Propyl Gallate Attenuates Methylglyoxal-Induced Alzheimer-like Cognitive Deficits and Neuroinflammation in Mice
Abstract
1. Introduction
2. Results
2.1. PG Improves Spatial Learning and Memory in MG-Treated Mice
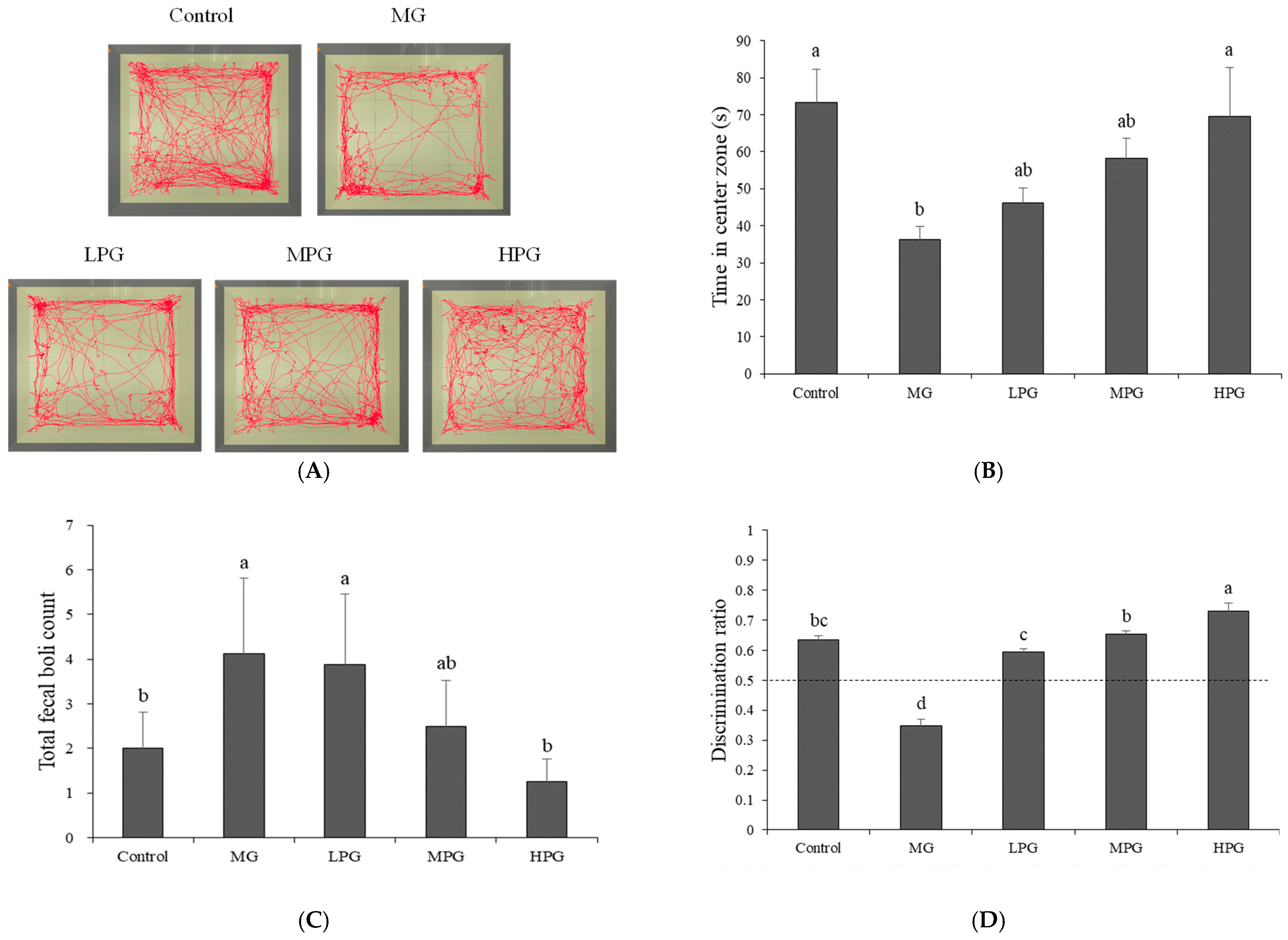
2.2. PG Alleviates Anxiety-like Behavior and Recognition-Memory Deficits
2.3. PG Mitigates Hippocampal Neuronal Damage and Tau Hyperphosphorylation
2.4. PG Reduces Hippocampal p-Tau and Aβ Accumulation
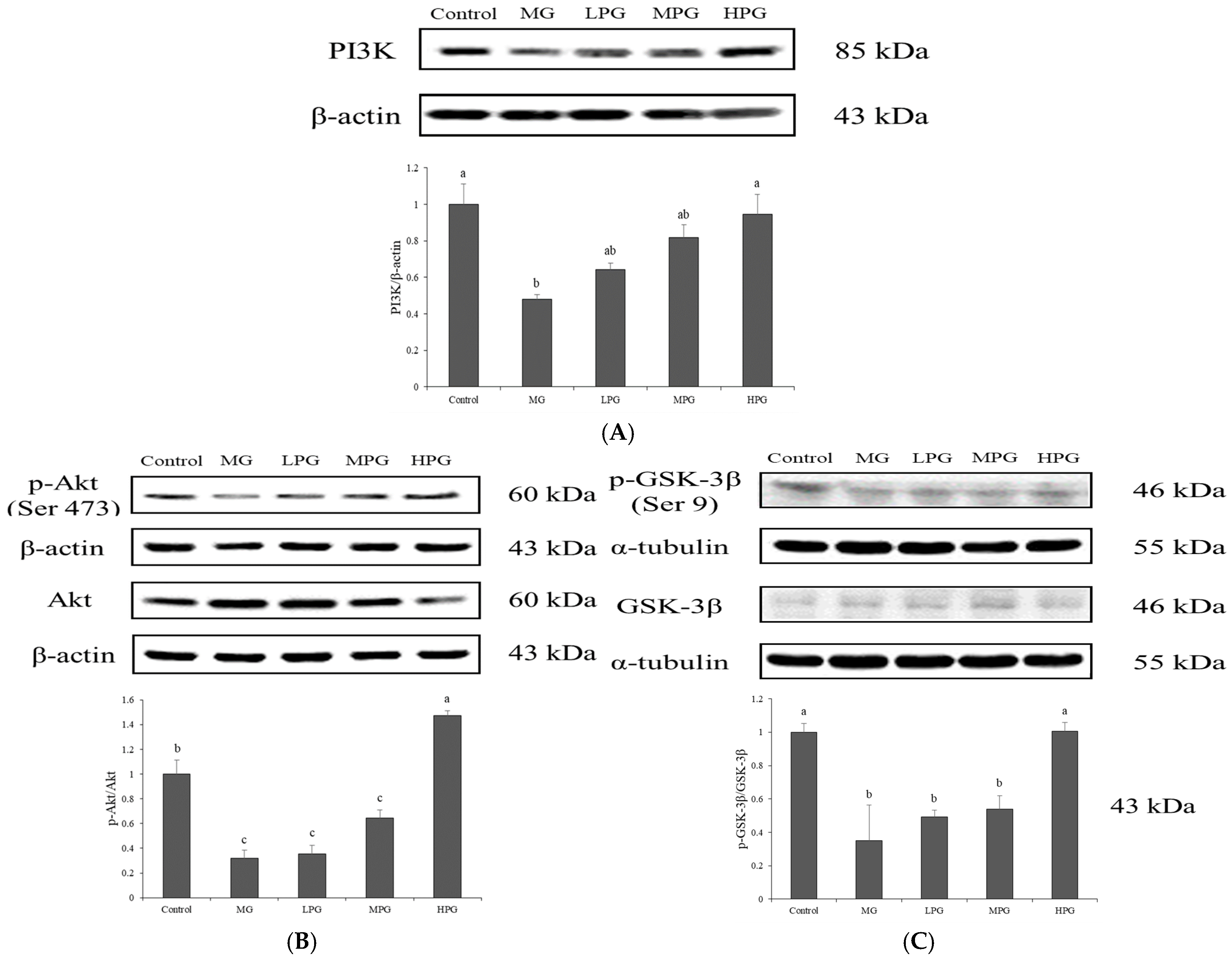
2.5. PG Restores PI3K/Akt/GSK-3β Signaling Disrupted by MG Exposure
2.6. PG Suppresses MG-Induced Hippocampal Inflammation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Experimental Design
4.3. Behavioral Assessments
4.3.1. Morris Water Maze Test
4.3.2. Open Field Test
4.3.3. Novel Object Recognition Test
4.4. Histological and Immunohistochemical Analyses
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef] [PubMed]
- Kellar, D.; Lockhart, S.N.; Aisen, P.; Raman, R.; Rissman, R.A.; Brewer, J.; Craft, S. Intranasal insulin reduces white matter hyperintensity progression in association with improvements in cognition and CSF biomarker profiles in mild cognitive impairment and Alzheimer’s disease. J. Prev. Alzheimers Dis. 2021, 8, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Kubis-Kubiak, A.M.; Rorbach-Dolata, A.; Piwowar, A. Crucial players in Alzheimer’s disease and diabetes mellitus: Friends or foes? Mech. Ageing Dev. 2019, 181, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.C.R.; Pereira, J.D.; Mamede, I.; Caramelli, P.; Silva, V.C.; Veloso, A.A.; Luizon, M.R.; Gomes, K.B. Common miRNAs, genes, and regulatory pathways in Alzheimer’s disease and type 2 diabetes mellitus: An integrative analysis of systematic reviews, bioinformatics and data mining. J. Neurochem. 2025, 169, e70196. [Google Scholar] [CrossRef]
- Amin, A.M.; Mostafa, H.; Khojah, H.M.J. Insulin resistance in Alzheimer’s disease: The genetics and metabolomics links. Clin. Chim. Acta 2023, 539, 215–236. [Google Scholar] [CrossRef]
- Bornemann, E.A.; Kamma, H.K.; Alabbas, M.; Elashahab, M.; Abid, N.; Manaye, S.; Cheran, K.; Murthy, C.; Giva, S.; Penumetcha, S.S. The effect of chronic inflammation and oxidative stress on Alzheimer’s disease progression: A systematic review. Cureus 2025, 17, e84057. [Google Scholar] [CrossRef]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef]
