Bone Metabolic Changes and Osteoporosis During Pregnancy and Lactation: A View from Dental Medicine
Abstract
1. Introduction
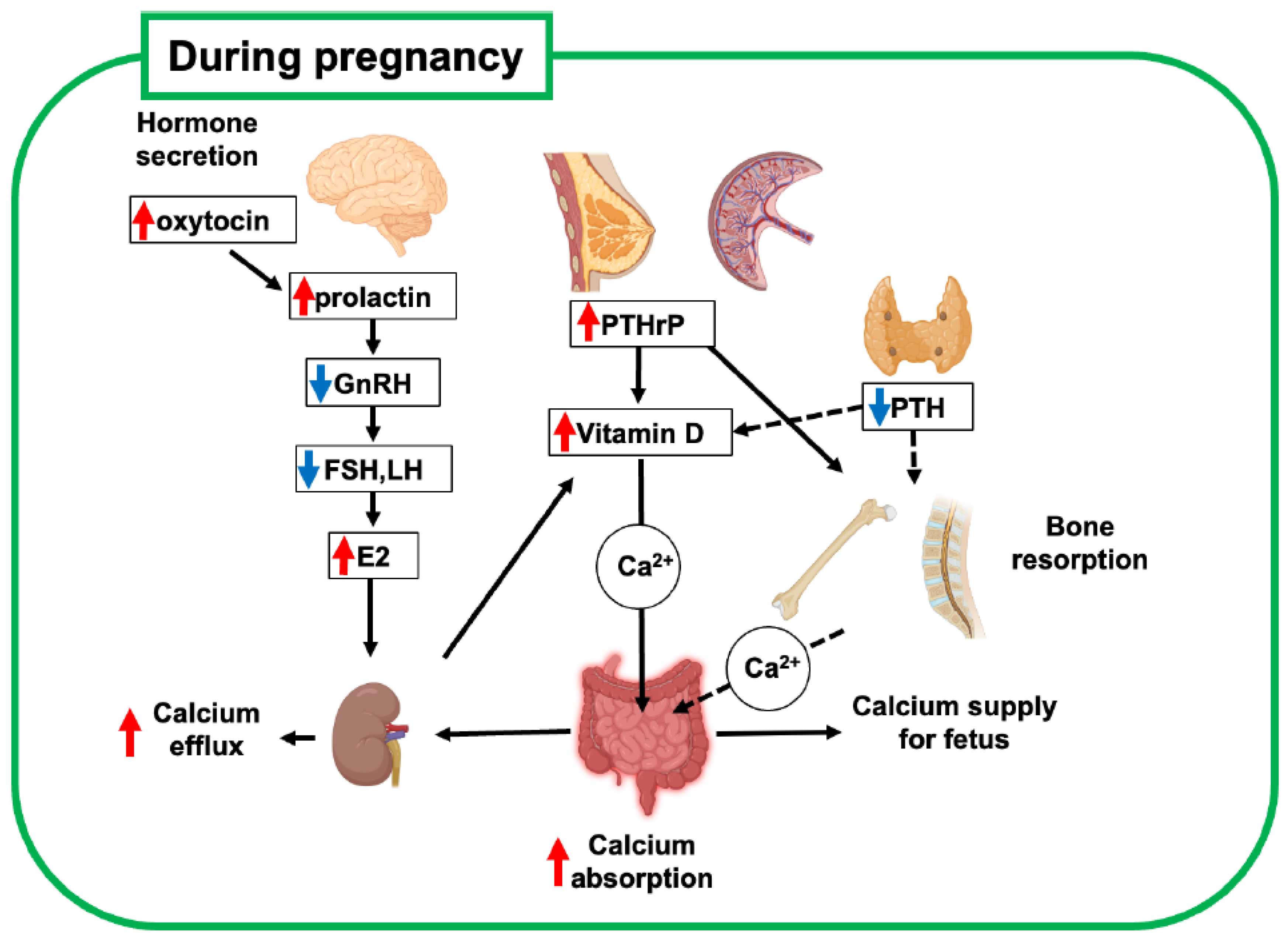
2. Bone Metabolism During Pregnancy
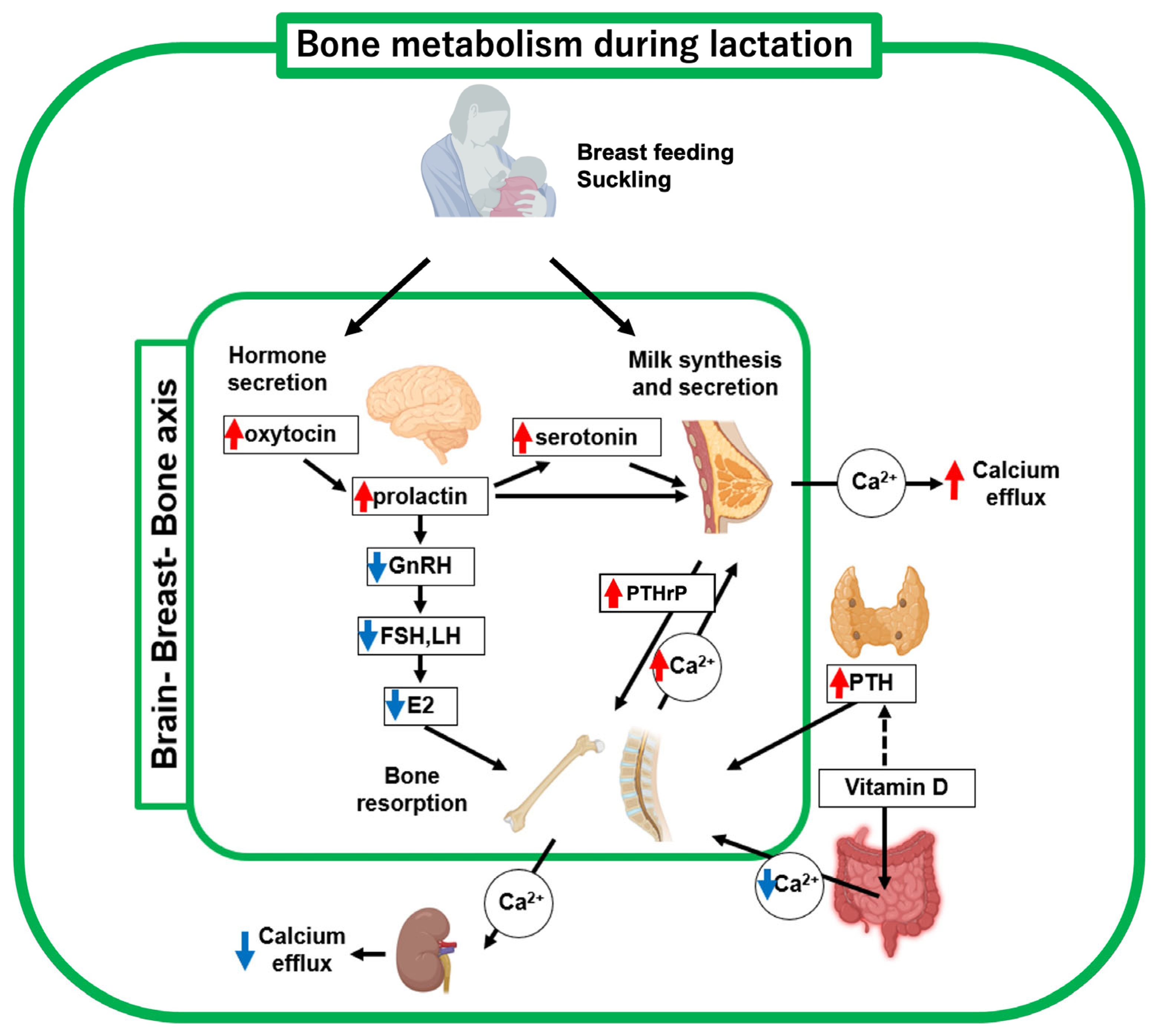
3. Brain-Breast-Bone Axis Regulates Bone Metabolism During Lactation
4. Physiological Recovery of Bone Metabolism After Pregnancy and Lactation
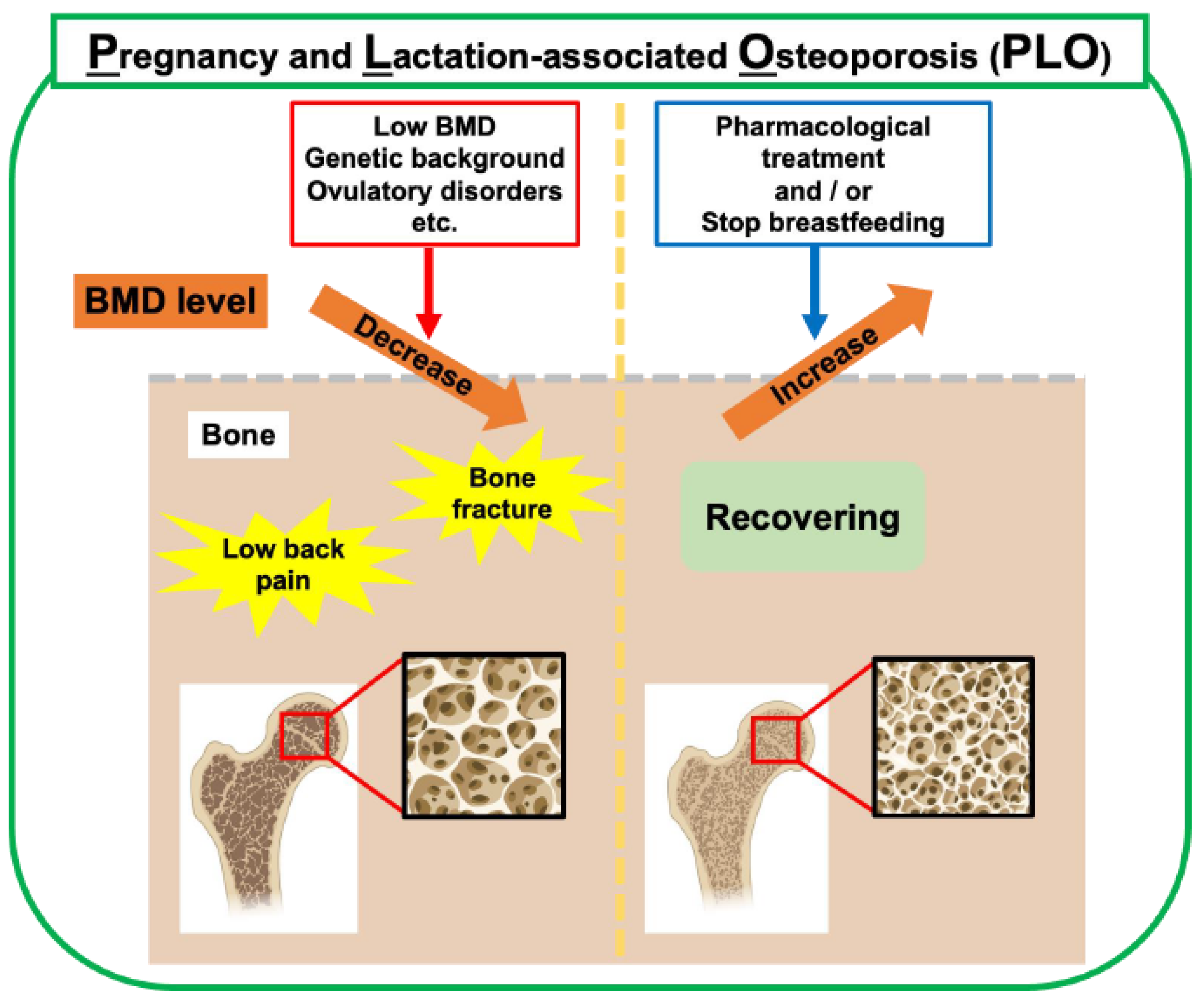
5. PLO
6. Breastfeeding and Development of PLO in Lactating Mothers
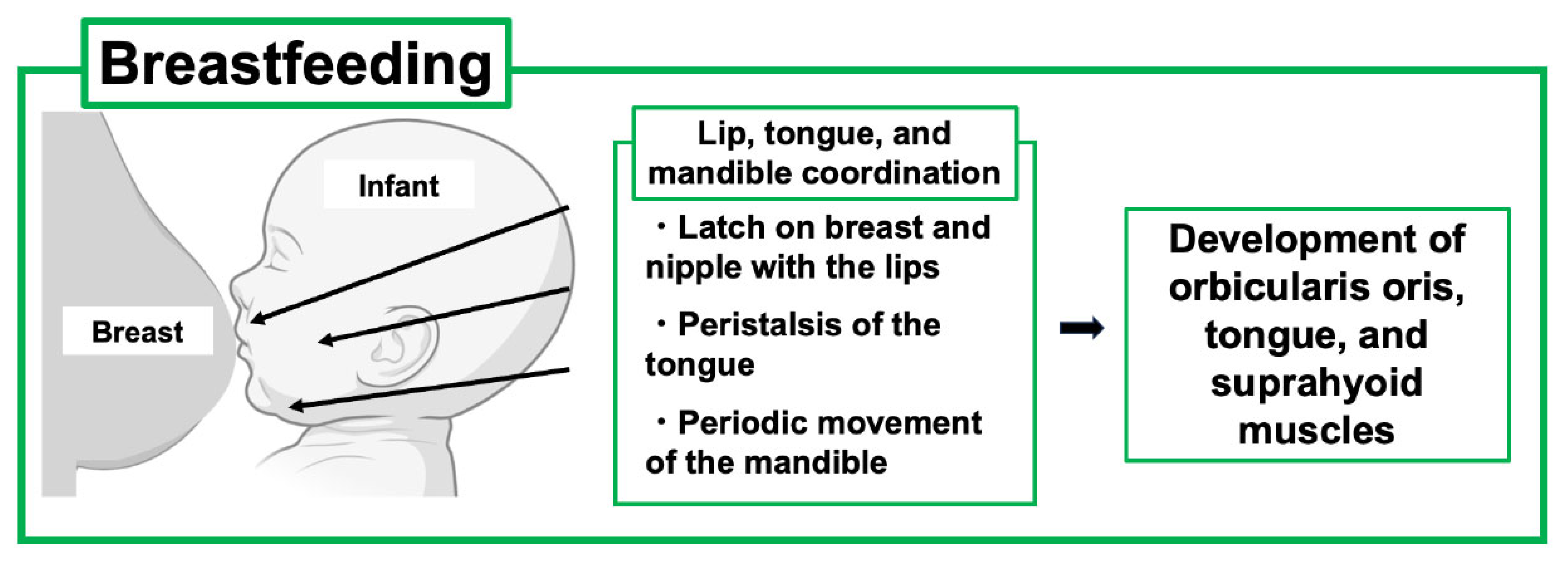
7. Breastfeeding and Infant Development, Including the Maxillofacial System
8. Pharmacotherapies for PLO
9. Consideration of Temporomandibular Joint Disorders During Pregnancy and Lactation
10. Consideration of Orthodontic Tooth Movement During Pregnancy and Lactation
11. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gak, N.; Abbara, A.; Dhillo, W.S.; Keen, R.; Comninos, A.N. Current and future perspectives on pregnancy and lactation-associated osteoporosis. Front. Endocrinol. 2024, 15, 1494965. [Google Scholar] [CrossRef]
- Anagnostis, P.; Lampropoulou-Adamidou, K.; Bosdou, J.K.; Trovas, G.; Galanis, P.; Chronopoulos, E.; Goulis, D.G.; Tournis, S. Comparative Effectiveness of Therapeutic Interventions in Pregnancy and Lactation-Associated Osteoporosis: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2024, 109, 879–901. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xia, B.; Ge, L. Effects of breast-feeding duration, bottle-feeding duration and non-nutritive sucking habits on the occlusal characteristics of primary dentition. BMC Pediatr. 2015, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.C.; Giugliani, E.R.; Caramez da Silva, F. Influence of the duration of breastfeeding on quality of muscle function during mastication in preschoolers: A cohort study. BMC Public Health 2012, 12, 934. [Google Scholar] [CrossRef] [PubMed]
- Hermont, A.P.; Martins, C.C.; Zina, L.G.; Auad, S.M.; Paiva, S.M.; Pordeus, I.A. Breastfeeding, bottle feeding practices and malocclusion in the primary dentition: A systematic review of cohort studies. Int. J. Environ. Res. Public Health 2015, 12, 3133–3151. [Google Scholar] [CrossRef]
- Robinson, J.L.; Johnson, P.M.; Kister, K.; Yin, M.T.; Chen, J.; Wadhwa, S. Estrogen signaling impacts temporomandibular joint and periodontal disease pathology. Odontology 2020, 108, 153–165. [Google Scholar] [CrossRef]
- Kovacs, C.S. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef]
- Athonvarangkul, D.; Wysolmerski, J.J. Crosstalk within a brain-breast-bone axis regulates mineral and skeletal metabolism during lactation. Front. Physiol. 2023, 14, 1121579. [Google Scholar] [CrossRef]
- Kirby, B.J.; Ma, Y.; Martin, H.M.; Buckle Favaro, K.L.; Karaplis, A.C.; Kovacs, C.S. Upregulation of calcitriol during pregnancy and skeletal recovery after lactation do not require parathyroid hormone. J. Bone Miner. Res. 2013, 28, 1987–2000. [Google Scholar] [CrossRef]
