Association of Lycopene and Male Reproductive Health: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. Study Selection
2.2. Summary of the Characteristics of the Included Studies and Patients
2.3. Primary and Secondary Outcomes
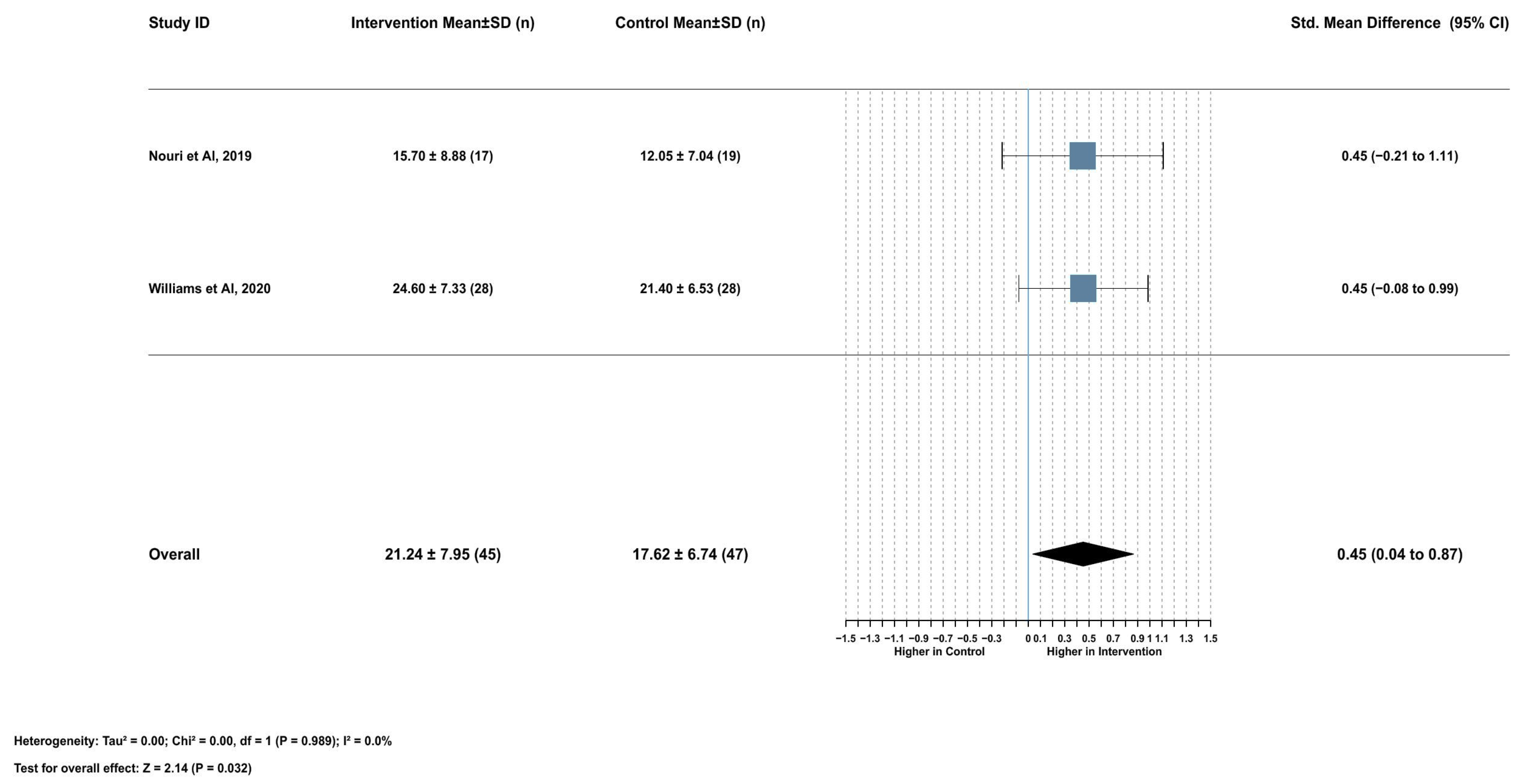
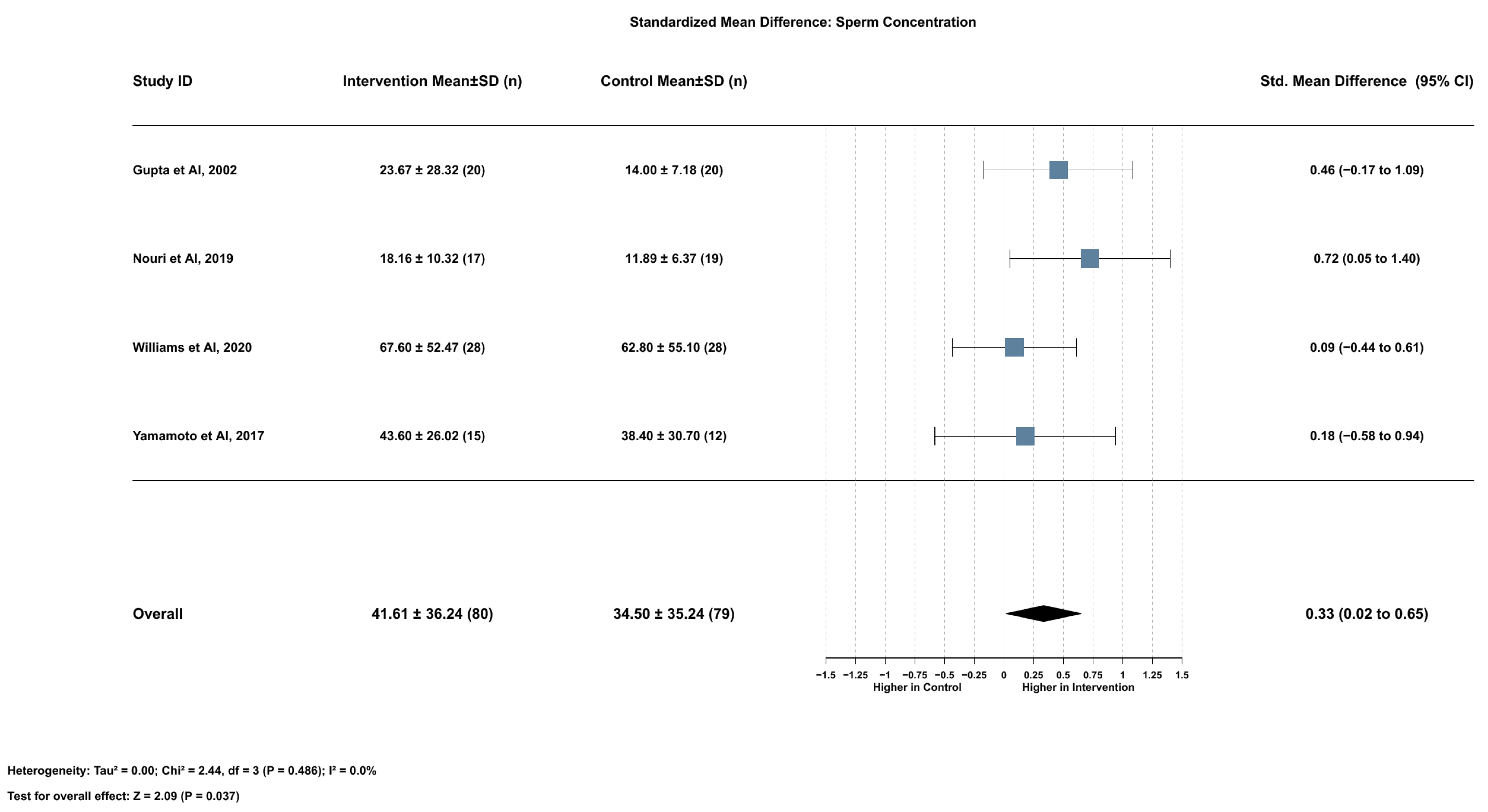
2.3.1. Association Between Serum Lycopene and Sperm Concentration
2.3.2. Association Between Serum Lycopene and Total Sperm Motility
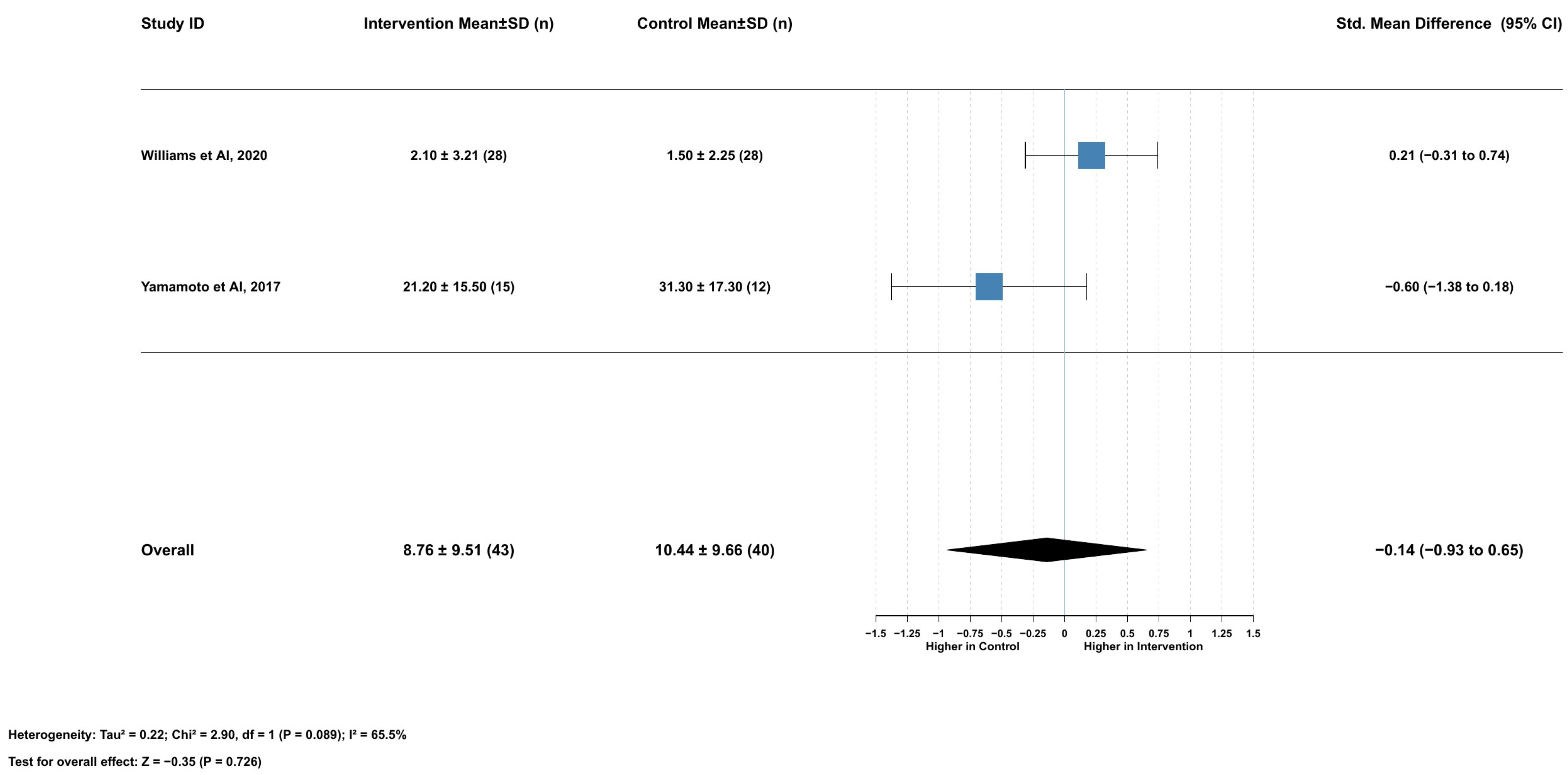
2.3.3. Association Between Serum Lycopene and Nonprogressive Motility
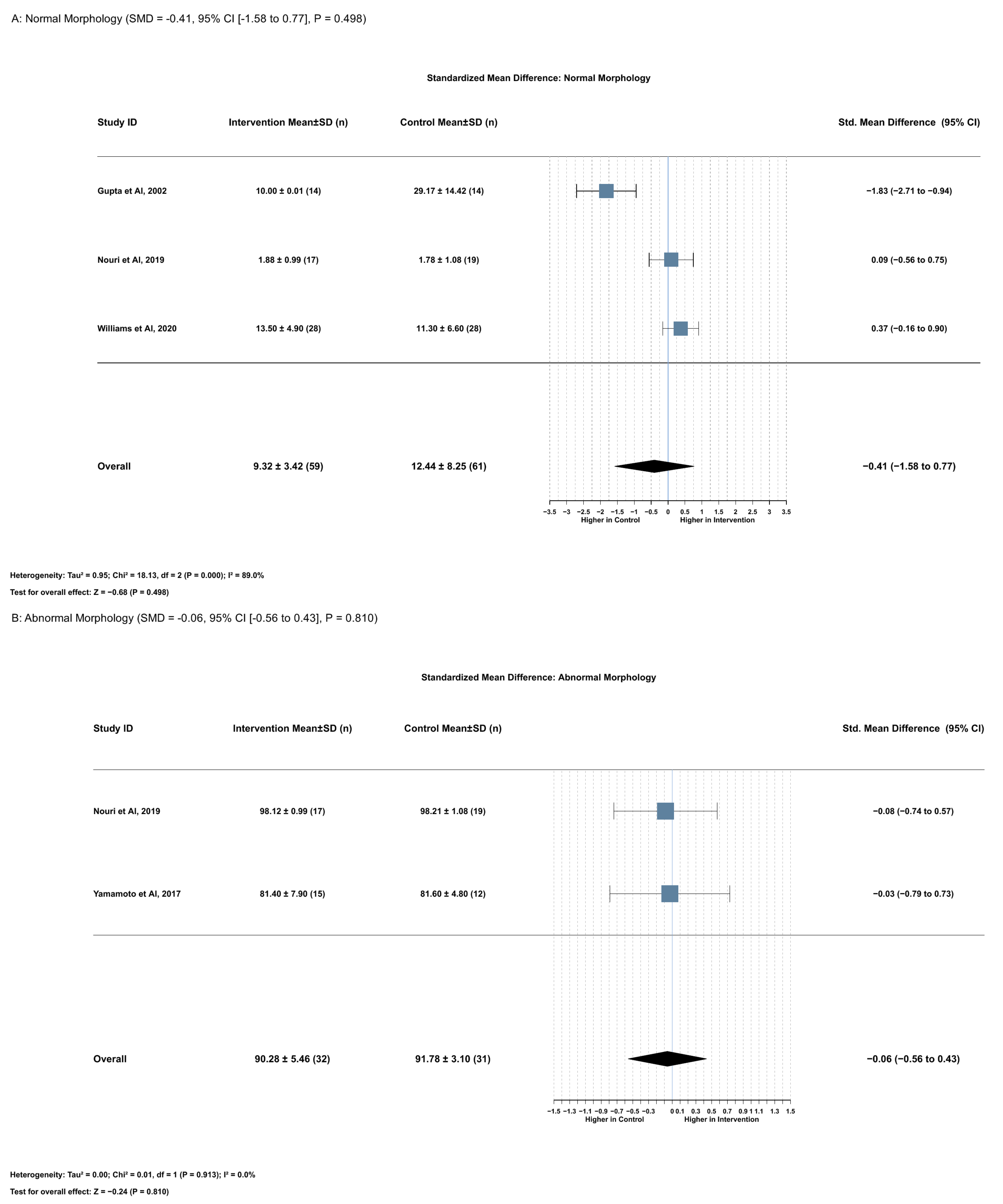
2.3.4. Association Between Serum Lycopene and Normal Morphology
2.3.5. Association Between Serum Lycopene and Abnormal Sperm Morphology
2.3.6. Association Between Serum Lycopene and DNA Damage
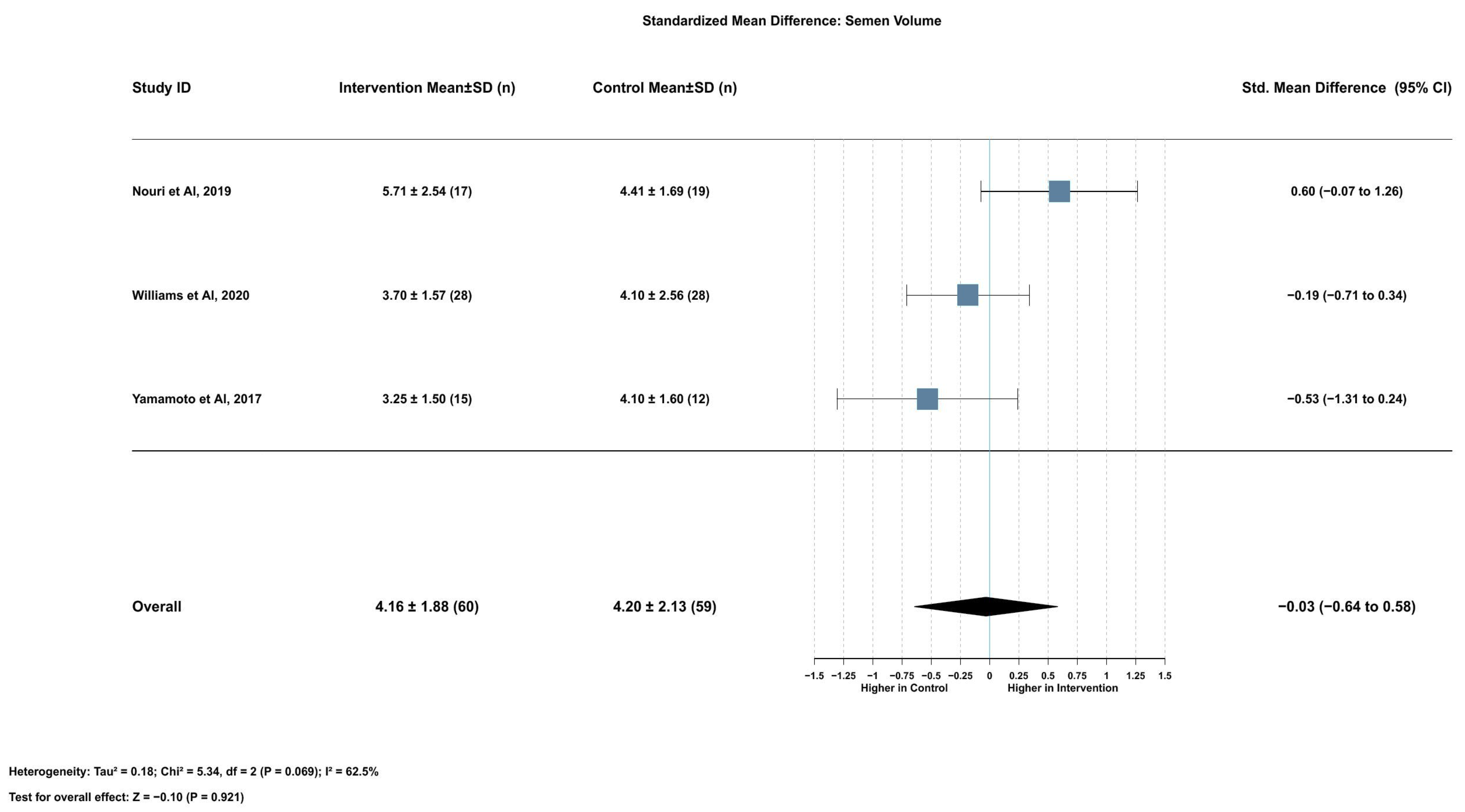
2.3.7. Association Between Serum Lycopene and Semen Volume
2.3.8. Association Between Serum Lycopene and Nonmotile Sperm
2.3.9. Association Between Serum Lycopene and Progressive Motility
2.3.10. Risk of Bias Assessment
3. Discussion
4. Materials and Methods
4.1. Study Protocol and Registration
