Abstract
Sulforaphane (SFN) is a promising molecule for developing phytopharmaceuticals due to its potential antioxidative and anti-inflammatory effects. A plethora of research conducted in vivo and in vitro reported the beneficial effects of SFN intervention and the underlying cellular mechanisms. Since SFN is a newly identified nutraceutical in sports nutrition, only some human studies have been conducted to reflect the effects of SFN intervention in exercise-induced inflammation and oxidative stress. In this review, we briefly discussed the effects of SFN on exercise-induced inflammation and oxidative stress. We discussed human and animal studies that are related to exercise intervention and mentioned the underlying cellular signaling mechanisms. Since SFN could be used as a potential therapeutic agent, we mentioned briefly its synergistic attributes with other potential nutraceuticals that are associated with acute and chronic inflammatory conditions. Given its health-promoting effects, SFN could be a prospective nutraceutical at the forefront of sports nutrition.
1. Introduction
Research with bioactive compounds is getting more attention due to their unique nutritional value and numerous health benefits. Fruits, vegetables, and whole grains are considered a good source of bioactive compounds, and they have many health benefits beyond fundamental nutritional values. In addition, various epidemiological studies reported the importance of bioactive compounds in diminishing the risk of life-threatening chronic diseases, for instance, cancer, diabetes, stroke, heart disease, obesity, and so on. Some bioactive compounds are more popular with certain consumers for sustainable personalized nutrition solutions [1,2,3,4].
Our immune system consistently maintains a sustainable homogenous condition by protecting us from any harmful or foreign substances, using a generic mechanism that involves the innate or non-specific immune system [5]. This system is also known as the first-line defense system, since it reacts very quickly. The innate immune system provides an immediate response against pathogens to prevent the spread of pathogens or foreign particles throughout the body. Additionally, it involves natural killer (NK) cells, which bind to the major histocompatibility complexes (MHCs) of affected cells [6]. The specialized or adaptive immune response is also known as the second-line defense system. It works on specific types of pathogens that cause infection. The adaptive immune system is slower compared with the innate immune system, since it needs to recognize the antigen first and then function to form new antibodies to neutralize the specific antigen. This immune system has the ability to remember specific types of antigens, so that it responds quickly the next time a similar antigen is encountered. Thus, the newly formed antibodies become a permanent component of the immune system inside the body [7].
Exercise has been considered well-structured and persistent body movement to maintain physical and mental well-being properly; however, its effects on overall health and wellness depend on its intensity and duration [8]. Regular exercise can exert anti-inflammatory effects by releasing anti-inflammatory cytokines [9]. On the other hand, intense exercise may induce the augmented production of pro-inflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, IL-1β, and reactive oxygen species (ROS), resulting in inflammation and oxidative stress [10,11]. Inflammation, characterized by fever, redness, pain, swelling, and dysfunction, may cause cellular proliferation and inhibition of apoptosis, consequently elevating the risk of cancer [12]. Also, oxidative stress (a disparity between the balance of oxidants (ROS) and antioxidants) occurs during the excess production of ROS that overwhelms the eliminatory effects of antioxidants, leading to a disturbance in the redox signaling and control and/or molecular damage [13]. Endogenous antioxidants work against the free radicals to neutralize the imbalanced status and maintain a sustainable physiological condition. In addition to that, food-derived natural antioxidants also promote immune functions by reducing oxidative stress [14].
GSLs (Glucosinolates) comprise a sulfur-bonded β-D-glucopyranose residue, a hydroxylamine sulfate ester, and a variable aglycon side chain that is derived from an α-amino acid (R-group). The R-group is derived from different amino acids based on which GSLs are classified as aliphatic (from alanine, leucine, isoleucine, methionine, or valine), indole (from tryptophan), and aromatic (from phenylalanine or tyrosine) [15]. GSLs were first introduced to the research community in 1831 by Robiquet and Boutrin, and around 137 GSLs have been identified up to date using modern spectroscopic methods [16,17]. GSLs are renowned secondary plant metabolites, particularly abundant in the Brassicaceae family and vegetables of the Brassica oleracea L. species, such as broccoli, cabbage, cauliflower, etc. [18]. Additionally, GSLs can be synthesized chemically, but the process is comparatively more expensive than obtaining the natural GSLs [19].
GSLs are biologically inactive and pass through enzymatic hydrolysis by a glycoprotein named myrosinase (thioglucosidase glucohydrolase) to produce a wide range of diverse biologically active substances, such as indoles, thiocyanates, isothiocyanates, and so on, with a rearrangement of their chemical structure [20]. The beneficial and adverse effects of GSLs in animal nutrition have been investigated. Nevertheless, for human nutrition, the harmful impacts of GSLs remain to be probed because of the low availability of evidence from the literature. However, the health-promoting effects of GSLs and their metabolites in humans are frequently mentioned, including immunomodulatory, cardioprotective, antibacterial, anticancer, chemopreventive, antioxidant, and anti-inflammatory functions [21]. Thus, the dietary intake of GSLs-rich foods has been identified as one of the more promising strategies to prevent or minimize inflammation and oxidative stress because of its role in activating detoxification enzymes, the scavenging of ROS, and inducing immune functions [22].
Sulforaphane (1-isothiocyanato-4-methylsulfinylbutane, SFN) is a naturally occurring isothiocyanate (ITC), which is currently the topic of active research due to its attribute as a critical regulator of cellular defenses through the activation or inactivation of vital transcription factors during any cellular stimulatory responses [23,24]. SFN could activate the nuclear factor erythroid 2-related factor 2 (Nrf2) and inhibit the nuclear factor-kappa-light-chain-enhancer of activated B cells (NF-ĸB), the major transcription factor in regulating cellular responses to inflammation and oxidative stress [20,23]. SFN, a hydrolytic product from glucoraphanin (4-methylsulfinylbutyl glucosinolate), attenuates the expression of pro-inflammatory cytokines (IL-6, TNF-α, and IL-1β) by downregulating the NF-ĸB protein expression and enhances the phase 2 enzymes such as heme oxygenase 1 (HO-1) by upregulating Nrf2, thereby reducing exercise-induced inflammation and oxidative stress [22,23]. In addition to that, SFN may mitigate exercise-induced excessive free radical production by creating an influx production of the endogenous antioxidant defense system [23]. In addition, SFN naturally induces phase 2 enzyme expression, which is important for cancer chemoprevention, and various epidemiological studies reported that sufficient absorption of SFN in the body also lowers the risk of cancer [25].
