Effects of Hormone Replacement Treatment with Estrogen and Progestins on the Vascular Renin–Angiotensin System of Ovariectomized Rats
Abstract
1. Introduction
2. Results
2.1. E2 and DRSP Treatments Prevent Body Mass Gain in Ovariectomized Rats
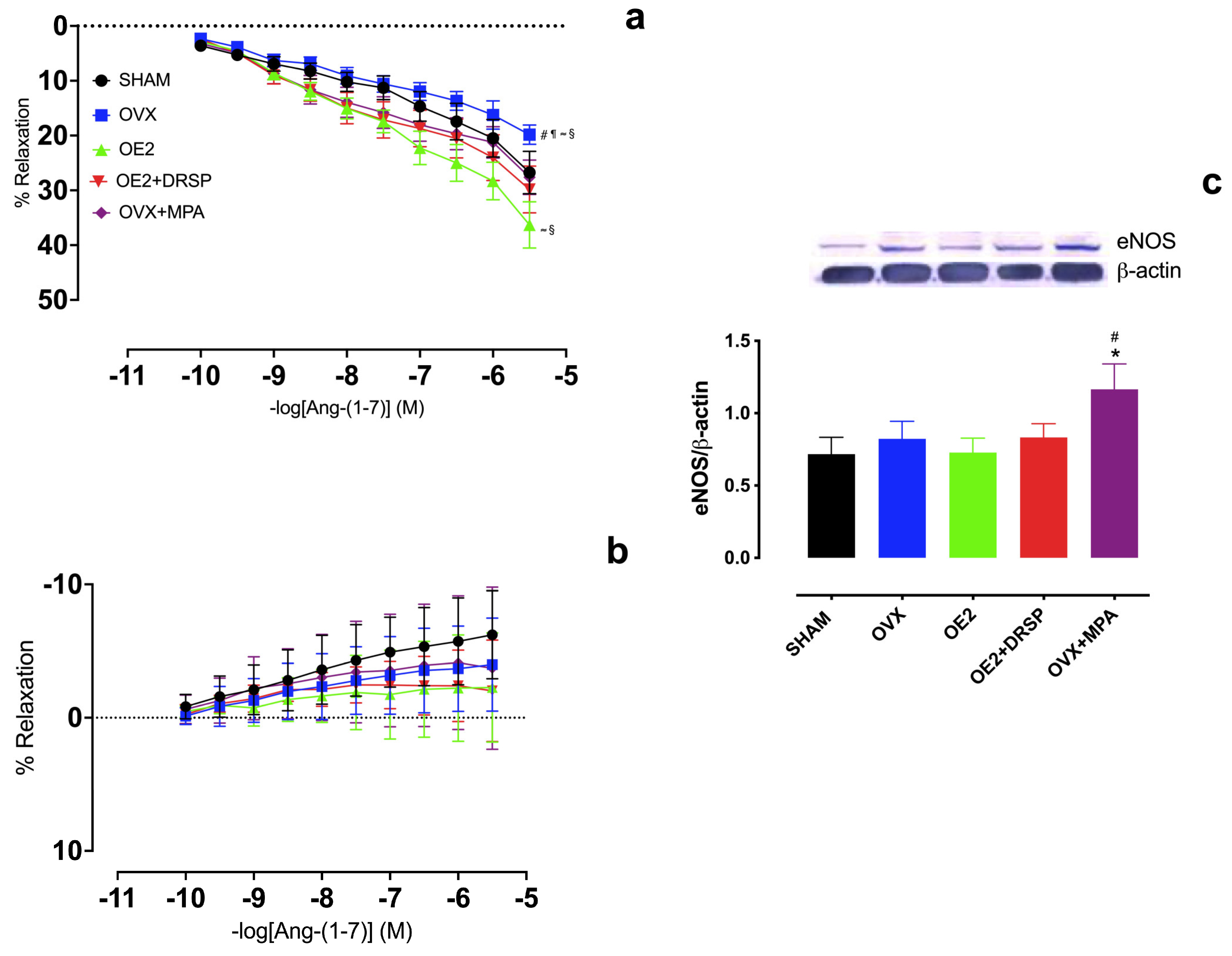
2.2. Hormonal Treatments Preserve Ang-(1-7)-Induced Vasodilation in an NO-Dependent Manner in the Aortic Rings of Ovariectomized Rats
2.3. MPA, but Not DRSP Treatment, Preserves Ang-(1-7)-Induced Vasodilation Independently of AT2R and Mas Receptors in the Aortic Rings of Ovariectomized Rats
3. Discussion
Clinical Perspectives
4. Materials and Methods
4.1. Experimental Animals
4.2. Ovariectomy
4.3. Hormonal Treatments
4.4. Estrous Cycle
4.5. Dissection and Vascular Reactivity of Isolated Thoracic Aorta Rings
4.6. Western Blot
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. Available online: https://www.jacc.org/doi/10.1016/j.jacc.2022.11.005 (accessed on 23 March 2024). [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef] [PubMed]
- Pardhe, B.D.; Ghimire, S.; Shakya, J.; Pathak, S.; Shakya, S.; Bhetwal, A.; Khana, P.R.; Parajuli, N.P. Elevated Cardiovascular Risks among Postmenopausal Women: A Community Based Case Control Study from Nepal. Biochem. Res. Int. 2017, 2017, 3824903. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5433412/ (accessed on 23 March 2024). [CrossRef] [PubMed]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Kristin, L.N.; Pinã, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update. Circulation 2011, 123, 1243–1262. [Google Scholar] [CrossRef]
- Davis, S.R.; Lambrinoudaki, I.; Lumsden, M.; Mishra, G.D.; Pal, L.; Rees, M.; Santoro, N.; Simoncini, T. Menopause. Nat. Rev. Dis. Primers 2015, 1, 15004. Available online: https://www.nature.com/articles/nrdp20154 (accessed on 23 March 2024). [CrossRef]
- Hernandez, I.; Delgado, J.L.; Diaz, J. 17β-Estradiol prevents oxidative stress and decreases blood pressure in ovariectomized rats. Am. J. Physiol. 2000, 279, 1599–1605. [Google Scholar] [CrossRef]
- Garcia, P.M.; Giménez, J.; Bonacasa, B.; Carbonell, L.F.; Miguel, S.G.; Quesada, T.; Hernández, I. 17β-Estradiol exerts a beneficial effect on coronary vascular remodeling in the early stages of hypertension in spontaneously hypertensive rats. Menopause 2005, 12, 453–459. [Google Scholar] [CrossRef]
- Grodstein, F.; Manson, J.E.; Colditz, G.A.; Willett, W.C.; Specizer, F.E.; Stampfer, M.J. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann. Intern. Med. 2000, 133, 933–941. [Google Scholar] [CrossRef]
- Grady, D.; Herrington, D.; Bittner, V.; Blumenthal, R.; Davidson, M.; Hlatky, M.; Hsia, J.; Hulley, S.; Herd, A.; Khan, S.; et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and estrogen/progestin replacement study follow-up (HERS II). JAMA 2002, 288, 49–57. [Google Scholar] [CrossRef]
- Hendrix, S.L.; Smoller, S.W.; Johnson, K.C.; Howard, B.V.; Kooperberg, C.; Rossouw, J.E.; Trevisan, M.; Aragaki, A.; Baird, A.E.; Bray, P.F.; et al. Effects of conjugated equine estrogen on stroke in the women’s health initiative. Circulation 2006, 113, 2425–2434. [Google Scholar] [CrossRef]
