a 1:1 mixture of toluene and CPME after one day at 80 °C (Scheme 2). Small adjustments to the conditions in Davies et al. [10], were made, slightly increasing the quantity of potassium trifluoroborate (1.2 equivalent here vs. 1.05) and the reaction time (24 h here vs. 16 h). An excellent yield of 93% was obtained with simple filtration as extraction/purification protocol to afford target compound 2.
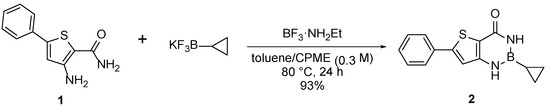
Scheme 2.
Synthesis of compound 2.
3. Discussion
The next step was to assess the antiplasmodial activity in vitro against P. falciparum strain, but this raised concerns as to the potential aqueous stability of compound 2. Indeed, benzo[d][1,3,2]diazaborin-4-ones obtained from anthranilamide are reported to be a protecting group for boronic acids and able to generate air-stable boronic acid precursors [11,12,13]. Davies et al. reported relative stability in neutral or acid pH for their benzo[d][1,3,2]diazaborin-4-ones derivatives, but with very small amounts of D2O (from 6 to 16 µL).
Therefore, a stability assay was performed on 2 (Figure 3): the compound was dissolved in DMSO-d6, a small amount of D2O was added and the stability of the compound was then monitored via 1H NMR (see Materials and Methods for complete procedure). Upon addition of D2O, signal changes can be seen on the two singlets around 9 ppm on the green spectra. After 16 h, 2 is completely degraded (Scheme 3, Supplementary Materials Figures S1, S4 and S5). New signals correspond to the starting material 1 [14] and cyclopropyl boronic acid [15] resulting from the hydrolysis of 2.
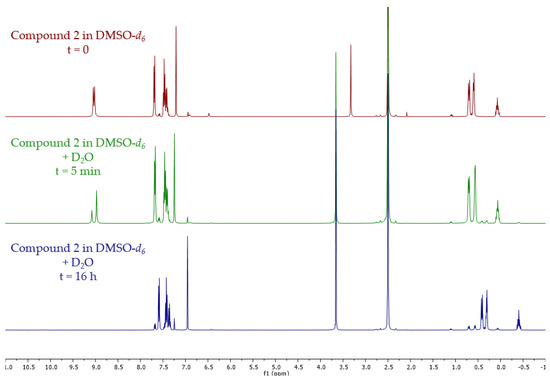
Figure 3.
Evolution of the NMR signals of compound 3 before and after addition of D2O.
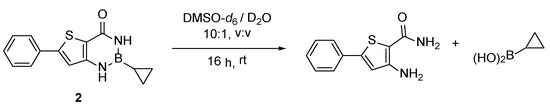
Scheme 3.
Observed degradation of 2 under neutral aqueous conditions.
Like benzo[d][1,3,2]diazaborinin-4-ones, 2 was found to be completely air stable up to one year after its synthesis when stored in a closed hemolysis tube. It is also stable in DMSO-d6 only up to 72 h after dissolution (Supplementary Materials Figures S6 and S7).
To the best of our knowledge, this is the first description of thieno[3,2-d][1,3,2]diazaborinin-4(1H)-one. However, its incompatibility with aqueous media makes it inappropriate as a potential drug candidate.
4. Materials and Methods
Starting materials were purchased from Sigma-Aldrich (Saint Louis, MO, USA) or Fluorochem (Derbyshire, UK). NMR spectra were recorded on a Bruker Avance NEO 400 MHz NanoBay spectrometer at the “Faculté de Pharmacie” of Marseille. The residual proton signal of the deuterated solvent was used as an internal reference: DMSO-d6 δ = 2.50 ppm for 1H and 39.52 ppm for 13C. Data for 1H NMR are reported as follows: chemical shifts (δ) in parts per million (ppm), multiplicity (described as follows: s, singlet; d, doublet; m, multiplet), coupling constants (J) in Hertz (Hz) and integration. Data for 13C NMR are reported as follows: chemical shifts (δ) in parts per million (ppm). HRMS spectrum (ESI) was recorded on a SYNAPT G2 HDMS (Waters) at the “Faculté des Sciences” of Marseille (St Jérôme campus). Melting points were determined on a Köfler melting point apparatus (Wagner & MunzGmbH, München, Germany) and are uncorrected.