This is a brief review of the immunomodulating effects of SFN in exercise-induced inflammation and oxidative stress. Here, we summarized previous studies using SFN-rich foods or supplementation to improve immune functions after intense exercise. The potential mechanisms/mode of actions of SFN to reduce inflammation and oxidative stress due to exhaustive exercise were also illustrated. Additionally, we briefly mentioned a few of the potential therapeutic effects of SFN in various diseased conditions.
2. Experimental Studies with SFN Intervention
The production of free radicals and oxidants is a normal physiological process, and when we exercise, an imbalance may be created between oxidant and antioxidant production levels [26]. Most free radicals are produced in the mitochondria via the electron transport chain [27]. Endogenous antioxidants work against the free radicals to neutralize the imbalanced status and maintain a sustainable physiological condition. However, an overwhelming production of ROS may hamper normal cellular metabolic processes [28]. Any electrophilic stimulation or stress activates Nrf2; SFN could be an efficient indirect antioxidant to help an individual to recover quickly from the stressed condition [29]. Since exercise training requires lots of muscle movement, nutrient supplements before and after exercise may ease muscle fatigue or muscle pain, providing efficient exercise-induced benefits [30,31,32]. In a randomized, double-blind, placebo-controlled, cross-over designed study, it was hypothesized that SFN may be used for extended periods as a therapeutic supplement for athletes for the prevention of muscle damage, since it involves high-intensity exercise [33]. In this study, young, healthy subjects were treated with SFN (30 mg/d) for four weeks in the first trial; then, after the four-week washout period, the SFN or placebo groups were changed to the opposite treatment in the second trial. Creatine kinase (CK) and pro-inflammatory cytokine IL-6 are the two most essential markers for exercise-induced muscle damage, and long-term oral intake of SFN suppresses both markers after a single bout of vigorous resistance exercise [33]. In rodents, SFN (25 mg/kg body weight) administration for three consecutive days reduced exhaustive exercise-induced muscle damage while increasing the total antioxidant capacity and attenuating plasma lactate dehydrogenase (LDH) and CK activities [34]. Additionally, single-dose SFN (50 mg/kg body weight) administration reduced plasma LDH, glutamic oxaloacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT) after a single-bout exhaustive exercise test in animals [35]. It minimized the gene expression of pro-inflammatory cytokines in the liver [35]. Pretreatment with SFN for three days (25 mg/kg body weight) also improved exercise endurance capacity [34]. Delayed-onset muscle soreness (DOMS) is a common phenomenon after eccentric exercise, while taking SFN prior to exercise may suppress DOMS after two days of eccentric exercise [36]. Exercise training positively affected endurance capacity, but SFN administration may accelerate the muscles’ antioxidant defense response, improving an individual’s running distance and duration [37]. SFN treatment ensured a safe and sound strategy to protect age-associated muscle and heart dysfunction. In an aged-mice model, it was reported that SFN-fed old mice were able to run longer than the control group [38]. In a cohort study, a 7-day intense training program supplemented with broccoli sprout juice reported that SFN mitigated several markers of oxidative stress, like the myeloperoxidase (MPO) level and lactate concentration in the blood, and improved the blood glucose profile and enhanced the physical performance and adaptation to intense exercise training [39]. Further, SFN contributed effectively in a muscular dystrophy x-linked (mdx) knock-out model; oral administration of SFN (2 mg/kg/day) for eight weeks, followed by an acute exercise protocol, protected dystrophic muscles from oxidative damage in mdx mice and improved the muscle function, ROS level, and inflammation and reduced immune cell infiltration [40]. Yang et al. conducted a study and reported that SFN protected the liver from exhaustive exercise-induced excessive ROS production [41]. We also reported that a single dose of SFN administration may protect the liver from exhaustive exercise-induced oxidative stress and inflammation [35]. So far, multiple independent studies have been conducted with SFN in both human and animal studies, and most of them reported that SFN can improve post-exercise inflammatory or oxidative stress parameters (Table 1).

Table 1.
Research elucidating the effect of SFN intervention on exercise-induced inflammation and oxidative stress.
3. How SFN Reacts within Cell Signaling Pathways
3.1. The Activation of Nrf2 Transcription Factor
Exercise significantly changes cellular activities in the organism while increasing oxidative stress and energetic stress. These changes must be addressed by activating or inactivating the modification of several vital transcription factors [43]. Nrf2 is an essential transcription factor that remains inactive while connected with the repressor protein Kelch-like ECH-associated protein 1 (Keap1) [44,45]. During cellular oxidative stress, Keap1 releases Nrf2 and translocates into the nucleus. It modulates gene expression by reacting with the promoter region of antioxidant-responsive elements (AREs) with the assistance of small musculoaponeurotic fibrosarcoma proteins (MAF) [45]. SFN is attributed with upregulating the expression of Nrf2-mediated phase 2 enzymes (including NADPH: quinone oxidoreductase 1 (NQO1) and heme oxygenase 1 (HO-1)) and the endogenous antioxidant enzyme gene expression (Figure 1) [35,46]. In an animal experiment using Nrf2 knock-out mice, intraperitoneal administration of SFN (25 mg/kg) reduced oxidative stress markers, i.e., TBARS and the GSSG/GSH ratio. In the Nrf2++ group, a reduced level of muscle damage markers (LDH and CK) and the downstream regulation of TBARS and the GSSG/GSH ratio lead to enhanced endurance exercise capacity from SFN-induced Nrf2 activation [42]. Changes in GSH homeostasis may be reflected in the ratio of GSSG/GSH, since the GSH concentration becomes low and the GSSG concentration is high during oxidative stress [47]. Many independent studies investigated the upsurge of the GSSG/GSH ratio due to exercise training, which may be correlated with the lactate/pyruvate ratio [48,49,50]. Monocarboxylate transporter (MCT) 1 and MCT 4 are engaged in the lactate–pyruvate interchange and metabolism. SFN pretreatment before hypoxic exercise increases the expression of the lactate transporter MCT1 and increases the running capacity, with elevated LDH activity [43]. Under stimulated conditions, Nrf2 activation may enhance the expression of MCT1 during muscle expression [51]. Bose et al. reported that an SFN diet increased the exercise capacity of old mice, which was almost similar to the young mice group fed with the non-SFN diet (regular mice diet). Additionally, SFN improved muscle strength and increased the number of stem cells with improved function in skeletal muscles. The probable mechanism was presented as the active function of Nrf2-ARE binding activity and improved skeletal muscle function in the SFN-fed old mice group. Additionally, genes involved in antioxidant, antielectrophile, and glutathione synthesis pathways play a crucial role during aging. At the same time, SFN increases the transcriptional activation of these essential genes’ expression by restoring the Keap1/Nrf2/ARE pathway [38]. Besides improving endurance capacity, SFN preintervention protects from exhaustive exercise-induced liver damage [41]. Yang et al. conducted an animal study with a mild and high dose of SFN and executed exhaustive exercise for seven consecutive days along with SFN treatment and reported that an SFN intervention improved the adequacy of antioxidative stress and reduced inflammation in the liver and therefore diminished liver damage and ameliorated exercise endurance [41].