- Khalil, R.A. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem. Pharmacol. 2013, 86, 1627–1642. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Imthurn, B.; Zacharia, L.C.; Jackson, E.K. Hormone replacement therapy and cardiovascular disease: What went wrong and where do we go from here? Hypertension 2004, 44, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Ghatge, R.P.; Jacobsen, B.M.; Schittone, S.A.; Horwitz, K.B. The progestational and androgenic properties of medroxyprogesterone acetate: Gene regulatory overlap with dihydrotestosterone in breast cancer cells. Breast. Cancer Res. 2005, 7, R1036. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawano, H.; Motoyama, T.; Hirai, N.; Yoshimura, T.; Kugiyama, K.; Ogawa, H.; Okamura, H.; Yasue, H. Effect of medroxyprogesterone acetate plus estradiol on endothelium-dependent vasodilation in post-menopausal women. Am. J. Cardiol. 2001, 87, 238–240. [Google Scholar] [CrossRef]
- Pedersena, S.H.; Nielsenb, L.B.; Mortensenc, A.; Sheykhzaded, M.; Nilase, L.; Ottesena, B. Medroxyprogesterone acetate attenuates long-term effects of 17-β-estradiol in coronary arteries fromnhyperlipidemic rabbits. Steroids 2006, 7, 834–842. [Google Scholar] [CrossRef]
- Williams, J.K.; Honore, E.K.; Washburn, S.A.; Clarkson, T.B. Effects of hormone replacement therapy on reactivity of atherosclerotic coronary arteries in cynomolgus monkeys. J. Am. Coll. Cardiol. 1994, 24, 1757–1761. [Google Scholar] [CrossRef]
- Mishra, R.G.; Hermsmeyer, R.K.; Miyagawa, K.; Sarrel, F.; Uchida, B.; Stanczyk, F.Z.; Burry, K.A.; Illingworth, D.R. Medroxyprogesterone acetate and dihydrotestosterone induce coronary hyperreactivity in intact male rhesus monkeys. J. Clin. Endocrinol. Metab. 2005, 90, 3706–3714. [Google Scholar] [CrossRef]
- Sitruk-Ware, R. Pharmacology of different progestogens: The special case of drospirenone. Climacteric 2005, 8, 4–12. [Google Scholar] [CrossRef]
- Arias-loza, P.A.; Hu, K.; Schafer, A.; Bauersachs, J.; Quaschning, T.; Galle, J.; Jazbutyte, V.; Neyses, L.; Ertl, J.; Fritzemeier, K.H.; et al. Medroxyprogesterone acetate but not drospirenone ablates the protective function of 17β -estradiol in aldosterone salt–treated rats. Hypertension 2006, 48, 994–1001. [Google Scholar] [CrossRef]
- Borgo, M.V.; Claudio, E.R.G.; Silva, F.B.; Romero, W.G.; Gouvea, S.A.; Moysés, M.R.; Santos, R.L.; Almeida, S.A.; Podratz, P.L.; Graceli, J.B.; et al. Hormonal therapy with estradiol and drospirenone improves endothelium-dependent vasodilation in the coronary bed of ovariectomized spontaneously hypertensive rats. Braz. J. Med. Biol. Res. 2016, 49, e4655. [Google Scholar] [CrossRef]