- Kovacs, C.S. The role of vitamin D in pregnancy and lactation: Insights from animal models and clinical studies. Annu. Rev. Nutr. 2012, 32, 97–123. [Google Scholar] [CrossRef]
- Kovacs, C.S. The Skeleton Is a Storehouse of Mineral That Is Plundered During Lactation and (Fully?) Replenished Afterwards. J. Bone Miner. Res. 2017, 32, 676–680. [Google Scholar] [CrossRef]
- Miyamoto, T.; Miyakoshi, K.; Sato, Y.; Kasuga, Y.; Ikenoue, S.; Miyamoto, K.; Nishiwaki, Y.; Tanaka, M.; Nakamura, M.; Matsumoto, M. Changes in bone metabolic profile associated with pregnancy or lactation. Sci. Rep. 2019, 9, 6787. [Google Scholar] [CrossRef]
- Honda, A.; Kurabayashi, T.; Yahata, T.; Tomita, M.; Matsushita, H.; Takakuwa, K.; Tanaka, K. Effects of pregnancy and lactation on trabecular bone and marrow adipocytes in rats. Calcif. Tissue Int. 2000, 67, 367–372. [Google Scholar] [CrossRef]
- Kent, G.N.; Price, R.I.; Gutteridge, D.H.; Smith, M.; Allen, J.R.; Bhagat, C.I.; Barnes, M.P.; Hickling, C.J.; Retallack, R.W.; Wilson, S.G.; et al. Human lactation: Forearm trabecular bone loss, increased bone turnover, and renal conservation of calcium and inorganic phosphate with recovery of bone mass following weaning. J. Bone Miner. Res. 1990, 5, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Parsons, C.; Maslin, K.; D’Angelo, S.; Moon, R.J.; Crozier, S.R.; Gossiel, F.; Bishop, N.J.; Kennedy, S.H.; Papageorghiou, A.T.; et al. Bone turnover in pregnancy, measured by urinary CTX, is influenced by vitamin D supplementation and is associated with maternal bone health: Findings from the Maternal Vitamin D Osteoporosis Study (MAVIDOS) trial. Am. J. Clin. Nutr. 2021, 114, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.S.; Schurman, C.A.; White, C.R.; Alliston, T. Investigating Osteocytic Perilacunar/Canalicular Remodeling. Curr. Osteoporos. Rep. 2019, 17, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Jahn, K.; Rauner, M.; Busse, B.; Bonewald, L.F. Physiological and pathological osteocytic osteolysis. J. Musculoskelet. Neuronal Interact. 2018, 18, 292–303. [Google Scholar]
- Lotinun, S.; Ishihara, Y.; Nagano, K.; Kiviranta, R.; Carpentier, V.T.; Neff, L.; Parkman, V.; Ide, N.; Hu, D.; Dann, P.; et al. Cathepsin K-deficient osteocytes prevent lactation-induced bone loss and parathyroid hormone suppression. J. Clin. Investig. 2019, 129, 3058–3071. [Google Scholar] [CrossRef]
- Qing, H.; Bonewald, L.F. Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int. J. Oral Sci. 2009, 1, 59–65. [Google Scholar] [CrossRef]
- Nakanishi-Kimura, A.; Takakura, A.; Hoshi-Numahata, M.; Watanabe, H.; Nishiura, M.; Sato, Y.; Takao-Kawabata, R.; Iimura, T. Dynamic morphometric changes in the mandibular osteocytic lacunae of ovariectomized rats in response to teriparatide, as revealed by three-dimensional fluorescence analyses: Possible involvement of osteocytic perilacunar remodeling. J. Oral Biosci. 2024, 66, 49–60. [Google Scholar] [CrossRef]