2-Cyclopropyl-6-phenyl-2,3-dihydrothieno[3,2-d][1,3,2]diazaborinin-4(1H)-one (2): Under nitrogen atmosphere, 3-amino-5-phenylthiophene-2-carboxamide (100 mg, 0.46 mmol), potassium cyclopropyltrifluoroborate (81 mg, 0.55 mmol) and boron trifluoride ethylamine complex (155 mg, 1.37 mmol) were suspended in toluene and cyclopentyl methyl ether (1:1 v:v, 0.3 mol L−1). The mixture was stirred for 24 h at 80 °C. Upon cooling, water (10 mL) was added, the mixture was filtered and the precipitate was washed with water affording 2-cyclopropyl-6-phenyl-2,3-dihydrothieno[3,2-d][1,3,2]diazaborinin-4(1H)-one as a white solid (115 mg, 93% yield). 1H NMR (DMSO-d6, 400 MHz) δ 9.05 (s, 1H), 9.01 (s, 1H), 7.69 (d, 3J = 7.3 Hz, 2H), 7.52-7.37 (m, 3H), 7.21 (s, 1H), 0.75-0.66 (m, 2H), 0.63-0.55 (m, 2H), 0.12-0.01 (m, 1H). 13C NMR (DMSO-d6, 100 MHz) δ 162.8, 150.9, 148.4, 133.0, 129.3 (2C), 129.2, 125.8, 115.2, 113.6, 5.3 (2C), 4.4. HRMS (ESI) m/z calculated for C14H14N2OSB [M+H]+ 269.0917, found 269.0915. mp = 249–251 °C (degradation).
Procedure for the aqueous stability test: 9 mg of 2 were dissolved in 500 µL of DMSO-d6. At t = 0.50 µL of D2O (10:1 v:v) were added. NMR analysis was then carried out on the sample resulting in the green spectra of Figure 3 (t ≈ 5 min). After 16 h (room temperature at 20 °C), another NMR analysis was performed resulting in the blue spectra of Figure 3 (t ≈ 16 h).
Supplementary Materials
The following are available online. Figure S1. 1H NMR spectra of 2-cyclopropyl-6-phenyl-2,3-dihydrothieno[3,2-d][1,3,2]diazaborinin-4(1H)-one, Figure S2. 13C NMR spectra of 2-cyclopropyl-6-phenyl-2,3-dihydrothieno[3,2-d][1,3,2]diazaborinin-4(1H)-one, Figure S3. HRMS (ESI) spectra of 2-cyclopropyl-6-phenyl-2,3-dihydrothieno[3,2-d][1,3,2]diazaborinin-4(1H)-one, Figure S4. 1H NMR spectra of 2-cyclopropyl-6-phenyl-2,3-dihydrothieno[3,2-d][1,3,2]diazaborinin-4(1H)-one after 5 min in DMSO-d6/D2O mixture (10:1 v:v), Figure S5. 1H NMR spectra of 2-cyclopropyl-6-phenyl-2,3-dihydrothieno[3,2-d][1,3,2]diazaborinin-4(1H)-one after 16 h in DMSO-d6/D2O mixture (10:1 v:v), Figure S6. 1H NMR spectra of 2-cyclopropyl-6-phenyl-2,3-dihydrothieno[3,2-d][1,3,2]diazaborinin-4(1H)-one after one year of storage, Figure S7. 1H NMR spectra of 2-cyclopropyl-6-phenyl-2,3-dihydrothieno[3,2-d][1,3,2]diazaborinin-4(1H)-one after 3 days in DMSO-d6
Author Contributions
Conceptualization, N.P.; formal analysis, R.M.; investigation, R.M.; resources, P.V. and N.P.; writing—original draft preparation, R.M.; writing—review and editing, P.V. and N.P.; supervision, P.V. and N.P.; project administration, N.P.; funding acquisition, N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Fondation pour la Recherche Médicale (FRM)”, project code DCM20181039565.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this study are available in this article and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- WHO. World Malaria Report 2020: 20 Years of Global Progress and Challenges; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-001579-1. [Google Scholar]
- Straimer, J.; Gnadig, N.F.; Witkowski, B.; Amaratunga, C.; Duru, V.; Ramadani, A.P.; Dacheux, M.; Khim, N.; Zhang, L.; Lam, S.; et al. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 2015, 347, 428–431. [Google Scholar] [CrossRef] [PubMed]