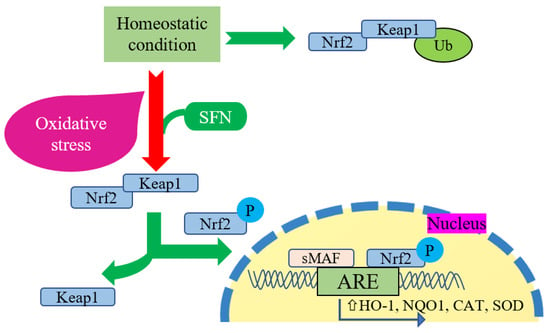
Figure 1.
Cellular interaction between Nrf2 and SFN. Keap1 inhibits Nrf2 degradation in homeostatic conditions by promoting Nrf2 ubiquitination (Ub) in the cytoplasm. During oxidative stress, Nrf2 phosphorylates and translocates into the nucleus to promote antioxidant response element (ARE) expression, as well as induces gene expression of HO-1, NQO1, CAT, and SOD. In this regard, SFN acts as a Nrf2 activator that promotes the dissociation of Nrf2 from its negative regulator Keap1 and upregulates this reaction comprehensively.
3.2. The Inhibition of NF-ĸB Activity
NF-ĸB is a prime protein transcription factor that efficiently controls the expression of genes that are involved in inflammatory responses. This protein complex consists of five precursors: NF-κB1 (or p50), NF-κB2 (or p52), and RelA (or p65), RelB, and c-Rel [52]. NF-ĸB is a heterodimer that is mainly composed of either p50 or p52 and p65. The NF-ĸB subunit p50 and p52 lacks a transactivation domain. Therefore, it needs to make a complex heterodimer with a subunit of the Rel family [53]. The NF-ĸB complex is activated by two pathways: the canonical and non-canonical, or alternative, pathway. Most of the inflammatory signal is mediated through canonical pathways [54].
SFN also protects cells from inflammatory reactions by interacting with key signaling pathways and inflammatory cytokines [23]. The NF-ĸB protein is relevant to inflammatory reactions, where the inhibitor of NF-ĸB kinase (IKK) is activated in response to any stimulation. Activated IKK then phosphorylates the NF-ĸB inhibitor, IĸB, causes proteasomal degradation, leaves NF-ĸB to enter into the nucleus, and commences transcription of genes, i.e., pro-inflammatory cytokines IL-6, IL-1β, and TNF-α. These pro-inflammatory markers are also known as secondary messengers and induce the function of NF-ĸB [55]. The activation of NF-ĸB in immune cells leads to an excessive production of pro-inflammatory mediators, caused by chronic inflammatory conditions and autoimmune diseases. Both the canonical and non-canonical pathways are involved in the activation of NF-ĸB [56]. The canonical pathway is dominant at the inflammatory site and is triggered by the production of pro-inflammatory cytokines [57].
SFN is familiar in this regard due to its anti-inflammatory properties. SFN-pretreated cells interfere with NF-ĸB nuclear translocation and IĸB degradation [58]. SFN may reduce inflammation by inhibiting NF-ĸB binding to DNA [59]. Various stimuli like lipopolysaccharide, hydrogen peroxide, acrolein, and TNF-α were used against various cell lines to assess the effective dose and concentration of SFN to minimize the inflammatory responses [29,60,61,62,63,64,65]. In addition, SFN may mitigate exercise-induced endotoxin production, which triggers the production of inducible nitric oxide synthase (iNOS) and nitrate production, as well as pro-inflammatory cytokines’ gene expression [29]. Sun et al. reported that a four-week SFN treatment alleviates muscle inflammation that is attributed to Nrf2-mediated inhibition of the NF-ĸB signaling pathway [66]. Few animal studies reported that selective doses of SFN are inversely associated with inflammatory responses [67,68]. Figure 2 briefly describes the inverse association between SFN and the NF-ĸB signaling system. NF-ĸB activation also acts as a key mediator for the priming signal of NLRP3 (nucleotide-binding oligomerization domain, leucine-rich repeat, and the pyrin domain containing 3) inflammasome activation [69]. Inflammasomes are multiprotein complexes that cause inflammatory reactions [70]. Prolonged inflammation causes sepsis, since muscle tissue is more prone to damage during sepsis, which results in sepsis-related pathogenesis [71,72]. Recently, it was reported that SFN attenuated the NLRP3 protein level in muscle myoblasts and reduced the secretion of inflammatory cytokine IL-1β and toll-like receptor 4 (TLR4) [73]. Moreover, SFN treatment also restores myogenic differentiation by repressing the activation of the TLR4 pathway [73].
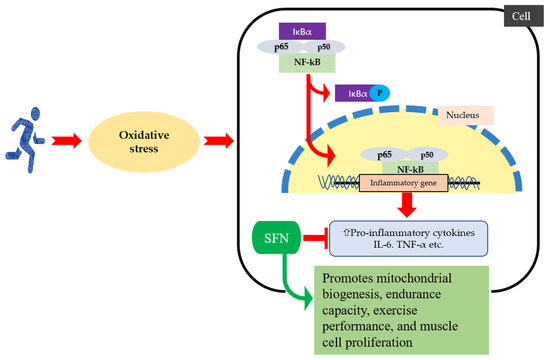
Figure 2.
