Abstract
Pregnancy- and lactation-associated osteoporosis (PLO) is receiving increasing attention. During pregnancy and lactation, bone metabolism is dramatically changed to supply minerals to the fetus and infant, which is a major cause of PLO. Weaning of lactation is clinically a primary choice to treat lactation-induced osteoporosis since breastfeeding is a key regulator of the pathophysiology during lactation. However, breastfeeding is beneficial to the physical and mental development of infants. We also discuss the beneficial effects of breastfeeding on the oral and maxillofacial development of infants. Pharmacological treatment of PLO is also discussed. This review also discusses how dynamic regulatory changes in bone metabolism during pregnancy and lactation affect homeostasis of the temporomandibular joint (TMJ) and alveolar bone in mothers, from the perspectives of TMJ diseases and orthodontic treatment.
1. Introduction
Pregnancy and lactation-associated osteoporosis (PLO) is defined as osteoporosis that occurs in mothers with rapidly increasing calcium demand from late pregnancy to postpartum lactation, resulting in back pain and vertebral compression fractures in the spine [1]. The prenatal and postpartum periods are critical for the health of mothers and children. Bone fractures and related pain resulting from PLO have a significant impact on the quality of life of women. As PLO is a relatively rare disease, its pathophysiological mechanisms and effective treatment strategies have not yet been fully established [1].
The pharmacological treatment of PLO in pregnant and lactating women should be considered with caution from the viewpoint of placental passage and milk transfer. Therefore, the first choice in PLO treatment is to restrict breastfeeding to limit calcium outflow. However, premature interruption of breastfeeding can cause malnutrition and increase the risk of infection, weakness, and obesity, as indicated by the WHO [2]. In addition, weaning affects the development of the oral function in infants [3,4,5]. Thus, maternal PLO can affect the growth and development of infants, including their oral development. Therefore, PLO is an important clinical issue in pediatric dentistry. This review discusses the potential impact of weaning lactation on the oral development of infants as a treatment for PLO. The pharmacological treatment of PLO is also discussed.
Dynamic hormonal changes during pregnancy and lactation are a major cause of the pathophysiology of PLO and are likely to be associated with skeletal metabolism in the oral and maxillofacial system of the mother. Therefore, it is important to consider how changes in bone metabolism during pregnancy and lactation affect temporomandibular joint (TMJ) homeostasis and alveolar bone metabolism.
In this review, we provide an overview of the current understanding of physiological changes in bone metabolism in mothers during pregnancy and lactation. We discuss how these changes influence the TMJ and orthodontic tooth movement in mothers from the perspectives of oral surgery, medicine, and orthodontics. Hormonal changes during pregnancy and lactation can alter the oral microbiome, thus promoting gingivitis and affecting bone repair [6], but these topics are beyond the scope of this review paper.
2. Bone Metabolism During Pregnancy
During fetal development, approximately 80% of calcium, phosphorus, and magnesium accumulate in the developing skeleton in late pregnancy. The fetal demand for calcium and phosphorus in late pregnancy is estimated to be equivalent to 5–10% of the maternal plasma [7]. Dramatic and reversible changes in bone metabolism occur differently in late gestational mothers [1].
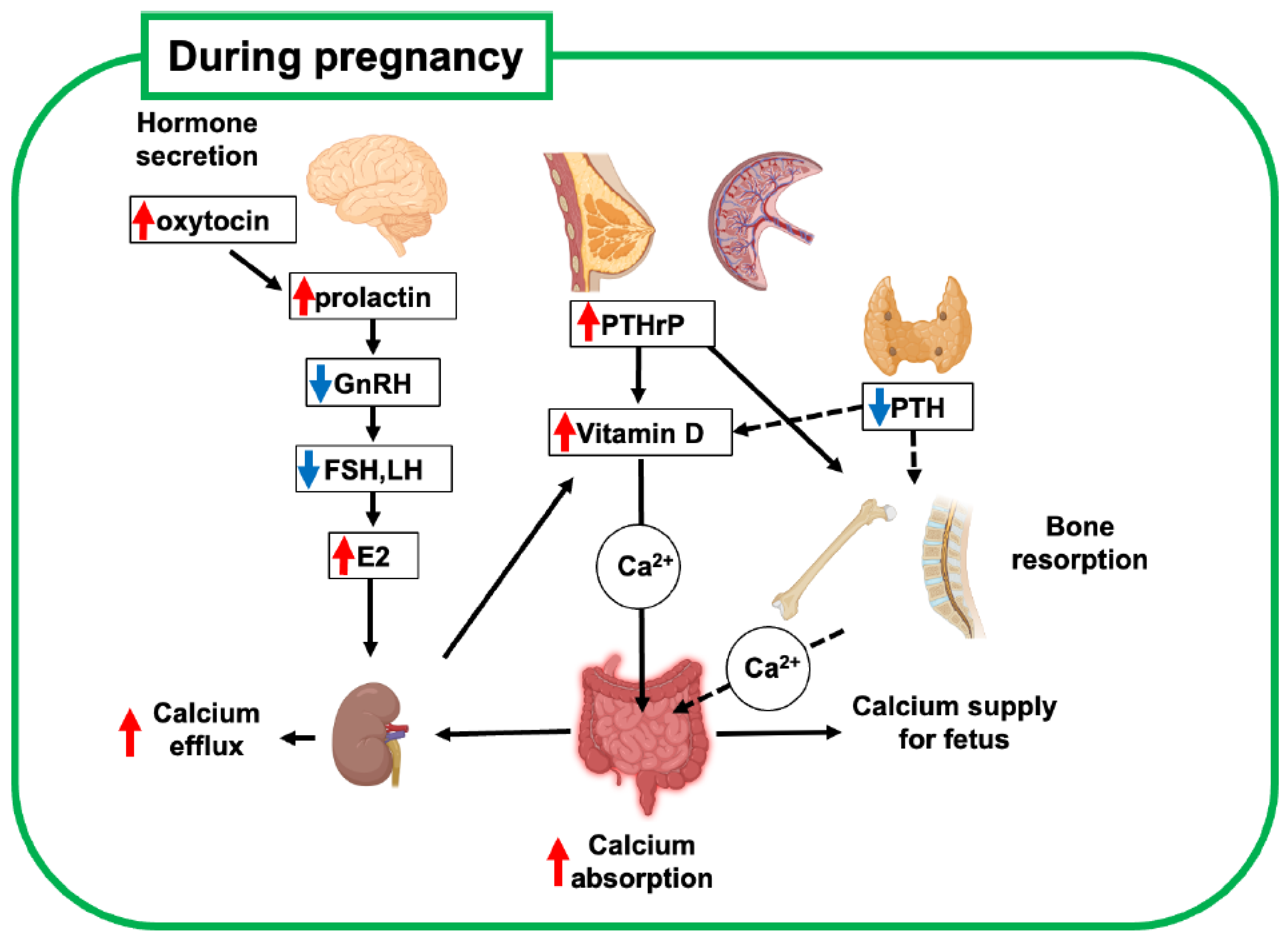
During late pregnancy, elevated levels of active vitamin D3 (1,25(OH)2D3) in blood circulation increase calcium absorption from the gastrointestinal tract in mothers compared with that before pregnancy [1,8] (Figure 1, Table 1). This increase in calcium absorption is driven by elevated levels of estradiol and prolactin, which upregulate maternal renal 1a-hydroxylase activity [9,10]. This is balanced by upregulated urinary calcium excretion. This suggests that the increasing calcium demand during gestation is compensated mainly by the calcium uptake in the gastrointestinal tract rather than by calcium derivation from the mother’s bone.
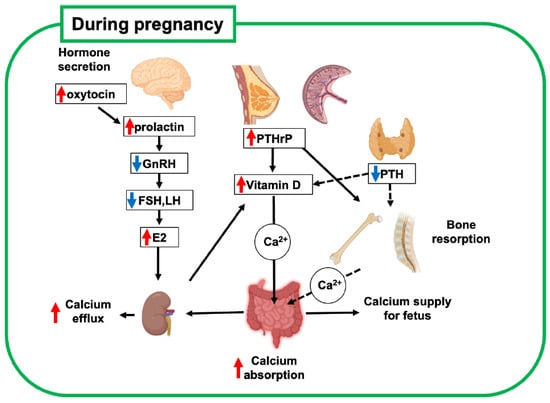
Figure 1.
Schematic diagram of bone metabolic changes in pregnant mother. Increased estrogen and participation of PTHrP in systemic regulation are key regulators of bone metabolism and mineral supply to the fetus. Red and blue arrows indicate upregulated and downregulated hormones, respectively. This figure was created with BioRender.com (accessed on 25 September 2025). PTH, parathyroid hormone; PTHrP, parathyroid hormone-related peptide; GnRH, gonadotropin-releasing hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol.