4.2. Search Strategy and Data Collection
4.3. Data Extraction and Outcome Measurements
4.4. Quality Assessment
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMDs | Standardized mean differences |
References
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Schagdarsurengin, U.; Paradowska, A.; Steger, K. Analysing the sperm epigenome: Roles in early embryogenesis and assisted reproduction. Nat. Rev. Urol. 2012, 9, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M.; Sundaram, R.; Sweeney, A.M.; Schisterman, E.F.; Maisog, J.; Kannan, K. Urinary bisphenol A, phthalates, and couple fecundity: The Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil. Steril. 2014, 101, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K. Oxidative stress and male infertility–a clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Panner Selvam, M.K.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA fragmentation: A new guideline for clinicians. World J. Mens. Health 2020, 38, 412–471. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kwon, W.S.; Lee, J.S.; Yoon, S.J.; Ryu, B.Y.; Pang, M.G. Bisphenol-A affects male fertility via fertility-related proteins in spermatozoa. Sci. Rep. 2015, 5, 9169. [Google Scholar] [CrossRef]
- Simon, L.; Zini, A.; Dyachenko, A.; Ciampi, A.; Carrell, D.T. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J. Androl. 2017, 19, 80–90. [Google Scholar] [CrossRef]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and Its Antioxidant Role in the Prevention of Cardiovascular Diseases-A Critical Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef]
- Moslemi, E.; Nouri, M.; Askari, G. Effect of lycopene supplementation on infertility in men: A systematic review on clinical trial studies. Qom Univ. Med. Sci. J. 2019, 12, 28–41. [Google Scholar] [CrossRef]
- de Lamirande, E.; O’Flaherty, C. Sperm activation: Role of reactive oxygen species and kinases. Biochim. Biophys. Acta 2008, 1784, 106–115. [Google Scholar] [CrossRef]
- Palozza, P.; Parrone, N.; Simone, R.E.; Catalano, A. Lycopene in atherosclerosis prevention: An integrated scheme of the potential mechanisms of action from cell culture studies. Arch. Biochem. Biophys. 