Abstract
The Mediterranean ecosystem is characterized by marked seasonality; it is composed of species such as shrublands that are subjected to high levels of water and thermal stress, making these species an important source of secondary metabolites of significant chemical and ecological interest. In this work, 21 plants were selected from the Mediterranean scrub. These abundant and characteristic representations of the ecosystem produce a total of 197 terpenes. The majority of these are monoterpenes (46.70%), followed by sesquiterpenes (38.07%), with a minority of diterpenes (5.53%) and triterpenes (10.15%). Tetraterpenes accounted for only 0.5% of the total compounds in the species studied, corresponding to only 1%. The major terpenes include 1,8-cineole, terpinen-4-ol, α-terpineol, borneol, camphor, γ-terpinene, limonene, linalool, o-cymene, α-tujene, α-pinene, β-pinene, sabinene, myrcene, β-phellandrene, and β-caryopylene. Species such as Pistacea terebinthus, Rosmarinus officinalis, Cistus ladanifer, Myrtus communis, Lavandula stoecha, and Thymus mastichina contain the most terpenic compounds in their chemical composition. Furthermore, these metabolites are involved in various biological functions, including antimicrobial, antioxidant, neuroprotective, antibacterial, cardiovascular, analgesic, antitumor, and insecticidal activities, among others. Various terpenes present in Mediterranean scrub species, such as 1,8-cineole, α-pinene, limonene, borneol, and terpinen-4-ol, have demonstrated synergistic effects that enhance their antimicrobial, insecticidal, and neuroprotective properties. These interactions between compounds make the natural extracts more effective than they would be individually, increasing their therapeutic and biotechnological value. The synergism among terpenes suggests a promising approach for developing more effective and sustainable phytotherapeutic products.
1. Introduction
Mediterranean ecosystems are distributed across various regions of the world, including the Iberian Peninsula [1], and characterized by warm, dry summers and humid, mild winters. Evapotranspiration involves three times the amount of precipitation, causing enormous water stress. These ecosystems have traditionally been considered marginal areas compared to more productive ecosystems. [2]. In the Iberian Peninsula, these ecosystems develop in soils of low fertility, with droughts in summer and frosts in winter [3]. Due to these stressful conditions, plants have developed specific morphological and physiological adaptive mechanisms [4].
Fragile Mediterranean landscapes have been used and transformed since ancient times for agricultural and livestock purposes [5,6]. Also, their species have been used for medicinal purposes by the inhabitants. Thus, medicinal plants have proven to be notable reservoirs of pharmacologically active secondary metabolites. Their relevance also extends to the food, cosmetic, and pharmaceutical industries. Currently, the abandonment of traditional agricultural practices, together with climate change, is altering the structure and dynamics of these ecosystems, with increasing amounts of scrubland and the appearance of invasive species [7]. These circumstances not only place traditional lifestyles based on the exploitation of Mediterranean species at risk, but also limit the potential uses of their bioactive molecules. The growing global demand for terpenes, driven by their application in sectors such as medicine, food, perfumery, and agriculture, has consolidated their commercial interest and potential as a strategic natural resource [8].
Plant molecules are divided into two categories based on their metabolic functions. On the one hand, there are primary metabolites, which participate in basic metabolic functions, and on the other hand, we have secondary metabolites, which perform specific functions in the plant [9]. The main groups of secondary metabolites in plants are terpenes, phenols, and alkaloids. Among the physiological adaptations of Mediterranean scrubland is the production of abundant secondary metabolites, mainly of terpene origin and in the form of essential oil. Essential oils are volatile and hydrophobic compounds produced by plants and composed of simple phenols, monoterpenes, and sesquiterpenes, the latter of which act as phytoalexins and antibiotic compounds produced by plants in response to the appearance of pathogenic microorganisms [10].
2. Chemical Structure of Terpenes
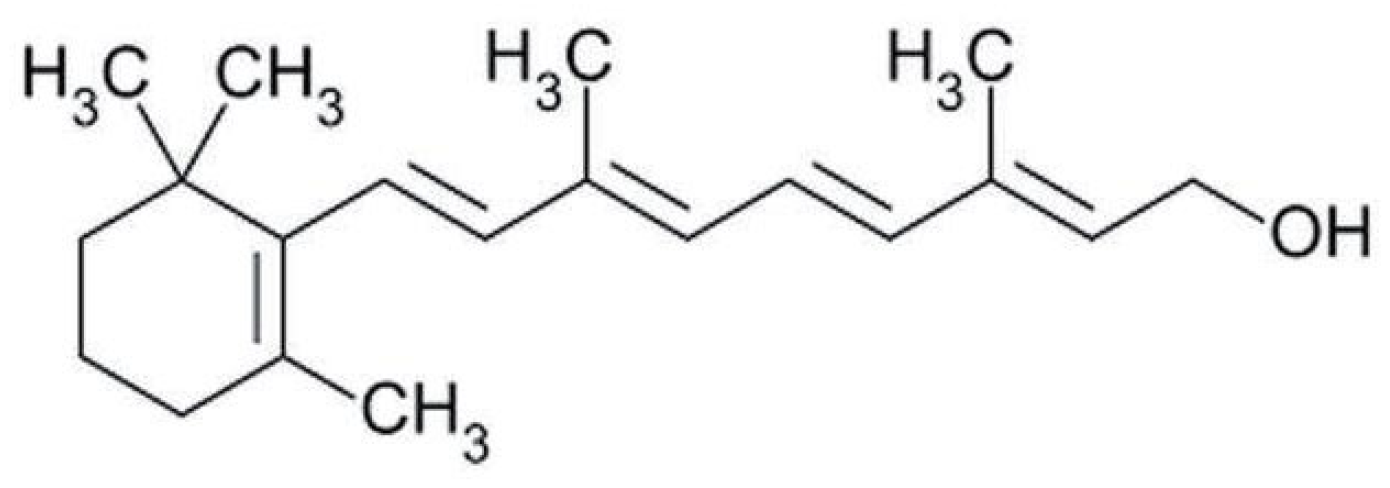
Terpenes are formed from isoprene units (2-methylbuta-1,3-diene) (Figure 1) and its double bond isomer, dimethyl pyrophosphate, and can form linear or cyclic structures through chemical pathways of mevalonate and methylerythriol phosphate [11,12].
Figure 1.
Basic structure of terpenes.
The classification of terpenes is carried out based on the number of carbon atoms present in the molecule, the basic unit of which is formed by five carbon atoms, called monoisoprene (C5). These compounds perform a protective function against animals and thermal stress conditions and are classified as monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), and tetratherpenes (C40) (Table 1) [13].

Table 1.
Classification of terpenes.
For the biosynthesis of terpenes, the mevalonate pathway is the only one that can produce precursors in animals and fungi. This occurs from the successive juxtaposition of their precursor molecules, isopentenyl diphosphate (IPP) and its isomer dimethylallyl di-phosphate (DMAPP), in a head-to-tail or tail-to-head fashion, which are produced in the cytosol from mevalonic acid (MVA) or in plastids from pyruvate and 3-phosphoglycerate (MEP) [14,15].
Different types of terpenes have diverse biological properties associated with them [9,16,17]. For example, monoterpenes play an important role in plants due to their antimicrobial, antifungal, and antiviral properties, and these types of compounds usually resist strains of S. aureus and E. coli [18]. On the other hand, sesquiterpenes help plants grow and present resistance to certain types of microorganisms such as Bacillus subtilis and Staphylococcus aureus [19]. It is important to highlight other types of terpenoids, such as sesquiterpenes; although uncommon, they present interesting biological activities [20,21,22,23,24].
For these reasons, this work aims to review the terpene composition of the main species composing Mediterranean scrub, as well as their biological potential. Their characterization will enable us to identify the true biotechnological potential of terpene compounds, including their antimicrobial, anti-inflammatory, antioxidant, and neuroprotective activity and potential application in the pharmaceutical, agri-food, and biotechnology sectors. Although studies on individual species exist, an integrative review analyzing the diversity of terpenes in the main plants of the Mediterranean scrubland is still lacking [4,8,17].
3. Species Studied
The most representative genera of Mediterranean scrub and trees were identified: Cistus, Cytisus, Rosmarinus, Lavandula, Thymus, Erica, Pistacea, Myrtus, Rubus, and Calluna. The Quercus genus was added because it is the most predominant and relevant in the tree stratum. In total, 21 species were selected for review. Most of these species are characterized as aromatic, with a high presence of terpenes: Cistus ladanifer, Cistus salvifolius, Cistus monspeliensis, Cistus albidus, Cytisus multiflorus, Cytisus scoparius, Erica manipuliflora, Erica scoparia, Calluna vulgaris, Myrtus communis, Pistacia lentiscus, Pistacia terebinthus, Rosmarius officinalis, Quercus ilex, Quercus suber, Arbutus unedo, Erica australis, Lavandula stoechas, Thymus mastichina, Thymus vulgaris, and Rubus ulmifolius. These Mediterranean plants were selected based on their promising profile of terpene compounds produced by the plant for various functions such as defense, communication, hormone production, and different biological and pharmacological activities [25]. A systematic bibliographic search was conducted on the databases Scopus, ScienceDirect, SciELO, PubMed, Web of Science, and ResearchGate, considering publications from the period 2010–2024. The search strategy combined terms related to the compounds of interest (terpenes, monoterpenes, sesquiterpenes, essential oils, and volatile organic compounds), the ecosystem (Mediterranean, maquis, matorral, garrigue, and shrubland), and the main plant genera characteristics of the Mediterranean scrub (Cistus, Rosmarinus, Thymus, Lavandula, among others). Boolean operators (‘AND’ and ‘OR’) and quotation marks for exact phrases were applied to optimize information retrieval. Studies were included if they provided data on the chemical composition of terpenes in Mediterranean species, as well as their biological activity and biotechnological potential. In total, 21 species were selected, based on their ecological abundance, taxonomic representativeness within the Mediterranean scrub ecosystem, and phytochemical relevance due to a high content of secondary metabolites of interest.
