Anti-Helicobacter pylori Activity and Gastroprotective Effects of Diacetylcurcumin and Four Metal Derivatives
Abstract
1. Introduction
2. Results
2.1. Anti-Helicobacter pylori Activity
Activity Versus Other Bacteria
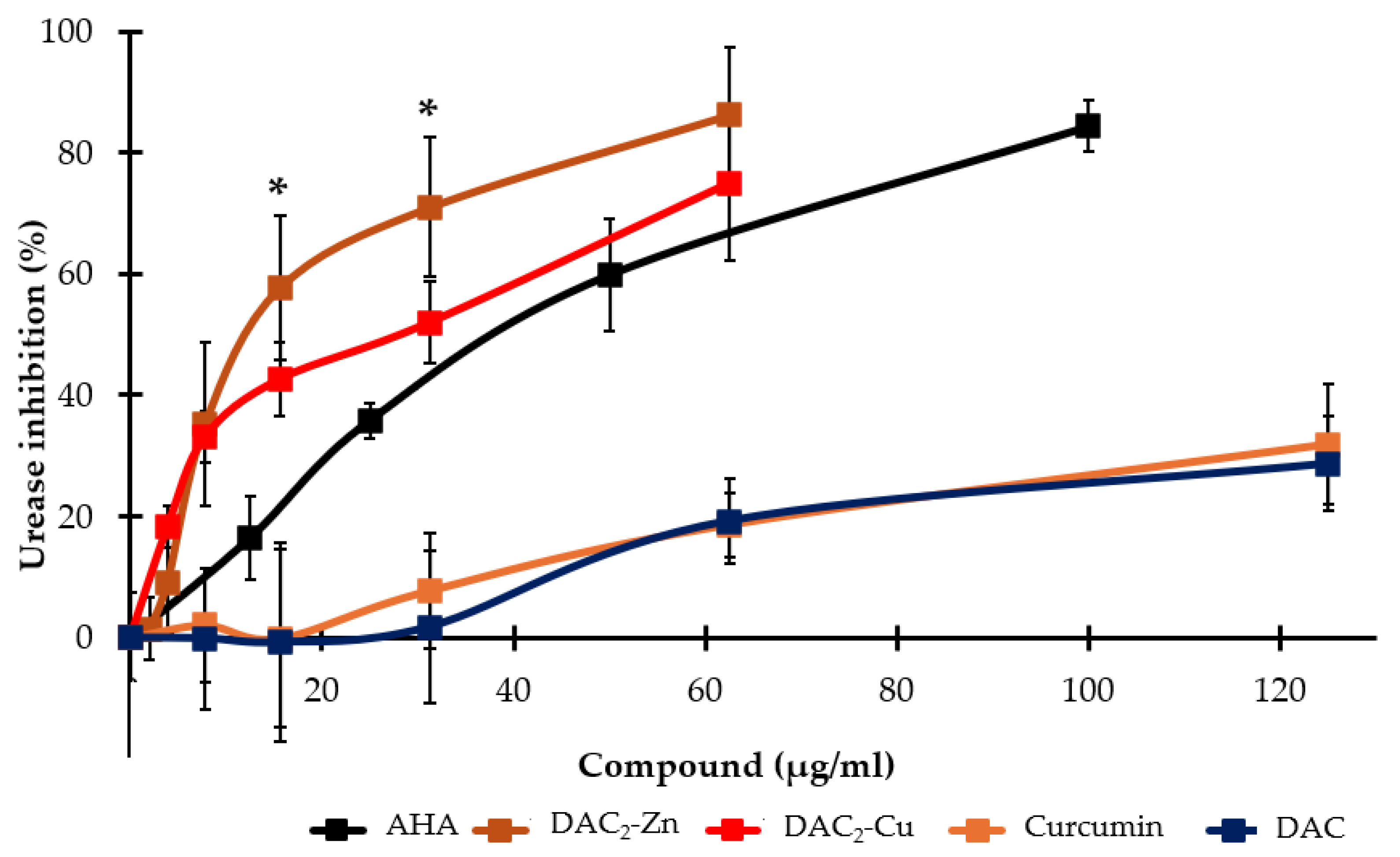
2.2. Helicobacter pylori Urease Inhibition
2.3. Effect on DNA Gyrase Activity
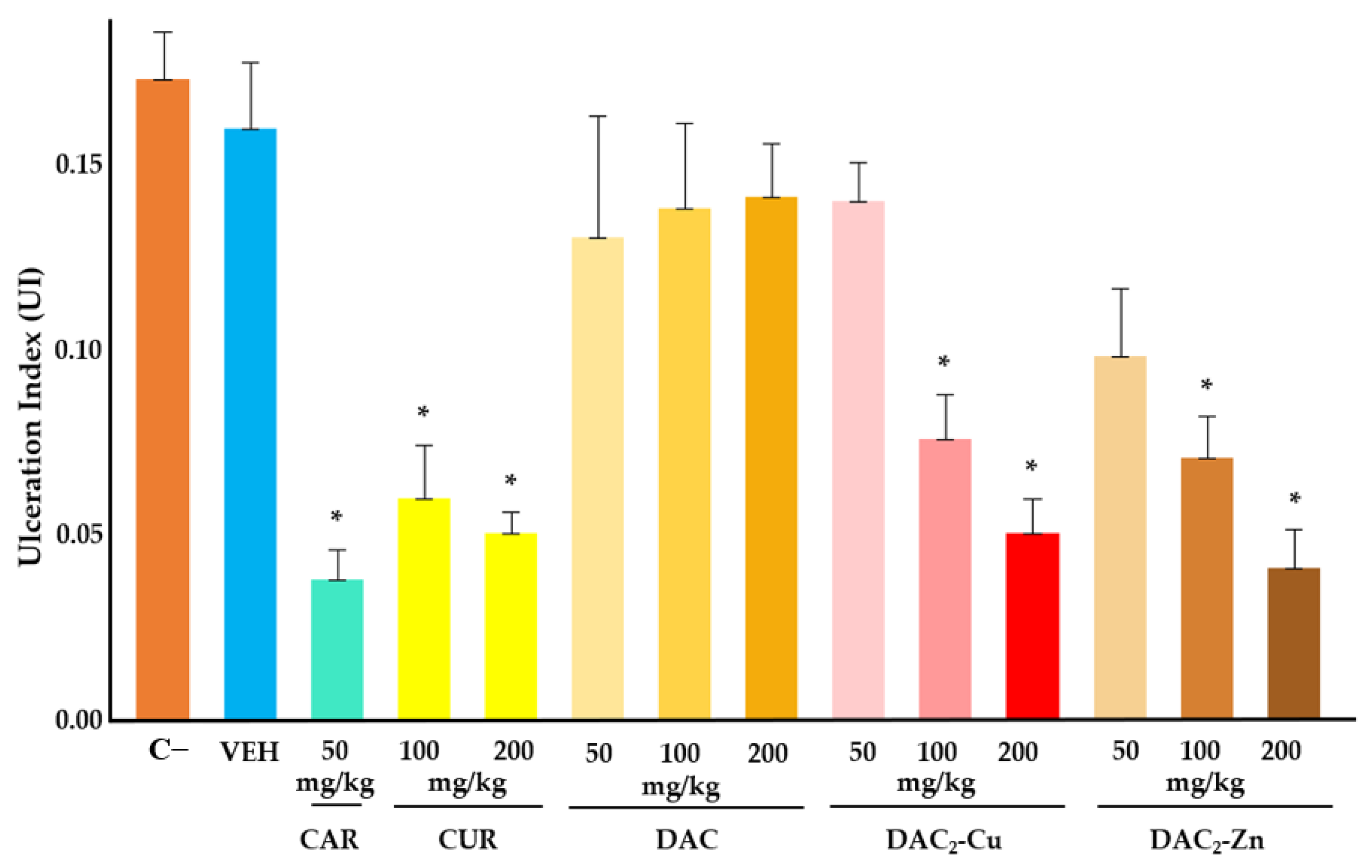
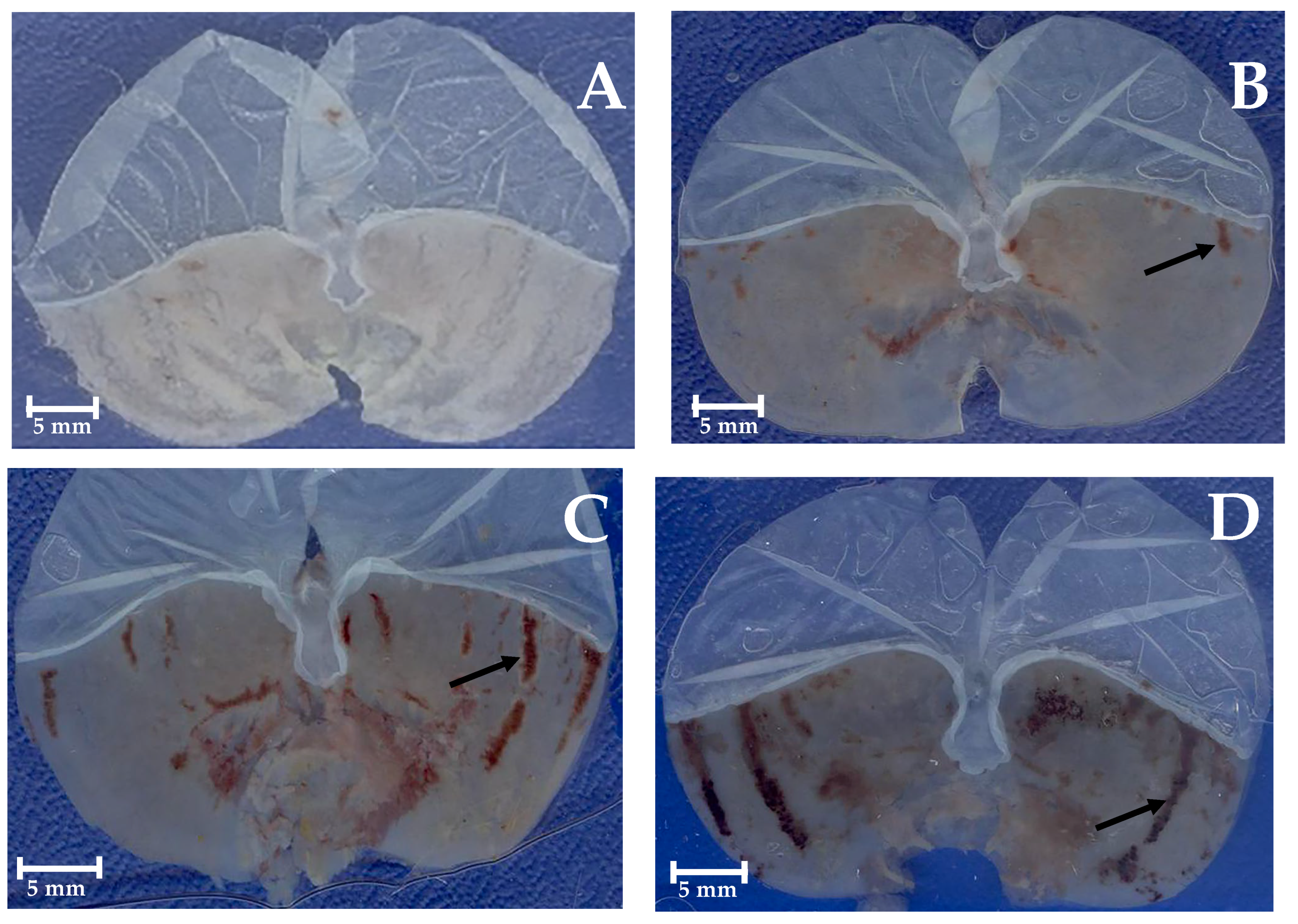
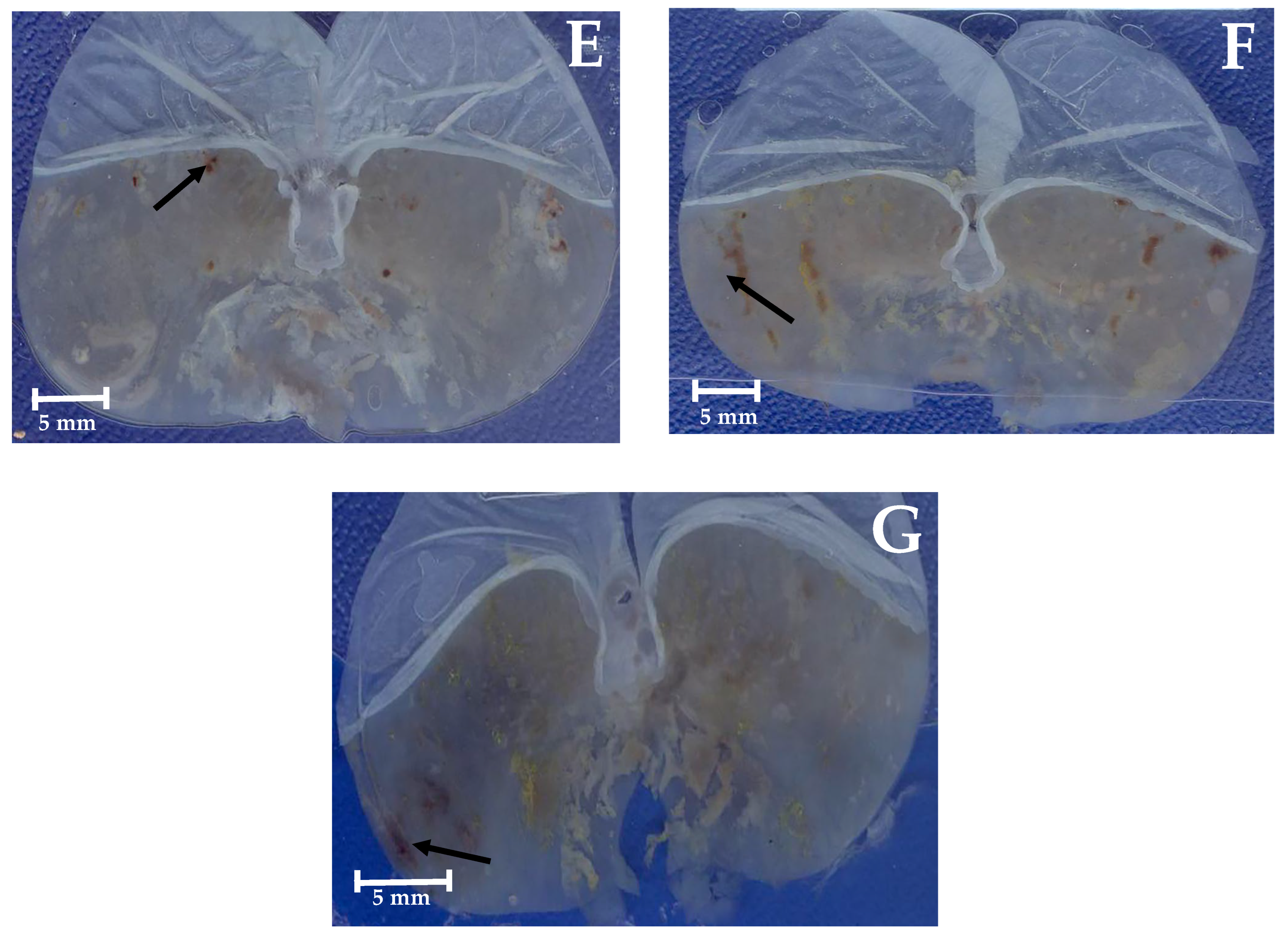
2.4. Gastroprotective Effect In Vivo
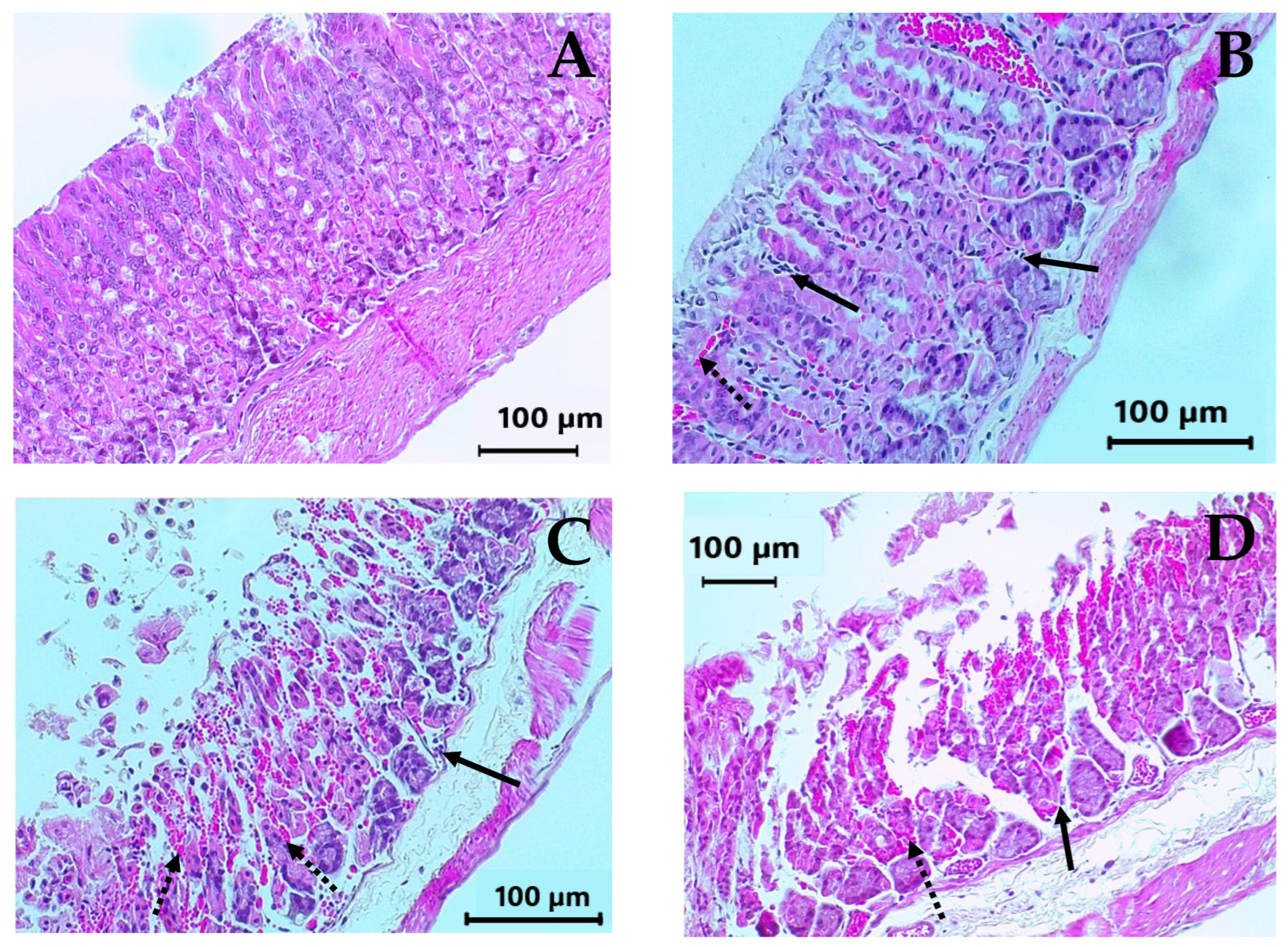
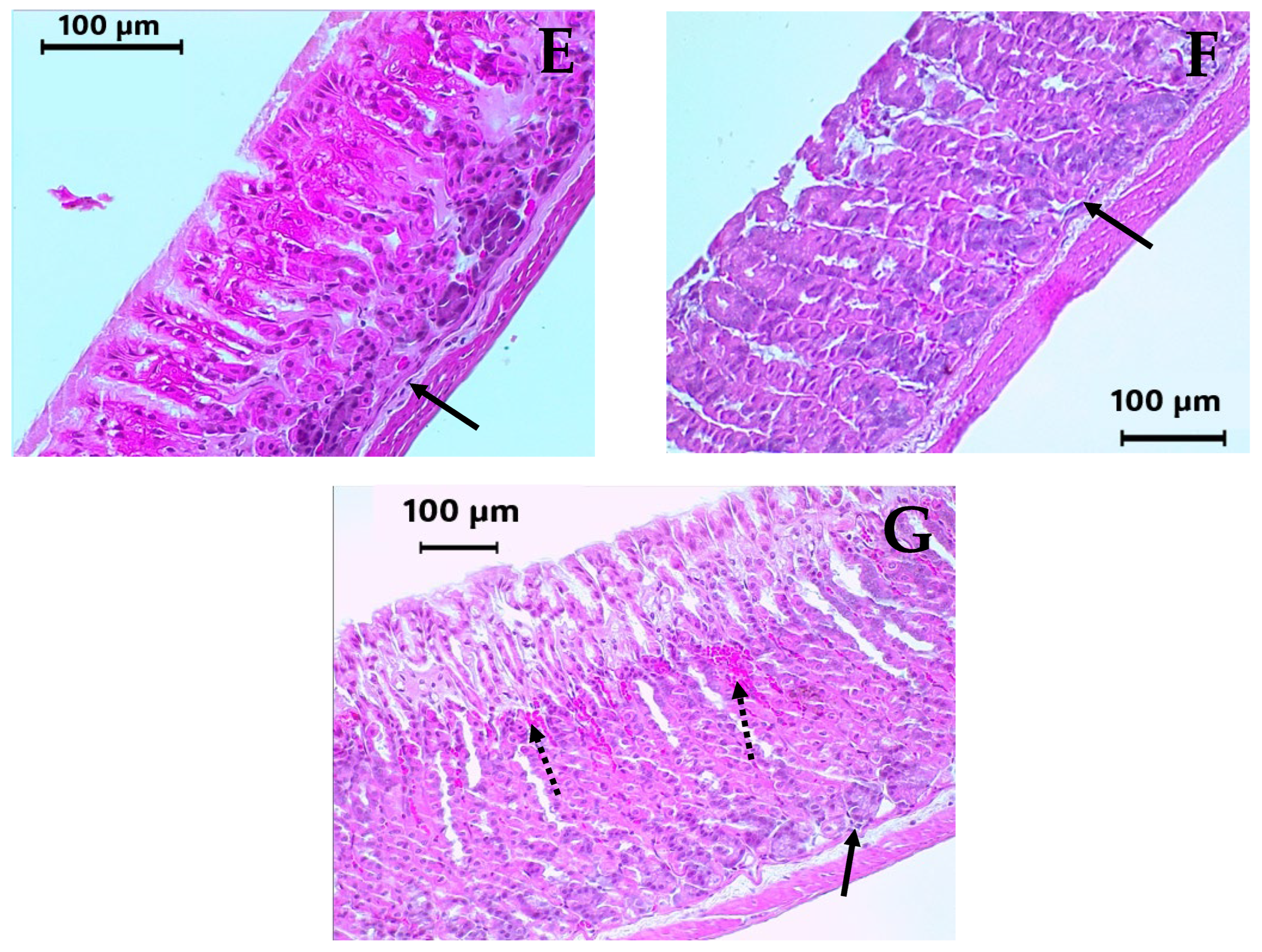
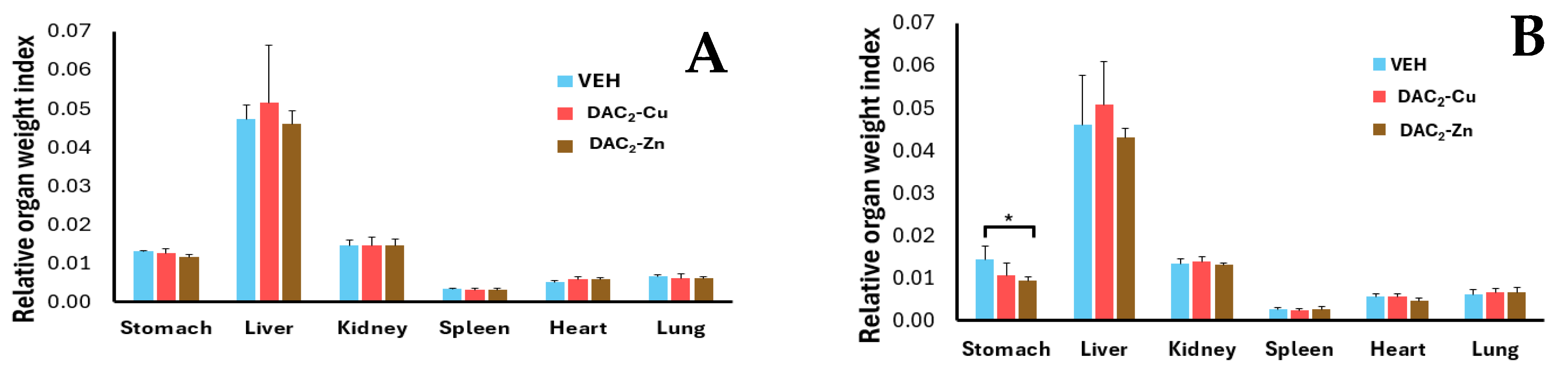
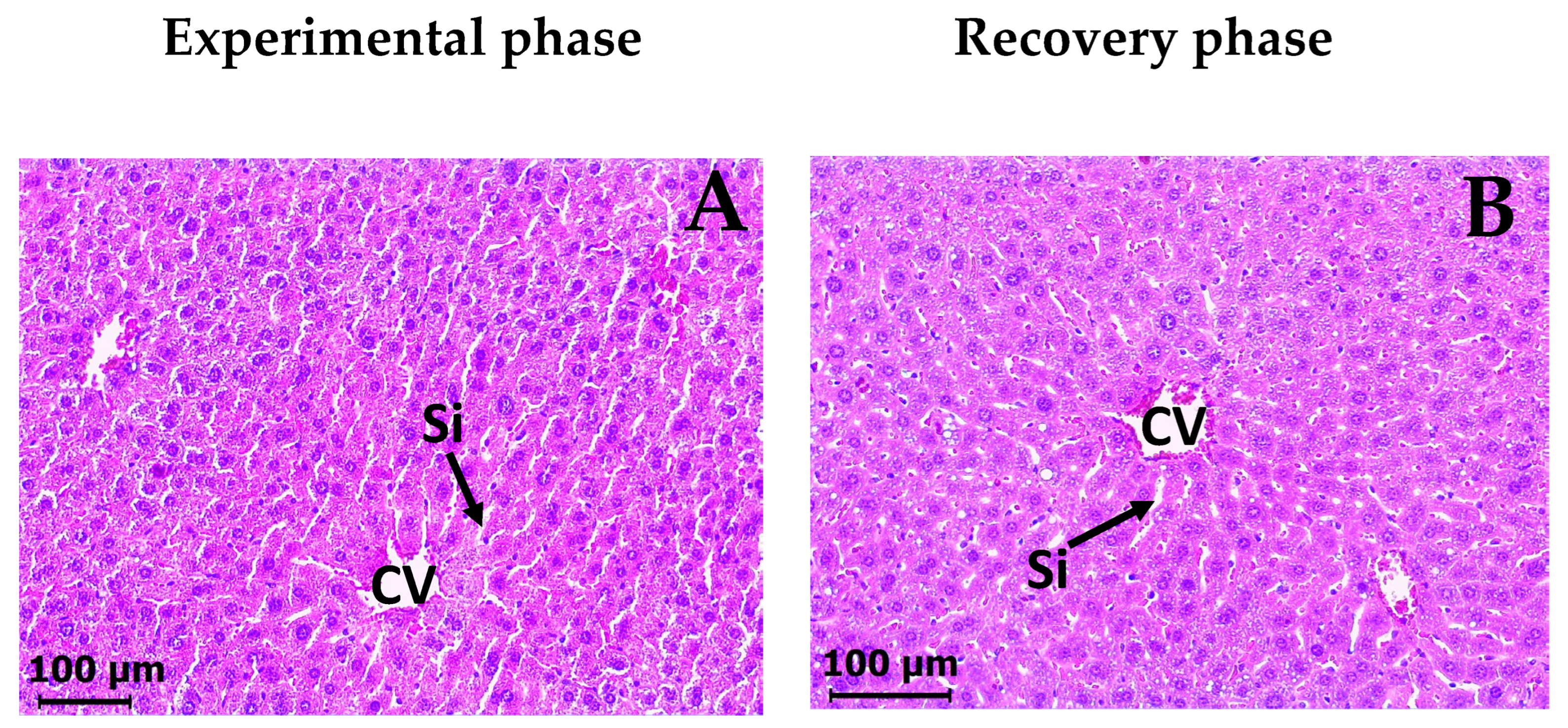
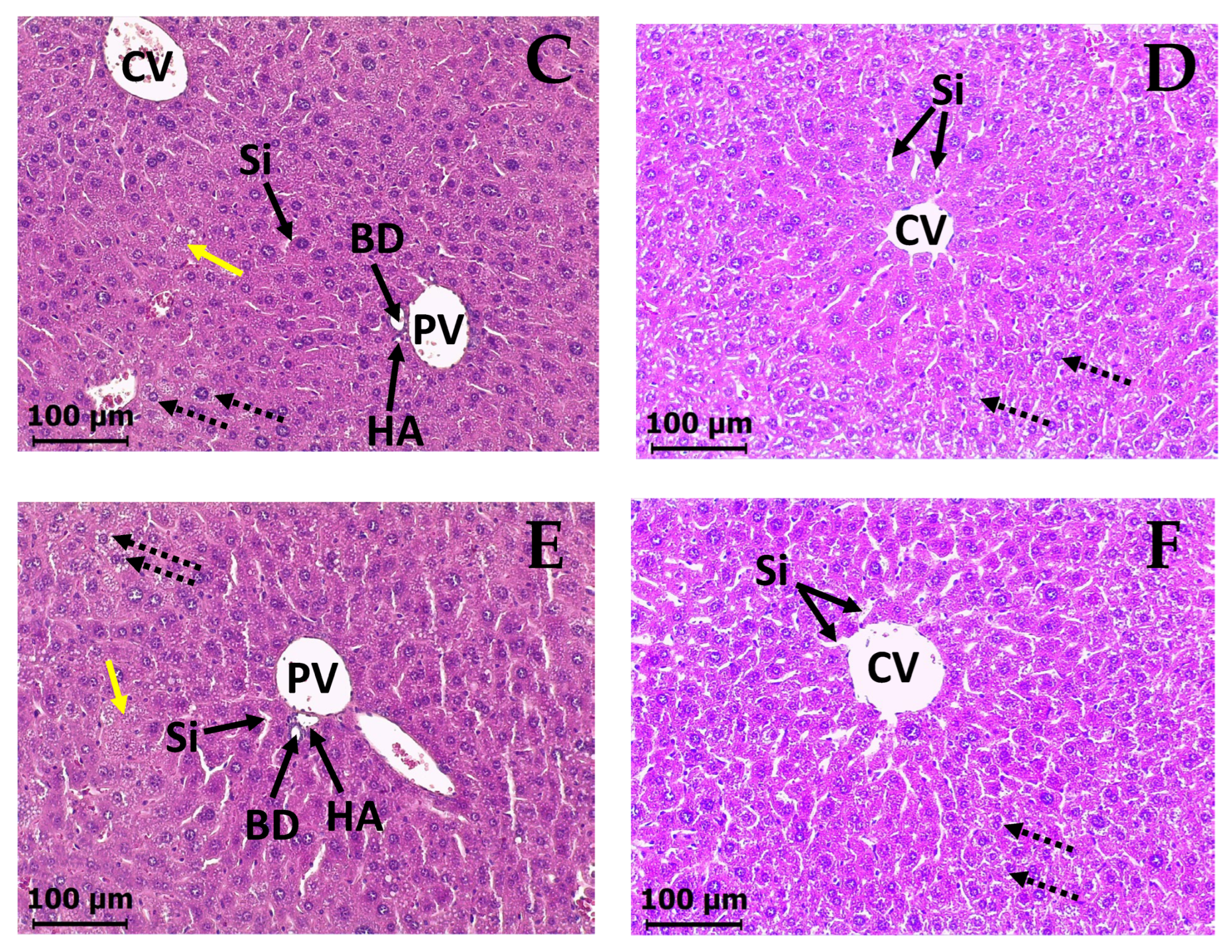
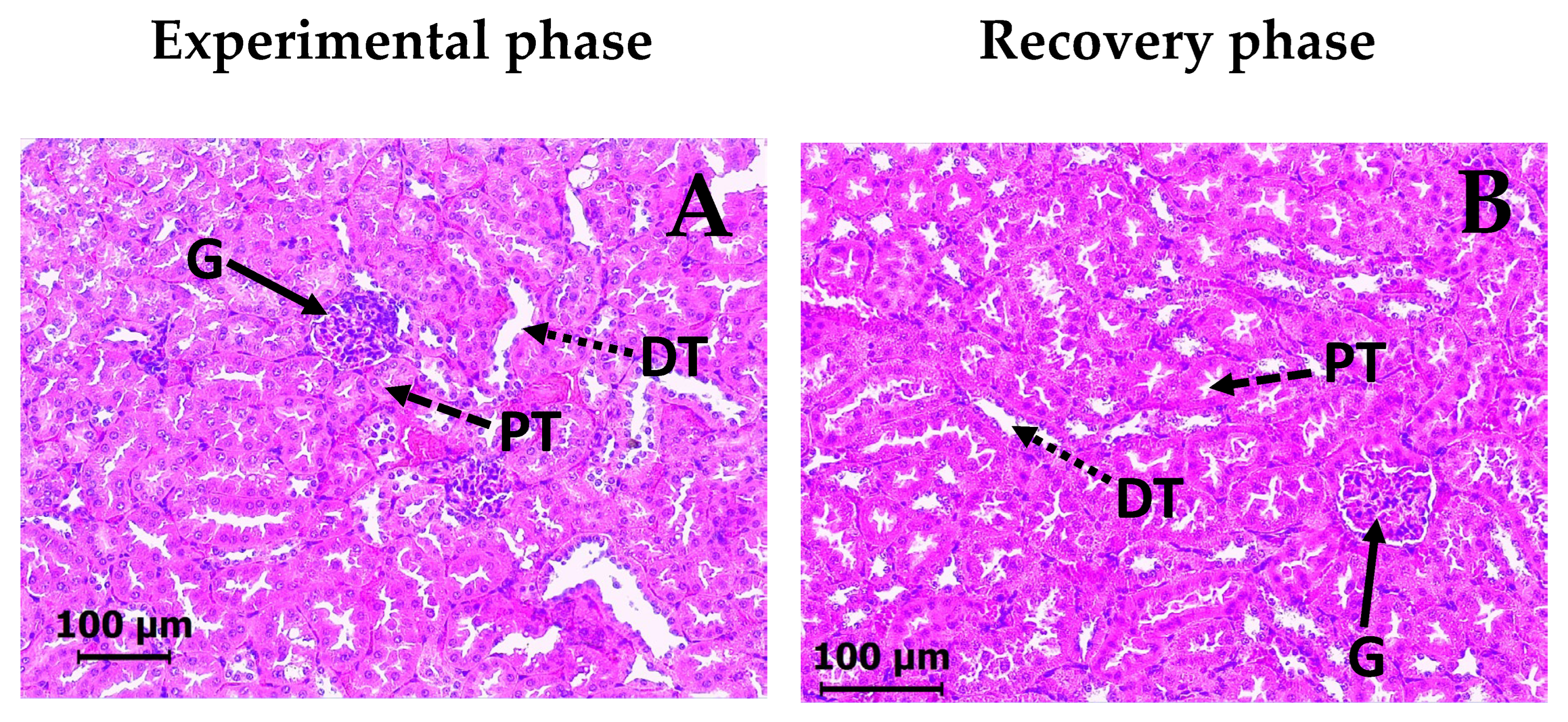
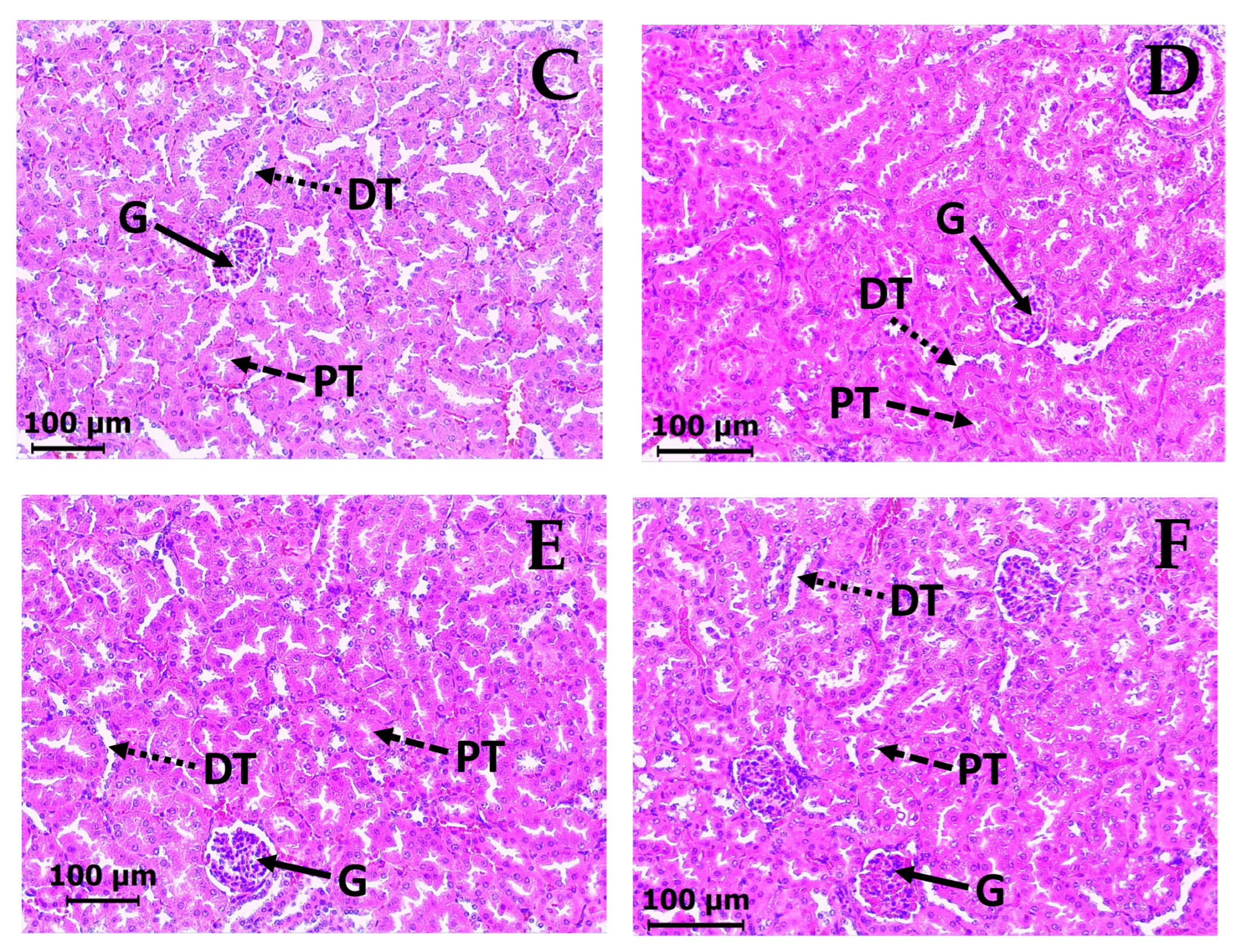
2.5. Subacute Toxicity Assay of DAC2-Cu and DAC2-Zn
3. Discussion
4. Materials and Methods
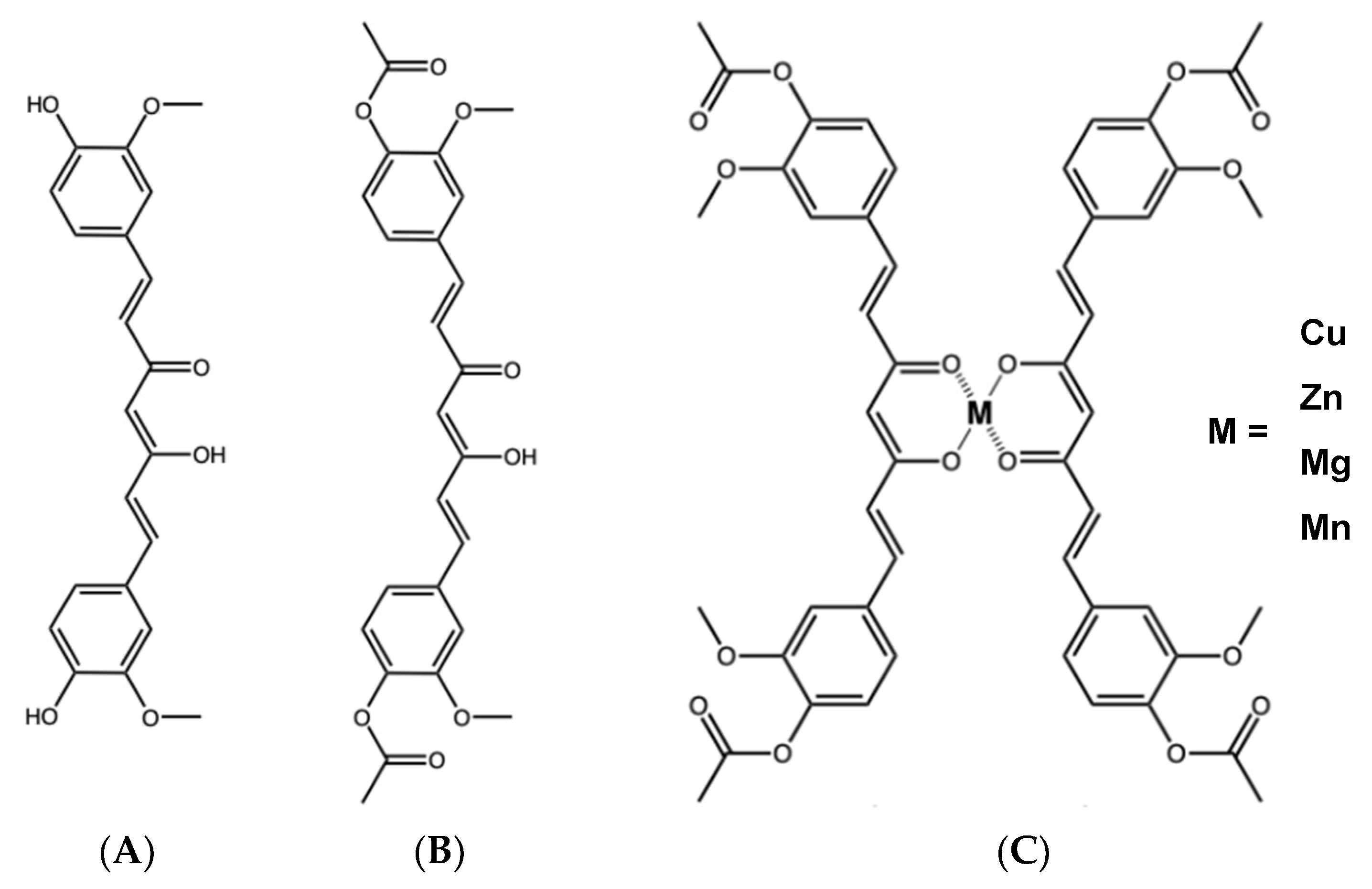
4.1. Curcumin, DAC, and Its Metal Derivatives
4.2. Strains and Growing Conditions
4.3. Antibacterial Activity
4.4. Urease Assay
4.4.1. Partial Purification of Urease
4.4.2. Urease Inhibition Assay
4.5. DNA Gyrase Supercoiling Assay
4.6. In Vivo Gastroprotective Activity
4.6.1. Animals
4.6.2. Anti-Ulcerogenic Activity
4.7. Subacute Toxicity Assay
4.7.1. Animals
4.7.2. Study Design
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAC | Diacetylcurcumin |
| MIC | Minimum Inhibitory Concentration |
| PPI | Proton Pump Inhibitor |
| LD | Lethal Dose |
| DNA | Deoxyribonucleic acid |
| IC50 | Half-maximal inhibitory concentration |