2010, 504, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.L.; Li, N.; Chen, Y.J.; Chen, X.S.; Yang, Z.; Xu, L.; Yang, H.M.; Wang, Z.Y. Protective effects of lycopene on mitochondrial oxidative injury and dysfunction in the liver of aflatoxin B1-exposed broilers. Poult. Sci. 2021, 100, 101441. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Guo, X.; Shang, J.; Li, Y.; Dong, W.; Peng, Q.; Xie, Z.; Chen, C. Effects of lycopene attenuating injuries in ischemia and reperfusion. Oxidative Med. Cell. Longev. 2022, 2022, 9309327. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C.; Dumont, U.; Halimi, C.; Lairon, D.; Page, D.; Sébédio, J.L.; Buisson, C.; Buffière, C.; Rémond, D. Dietary calcium impairs tomato lycopene bioavailability in healthy humans. Br. J. Nutr. 2016, 116, 2091–2096. [Google Scholar] [CrossRef]
- Van Steenwijk, H.P.; Bast, A.; de Boer, A. The role of circulating lycopene in low-grade chronic inflammation: A systematic review of the literature. Molecules 2020, 25, 4378. [Google Scholar] [CrossRef]
- Williams, E.A.; Parker, M.; Robinson, A.; Pitt, S.; Pacey, A.A. A randomized placebo-controlled trial to investigate the effect of lactolycopene on semen quality in healthy males. Eur. J. Nutr. 2020, 59, 825–833. [Google Scholar] [CrossRef]