4. Identified Terpenes
In total, 197 terpene compounds have been identified in Mediterranean scrub plants (Table 2). As can be seen, their presence varies considerably between species. Monoterpenes comprise the majority of compounds, with a total of 92 monoterpene compounds representing 46.70% of the compounds studied. Sesquiterpenes follow with 75 compounds (38.07%). In order of abundance, these are followed by triterpenes, with 20 compounds and 10.15% of the total. Finally, the minority of terpene families found are diterpenes, with only five compounds representing 2.53% of the total, and only one tetraterpene was identified, representing 0.5% of the total.

Table 2.
Terpenes found in the Mediterranean scrub plants studied.
Analyzing the distribution of compounds by species (Table 3), monoterpenes and sesquiterpenes comprise the majority of compounds in 12 of the species, with 100% of monoterpenic compounds for Q.i. The percentage of plants with terpenic compounds decreases from 100% for Q.i to 42.85% for E.a., and sesquiterpenic compounds reached the highest percentage for C.a., where all of the identified compounds were sesquitermenes. Values of 59.2 were adopted for the species C.m. for low quantities of this family of compounds for C.sc., constituting 4.76%. On the other hand, diterpenic compounds were only found in four of the studied species and were found at percentages of 23.56% for C.mo, comprising only 2.39% of said compounds for C.l. Triperpenes are the main compounds for the species C.v., with 11 compounds found, constituting 78.57% of the total; teraterpenes constituted 21.43% of the total for the same species. They were only identified in three species. Conversely, in A.u., a tetraterpene compound was found constituting 3.59% of the total compounds found in this plant.

Table 3.
Number of terpenes per group and percentage of the total for each species.
As can be seen in Table 2 and Table 3, the presence of these compounds varies between species, resulting in a high qualitative variation. Some compounds were only identified in one species, while others were identified in most species. In this review, we describe the biological and biotechnological functions of these major compounds. Limonene was the most frequently identified compound, found in 13 species, followed by the two isomers of pinene (α-pinene and β-pinene), found in a total of 12 species. Next, borneol was found in 11 species; other terpenes such as 1,8-cineole, terpinen-4-ol, p-cymene, and sabinene appeared in 10 species. α-terpineol and the sesquiterpene caryopylene were found in nine of the species studied. The compounds camphor and α-thujene were found in eight of the species studied; the terpenes myrcene, β-phellandrene, γ-terpinene, and linalool were found in seven of the species studied. We did not consider terpenes present in less than seven species in this work (a third of the species analyzed).
There were variations between species regarding both the type and number of compounds, with large differences in their concentrations. Table 4 contains the variations in concentrations of major terpenes measured as a percentage in two-thirds of the Mediterranean scrub species studied. The most frequent compound among the Mediterranean scrubs studied was limonene; however, this was not the main compound in most of the species where it was present, ranging from trace concentrations in R.u. [58] to concentrations of 17.8% in M.c. [54,55]. Of the two pinene isomers, α-pinene was found mostly at concentrations of up to 49.65% in C.l. [47,48,49,50]. β-pinene was found at lower concentrations compared to α-pinene, with the highest concentration identified in E.s. (26.1%) [45]. Borneol, another of the major terpenes, was found at significant concentrations in P.l. (50.6%) and concentrations of 4.8% in A.u. [38,39,40]. 1,8-cineole was present in eight of the species studied, with varying concentrations from 0.3% for C.l. [47,48,49,50] to 33.8% for R.o. One of the major compounds found was terpinel-4-ol, at concentrations ranging from 0.2% for E.s. to 0.38% for R.o. [46] p-cymene was also found at low concentrations in eight of the species studied, with the exception of P.l. (22.1%) [56]. Sabinene, found in 11 of the species, presented a higher concentration of 32.7% in P.l., similar to myrcene, the majority of which was found in this plant (16.9%) [57]. α-thujene was found in five of the species at concentrations lower than 1% with the exception of P.t. (1.18%) [36,37] and P.l. (11.6%) [56]. Other terpenes present in 10 of the species included α-terpinene, varying from 0.06% for L.s. [59,60,61,62] to 3.53% in C.mo. [32]. Camphor had the highest concentration in P.l. (41.6%) [56] and the lowest concentration in P.t., ranging between 0.8 and 3.4% [36,37]. On the other hand, linalool was only found in five of the plants studied and at very low percentages, compared to its higher concentration in C.sc. (3.08%) [63,64,65]. Finally, β-phellandrene was found at a higher concentration in C.a. (7.48–36.9%) compared to the other Mediterranean plants studied [41,42].

Table 4.
Concentration of the majority terpenes expressed as a percentage identified in the different species.
4.1. Activity of the Most Common Terpenes Found in Mediterranean Scrub Plants
Most terpenes with common biological activities have antiviral, antibacterial, antifungal, insecticidal, antitumor, anti-inflammatory, neuroprotective, cardioprotective, gastroprotective, and analgesic properties, among others. Table 5 shows the biological activities attributed to each of the most frequently analyzed terpene compounds present in the selected Mediterranean scrub species [65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136].

Table 5.
Functions of the major terpenes found in this work.
4.1.1. Antimicrobial Activity
One of the main properties of essential oils is their antimicrobial activity, which is being increasingly used in the biotechnology industry. The antimicrobial activity of essential oils from aromatic plants stems from the set of terpenes that make up their chemical composition [137]. The terpene compounds studied in this work all showed antimicrobial activity. Thus, plant extracts with these compounds also have this property. T.v. exhibits antibacterial action against Gram-positive and Gram-negative bacterial strains [137] as well as other pathogenic bacteria such as Staphylococcus aureus, Bacillus cereus, Escherichia coli, Proteus vulgaris, Proteus mirabilis, Salmonella typhi, Salmonella typhimurium, Klebsiella pneumoniae, and Pseudomonas aeruginosa [138]. The species C.sc. also exhibits antimicrobial activity against large-sized positive bacteria such as Staphylococcus aureus and Bacillus spp. [139,140]. Terpenic extracts of L.s. undertake activity against Gram-positive and Gram-negative bacteria, and have susceptibility against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, and Staphylococcus. The terpenes with this property present in Mediterranean scrub plants include the following: 1,8-cineole [65,66,67,68,69,70], terpinen-4-ol [75,76,77,78,79,80,81], α-terpineol [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74], borneol [66,82,83,84,85,86], camphor [87,88], χ-terpinene [89,90,91,92], limonene [93,94,95,96,97,98,99], linalool [100,101,102,103,104,105], o-cymene [106,107,108,109,110,111], α-thujene [112,113], α-pinene [114,115,116,117,118,119], sabinene [132,133,134], myrcene [125,126,127,128,129], β-phellandrene [130,131], and β-caryopillene [132,133,134,135,136].
4.1.2. Anti-Inflammatory Activity
One of the main properties of the terpenes present in Table 4 is their anti-inflammatory characteristic. Inflammation is a defense response triggered in a part of the body to various inflammatory agents as a result of an immunological process, whether due to microbial conditions, physicochemical factors, or foreign bodies. In response to this inflammation, species experience high fever and leukocytosis [42,76]. The anti-inflammatory response of terpenoids originates from the inhibition of the nuclear transcription factor-kB (NF-kB) and mitogen-activated protein kinase (MAPK) signaling pathways [138,139].
In addition, they modulate the generation of cytokines, chemokines, and molecular cell adhesion, while targeting cell signaling pathways [141]. Among the plants included in the Mediterranean scrub with these types of properties, E.s. stands out, traditionally used for bladder inflammation, kidney disorders, and urinary tract inflammation [142]. On the other hand, C.l. has anti-inflammatory properties and a healing action [143]. A complete list of compounds identified in Table 4 with these properties is 1,8-cineole, terpinen-4-ol, α-terpineol, camphor, γ-pinene, linalool, o-cymene, β-pinene, and myrcene.