| AHA | Acetohydroxamic acid |
| CUR | Curcumin |
| SD | Standard Deviation |
| BW | Body weight |
| NSS | Isotonic sodium saline solution |
| VEH | Vehicle |
| CMC | carboxymethylcellulose |
| CAR | Carbenoxolone |
| SEM | Standard Error of the Mean |
| H&E | Hematoxylin & Eosin |
| ALT | Alanine aminotransferase |
| LDH | Lactate dehydrogenase |
| SDs | Solid dispersions |
| OECD | Organisation for Economic Co-operation and Development |
| UNAM | Universidad Nacional Autónoma de México |
| ATCC | American Type Culture Collection |
| LB | Luria–Bertani |
| CLSI | Clinical and Laboratory Standards Institute |
| DMSO | Dimethyl sulfoxide |
| CFU | Colony Forming Unit |
| CICUAL | Committee for the Care and Use of Laboratory Animals |
References
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Kusters, J.G.; van Vliet, A.H.M.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef]
- Suzuki, S.; Kusano, C.; Horii, T.; Ichijima, R.; Ikehara, H. The Ideal Helicobacter pylori Treatment for the Present and the Future. Digestion 2022, 103, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Qureshi, Z.A.; Khattak, A.L.; Saeed, F.; Asghar, A.; Azam, K.; Khan, M.A. Helicobacter pylori Eradication Therapy: Still a Challenge. Cureus 2021, 13, e14872. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [CrossRef]
- Mahady, G.B.; Pendland, S.L.; Yun, G.; Lu, Z.Z. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002, 22, 4179–4181. [Google Scholar]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef]
