Regulation of Follicular Development in Chickens: WIF1 Modulates Granulosa Cell Proliferation and Progesterone Synthesis via Wnt/β-Catenin Signaling Pathway
Abstract
1. Introduction
2. Results
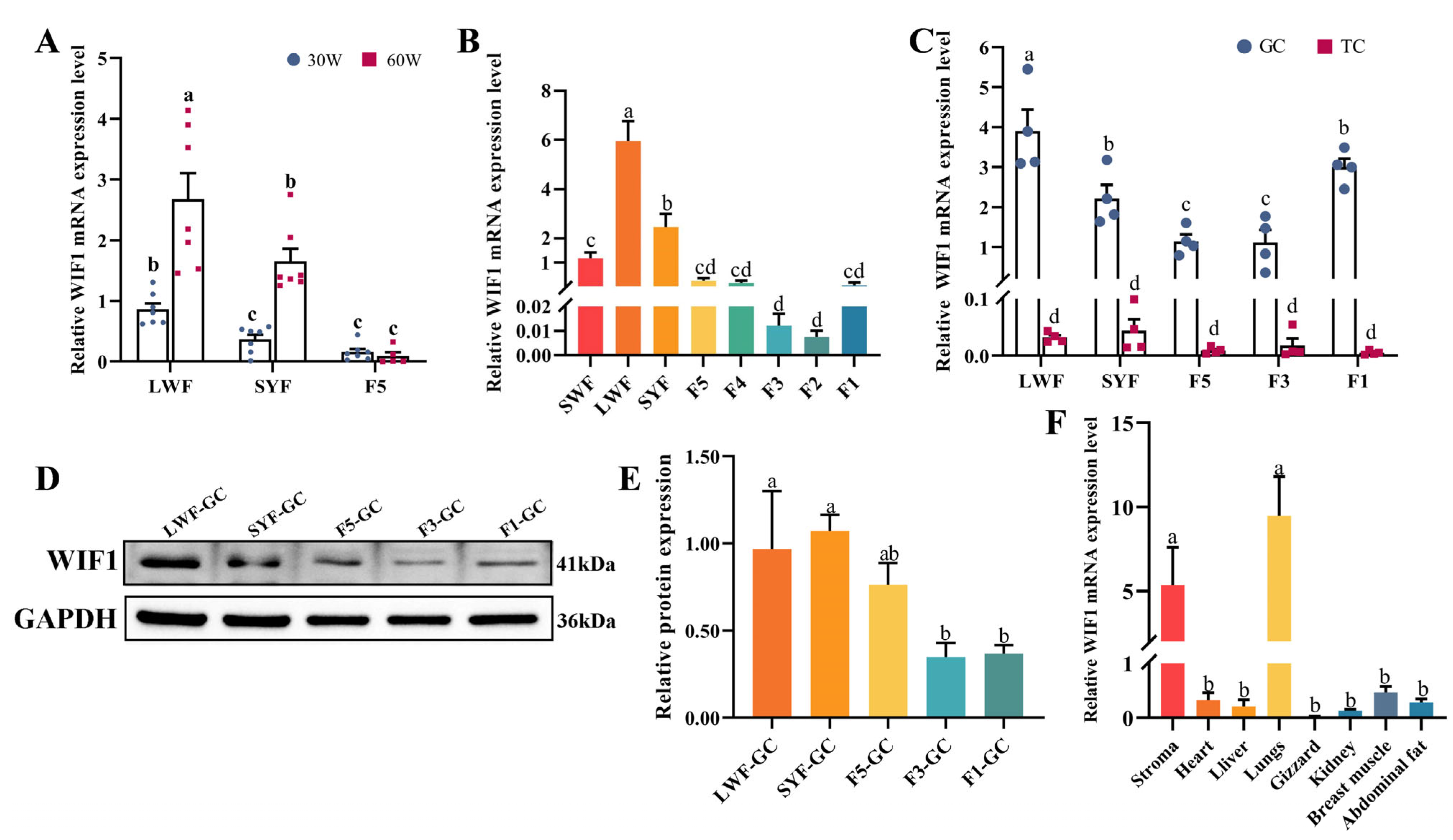
2.1. Expression Characteristics of WIF1 in Chickens
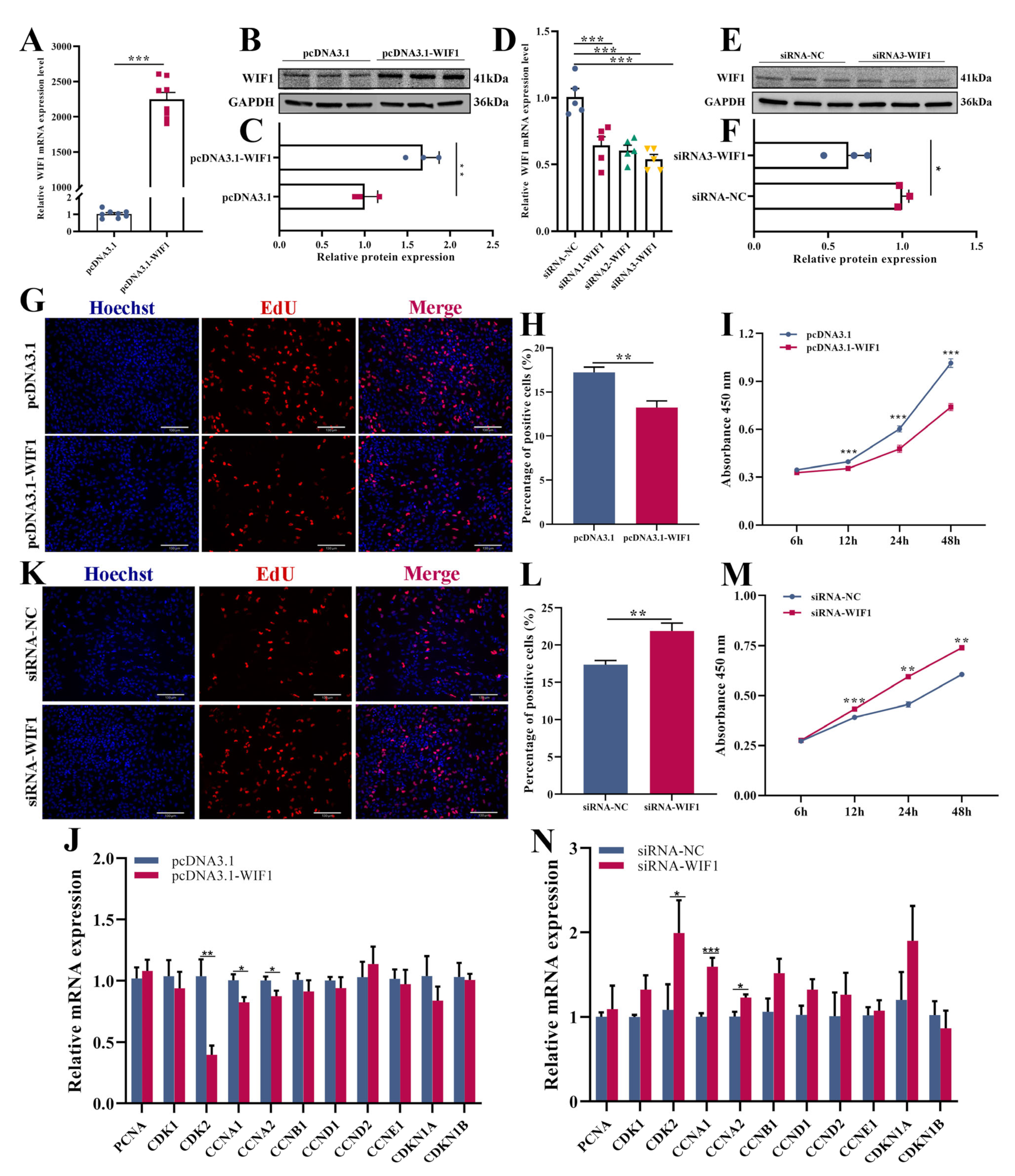
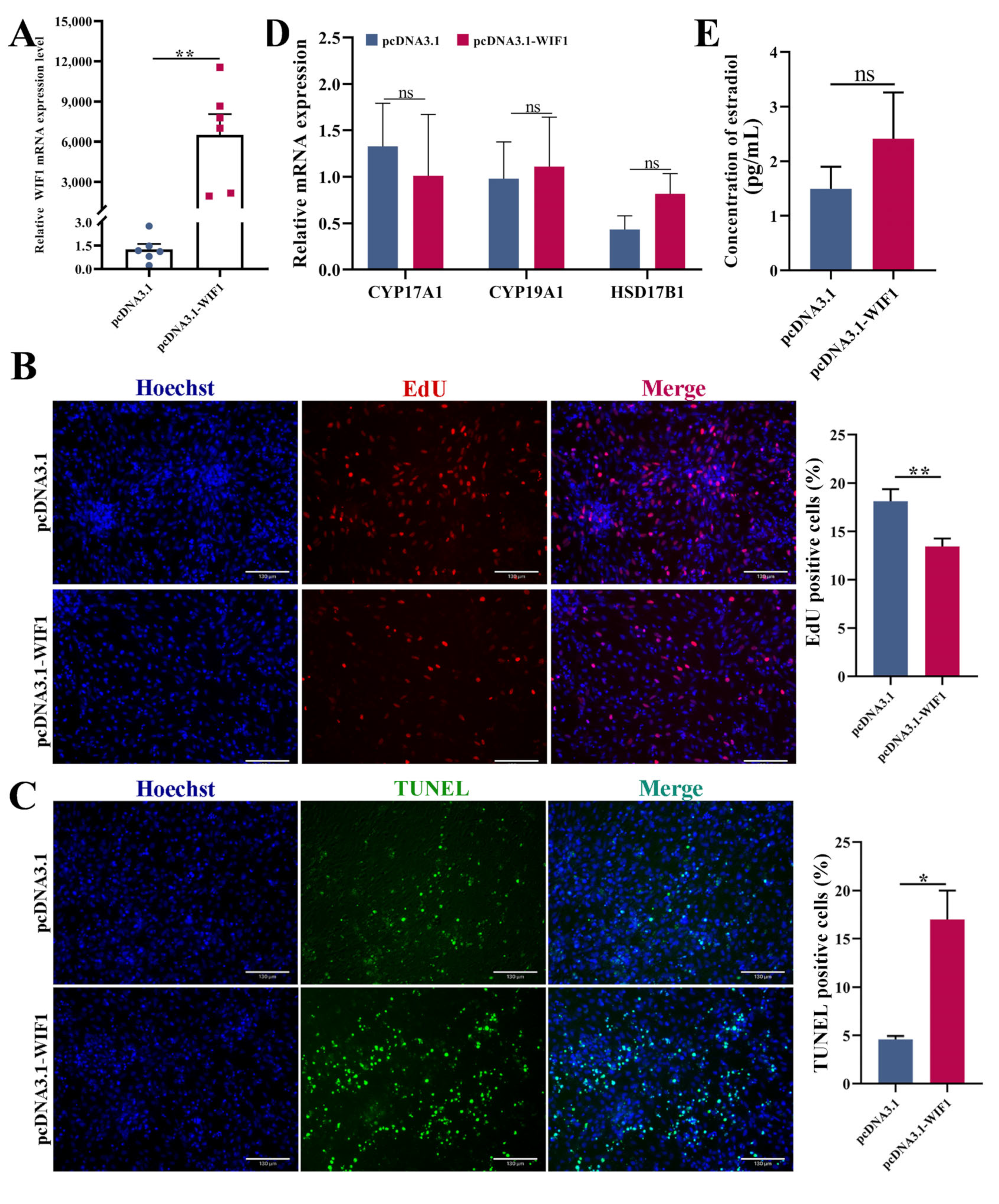
2.2. WIF1 Inhibits Proliferation of Chicken GCs from Prehierarchal Follicles
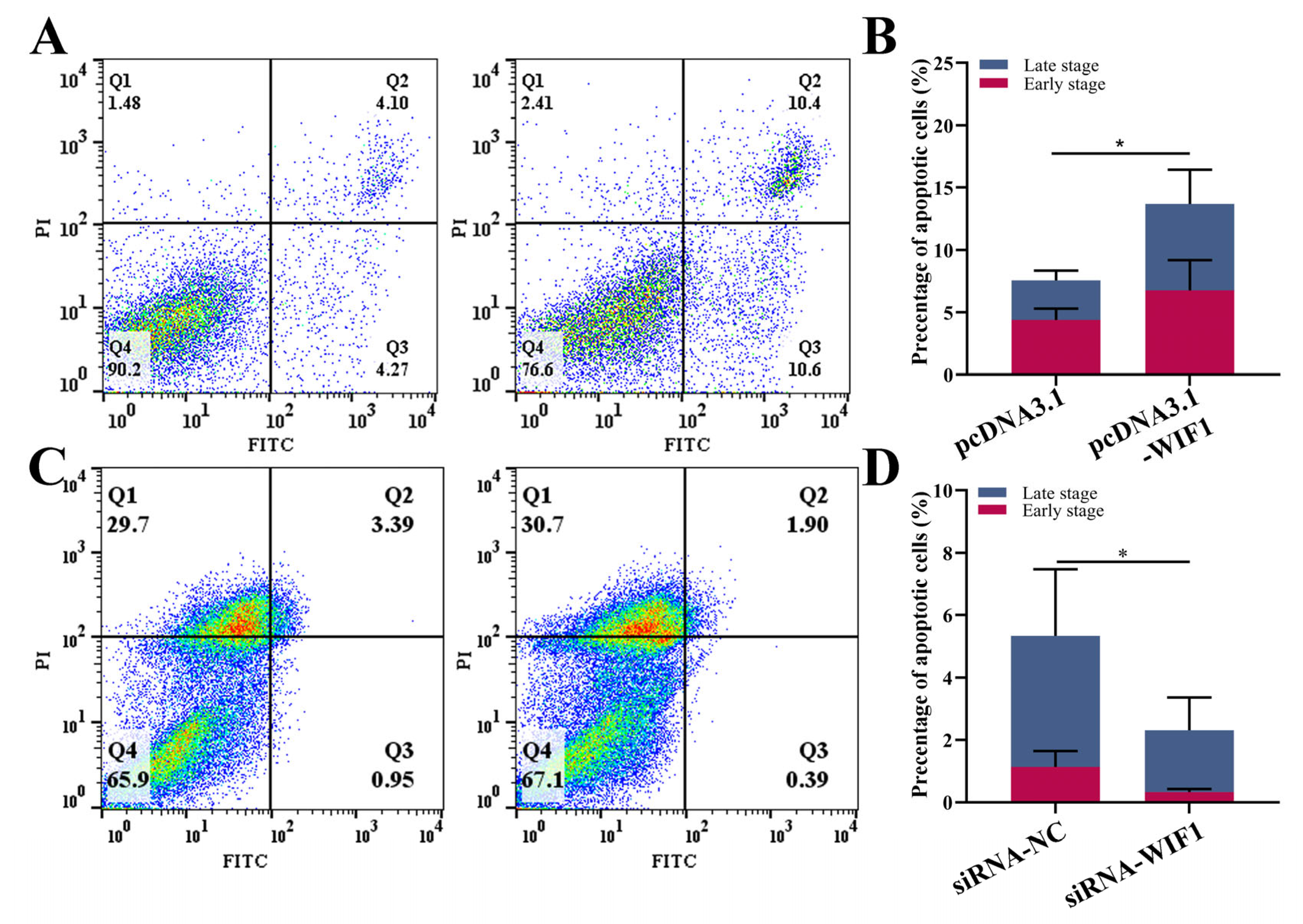
2.3. WIF1 Promotes the Apoptosis of Chicken Prehierarchal GCs
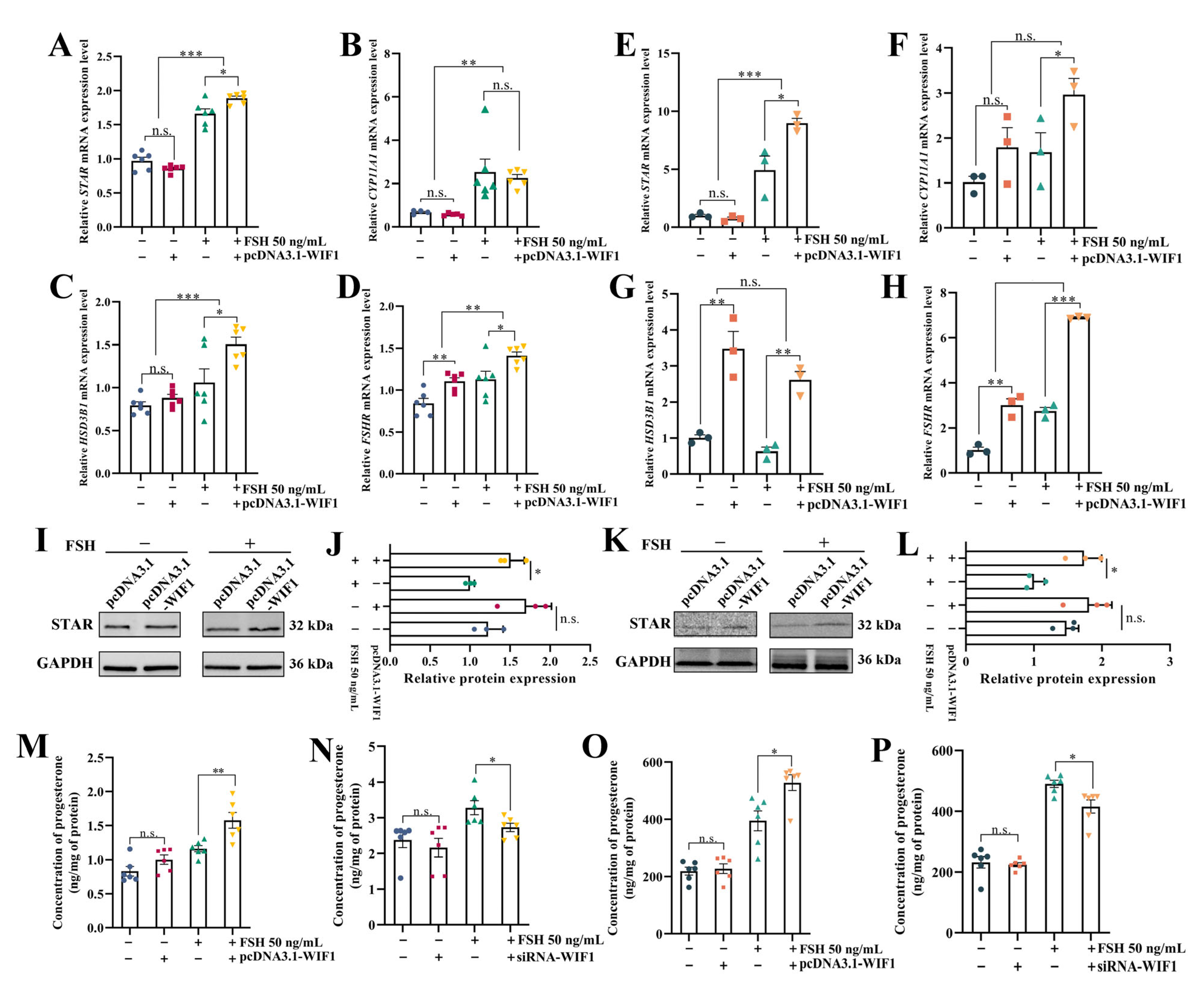
2.4. WIF1 Promotes the Secretion of Progesterone with the Participation of FSH in Chicken GCs
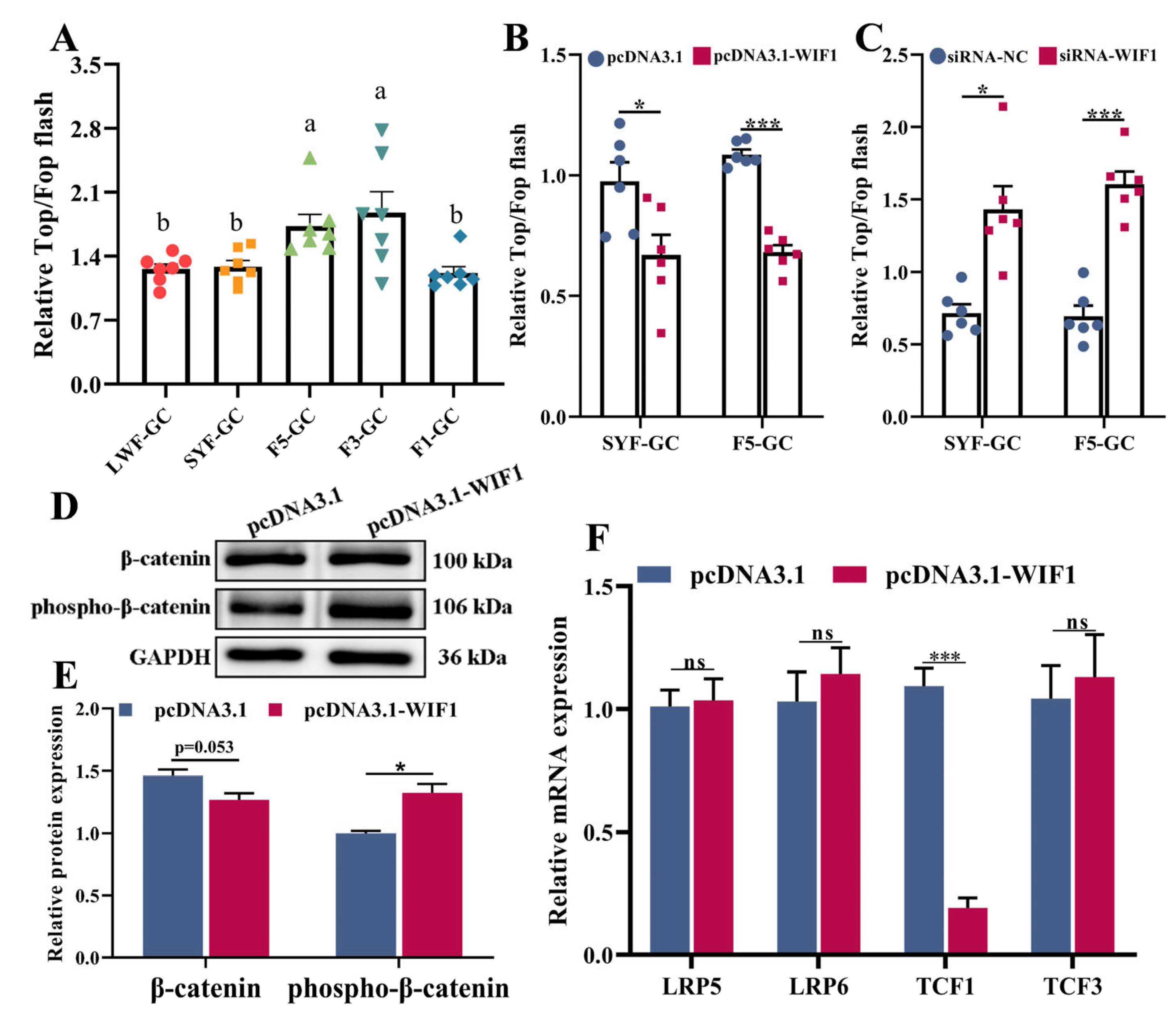
2.5. WIF1 Inhibits Wnt Signaling Activity in Chicken GCs
2.6. WIF1 Stimulates Metabolic Activity of Chicken Prehierarchal GCs
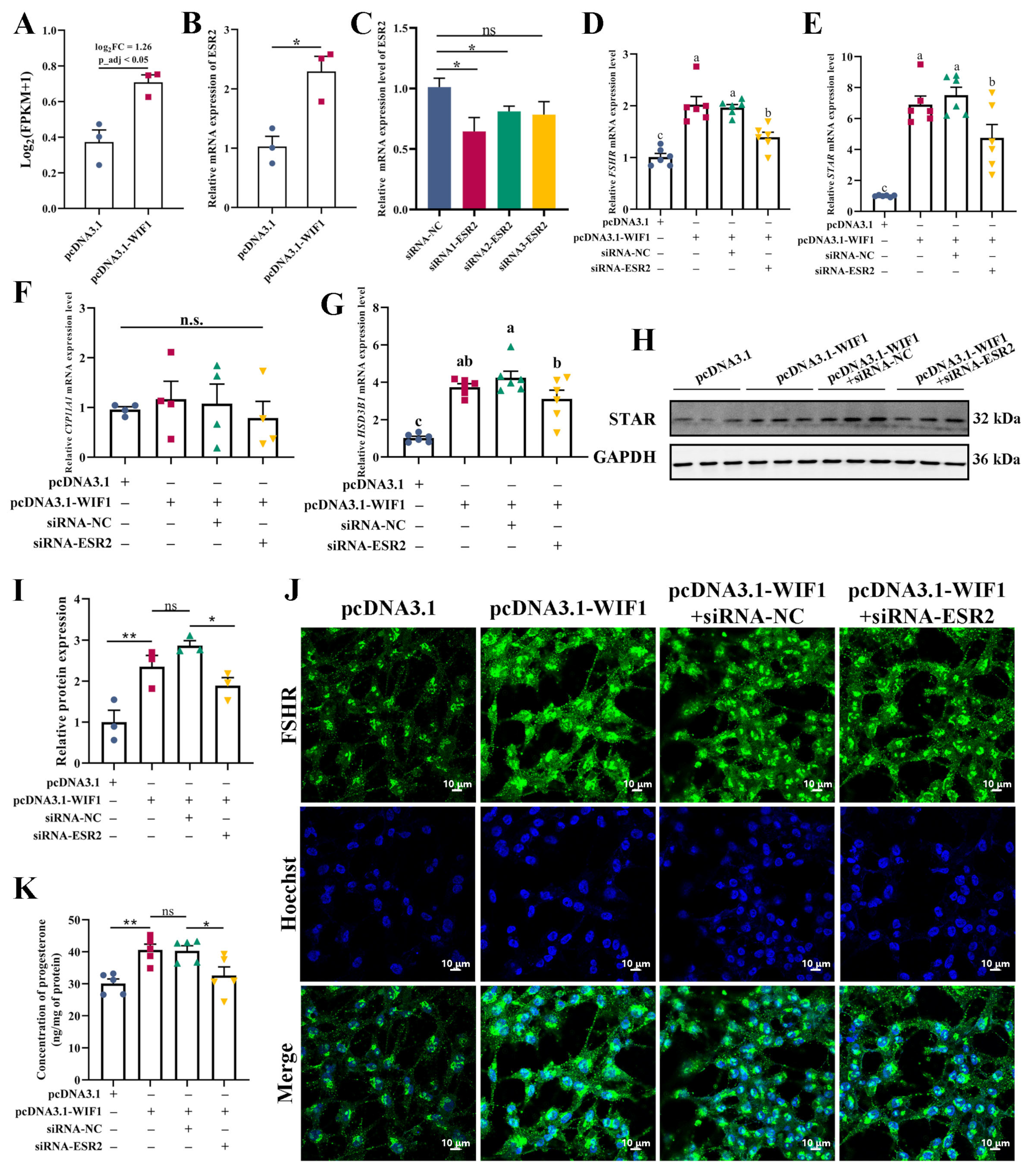
2.7. WIF1 Promotes Progesterone Synthesis through Upregulation of ESR2 in GCs
2.8. WIF1 Suppresses TC Proliferation and Promotes Apoptosis
3. Discussion
4. Materials and Methods
4.1. Ethics Statement and Experimental Animals
4.2. Cell Isolation and Culture
4.3. Plasmid Construction, RNA Oligonucleotide Synthesis, and Cell Transfection
4.4. Quantitative Real-Time PCR (qRT-PCR)
4.5. Western Blotting
4.6. 5-Ethynyl-2′-Deoxyuridine (EdU) Assay
4.7. Cell Counting Kit-8 (CCK 8) Assay
4.8. Flow Cytometry
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. Dual-Luciferase Reporter Assay
4.11. RNA-seq and Bioinformatics Analysis
4.12. Co-Transfection of Plasmid DNA and Small Interfering RNA (siRNA)
4.13. Immunofluorescence Staining
4.14. TdT-Mediated dUTP Nick-End Labeling (TUNEL) Assay