- Takakura, A.; Sato, T.; Lee, J.W.; Hirano, K.; Takao-Kawabata, R.; Ishizuya, T.; Iimura, T. Expansion of the osteocytic lacunar-canalicular system involved in pharmacological action of PTH revealed by AI-driven fluorescence morphometry in female rabbits. Sci. Rep. 2022, 12, 16799. [Google Scholar] [CrossRef] [PubMed]
- de Bakker, C.M.J.; Tseng, W.J.; Li, Y.; Zhao, H.; Altman-Singles, A.R.; Jeong, Y.; Robberts, J.; Han, L.; Kim, D.G.; Sherry Liu, X. Reproduction Differentially Affects Trabecular Bone Depending on Its Mechanical Versus Metabolic Role. J. Biomech. Eng. 2017, 139, 1110061–11100610. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Basta-Pljakic, J.; Seref-Ferlengez, Z.; Majeska, R.J.; Cardoso, L.; Bromage, T.G.; Zhang, Q.; Flach, C.R.; Mendelsohn, R.; Yakar, S.; et al. Lactation-Induced Changes in the Volume of Osteocyte Lacunar-Canalicular Space Alter Mechanical Properties in Cortical Bone Tissue. J. Bone Miner. Res. 2017, 32, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; Bowman, B.M. Rapid inactivation and apoptosis of osteoclasts in the maternal skeleton during the bone remodeling reversal at the end of lactation. Anat. Rec. 2007, 290, 65–73. [Google Scholar] [CrossRef]
- Ardeshirpour, L.; Dann, P.; Adams, D.J.; Nelson, T.; VanHouten, J.; Horowitz, M.C.; Wysolmerski, J.J. Weaning triggers a decrease in receptor activator of nuclear factor-kappaB ligand expression, widespread osteoclast apoptosis, and rapid recovery of bone mass after lactation in mice. Endocrinology 2007, 148, 3875–3886. [Google Scholar] [CrossRef]
- Kasahara, K.; Tanaka-Mizuno, S.; Tsuji, S.; Ohashi, M.; Kasahara, M.; Kawasaki, T.; Murakami, T. Pregnancy and lactation-associated osteoporosis as a major type of premenopausal osteoporosis: A retrospective cohort study based on real-world data. BMC Pregnancy Childbirth 2024, 24, 301. [Google Scholar] [CrossRef]
- Hadji, P.; Mouzakiti, N.; Kyvernitakis, I. Effect of Teriparatide on Subsequent Fracture and Bone Mineral Density in 47 Women with Pregnancy- and Lactation-associated Osteoporosis and Vertebral Fractures. Geburtshilfe Frauenheilkd. 2022, 82, 619–626. [Google Scholar] [CrossRef]
- Bonfanti, R.C.; Melchiori, F.; Teti, A.; Albano, G.; Raffard, S.; Rodgers, R.; Lo Coco, G. The association between social comparison in social media, body image concerns and eating disorder symptoms: A systematic review and meta-analysis. Body Image 2025, 52, 101841. [Google Scholar] [CrossRef]
- Dobranowska, K.; Plinska, S.; Dobosz, A. Dietary and Lifestyle Management of Functional Hypothalamic Amenorrhea: A Comprehensive Review. Nutrients 2024, 16, 2967. [Google Scholar] [CrossRef]
- Peris, P.; Guanabens, N.; Monegal, A.; Pons, F.; Martinez de Osaba, M.J.; Ros, I.; Munoz-Gomez, J. Pregnancy associated osteoporosis: The familial effect. Clin. Exp. Rheumatol. 2002, 20, 697–700. [Google Scholar]