- van der Pluijm, R.W.; Imwong, M.; Chau, N.H.; Hoa, N.T.; Thuy-Nhien, N.T.; Thanh, N.V.; Jittamala, P.; Hanboonkunupakarn, B.; Chutasmit, K.; Saelow, C.; et al. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: A prospective clinical, pharmacological, and genetic study. Lancet Infect. Dis. 2019, 19, 952–961. [Google Scholar] [CrossRef]
- Uwimana, A.; Legrand, E.; Stokes, B.H.; Ndikumana, J.-L.M.; Warsame, M.; Umulisa, N.; Ngamije, D.; Munyaneza, T.; Mazarati, J.-B.; Munguti, K.; et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 2020, 26, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Uwimana, A.; Umulisa, N.; Venkatesan, M.; Svigel, S.S.; Zhou, Z.; Munyaneza, T.; Habiman, R.M.; Rucogoza, A.; Moriarty, L.F.; Sandford, R.; et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: An open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect. Dis. 2021, 8. [Google Scholar] [CrossRef]
- Cohen, A.; Suzanne, P.; Lancelot, J.-C.; Verhaeghe, P.; Lesnard, A.; Basmaciyan, L.; Hutter, S.; Laget, M.; Dumètre, A.; Paloque, L.; et al. Discovery of new thienopyrimidinone derivatives displaying antimalarial properties toward both erythrocytic and hepatic stages of Plasmodium. Eur. J. Med. Chem. 2015, 95, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.H.; Abdel-Maksoud, M.S.; Oh, C.-H. Thieno[2,3-d]pyrimidine as a promising scaffold in medicinal chemistry: Recent advances. Bioorganic Med. Chem. 2019, 27, 1159–1194. [Google Scholar] [CrossRef] [PubMed]
- Kurasawa, O.; Miyazaki, T.; Homma, M.; Oguro, Y.; Imada, T.; Uchiyama, N.; Iwai, K.; Yamamoto, Y.; Ohori, M.; Hara, H.; et al. Discovery of a novel, highly potent, and selective thieno[3,2-d]pyrimidinone-based Cdc7 inhibitor with a quinuclidine moiety (TAK-931) as an orally active investigational antitumor agent. J. Med. Chem. 2020, 21, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhu, M.; Zhang, J.; Zhou, H. Synthesis of biologically active boron-containing compounds. MedChemComm 2018, 11, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.H.M.; Mukhtar, A.; Saeednia, B.; Sherafat, F.; Kelly, C.B.; Molander, G.A. Azaborininones: Synthesis and structural analysis of a carbonyl-containing class of azaborines. J. Org. Chem. 2017, 82, 5380–5390. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Eichenauer, N.; Ihara, H.; Yamamoto, T.; Suginome, M. Anthranilamide-masked o-iodoarylboronic acids as coupling modules for iterative synthesis of ortho-linked oligoarenes. Chem. Lett. 2013, 42, 541–543. [Google Scholar] [CrossRef]
- Kamio, S.; Kageyuki, I.; Osaka, I.; Hatano, S.; Abe, M.; Yoshida, H. Anthranilamide (aam)-substituted diboron: Palladium-catalyzed selective b(aam) transfer. Chem. Commun. 2018, 54, 9290–9293. [Google Scholar] [CrossRef] [PubMed]
- Kamio, S.; Kageyuki, I.; Osaka, I.; Yoshida, H. Anthranilamide (aam)-substituted arylboranes in direct carbon–carbon bond-forming reactions. Chem. Commun. 2019, 55, 2624–2627. [Google Scholar] [CrossRef] [PubMed]
- Morwick, T.; Berry, A.; Brickwood, J.; Cardozo, M.; Catron, K.; DeTuri, M.; Emeigh, J.; Homon, C.; Hrapchak, M.; Jacober, S.; et al. Evolution of the thienopyridine class of inhibitors of IκB kinase-β: Part I: Hit-to-lead strategies. J. Med. Chem. 2006, 49, 2898–2908. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.M.; Gillis, E.P.; Burke, M.D. A general solution for unstable boronic acids: Slow-release ross-coupling from air-stable MIDA boronates. J. Am. Chem. Soc. 2009, 131, 6961–6963. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).