4.1.3. Neuroprotective Activity
Some of the compounds studied are attributed with a neuroprotective function. One of these is terpinen-4-ol, which, due to its antioxidant capacity, is capable of eliminating free radicals and reactive oxygen species, in addition to forming chelates with metal ions to reduce the effects of oxidative stress on the nervous system, among others [144]. Among the neuroprotective activities of this compound, enzymes such as acetylcholinesterase, butylhydroxycholinesterase, and urease are inhibited and involved in the synthesis of neurotransmitters [145]. For their part, α-pinene and 1,8-cinelol act as regulators of gene expression, protecting PC12 cells against apoptosis induced by oxidative stress in nervous system cells [146]. α-pinene acts as a protector against apoptotic cell death and brain diseases [147]. Another terpene with neuroprotective action is borneol, which has anti-amyloid effects, both in SH-SY5Y cells and motor neurons [148]. p-cymene also has neuroprotective effects due to its cholinergic effect when regulating gene expression in Caenorhabditis elegans [149]. Limonene, among its biological properties, also has a neuroprotective role in neurodegenerative diseases such as Alzheimer’s, multiple sclerosis, epilepsy, anxiety, and stroke [150]. In addition, limonene induces neurotoxicity against Aβ42 in droscophila, a model developed in the fruit fly [151]. Finally, another compound that has neurotoxic activity is myrcene [130]. This leads us to propose that the species P. lentiscus, L. stoechas, and T. vulgaris are reservoirs of compounds involved in neuroprotective function [152].
4.1.4. Gastroprotective Activity
Another therapeutic application of terpenes is their gastroprotective function against gastric ulcers, a pathology that affects more than 14.5 million people worldwide [152]. In addition, they have been shown to be effective against one of the most important gastrointestinal pathogens, the Gram-negative bacterium Helicobacter pylori (H. pylori), which infects almost 4.4 billion people worldwide [125,153,154]. They also reduce the adverse effects caused by the prolonged use of certain medications, such as stomach cancer [155]. There is an urgent need to search for new and effective but non-toxic, easily accessible, and affordable medications [155]. One compound that has this potential is α-terpineol, which is present in the essential oils of C.s., A.u., E.a.s and L.s.. α-terpineol and stands out for its antispasmodic, contraceptive, and immunostimulant properties [82]. Because this compound is common in flowers, herbs, leaves, and fruits, its biomolecule is easy to extract. Its only limitation is that it is a volatile compound, insoluble in water, and has a short life in the bloodstream of only 12.5 min [156]. Therefore, to improve its solubility, reduce its volatility, and enhance bioavailability, encapsulation techniques are used to ensure that it reaches the digestive tract with a controlled and prolonged release to its target region [157,158].
4.1.5. Cardioprotective Activity
Cardioprotection involves all mechanisms that contribute to preventing or correcting myocardial damage [75,159,160,161,162,163]. The main compounds listed in Table 4 associated with this property are terpinen-4-ol, 1,8-cineole, borneol, β-pinene, and β-caryopylene. These compounds are present in plants such as C.s., C.m., P.t., E.s., R.o., C.l., C.m., and L.s.. Terpinel-4-ol has been studied both individually and as a constituent of essential oils, and has been used in cardiovascular treatment in animals with arterial hypertension. It results in a significant expansion of the blood in addition to improving muscular relaxation of the blood vessels. This compound can also act as an antioxidant by decreasing LPO levels and reducing metal ions such as Ca2+ and Fe3+ while mitigating the effects of free radicals, and increasing the levels of glutathione transferase and superoxide dismutase activity [76,145]. Borneol also shows cardioprotective activity [85,87]. Borneol, a highly lipid-soluble bicyclic terpenoid, is widely used in oriental medicine due to its various therapeutic actions, including, among other cardioprotective properties, the alleviation of acute heart diseases [66]. In addition, it suppresses inflammatory responses by downregulating the mRNA expression of interleukin-1β and interleukin-6. It also promotes angiogenesis by upregulating hypoxia-induced factor 1α and CD34, which exerts positive cardiovascular effects and alleviates pathological damage [163].
4.1.6. Insecticidal Activity
Commercial formulations of terpene-based pesticides are currently being used [164,165,166,167,168]. In particular, α, β-unsaturated terpene ketones, which are produced naturally by some plants, have a potent fumigant effect and toxicity against certain insect pests [169]. Essential oils can be used as an alternative to synthetic pesticides due to their low toxicity and high structural diversity. The main terpenes found in Mediterranean scrub plants with insecticidal properties are 1,8-cineole, α-terpineol, limonene, o-cymene, and α-pinene. There is evidence that not all terpenes have the same insecticidal power, with monoterpenes containing carbonyl groups that are more toxic than molecules containing alcohols or hydrocarbons [170,171,172]. Among the plants studied, C.m., E.a., R.o., C.l., C.m., and L.s. stand out with greater insecticidal potential. Essential oils with a high presence of limonene are toxic to numerous insect pests [171,172,173]. α-pinene also has insecticidal properties in synergism with other terpenes such as β-pinene, sabinene, and γ-terpinene, against Musca domestica larvae [90,93,114,115,133,134].
4.1.7. Synergistic Interactions Between Terpenes
In addition to their individual biological activity, several studies highlight that many terpenes present in Mediterranean scrub species can act synergistically, enhancing their therapeutic and ecological efficacy. This synergy refers to the interaction between two or more compounds that, when combined, generate an effect greater than the sum of their individual effects. In the case of the compounds identified in Table 4, it has been observed that multiple combinations can present enhanced effects on various biological activities, especially in antimicrobial, anti-inflammatory, insecticidal, and neuroprotective contexts. The combination of 1,8-cineole with terpinen-4-ol enhances its antimicrobial capacity, complementing the destabilization of cell membranes and the inhibition of bacterial enzymatic activity [9]. Similarly, α-terpineol and linalool, which are both present in several species of the Mediterranean scrub, have synergy in their antioxidant and antimicrobial properties, improving efficacy against pathogens by combining bacteriostatic and bactericidal properties [18].
In the case of insecticidal activity, limonene, γ-terpinene, α-pinene, and sabinene have synergistic effects when used in mixtures, increasing toxicity against pest insects such as Musca domestica [173]. This interaction improves the ability of the compound to penetrate the insect and enhances its neurotoxic action. In the field of neuroprotection, the combination of borneol and linalool exerts beneficial effects by reducing neuronal oxidative stress and modulating the activity of enzymes such as acetylcholinesterase, with implications for neurodegenerative diseases such as Alzheimer’s [149]. Likewise, α-pinene, in the presence of other monoterpenes such as limonene and sabinene, can act as a cofactor in the protection of cells in the nervous system [145,151]. On the other hand, it has been suggested that β-caryophyllene, together with linalool or limonene, enhances anti-inflammatory potential by acting on different cellular signaling pathways, including NF-κB and MAPK, suggesting a broader therapeutic use through terpene mixtures [16].
From an ecological perspective, this chemical synergy could represent an adaptive strategy for Mediterranean plants, enhancing their defense against extreme conditions and pathogens through specific combinations of terpenes with complementary or enhanced activity. This metabolic cooperation allows for more effective responses to different types of biotic or abiotic stress, enhancing the biotechnological potential of these species. From all this, it can be concluded that Mediterranean scrub plant extracts should not be considered solely for the presence of individual compounds, but rather for the complete terpene profile and the interactions between them, which may be decisive for their application in phytotherapy, pharmacological formulations, natural cosmetics, and biological control.
5. Conclusions
In this review, phytochemical information was compiled and analyzed for representative species of the Mediterranean scrubland, revealing their richness in terpene compounds with relevant biological activities, such as carvacrol, 1,8-cineole, borneol, and thymol. These volatile profiles not only reflect the pharmacological and cosmetic potential of these plants but also suggest potential synergistic effects between terpenes that may enhance their efficacy. Revaluing these species as a source of bioactive molecules not only has a scientific and ecological impact but also an economic one, opening up opportunities for the development of natural products with added value. This perspective can promote the sustainable use of Mediterranean biodiversity, especially in rural areas, contributing to the revitalization of local economies through their integration into sectors such as phytotherapy, agroindustry, and natural cosmetics.
Author Contributions
Conceptualization, I.M.-F. and N.C.L.; data curation, I.M.-F., N.C.L. and J.C.A.G.; funding acquisition, N.C.L.; investigation, I.M.-F., N.C.L., J.C.A.G., L.N.G., and J.B.-S.; methodology, I.M.-F. and N.C.L.; project administration, N.C.L.; resources, N.C.L. and I.M.-F.; supervision, J.C.A.G.; validation and visualization, J.C.A.G., L.N.G., and J.B.-S.; writing—original draft, I.M.-F.; writing—review and editing, I.M.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been financed by the project titled “Direct subsidy to the University of Extremadura for the implementation of the LA4 lines of action of the I+D+i program in the area of Biodiversity. LIA4: Evaluation and mitigation of the impact of global change on biodiversity—FEDER Funds”/LIA4 Complementary Plan, co-financed by the Ministry of Economy, Science and Digital Agenda of the Government of Extremadura and by the European Regional Development Fund (FEDER) of Extremadura corresponding to the 2021–2027 programming period.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Casadesús, A.; Mumné-Bosch, S. Holoparasitic plant-host interactions and their impact on Mediterranean ecosystems. Plant Physiol. 2021, 185, 1325–1338. [Google Scholar] [CrossRef]
- Ramos-Rodríguez, E.; López-RodrÍguez, M.J. Manual de Prácticas de Ecología de Organismos y Poblaciones. Available online: https://digibug.ugr.es/bitstream/handle/10481/80083/MANUAL%20PR%C3%81CTICAS%20%20EOP%20CCAA%202223.pdf?sequence=1 (accessed on 23 September 2025).