Table 1.
Physiological changes in the mother during pregnancy and lactation. PTH: Parathyroid hormone, PTHrP: Parathyroid hormone-related peptide, GnRH: Gonadotropic releasing hormone, FSH: Follicle-stimulating hormone, LH: Luteinizing hormone, E2: estradiol, CTX: collagen type 1 c-telopeptide, NTX: collagen type 1 n-telopeptide, P1NP: procollagen type 1 amino-terminal propeptide, BMD: bone mineral density.
The secretion of parathyroid hormone (PTH) decreases during pregnancy, while the secretion of parathyroid hormone-related peptide (PTHrP) increases [7]. PTHrP is not measurable in the serum of non-pregnant women, and because of its relatively short half-life as a peptide factor, it acts as a local hormone in the developmental and homeostatic regulation of tissues, such as cartilage and mammary glands. Importantly, PTHrP levels are elevated during pregnancy because of increased production from the placenta, breasts, uterus, and embryonic tissues, and this phenomenon reduces the blood level of PTH as a hormonal regulator [7]. These findings indicate that PTHrP functions as a systemic hormone for bone and calcium metabolism, similar to PTH, which drives the unique regulation of bone metabolism during pregnancy. Therefore, upregulated PTHrP also contributes to increased calcium absorption during lactation by enhancing active vitamin D3 synthesis, similar to PTH in non-pregnant women.
3. Brain-Breast-Bone Axis Regulates Bone Metabolism During Lactation
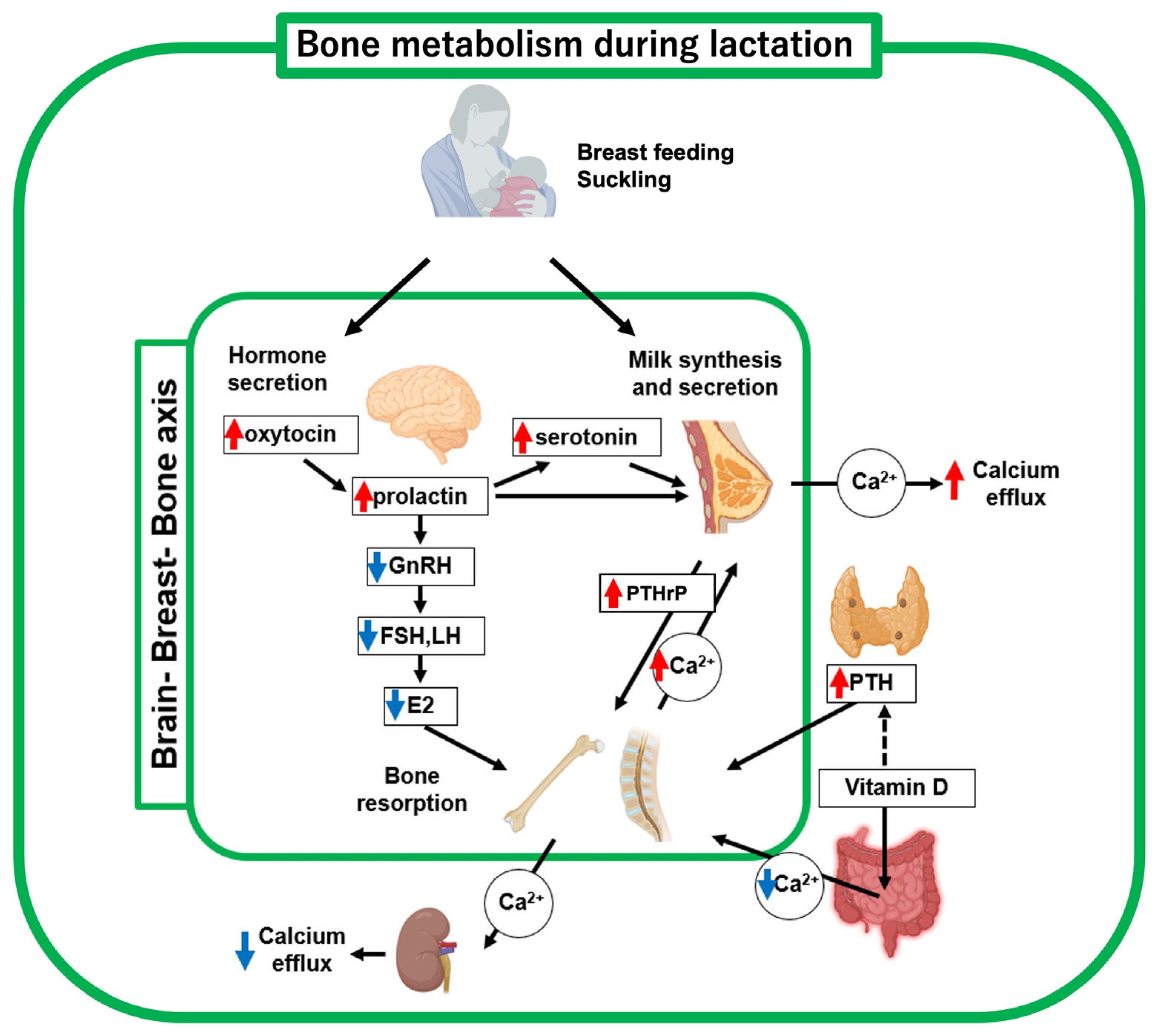
During lactation, coordination of the brain-breast-bone axis of maternal calcium regulation contributes to an adequate supply of calcium required for milk production in which upregulated neural hormones such as oxytocin, prolactin and serotonin regulate bone metabolic molecules [8] (Figure 2, Table 1). The proper functioning of this brain-breast-bone axis regulatory system enables increased calcium demand during lactation. Breastfeeding is a critical driver of this axis, which is further discussed in a later section (see Section 6). In a lactating mother, the calcium uptake from the gastrointestinal tract is unchanged compared with that before pregnancy [11]. Hormonally, PTH, PTHrP, and prolactin secretion increase, while estradiol levels drop sharply [12]. The serum vitamin D level has been reported to be inversely correlated with serum PTH levels. A decrease and an increase in blood levels of 1,25(OH)2D3 and PTH, respectively, were observed after delivery [12]. These hormonal changes during lactation promote osteoclast functions. Therefore, bone resorption by osteoclasts is believed to be the main response to the rapid increase in calcium demand for milk secretion.
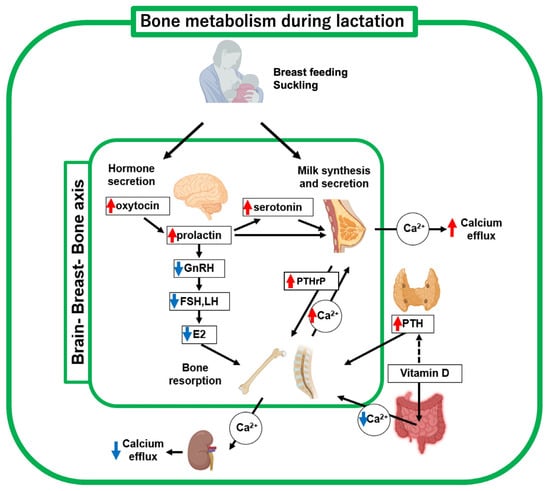
Figure 2.
Schematic diagram of bone metabolic changes in lactating mother. Decreased estrogen and increased PTHrP and PTH levels, respectively, are key regulators of bone metabolism and mineral supply to breast milk. Suckling (breast-feeding) upregulates oxytocin, prolactin, and serotonin, thus driving the brain-breast-bone axis. Red and blue arrows indicate upregulated and downregulated hormones, respectively. This figure was created with BioRender.com (accessed on 25 September 2025).
Increased bone resorption mainly affects the trabecular and endocortical surfaces [13,14]. In nursing women, bone turnover is thought to be promoted, since bone formation marker levels, such as procollagen 1 Intact N-terminal propeptide (P1NP) and osteocalcin, as well as bone resorption marker levels, such as collagen type 1 c-telopeptide (CTX) and n-telopeptide (NTX), have been shown to be increased [15].