- Atabi, F.; Moassesfar, M.; Nakhaie, T.; Bagherian, M.; Hosseinpour, N.; Hashemi, M. A systematic review on type 3 diabetes: Bridging the gap between metabolic dysfunction and Alzheimer’s disease. Diabetol. Metab. Syndr. 2025, 17, 356. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y. Tau and neuroinflammation in Alzheimer’s disease: Interplay mechanisms and clinical translation. J. Neuroinflamm. 2023, 20, 165. [Google Scholar] [CrossRef]
- Luo, Y.; Yu, H.; Ye, K. Distinct factors drive the progression of tau pathology in Alzheimer’s disease. Fundam. Res. 2025; in press. [Google Scholar] [CrossRef]
- Malafaia, D.; Albuquerque, H.M.T.; Silva, A.M.S. Amyloid-β and tau aggregation dual-inhibitors: A synthetic and structure-activity relationship focused review. Eur. J. Med. Chem. 2021, 214, 113209. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.H.; Park, J.-B. Molecular mechanisms of Alzheimer’s disease induced by amyloid-β and tau phosphorylation along with RhoA activity: Perspective of RhoA/Rho-associated protein kinase inhibitors for neuronal therapy. Cells 2025, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Crick, S.L.; Bu, G.; Frieden, C.; Pappu, R.V.; Lee, J.M. Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc. Natl. Acad. Sci. USA 2009, 106, 20324–20329. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Boccardi, V.; Mancinetti, F.; Mecocci, P. Oxidative stress, advanced glycation end products (AGEs), and neurodegeneration in Alzheimer’s disease: A metabolic perspective. Antioxidants 2025, 14, 1044. [Google Scholar] [CrossRef]
- Raza, A.; Saleem, S.; Imran, S.; Rahman, S.; Haroon, M.; Razzaq, A.; Hussain, A.; Iqbal, J.; Sathian, B. From metabolic dysregulation to neurodegenerative pathology: The role of hyperglycemia, oxidative stress, and blood-brain barrier breakdown in T2D-driven Alzheimer’s disease. Metab. Brain Dis. 2025, 40, 276. [Google Scholar] [CrossRef]
- Lai, S.W.T.; Lopez Gonzalez, E.D.J.; Zoukari, T.; Ki, P.; Shuck, S.C. Methylglyoxal and its adducts: Induction, repair, and association with disease. Chem. Res. Toxicol. 2022, 35, 1720–1746. [Google Scholar] [CrossRef]