- Nouri, M.; Amani, R.; Nasr-Esfahani, M.; Tarrahi, M.J. The effects of lycopene supplement on the spermatogram and seminal oxidative stress in infertile men: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2019, 33, 3203–3211. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Aizawa, K.; Mieno, M.; Karamatsu, M.; Hirano, Y.; Furui, K.; Miyashita, T.; Yamazaki, K.; Inakuma, T.; Sato, I.; et al. The effects of tomato juice on male infertility. Asia Pac. J. Clin. Nutr. 2017, 26, 65–71. [Google Scholar] [CrossRef]
- Gupta, N.P.; Kumar, R. Lycopene therapy in idiopathic male infertility—A preliminary report. Int. Urol. Nephrol. 2002, 34, 369–372. [Google Scholar] [CrossRef]
- Sadeghi, N.; Boissonneault, G.; Tavalaee, M.; Nasr-Esfahani, M.H. Oxidative versus reductive stress: A delicate balance for sperm integrity. Syst. Biol. Reprod. Med. 2023, 69, 20–31. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.; Li, H. Mechanisms of oxidative stress-induced sperm dysfunction. Front. Endocrinol. 2025, 16, 1520835. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, J.; Li, L.; Li, T.; Wang, J. Lycopene ameliorates D-galactose-induced hepatic senescence in CD-1 female mice through fibroblast growth factor 21/mitochondrial dynamics mediation. Wei Sheng Yan Jiu 2025, 54, 265–272. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Chang, Y.H.; Wang, X.C.; Liu, R.Q.; Yang, S.J.; Hu, Z.Y.; Jiang, F.W.; Chen, M.S.; Wang, J.X.; Liu, S.; et al. SIRT1 Regulates Fumonisin B1-Induced LMH Cell PANoptosis and Antagonism of Lycopene. J. Agric. Food Chem. 2025, 73, 4923–4935. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2019; Available online: https://www.cochrane.org/handbook (accessed on 13 March 2025).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016, 355, i4919. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021, 372, n71. [Google Scholar] [CrossRef]
- Boutron, I.; Page, M.J.; Higgins, J.P.T.; Altman, D.G.; Lundh, A.; Hróbjartsson, A. Considering bias and conflicts of interest among the included studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Cochrane: London, UK, 2024; Available online: https://www.cochrane.org/handbook (accessed on 13 March 2025).