- Kundu, P.; De, R.; Pal, I.; Mukhopadhyay, A.K.; Saha, D.R.; Swarnakar, S. Curcumin alleviates matrix metalloproteinase-3 and -9 activities during eradication of Helicobacter pylori infection in cultured cells and mice. PLoS ONE 2011, 6, e16306. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Koosirirat, C.; Linpisarn, S.; Changsom, D.; Chawansuntati, K.; Wipasa, J. Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int. Immunopharmacol. 2010, 10, 815–818. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Meza-Morales, W.; Estévez-Carmona, M.M.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Cassani, J.; Ramírez-Apan, M.T.; Escobedo-Martínez, C.; Soriano-García, M.; Reynolds, W.F.; Enríquez, R.G. Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-level Cytotoxicity in vitro with Minimal Acute Toxicity in vivo. Molecules 2019, 24, 1598. [Google Scholar] [CrossRef]
- Clyne, M.; Cróinín, T.Ó. Pathogenicity and virulence of Helicobacter pylori: A paradigm of chronic infection. Virulence 2025, 16, 2438735. [Google Scholar] [CrossRef]
- Ruan, S.; Tu, C.H.; Bourne, C.R. Friend or Foe: Protein Inhibitors of DNA Gyrase. Biology 2024, 13, 84. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J.; Fernandez-Botran, R. Effects of curcumin on Helicobacter pylori infection. Ann. Transl. Med. 2016, 4, 479. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Lehene, S.; Lasnapure, B.; Pawar, S.; Kandipati, D.; Panchal, P. Investigation of antioxidant, anti-ulcer, and analgesic potential of a metal-curcumin complex. Naunyn-Schmiedebergs Arch. Pharmacol. 