4.15. Statistical Analysis and Data Visualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, A.L. Ovarian Follicle Selection and Granulosa Cell Differentiation. Poult. Sci. 2015, 94, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Lovell, T.M.; Gladwell, R.T.; Groome, N.P.; Knight, P.G. Ovarian Follicle Development in the Laying Hen Is Accompanied by Divergent Changes in Inhibin A, Inhibin B, Activin A and Follistatin Production in Granulosa and Theca Layers. J. Endocrinol. 2003, 177, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L. Intracellular Mechanisms Regulating Cell Survival in Ovarian Follicles. Anim. Reprod. Sci. 2003, 78, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Q.; Liu, Z.; Guo, X.; Du, Y.; Yuan, Z.; Guo, M.; Kang, L.; Sun, Y.; Jiang, Y. Transcriptome Analysis on Single Small Yellow Follicles Reveals That Wnt4 Is Involved in Chicken Follicle Selection. Front. Endocrinol. 2017, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Elis, S.; Dupont, J.; Couty, I.; Persani, L.; Govoroun, M.; Blesbois, E.; Batellier, F.; Monget, P. Expression and Biological Effects of Bone Morphogenetic Protein-15 in the Hen Ovary. J. Endocrinol. 2007, 194, 485–497. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, C.Y.; Kwok, A.H.Y.; Leung, F.C. Epidermal Growth Factor (EGF) Receptor Ligands in the Chicken Ovary: I. Evidence for Heparin-Binding EGF-Like Growth Factor (HB-EGF) as a Potential Oocyte-Derived Signal to Control Granulosa Cell Proliferation and HB-EGF and Kit Ligand Expression. Endocrinology 2007, 148, 3426–3440. [Google Scholar] [CrossRef]
- Yu, Y.S.; Sui, H.S.; Han, Z.B.; Li, W.; Luo, M.J.; Tan, J.H. Apoptosis in Granulosa Cells During Follicular Atresia: Relationship with Steroids and Insulin-Like Growth Factors. Cell Res. 2004, 14, 341–346. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, Z.; Li, D.; Kang, L.; Jiang, Y. Long-Read Sequencing Reveals the Effect of Follicle-Stimulating Hormone on the mRNA Profile of Chicken Granulosa Cells from Prehierarchical Follicles. Poult. Sci. 2023, 102, 102600. [Google Scholar] [CrossRef]