In addition to bone resorption by osteoclasts on the bone surface, increased osteocyte lacunar-canalicular bone remodeling, known as osteocytic osteolysis, has been reported during lactation [16,17]. Osteocytes express osteoclast-like bone resorptive enzymes such as cathepsin K and MMP13, resulting in osteocytic bone resorption that demineralizes and resorbs bone around osteocyte lacunar and luminal networks [18,19]. These findings demonstrate that osteocytic perilacunar bone resorption and osteoclastic bone resorption participate in changes in bone metabolism during lactation. In animal model studies using rabbits and rats, the administration of teriparatide, an active peptide of human PTH, changed the size of osteocyte lacunae [20,21]. Therefore, augmented PTH and PTHrP levels during lactation are likely to regulate osteocytic perilacunar bone remodeling directly.
It has also been shown that osteocytes respond to mechanical forces and regulate the activity of bone surface cells, which affects overall bone turnover. It is important to determine how changes in lacunar volume due to osteocytic osteolysis during lactation and load changes that occur during pregnancy and postpartum affect the balance of bone metabolism through the regulation of osteoclasts and osteoblasts. These dramatic changes in bone metabolism in lactating women are likely to be associated with reduced material properties and mechanical stress of the bone, as suggested by animal models [22,23], although no direct measurement of bone strength in women has been reported. Nevertheless, nursing women do not typically have fractures, except in very rare cases of PLO.
4. Physiological Recovery of Bone Metabolism After Pregnancy and Lactation
Bone loss associated with pregnancy and lactation usually returns to the pre-pregnancy state within a few months [1]. The anabolic phase of bone after lactation is thought to play a major role in metabolic recovery. During the bone anabolic phase, the menstrual cycle resumes, which induces estradiol by prolactin and decreases PTHrP levels. These hormonal changes contribute to the induction of osteoclast apoptosis and decrease in bone resorption [24]. Bone formation during the anabolic phase is rapidly accelerated by the induction of osteoblast progenitor cell differentiation into osteoblasts in the bone marrow [25]. Therefore, in the post-lactation phase, bone formation overcomes bone resorption, which is due to dramatic hormonal changes. The changes that occur because of osteocytic osteolysis are also reversed. Osteocytes rapidly lose their osteoclast-like properties and return to an osteoblast-like phenotype with the ability to remineralize lacuna; thus, the hypertrophic osteocyte lacuna fully returns to its baseline volume approximately one week after weaning [19,23].
5. PLO
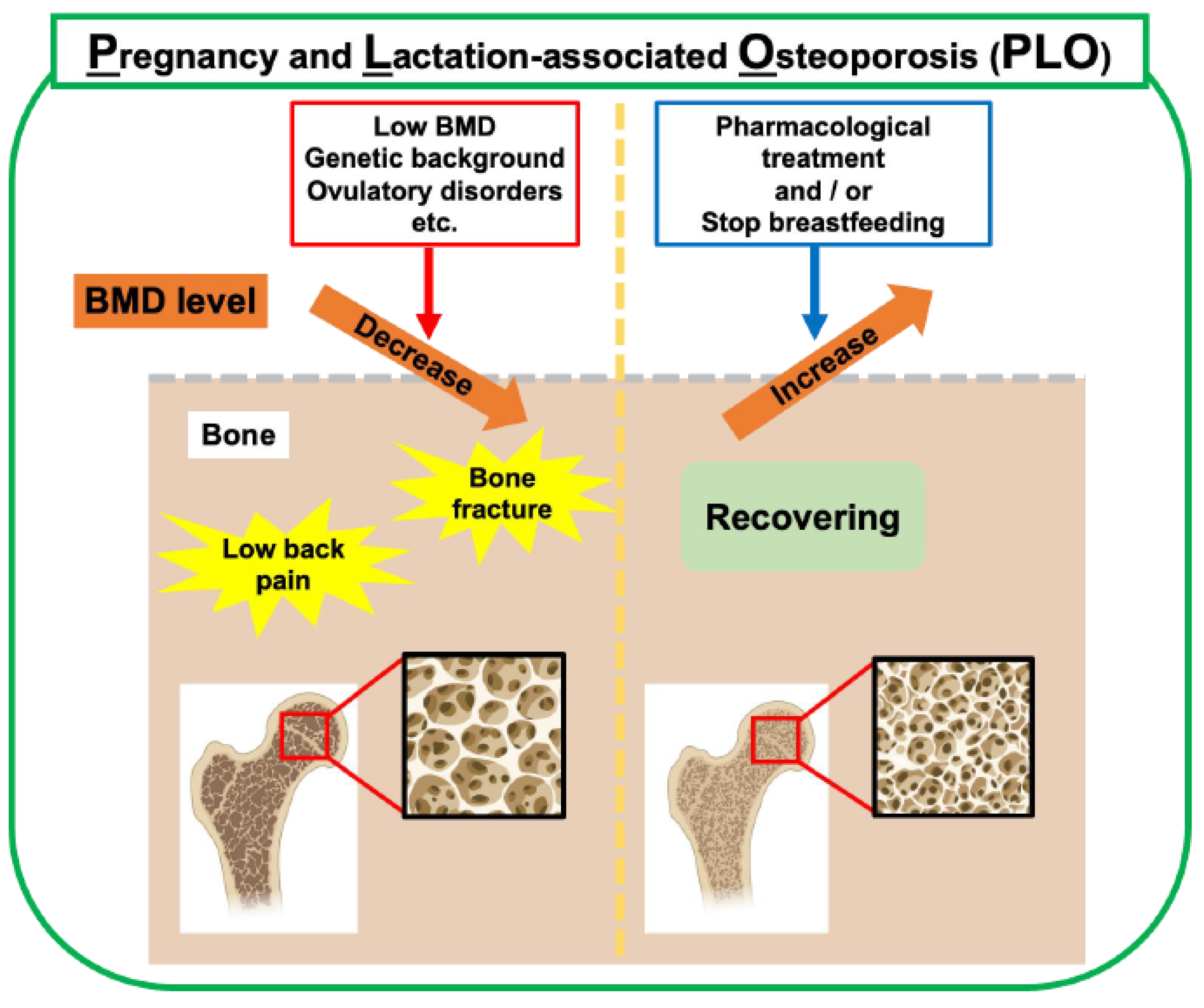
Osteoporosis is a metabolic bone disease characterized by decreased bone mass and quality, which makes bones fragile and easily broken. Osteoporosis can be classified as primary osteoporosis, such as age-related osteoporosis and postmenopausal osteoporosis, with a rapid decrease in estrogen levels, and secondary osteoporosis caused by endocrine factors, such as hyperthyroidism and Cushing’s syndrome, or drug-related factors, such as long-term steroid use, and consequences of digestive diseases, such as gastrectomy. Furthermore, PLO occurs in mothers with a rapidly increasing calcium demand from late pregnancy to postpartum lactation (Figure 3). PLO can cause back pain and vertebral compression fractures in the spine, significantly affecting the quality of life of the mother and child before and after childbirth.
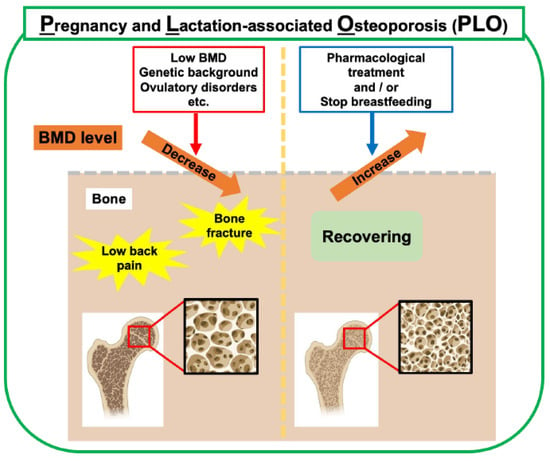
Figure 3.
Risk factors and management of PLO. In addition to bone metabolic changes during pregnancy and lactation, low BMD, genetic background, and ovulatory disorders are risk factors. Weaning breastfeeding is the primary choice for treating PLO in lactating mothers. Pharmacological treatment with anti-osteoporosis drugs is clinically used to treat PLO. This figure was created with BioRender.com (accessed on 25 September 2025).
The incidence rate of PLO has been reported to be 4–8 cases per million. However, a recent report by Kasahara et al. in 2024 estimated the incidence rate in Japan to be 460 cases per million. Although the number of PLO patients appears to have rapidly increased, it can be considered that potential PLO patients who have not been previously diagnosed are being increasingly frequently and precisely diagnosed with PLO because PLO has become more widely recognized [26].
Many risk factors for PLO have been proposed, such as a low bone mineral density (BMD), low body mass index (BMI), infrequent exercise, ovulatory disorders, high maternal age, and a strong family history of osteoporosis [1,12,26] (Figure 3). Although PLO is a relatively rare disease, awareness of PLO has increased in recent years, and it is important to understand the pathophysiology of PLO and establish a treatment for the disease.