- Nickenig, G.; Baumer, E.; Grohè, C.; Kahlert, S.; Strehlow, K.; Rosenkranz, S.; Stablein, U.; Bechers, F.; Smits, J.; Daemen, M.; et al. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation 1998, 97, 2197–2201. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, S.; Baumer, A.T.; Strehlow, K.; Eickels, M.V.; Grohè, C.; Ahlbory, K.; Rosen, R.; Bohm, M.; Nickening, G. Endothelial dysfunction and oxidative stress during estrogen deficiency in spontaneously hypertensive rats. Circulation 2001, 103, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Dalpiaz, P.L.; Lamas, A.Z.; Caliman, I.F.; Ribeiro, R.F., Jr.; Abreu, G.R.; Moyses, M.R.; Andrade, T.U.; Gouvea, S.A.; Alves, M.F.; Carmona, A.K.; et al. Sex Hormones Promote Opposite Effects on ACE and ACE2 Activity, Hypertrophy and Cardiac Contractility in Spontaneously Hypertensive Rats. PLoS ONE 2015, 10, e0127515, Erratum in PLoS ONE 2015, 10, e0133225. [Google Scholar] [CrossRef]
- Melo Junior, A.F.; Dalpiaz, P.L.M.; Escouto, L.D.S.; Sousa, G.J.; Aires, R.; Oliveira, N.D.; Carmona, A.K.; Gava, Á.L.; Bissoli, N.S. Involvement of sex hormones, oxidative stress, ACE and ACE2 activity in the impairment of renal function and remodelling in SHR. Life Sci. 2020, 257, 118138. [Google Scholar] [CrossRef]
- Neves, L.A.; Averill, D.B.; Ferrario, C.M.; Aschner, J.L.; Brosnihan, K.B. Vascular responses to Angiotensin-(1-7) during the estrous cycle. Endocrine 2004, 24, 161–165. [Google Scholar] [CrossRef]
- Bosnyak, S.; Jones, E.S.; Christopoulos, A.; Aguilar, M.-I.; Thomas, W.G.; Widdop, R.E. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin. Sci. 2011, 121, 297–303. [Google Scholar] [CrossRef]
- Hallberg, M.; Sävmarker, J.; Hallberg, A. Angiotensin peptides as AT2 receptor agonists. Curr. Protein Pept. Sci. 2017, 18, 809–818. [Google Scholar] [CrossRef]
- Sampaio, W.O.; Henrique de Castro, C.; Santos, R.A.S.; Schiffrin, E.L.; Touyz, R.M. Angiotensin-(1–7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 2007, 50, 1093–1098. [Google Scholar] [CrossRef]
- Karnik, S.S.; Singh, K.D.; Tirupula, K.; Unal, H. Significance of angiotensin 1-7 coupling with MAS1 receptor and other GPCRs to the renin-angiotensin system: IUPHAR Review 22. Br. J. Pharmacol. 2017, 174, 737–753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sampson, A.K.; Hilliard, L.M.; Moritz, K.M.; Thomas, M.C.; Tikellis, C.; Widdop, R.E.; Denton, K.M. The arterial depressor response to chronic low-dose angiotensin II infusion in female rats is estrogen dependent. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R159–R165. [Google Scholar] [CrossRef]
- Yung, L.M.; Wong, W.T.; Tian, X.Y.; Leung, F.P.; Yung, L.H.; Chen, Z.Y.; Yao, X.; Lau, C.W.; Huang, Y. Inhibition of renin-angiotensin system reverses endothelial dysfunction and oxidative stress in estrogen-deficient rats. PLoS ONE 2011, 6, e17437. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.M.; Wade, G.N. Food intake, body weight, and adiposity in female rats: Actions and interactions of progestins and antiestrogens. Am. J. Physiol. 1981, 240, E474–E481. [Google Scholar] [CrossRef] [PubMed]