2023, 396, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Medeiros, J.; Yordanov, D.; Gergova, R.; Markovska, R. Turmeric and curcumin as adjuncts in controlling Helicobacter pylori-associated diseases: A narrative review. Lett. Appl. Microbiol. 2024, 77, ovae049. [Google Scholar] [CrossRef]
- Wang, Y.C. Medicinal plant activity on Helicobacter pylori related diseases. World J. Gastroenterol. 2014, 20, 10368–10382. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Mohammadi, K.; Deilami, I.; Zandi, K.; Fouladvand, M.; Ramedani, E.; Asayesh, G. Antibacterial activity of indium curcumin and indium diacetylcurcumin. Afr. J. Biotechnol. 2008, 7, 3832–3835. [Google Scholar]
- Changtam, C.; Hongmanee, P.; Suksamrarn, A. Isoxazole analogs of curcuminoids with highly potent multidrug-resistant antimycobacterial activity. Eur. J. Med. Chem. 2010, 45, 4446–4457. [Google Scholar] [CrossRef]
- Souza, V.; Polaquini, C.R.; de Moraes, G.R.; Oliveira Braga, A.R.; da Silva, P.V.; da Silva, D.R.; Ribeiro Lima, F.R.; Regasini, L.O.; Cássia Orlandi Sardi, J. Diacetylcurcumin: A novel strategy against Enterococcus faecalis biofilm in root canal disinfection. Future Microbiol. 2024, 19, 647–654. [Google Scholar] [CrossRef]
- Sanches, C.V.G.; Sardi, J.C.O.; Terada, R.S.S.; Lazarini, J.G.; Freires, I.A.; Polaquini, C.R.; Torrezan, G.S.; Regasini, L.O.; Fujimaki, M.; Rosalen, P.L. Diacetylcurcumin: A new photosensitizer for antimicrobial photodynamic therapy in Streptococcus mutans biofilms. Biofouling 2019, 35, 340–349. [Google Scholar] [CrossRef]