- Samardzija, D.; Pogrmic-Majkic, K.; Fa, S.; Stanic, B.; Jasnic, J.; Andric, N. Bisphenol A Decreases Progesterone Synthesis by Disrupting Cholesterol Homeostasis in Rat Granulosa Cells. Mol. Cell. Endocrinol. 2018, 461, 55–63. [Google Scholar] [CrossRef]
- Miller, W.L. Steroidogenesis: Unanswered Questions. Trends Endocrinol. Metab. 2017, 28, 771–793. [Google Scholar] [CrossRef]
- Nie, R.; Zheng, X.; Zhang, W.; Zhang, B.; Ling, Y.; Zhang, H.; Wu, C. Morphological Characteristics and Transcriptome Landscapes of Chicken Follicles During Selective Development. Animals 2022, 12, 713. [Google Scholar] [CrossRef]
- Hsieh, J.C.; Kodjabachian, L.; Rebbert, M.L.; Rattner, A.; Smallwood, P.M.; Samos, C.H.; Nusse, R.; Dawid, I.B.; Nathans, J. A New Secreted Protein That Binds to Wnt Proteins and Inhibits Their Activities. Nature 1999, 398, 431–436. [Google Scholar] [CrossRef]
- Wissmann, C.; Wild, P.J.; Kaiser, S.; Roepcke, S.; Stoehr, R.; Woenckhaus, M.; Kristiansen, G.; Hsieh, J.C.; Hofstaedter, F.; Hartmann, A.; et al. WIF1, a Component of the Wnt Pathway, Is Down-Regulated in Prostate, Breast, Lung, and Bladder Cancer. J. Pathol. 2003, 201, 204–212. [Google Scholar] [CrossRef]
- Becker, M.; Bauer, J.; Pyczek, J.; König, S.; Müllen, A.; Rabe, H.; Schön, M.P.; Uhmann, A.; Hahn, H. WIF1 Suppresses the Generation of Suprabasal Cells in Acanthotic Skin and Growth of Basal Cell Carcinomas upon Forced Overexpression. J. Investig. Dermatol. 2020, 140, 1556–1565.e11. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, S.; Tan, L.; Wu, X.; Wu, Z.; Ran, R. Clinicopathological Significance of WIF1 Hypermethylation in NSCLC, a Meta-analysis and Literature Review. Oncotarget 2017, 8, 2550–2557. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Luo, D.; Sun, X.; Li, H.; Lu, Y.; Wang, J.; Zhang, M.; Lin, N.; Yin, C.; et al. Aberrant Promoter Methylation of Wnt Inhibitory Factor-1 Gene Is a Potential Target for Treating Psoriasis. Clin. Immunol. 2023, 250, 109294. [Google Scholar] [CrossRef]