- Vašková, J.; Kováčová, G.; Pudelský, J.; Palenčár, D.; Mičková, H. Methylglyoxal formation-metabolic routes and consequences. Antioxidants 2025, 14, 212. [Google Scholar] [CrossRef]
- Maessen, D.E.; Stehouwer, C.D.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015, 128, 839–861. [Google Scholar] [CrossRef]
- Abordo, E.A.; Minhas, H.S.; Thornalley, P.J. Accumulation of alpha-oxoaldehydes during oxidative stress: A role in cytotoxicity. Biochem. Pharmacol. 1999, 58, 641–648. [Google Scholar] [CrossRef]
- Alomar, F.A.; Alshakhs, M.N.; Abohelaika, S.; Almarzouk, H.M.; Almualim, M.; Al-Ali, A.K.; Al-Muhanna, F.; Alomar, M.F.; Alhaddad, M.J.; Almulaify, M.S.; et al. Elevated plasma level of the glycolysis byproduct methylglyoxal on admission is an independent biomarker of mortality in ICU COVID-19 patients. Sci. Rep. 2022, 12, 9510. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, B.; Lüth, H.J.; Haferburg, D.; Boeck, K.; Arendt, T.; Münch, G. Methylglyoxal, glyoxal, and their detoxification in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2005, 1043, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Zambonin, L.; Hrelia, S. Role of methylglyoxal in Alzheimer’s disease. Biomed. Res. Int. 2014, 2014, 238485. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Chen, Z.; Wang, Y.; Liu, X.; Liu, X.; Ke, L.; Zheng, Z.; Lin, X.; Zhou, Y.; Wu, L.; et al. Subcutaneous liraglutide ameliorates methylglyoxal-induced Alzheimer-like tau pathology and cognitive impairment by modulating tau hyperphosphorylation and glycogen synthase kinase-3β. Am. J. Transl. Res. 2017, 9, 247–260. [Google Scholar]
- EFSA Panel on Food additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of propyl gallate (E 310) as a food additive. EFSA J. 2014, 12, 3642. [Google Scholar] [CrossRef]
- Cui, H.; Tao, F.; Hou, Y.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Dual effects of propyl gallate and its methylglyoxal adduct on carbonyl stress and oxidative stress. Food Chem. 2018, 265, 227–232. [Google Scholar] [CrossRef]
- Kawano, Y.; Kawaguchi, M.; Hirota, K.; Kai, S.; Konishi, N.; Furuya, H. Effects of n-propyl gallate on neuronal survival after forebrain ischemia in rats. Resuscitation 2012, 83, 249–252. [Google Scholar] [CrossRef]