- Jackson, L.E.; Bowles, T.M.; Ferris, H.; Margenot, A.J.; Hollander, A.; García-Palacios, P.; Daufresne, T.; Sánchez-Moreno, S. Plant and solil microfaunal biodiversity across the borders between arable and forest ecosistems in a Mediterranean landscape. Appl. Soil Ecol. 2019, 136, 122–138. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Díaz-Barradas, M.C.; Jauregui, J.; Rodríguez, H.; Alvarez-Cansino, L. Season-dependent and independent responses of Mediterranean scrub to light condictions. Plant Physiol. Biochem. 2016, 102, 80–91. [Google Scholar] [CrossRef]
- Navarro, L.M.; Pereira, H.M. Rewilding abandoned landscapes in Europe. In Rewilding European Landscapes; Pereira, H.M., Navarro, L.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 3–23. [Google Scholar] [CrossRef]
- García, R.R.; Fraser, M.D.; Celaya, R.; Ferreira, L.M.M.; García, U.; Osoro, K. Grazing land management and biodiversity in the Atlantic European heathlands: A review. Agrof. Syst. 2013, 87, 19–43. [Google Scholar] [CrossRef]
- Fernández-Manjarrés, J.F.; Ruiz-Benito, P.; Zavala, M.A.; Camarero, J.J.; Pulido, F.; Proença, V.; Navarro, L.; Sansilvestri, R.; Granda, E.; Marqués, L.; et al. Forest Adaptation to Climate Change along Steep Ecological Gradients: The Case of the Mediterranean-Temperate Transition in South-Western Europe. Sustainability 2018, 10, 3065. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. In Advances in Biochemical Engineering/Biotechnology; Schrader, J., Bohlmann, J., Eds.; Springer: Cham, Switzerland, 2015; Volume 148, pp. 63–106. [Google Scholar] [CrossRef]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.L.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Lin, S.H.E.; Lai, K.S. Terpene derivates as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J.M. Chitosan nanoparticles encapsulating lemongrass (Cymbopogon commutatus) essential oil: Physicochemical, structural, antimicrobial and in-vitro release properties. Int. J. Biol. Macromol. 2021, 192, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Croteau, R.; Johnsos, M.A. Biosynthesis of terpenoid wood extractives. In Biosynthesis and Biodegradation of Wood Components. Orlando; Higucho, T., Ed.; Academic Press: Cambridge, MA, USA, 1985; pp. 379–439. [Google Scholar] [CrossRef]
- Mabou, F.D.; Yossa, I.B.N. Terpenes; structural classification and biological activities. J. Pharm. Biol. Sci. 2021, 16, 25–40. [Google Scholar] [CrossRef]
- Rodríguez, R.; Johnson, J.J. Modulating anti-inflamatory signaling in inflammatory bowel disease. Pharmacol. Ther. 2023, 248, 108456. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Cassuriaga, A.P.A.; Moraes, L.; Morais, M.G. Biosynthesis and potential applications of terpenes produced from microalgae. Biorescurse Technol. Rep. 2022, 19, 101166. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho, A.J.S.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Obafemi, C.A.; Sulaimon, T.O.; Akinpelu, D.A.; Olugbade, T.A. Antimicrobial activity of extracts and a germacranolidetype sesquiterpene lactone from Tithonia diversifolia leaf extract. Afr. J. Biotechnol. 2006, 5, 1254–1258. [Google Scholar]
- Huang, A.C.; Kautsar, S.A.; Hong, Y.J.; Medene, M.M.; Bond, A.B. Unearthing a sesterpene biosynthetic repertoire in the Brassicaceae through genome mining receals conversent evolution. Proc. Natl. Acad. Sci. USA 2017, 114, E6005–E6014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Z.; Li, C.; Yu, X.; He, Q.; You, C.; Li, D.; Liu, Q.; Zhang, J. Sesquiterpenes from two Compositae plants as promising inhibitions to nuclear hormone receptor 3 of Tribolium castaneum. Pestic. Biochem. Physiol. 2023, 195, 105578. [Google Scholar] [CrossRef]
- Salazar-Gómez, A.; Ontiveros-Rodríguez, J.A.; Pablo-Pérez, S.; Vargas-Díaz, E.; Garduno-Siciliano, L. The potential role of sesquiterpene lactones isolated from medicinal plants in the trearment of the metabolic syndrome—A review. S. Afr. J. Bot. 2020, 135, 240–251. [Google Scholar] [CrossRef]
- Kokilananthan, S.; Bulugahapitiya, V.P.; Manawadu, H.; Gangabadage, S. Sesquiterpenes and monoterpenes from different varieties of guava leaf essential oil and their antioxidant potentital. Heliyon 2022, 8, e12104. [Google Scholar] [CrossRef]
- Xu, Y.-Z.; Gu, X.-Y.; Peng, S.-J.; Fang, J.-G.; Zhang, Y.-M.; Huang, D.-J.; Chen, J.-J.; Gao, K. Design, synthesis and biological evaluation of novel sesquiterpene mustards as potential anticancer agents. Eur. J. Med. Chem. 2015, 94, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.; Borges, B.A.; Veloso, M.P.; Chagas-Paula, D.A.; Gonçalves, R.V.; Novaes, R.D. Impacto of sesquiterpene lactones on the skin and skin-related cells? A sustematic review of in vitro and in vivo evidence. Life Sci. 2021, 15, 118815. [Google Scholar] [CrossRef]
- Abdelli, W.; Bahri, F.; Romane, A.; Höferl, M.; Wanner, J.; Schmidt, E.; Jirovetz, L. Chemical Composition and Anti-inflammatory Activity of Algerian Thymus vulgaris Essential Oil. Nat. Prod. Commun. 2017, 12, 611–614. [Google Scholar] [CrossRef]
- Shabnum, S.; Wagay, M.G. Essential Oil Composition of Thymus vulgaris L. and their Uses. J. Res. Dev. 2011, 11, 83–94. [Google Scholar] [CrossRef]
- Boruga, O.; Jianu, C.; Misca, C.; Golet, I.; Gruia, A.T.; Horthat, F.G. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56–60. [Google Scholar] [PubMed]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Čmiková, N.; Kačániová, M. Thymus vulgaris Essential Oil and Its Biological Activity. Plants 2021, 10, 1959. [Google Scholar] [CrossRef]
- Demetzos, C.; Angelopoulou, D.; Perdetzoglou, D. A comparative study of the essential oils of Cistus salvifolius in several populations of Crete (Greece). Biochem. Syst. Ecol. 2002, 30, 651–665. [Google Scholar] [CrossRef]
- Hitl, M.; Bijelic, K.; Stilinovic, N.; Bozin, B.; Srdenovic-Conic, B.; Torovic, L.; Kladar, N. Phytochemistry and Antihyperglycemic Potential of Cistus salvifolius, L., Cistaceae. Molecules 2022, 27, 8003. [Google Scholar] [CrossRef]
- Daghbouche, S.; Ammar, I.; Rekik, D.M.; Djazouli, E.; Zebib, B.; Merah, O. Effect of phenological stages on essential oil composition of Cytisus triflorus L’Her. J. King Saud Univ.-Sci. 2020, 32, 2383–2387. [Google Scholar] [CrossRef]
- Geraci, A.; Schicchi, R.; Sgadari, F.; Badalamenti, N.; Bruno, M. The essential oil compositions of three Sicilian accessions of Viscum album L. growing on three different host trees. Nat. Prod. Res. 2023, 37, 2623–2627. [Google Scholar] [CrossRef]
- Welter, S.; Bracho-Nuñez, A.; Mir, C.; Zimmer, I.; Kesselmeier, J.; Lumaret, R.; Schnitzler, J.P.; Staudt, M. The diversification of terpene emissions in Mediterranean oaks: Lessons from a study of Quercus suber, Quercus canariensis and its hybrid Quercus afares. Tree Physiol. 2012, 32, 1082–1091. [Google Scholar] [CrossRef]
- Kelmeier, J.; Schäfer, L.; Ciccioli, P.; Brancaleoni, E.; Cecinato, A.; Frattoni, M.; Foster, P.; Jacob, V.; Denis, J.; Fugit, J.L.; et al. Emission of monoterpenes and isoprone from a Mediterranean oak species Quercus ilex L. measured within the BEMA (Biogenic Emissions in the Mediterranean Area) project. Atmos. Environ. 1996, 30, 1841–1850. [Google Scholar] [CrossRef]
- Pulaj, B.; Mustafa, B.; Nelson, K.; Quave, C.L.; Hajdari, A. Chemical composition and in vitro antibacterial activity of Pistacia terebinthus essential oils derived from wild populations in Kosovo. Complement. Altern. Med. 2016, 16, 147. [Google Scholar] [CrossRef]
- Schoina, V.; Terpou, A.; Bosnea, L.; Kanellaki, M.; Nigam, P.S. Entrapment of Lactobacillus casei ATCC393 in the viscus matrix of Pistacia terebinthus resin form functional mizithra cheese manufacture. LWT-Food Sci. Technol. 2018, 89, 441–448. [Google Scholar] [CrossRef]
- Kivcak, B.; Mert, T.; Demirci, B.; Baser, K. Composition of the essential oil of Arbutus unedo. Chem. Nat. Compd. 2001, 37, 445–446. [Google Scholar] [CrossRef]