- Malínská, H.; Hüttl, M.; Miklankova, D.; Trnovska, J.; Zapletalová, I.; Poruba, M.; Marková, I. Ovariectomy-Induced Hepatic Lipid and Cytochrome P450 Dysmetabolism Precedes Serum Dyslipidemia. Int. J. Mol. Sci. 2021, 22, 527. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xiao, J.C.; Luo, L.F.; Wang, S.; Zhang, J.P.; Huang, J.J.; Liu, M.L.; Liu, C.G.; Xu, K.Q.; Li, Y.J.; et al. Effects of ovariectomy and 17beta-estradiol treatment on the renin-angiotensin system, blood pressure, and endothelial ultrastructure. Int. J. Cardiol. 2008, 130, 196–204. [Google Scholar] [CrossRef]
- Fontana, R.; Torre, S.D.; Meda, C.; Longo, A.; Eva, C.; Maggi, A.C. Estrogen replacement therapy regulation of energy metabolism in female mouse hypothalamus. Endocrinology 2014, 155, 2213–2221. [Google Scholar] [CrossRef]
- Gravena, A.A.; Brischilari, S.C.; Lopes, T.C.; Agnolo, C.M.; Carvalho, M.D.; Pelloso, S.M. Excess weight and abdominal obesity in postmenopausal Brazilian women: A population-based study. BMC Women’s Health 2013, 13, 46. [Google Scholar] [CrossRef]
- Archer, D.F.; Thorneycroft, I.H.; Foegh, M.; Hanes, V.; Glant, M.D.; Bitterman, P.; Kempson, R. Long-term safety of drospirenone-estradiol for hormone therapy: A randomized, double-blind, multicenter trial. Menopause 2005, 12, 716–727. [Google Scholar] [CrossRef]
- Huber, J.; Foidart, J.M.; Wuttke, W.; Feld, G.M.; The, H.S.; Gerlinger, C.; Schellschmidt, E.; Heithecker, R. Efficacy and tolerability of a monophasic oral contraceptive containing ethinylestradiol and drospirenone. Eur. J. Contracept. Reprod. Health Care 2000, 5 (Suppl. S3), 25–34. [Google Scholar] [CrossRef]
- Foidart, J.M. The contraceptive profile of a new oral contraceptive with antimineralocorticoid and antiandrogenic effects. Eur. J. Contracept. Reprod. Health Care 2000, 5, 25–33. [Google Scholar] [CrossRef]
- Oelkers, W.; Foidart, J.M.; Dombrovicz, N.; Welter, A.; Heithecker, R. Effects of a new oral contraceptive containing an antimineralocorticoid progestogen, drospirenone, on the renin-aldosterone system, body weight, blood pressure, glucose tolerance, and lipid metabolism. J. Clin. Endocrinol. Metab. 1995, 80, 1816–1821. [Google Scholar] [CrossRef]
- Ferreira, A.J.; Santos, R.A.S. Cardiovascular actions of angiotensin-(1,7). Braz. J. Med. Biol. Res. 2005, 38, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Laborde, C.; Craig, T.; Zheng, W.; Ji, H.; Haywood, J.R.; Sandberg, K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 2004, 44, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, N.; Sumino, H.; Ichikawa, S.; Kumakura, H.; Takayama, Y.; Nakamura, T.; Kanda, T.; Mizunuma, H.; Kurabayashi, M. Effect of long-term hormone replacement therapy on angiotensin-converting enzyme activity and bradykinin in postmenopausal women with essential hypertension and normotensive postmenopausal women. Menopause 2001, 8, 210–215. [Google Scholar] [CrossRef]
