In Vitro Analysis of the Dynamic Role of the Bacterial Virulence Factors in Skin Wound Healing
Abstract
1. Introduction
2. Results
2.1. Bacterial Culture and Quantification of Virulence Factor Secretion
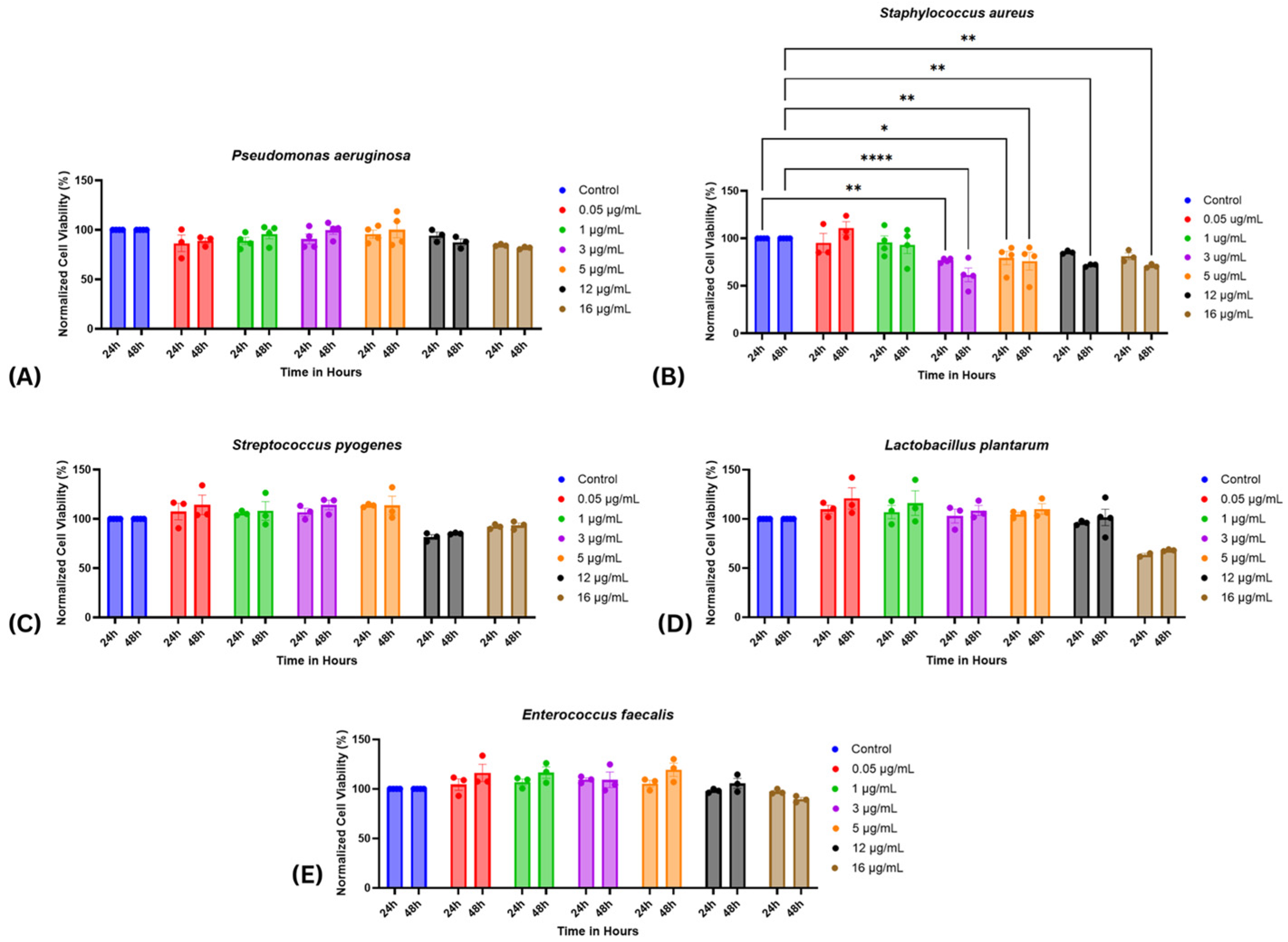
2.2. Cell Viability Assessment
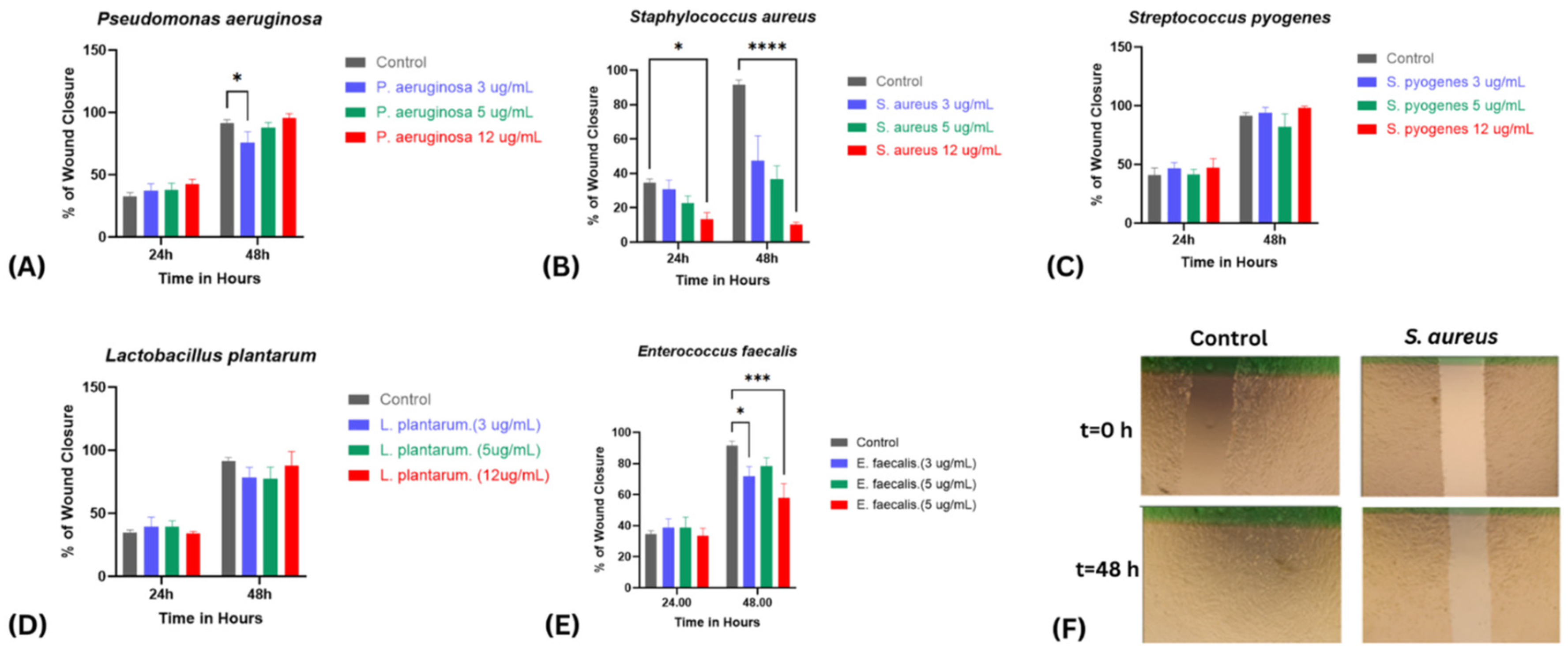
2.3. Wound Healing Assay
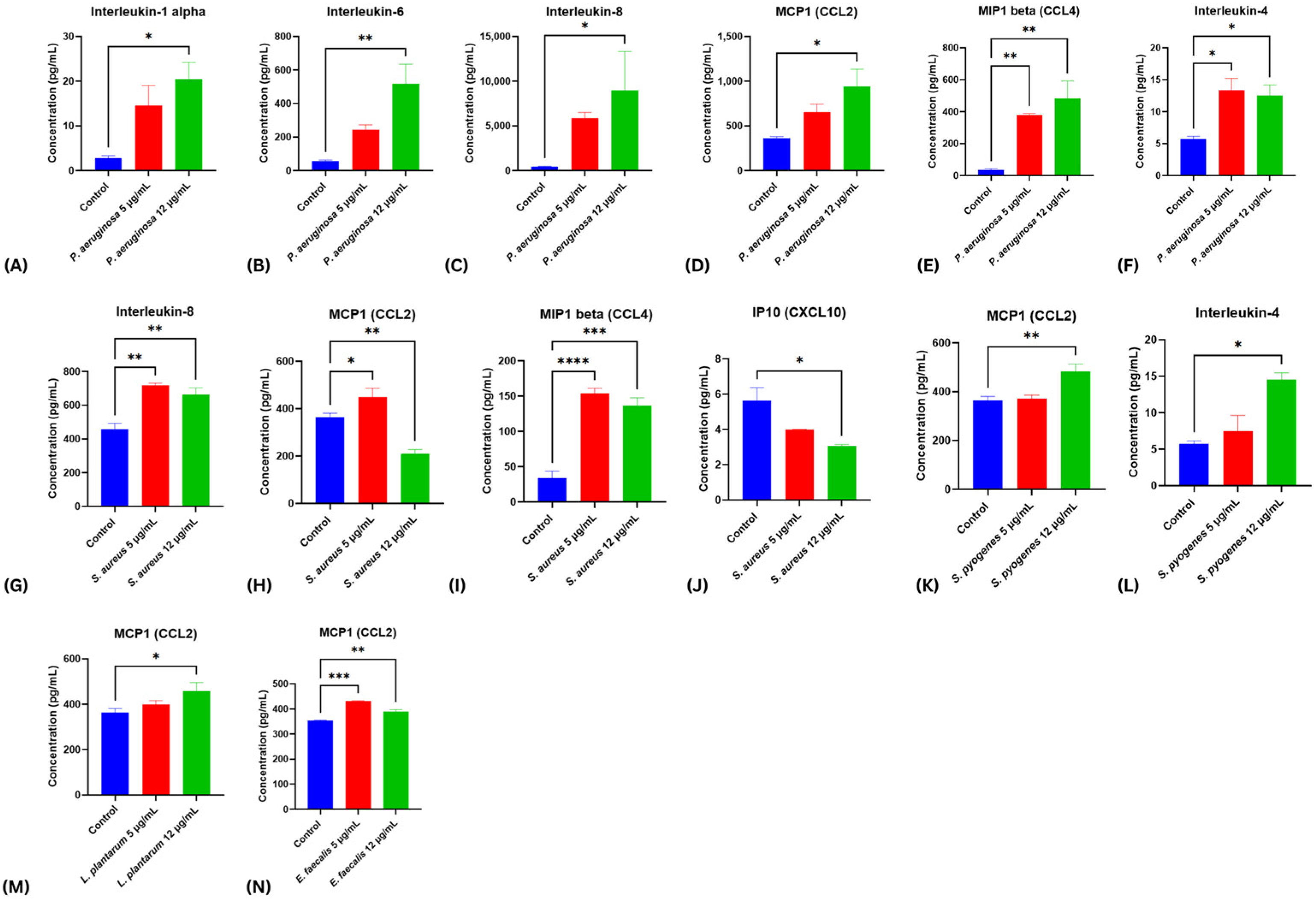
2.4. Modulatory Effects of Bacterial Virulence Factors on Cytokine Release from Stimulated Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.1.1. Bacterial Strains, and Culture
4.1.2. Preparation of Bacterial Supernatants
4.1.3. Total Protein Quantification
4.1.4. Preparation of Gradient Virulence Factor Concentrations
4.2. In Vitro Cellular Responses to Bacterial Virulence Factors
4.2.1. Cell Culture
4.2.2. Cellular Viability Assay
4.3. Wound Healing (Scratch) Assay
4.4. Analysis of Cytokine Release Following Bacterial Virulence Factor Exposure
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Odhav, A.; Kollipara, R.; Fike, J.; Stanford, C.; Hall, J.C. Acute Cutaneous Necrosis: A Guide to Early Diagnosis and Treatment. J. Cutan. Med. Surg. 2017, 21, 425–437. [Google Scholar] [CrossRef]
- Orgill, D.; Blanco, C. Summary: Biomaterials for treating skin loss. In Biomaterials for Treating Skin Loss; Elsevier: Amsterdam, The Netherlands, 2009; pp. 231–235. [Google Scholar]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Shah, J.M.; Omar, E.; Pai, D.R.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012, 45, 220–228. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef]
- Karppinen, S.M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research 2019, 8, 787. [Google Scholar] [CrossRef]
- Parani, M.; Lokhande, G.; Singh, A.; Gaharwar, A.K. Engineered Nanomaterials for Infection Control and Healing Acute and Chronic Wounds. ACS Appl. Mater. Interfaces 2016, 8, 10049–10069. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Wang, S.; Tan, Q.; Maitz, J.; Maitz, P.K.; et al. Challenges and innovations in treating chronic and acute wound infections: From basic science to clinical practice. Burn Trauma 2022, 10, tkac014. [Google Scholar] [CrossRef]