When oxidative stress increases during exercise, IκBα is phosphorylated, and an released activated NF-κB that entered into the nucleus, which increases production of pro-inflammatory cytokines and chemokine expression. SFN intervention improves exercise performance by reducing reactive oxygen species, inflammatory cytokines, and chemokines, as well as inactivated NF-ĸB signaling pathway.
4. The Therapeutic Attributes of SFN in Combination with Other Nutrients
4.1. The Synergistic Effect of SFN and Other Nutraceuticals
Several clinical and preclinical studies have reported the therapeutic effects of SFN in many diseases that are related to inflammation. Subsequently, few studies have been performed on the combined effect of SFN with different nutrients like vitamin D, nobiletin (NBN), and curcumin (CUR) [74,75,76]. SFN combined with vitamin D upregulated Nrf2 expression [76]. Moreover, CUR and SFN are more effective in preventing inflammation-associated diseases. Both CUR and SFN have some efficacy to induce the Nrf2/ARE signaling pathway; however, CUR and SFN become more effective, even at a lower concentration. Cheung et al. reported that CUR and SFN synergistically induce HO-1 expression and simultaneously reduce iNOS and cyclooxygenase (COX)-2 protein expression and related inflammatory markers [74].
4.2. SFN as a Cancer Chemopreventive Nutraceutical
SFN is also known for significant cancer chemopreventive benefits, and a plethora of research was performed regarding the affectivity of SFN against different types of cancer like liver cancer, prostate cancer, breast cancer, ovarian cancer, pancreatic cancer, and colorectal cancer [77,78,79,80,81,82]. Cornblatt et al. performed a study to extrapolate the practical dose of SFN as a cancer chemopreventive agent and reported that SFN metabolites are readily available in the mammary tissue after receiving a single dose of SFN (200 μmole), which is prepared from myrosinase-active broccoli sprout powder [78]. This dose is equivalent to 35 mg of SFN (molecular weight of SFN: 177.29). After thirty minutes of a single dose of SFN (150 μmol), significant induction was noticed with two important cryoprotective enzymes, HO-1 and detoxification enzyme NQO1, in the mammary tissue [78]. After 12 h of SFN ingestion, a maximum of 12-fold of the induction of NQO1 was observed in the mammary tissue, while significant induction was found after 2 h. Similarly, HO-1 induction was significantly observed within one hour of ingestion [78]. Moreover, another animal study reported that SFN administration prevented tumor formation in rats who were treated with the carcinogen 9,10-dimethyl-1,2-benzanthracene [83]. SFN modulates our immune system by regulating T-cell and B-cell proliferation and phagocytic activity and by influencing the cytotoxicity in NK cells. The NK cells are critically important in controlling carcinogenesis [84]. Due to the chemopreventive effect, SFN can readily block and suppress the carcinogen [85]. Numerous mechanisms of SFN are being investigated to target multiple carcinogenetic cells. Many suggested that SFN exerted a chemopreventive function by preventing the phase 1 enzymes’ activation, along with the induction of detoxification enzymes, therefore suppressing pro-inflammatory responses within the cells [86]. The NF-ĸB signaling pathway is a critical part of the innate immune system and plays a vital role in cancer initiation and progression. The active form of NF-ĸB upregulates the anti-apoptotic gene expression, therefore, it indirectly upregulates cell proliferation [87]. In cancer patients, NF-ĸB remains active, and SFN administration may downregulate the NF-ĸB expression in prostate cancer cells [88]. Heiss et al. reported that SFN can directly inhibit the activation of the NF-ĸB subunit and reduce the DNA-binding capacity without interfering with the endotoxin-induced breakdown of the inhibitor of NF-ĸB and the nuclear translocation of NF-ĸB [59].
4.3. SFN and Other Chronic Diseases
Considering broccoli sprout powder (BSP) to be a rich source of SFN, a randomized, double-blind and placebo-controlled clinical trial was conducted among type 2 diabetic patients. A four-week intervention of BSP (the SFN content of BSP was determined to be ~22.5 μmol/g) resulted in the lowering of the inflammatory mediator IL-6 concentration in type 2 diabetic patients compared to the control [89]. Additionally, SFN administration (100 μmol per kg/body weight) also upregulates the insulin signaling pathway, as well as improves the glucose tolerance (Figure 3) [90]. In 2021, a clinical trial was conducted with type 2 diabetes mellitus (T2D) patients with an intervention of aerobic resistance training and broccoli supplementation (10 g/day; 22.5 mmol/g SFN) for 12 weeks [91]. They reported that broccoli supplementation with exercise training improved the lipid profile, body composition variables, and insulin level among the diabetes group compared to broccoli supplementation alone or exercise training alone [91]. Additionally, several studies reported that SFN intervention can reduce obesity through various mechanisms like the browning of fat, altering leptin resistance, and promoting lipolysis [92,93,94,95]. However, SFN showed no anti-inflammatory or antioxidative effects in patients with chronic kidney diseases (CKDs). A cross-over, randomized, double-blind study was performed with CKD patients, providing 150 μmol of SFN for two months and showing no effect in terms of Nrf2 and NF-ĸB expression or inflammatory markers [96]. An acute toxic dose of SFN (300 mg/kg body weight) causes pro-convulsion, hypothermia (150–300 mg/kg), impaired motor coordination (200–300 mg/kg), and reduced muscle strength (200–250 mg/kg) [97].
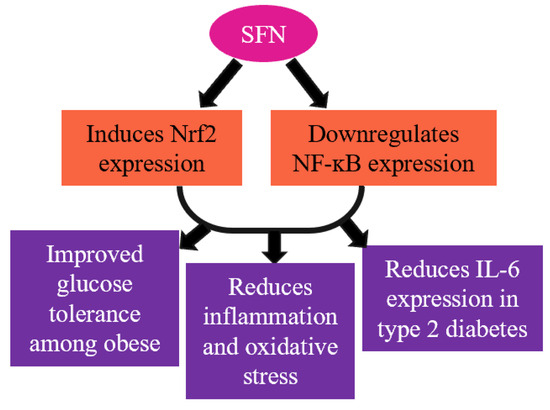
Figure 3.
SFN mediates vital transcription factors Nrf2 and NF-ĸB and reduces inflammation and oxidative stress.