- Rolvien, T.; Stürznickel, J.; Schmidt, F.N.; Butscheidt, S.; Schmidt, T.; Busse, B.; Mundlos, S.; Schinke, T.; Kornak, U.; Amling, M.; et al. Comparison of Bone Microarchitecture Between Adult Osteogenesis Imperfecta and Early-Onset Osteoporosis. Calcif. Tissue Int. 2018, 103, 512–521. [Google Scholar] [CrossRef]
- Butscheidt, S.; Tsourdi, E.; Rolvien, T.; Delsmann, A.; Sturznickel, J.; Barvencik, F.; Jakob, F.; Hofbauer, L.C.; Mundlos, S.; Kornak, U.; et al. Relevant genetic variants are common in women with pregnancy and lactation-associated osteoporosis (PLO) and predispose to more severe clinical manifestations. Bone 2021, 147, 115911. [Google Scholar] [CrossRef] [PubMed]
- Kalkwarf, H.J.; Specker, B.L.; Bianchi, D.C.; Ranz, J.; Ho, M. The effect of calcium supplementation on bone density during lactation and after weaning. N. Engl. J. Med. 1997, 337, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Brembeck, P.; Lorentzon, M.; Ohlsson, C.; Winkvist, A.; Augustin, H. Changes in cortical volumetric bone mineral density and thickness, and trabecular thickness in lactating women postpartum. J. Clin. Endocrinol. Metab. 2015, 100, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Bjornerem, A.; Ghasem-Zadeh, A.; Wang, X.; Bui, M.; Walker, S.P.; Zebaze, R.; Seeman, E. Irreversible Deterioration of Cortical and Trabecular Microstructure Associated with Breastfeeding. J. Bone Miner. Res. 2017, 32, 681–687. [Google Scholar] [CrossRef]
- More, C.; Bettembuk, P.; Bhattoa, H.P.; Balogh, A. The effects of pregnancy and lactation on bone mineral density. Osteoporos. Int. 2001, 12, 732–737. [Google Scholar] [CrossRef]
- Elad, D.; Kozlovsky, P.; Blum, O.; Laine, A.F.; Po, M.J.; Botzer, E.; Dollberg, S.; Zelicovich, M.; Ben Sira, L. Biomechanics of milk extraction during breast-feeding. Proc. Natl. Acad. Sci. USA 2014, 111, 5230–5235. [Google Scholar] [CrossRef]
- Geddes, D.T.; Sakalidis, V.S.; Hepworth, A.R.; McClellan, H.L.; Kent, J.C.; Lai, C.T.; Hartmann, P.E. Tongue movement and intra-oral vacuum of term infants during breastfeeding and feeding from an experimental teat that released milk under vacuum only. Early Hum. Dev. 2012, 88, 443–449. [Google Scholar] [CrossRef]
- Yonezu, T.; Kadoya, M.; Yakushiji, M. Effects of prolonged breast- and bottle-feeding on occlusal characteristics in the primary dentition. Pediatr. Dent. J. 2005, 15, 176–179. [Google Scholar] [CrossRef][Green Version]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Black, D.M.; Reid, I.R.; Boonen, S.; Bucci-Rechtweg, C.; Cauley, J.A.; Cosman, F.; Cummings, S.R.; Hue, T.F.; Lippuner, K.; Lakatos, P.; et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: A randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J. Bone Miner. Res. 2012, 27, 243–254, Erratum in J. Bone Miner. Res. 2012, 27, 2612. [Google Scholar] [CrossRef]