- Schunkert, H.; Danser, A.H.; Hense, H.W.; Derkx, F.H.; Kurzinger, S.; Riegger, G.A. Effects of estrogen replacement therapy on the renin–angiotensin system in post-menopausal women. Circulation 1997, 95, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Endlich, P.W.; Cláudio, E.R.G.; Lima, L.C.; Júnio, R.F.R.; Peluso, A.A.B.; Stefanon, I.; Bissoli, N.S.; Lemos, V.S.; Santos, R.A.S.; Abreu, G.R. Exercise modulates the aortic renin-angiotensin system independently of estrogen therapy in ovariectomized hypertensive rats. Peptides 2017, 87, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Silva, J.; Tavares, R.; Ferreira, M.; Honorato-Sampaio, K.; Cavallo, I.; Santos, R.; Reis, A.; Reis, F. Tissue specific localization of angiotensin-(1–7) and its receptor Mas in the uterus of ovariectomized rats. J. Mol. Histol. 2012, 43, 597–602. [Google Scholar] [CrossRef]
- Acs, N.; Székács, B.; Nádasy, G.; Várbiró, S.; Kabucs, R.; Monos, E. The effect of ovariectomy and oestrogen replacement on small artery biomechanics in the rat. Br. J. Obs. Gynecol. 1999, 106, 148–154. [Google Scholar] [CrossRef]
- Brosnihan, K.B.; Li, P.; Gaten, D.; Ferrario, C.M. Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS. Am. J. Physiol. 1997, 273, 1908–1915. [Google Scholar] [CrossRef]
- Lemos, V.S.; Silva, D.M.; Walther, T.; Alenina, N.; Bader, M.; Santos, R.A.S. The endothelium-dependent vasodilator effect of the nonpeptide Ang(1–7) mimic AVE 0991 is abolished in the aorta of Mas-knockout mice. J. Cardiovasc. Pharmacol. 2005, 46, 274–279. [Google Scholar] [CrossRef]
- Silva, D.M.R.; Filho, A.G.; Olivon, V.C.; Santos, T.M.; Becker, L.K.; Santos, R.A.; Lemos, V.S. Swimming training improves the vasodilator effect of angiotensin-(1-7) in the aorta of spontaneously hypertensive rat. J. Appl. Physiol. 2011, 111, 1272–1277. [Google Scholar] [CrossRef]
- Walters, P.E.; Gaspari, T.A.; Widdop, R.E. Angiotensin-(1-7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension. 2005, 45, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.S.; Ferreira, A.J.; Verano-Braga, T.; Bader, M. Angiotensin-converting enzyme 2, angiotensin-(1–7) and Mas: New players of the renin-angiotensin system. J. Endocrinol. 2013, 216, R1–R17. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.H.; Santos, R.A.; Ferreira, A.J.; Bader, M.; Alenina, N.; Almeida, A.P. Evidence for a functional interaction of the angiotensin-(1-7) receptor Mas with AT1 ou AT2 receptors in the mouse heart. Hypertension 2005, 46, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.M.; Vianna, H.R.; Cortes, S.F.; Campagnole-Santos, M.J.; Santos, R.A.S.; Lemos, V.S. Evidence for a new angiotensin-(1-7) receptor subtype in the aorta of Sprague-Dawley rats. Peptides 2007, 28, 702–707. [Google Scholar] [CrossRef]
- Lautner, R.Q.; Villela, D.C.; Fraga-Silva, R.A.; Silva, N.; Verano-Braga, T.; Costa-Fraga, F.; Jankowski, J.; Jankowski, V.; Sousa, F.; Alzamora, A.; et al. Discovery and characterization of alamandine: A novel component of the renin-angiotensin system. Circ. Res. 2013, 112, 1104–1111, Erratum in Circ. Res. 2013, 112, e156. [Google Scholar] [CrossRef] [PubMed]