A large case–control study showed that pre-pregnancy low BMD, reduced physical activity in childhood or adolescence, and dental problems in childhood increase the likelihood of PLO [27]. In recent years, particularly among young women, an increase in acceptance of the societal value that “slender is beautiful” has been proposed to contribute to the increasing occurrence of PLO. An excessive desire to be slender, like fashion models and influencers, through fashion trends and social media, is accelerating excessive dieting behavior [28]. Excessive dieting can be associated with low pre-pregnancy BMD and ovulatory disorders [29]. Ovulatory disorders, including absent, scanty, and rare menstruation, as well as anovulation, are linked to PLO occurrence, which is not unexpected, as low estrogen levels result in net bone loss [26].
A family history as a background of PLO suggests that genetic factors also play a role in its pathogenesis [30,31]. Commonly observed genetic variants in patients with PLO have been reported to involve WNT signaling-related genes (LRP5 and WNT1), osteogenesis imperfecta and dentinogenesis imperfecta-related genes (COL1A1 and COL1A2), and hypophosphatemia-related genes (ALPL and SLC34A3) [32]. Notably, these genes are also involved in bone metabolism and primary osteoporosis and are not specific to PLO.
6. Breastfeeding and Development of PLO in Lactating Mothers
The magnitude of bone loss during lactation was positively correlated with the amount of milk produced (Figure 2). Women nursing twins or triplets experience greater bone loss than women nursing one baby [33,34,35]. Exclusive breastfeeding causes greater bone loss than intermittent breastfeeding [35]. Furthermore, a longer breastfeeding duration results in greater bone loss [34,36]. A clinical study compared groups of fully breastfed and formula-fed infants [12]. In the breastfed group, bone resorption markers, such as serum TRACP5b, PTH, uNTX, and TG, were all significantly increased. In contrast, bone formation markers, such as osteocalcin, did not change markedly with breastfeeding. These observations suggest that increased bone resorption occurs only when breastfeeding. No significant difference in BMD was observed when comparing the breastfed and formula-fed groups. However, when comparing non-fracture and fracture groups observed at the lumbar spine and femoral neck, a significant decrease in BMD was observed. Therefore, decreased BMD due to breastfeeding is a critical risk factor for the development of PLO in feeding mothers. These findings support the clinical decision that early weaning from breastfeeding is often the first choice of treatment.
7. Breastfeeding and Infant Development, Including the Maxillofacial System
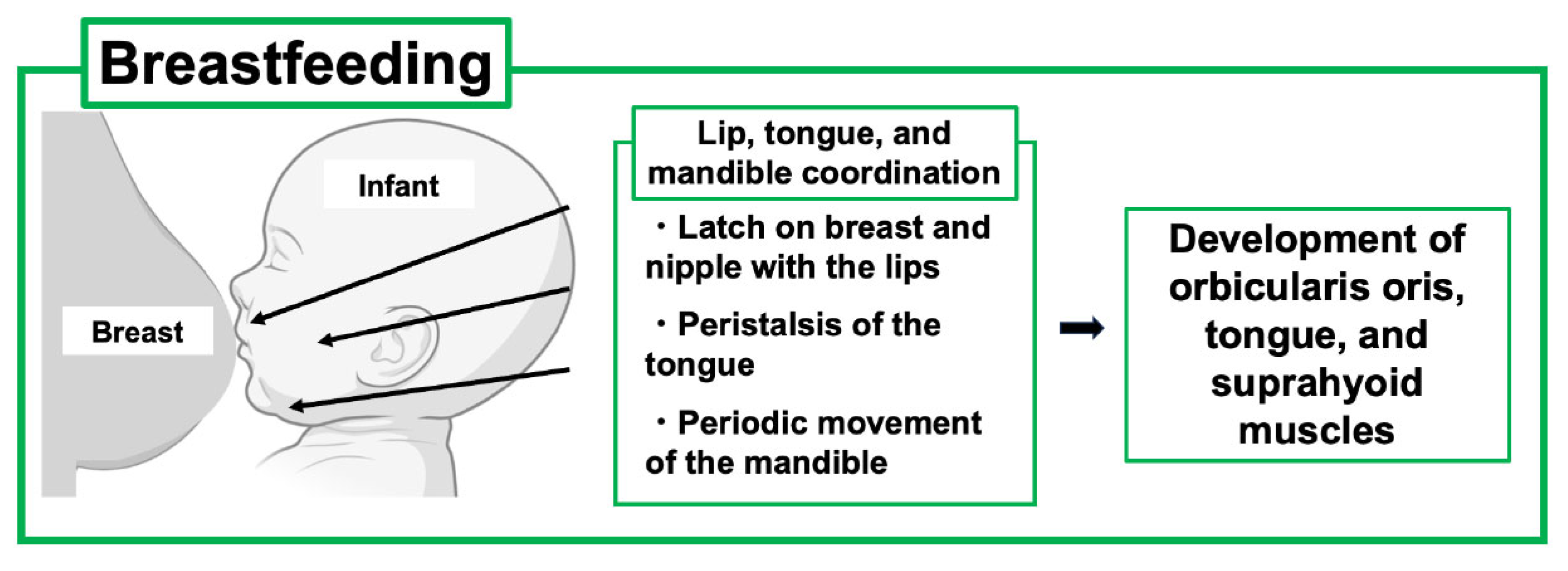
As PLO develops during pregnancy and lactation, treatment strategies including the use of anti-osteoporosis drugs must be carefully considered. As discussed above, early weaning from breastfeeding can be the first choice of PLO treatment in feeding mothers; however, this leads to concern about the impact on the infant because the lactation period is not only a period of nutritional support but also an important time for the newborn to acquire physiological functions through interaction with the mother. Furthermore, recent reports have mentioned that suckling movements are related to the development of the oral function in infants [3,5] (Figure 4).
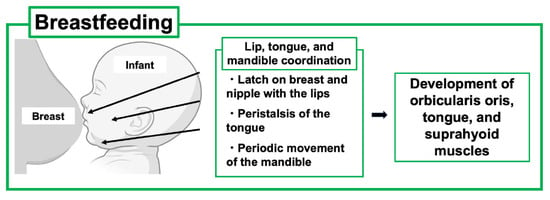
Figure 4.
Effects of breastfeeding on the development of the oral maxillofacial system of the child. Breastfeeding is beneficial for infant development of oral and maxillofacial systems through lip, tongue, and mandible coordination. This figure was created with BioRender.com (accessed on 25 September 2025).
Suckling is a dynamic process that promotes jaw-oral movements in infants, leading to the proper acquisition of coordinated movements of the lips, tongue, and jaws. Specifically, the infant first latches on to the breast and nipple and draws the nipple tip down to the hard-soft palate junction. Such feeding behavior requires coupling between periodic movements of the infant’s jaws and undulation of the tongue, known as “peristalsis”. In addition, mandibular and peristaltic movements have been shown to generate constant negative pressure in the infant’s oral cavity to induce milk ejection [37,38].
It has also been reported that long-term bottle feeding may lead to malocclusion of the primary dentition. Yonezu et al. reported that bottle-feeding may lead to incompetent lip seal and non-nutritive sucking habits (pacifier and finger sucking), thus affecting occlusal characteristics in primary dentition [39]. Chen et al. reported that a shorter duration of breastfeeding was indirectly associated with the development of permanent dentition and occlusion [3]. Children breastfed for less than six months were four times more likely to develop the habit of sucking pacifiers than those breastfed for six months. Prolonged finger sucking habits increase the probability of an anterior open bite, and pacifier habits are associated with excessive overjet and the absence of developmental space in the lower arch [3]. These findings suggest that the sucking mechanism differs greatly between artificial nipples and the breast and may contribute to the development of the maxillofacial system in infants.
Breastfeeding is also an important event in the development of the microbiome in infants, which can be associated with oral infectious diseases, including periodontitis [40]. This subject is beyond the scope of this review.
8. Pharmacotherapies for PLO
Drug administration to pregnant and nursing women is often contraindicated or not recommended in principle because of teratogenicity caused by placental passage and adverse events in infants caused by breast milk transfer. Therefore, the establishment of safe treatment methods is required. The first choice of treatment during the lactation period is to stop calcium efflux by breastfeeding. The drug therapy used in PLO patients may include vitamin D and calcium replacement therapy, as well as osteoporosis medications, such as teriparatide (TPTD) and bisphosphonates (BPs) [2]. The effective treatment of PLO is controversial, as there are few reported cases in clinical trials.