During the COVID-19 pandemic, caused by the SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) virus, SFN was used as a drug to treat the immune cells in the lungs, which results in a reduction in T-cell activation and cytokine production [98]. Since SARS-CoV-2 directly promotes NLRP3 inflammasome activation, and SFN was reported to inhibit the inflammasome activation via Nrf2 activation, SFN may indirectly contribute to diminishing the cytokine storm in patients with COVID-19 [99,100]. However, direct studies regarding exercise and SFN intervention in COVID-19 patients are yet to be published.
5. Discussion and Further Perspectives
Numerous research articles have reported the interplay between SFN and its activity at the transcriptional level. In this review, we discussed mainly the impact of SFN on exercise capacity and the inherent physiological changes after an SFN intervention. SFN could be a sustainable intervention to improve exercise endurance capacity and elevate the mitochondrial function and cellular antioxidant responses. It has been reported that SFN showed its protective effect against exercise-induced ROS production with the induction of the Nrf2 pathway, which further activates several genes that are related to antioxidant and anti-inflammatory responses. SFN also protects organisms by increasing the activity of endogenous antioxidants, i.e., SOD, CAT, GPx, HO-1, and NQO1. Inflammation is a significant cause of the progression of several chronic diseases; SFN interferes with the regulation of the NF-ĸB pathway and, therefore, reduces the secretion of pro-inflammatory cytokines and other inflammatory markers’ expression. Since an excessive production of ROS impairs redox homeostasis, SFN intervention may improve the imbalanced condition by modulating several major transcription factors, like Nrf2. Moreover, SFN is thought to have an anti-inflammatory role, in conjunction with its other chemopreventive properties. This review mainly mentioned published research articles on SFN intervention and exercise outcomes. Hence, limited research articles on exercise and SFN were presented; we expanded the article with primary research on the SFN mechanisms and also briefly mentioned the therapeutic and chemopreventive role of SFN in different diseased conditions. Although various randomized control trials with different protocols were conducted and reported, a generally acceptable guideline for the intake of SFN is yet to be declared. To our knowledge, SFN is one of the most studied phytochemicals among ITCs and has shown plenty of health benefits. In order to gain a precise understanding of SFN consumption, more preclinical and clinical studies are required.
Author Contributions
R.T.R. conceived the review and drafted the manuscript. K.S. made some additions to the text, revised the manuscript, and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We gratefully appreciate our colleagues and laboratory team for research progress and discussion. Also, we appreciate Marjia Sultana, Noakhali Science and Technology University, Bangladesh, for content support and English editing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef]
- Hooper, L.; Cassidy, A. A review of the health care potential of bioactive compounds. J. Sci. Food Agric. 2006, 86, 1805–1813. [Google Scholar] [CrossRef]
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78, A18–A25. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Czubacka, A.; Oleszek, W. Dietary phytochemicals and human health. In Bio-Farms for Nutraceuticals: Functional Food and Safety Control by Biosensors; Springer: New York, NY, USA, 2010; Volume 698, pp. 74–98. [Google Scholar]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Principles of innate and adaptive immunity. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Hamerman, J.A.; Ogasawara, K.; Lanier, L.L. NK cells in innate immunity. Curr. Opin. Immunol. 2005, 17, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.; Lim, C.L.; Suzuki, K. Effects of endurance-, strength-, and concurrent training on cytokines and inflammation. In Concurrent Aerobic and Strength Training; Springer: Cham, Switzerland, 2019; pp. 25–138. [Google Scholar]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Davison, G.W.; McClean, C. Oxidative Stress and Exercise. Antioxidants 2022, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Cytokine response to exercise and its modulation. Antioxidants 2018, 7, 17. [Google Scholar] [CrossRef]
- Ran, D.; Hong, W.; Yan, W.; Mengdie, W. Properties and molecular mechanisms underlying geniposide-mediated therapeutic effects in chronic inflammatory diseases. J. Ethnopharmacol. 2021, 273, 113958. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Bischoff, K. Glucosinolates and organosulfur compounds. In Nutraceuticals in Veterinary Medicine; Springer: Cham, Switzerland, 2019; pp. 113–119. [Google Scholar]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Blazevic, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Martini, D.; Venturi, S.; Tucci, M.; Porrini, M.; Riso, P.; Del Bo, C. An overview of registered clinical trials on glucosinolates and human health: The current situation. Front. Nutr. 2021, 8, 730906. [Google Scholar] [CrossRef]
- Wu, J.; Cui, S.; Liu, J.; Tang, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. The recent advances of glucosinolates and their metabolites: Metabolism, physiological functions and potential application strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 4217–4234. [Google Scholar] [CrossRef]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural occurrence, biosynthesis, accessibility, isolation, structures, and biological activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.; Suzuki, K. Organosulfur compounds: A review of their anti-inflammatory effects in human health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Suzuki, K. The integrative role of sulforaphane in preventing inflammation, oxidative stress and fatigue: A review of a potential protective phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef]