- Ito, E.; Sato, Y.; Kobayashi, T.; Soma, T.; Matsumoto, T.; Kimura, A.; Miyamoto, K.; Matsumoto, H.; Matsumoto, M.; Nakamura, M.; et al. Transient alendronate administration to pregnant or lactating mothers prevents bone loss in mice without adverse effects on offspring. Bone 2021, 153, 116133. [Google Scholar] [CrossRef]
- Bone, H.G.; Bolognese, M.A.; Yuen, C.K.; Kendler, D.L.; Miller, P.D.; Yang, Y.C.; Grazette, L.; San Martin, J.; Gallagher, J.C. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J. Clin. Endocrinol. Metab. 2011, 96, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Taylor, V.E.; Castro-Martinez, A.; Dhakal, S.; Zamerli, A.; Mohanty, S.; Xiao, Y.; Simic, M.K.; Wen, J.; Chai, R.; et al. Temporal patterns of osteoclast formation and activity following withdrawal of RANKL inhibition. J. Bone Miner. Res. 2024, 39, 484–497. [Google Scholar] [CrossRef]
- Ishikawa, K.; Tani, S.; Sakai, N.; Kudo, Y.; Horiuchi, H.; Kimura-Suda, H.; Takami, M.; Tsuji, M.; Inagaki, K.; Kiuchi, Y.; et al. Mouse model of anti-RANKL discontinuation reveals reduced bone mass and quality through disruption of bone remodeling. Bone Res. 2025, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Takami, M.; Azetsu, Y.; Karakawa, A.; Chatani, M.; Funatsu, T.; Sakai, N. Effects of anti-RANKL antibodies administered to pregnant mice on bone and tooth development in neonates. J. Oral. Biosci. 2023, 65, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Satterwhite, J.; Heathman, M.; Miller, P.D.; Marin, F.; Glass, E.V.; Dobnig, H. Pharmacokinetics of teriparatide (rhPTH[1–34]) and calcium pharmacodynamics in postmenopausal women with osteoporosis. Calcif. Tissue Int. 2010, 87, 485–492. [Google Scholar] [CrossRef]
- Hellmeyer, L.; Boekhoff, J.; Hadji, P. Treatment with teriparatide in a patient with pregnancy-associated osteoporosis. Gynecol. Endocrinol. 2010, 26, 725–728. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, W.; Zhao, S.; Mo, X.; Yuan, W.; Cheung, W.H.; Fu, D.; Chen, B. Effect of Teriparatide on pain relief, and quality of life in postmenopausal females with osteoporotic vertebral compression fractures, a retrospective cohort study. Ann. Palliat. Med. 2021, 10, 4000–4007. [Google Scholar] [CrossRef]
- Dore, R.K.; Chen, P.Q.; Glass, E.V.; Krege, J.H. Reduced risk of back pain following teriparatide treatment: A meta-analysis. Arthritis Rheum. 2004, 50, 273–280. [Google Scholar]
- Nevitt, M.C.; Chen, P.Q.; Dore, R.K.; Reginster, J.Y.; Kiel, D.P.; Zanchetta, J.R.; Glass, E.V.; Krege, J.H. Reduced risk of back pain following teriparatide treatment: A meta-analysis. Osteoporos. Int. 2006, 17, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takao-Kawabata, R.; Takakura, A.; Shimazu, Y.; Nakatsugawa, M.; Ito, A.; Lee, J.W.; Kawasaki, K.; Iimura, T. Teriparatide relieves ovariectomy-induced hyperalgesia in rats, suggesting the involvement of functional regulation in primary sensory neurons by PTH-mediated signaling. Sci. Rep. 2020, 10, 5346. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.Y.; Song, J.E.; Park, K.H.; Seok, H.; Lee, E.J.; Lim, S.K.; Rhee, Y. Effect of teriparatide on pregnancy and lactation-associated osteoporosis with multiple vertebral fractures. J. Bone Miner. Metab. 2012, 30, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, N.; Kim, K.J.; Park, C.H.; Lee, J.; Rhee, Y. Bone Density After Teriparatide Discontinuation With or Without Antiresorptive Therapy in Pregnancy- and Lactation-Associated Osteoporosis. Calcif. Tissue Int. 2021, 109, 544–553. [Google Scholar] [CrossRef]
- Lampropoulou-Adamidou, K.; Trovas, G.; Triantafyllopoulos, I.K.; Yavropoulou, M.P.; Anastasilakis, A.D.; Anagnostis, P.; Toulis, K.A.; Makris, K.; Gazi, S.; Balanika, A.; et al. Teriparatide Treatment in Patients with Pregnancy- and Lactation-Associated Osteoporosis. Calcif. Tissue Int. 2021, 109, 554–562. [Google Scholar] [CrossRef]
- Ali, D.S.; Khan, A.A.; Brandi, M.L. Effective strategies for pregnancy and lactation-associated osteoporosis: Teriparatide use in focus. Endocrine 2024, 86, 459–469. [Google Scholar] [CrossRef]
- Zhao, Y.; Qian, S.; Zheng, Z.; Peng, J.; Liu, J.; Guan, X.; Liao, C. Consideration of hormonal changes for orthodontic treatment during pregnancy and lactation—A review. Reprod. Biol. Endocrinol. 2024, 22, 106. [Google Scholar] [CrossRef]
- Iodice, G.; Cimino, R.; Vollaro, S.; Lobbezoo, F.; Michelotti, A. Prevalence of temporomandibular disorder pain, jaw noises and oral behaviours in an adult Italian population sample. J. Oral. Rehabil. 2019, 46, 691–698. [Google Scholar] [CrossRef]
- LeResche, L. Epidemiology of temporomandibular disorders: Implications for the investigation of etiologic factors. Crit. Rev. Oral. Biol. Med. 1997, 8, 291–305. [Google Scholar] [CrossRef]
- Watanabe, H.; Iori, T.; Lee, J.W.; Kajii, T.S.; Takakura, A.; Takao-Kawabata, R.; Kitagawa, Y.; Maruoka, Y.; Iimura, T. Association between an Increased Serum CCL5 Level and Pathophysiology of Degenerative Joint Disease in the Temporomandibular Joint in Females. Int. J. Mol. Sci. 2023, 24, 2775. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yoo, D.M.; Kwon, M.J.; Kim, J.H.; Kim, J.H.; Byun, S.H.; Park, B.; Lee, H.J.; Choi, H.G. Increased Risk of Temporomandibular Joint Disorder in Osteoporosis Patients: A Longitudinal Study. Front. Endocrinol. 2022, 13, 835923. [Google Scholar] [CrossRef]
- Iwasaki, T.; Takahara, N.; Duc, V.V.; Tomomatsu, N.; Tabata, M.J.; Yoda, T. Effect of anterior disc displacement and estrogen deficiency on rabbit mandibular condyle. J. Oral. Biosci. 2025, 67, 100599. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciu, M. Prevalence of temporomandibular disorders (TMD) in pregnancy: A systematic review with meta-analysis. J. Oral. Rehabil. 2023, 50, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Marrapodi, M.M.; La Verde, M.; Meto, A.; Siurkel, Y.; Cicciu, M.; Russo, D. The relationship between pregnancy and temporomandibular disorder (TMD) through diagnostic criteria for temporomandibular disorders (DC/TMD) axis II evaluation: A case-control cross-sectional study. BMC Oral Health 2024, 24, 342. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Basoa, M.E.; Gintzler, A.R. Estrogen and progesterone activate spinal kappa-opiate receptor analgesic mechanisms. Pain 1996, 64, 608–615. [Google Scholar] [CrossRef]