A previous clinical study in which mothers were treated with calcium replacement therapy reported a slight increase in lumbar spine BMD in mothers with moderate dietary calcium intake. However, this therapy did not significantly affect the overall BMD to compensate for the difference between nursing and non-nursing mothers [33].
BPs are synthetic compounds with a common phosphorus–carbon–phosphorus bond and have a high affinity for calcium hydroxyapatite in the bone. They are a class of drugs that prevent loss of bone density to treat osteoporosis. There are several concerns regarding the use of BPs in PLO patients, including the lack of long-term results with BPs in premenopausal females and placental passage of BPs accumulated in the bone [41]. In a mouse model study, administration of low-dose alendronate to lactating mother mice twice a week for two and four weeks showed increased BMD in the mothers without affecting the pups [42]. If applicable to humans, BPs may be able to protect mothers during pregnancy and lactation from bone loss and fragility without affecting the fetus or infants.
Denosumab is an anti-RANK ligand (RANKL) antibody that blocks the differentiation and function of osteoclasts, thus categorized as an anti-bone resorptive agent as well as BPs. Denosumab is an attractive drug for treating osteoporosis owing to its quick offset time. However, it has to be acknowledged that discontinuation of denosumab causes rebound and sudden stimulation of bone resorption because of accumulated osteoclast precursors in the treated bone [43,44,45]. Denosumab has a 25.4-day half-life, resulting in concentrations gradually decreasing over 4–5 months, not accumulating directly in bone like BPs, which may be an appealing point in women with childbearing potential. However, the benefits of dramatic augmentation of BMD and potential rebound-related bone fractures after drug cessation have to be well considered. A study using pregnant mice demonstrated that administration of anti-RANKL antibodies to mice in the late stage of pregnancy resulted in adverse events in their neonatal offspring [46]. This suggests that administering denosumab to pregnant women may affect fetal development and growth after birth.
Teriparatide (TPTD) is a human parathyroid hormone (1–34) with an anabolic effect on bones and is frequently the preferred choice for treating women with PLO [47]. PLO patients with vertebral fractures treated with TPTD for 24 months reportedly show increased BMD in the lumbar spine, femoral neck, and hip [27,48]. In addition to its increasing effect on BMD, clinical studies have demonstrated that chronic skeletal pain is relieved by TPTD treatment [49,50,51]. Supportively, this anti-pain effect is demonstrated to be exerted directly through peripheral sensory neurons in ovariectomized rats [52]. The pain-relieving effect of TPTD is beneficial for PLO patients with pain and may not require another pain drug. TPTD is rapidly absorbed and has a half-life of approximately 1 h; therefore, if pregnancy is desired after treatment, the effect on the fetus is expected to be minimal if quit beforehand [53]. Furthermore, a clinical study demonstrated that PLO patients treated with TPTD maintained their BMD even 12 months after cessation of treatment [54]. This study suggests that TPTD should be the preferred therapy for patients with PLO planning to have another pregnancy [55,56]. PTH and PTHrP play key roles in bone metabolism during pregnancy and lactation. It is necessary to elucidate how exogenous PTH1R agonists, such as TPTD and abaloparatide (a PTHrP derivative), affect bone metabolism in pregnant or nursing women.
9. Consideration of Temporomandibular Joint Disorders During Pregnancy and Lactation
Hormonal changes during pregnancy and lactation may impact the TMJ and associated tissues [57]. TMJ disorders encompass a variety of clinical conditions affecting the TMJ and its associated muscles, such as jaw discomfort, dysfunction, earache, facial pain, and headaches. These factors mainly affect women of reproductive age [58,59]. In severe cases of TMJ disorders, such as degenerative joint disease of the temporomandibular joints (DJD-TMJ), which involves progressive condylar resorption (PCR) and idiopathic condylar resorption (ICR), a clinical study reported that DJD-TMJ mainly affects women of both reproductive and perimenopausal ages [60].
Fluctuating levels of estrogen during pregnancy and post-gestation have been discussed as key regulators of oral diseases, including TMJ disorders, since estrogen is essential for the structural maintenance of joints and bones [6]. A clinical study reported that postmenopausal women with osteoporosis had a higher risk of developing TMJ disorders [61]. A study using rabbits demonstrated that estrogen deficiency did not cause mandibular resorption but did carry a risk of anterior displacement of the joint [62]. These clinical and animal studies indicate that an appropriate level of estrogen is essential for TMJ homeostasis.
However, a systematic review and meta-analysis found no significant differences in the prevalence of TMJ disorders between pregnant and non-pregnant women of reproductive age [63]. A recent case–control cross-sectional study suggested that pregnancy is neither a risk nor protective factor for temporomandibular disorder (TMD) [64]. How hormonal and bone metabolic changes other than estrogen during pregnancy and lactation affect TMJ disorders remains unclear.
Studies have shown that TMJ-related pain decreases while TMJ relaxation increases during pregnancy [59]. An experimental study has demonstrated that high levels of estrogen and progesterone have antinociceptive properties [65]. A prospective study showed that TMJ-related pain and other symptoms appear to improve over the course of pregnancy [66]. These findings suggest that elevated estrogen levels during pregnancy reduce orofacial pain and that reduced estrogen levels during lactation may promote chronic pain [57].
Accumulated clinical and preclinical animal model studies have demonstrated that pharmacological administration of PTH not only protects bone and joint degeneration but also reduces chronic skeletal pain [52,67,68,69]. It is likely that dynamic changes in PTH and PTHrP levels during lactation and pregnancy are involved in the pathophysiological control of the TMJ and orofacial pain. It will be relevant to conduct clinical and preclinical studies to determine how PTH and PTHrP levels are associated with TMJ disorders and orofacial pain.
10. Consideration of Orthodontic Tooth Movement During Pregnancy and Lactation
Dynamic changes in the hormonal regulation of bone metabolism during pregnancy and lactation are likely to affect the homeostatic regulation of maxillofacial bones. Because of the increasing demand for orthodontic treatment for aesthetic reasons, it is important to consider how bone remodeling induced by orthodontic tooth movement (OTM) is affected in pregnant and feeding mothers [57]. During pregnancy, upregulated serum levels of estrogen and PTHrP are key regulators of bone remodeling (Figure 1, Table 1), which lays the foundation for the rate of OTM. It positively regulates bone formation by promoting osteogenic precursors and osteoblast differentiation while suppressing osteoclast formation and function. Therefore, estrogen is fundamentally favorable for bone anabolism, which is supported by the fact that estrogen deficiency causes osteoporosis [69]. PTHrP upregulated to detectable levels in serum is a characteristic feature of bone metabolism during pregnancy and lactation and is likely to function like PTH through its common receptor of PTH1R in osteoblast lineage cells. Since PTH stimulates both bone formation and resorption in trabecular and cortical bones [21,70,71,72], augmented PTHrP during pregnancy is suggested to promote bone turnover, although downregulated PTH may compensate for the over-function of PTHrP.
Seeing the upregulated serum levels of estrogen and PTHrP during pregnancy, the acceleration of OTM sounds reasonable. Animal model studies tested the rate of OTM during pregnancy [73,74,75], which found no statistically significant difference in the rate of OTM between the pregnant and non-pregnant groups. In addition to estrogen and PTHrP, many other hormonal and biological factors are involved in bone remodeling during pregnancy, which may alter the rate of OTM. Verification with large-scale clinical studies will be needed.
Bone metabolism during lactation is characterized by decreased estrogen and increased PTHrP and PTH levels (Figure 2, Table 1), which enhances bone turnover [76]. A mouse study observed that lactation resulted in a significantly increased rate of OTM compared to that in the non-lactating group [77]. However, large-scale clinical studies and animal studies are needed to verify this observation [75,78].
11. Conclusions and Perspective
During late pregnancy and lactation, dramatic changes occur in maternal bone metabolism, resulting in an adequate supply of calcium required for fetal development and milk production [8]. Although PLO is relatively rare, its prevention and treatment are critical for proper infant development and improving the maternal quality of life. Although accumulated evidence suggests early weaning from breastfeeding as a primary choice to treat PLO in lactating mothers, this often burdens mothers’ mental health and may affect infant development. Therefore, the establishment of a safe pharmacotherapy is required. It is also worth noting that dramatic changes in maternal bone metabolism during pregnancy and lactation may affect the homeostasis of the TMJ and alveolar bone in mothers. Therefore, it may be an effective approach to confirm the mother’s medical history of PLO, whether the child is breastfed or bottle-fed, and the age of weaning of the child as a medical interview to monitor oral and maxillofacial health both in children and mothers. The strength of this review is proposing the relevance of knowing the changes in maternal bone metabolism for dentists as well as related medical fields. However, there are still some limitations in this subject. Large-scale clinical studies and accumulating further mechanical analysis using animal studies are needed. We still need a life-span view of premenopausal and postmenopausal pathophysiology of bone metabolism [79].