- Tomic-Canic, M.; Burgess, J.L.; O’Neill, K.E.; Strbo, N.; Pastar, I. Skin Microbiota and its Interplay with Wound Healing. Am. J. Clin. Dermatol. 2020, 21 (Suppl. 1), 36–43. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Kalan, L.R.; Meisel, J.S.; Loesche, M.A.; Horwinski, J.; Soaita, I.; Chen, X.; Uberoi, A.; Gardner, S.E.; Grice, E.A. Strain- and Species-Level Variation in the Microbiome of Diabetic Wounds Is Associated with Clinical Outcomes and Therapeutic Efficacy. Cell Host Microbe 2019, 25, 641–655.e645. [Google Scholar] [CrossRef] [PubMed]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.B.; Shruptha, P.; Prabhu, V.; Srujan, C.; Nayak, U.Y.; Anuradha, C.K.R.; Ramachandra, L.; Keerthana, P.; Joshi, M.B.; Murali, T.S.; et al. Pseudomonas aeruginosa virulence proteins pseudolysin and protease IV impede cutaneous wound healing. Lab. Investig. 2020, 100, 1532–1550. [Google Scholar] [CrossRef]
- Eldeen, M.; Elnahal, A.; Ads, A.; El-Sawaf, M.; Samy, S. Effect of Lactobacillus Plantarum on virulence factors of Pseudomonas aeruginosa isolated from wound infection. Microbes Infect. Dis. 2021, 2, 790–796. [Google Scholar]
- Shettigar, K.; Murali, T.S. Virulence factors and clonal diversity of Staphylococcus aureus in colonization and wound infection with emphasis on diabetic foot infection. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2235–2246. [Google Scholar] [CrossRef]
- Petkovšek, Ž.; Eleršič, K.; Gubina, M.; Žgur-Bertok, D.; Starčič Erjavec, M. Virulence Potential of Escherichia coli Isolates from Skin and Soft Tissue Infections. J. Clin. Microbiol. 2009, 47, 1811–1817. [Google Scholar] [CrossRef]