- AlRuwaili, R.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Alexiou, A.; Papadakis, M.; Fetoh, M.E.A.-E.; Batiha, G.E.-S. Targeting of the PI3K/AKT/GSK3β pathway in Parkinson’s disease: A therapeutic blueprint. Mol. Neurobiol. 2025, 62, 15108–15131. [Google Scholar] [CrossRef]
- Pan, J.; Yao, Q.; Wang, Y.; Chang, S.; Li, C.; Wu, Y.; Shen, J.; Yang, R. The role of PI3K signaling pathway in Alzheimer’s disease. Front. Aging Neurosci. 2024, 16, 1459025. [Google Scholar] [CrossRef]
- Rekha, A.; Afzal, M.; Babu, M.A.; Menon, S.V.; Nathiya, D.; Supriya, S.; Mishra, S.B.; Gupta, S.; Goyal, K.; Rana, M.; et al. GSK-3β dysregulation in aging: Implications for tau pathology and Alzheimer’s disease progression. Mol. Cell. Neurosci. 2025, 133, 104005. [Google Scholar] [CrossRef]
- Peng, X.; Guo, H.; Zhang, X.; Yang, Z.; Ruganzu, J.B.; Yang, Z.; Wu, X.; Bi, W.; Ji, S.; Yang, W. TREM2 inhibits tau hyperphosphorylation and neuronal apoptosis via the PI3K/Akt/GSK-3β signaling pathway in vivo and in vitro. Mol. Neurobiol. 2023, 60, 2470–2485. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, Y.; Xu, Q.Q.; Xian, Y.F.; Lin, Z.X. Sulforaphene ameliorates neuroinflammation and hyperphosphorylated tau protein via regulating the PI3K/Akt/GSK-3β pathway in experimental models of Alzheimer’s disease. Oxid. Med. Cell Longev. 2020, 2020, 4754195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, P.; Sang, S. Dietary genistein inhibits methylglyoxal-induced advanced glycation end product formation in mice fed a high-fat diet. J. Nutr. 2019, 149, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.B.; Velosky, A.G.; McCabe, J.T. Applications of the Morris water maze in translational traumatic brain injury research. Neurosci. Biobehav. Rev. 2018, 88, 187–200. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, H.; Ou, J.; Liu, P.; Huang, C.; Wang, M.; Simal-Gandara, J.; Battino, M.; Jafari, S.M.; Zou, L.; et al. Benefits, deleterious effects and mitigation of methylglyoxal in foods: A critical review. Trends Food Sci. Technol. 2021, 107, 201–212. [Google Scholar] [CrossRef]
- Pucci, M.; Aria, F.; Premoli, M.; Maccarinelli, G.; Mastinu, A.; Bonini, S.; Memo, M.; Uberti, D.; Abate, G. Methylglyoxal affects cognitive behaviour and modulates RAGE and Presenilin-1 expression in hippocampus of aged mice. Food Chem. Toxicol. 2021, 158, 112608. [Google Scholar] [CrossRef]