- Khristi, V.; Chakravarthi, V.P.; Singh, P.; Ghosh, S.; Pramanik, A.; Ratri, A.; Borosha, S.; Roby, K.F.; Wolfe, M.W.; Rumi, M.A.K. ESR2 Regulates Granulosa Cell Genes Essential for Follicle Maturation and Ovulation. Mol. Cell. Endocrinol. 2018, 474, 214–226. [Google Scholar] [CrossRef]
- Couse, J.F.; Hewitt, S.C.; Bunch, D.O.; Sar, M.; Walker, V.R.; Davis, B.J.; Korach, K.S. Postnatal Sex Reversal of the Ovaries in Mice Lacking Estrogen Receptors Alpha and Beta. Science 1999, 286, 2328–2331. [Google Scholar] [CrossRef] [PubMed]
- Poggi, L.; Casarosa, S.; Carl, M. An Eye on the Wnt Inhibitory Factor Wif1. Front. Cell Dev. Biol. 2018, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chen, C.; Chen, H.; Zheng, S.G.; Bringas, P.; Xu, M.; Zhou, X.H.; Chen, D.; Umans, L.; Zwijsen, A.; et al. Smad1 and Its Target Gene Wif1 Coordinate BMP and Wnt Signaling Activities to Regulate Fetal Lung Development. Development 2011, 138, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Ortego, J.; Escribano, J.; Coca-Prados, M. Cloning and Characterization of Subtracted cDNAs from a Human Ciliary Body Library Encoding TIGR, a Protein Involved in Juvenile Open Angle Glaucoma with Homology to Myosin and Olfactomedin. FEBS Lett. 1997, 413, 349–353. [Google Scholar] [CrossRef]
- Kwon, H.S.; Lee, H.S.; Ji, Y.; Rubin, J.S.; Tomarev, S.I. Myocilin Is a Modulator of Wnt Signaling. Mol. Cell. Biol. 2009, 29, 2139–2154. [Google Scholar] [CrossRef]
- Menaa, F.; Braghini, C.A.; Vasconcellos, J.P.; Menaa, B.; Costa, V.P.; Figueiredo, E.S.; Melo, M.B. Keeping an Eye on Myocilin: A Complex Molecule Associated with Primary Open-Angle Glaucoma Susceptibility. Molecules 2011, 16, 5402–5421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Wang, Y.Y.; Liu, Z.M.; Guo, X.L.; Sun, Y.; Kang, L.; Jiang, Y.L. Transcriptomic and Proteomic Analyses of Ovarian Follicles Reveal the Role of VLDLR in Chicken Follicle Selection. BMC Genom. 2020, 21, 486. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Jung, E.; Heur, M. Fibroblast Growth Factor 2 Induces Proliferation and Fibrosis via SNAI1-Mediated Activation of CDK2 and ZEB1 in Corneal Endothelium. J. Biol. Chem. 2018, 293, 3758–3769. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, F.; Liu, Z.; Zhao, Y.; Jiang, Y.; Chen, L.; Li, C.; Zhou, X. Brain-Derived Neurotrophic Factor Promotes Proliferation and Progesterone Synthesis in Bovine Granulosa Cells. J. Cell. Physiol. 2019, 234, 8776–8787. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Li, Q.; Yang, L.; Li, X.; Sun, C.; Liu, Y.; Liu, H.; Pan, Z.; Li, Q.; Du, X. SDNOR, a Novel Antioxidative lncRNA, Is Essential for Maintaining the Normal State and Function of Porcine Follicular Granulosa Cells. Antioxidants 2023, 12, 799. [Google Scholar] [CrossRef] [PubMed]
- Lambiv, W.L.; Vassallo, I.; Delorenzi, M.; Shay, T.; Diserens, A.C.; Misra, A.; Feuerstein, B.; Murat, A.; Migliavacca, E.; Hamou, M.F.; et al. The Wnt Inhibitory Factor 1 (WIF1) Is Targeted in Glioblastoma and Has a Tumor Suppressing Function Potentially by Induction of Senescence. Neuro Oncol. 2011, 13, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, I.; Thavathiru, E.; Ramalingam, S.; Natarajan, G.; Mills, W.K.; Benbrook, D.M.; Zuna, R.; Lightfoot, S.; Reis, A.; Anant, S.; et al. Wnt Inhibitory Factor 1 Induces Apoptosis and Inhibits Cervical Cancer Growth, Invasion and Angiogenesis In Vivo. Oncogene 2012, 31, 2725–2737. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Wang, Z.; Sun, Y.; Wei, H.; Cui, Y.; Wu, Z.; Zhang, C.; Xie, K.P.; Wang, F.; Zhang, M. Sgpl1 Deletion Elevates S1P Levels, Contributing to NPR2 Inactivity and p21 Expression That Block Germ Cell Development. Cell Death Dis. 2021, 12, 574. [Google Scholar] [CrossRef]