- Wakasugi-Onogi, S.; Ma, S.; Ruhee, R.T.; Tong, Y.; Seki, Y.; Suzuki, K. Sulforaphane Attenuates Neutrophil ROS Production, MPO Degranulation and Phagocytosis, but Does Not Affect NET Formation Ex Vivo and In Vitro. Int. J. Mol. Sci. 2023, 24, 8479. [Google Scholar] [CrossRef]
- Khan, S.; Awan, K.A.; Iqbal, M.J. Sulforaphane as a potential remedy against cancer: Comprehensive mechanistic review. J. Food Biochem. 2022, 46, e13886. [Google Scholar]
- Nikolaidis, M.G.; Margaritelis, N.V. Free radicals and antioxidants: Appealing to magic. Trends Endocrinol. Metab. 2023, 34, 503–504. [Google Scholar] [CrossRef]
- Munro, D.; Treberg, J.R. A radical shift in perspective: Mitochondria as regulators of reactive oxygen species. J. Exp. Biol. 2017, 220, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane protects cells against lipopolysaccharide-stimulated inflammation in murine macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B. Dietary supplements and the promotion of muscle growth with resistance exercise. Sports Med. 1999, 27, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Recent Progress in Applicability of Exercise Immunology and Inflammation Research to Sports Nutrition. Nutrients 2021, 13, 4299. [Google Scholar] [CrossRef]
- Tanabe, Y.; Fujii, N.; Suzuki, K. Dietary supplementation for attenuating exercise-induced muscle damage and delayed-onset muscle soreness in humans. Nutrients 2021, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kihara, H.; Kumazawa, Y.; Tatara, K. Oral chronic sulforaphane effects on heavy resistance exercise: Implications for inflammatory and muscle damage parameters in young practitioners. Nutrition 2021, 90, 111266. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, M.; Angeloni, C.; Garatachea, N.; Baldini, M.; Leoncini, E.; Collado, P.S.; Teti, G.; Falconi, M.; Gonzalez-Gallego, J.; Hrelia, S. Sulforaphane treatment protects skeletal muscle against damage induced by exhaustive exercise in rats. J. Appl. Physiol. 2009, 107, 1028–1036. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Protective effects of sulforaphane on exercise-induced organ damage via inducing antioxidant defense responses. Antioxidants 2020, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Komine, S.; Miura, I.; Miyashita, N.; Oh, S.; Tokinoya, K.; Shoda, J.; Ohmori, H. Effect of a sulforaphane supplement on muscle soreness and damage induced by eccentric exercise in young adults: A pilot study. Physiol. Rep. 2021, 9, e15130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, Y.; Wang, R.; Li, X.; Wang, J.; Yu, S.; Zhang, Y. Sulforaphane enhances Nrf2-mediated antioxidant responses of skeletal muscle induced by exhaustive exercise in HIIT mice. Food Sci. Hum. Wellness 2022, 11, 1355–1361. [Google Scholar] [CrossRef]
- Bose, C.; Alves, I.; Singh, P.; Palade, P.T.; Carvalho, E.; Børsheim, E.; Jun, S.R.; Cheema, A.; Boerma, M.; Awasthi, S. Sulforaphane prevents age-associated cardiac and muscular dysfunction through Nrf2 signaling. Aging Cell 2020, 19, e13261. [Google Scholar] [CrossRef]
- Flockhart, M.; Nilsson, L.; Tillqvist, E.; Vinge, F.; Millbert, F.; Lännerström, J.; Nilsson, P.H.; Samyn, D.; Apró, W.; Sundqvist, M.L. Glucosinolate-rich broccoli sprouts protect against oxidative stress and improve adaptations to intense exercise training. Redox Biol. 2023, 67, 102873. [Google Scholar] [CrossRef]
- Sun, C.; Yang, C.; Xue, R.; Li, S.; Zhang, T.; Pan, L.; Ma, X.; Wang, L.; Li, D. Sulforaphane alleviates muscular dystrophy in mdx mice by activation of Nrf2. J. Appl. Physiol. 2015, 118, 224–237. [Google Scholar] [CrossRef]
- Yang, J.; Guo, X.; Li, T.; Xie, Y.; Wang, D.; Yi, L.; Mi, M. Sulforaphane Inhibits Exhaustive Exercise-Induced Liver Injury and Transcriptome-Based Mechanism Analysis. Nutrients 2023, 15, 3220. [Google Scholar] [CrossRef]
- Oh, S.; Komine, S.; Warabi, E.; Akiyama, K.; Ishii, A.; Ishige, K.; Mizokami, Y.; Kuga, K.; Horie, M.; Miwa, Y. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci. Rep. 2017, 7, 12902. [Google Scholar] [CrossRef]
- Radak, Z.; Torma, F.; Berkes, I.; Goto, S.; Mimura, T.; Posa, A.; Balogh, L.; Boldogh, I.; Suzuki, K.; Higuchi, M.; et al. Exercise effects on physiological function during aging. Free Radic. Biol. Med. 2019, 132, 33–41. [Google Scholar] [CrossRef]
- Taguchi, K.; Yamamoto, M. The KEAP1–NRF2 system in cancer. Front. Oncol. 2017, 7, 85. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Philbrook, N.A.; Winn, L.M. Sub-chronic sulforaphane exposure in CD-1 pregnant mice enhances maternal NADPH quinone oxidoreductase 1 (NQO1) activity and mRNA expression of NQO1, glutathione S-transferase, and glutamate-cysteine ligase: Potential implications for fetal protection against toxicant exposure. Reprod. Toxicol. 2014, 43, 30–37. [Google Scholar]
- Huynh, T.P.N.; Bowater, R.P.; Bernuzzi, F.; Saha, S.; Wormstone, I.M. GSH levels serve as a biological redox switch regulating sulforaphane-induced cell fate in human lens cells. Investig. Ophthalmol. Vis. Sci. 2021, 62, 2. [Google Scholar] [CrossRef]