- Miguel, M.G.; Faleiro, M.L.; Guerreiro, A.C.; Antunes, M.D. Arbutus unedo L.: Chemical and Biological Properties. Molecules 2014, 19, 15799–15823. [Google Scholar] [CrossRef]
- Alexandre, A.M.R.C.; Serra, A.T.; Matias, A.A.; Duarte, C.M.M.; Bronze, M.R. Supercritical fluid extraction of Arbutus unedo distillate residues-Impact of process conditions on antiproliferative response of extracts. J. CO2 Util 2020, 37, 29–38. [Google Scholar] [CrossRef]
- Fadel, H.; Kebbi, S.; Chalchat, J.C.; Figueredo, G.; Chalard, P.; Benayache, F.; Ghedadba, N.; Benayache, S. Identification of Volatile Components and Antioxidant Assessment of the aerial Part Extracts From An Algerian Cistus albidus L. of the aures region. J. New Technol. Mater. 2020, 10, 38–46. [Google Scholar] [CrossRef]
- Khadijah, A.J.; Pigott, M.; Sheridan, H.; Walsh, J.J. Mediterranean Basin Erica Species: Traditional Uses, Phytochemistry and Pharmacological. Molecules 2025, 30, 2616. [Google Scholar] [CrossRef]
- Días, P.; Alice, M.; Figueiredo, A.C.; Rauter, A.P. Flower Colour and Essential Oil Composition in Erica australis L. Grown in Portugal. J. Essent. Oil-Bear. Plants 2016, 19, 1013–1018. [Google Scholar] [CrossRef]
- Ickovski, J.; Stepic, K.D.; Stojanovic, G.S. Composition of essential oils and headspace constituients of Artemisia annua L. and A. scoparia Waldst. Et Kit. J. Serbian Chem. Soc. 2020, 85, 1565–1575. [Google Scholar] [CrossRef]
- Hcini, K.; Sotomayor, J.A.; Jordan, M.J.; Bouzid, S. Chemical Composition of the Essential Oil of Rosemary (Rosmarinus officinalis L.) of Tunisian Origin. Asian J. Chem. 2013, 25, 2601–2603. [Google Scholar] [CrossRef]
- Zidane, H.; Elmiz, M.; Aouinti, F.; Tahani, A.; Wathelet, J.; Sindic, M.; Elbachiri, A. Chemical composition and antioxidant activity of essential oil, various organic extracts of Cistus ladanifer and Cistus libanotis growing in Eastern Morocco. Afr. J. Biotechnol. 2013, 12, 5314–5320. [Google Scholar] [CrossRef]
- Mediavilla, I.; Blázquez, M.A.; Ruiz, A.; Esteban, L.S. Influence of the Storage of Cistus ladanifer L. Bales from Mechanised Harvesting on the Essential Oil Yield and Qualitative Composition. Molecules 2021, 26, 2379. [Google Scholar] [CrossRef]
- Pérez-Izquierdo, C.; Serrano-Pérez, P.; Rodríguez-Molina, M.C. Chemical composition, antifungal and phytotoxic activities of Cistus ladanifer L. essential oil and hydrolate. Biocatal. Agric. Biotechnol. 2022, 45, 102527. [Google Scholar] [CrossRef]
- Gomes, P.B.; Mata, V.G.; Rodrigues, A.E. Characterization of the Portuguese-Grown Cistus ladanifer Essential Oil. J. Essent. Oil Res. 2005, 17, 160–165. [Google Scholar] [CrossRef]
- Robles, C.; Garzino, S. Essential oil composition of Cistus albidus leaves. Phytochemistry 1998, 48, 1341–1345. [Google Scholar] [CrossRef]
- Angelopoulou, D.; Demetzos, C.; Perdetzoglou, D. Diurnal and seasonal variation of the essential oil labdanes and clerodanes from Cistus monospeliensis L. leaves. Biochem. Syst. Ecol. 2002, 30, 189–203. [Google Scholar] [CrossRef]
- Cucu, A.-A.; Baci, G.-M.; Cucu, A.-B.; Dezsi, Ş.; Lujerdean, C.; Hegeduş, I.C.; Bobiş, O.; Moise, A.R.; Dezmirean, D.S. Calluna vulgaris as a Valuable Source of Bioactive Compounds: Exploring Its Phytochemical Profile, Biological Activities and Apitherapeutic Potential. Plants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Zhao, J. The Extraction of High Value Chemicals from Heather (Calluna vulgaris) and Bracken (Pteridium aquilinum). Available online: https://etheses.whiterose.ac.uk/id/eprint/2019/2/Final_Thesis_of_Jiewen_Zhao.pdf (accessed on 12 July 2025).
- Mimica-Dukić, N.; Bugarin, D.; Grbović, S.; Mitić-Ćulafić, D.; Vuković-Gačić, B.; Orčić, D.; Jovin, E.; Couladis, M. Essential Oil of Myrtus communis L. as a Potential Antioxidant and Antimutagenic Agents. Molecules 2010, 15, 2759–2770. [Google Scholar] [CrossRef]
- Gardeli, C.; Papageorgiou, V.; Mallouchos, A.; Theodosis, K.; Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P.; Angioni, A. Characterization of the Volatile Constituents in the Essential Oil of Pistacia lentiscus L. from different Origins and its Antifungal and Antioxidant Activity. J. Agric. Food Chem. 2007, 55, 7093–7098. [Google Scholar] [CrossRef]
- Bandeira Reidel, R.V.; Melai, B.; Cioni, P.; Flamini, G.; Pistelli, L. Aroma profile of Rubus ulmifolius flowers and fruits during different ontogenetic phases. Chem. Biodivers. 2016, 13, 1776–1784. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Benzarti, A.; Marongiu, B.; Maxia, A.; Piras, A.; Salgueiro, L. Antifungal and anti-inflammatory potential of Lavandula stoechas and Thymus herba-barona essential oils. Ind. Crops Prod. 2013, 44, 97–103. [Google Scholar] [CrossRef]
- Dob, T.; Dahmane, D.; Agli, M.; Chelghoum, C. Essential Oil Composition of Lavandula stoechas from Algeria. Pharm. Biol. 2006, 44, 60–64. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical Composition, Seasonal Variability, and Antifungal Activity of Lavandula stoechas L. ssp. Stoechas Essential Oils from Stem/Leaves and Flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [CrossRef]
- Hassiotis, C.N. Chemical compounds and essential oil release through decomposition process from Lavandula stoechas in Mediterranean region. Biochem. Syst. Ecol. 2010, 38, 493–501. [Google Scholar] [CrossRef]
- Delgado-Adámez, J.; Garrido, M.; Bote, M.E.; Fuentes-Pérez, M.C.; Espino, J.; Martín-Vertedor, D. Chemical composition and bioactivity of essential oils from flower and fruit of Thymbra capitata and Thymus species. J. Food Sci. Technol. 2017, 54, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Cutillas, A.B.; Carrasco, A.; Martínez-Gutiérrez, R.; Tomas, V.; Tudela, J. Thymus mastichina L. essential oils from Murcia (Spain): Composition and antioxidant antienzymatic and antimicrobial bioactivities. PLoS ONE 2018, 13, e0190790. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Muras, M.; Puig, C.G.; López-Nogueira, A.; Cavalereiro, C.; Pedrol, N. On the bioherbicide potential of Ulex europaeus and Cytisus scoparius: Profile of volatile organic compounds and their phytotoxic effects. PLoS ONE 2018, 13, e0205997. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hu, Q.; Wang, S.; Tao, L.; Hu, X.; Shen, X. 1,8-Cineole ameliorates endothelial injury and hypertension induced by L-NAME through regulation of autophagy via PI3K/mTOR signaling pathway. Eur. J. Pharmacol. 2023, 954, 175863. [Google Scholar] [CrossRef]
- Ma, R.; Lu, D.; Wang, J.; Xie, Q.; Guo, J. Comparation of pharmacological activity and safety of different sterochemical configurations of borneol: L-borneol, D-borneol, and synthetic borneol. Biomed. Pharmacother. 2023, 164, 114668. [Google Scholar] [CrossRef] [PubMed]
- Hoch, C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Dezfouli, A.B.; Wollenberg, B. 1,8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef]
- Sobhy, M.; Ali, S.S.; Cui, H.; Lin, L.; El-Sapagh, S. Exploring the potential of 1,8-cineole from cardamom oil against food-borne pathogens: Antibacterial mechanisms and its application in meat preservzation. Microb. Pathog. 2023, 184, 106375. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Zuzarte, M.; Marques, C.; Viana, S.; Preguiça, I.; Baptista, R.; Ferreira, C.; Cabaleiro, C.; Domingues, N.; Sardao, V.; et al. 1,8-Cineole ameliorates right ventricle dysfunctions associated with pulmonary arterial hypertension by restoring connexin43 and mitochondrial homeostasis. Pharmacol. Res. 2022, 180, 106151. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.A.; Baptista, N.M.Q.; de Oliveira, A.P.S.; da Silve, P.A.; Gusmao, N.B.; Correia, M.T.d.S.; Napoleao, T.H.; da Silva, M.V.; Paiva, P.M.G. Insecticidal activity of a chemotype VI essential oil from Lippia alba leaves collected ad Caatinga and the major compound (1,8-cineole) against Nasutitermes corniger and Sitophilus zeamais. Pestic. Biochem. Physiol. 2021, 177, 104901. [Google Scholar] [CrossRef]