Author Contributions
M.N., H.W., A.N.-K., M.H.-N. and T.I. designed the entire study project and wrote the manuscript. S.N., F.U., R.K., R.T., Y.K. and L.B. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was mainly supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI) (grant numbers 25K24119 to M.N., 25K20391 to H.W., 24K20047 to A.N.-K., 24K23572 to M.H.-N., and 25K22680, 24KK0165 and 23K18347 to T.I.). This work was partially supported by JST SPRING, Grant Number JPMJSP2119 to M.N., H.W., A.N.-K., M.H.-N., S.N., F.U. and R.K.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank all of the lab members in the department of Pharmacology, Faculty and Graduate School of Dental Medicine, Hokkaido University for their constructive discussion of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gak, N.; Abbara, A.; Dhillo, W.S.; Keen, R.; Comninos, A.N. Current and future perspectives on pregnancy and lactation-associated osteoporosis. Front. Endocrinol. 2024, 15, 1494965. [Google Scholar] [CrossRef]
- Anagnostis, P.; Lampropoulou-Adamidou, K.; Bosdou, J.K.; Trovas, G.; Galanis, P.; Chronopoulos, E.; Goulis, D.G.; Tournis, S. Comparative Effectiveness of Therapeutic Interventions in Pregnancy and Lactation-Associated Osteoporosis: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2024, 109, 879–901. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xia, B.; Ge, L. Effects of breast-feeding duration, bottle-feeding duration and non-nutritive sucking habits on the occlusal characteristics of primary dentition. BMC Pediatr. 2015, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.C.; Giugliani, E.R.; Caramez da Silva, F. Influence of the duration of breastfeeding on quality of muscle function during mastication in preschoolers: A cohort study. BMC Public Health 2012, 12, 934. [Google Scholar] [CrossRef] [PubMed]
- Hermont, A.P.; Martins, C.C.; Zina, L.G.; Auad, S.M.; Paiva, S.M.; Pordeus, I.A. Breastfeeding, bottle feeding practices and malocclusion in the primary dentition: A systematic review of cohort studies. Int. J. Environ. Res. Public Health 2015, 12, 3133–3151. [Google Scholar] [CrossRef]
- Robinson, J.L.; Johnson, P.M.; Kister, K.; Yin, M.T.; Chen, J.; Wadhwa, S. Estrogen signaling impacts temporomandibular joint and periodontal disease pathology. Odontology 2020, 108, 153–165. [Google Scholar] [CrossRef]
- Kovacs, C.S. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef]
- Athonvarangkul, D.; Wysolmerski, J.J. Crosstalk within a brain-breast-bone axis regulates mineral and skeletal metabolism during lactation. Front. Physiol. 2023, 14, 1121579. [Google Scholar] [CrossRef]
- Kirby, B.J.; Ma, Y.; Martin, H.M.; Buckle Favaro, K.L.; Karaplis, A.C.; Kovacs, C.S. Upregulation of calcitriol during pregnancy and skeletal recovery after lactation do not require parathyroid hormone. J. Bone Miner. Res. 2013, 28, 1987–2000. [Google Scholar] [CrossRef]
- Kovacs, C.S. The role of vitamin D in pregnancy and lactation: Insights from animal models and clinical studies. Annu. Rev. Nutr. 2012, 32, 97–123. [Google Scholar] [CrossRef]
- Kovacs, C.S. The Skeleton Is a Storehouse of Mineral That Is Plundered During Lactation and (Fully?) Replenished Afterwards. J. Bone Miner. Res. 2017, 32, 676–680. [Google Scholar] [CrossRef]
- Miyamoto, T.; Miyakoshi, K.; Sato, Y.; Kasuga, Y.; Ikenoue, S.; Miyamoto, K.; Nishiwaki, Y.; Tanaka, M.; Nakamura, M.; Matsumoto, M. Changes in bone metabolic profile associated with pregnancy or lactation. Sci. Rep. 2019, 9, 6787. [Google Scholar] [CrossRef]
- Honda, A.; Kurabayashi, T.; Yahata, T.; Tomita, M.; Matsushita, H.; Takakuwa, K.; Tanaka, K. Effects of pregnancy and lactation on trabecular bone and marrow adipocytes in rats. Calcif. Tissue Int. 2000, 67, 367–372. [Google Scholar] [CrossRef]
- Kent, G.N.; Price, R.I.; Gutteridge, D.H.; Smith, M.; Allen, J.R.; Bhagat, C.I.; Barnes, M.P.; Hickling, C.J.; Retallack, R.W.; Wilson, S.G.; et al. Human lactation: Forearm trabecular bone loss, increased bone turnover, and renal conservation of calcium and inorganic phosphate with recovery of bone mass following weaning. J. Bone Miner. Res. 1990, 5, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Parsons, C.; Maslin, K.; D’Angelo, S.; Moon, R.J.; Crozier, S.R.; Gossiel, F.; Bishop, N.J.; Kennedy, S.H.; Papageorghiou, A.T.; et al. Bone turnover in pregnancy, measured by urinary CTX, is influenced by vitamin D supplementation and is associated with maternal bone health: Findings from the Maternal Vitamin D Osteoporosis Study (MAVIDOS) trial. Am. J. Clin. Nutr. 2021, 114, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.S.; Schurman, C.A.; White, C.R.; Alliston, T. Investigating Osteocytic Perilacunar/Canalicular Remodeling. Curr. Osteoporos. Rep. 2019, 17, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Jahn, K.; Rauner, M.; Busse, B.; Bonewald, L.F. Physiological and pathological osteocytic osteolysis. J. Musculoskelet. Neuronal Interact. 2018, 18, 292–303. [Google Scholar]
- Lotinun, S.; Ishihara, Y.; Nagano, K.; Kiviranta, R.; Carpentier, V.T.; Neff, L.; Parkman, V.; Ide, N.; Hu, D.; Dann, P.; et al. Cathepsin K-deficient osteocytes prevent lactation-induced bone loss and parathyroid hormone suppression. J. Clin. Investig. 2019, 129, 3058–3071. [Google Scholar] [CrossRef]
- Qing, H.; Bonewald, L.F. Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int. J. Oral Sci. 2009, 1, 59–65. [Google Scholar] [CrossRef]
- Nakanishi-Kimura, A.; Takakura, A.; Hoshi-Numahata, M.; Watanabe, H.; Nishiura, M.; Sato, Y.; Takao-Kawabata, R.; Iimura, T. Dynamic morphometric changes in the mandibular osteocytic lacunae of ovariectomized rats in response to teriparatide, as revealed by three-dimensional fluorescence analyses: Possible involvement of osteocytic perilacunar remodeling. J. Oral Biosci. 2024, 66, 49–60. [Google Scholar] [CrossRef]
- Takakura, A.; Sato, T.; Lee, J.W.; Hirano, K.; Takao-Kawabata, R.; Ishizuya, T.; Iimura, T. Expansion of the osteocytic lacunar-canalicular system involved in pharmacological action of PTH revealed by AI-driven fluorescence morphometry in female rabbits. Sci. Rep. 2022, 12, 16799. [Google Scholar] [CrossRef] [PubMed]
- de Bakker, C.M.J.; Tseng, W.J.; Li, Y.; Zhao, H.; Altman-Singles, A.R.; Jeong, Y.; Robberts, J.; Han, L.; Kim, D.G.; Sherry Liu, X. Reproduction Differentially Affects Trabecular Bone Depending on Its Mechanical Versus Metabolic Role. J. Biomech. Eng. 2017, 139, 1110061–11100610. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Basta-Pljakic, J.; Seref-Ferlengez, Z.; Majeska, R.J.; Cardoso, L.; Bromage, T.G.; Zhang, Q.; Flach, C.R.; Mendelsohn, R.; Yakar, S.; et al. Lactation-Induced Changes in the Volume of Osteocyte Lacunar-Canalicular Space Alter Mechanical Properties in Cortical Bone Tissue. J. Bone Miner. Res. 2017, 32, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; Bowman, B.M. Rapid inactivation and apoptosis of osteoclasts in the maternal skeleton during the bone remodeling reversal at the end of lactation. Anat. Rec. 2007, 290, 65–73. [Google Scholar] [CrossRef]