- Linehan, J.L.; Harrison, O.J.; Han, S.J.; Byrd, A.L.; Vujkovic-Cvijin, I.; Villarino, A.V.; Sen, S.K.; Shaik, J.; Smelkinson, M.; Tamoutounour, S.; et al. Non-classical Immunity Controls Microbiota Impact on Skin Immunity and Tissue Repair. Cell 2018, 172, 784–796.e18. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair. Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, J.; Tao, Y.; Wang, T.; Li, L. Lactobacillus Plantarum Promotes Wound Healing by Inhibiting the NLRP3 Inflammasome and Pyroptosis Activation in Diabetic Foot Wounds. J. Inflamm. Res. 2024, 17, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized control of skin immunity by resident commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Bouladoux, N.; Linehan, J.L.; Han, S.J.; Harrison, O.J.; Wilhelm, C.; Conlan, S.; Himmelfarb, S.; Byrd, A.L.; Deming, C.; et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015, 520, 104–108. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Vasquez, K.S.; Truong, H.A.; Gearty, S.V.; Pauli, M.L.; Nosbaum, A.; Gratz, I.K.; Otto, M.; Moon, J.J.; Liese, J.; et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity 2015, 43, 1011–1021. [Google Scholar] [CrossRef]
- Constantinides, M.G.; Link, V.M.; Tamoutounour, S.; Wong, A.C.; Perez-Chaparro, P.J.; Han, S.J.; Chen, Y.E.; Li, K.; Farhat, S.; Weckel, A.; et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 2019, 366, 6464. [Google Scholar] [CrossRef]
- Jacobsen, J.N.; Andersen, A.S.; Krogfelt, K.A. Impact of Pseudomonas aeruginosa quorum sensing on cellular wound healing responses in vitro. Scand. J. Infect. Dis. 2012, 44, 615–619. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Phan, S.; Feng, C.H.; Huang, R.; Lee, Z.X.; Moua, Y.; Phung, O.J.; Lenhard, J.R. Relative Abundance and Detection of Pseudomonas aeruginosa from Chronic Wound Infections Globally. Microorganisms 2023, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Iocono, J.A.; Colleran, K.R.; Remick, D.G.; Gillespie, B.W.; Ehrlich, H.P.; Garner, W.L. Interleukin-8 levels and activity in delayed-healing human thermal wounds. Wound Repair. Regen. 