- Laaksonen, D.E.; Atalay, M.; Niskanen, L.; Uusitupa, M.; Hänninen, O.; Sen, C.K. Blood glutathione homeostasis as a determinant of resting and exercise-induced oxidative stress in young men. Redox Rep. 1999, 4, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Urso, M.L.; Clarkson, P.M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Asensi, M.; Gasco, E.; Pallardo, F.V.; Ferrero, J.; Furukawa, T.; Vina, J. Exhaustive physical exercise causes oxidation of glutathione status in blood: Prevention by antioxidant administration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992, 263, R992–R995. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, R.; Wang, J.; Yu, S.; Zhang, Y. Nrf2 Activation Enhances Muscular MCT1 Expression and Hypoxic Exercise Capacity. Med. Sci. Sports Exerc. 2020, 52, 1719–1728. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Kravtsova-Ivantsiv, Y.; Goldhirsh, G.; Ivantsiv, A.; Ben Itzhak, O.; Kwon, Y.T.; Pikarsky, E.; Ciechanover, A. Excess of the NF-ĸB p50 subunit generated by the ubiquitin ligase KPC1 suppresses tumors via PD-L1–and chemokines-mediated mechanisms. Proc. Natl. Acad. Sci. USA 2020, 117, 29823–29831. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, Y.; Wang, W.; Bai, Y.; Jia, H.; Yuan, Z.; Yang, Z. Role and mechanisms of the NF-ĸB signaling pathway in various developmental processes. Biomed. Pharmacother. 2022, 153, 113513. [Google Scholar] [CrossRef]
- Grimm, S.; Baeuerle, P.A. The inducible transcription factor NF-kappa B: Structure-function relationship of its protein subunits. Biochem. J. 1993, 290, 297. [Google Scholar] [CrossRef]
- Ahn, K.S.; Aggarwal, B.B. Transcription factor NF-κB: A sensor for smoke and stress signals. Ann. N. Y. Acad. Sci. 2005, 1056, 218–233. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Nallasamy, P.; Si, H.; Babu, P.V.A.; Pan, D.; Fu, Y.; Brooke, E.A.; Shah, H.; Zhen, W.; Zhu, H.; Liu, D. Sulforaphane reduces vascular inflammation in mice and prevents TNF-α-induced monocyte adhesion to primary endothelial cells through interfering with the NF-κB pathway. J. Nutr. Biochem. 2014, 25, 824–833. [Google Scholar] [CrossRef]
- Heiss, E.; Herhaus, C.; Klimo, K.; Bartsch, H.; Gerhäuser, C. Nuclear factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 2001, 276, 32008–32015. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; Brasil, F.B.; Fürstenau, C.R. Sulforaphane attenuated the pro-inflammatory state induced by hydrogen peroxide in SH-SY5Y cells through the Nrf2/HO-1 signaling pathway. Neurotox. Res. 2018, 34, 241–249. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R.; de Bittencourt Brasil, F.; Fürstenau, C.R. Sulforaphane promotes mitochondrial protection in SH-SY5Y cells exposed to hydrogen peroxide by an Nrf2-dependent mechanism. Mol. Neurobiol. 2018, 55, 4777–4787. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.-S.; Deng, Y.-H.; Cui, F.-C. Sulforaphane protects against acrolein-induced oxidative stress and inflammatory responses: Modulation of Nrf-2 and COX-2 expression. Arch. Med. Sci. 2016, 12, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-inflammatory effect of sulforaphane on LPS-activated microglia potentially through JNK/AP-1/NF-κB inhibition and Nrf2/HO-1 activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef]
- Folkard, D.L.; Melchini, A.; Traka, M.H.; Al-Bakheit, A.a.; Saha, S.; Mulholland, F.; Watson, A.; Mithen, R.F. Suppression of LPS-induced transcription and cytokine secretion by the dietary isothiocyanate sulforaphane. Mol. Nutr. Food Res. 2014, 58, 2286–2296. [Google Scholar] [CrossRef]
- Moon, D.-O.; Kim, M.-O.; Kang, S.-H.; Choi, Y.H.; Kim, G.-Y. Sulforaphane suppresses TNF-α-mediated activation of NF-κB and induces apoptosis through activation of reactive oxygen species-dependent caspase-3. Cancer Lett. 2009, 274, 132–142. [Google Scholar] [CrossRef]
- Sun, C.-C.; Li, S.-J.; Yang, C.-L.; Xue, R.-L.; Xi, Y.-Y.; Wang, L.; Zhao, Q.-L.; Li, D.-J. Sulforaphane attenuates muscle inflammation in dystrophin-deficient mdx mice via NF-E2-related factor 2 (Nrf2)-mediated inhibition of NF-κB signaling pathway. J. Biol. Chem. 2015, 290, 17784–17795. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C. Anti-nociceptive and anti-inflammatory actions of sulforaphane in chronic constriction injury-induced neuropathic pain mice. Inflammopharmacology 2017, 25, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Xu, F.; Yan, X.; Li, S.; Li, H. Sulforaphane exerts anti-inflammatory effects against lipopolysaccharide-induced acute lung injury in mice through the Nrf2/ARE pathway. Int. J. Mol. Med. 2016, 37, 182–188. [Google Scholar] [CrossRef]
- Boaru, S.G.; Borkham-Kamphorst, E.; Van de Leur, E.; Lehnen, E.; Liedtke, C.; Weiskirchen, R. NLRP3 inflammasome expression is driven by NF-κB in cultured hepatocytes. Biochem. Biophys. Res. Commun. 2015, 458, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Li, T.; Yang, F.; Li, Z.; Bai, X.; Wang, Y. The role of NLRP3 inflammasome in inflammation-related skeletal muscle atrophy. Front. Immunol. 2022, 13, 1035709. [Google Scholar] [CrossRef]
- Hung, Y.-L.; Suzuki, K. The pattern recognition receptors and lipopolysaccharides (LPS)-induced systemic inflammation. Int. J. Res. Stud. Med. Health Sci. 2017, 2, 1–7. [Google Scholar]
- Wang, M.; Liu, M.; Xu, W.; Teng, Z.; Wu, X.; Gan, L.; Zhang, Y. Sulforaphane reduces lipopolysaccharide-induced inflammation and enhances myogenic differentiation of mouse embryonic myoblasts via the toll-like receptor 4 and NLRP3 pathways. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2023, 32, 457–467. [Google Scholar] [CrossRef]
- Cheung, K.L.; Khor, T.O.; Kong, A.-N. Synergistic effect of combination of phenethyl isothiocyanate and sulforaphane or curcumin and sulforaphane in the inhibition of inflammation. Pharm. Res. 2009, 26, 224–231. [Google Scholar] [CrossRef]
- Guo, S.; Qiu, P.; Xu, G.; Wu, X.; Dong, P.; Yang, G.; Zheng, J.; McClements, D.J.; Xiao, H. Synergistic anti-inflammatory effects of nobiletin and sulforaphane in lipopolysaccharide-stimulated RAW 264.7 cells. J. Agric. Food Chem. 2012, 60, 2157–2164. [Google Scholar] [CrossRef]
- Tuttis, K.; Machado, A.R.T.; Santos, P.W.d.S.; Antunes, L.M.G. Sulforaphane Combined with Vitamin D Induces Cytotoxicity Mediated by Oxidative Stress, DNA Damage, Autophagy, and JNK/MAPK Pathway Modulation in Human Prostate Tumor Cells. Nutrients 2023, 15, 2742. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Orsulic, S.; Ashok, B.T. Antiproliferative activity of sulforaphane in Akt-overexpressing ovarian cancer cells. Mol. Cancer Ther. 2007, 6, 334–345. [Google Scholar] [CrossRef]