- Ardeshirpour, L.; Dann, P.; Adams, D.J.; Nelson, T.; VanHouten, J.; Horowitz, M.C.; Wysolmerski, J.J. Weaning triggers a decrease in receptor activator of nuclear factor-kappaB ligand expression, widespread osteoclast apoptosis, and rapid recovery of bone mass after lactation in mice. Endocrinology 2007, 148, 3875–3886. [Google Scholar] [CrossRef]
- Kasahara, K.; Tanaka-Mizuno, S.; Tsuji, S.; Ohashi, M.; Kasahara, M.; Kawasaki, T.; Murakami, T. Pregnancy and lactation-associated osteoporosis as a major type of premenopausal osteoporosis: A retrospective cohort study based on real-world data. BMC Pregnancy Childbirth 2024, 24, 301. [Google Scholar] [CrossRef]
- Hadji, P.; Mouzakiti, N.; Kyvernitakis, I. Effect of Teriparatide on Subsequent Fracture and Bone Mineral Density in 47 Women with Pregnancy- and Lactation-associated Osteoporosis and Vertebral Fractures. Geburtshilfe Frauenheilkd. 2022, 82, 619–626. [Google Scholar] [CrossRef]
- Bonfanti, R.C.; Melchiori, F.; Teti, A.; Albano, G.; Raffard, S.; Rodgers, R.; Lo Coco, G. The association between social comparison in social media, body image concerns and eating disorder symptoms: A systematic review and meta-analysis. Body Image 2025, 52, 101841. [Google Scholar] [CrossRef]
- Dobranowska, K.; Plinska, S.; Dobosz, A. Dietary and Lifestyle Management of Functional Hypothalamic Amenorrhea: A Comprehensive Review. Nutrients 2024, 16, 2967. [Google Scholar] [CrossRef]
- Peris, P.; Guanabens, N.; Monegal, A.; Pons, F.; Martinez de Osaba, M.J.; Ros, I.; Munoz-Gomez, J. Pregnancy associated osteoporosis: The familial effect. Clin. Exp. Rheumatol. 2002, 20, 697–700. [Google Scholar]
- Rolvien, T.; Stürznickel, J.; Schmidt, F.N.; Butscheidt, S.; Schmidt, T.; Busse, B.; Mundlos, S.; Schinke, T.; Kornak, U.; Amling, M.; et al. Comparison of Bone Microarchitecture Between Adult Osteogenesis Imperfecta and Early-Onset Osteoporosis. Calcif. Tissue Int. 2018, 103, 512–521. [Google Scholar] [CrossRef]
- Butscheidt, S.; Tsourdi, E.; Rolvien, T.; Delsmann, A.; Sturznickel, J.; Barvencik, F.; Jakob, F.; Hofbauer, L.C.; Mundlos, S.; Kornak, U.; et al. Relevant genetic variants are common in women with pregnancy and lactation-associated osteoporosis (PLO) and predispose to more severe clinical manifestations. Bone 2021, 147, 115911. [Google Scholar] [CrossRef] [PubMed]
- Kalkwarf, H.J.; Specker, B.L.; Bianchi, D.C.; Ranz, J.; Ho, M. The effect of calcium supplementation on bone density during lactation and after weaning. N. Engl. J. Med. 1997, 337, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Brembeck, P.; Lorentzon, M.; Ohlsson, C.; Winkvist, A.; Augustin, H. Changes in cortical volumetric bone mineral density and thickness, and trabecular thickness in lactating women postpartum. J. Clin. Endocrinol. Metab. 2015, 100, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Bjornerem, A.; Ghasem-Zadeh, A.; Wang, X.; Bui, M.; Walker, S.P.; Zebaze, R.; Seeman, E. Irreversible Deterioration of Cortical and Trabecular Microstructure Associated with Breastfeeding. J. Bone Miner. Res. 2017, 32, 681–687. [Google Scholar] [CrossRef]
- More, C.; Bettembuk, P.; Bhattoa, H.P.; Balogh, A. The effects of pregnancy and lactation on bone mineral density. Osteoporos. Int. 2001, 12, 732–737. [Google Scholar] [CrossRef]
- Elad, D.; Kozlovsky, P.; Blum, O.; Laine, A.F.; Po, M.J.; Botzer, E.; Dollberg, S.; Zelicovich, M.; Ben Sira, L. Biomechanics of milk extraction during breast-feeding. Proc. Natl. Acad. Sci. USA 2014, 111, 5230–5235. [Google Scholar] [CrossRef]
- Geddes, D.T.; Sakalidis, V.S.; Hepworth, A.R.; McClellan, H.L.; Kent, J.C.; Lai, C.T.; Hartmann, P.E. Tongue movement and intra-oral vacuum of term infants during breastfeeding and feeding from an experimental teat that released milk under vacuum only. Early Hum. Dev. 2012, 88, 443–449. [Google Scholar] [CrossRef]
- Yonezu, T.; Kadoya, M.; Yakushiji, M. Effects of prolonged breast- and bottle-feeding on occlusal characteristics in the primary dentition. Pediatr. Dent. J. 2005, 15, 176–179. [Google Scholar] [CrossRef][Green Version]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Black, D.M.; Reid, I.R.; Boonen, S.; Bucci-Rechtweg, C.; Cauley, J.A.; Cosman, F.; Cummings, S.R.; Hue, T.F.; Lippuner, K.; Lakatos, P.; et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: A randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J. Bone Miner. Res. 2012, 27, 243–254, Erratum in J. Bone Miner. Res. 2012, 27, 2612. [Google Scholar] [CrossRef]
- Ito, E.; Sato, Y.; Kobayashi, T.; Soma, T.; Matsumoto, T.; Kimura, A.; Miyamoto, K.; Matsumoto, H.; Matsumoto, M.; Nakamura, M.; et al. Transient alendronate administration to pregnant or lactating mothers prevents bone loss in mice without adverse effects on offspring. Bone 2021, 153, 116133. [Google Scholar] [CrossRef]
- Bone, H.G.; Bolognese, M.A.; Yuen, C.K.; Kendler, D.L.; Miller, P.D.; Yang, Y.C.; Grazette, L.; San Martin, J.; Gallagher, J.C. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J. Clin. Endocrinol. Metab. 2011, 96, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Taylor, V.E.; Castro-Martinez, A.; Dhakal, S.; Zamerli, A.; Mohanty, S.; Xiao, Y.; Simic, M.K.; Wen, J.; Chai, R.; et al. Temporal patterns of osteoclast formation and activity following withdrawal of RANKL inhibition. J. Bone Miner. Res. 2024, 39, 484–497. [Google Scholar] [CrossRef]
- Ishikawa, K.; Tani, S.; Sakai, N.; Kudo, Y.; Horiuchi, H.; Kimura-Suda, H.; Takami, M.; Tsuji, M.; Inagaki, K.; Kiuchi, Y.; et al. Mouse model of anti-RANKL discontinuation reveals reduced bone mass and quality through disruption of bone remodeling. Bone Res. 2025, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Takami, M.; Azetsu, Y.; Karakawa, A.; Chatani, M.; Funatsu, T.; Sakai, N. Effects of anti-RANKL antibodies administered to pregnant mice on bone and tooth development in neonates. J. Oral. Biosci. 2023, 65, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Satterwhite, J.; Heathman, M.; Miller, P.D.; Marin, F.; Glass, E.V.; Dobnig, H. Pharmacokinetics of teriparatide (rhPTH[1–34]) and calcium pharmacodynamics in postmenopausal women with osteoporosis. Calcif. Tissue Int. 2010, 87, 485–492. [Google Scholar] [CrossRef]
- Hellmeyer, L.; Boekhoff, J.; Hadji, P. Treatment with teriparatide in a patient with pregnancy-associated osteoporosis. Gynecol. Endocrinol. 2010, 26, 725–728. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, W.; Zhao, S.; Mo, X.; Yuan, W.; Cheung, W.H.; Fu, D.; Chen, B. Effect of Teriparatide on pain relief, and quality of life in postmenopausal females with osteoporotic vertebral compression fractures, a retrospective cohort study. Ann. Palliat. Med. 2021, 10, 4000–4007. [Google Scholar] [CrossRef]
- Dore, R.K.; Chen, P.Q.; Glass, E.V.; Krege, J.H. Reduced risk of back pain following teriparatide treatment: A meta-analysis. Arthritis Rheum. 2004, 50, 273–280. [Google Scholar]