- Tetzner, A.; Gebolys, K.; Meinert, C.; Klein, S.; Uhlich, A.; Trebicka, J.; Villacañas, Ó.; Walther, T. G-Protein-Coupled Receptor MrgD Is a Receptor for Angiotensin-(1-7) Involving Adenylyl Cyclase, cAMP, and Phosphokinase A. Hypertension 2016, 68, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Sampson, A.K.; Moritz, K.M.; Jones, E.S.; Flower, R.L.; Widdop, R.E.; Denton, K.M. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 2008, 52, 666–671. [Google Scholar] [CrossRef]
- Hudson, M.; Rahme, E.; Behlouli, H.; Sheppard, R.; Pilote, L. Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin-converting enzyme inhibitors in patients with congestive heart failure–A population study. Eur. J. Heart. Fail. 2007, 9, 602–609. [Google Scholar] [CrossRef]
- Ramos-Filho, A.C.; Faria, J.A.; Calmasini, F.B.; Teixeira, S.A.; Monica, F.Z.; Muscará, M.N.; Gontijo, J.A.R.; Anhê, G.F.; Zanesco, A.; Antunes, E. The renin-angiotensin system plays a major role in voiding dysfunction of ovariectomized rats. Life Sci. 2013, 93, 820–829. [Google Scholar] [CrossRef]
- Shenoy, V.; Grobe, J.L.; Qi, Y.; Ferreira, A.J.; Silva, R.A.; Collamat, G.; Bruce, E.; Katovich, M.J. 17b-Estradiol modulates local cardiac renin-angiotensin system to prevent cardiac remodeling in the DOCA-salt model of hypertension in rats. Peptides 2009, 30, 2309–2315. [Google Scholar] [CrossRef]
- Ji, H.; Menini, S.; Zheng, W.; Pesce, C.; Wu, X.; Sandberg, K. Role of angiotensin-converting enzyme 2 and angiotensin (1–7) in 17β-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp. Physiol. 2008, 93, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Schleifenbaum, J. Alamandine and Its Receptor MrgD Pair Up to Join the Protective Arm of the Renin-Angiotensin System. Front. Med. 2019, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Qaradakhi, T.; Matsoukas, M.; Hayes, A.; Rybalka, E.; Čaprnda, M.; Rimárová, K.; Sepsi, M.; Büsselberg, D.; Kruzliak, P.; Matsoukas, J.; et al. Alamandine reverses hyperhomocysteinemia-induced vascular dysfunction via PKA-dependent mechanisms. Cardiovasc. Ther. 2017, 35, e12306. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Fortes, Z.; Nugro, D.; Tostes, R.; Santos, R.; Carvalho, M.C. Potentiation of bradykinin by angiotensin-(1–7) on arterioles of spontaneously hypertensive rats studied in vivo. Hypertension 2001, 37, 703–709. [Google Scholar] [CrossRef]
- Sampaio, W.O.; Souza dos Santos, R.A.; Faria-Silva, R.; da Mata Machado, L.T.; Schiffrin, E.L.; Touyz, R.M. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 2007, 49, 185–192. [Google Scholar] [CrossRef]
- Carey, R.M.; Siragy, H.M. Newly components of the renin-angiotensin system: Potential roles in cardiovascular and renal regulation. Endocr. Rev. 2003, 24, 261–271. [Google Scholar] [CrossRef]
- Simoncini, T.; Mannella, P.; Fornari, L.; Caruso, A.; Willis, M.Y.; Garibaldi, S.; Baldacci, C.; Genazzani, A.R. Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology 2004, 145, 5745–5756. [Google Scholar] [CrossRef]
- Liao, J.K. Endothelial nitric oxide and vascular inflammation. In Endothelium, Nitric Oxide and Atherosclerosis; Panza, J.A., Cannon, R.O.I., Eds.; Futura Publishing Co.: Armonk, NY, USA, 1999; pp. 119–132. [Google Scholar]