2000, 8, 216–225. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Montgomery, C.P.; Daniels, M.D.; Zhao, F.; Spellberg, B.; Chong, A.S.; Daum, R.S. Local inflammation exacerbates the severity of Staphylococcus aureus skin infection. PLoS ONE 2013, 8, e69508. [Google Scholar] [CrossRef]
- Fournier, B.; Philpott, D.J. Recognition of Staphylococcus aureus by the innate immune system. Clin. Microbiol. Rev. 2005, 18, 521–540. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Hamed, M.I.; Panitch, A.; Seleem, M.N. Targeting methicillin-resistant Staphylococcus aureus with short salt-resistant synthetic peptides. Antimicrob. Agents Chemother. 2014, 58, 4113–4122. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.C.; Hulten, K.G.; Kaplan, S.L.; Mason, E.O. Mupirocin resistance in Staphylococcus aureus causing recurrent skin and soft tissue infections in children. Antimicrob. Agents Chemother. 2011, 55, 2431–2433. [Google Scholar] [CrossRef]
- Farrell, D.J.; Castanheira, M.; Chopra, I. Characterization of global patterns and the genetics of fusidic acid resistance. Clin. Infect. Dis. 2011, 52 (Suppl. 7), S487–S492. [Google Scholar] [CrossRef]
- Reglinski, M.; Sriskandan, S. Chapter 38—Streptococcus pyogenes. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.-W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 675–716. [Google Scholar]
- Jasim, S.; Hatem, Z.; Abd Mohammed, Z. Virulence Factors and Clinical Features of Streptococcus Pyogenes: Overview. Ann. Rom. Soc. Cell Biol. 2021, 25, 603–614. [Google Scholar]
- Yin, Z.; Qiu, Y.; Han, Y.; Li, K. Topical probiotics in wound care: A review of effects, mechanisms, and applications. Interdiscip. Nurs. Res. 2024, 3, 63–71. [Google Scholar] [CrossRef]