- LeResche, L.; Sherman, J.J.; Huggins, K.; Saunders, K.; Mancl, L.A.; Lentz, G.; Dworkin, S.F. Musculoskeletal orofacial pain and other signs and symptoms of temporomandibular disorders during pregnancy: A prospective study. J. Orofac. Pain. 2005, 19, 193–201. [Google Scholar]
- Sun, Q.; Zhen, G.; Li, T.P.; Guo, Q.; Li, Y.; Su, W.; Xue, P.; Wang, X.; Wan, M.; Guan, Y.; et al. Parathyroid hormone attenuates osteoarthritis pain by remodeling subchondral bone in mice. eLife 2021, 10, e66532. [Google Scholar] [CrossRef]
- Ling, Z.; Crane, J.; Hu, H.; Chen, Y.; Wan, M.; Ni, S.; Demehri, S.; Mohajer, B.; Peng, X.; Zou, X.; et al. Parathyroid hormone treatment partially reverses endplate remodeling and attenuates low back pain in animal models of spine degeneration. Sci. Transl. Med. 2023, 15, eadg8982. [Google Scholar] [CrossRef]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef]
- Yamane, H.; Takakura, A.; Shimadzu, Y.; Kodama, T.; Lee, J.W.; Isogai, Y.; Ishizuya, T.; Takao-Kawabata, R.; Iimura, T. Acute development of cortical porosity and endosteal naive bone formation from the daily but not weekly short-term administration of PTH in rabbit. PLoS ONE 2017, 12, e0175329. [Google Scholar] [CrossRef]
- Takakura, A.; Lee, J.W.; Hirano, K.; Isogai, Y.; Ishizuya, T.; Takao-Kawabata, R.; Iimura, T. Administration frequency as well as dosage of PTH are associated with development of cortical porosity in ovariectomized rats. Bone Res. 2017, 5, 17002. [Google Scholar] [CrossRef]
- Hoshi-Numahata, M.; Takakura, A.; Nakanishi-Kimura, A.; Watanabe, H.; Takada, K.; Nishiura, M.; Sato, Y.; Takao-Kawabata, R.; Iimura, T. Evaluation of cortical bone remodeling in canines treated with daily and weekly administrations of teriparatide by establishing AI-driven morphometric analyses and GIS-based spatial mapping. Bone Rep. 2023, 19, 101720. [Google Scholar] [CrossRef] [PubMed]
- Hellsing, E.; Hammarstrom, L. The effects of pregnancy and fluoride on orthodontic tooth movements in rats. Eur. J. Orthod. 1991, 13, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, K.; Olyaee, P.; Mirzakouchaki, B.; Ghahremani, L.; Garjani, A.; Dadgar, E.; Marjani, S. The effect of pregnancy on orthodontic tooth movement in rats. Med. Oral. Patol. Oral. Cir. Bucal 2013, 18, e351–e355. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Kaklamanos, E.G. Does the rate of orthodontic tooth movement change during pregnancy and lactation? A systematic review of the evidence from animal studies. BMC Oral Health 2020, 20, 237. [Google Scholar] [CrossRef]