- Szczepanik, J.C.; de Almeida, G.R.L.; Cunha, M.P.; Dafre, A.L. Repeated methylglyoxal treatment depletes dopamine in the prefrontal cortex, and causes memory impairment and depressive-like behavior in mice. Neurochem. Res. 2020, 45, 354–370. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Rahaman, M.M.; Islam, T.; Bappi, M.H.; Sikder, M.I.; Hossain, K.N.; Akter, F.; Al Shamsh Prottay, A.; Rokonuzzman, M.; Gürer, E.S.; et al. Neurobiological effects of gallic acid: Current perspectives. Chin. Med. 2023, 18, 27. [Google Scholar] [CrossRef]
- Kokras, N.; Poulogiannopoulou, E.; Sotiropoulos, M.G.; Paravatou, R.; Goudani, E.; Dimitriadou, M.; Papakonstantinou, E.; Doxastakis, G.; Perrea, D.N.; Hloupis, G.; et al. Behavioral and neurochemical effects of extra virgin olive oil total phenolic content and Sideritis extract in female mice. Molecules 2020, 25, 5000. [Google Scholar] [CrossRef]
- Patil, G.; Kulsange, S.; Kazi, R.; Chirmade, T.; Kale, V.; Mote, C.; Aswar, M.; Koratkar, S.; Agawane, S.; Kulkarni, M. Behavioral and proteomic studies reveal methylglyoxal activate pathways associated with Alzheimer’s disease. ACS Pharmacol. Transl. Sci. 2023, 6, 65–75. [Google Scholar] [CrossRef]
- Li, X.H.; Lv, B.L.; Xie, J.Z.; Liu, J.; Zhou, X.W.; Wang, J.Z. AGEs induce Alzheimer-like tau pathology and memory deficit via RAGE-mediated GSK-3 activation. Neurobiol. Aging 2012, 33, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; van der Flier, W.M.; Jessen, F.; Hoozemanns, J.; Thal, D.R.; Boche, D.; Brosseron, F.; Teunissen, C.; Zetterberg, H.; Jacobs, A.H.; et al. Neuroinflammation in Alzheimer disease. Nat. Rev. Immunol. 2025, 25, 321–352. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef] [PubMed]
- Boronat, A.; Serreli, G.; Rodríguez-Morató, J.; Deiana, M.; de la Torre, R. Olive oil phenolic compounds’ activity against age-associated cognitive decline: Clinical and experimental evidence. Antioxidants 2023, 12, 1472. [Google Scholar] [CrossRef]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic acids and prevention of cognitive decline: Polyphenols with a neuroprotective role in cognitive disorders and Alzheimer’s disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, J.; Andersen, M.L.; Peters, G.H.J.; Lund, M.N. Predicting the reaction rates between flavonoids and methylglyoxal by combining molecular properties and machine learning. Food Biosci. 2023, 54, 102890. [Google Scholar] [CrossRef]