- Li, M.; Xiao, H.; Su, Y.; Cheng, D.; Jia, Y.; Li, Y.; Yin, Q.; Gao, J.; Tang, Y.; Bai, Q. Synergistic Inhibitory Effect of Honey and Lactobacillus plantarum on Pathogenic Bacteria and Their Promotion of Healing in Infected Wounds. Pathogens 2023, 12, 501. [Google Scholar] [CrossRef]
- Mohtashami, M.; Mohamadi, M.; Azimi-Nezhad, M.; Saeidi, J.; Nia, F.F.; Ghasemi, A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol. Appl. Biochem. 2021, 68, 1421–1431. [Google Scholar] [CrossRef]
- Valdéz, J.C.; Peral, M.C.; Rachid, M.; Santana, M.; Perdigón, G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: The potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 2005, 11, 472–479. [Google Scholar] [CrossRef]
- Sesto Cabral, M.E.; Ramos, A.N.; Macedo, A.J.; Trentin, D.S.; Treter, J.; Manzo, R.H.; Valdez, J.C. Formulation and quality control of semi-solid containing harmless bacteria by-products: Chronic wounds pro-healing activity. Pharm. Dev. Technol. 2015, 20, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Soleymanzadeh Moghadam, S.; Mohammad, N.; Ghooshchian, M.; FathiZadeh, S.; Khodaii, Z.; Faramarzi, M.; Fagheei Aghmiyuni, Z.; Roudbari, M.; Pazouki, A.; Mousavi Shabestari, T. Comparison of the effects of Lactobacillus plantarum versus imipenem on infected burn wound healing. Med. J. Islam. Repub. Iran 2020, 34, 94. [Google Scholar] [CrossRef] [PubMed]
- Gudadappanavar, A.M.; Hombal, P.R.; Timashetti, S.S.; Javali, S.B. Influence of Lactobacillus acidophilus and Lactobacillus plantarum on wound healing in male Wistar rats—An experimental study. Int. J. Appl. Basic. Med. Res. 2017, 7, 233–238. [Google Scholar] [CrossRef]
- Celik, C.; Lee, S.Y.T.; Tanoto, F.R.; Veleba, M.; Kline, K.A.; Thibault, G. Decoding the Complexity of Delayed Wound Healing Following Enterococcus faecalis Infection; Cold Spring Harbor Laboratory: Laurel Hollow, NY, USA, 2023. [Google Scholar]
- Chong, K.K.L.; Tay, W.H.; Janela, B.; Yong, A.M.H.; Liew, T.H.; Madden, L.; Keogh, D.; Barkham, T.M.S.; Ginhoux, F.; Becker, D.L.; et al. Enterococcus faecalis Modulates Immune Activation and Slows Healing During Wound Infection. J. Infect. Dis. 2017, 216, 1644–1654. [Google Scholar] [CrossRef]