- Arias-loza, P.; Hu, K.; Frantz, S.; Dienesch, C.; Bayer, B.; Wu, R.; Ertl, J.; Pelzer, T. Medroxyprogesterone acetate aggravates oxidative stress and left ventricular dysfunction in rats with chronic myocardial infarction. Toxicol. Pathol. 2011, 39, 867–878. [Google Scholar] [CrossRef]
- Harman, S.M.; Black, D.M.; Naftolin, F.; Brinton, E.; Budoff, M.J.; Cedros, M.I.; Hopkins, P.N.; Lobo, R.A.; Manson, J.E.; Merriam, G.R.; et al. Arterial Imaging and Cardiovascular Risk Factors in Recently Menopausal Women: A Randomized Trial. Ann. Intern. Med. 2014, 161, 249–260. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Almeida, S.A.; Claudio, E.R.; Mengal, V.; Oliveira, S.G.; Merlo, E.; Podratz, P.L.; Gouvêa, S.A.; Graceli, J.B.; de Abreu, G.R. Exercise training reduces cardiac dysfunction and remodeling in ovariectomized rats submitted to myocardial infarction. PLoS ONE 2014, 9, e115970, Erratum in PLoS ONE 2015, 10, e0118246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petanceska, S.S.; Nagy, V.; Frail, D.; Gandy, S. Ovariectomy and 17beta-estradiol modulate the levels of Alzheimer’s amyloid beta peptides in brain. Neurology 2000, 54, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.M.; Borges, P.P.; Lisboa, P.C.; Curty, F.H.; Moura, E.G.; Pazos-Moura, C.C. Effect of medroxyprogesterone acetate on thyrotropin secretion in adult and old female rats. Braz. J. Med. Biol. Res. 2000, 33, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.B.; Romero, W.G.; Rouver, W.D.N.; Silva, K.; de Almeida, S.A.; Mengal, V.; Peluso, A.A.; Endlich, P.W.; Bissoli, N.S.; Claudio, E.R.G.; et al. Ellagic Acid prevents vascular dysfunction in small mesenteric arteries of ovariectomized hypertensive rats. J. Nutr. Biochem. 2022, 105, 108995. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef]
- Dalle Lucca, J.J.; Adeagbo, A.S.O.; Alsip, N.L. Influence of oestrous cycle and pregnancy on the reactivity of the rat mesenteric vascular bed. Hum. Reprod. 2000, 15, 961–968. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Grant, G.; Bernadoni, J.; Soh, H.; Walther, T. A role for Angiotensin-(1-7) and its receptors in sprouting angiogenesis and vascular homeostasis. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Tetzner, A.; Naughton, M.; Gebolys, K.; Sala, E.; Villacañas, Ó.; Walther, T. Abstract P237: The At2 Receptor Agonist, C21, Can Also Stimulate Mas and Mrgd Receptors. Hypertension 2017, 70, AP237. [Google Scholar] [CrossRef]
- Resende, G.; Silva, N.; Villela, D.; Santos, R. Abstract 484: The Vasorelaxing No Release Effect of Angiotensin-(1-9) Is Independent of At2, Mas or Mrgd Receptors. Hypertension 2014, 64, A484. [Google Scholar] [CrossRef]

| SHAM (n) | OVX (n) | OE2 (n) | OE2 + DRSP (n) | OVX + MPA (n) | |
|---|---|---|---|---|---|
| Initial Body Mass (g) | 242.2 ± 5.4 (11) | 247.3 ± 5.5 (15) | 252.2 ± 5.3 (16) | 251.0 ± 5.6 (16) | 257.0 ± 6.1 (15) |
| Final Body Mass (g) | 271.3 ± 5.8 (11) | 312.0 ±7.8 *#≈ (15) | 287.8 ± 6.0 (16) | 289.3 ± 4.8 (16) | 317.4 ±8.5 *#≈ (15) |