- Paoline, J.; Tomi, P.; Bernardini, F.; Bradesi, P.; Casanova, J.; Kaloustian, J. Detailed analysis of the essential oil from Cistus albidus L. by combination of GC/RI, GC/MS, and 13C-NMR. Prod. Res. 2008, 22, 1270–1278. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, T.; Chen, Y.; Yan, X.; Wu, W.; Zhang, S.; Li, Z. α-Terpineol affects social inmmunity, increasing the pathogenicity of entomopathogenic nematodes to subterranean termites (Isoptera). Pestic. Biochem. Physiol. 2023, 196, 105621. [Google Scholar] [CrossRef]
- Ding, Q.; Zhuang, T.; Fu, P.; Zhou, Q.; Luo, L.; Dong, Z.; Li, H.; Tang, S. Alpha-terpineol grafted acetylated lentinan as an anti-bacterial adhesion agent. Carbohydr. Polym. 2022, 277, 118825. [Google Scholar] [CrossRef]
- An, P.; Yang, X.; Yu, Y.J.; Qi, J.; Ren, X.; Kong, Q. α-terpineol and terpene-4-ol, the critical components of tree oil, exert antifungal activities in vitro and in vivo against Aspergillus niger in grapes by inducing morphous damage and metabolic changes of fungus. Food Control. 2019, 98, 42–53. [Google Scholar] [CrossRef]
- Deen, J.I.; Zawad, A.N.M.S.; Uddin, M.; Chowdhury, M.A.H.; Al Araby, S.Q.; Rahman, M.A. Terpinen-4-ol, A volatile terpene molecule, extensively electrifies the biological systems against the oxidative stress-linked pathogenesis. Adv. Redox Res. 2023, 9, 100082. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Yue, S.; Chen, X.; Huang, Y.; Cao, H.; Liao, M. Role of CYP6MS subfamily in detoxification of Sitophilus zeamais after exposure to terpinene-4-ol and limonene. Pestic. Biochem. Physiol. 2023, 193, 105426. [Google Scholar] [CrossRef]
- Gao, S.S.; Zhang, Y.; Zhang, K.; Wang, X.; Tang, Q.; Zhang, Y. Contact toxicology and transcriptomic analysis of terpinene-4-ol exposure in Tribolium casteneum. J. Asia-Pac. Entomol. 2022, 25, 101950. [Google Scholar] [CrossRef]
- Yousafi, Q.; Shahzad, M.S.; Saleem, S.; Sajid, M.W.; Hussain, A.; Mehmood, A.; Abid, A.D.; Qandel, A.; Shahid, A.; Khan, M.S.; et al. Terpinen-4-ol from Trachypermum ammi is a potential and safer candidate molecule for fungicide development against Alternaria solani. J. King Saud Univ. 2022, 34, 101747. [Google Scholar] [CrossRef]
- Gondim, A.N.S.; Lara, A.; Santos-Miranda, A.; Roman-Campos, D.; Lauton-Santos, S.; Menezes-Filho, J.E.R.; de Vasconcelos, C.M.L.; Conde-Garcia, E.A.; Guatimosim, S.; Cruz, J.S. (-)-Terpinen-4-ol changes intracelular Ca2+ handling and induces pacing disturbace in rato hearts. Eur. J. Pharmacol. 2017, 807, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Benelli, G. Larvicidal potential of carvacrol and terpinene-4-ol from the essential oil of Origanum vulgare (Lamiaceae) against Anopheles stephensi, Anopheles subpictus, Culex quinquefasciatus and Culex tritaeniorhynchus (Diptera: Culicidae). Res. Vet. 2016, 104, 77–82. [Google Scholar] [CrossRef]
- Fernandes, H.d.B.; Ciriaco, S.L.; Filgueiras, L.A.; Barros, I.C.; Carvalho, A.L.M.; Rolim, H.M.L.; de Souza, M.N.; Pinto, J.C.C.d.S.; Mendes, A.N.; Oliveira, R.d.C.M. Gastrotective effect of α-terpineol-loaded polymethyl methacrylate particles on gastric injury model. J. Drug Deliv. Sci. Technol. 2022, 67, 102989. [Google Scholar] [CrossRef]
- Kim, I.-K.; Kim, B.; Song, B.-W.; Kim, S.W.; Kim, D.; Kang, J.H.; Hwang, S.H.; Hwang, K.; Lee, S. Borneol facilitates the whitening and anti-wrinkle effect of the essential oil extracted from Abies koreana needles. J. King Saud Univ. 2023, 35, 102886. [Google Scholar] [CrossRef]
- Li, H.; Liao, H.; Li, Y.; Qi, Y.; Ni, H.; Zou, Z.; Liu, Z. Chemical composition and antifungal activity of Cinnamomum camphora chvar. Borneol essential oil obtained using solvent-free microwave-assisted method. Arab. J. Chem. 2023, 16, 104996. [Google Scholar] [CrossRef]
- Mei, Y.; Li, L.; Fan, L.; Fan, W.; Liu, L.; Zhang, F.; Hu, Z.; Wang, K.; Yang, L.; Wang, Z. The history, stereochemistry, ethnopharmacology and quality assessment of borneol. J. Ethnopharmacol. 2023, 300, 115697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, S.-L.; Hui, W.; Shi, L.-Y.; Li, J.-P.; Jia, L.-J.; Xie, B.-P. The effects of borneol on the pharmacokinetics and brain distribution of tanshione IIA, salvianolic acid B and ginsenoside Rg1 in Fufang Danshen preparation in rats. Chem. J. Nat. Med. 2021, 19, 153–160. [Google Scholar] [CrossRef]
- Liu, S.; Long, Y.; Yu, S.; Zhang, D.; Yang, O.; Ci, Z.; Cui, M.; Zhang, Y.; Wan, J.; Li, D.; et al. Borneol in cardio-cerebrovascular diseses: Pharmacological actions, mechanisms, and therapeutics. Pharmacol. Res. 2021, 169, 105627. [Google Scholar] [CrossRef]
- Schandry, R.; Duschek, S. The effect of Camphor-Crataegus berry extract combination on blood pressure and mental functions in chronic hypotension-A randomized placebo controlled double blind design. Phytomedicine 2008, 15, 914–922. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M. Camphor (Cinnamomum camphora), a traditional remedy with the history of treating several diseases. Int. J. Case Rep. Images 2013, 4, 86–89. [Google Scholar] [CrossRef]
- Zochedh, A.; Priya, M.; Shunmuganarayanan, A.; Thandavarayan, K.; Sultan, A.B. Investigation on structural, spectrocopic, DFT, biological activity and molecular docking simulation of essential oil Gamma-Terpinene. J. Mol. Struct. 2022, 1268, 133651. [Google Scholar] [CrossRef]
- Qi, C.; Zhao, H.; Li, W.; Haiying, X.; Zhang, G.; Liu, H.; Wanf, Q.; Wang, Y.; Xian, M.; Zhang, H. Production of γ-terpinene by metabolically engineered Escherichia coli using glycerol as feedstock. RCS Adv. 2018, 8, 30851–30859. [Google Scholar] [CrossRef]
- Irwan, A.; Humaida, N.; Nur, H.S. Antibacterial activity assay of essential oils from limau kuit peel against Staphylococcus aureus. IOP Conf. Ser. Mater. Eng. 2020, 980, 012026. [Google Scholar] [CrossRef]
- Bailén, M.; Díaz-Castellanos, I.; Azami-Conesa, I.; Fernández, S.A.; Martínez-Díaz, R.A.; Nvarro-Rocha, J.; Gómez-Muñoz, T.; González-Coloma, A. Anti-Trichomonas gallinae activity of essential oils and main compounds from Lamiaceae and Asterqaceae plants. Front. Vet. Sci. 2022, 9, 981763. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, H.; Chen, H.; Zhong, B.; Luo, X.; Chun, J. Antioxidant and anticancer activities of essential oil from gannan navel orange peel. Molecules 2017, 22, 1391. [Google Scholar] [CrossRef]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Quian, B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef]
- Diptikanta, P.; Smaranika, P.A.K.B. Growth-arresting Activity of Acmella Essential Oil and its Isolated Component D-Limonene (1,8 p-mentha diene) against Trichophyton rubrum (microbial Type Culture Collection 296). Pharmacogn. Mag. 2019, 15, 38–46. [Google Scholar] [CrossRef]
- Alexa, V.T.; Szuhanek, C.; Cozma, A.; Galuscan, A.; Borcan, F.; Obistioiu, D.; Dehelean, C.A.; Jumanca, D. Natural Preparations Based on Orange, Bergamot and Clove Essential Oils and Their Chemical Compounds as Antimicrobial Agents. Molecules 2020, 25, 5502. [Google Scholar] [CrossRef]
- Durço, A.O.; de Souza, D.S.; Heimfarth, L.; Miguel-dos Santos, R.; Rabelo, T.K.; de Oliveira Barreto, T.; Rhana, P.; Santos, S.M.N.; Braga, W.F.; Santos, C.J.; et al. D-Limonene Ameliorates in a Murine Model. J. Nat. Prod. 2019, 82, 3010–3019. [Google Scholar] [CrossRef]
- Praveen, K.M.; Poornima, M.E.; Al-Ghanim, K.; AL-Misned, F.; Ahmed, Z.; Mahboob, S. Effects of D-Limonene on aldose reductase and protein glycation in diabetic rats. J. King Saud Univ.-Sci. 2020, 32, 1953–1958. [Google Scholar] [CrossRef]
- Lorigooini, Z.; Boroujeni, S.N.; Sayyadi-Shahraki, M.; Rahimi-Madiseh, M.; Bijad, E.; Amini-khoei, H. Limonene through Attenuation of Neuroinflammation and Nitrite Level Exerts Antidepressant-Like Effect on Mouse Model of Maternal Separation Stress. Behav. Neurol. 2021, 8817309. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, V.; Sharmin, A.M.; Anand, M.A.V.; Sivakumar, S.; Surendhiran, D.; Sharesh, S.K. Fabrication and bacterial inhibitory activity of essential oil linalool loaded biocapsules against Escherichia coli. J. Drug Deliv. Sci. Technol. 2002, 74, 103495. [Google Scholar] [CrossRef]