- Sachs, G.; Weeks, D.L.; Wen, Y.; Marcus, E.A.; Scott, D.R.; Melchers, K. Acid acclimation by Helicobacter pylori. Physiology 2005, 20, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Wirth, H.P.; Beins, M.H.; Yang, M.; Tham, K.T.; Blaser, M.J. Experimental infection of Mongolian gerbils with wild-type and mutant Helicobacter pylori strains. Infect. Immun. 1998, 66, 4856–4866. [Google Scholar] [CrossRef]
- Eaton, K.A.; Brooks, C.L.; Morgan, D.R.; Krakowka, S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect. Immun. 1991, 59, 2470–2475. [Google Scholar] [CrossRef] [PubMed]
- Schoep, T.D.; Fulurija, A.; Good, F.; Lu, W.; Himbeck, R.P.; Schwan, C.; Choi, S.S.; Berg, D.E.; Mittl, P.R.; Benghezal, M.; et al. Surface properties of Helicobacter pylori urease complex are essential for persistence. PLoS ONE 2010, 5, e15042. [Google Scholar] [CrossRef] [PubMed]
- Nugrahaeningtyas, E.; Lee, D.J.; Song, J.I.; Kim, J.K.; Park, K.H. Potential application of urease and nitrification inhibitors to mitigate emissions from the livestock sector: A review. J. Anim. Sci. Technol. 2022, 64, 603–620. [Google Scholar] [CrossRef]
- Spencer, A.C.; Panda, S.S. DNA Gyrase as a Target for Quinolones. Biomedicines 2023, 11, 371. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Suzuki, H.; Tsugawa, H.; Nishizawa, T.; Hibi, T. Homology model of the DNA gyrase enzyme of Helicobacter pylori, a target of quinolone-based eradication therapy. J. Gastroenterol. Hepatol. 2010, 25, S7–S10. [Google Scholar] [CrossRef]