- Cornblatt, B.S.; Ye, L.; Dinkova-Kostova, A.T.; Erb, M.; Fahey, J.W.; Singh, N.K.; Chen, M.-S.A.; Stierer, T.; Garrett-Mayer, E.; Argani, P. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 2007, 28, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Chiao, J.; Chung, F.-L.; Kancherla, R.; Ahmed, T.; Mittelman, A.; Conaway, C. Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int. J. Oncol. 2002, 20, 631–636. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Robertson, A.S.; Chao, A.; Jacobs, E.J.; Stampfer, M.J.; Jacobs, D.R.; Diver, W.R.; Calle, E.E.; Thun, M.J. A prospective study of whole grains, fruits, vegetables and colon cancer risk. Cancer Causes Control. 2003, 14, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Darkwa, M.; Burkhardt, C.; Tsuji, P. Dietary Polyphenols and Sulforaphane: Impact on Hallmarks of Colon Cancer (P06-045-19). Curr. Dev. Nutr. 2019, 3, nzz031. [Google Scholar] [CrossRef]
- Sato, S.; Moriya, K.; Furukawa, M.; Saikawa, S.; Namisaki, T.; Kitade, M.; Kawaratani, H.; Kaji, K.; Takaya, H.; Shimozato, N. Sulforaphane Inhibits Liver Cancer Cell Growth and Angiogenesis. Arch. Can. Res. 2018, 6. [Google Scholar]
- Zhang, Y.; Kensler, T.W.; Cho, C.-G.; Posner, G.H.; Talalay, P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. USA 1994, 91, 3147–3150. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Wu, L.Y.; Lee, C.H.; Chen, Y.L.; Hsueh, S.C.; Lu, H.F.; Liao, N.C.; Chung, J.G. Sulforaphane promotes immune responses in a WEHI-3-induced leukemia mouse model through enhanced phagocytosis of macrophages and natural killer cell activities in vivo. Mol. Med. Rep. 2016, 13, 4023–4029. [Google Scholar] [CrossRef]
- Li, Y.; Buckhaults, P.; Li, S.; Tollefsbol, T. Temporal efficacy of a sulforaphane-based broccoli sprout diet in prevention of breast cancer through modulation of epigenetic mechanisms. Cancer Prev. Res. 2018, 11, 451–464. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Baluch, N.; Homayouni, T.S.; Morgatskaya, E.; Kumar, S.; Kazemi, P.; Yeger, H. The role of Sulforaphane in cancer chemoprevention and health benefits: A mini-review. J. Cell Commun. Signal. 2018, 12, 91–101. [Google Scholar] [CrossRef]
- Jost, P.J.; Ruland, J.r. Aberrant NF-κB signaling in lymphoma: Mechanisms, consequences, and therapeutic implications. Blood 2007, 109, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lew, K.L.; Xiao, H.; Herman-Antosiewicz, A.; Xiao, D.; Brown, C.K.; Singh, S.V. D,L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis 2007, 28, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Hosseinpanah, F.; Keyzad, A.; Azizi, F. Effects of broccoli sprout with high sulforaphane concentration on inflammatory markers in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. J. Funct. Foods 2012, 4, 837–841. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, J.-F.; Chen, J.-H.; Zhang, Z.-W.; Zou, Z.-Q.; Han, L.-Y.; Hua, Q.-H.; Zhao, J.-S.; Zhang, X.-H.; Shan, Y.-J. Sulforaphane ameliorates glucose intolerance in obese mice via the upregulation of the insulin signaling pathway. Food Funct. 2018, 9, 4695–4701. [Google Scholar] [CrossRef]
- Saeidi, A.; Soltani, M.; Daraei, A.; Nohbaradar, H.; Haghighi, M.M.; Khosravi, N.; Johnson, K.E.; Laher, I.; Hackney, A.C.; VanDusseldorp, T.A. The effects of aerobic-resistance training and broccoli supplementation on plasma dectin-1 and insulin resistance in males with type 2 diabetes. Nutrients 2021, 13, 3144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Chen, S.Y.; Wang, A.S.; Yao, A.J.; Fu, J.F.; Zhao, J.S.; Chen, F.; Zou, Z.Q.; Zhang, X.H.; Shan, Y.J. Sulforaphane induces adipocyte browning and promotes glucose and lipid utilization. Mol. Nutr. Food Res. 2016, 60, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, X.; Chen, Z.; Luo, T.; Zhu, C.; Ji, Y.; Bian, Z. The protective effects of sulforaphane on high-fat diet-induced obesity in mice through browning of white fat. Front. Pharmacol. 2021, 12, 665894. [Google Scholar] [CrossRef]
- Cakir, I.; Lining Pan, P.; Hadley, C.K.; El-Gamal, A.; Fadel, A.; Elsayegh, D.; Mohamed, O.; Rizk, N.M.; Ghamari-Langroudi, M. Sulforaphane reduces obesity by reversing leptin resistance. eLife 2022, 11, e67368. [Google Scholar] [CrossRef]
- Tian, S.; Wang, Y.; Li, X.; Liu, J.; Wang, J.; Lu, Y. Sulforaphane regulates glucose and lipid metabolisms in obese mice by restraining JNK and activating insulin and FGF21 signal pathways. J. Agric. Food Chem. 2021, 69, 13066–13079. [Google Scholar] [CrossRef]
- Ribeiro, M.; Cardozo, L.F.M.F.; Paiva, B.R.; Baptista, B.G.; Fanton, S.; Alvarenga, L.; Lima, L.S.; Britto, I.; Nakao, L.S.; Fouque, D.; et al. Sulforaphane Supplementation Did Not Modulate NRF2 and NF-kB mRNA Expressions in Hemodialysis Patients. J. Ren. Nutr. 2023. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Nieoczym, D.; Kowalczuk-Vasilev, E.; Wyska, E.; Wlaź, P. Increased seizure susceptibility and other toxicity symptoms following acute sulforaphane treatment in mice. Toxicol. Appl. Pharmacol. 2017, 326, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, A.A.; Bullen, C.K.; Villabona-Rueda, A.F.; Thompson, E.A.; Turner, M.L.; Davis, S.L.; Komm, O.; Powell, J.D.; D’Alessio, F.R.; Yolken, R.H. Sulforaphane exhibits in vitro and in vivo antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kow, C.S.; Ramachandram, D.S.; Hasan, S.S. Use of sulforaphane in COVID-19: Clinical trials are needed. Mol. Immunol. 2022, 145, 78–79. [Google Scholar] [CrossRef]
- Pan, P.; Shen, M.; Yu, Z.; Ge, W.; Chen, K.; Tian, M.; Xiao, F.; Wang, Z.; Wang, J.; Jia, Y. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat. Commun. 2021, 12, 4664. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).