- Han, S.; Wang, J.; Cui, C.; Yu, C.; Zhang, Y.; Li, D.; Ma, M.; Du, H.; Jiang, X.; Zhu, Q.; et al. Fibromodulin Is Involved in Autophagy and Apoptosis of Granulosa Cells Affecting the Follicular Atresia in Chicken. Poult. Sci. 2022, 101, 101524. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Bai, X.; Wang, H.; Li, X.; Sun, G.; Zhang, P. A Study on the Mechanism of Wnt Inhibitory Factor 1 in Osteoarthritis. Arch. Med. Sci. 2020, 16, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M.; Wang, X.; Jo, Y.; Manna, P.R. Multiple Signaling Pathways Regulating Steroidogenesis and Steroidogenic Acute Regulatory Protein Expression: More Complicated than We Thought. Mol. Endocrinol. 2005, 19, 2647–2659. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Dyson, M.T.; Eubank, D.W.; Clark, B.J.; Lalli, E.; Sassone-Corsi, P.; Zeleznik, A.J.; Stocco, D.M. Regulation of Steroidogenesis and the Steroidogenic Acute Regulatory Protein by a Member of the cAMP Response-Element Binding Protein Family. Mol. Endocrinol. 2002, 16, 184–199. [Google Scholar] [CrossRef]
- Zhou, X.; He, Y.; Pan, X.; Quan, H.; He, B.; Li, Y.; Bai, G.; Li, N.; Zhang, Z.; Zhang, H.; et al. DNMT1-Mediated lncRNA IFFD Controls the Follicular Development via Targeting GLI1 by Sponging miR-370. Cell Death Differ. 2023, 30, 576–588. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Zhang, Z.; Xu, D.; Duan, J.; Li, X.; Yang, L.; Hua, R.; Cheng, J.; Li, Q. Isorhamnetin Promotes Estrogen Biosynthesis and Proliferation in Porcine Granulosa Cells via the PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2021, 69, 6535–6542. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Pangas, S.A. The Ovary: Basic Biology and Clinical Implications. J. Clin. Investig. 2010, 120, 963–972. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt Signaling Pathway in Development and Disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Song, J.; Xie, C.; Jiang, L.; Wu, G.; Zhu, J.; Zhang, S.; Tang, M.; Song, L.; Li, J. Transcription Factor AP-4 Promotes Tumorigenic Capability and Activates the Wnt/β-Catenin Pathway in Hepatocellular Carcinoma. Theranostics 2018, 8, 3571–3583. [Google Scholar] [CrossRef]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a Target of the APC Pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef]