- Gao, Y.; Tan, L.; Yu, J.T.; Tan, L. Tau in Alzheimer’s disease: Mechanisms and therapeutic strategies. Curr. Alzheimer Res. 2018, 15, 283–300. [Google Scholar] [CrossRef]
- Plascencia-Villa, G.; Perry, G. Roles of oxidative stress in synaptic dysfunction and neuronal cell death in Alzheimer’s disease. Antioxidants 2023, 12, 1628. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, D.M. Antioxidant therapies for neuroprotection—A review. J. Clin. Med. 2019, 8, 1659. [Google Scholar] [CrossRef]
- Basha, S.; Ks, P.; Pai, A.R.; Mahato, K.K. Citrus phytochemicals in neurodegenerative diseases: Preclinical evidence and clinical potential. Trends Food Sci. Technol. 2025, 166, 105390. [Google Scholar] [CrossRef]
- Nakadate, K.; Ito, N.; Kawakami, K.; Yamazaki, N. Anti-inflammatory actions of plant-derived compounds and prevention of chronic diseases: From molecular mechanisms to applications. Int. J. Mol. Sci. 2025, 26, 5206. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Tsai, H.-Y.; Qiu, J.; Liao, H.-W.; Chang, C.-I.; Chen, Y.-H.; Ho, C.-T.; Chen, Y.-K. Propyl Gallate Attenuates Methylglyoxal-Induced Alzheimer-like Cognitive Deficits and Neuroinflammation in Mice. Int. J. Mol. Sci. 2026, 27, 511. https://doi.org/10.3390/ijms27010511
Tsai H-Y, Qiu J, Liao H-W, Chang C-I, Chen Y-H, Ho C-T, Chen Y-K. Propyl Gallate Attenuates Methylglyoxal-Induced Alzheimer-like Cognitive Deficits and Neuroinflammation in Mice. International Journal of Molecular Sciences. 2026; 27(1):511. https://doi.org/10.3390/ijms27010511
Chicago/Turabian StyleTsai, Hui-Yun, Jing Qiu, Han-Wei Liao, Chi-I Chang, Yu-Hsiang Chen, Chi-Tang Ho, and Yu-Kuo Chen. 2026. "Propyl Gallate Attenuates Methylglyoxal-Induced Alzheimer-like Cognitive Deficits and Neuroinflammation in Mice" International Journal of Molecular Sciences 27, no. 1: 511. https://doi.org/10.3390/ijms27010511
APA StyleTsai, H.-Y., Qiu, J., Liao, H.-W., Chang, C.-I., Chen, Y.-H., Ho, C.-T., & Chen, Y.-K. (2026). Propyl Gallate Attenuates Methylglyoxal-Induced Alzheimer-like Cognitive Deficits and Neuroinflammation in Mice. International Journal of Molecular Sciences, 27(1), 511. https://doi.org/10.3390/ijms27010511