- Carneiro, R.M.; Prebehalla, L.; Tedesco, M.B.; Sereika, S.M.; Hugo, M.; Hollis, B.W.; Gundberg, C.M.; Stewart, A.F.; Horwitz, M.J. Lactation and bone turnover: A conundrum of marked bone loss in the setting of coupled bone turnover. J. Clin. Endocrinol. Metab. 2010, 95, 1767–1776. [Google Scholar] [CrossRef]
- Macari, S.; Sharma, L.A.; Wyatt, A.; da Silva, J.M.; Dias, G.J.; Silva, T.A.; Szawka, R.E.; Grattan, D.R. Lactation induces increases in the RANK/RANKL/OPG system in maxillary bone. Bone 2018, 110, 160–169. [Google Scholar] [CrossRef]
- Iglesias-Linares, A.; Morford, L.A.; Hartsfield, J.K., Jr. Bone Density and Dental External Apical Root Resorption. Curr. Osteoporos. Rep. 2016, 14, 292–309. [Google Scholar] [CrossRef]
- Agarwal, K.; Nagendra, L.; Bhattacharya, S. Approach to premenopausal osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2025. [Google Scholar] [CrossRef]
| Pregnancy | Lactation | |
|---|---|---|
| Brain | ↑ oxytocin, prolactin | ↑ oxytocin, prolactin |
| ↓ GnRH, FSH, LH | ↓ GnRH, FSH, LH | |
| Parathyroid gland | ↓ PTH | ↑ PTH |
| Breast | ↑ PTHrP | ↑ PTHrP |
| Intestine | ↑ calcium absorption | Normal calcium absorption |
| Kidney | ↑ urinary calcium excretion | ↓ urinary calcium excretion |
| Ovary | ↑ E2 | ↓ E2 |
| Blood serum | ↑ CTX, NTX | ↑ CTX, NTX |
| ↑ P1NP | ↑ P1NP, osteocalcin | |
| Bone | ↓ BMD spine up to −4.6% | ↓ BMD spine up to −7% |
| ↓ BMD hip up to −4.2% | ↓ BMD hip up to −3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiura, M.; Watanabe, H.; Nakanishi-Kimura, A.; Hoshi-Numahata, M.; Nishimoto, S.; Ueno, F.; Koguchi, R.; Takemoto, R.; Kurakane, Y.; Bao, L.; et al. Bone Metabolic Changes and Osteoporosis During Pregnancy and Lactation: A View from Dental Medicine. Int. J. Mol. Sci. 2025, 26, 10476. https://doi.org/10.3390/ijms262110476
Nishiura M, Watanabe H, Nakanishi-Kimura A, Hoshi-Numahata M, Nishimoto S, Ueno F, Koguchi R, Takemoto R, Kurakane Y, Bao L, et al. Bone Metabolic Changes and Osteoporosis During Pregnancy and Lactation: A View from Dental Medicine. International Journal of Molecular Sciences. 2025; 26(21):10476. https://doi.org/10.3390/ijms262110476
Chicago/Turabian StyleNishiura, Mai, Haruhisa Watanabe, Atsuko Nakanishi-Kimura, Marie Hoshi-Numahata, Shinnosuke Nishimoto, Fumi Ueno, Riyu Koguchi, Ryutaro Takemoto, Yusuke Kurakane, Lang Bao, and et al. 2025. "Bone Metabolic Changes and Osteoporosis During Pregnancy and Lactation: A View from Dental Medicine" International Journal of Molecular Sciences 26, no. 21: 10476. https://doi.org/10.3390/ijms262110476
APA StyleNishiura, M., Watanabe, H., Nakanishi-Kimura, A., Hoshi-Numahata, M., Nishimoto, S., Ueno, F., Koguchi, R., Takemoto, R., Kurakane, Y., Bao, L., & Iimura, T. (2025). Bone Metabolic Changes and Osteoporosis During Pregnancy and Lactation: A View from Dental Medicine. International Journal of Molecular Sciences, 26(21), 10476. https://doi.org/10.3390/ijms262110476