- Duprey, A.; Groisman, E.A. The regulation of DNA supercoiling across evolution. Protein Sci. 2021, 30, 2042–2056. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, A.; Skliar, M.I. Medicinal plants from Argentina with gastroprotective activity. Ars Pharm. 2007, 48, 361–369. [Google Scholar]
- Chattopadhyay, I.; Bandyopadhyay, U.; Biswas, K.; Maity, P.; Banerjee, R.K. Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic. Biol. Med. 2006, 40, 1397–1408. [Google Scholar] [CrossRef]
- Morsy, M.A.; El-Moselhy, M.A. Mechanisms of the protective effects of curcumin against indomethacin-induced gastric ulcer in rats. Pharmacology 2013, 91, 267–274. [Google Scholar] [CrossRef]
- Czekaj, R.; Majka, J.; Magierowska, K.; Sliwowski, Z.; Magierowski, M.; Pajdo, R.; Ptak-Belowska, A.; Surmiak, M.; Kwiecien, S.; Brzozowski, T. Mechanisms of curcumin-induced gastroprotection against ethanol-induced gastric mucosal lesions. J. Gastroenterol. 2018, 53, 618–630. [Google Scholar] [CrossRef]
- Kwiecien, S.; Magierowski, M.; Majka, J.; Ptak-Belowska, A.; Wojcik, D.; Sliwowski, Z.; Magierowska, K.; Brzozowski, T. Curcumin: A Potent Protectant against Esophageal and Gastric Disorders. Int. J. Mol. Sci. 2019, 20, 1477. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Luo, X.; Xu, S.; Xu, D.; Zheng, Y.; Xu, S.; Lv, J. Gastroprotective effects of a new zinc (II)-curcumin complex against pylorus-ligature-induced gastric ulcer in rats. Chem. Biol. Interact. 2009, 181, 316–321. [Google Scholar] [CrossRef]