- Tetsu, O.; McCormick, F. Beta-Catenin Regulates Expression of Cyclin D1 in Colon Carcinoma Cells. Nature 1999, 398, 422–426. [Google Scholar] [CrossRef]
- Lin, Z.; Gong, Y.; Sun, H.; Yang, C.; Tang, Y.; Yin, L.; Zhang, D.; Wang, Y.; Yu, C.; Liu, Y. Lipid Deposition and Progesterone Synthesis Are Increased by miR-181b-5p Through RAP1B/ERK1/2 Pathway in Chicken Granulosa Cells. J. Agric. Food Chem. 2023, 71, 12910–12924. [Google Scholar] [CrossRef]
- Ning, Z.; Deng, X.; Li, L.; Feng, J.; Du, X.; Amevor, F.K.; Tian, Y.; Li, L.; Rao, Y.; Yi, Z.; et al. miR-128-3p Regulates Chicken Granulosa Cell Function via 14-3-3β/FoxO and PPAR-γ/LPL Signaling Pathways. Int. J. Biol. Macromol. 2023, 241, 124654. [Google Scholar] [CrossRef] [PubMed]
- Mitani, S.; Takayama, K.; Nagamoto, Y.; Imagawa, K.; Sakurai, F.; Tachibana, M.; Sumazaki, R.; Mizuguchi, H.; Human, E.S.C. Human ESC/iPSC-Derived Hepatocyte-Like Cells Achieve Zone-Specific Hepatic Properties by Modulation of WNT Signaling. Mol. Ther. 2017, 25, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, I.; Zinn, P.; Lai, M.; Rajakannu, P.; Hamou, M.F.; Hegi, M.E. WIF1 Re-expression in Glioblastoma Inhibits Migration Through Attenuation of Non-canonical WNT Signaling by Downregulating the lncRNA MALAT1. Oncogene 2016, 35, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.E.; Hargis, B.M.; Silsby, J.L.; el Halawani, M.E. Differential Steroid Production Between Theca Interna and Theca Externa Cells: A Three-Cell Model for Follicular Steroidogenesis in Avian Species. Endocrinology 1989, 125, 109–116. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript Assembly and Quantification by RNA-Seq Reveals Unannotated Transcripts and Isoform Switching During Cell Differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Ginestet, C. ggplot2: Elegant Graphics for Data Analysis. J. R. Stat. Soc. A 2011, 174, 245–246. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, H.; Liu, Y.; Liu, Y.X.; Huang, L. EVenn: Easy to Create Repeatable and Editable Venn Diagrams and Venn Networks Online. J. Genet. Genom. 2021, 48, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.E.; Reinaltt, K.; Civelek, M.; Anderson, W.D. GSEAplot: A Package for Customizing Gene Set Enrichment Analysis in R. J. Comput. Biol. 2021, 28, 629–631. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, R.; Zhang, W.; Tian, H.; Li, J.; Ling, Y.; Zhang, B.; Zhang, H.; Wu, C. Regulation of Follicular Development in Chickens: WIF1 Modulates Granulosa Cell Proliferation and Progesterone Synthesis via Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 1788. https://doi.org/10.3390/ijms25031788
Nie R, Zhang W, Tian H, Li J, Ling Y, Zhang B, Zhang H, Wu C. Regulation of Follicular Development in Chickens: WIF1 Modulates Granulosa Cell Proliferation and Progesterone Synthesis via Wnt/β-Catenin Signaling Pathway. International Journal of Molecular Sciences. 2024; 25(3):1788. https://doi.org/10.3390/ijms25031788
Chicago/Turabian StyleNie, Ruixue, Wenhui Zhang, Haoyu Tian, Junying Li, Yao Ling, Bo Zhang, Hao Zhang, and Changxin Wu. 2024. "Regulation of Follicular Development in Chickens: WIF1 Modulates Granulosa Cell Proliferation and Progesterone Synthesis via Wnt/β-Catenin Signaling Pathway" International Journal of Molecular Sciences 25, no. 3: 1788. https://doi.org/10.3390/ijms25031788
APA StyleNie, R., Zhang, W., Tian, H., Li, J., Ling, Y., Zhang, B., Zhang, H., & Wu, C. (2024). Regulation of Follicular Development in Chickens: WIF1 Modulates Granulosa Cell Proliferation and Progesterone Synthesis via Wnt/β-Catenin Signaling Pathway. International Journal of Molecular Sciences, 25(3), 1788. https://doi.org/10.3390/ijms25031788