- Nevitt, M.C.; Chen, P.Q.; Dore, R.K.; Reginster, J.Y.; Kiel, D.P.; Zanchetta, J.R.; Glass, E.V.; Krege, J.H. Reduced risk of back pain following teriparatide treatment: A meta-analysis. Osteoporos. Int. 2006, 17, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takao-Kawabata, R.; Takakura, A.; Shimazu, Y.; Nakatsugawa, M.; Ito, A.; Lee, J.W.; Kawasaki, K.; Iimura, T. Teriparatide relieves ovariectomy-induced hyperalgesia in rats, suggesting the involvement of functional regulation in primary sensory neurons by PTH-mediated signaling. Sci. Rep. 2020, 10, 5346. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.Y.; Song, J.E.; Park, K.H.; Seok, H.; Lee, E.J.; Lim, S.K.; Rhee, Y. Effect of teriparatide on pregnancy and lactation-associated osteoporosis with multiple vertebral fractures. J. Bone Miner. Metab. 2012, 30, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, N.; Kim, K.J.; Park, C.H.; Lee, J.; Rhee, Y. Bone Density After Teriparatide Discontinuation With or Without Antiresorptive Therapy in Pregnancy- and Lactation-Associated Osteoporosis. Calcif. Tissue Int. 2021, 109, 544–553. [Google Scholar] [CrossRef]
- Lampropoulou-Adamidou, K.; Trovas, G.; Triantafyllopoulos, I.K.; Yavropoulou, M.P.; Anastasilakis, A.D.; Anagnostis, P.; Toulis, K.A.; Makris, K.; Gazi, S.; Balanika, A.; et al. Teriparatide Treatment in Patients with Pregnancy- and Lactation-Associated Osteoporosis. Calcif. Tissue Int. 2021, 109, 554–562. [Google Scholar] [CrossRef]
- Ali, D.S.; Khan, A.A.; Brandi, M.L. Effective strategies for pregnancy and lactation-associated osteoporosis: Teriparatide use in focus. Endocrine 2024, 86, 459–469. [Google Scholar] [CrossRef]
- Zhao, Y.; Qian, S.; Zheng, Z.; Peng, J.; Liu, J.; Guan, X.; Liao, C. Consideration of hormonal changes for orthodontic treatment during pregnancy and lactation—A review. Reprod. Biol. Endocrinol. 2024, 22, 106. [Google Scholar] [CrossRef]
- Iodice, G.; Cimino, R.; Vollaro, S.; Lobbezoo, F.; Michelotti, A. Prevalence of temporomandibular disorder pain, jaw noises and oral behaviours in an adult Italian population sample. J. Oral. Rehabil. 2019, 46, 691–698. [Google Scholar] [CrossRef]
- LeResche, L. Epidemiology of temporomandibular disorders: Implications for the investigation of etiologic factors. Crit. Rev. Oral. Biol. Med. 1997, 8, 291–305. [Google Scholar] [CrossRef]
- Watanabe, H.; Iori, T.; Lee, J.W.; Kajii, T.S.; Takakura, A.; Takao-Kawabata, R.; Kitagawa, Y.; Maruoka, Y.; Iimura, T. Association between an Increased Serum CCL5 Level and Pathophysiology of Degenerative Joint Disease in the Temporomandibular Joint in Females. Int. J. Mol. Sci. 2023, 24, 2775. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yoo, D.M.; Kwon, M.J.; Kim, J.H.; Kim, J.H.; Byun, S.H.; Park, B.; Lee, H.J.; Choi, H.G. Increased Risk of Temporomandibular Joint Disorder in Osteoporosis Patients: A Longitudinal Study. Front. Endocrinol. 2022, 13, 835923. [Google Scholar] [CrossRef]
- Iwasaki, T.; Takahara, N.; Duc, V.V.; Tomomatsu, N.; Tabata, M.J.; Yoda, T. Effect of anterior disc displacement and estrogen deficiency on rabbit mandibular condyle. J. Oral. Biosci. 2025, 67, 100599. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciu, M. Prevalence of temporomandibular disorders (TMD) in pregnancy: A systematic review with meta-analysis. J. Oral. Rehabil. 2023, 50, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Marrapodi, M.M.; La Verde, M.; Meto, A.; Siurkel, Y.; Cicciu, M.; Russo, D. The relationship between pregnancy and temporomandibular disorder (TMD) through diagnostic criteria for temporomandibular disorders (DC/TMD) axis II evaluation: A case-control cross-sectional study. BMC Oral Health 2024, 24, 342. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Basoa, M.E.; Gintzler, A.R. Estrogen and progesterone activate spinal kappa-opiate receptor analgesic mechanisms. Pain 1996, 64, 608–615. [Google Scholar] [CrossRef]
- LeResche, L.; Sherman, J.J.; Huggins, K.; Saunders, K.; Mancl, L.A.; Lentz, G.; Dworkin, S.F. Musculoskeletal orofacial pain and other signs and symptoms of temporomandibular disorders during pregnancy: A prospective study. J. Orofac. Pain. 2005, 19, 193–201. [Google Scholar]
- Sun, Q.; Zhen, G.; Li, T.P.; Guo, Q.; Li, Y.; Su, W.; Xue, P.; Wang, X.; Wan, M.; Guan, Y.; et al. Parathyroid hormone attenuates osteoarthritis pain by remodeling subchondral bone in mice. eLife 2021, 10, e66532. [Google Scholar] [CrossRef]
- Ling, Z.; Crane, J.; Hu, H.; Chen, Y.; Wan, M.; Ni, S.; Demehri, S.; Mohajer, B.; Peng, X.; Zou, X.; et al. Parathyroid hormone treatment partially reverses endplate remodeling and attenuates low back pain in animal models of spine degeneration. Sci. Transl. Med. 2023, 15, eadg8982. [Google Scholar] [CrossRef]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef]
- Yamane, H.; Takakura, A.; Shimadzu, Y.; Kodama, T.; Lee, J.W.; Isogai, Y.; Ishizuya, T.; Takao-Kawabata, R.; Iimura, T. Acute development of cortical porosity and endosteal naive bone formation from the daily but not weekly short-term administration of PTH in rabbit. PLoS ONE 2017, 12, e0175329. [Google Scholar] [CrossRef]
- Takakura, A.; Lee, J.W.; Hirano, K.; Isogai, Y.; Ishizuya, T.; Takao-Kawabata, R.; Iimura, T. Administration frequency as well as dosage of PTH are associated with development of cortical porosity in ovariectomized rats. Bone Res. 2017, 5, 17002. [Google Scholar] [CrossRef]
- Hoshi-Numahata, M.; Takakura, A.; Nakanishi-Kimura, A.; Watanabe, H.; Takada, K.; Nishiura, M.; Sato, Y.; Takao-Kawabata, R.; Iimura, T. Evaluation of cortical bone remodeling in canines treated with daily and weekly administrations of teriparatide by establishing AI-driven morphometric analyses and GIS-based spatial mapping. Bone Rep. 2023, 19, 101720. [Google Scholar] [CrossRef] [PubMed]
- Hellsing, E.; Hammarstrom, L. The effects of pregnancy and fluoride on orthodontic tooth movements in rats. Eur. J. Orthod. 1991, 13, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, K.; Olyaee, P.; Mirzakouchaki, B.; Ghahremani, L.; Garjani, A.; Dadgar, E.; Marjani, S. The effect of pregnancy on orthodontic tooth movement in rats. Med. Oral. Patol. Oral. Cir. Bucal 2013, 18, e351–e355. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Kaklamanos, E.G. Does the rate of orthodontic tooth movement change during pregnancy and lactation? A systematic review of the evidence from animal studies. BMC Oral Health 2020, 20, 237. [Google Scholar] [CrossRef]
- Carneiro, R.M.; Prebehalla, L.; Tedesco, M.B.; Sereika, S.M.; Hugo, M.; Hollis, B.W.; Gundberg, C.M.; Stewart, A.F.; Horwitz, M.J. Lactation and bone turnover: A conundrum of marked bone loss in the setting of coupled bone turnover. J. Clin. Endocrinol. Metab. 2010, 95, 1767–1776. [Google Scholar] [CrossRef]
- Macari, S.; Sharma, L.A.; Wyatt, A.; da Silva, J.M.; Dias, G.J.; Silva, T.A.; Szawka, R.E.; Grattan, D.R. Lactation induces increases in the RANK/RANKL/OPG system in maxillary bone. Bone 2018, 110, 160–169. [Google Scholar] [CrossRef]
- Iglesias-Linares, A.; Morford, L.A.; Hartsfield, J.K., Jr. Bone Density and Dental External Apical Root Resorption. Curr. Osteoporos. Rep. 2016, 14, 292–309. [Google Scholar] [CrossRef]
- Agarwal, K.; Nagendra, L.; Bhattacharya, S. Approach to premenopausal osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).