- Mei, X.; Xu, D.; Xu, S.; Zheng, Y.; Xu, S. Gastroprotective and antidepressant effects of a new zinc (II)-curcumin complex in rodent models of gastric ulcer and depression induced by stresses. Pharmacol. Biochem. Behav. 2011, 99, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Xu, D.; Xu, S.; Zheng, Y.; Xu, S. Novel role of Zn (II)-curcumin in enhancing cell proliferation and adjusting proinflammatory cytokine-mediated oxidative damage of ethanol-induced acute gastric ulcers. Chem. Biol. Interact. 2012, 197, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development (OECD). Test No. 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2025. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing: Seventeenth Informational Supplement (Document M100-S17); CLSI: Wayne, PA, USA, 2007. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1976, 39, 971–974. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
| Compound | MIC 1 | |
|---|---|---|
| µg/mL | µM | |
| Curcumin | 1.95 | 5.29 |
| DAC | 62.50 | 138.12 |
| DAC2-Cu | 15.62 | 15.00 |
| DAC2-Zn | 31.25 | 29.86 |
| DAC2-Mn | 15.62 | 14.90 |
| DAC2-Mg | 31.25 | 28.84 |
| Metronidazole 2 | 300.00 | 1752.84 |
| Clarithromycin 2 | 0.05 | 0.07 |
| Compound (62.5 µg/mL) | E. coli EPEC | S. enterica | S. aureus |
|---|---|---|---|
| Inhibition (%) | |||
| Curcumin (170 µM) | 25.7 ± 3.3 | 47.5 ± 10.1 | 67.2 ± 4.0 |
| DAC (138 µM) | 21.3 ± 3.0 | 15.2 ± 2.4 | 16.7 ± 6.9 |
| DAC2-Cu (60 µM) | 10.7 ± 2.8 | 3.9 ± 1.2 | 10.9 ± 1.8 |
| DAC2-Zn (60 µM) | 19.3 ± 4.5 | 10.0 ± 3.1 | 23.7 ± 6.3 |