- Azirak, S.; Özgöçmen, M. Linalool prevents kidney damage by inhibiting rifampicin-induced oxidative stress and apoptosis. Tissue Cell 2023, 82, 102097. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, S.; Deng, W. Dual responsive linallol capsules with high loading ratio form excellent antioxidant and antibacterial efficiency. Colloids Surf. B Biointerfaces 2020, 190, 110978. [Google Scholar] [CrossRef]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool bioactive properties and potential applicability in drug delivery sustems. Colloids Surf. B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, S.M.; Min, J.H.; Kwon, O.K.; Park, M.H.; Park, J.W.; Ahn, H.I.; Hwang, J.Y.; Oh, S.R.; Lee, J.W.; et al. Anti-inflamatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 2019, 74, 105706. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Hobbie, F.; Keerthi, A.; Oun, A.; Kortholt, A.; Boddeke, E.; Dolga, A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed. Pharmacother. 2019, 118, 109295. [Google Scholar] [CrossRef]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El MEnyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef]
- Formiga, R.d.O.; Júnior, E.B.A.; Vasconcelos, R.C.; Araújo, A.A.; de Carvalho, T.G.; Junior, R.F.d.A.; Guerra, G.B.C.; Vieira, G.C.; de Oliveira, K.M.; Diniz, M.d.F.F.; et al. Effect of p-cymene and rosmarinic acid on gastric ulcer healing-Involvement of multiple endogenous curative mechanisms. Phytomedicine 2021, 86, 153497. [Google Scholar] [CrossRef]
- do Rosario, V.G.B.; Marszaukowski, F.; Guimaraes, I.D.; Maranha, F.G.; Mika, B.F.; Rosa, G.B.; Pessoa, C.A.; Ribeiro, R.A.P.; Inaba, J.; Boeré, R.T.; et al. Synthesis, structure and biological evaluation as antibacterial agents of Ru (II)-p-cymene-aruldicyclohexulphospine complexes. Inorganica Chim. Acta 2023, 558, 121749. [Google Scholar] [CrossRef]
- Feng, Y.X.; Zhang, X.; Wang, Y.; Chen, Z.Y.; Lu, X.X.; Du, Y.S.; Du, S.S. The potential contribution of cymene isomers to insecticidal and repellent acivities of the essential oil from Alpine zerumbet. Int. Biodeterior. Biodegrad. 2021, 157, 105138. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, B.; Dasgupta, S.; Seal, I.; Sil, S.; Roy, S. Inhibition of stemness and EMT by taxifolin ruthenium-p-cymene complex via downregulating the SOX2 and OCT4 expression on lung cancer. Arab. J. Chem. 2023, 16, 104995. [Google Scholar] [CrossRef]
- Yilmazoglu, E.; Akgüen, M. p-cymene production from orange peel oil using metal catalyst in supercritical alcohols. J. Supercrit. Fluids 2018, 131, 37–46. [Google Scholar] [CrossRef]
- Pelkonen, O.; Abass, K.; Wiesner, J. Thujone and thujone-containing herbal medicinal and botanical products: Toxicological assessment. Regul. Toxicol. Pharmacol. 2013, 65, 100–107. [Google Scholar] [CrossRef]
- Németh, E.Z.; Nguyen, H.T. Thujone, a widely debated volatile compound: What do we know about it? Phytochem. Rev. 2020, 19, 405–423. [Google Scholar] [CrossRef]
- Rahimi, K.; Zalaghi, M.; Shehnizad, E.G.; Salari, G.; Baghdezfoli, F.; Ebrahimifar, A. The effects of alpha-pinene on inflammatory responses and oxidative stress in the formalin test. Brain Res. Bull. 2023, 203, 110774. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Wang, X.; Wang, F.; Li, X. Efficient production of α-pinene through identifying the rate-limiting enzymes and tailoring inactive terminal of pinene synthase in Escherichia coli. Fuel 2023, 343, 127872. [Google Scholar] [CrossRef]
- Santos, E.S.; de Sousa Machado, S.T.; Rodrigues, F.B.; da Silva, L.C.X.; Lopes, M.J.P.; Gomes, A.D.S.; Ribeiro, T.F.; Garcia, F.A.d.O.; Coutinho, H.D.M.; Felipe, C.F.B.; et al. Potential anti-inflamatory, hypoglycemic, and hypolipidemic activities of alpha-pinene in diabetic rats. Process Biochem. 2023, 126, 80–86. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef] [PubMed]
- Ensaka, N.; Sakamoto, K. α-Pinene odor exposure enhances heat stress tolerance through Daf-16 in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2020, 528, 726–731. [Google Scholar] [CrossRef]
- Rodrigues, P.R.; Wang, X.; Li, Z.; Wang, W.; Vieira, R. A new nano hyperbranched β-pinene polymer: Controlled synthesis and nonviral gene delivery. Colloids Surf. 2023, 222, 113032. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; L.D. Jayaweera, S.; A. Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Kovac, J.; Simunovic, K.; Wu, Z.; Klancnik, A.; Bucar, F.; Zhang, Q.; Mozina, S.S. Antibiotic Resistence Modulation and Modes of Action of (-)-α-Pinene in Campylobacter jejuni. PLoS ONE 2015, 10, e0122871. [Google Scholar] [CrossRef]
- Lakhdari, A.; Sakhri, L.; Khane, Y.; Lakhdar, A.M.; Kemassi, A.; Bouras, N. Evaluation of drying effect on the composition of the essential oil isolated from aerial parts of Pituranthos chloranthus from southern Algeria and ther biological activities. Biocatal. Agric. Biotechnol. 2020, 30, 101844. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Cao, Y.; Feng, X.; Zheng, Y.; Zou, H.; Liu, H.; Yang, J.; Xian, M. Microbial productions of sabinene-a new terpene-based precursor of advanced biofuel. Microb. Cell Factories 2014, 13, e0122871. [Google Scholar] [CrossRef]
- Wu, T.; Liu, J.; Li, M.; Zhang, G.; Liu, L.; Li, X.; Men, X.; Xian, M.; Zhang, H. Improvement of sabinene tolerance of Escherichia coli usig adaptive laboratory. Biotechnol. Biofuels 2020, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene-what are the potential Health Benefits of this Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef] [PubMed]
- López, P.L.; Guerberoff, G.K.; Grosso, N.R.; Olmedo, R.H. Antioxidant-efficient indicator determinate by the relationship between β-myrcene/caryophyllene (α,β) on hop (humulus lupulus) essential oils under an accelerated oxidation test. Ind. Crops Prod. 2023, 205, 117399. [Google Scholar] [CrossRef]
- Che, J.; Chen, Y.; Li, L.; Nengguo, T. Metabolomics analysis reveals that myrcene stimulates the spore germination of Penicillium digitatum via the upregulation of central carbon and energy metabolism. Posthaverst Biol. Technol. 2020, 170, 111329. [Google Scholar] [CrossRef]
- Poléc, K.; Broniatowski, M.; Wydro, P.; Hac-Wydro, K. The impacto of β-myrcene-the main component of the hop essential oil-on the lipid films. J. Mol. Liq. 2020, 308, 113028. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Sharna, N.; Khurana, N. Ameliorative effect of myrcene in mouse model of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 911, 174529. [Google Scholar] [CrossRef]
- Iscan, G.; Kirimer, N.; Demirci, F.; Base, K.H.C. Microbial Transformation of β-phellandrene. Planta Medica 2011, 77, 1278. [Google Scholar] [CrossRef]
- Buitrago, D.; Velasco, J.; Díaz, T.; Morales, A. Chemical composition and antibacterial activity of the essential oil of Monticalia Imbricatifolia Schultz (Asteraceae). Rev. Latinoam. Química 2012, 40, 13–18. [Google Scholar]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Sharma, C.; Kaabi, J.M.A.; Nurulain, S.M.; Goyal, S.N.; Amjad, K.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of β-caryophyllene: A dietary phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 1–28. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strzadala, L.; Szumny, A. β-caryophyllene and β-caryophylleneoxide-natural compunds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Serra, M.P.; Boi, M.; Carta, A.; Murru, E.; Carta, G.; Banni, S.; Quartu, M. Anti-Inflammatory Effect of Beta-Caryophyllene Mediated by the Involvement of TRPV1, BDNF and trkB in the Rat Cerebral Cortex after Hypoperfusion/Reperfusion. Int. J. Mol. Sci. 2022, 23, 3633. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef] [PubMed]