- Schwarzer, G. meta: An R package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Soft. 2010, 36, 1–48. Available online: https://www.jstatsoft.org/index.php/jss/article/view/v036i03 (accessed on 13 March 2025). [CrossRef]
- Gordon, M.; Lumley, T. forestplot: Advanced forest plot using ‘grid’graphics. Package Version 2017, 1, 70. [Google Scholar]
- Neuwirth, E. RColorBrewer: Colorbrewer Palettes. 2014. Available online: https://CRAN.R-project.org/package=RColorBrewer (accessed on 10 March 2025).


| Study ID | Setting (Country) | Study Design | Sample Size | Condition of the Included Patients (PC/BPH/Healthy) | Form of Intervention | Duration of Follow-Up | Baseline Data | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Controls | Intervention | Controls | Lycopene Supplement (dose) | Control | Age (Mean ± SD)/Age Group, Years | Smoking Status (%) | BMI Mean (sd) | % of Body Fat | No. of Intervention Days | Total Lycopene Consumed During the Study, mg/day | |||||||||
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | ||||||||||
| Williams et al., 2020 [16] | UK | Randomized, double-blind, placebo-controlled clinical trial | 28 | 28 | Healthy | Healthy | 7 mg twice/day | Placebo | 3 months | 23.4 ± 3.22 | 23.3 ± 2.58 | 14.30% | 14.30% | 25.2 ± 3.08 | 23.5 ± 3.11 | NA | NA | 3 months | 3 months | 14 mg/day |
| Nouri et al., 2019 [17] | Iran | Randomized, double-blind, placebo-controlled clinical trial | 17 | 19 | Primary/secondary infertility | Primary/secondary infertility | 25 mg once a day and a second | Placebo | 3 months | 32.89 ± 2.33 | 32.15 ± 2.16 | 6 (35.29%) | 8 (42.1%) | 26.94 ± 1.57 | 26.53 ± 1.53 | 28.35 ± 3.23 | 27.98 ± 3.69 | 3 months | 3 months | 25 mg/day and a second |
| Yamamoto et al., 2017 [18] | Japan | Randomized clinical trial | 17 | 12 | Infertility | Infertility | 30 mg once/day | NA | 3 months | 38.1 ± 1.76 | 36.2 ± 1.91 | NA | NA | NA | NA | NA | NA | 3 months | 3 months | 30 mg |
| Gupta et al., 2002 [19] | India | Single arm clinical trial | 30 | Idiopathic infertility | 2000 mcg twice/day | 3 months | NA | NA | NA | NA | NA | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viña, I.; Viña, J.R. Association of Lycopene and Male Reproductive Health: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 7224. https://doi.org/10.3390/ijms26157224
Viña I, Viña JR. Association of Lycopene and Male Reproductive Health: Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(15):7224. https://doi.org/10.3390/ijms26157224
Chicago/Turabian StyleViña, Isabel, and Juan R. Viña. 2025. "Association of Lycopene and Male Reproductive Health: Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 15: 7224. https://doi.org/10.3390/ijms26157224
APA StyleViña, I., & Viña, J. R. (2025). Association of Lycopene and Male Reproductive Health: Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(15), 7224. https://doi.org/10.3390/ijms26157224