| Body Mass Gain (Δ%) | 10.6 ± 1.0 (11) | 26.1 ± 1.7 *#≈ (15) | 12.2 ± 1.3 (16) | 13.2 ± 1.3 (16) | 23.5 ± 1.7 *#≈ (15) |
| Dry Uterus Weight (g) | 12.3 ± 0.7 (11) | 3.4 ± 0.1 *#≈§ (15) | 6.0 ± 0.4 * (16) | 5.8 ± 0.3 * (16) | 4.8 ± 0.3 *# (15) |
| Uterus Weight/Tibia Length (g/cm) | 3.1 ± 0.3 (11) | 0.9 ± 0.03 *#≈§ (15) | 1.6 ± 0.1 * (16) | 1.5 ± 0.08 * (16) | 1.2 ± 0.08 *#≈ (15) |
| Parametrial Fat (g) | 4.5 ± 0.4 (9) | 5.0 ± 0.5 (9) | 4.5 ± 0.5 (9) | 5.6 ± 0.8 (9) | 6.9 ± 0.8 *$# (9) |
| Retroperitoneal Fat (g) | 3.3 ± 0.5 (9) | 4.2 ± 0.4 (9) | 4.1 ± 0.4 (9) | 4.6 ± 0.5 (9) | 6.2 ± 0.5 *$#≈ (9) |
| Mesenteric Fat (g) | 2.7 ± 0.1 (9) | 3.2 ± 0.2 (9) | 3.0 ± 0.2 (9) | 3.1 ± 0.1 (9) | 3.8 ± 0.3 *#≈ (9) |
| Total Fat (g) | 10.6 ± 0.9 (9) | 12.4 ± 1.0 (9) | 11.7 ± 1.1 (9) | 13.44 ± 1.5 (9) | 17.0 ± 1.4 *$# (9) |
| Primary Antibodies | Dilution | Source | Catalog Number | Brand |
| Anti-AT1R | 1: 500 | Mouse | SC-515884 | Santa Cruz Biotechnology |
| Anti-AT2R | 1: 500 | Mouse | SC156014 | Santa Cruz Biotechnology |
| Anti-eNOS | 1: 2500 | Mouse | BD554002 | Transduction Laboratories |
| Anti-ACE2 | 1: 100 | Rabbit | SC390851 | Santa Cruz Biotechnology |
| Anti-Mas | 1: 100 | Mouse | SC390453 | Santa Cruz Biotechnology |
| Secondary Antibodies | Dilution | Source | Catalog Number | Brand |
| Anti-rabbit | 1: 2500 | Rabbit | A0545 | Sigma-Aldrich |
| Anti-mouse | 1: 7000 | Mouse | A2554 | Sigma-Aldrich |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes, L.A.; Endlich, P.W.; Lima, D.S.S.; Peluso, A.A.; de Almeida, S.A.; Borgo, M.V.; Santos, R.A.S.; de Abreu, G.R. Effects of Hormone Replacement Treatment with Estrogen and Progestins on the Vascular Renin–Angiotensin System of Ovariectomized Rats. Int. J. Mol. Sci. 2025, 26, 4930. https://doi.org/10.3390/ijms26104930
Menezes LA, Endlich PW, Lima DSS, Peluso AA, de Almeida SA, Borgo MV, Santos RAS, de Abreu GR. Effects of Hormone Replacement Treatment with Estrogen and Progestins on the Vascular Renin–Angiotensin System of Ovariectomized Rats. International Journal of Molecular Sciences. 2025; 26(10):4930. https://doi.org/10.3390/ijms26104930
Chicago/Turabian StyleMenezes, Laís Almeida, Patrick Wander Endlich, Deiviany Santana Santos Lima, A. Augusto Peluso, Simone Alves de Almeida, Mariana Veronez Borgo, Robson Augusto Souza Santos, and Glaucia Rodrigues de Abreu. 2025. "Effects of Hormone Replacement Treatment with Estrogen and Progestins on the Vascular Renin–Angiotensin System of Ovariectomized Rats" International Journal of Molecular Sciences 26, no. 10: 4930. https://doi.org/10.3390/ijms26104930
APA StyleMenezes, L. A., Endlich, P. W., Lima, D. S. S., Peluso, A. A., de Almeida, S. A., Borgo, M. V., Santos, R. A. S., & de Abreu, G. R. (2025). Effects of Hormone Replacement Treatment with Estrogen and Progestins on the Vascular Renin–Angiotensin System of Ovariectomized Rats. International Journal of Molecular Sciences, 26(10), 4930. https://doi.org/10.3390/ijms26104930