- Alkufeidy, M.; Dunia, A.; Farrai, A.; Reen, M.; Aljowaie, M.; Ajmal, A.; Elshikh, M.S. Chemical composition of Thymus vulgaris extracts and antibacterial activity against pathogenic multidrug resistance bacteria Roua. Physiol. Mol. Plant Pathol. 2022, 117, 101745. [Google Scholar] [CrossRef]
- Al-Bayati, F.A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J. Ethnopharmacol. 2008, 116, 403–406. [Google Scholar] [CrossRef]
- Caramelo, D.; Barroca, C.; Guiné, R.; Gallardo, E.; Anjos, O.; Gominho, J. Potential Applications of the Cytisus Shrub Species: Cytisus multiflorus, Cytisus scoparius, and Cytisus striatus. Processes 2022, 10, 1287. [Google Scholar] [CrossRef]
- Araruna, M.E.; Serafim, C.; Alves Júnior, E.; Hiruma-Lima, C.; Diniz, M.; Batista, L. Intestinal anti-inflammatory activity of terpenes in experimental models (2010–2020): A review. Molecules 2020, 25, 5430. [Google Scholar] [CrossRef]
- Martínez-Solis, I.; Sanahuja, M.A.; Moreno, L.; Olivar, T.; Castillo, E.; Zagotto, G.; Soriano, P. Medicinal Potential from Plant Biodiversity in a Mediterranean Scrubland. J. Biodivers. Bioprospecting J. Dev. 2014, 1, 1000111. [Google Scholar] [CrossRef]
- Abadi, I.; El Ayadi, R.; Bentayeb, A.; Aaziz, H.; Bouymajane, A.; Alemini, A.B.; Cacciola, F.; El Ibaoui, H. Phytochemical profile, in vivo anti-inflamatory and wound healing activities of the acqueous extract from aerial parts of Cistus ladanifer L. J. Pharm. Biomed. Anal. 2022, 249, 114960. [Google Scholar] [CrossRef]
- Aslam, S.; Younis, W.; Malik, M.N.N.; Jahan, S.; Alamgeer; Uttra, A.M.; Munir, M.U.; Roman, M. Pharmacological evaluation of anti-arthritic potential of terpinene-4-ol using in vitro and in vivo assays. Inflammopharmacology 2022, 30, 945–959. [Google Scholar] [CrossRef]
- Ngenge, A.T.; Kucukaydin, S.; Ceylan, O.; Duru, M.E. Evaluation of enzyme inhibition and anti-quorum sensing potentials of Melaleuca alternifolia and Citrus sinensis essential oils. Nat. Prod. Commun. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. In vitro neuroprotective potential of the monoterpenes a-pinene and 1,8-cineole against H2O2-induced stress in PC12 cells. Z. Naturforschung C 2016, 71, 191–199. [Google Scholar] [CrossRef]
- Khoshnazar, M.; Parvardeh, S.; Bigdeli, R. Alpha-pinene exerts neuroprotective effects via anti-inflamatory and antiapoptotic mechanisms in a rat model of focal cerebral ischemia-reperfusion. J. Stroke Cerebrovasc. Dis. 2020, 29, 104997. [Google Scholar] [CrossRef]
- Han, M.; Liu, Y.; Zhang, B.; Qiao, J.; Lu, W.; Zhu, Y.; Wang, Y.; Zhao, C. Salvianic borneol ester reduces b-amyloid oligomers and prevents cytoxicity. Pharm. Biol. 2011, 49, 1008–1013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sammi, S.R.; Trivedi, S.; Rath, S.K.; Nagar, A.; Tandon, S.; Kalra, A.; Pandey, R. 1-Methyl-4-propan-2-ylbenzene from Thymus vulgaris Attenuates Cholinergic Dysfunction. Mol. Neurobiol. 2017, 54, 5468–5481. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef]
- Chaves, N.; Nogales, L.; Montero-Fernández, I.; Blanco-Salas, J.; Alías, J.C. Mediterranean Shrub Species as a Source of Biomolecules against Neurodegenerative Diseases. Molecules 2023, 28, 8133. [Google Scholar] [CrossRef]
- Sales, A.; de Oliveira Felipe, L.; Bicas, J.L. Production, properties and applications of α-terpineol. Food Bioprocess. Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Charitos, I.A.; D’Agostino, D.; Topi, S.; Bottalico, L. 40 Years of Helicobacter pylori: A revolution in biomedical thought. Gastroenterol. Insights 2021, 12, 111–135. [Google Scholar] [CrossRef]
- Oztekin, M.; Yilmaz, B.; Agagunduz, D.; Capasso, R. Overview of Helicobacter pylori infection: Clinical features, treatment and nutritional aspects. Diseases 2021, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hurtado, P.A.; Garduno-Siciliano, L.; Domínguez-Verano, P.; Martinez-Calero, E.; Canales-Martínez, M.A.; Rodríguez-Monroy, M.A. Evaluation of the gastroprotective effects of Chihuahua propolis on indomethacin induced gastric ulcers in mouse. Biomed. Pharmacother 2021, 137, 111345. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Chellappon, D.K.; Kikuchi, I.S.; Pinto, T.J.A.; Pabreja, K.; Agrawal, M.; Yogendra, S.; Tiwari, J.; Dua, K. Nephrotoxicity in Rats Exposed to Paracetamol: The Protective Role of Moralbosteroid, a Steroidal Glycoside. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 113–119. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Weiss, B.D.; Weiss, E.C.; Haggard, W.O.; Evans, R.P.; McLaren, S.G.; Smeltzer, M.S. Optimized elution of daptomycin from polymethylmethacrylate beads. Antimicrob. Agents Chemother. 2009, 53, 254–266. [Google Scholar] [CrossRef]
- Neto, J.C.; Tenorio, P.A.; Rodrigues, A.K.B.F.; Galvao, J.C.; Bernardino, A.C.; Oliveira, J.M.S.; Nascimento, T.G.; Oliveira, W.S.; Santos, J.C.C.; Smaniotto, S.; et al. Cardioprotective effect of hydroalcoholic extract of Brazilian red propolis against isoproterenol-induced myocardial infarction in rats. Phytomedicine Plus 2022, 2, 100190. [Google Scholar] [CrossRef]
- Kübler, W.; Haass, M. Cardioprotection: Definition, classification and fundamental principles. Heart 1996, 75, 330–333. [Google Scholar] [CrossRef]
- Fan, Y.H.; Xie, H.X.; Xin, P.; Mao, J.X.; Zhang, Y.Y.; Li, X.J. Analysis of the rule of medication for internal administration of gout by traditional Chinese medicine based on Traditional Chinese Medicine Inheritance System. Food Ther. Health Care 2020, 2, 64–78. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kang, J.; Park, I.K. Fumigant toxicity of Apiaceae essential oils and their constituents against Sitophilus oryzae and their acetylcholinesterase inhibitory activity. J. Asia-Pac. Entomol. 2013, 16, 443–448. [Google Scholar] [CrossRef]
- Smith, G.H.; Roberts, J.M.; Pope, T. Terpene based biopesticides as potential alternatives to synthesis insecticides for control of aphid on protected ornamentals. Crop Prot. 2018, 110, 125–130. [Google Scholar] [CrossRef]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopest. Int. 2008, 4, 63–84. [Google Scholar]
- Herrera, J.M.; Pizzolitto, R.P.; Zunino, M.P.; Dambolena, J.S.; Zygadlo, J.A. Effect of fungal volatile organic compounds on a fungus and an insect that damage stored maize. J. Stored Prod. Res 2015, 62, 74–80. [Google Scholar] [CrossRef]
- Yildirim, E.; Emsen, B.; Kordali, S. Insecticidal effects of monoterpenes on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Appl. Bot. Food Qual. 2013, 86, 198–204. [Google Scholar] [CrossRef]
- Chaubey, M.K. Fumigant toxicity of essential oils and pure compounds against Sitophilus oryzae L. (Coleoptera: Curculionidae). Biol. Agric. Hortic. 2012, 28, 111–119. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.; John, B.; Mark, D.; Alan, L.; Raj, B.; Michael, F. Effect of orange oil extract on the Formosan subterranean termite (Isoptera: Rhinotermitidae). J. Econ. Entomol. 2007, 3, 880–885. [Google Scholar] [CrossRef]
- Hollingswort, R.G. Limonene, a Citrus Extract, for Control of Mealybugs and Scale Insects. J. Econ. Entomol. 2005, 3, 772–779. [Google Scholar] [CrossRef]
- Nirmal, P.; Mereddy, R.; Sultanbawa, Y. Formulation, characterization and antibacterial activity of lemon myrtle and anise myrtle essential oil in water nanoemulsion. Food Chem. 2018, 254, 1–7. [Google Scholar] [CrossRef]
- Fei, T.; Gwinn, K.; Leyva-Gutierrrez, F.M.A.; Wang, T. Nanoemulsions of terpene by-products from cannabidiol production have promising insecticidal effect on Callosobruchus maculatus. Heliyon 2023, 9, e15101. [Google Scholar] [CrossRef]
- Levchenko, M.A.; Silivanova, E.A.; Khodakov, P.E.; Gholizadeh, S. Insecticidal efficacy of some essential oils against adults of Musca domestica L. (Diptera: Muscidae). Int. J. Trop. Insect Sci. 2021, 41, 2669–2677. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).