- Kao, P.H.; Ch’ng, J.H.; Chong, K.K.L.; Stocks, C.J.; Wong, S.L.; Kline, K.A. Enterococcus faecalis suppresses Staphylococcus aureus-induced NETosis and promotes bacterial survival in polymicrobial infections. FEMS Microbes 2023, 4, xtad019. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hammad, A.S.; Al Kaabi, A.S.; Alawi, H.H.; Khatoon, S.; Al-Asmakh, M. Evaluating the Effects of BSA-Coated Gold Nanorods on Cell Migration Potential and Inflammatory Mediators in Human Dermal Fibroblasts. J. Funct. Biomater. 2024, 15, 284. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad, A.S.; Zahedy, S.H.; Elqasass, S.S.; Said, S.S.; Elgamal, A.M.; Mahmoud, N.N.; Al-Asmakh, M. In Vitro Analysis of the Dynamic Role of the Bacterial Virulence Factors in Skin Wound Healing. Int. J. Mol. Sci. 2025, 26, 10472. https://doi.org/10.3390/ijms262110472
Hammad AS, Zahedy SH, Elqasass SS, Said SS, Elgamal AM, Mahmoud NN, Al-Asmakh M. In Vitro Analysis of the Dynamic Role of the Bacterial Virulence Factors in Skin Wound Healing. International Journal of Molecular Sciences. 2025; 26(21):10472. https://doi.org/10.3390/ijms262110472
Chicago/Turabian StyleHammad, Ayat S, Sarah H. Zahedy, Shatha S. Elqasass, Sawsan Sudqi Said, Abdelrahman M. Elgamal, Nouf N Mahmoud, and Maha Al-Asmakh. 2025. "In Vitro Analysis of the Dynamic Role of the Bacterial Virulence Factors in Skin Wound Healing" International Journal of Molecular Sciences 26, no. 21: 10472. https://doi.org/10.3390/ijms262110472
APA StyleHammad, A. S., Zahedy, S. H., Elqasass, S. S., Said, S. S., Elgamal, A. M., Mahmoud, N. N., & Al-Asmakh, M. (2025). In Vitro Analysis of the Dynamic Role of the Bacterial Virulence Factors in Skin Wound Healing. International Journal of Molecular Sciences, 26(21), 10472. https://doi.org/10.3390/ijms262110472