| DAC2-Mn (56 µM) | 8.6 ± 3.4 | 6.5 ± 4.1 | 10.9 ± 2.0 |
| DAC2-Mg (58 µM) | 14.2 ± 2.3 | 11.0 ± 8.7 | 17.4 ±1.7 |
| Ampicillin (40 µM) (14 µg/mL) | 100% | ||
| Treatment | 50 mg/kg | 100 mg/kg | 200 mg/kg |
|---|---|---|---|
| Carbenoxolone | 78.3 ± 4.8 | ||
| Curcumin | - | 65.5 ± 8.4 | 71.1 ± 3.4 |
| DAC | 24.8 ± 19.0 | 20.3 ± 13.4 | 18.6 ± 8.6 |
| DAC2-Cu | 19.0 ± 6.1 | 56.5 ± 7.0 * | 71.2 ± 5.5 * |
| DAC2-Zn | 43.6 ± 10.8 | 59.3 ± 6.5 | 76.6 ± 6.3 |
| DAC2-Mn | 20.8 ± 11.6 | 21.1 ± 16.5 | 24.0 ± 15.6 |
| DAC2-Mg | 0 ± 10.0 | 0.2 ± 19.5 | 11.4 ± 14.3 |
| Experimental Phase | Recovery Phase | |||||
|---|---|---|---|---|---|---|
| Serum | Vehicle | DAC2-Cu | DAC2-Zn | Vehicle | DAC2-Cu | DAC2-Zn |
| Total protein | 5.3 ± 0.3 | 5.5 ± 0.4 | 5.4 ± 0.4 | 5.2 ± 0.5 | 5.8 ± 1.0 | 5.6 ± 0.4 |
| Albumin | 3.5 ± 0.5 | 3.5 ± 0.4 | 3.5 ± 0.4 | 3.1 ± 1.01 | 3.4 ± 0.3 | 3.5 ± 0.7 |
| Creatinine | 0.37 ± 0.08 | 0.30 ± 0.06 | 0.34 ± 0.04 | 0.44 ± 0.18 | 0.37 ± 0.10 | 0.31 ± 0.14 |
| Total bilirubin | 0.17 ± 0.05 | 0.19 ± 0.062 | 0.16 ± 0.03 | 0.25 ± 0.04 | 0.34 ± 0.04 | 0.29 ± 0.05 |
| Glucose | 164.9 ± 52.8 | 178.7 ± 41.1 | 147.3 ± 14.0 | 200.6 ± 25.6 | 232.5 ± 29.2 | 203.6 ± 56.9 |
| ALT | 25.0 ± 14.8 | 12.3 ± 9.0 | 24.0 ± 8.2 | 21.5 ± 8.7 | 23.4 ± 12.0 | 20.0 ± 5.2 |
| LDH | 891.8 ± 395.8 | 1465.1 ± 636.7 | 1051.1 ± 220.2 | 1945.1 ± 1399.5 | 1789.7 ± 916.3 | 1205.8 ± 283.5 |
| Urine | ||||||
| Total protein | 3.9 ± 0.2 | 3.8 ± 0.2 | 4.1 ± 0.2 | 5.1 ± 0.3 | 5.6 ± 0.06 | 5.4 ± 0.3 |
| Creatinine | 25.56 ± 1.9 | 22.17 ± 5.3 | 28.16 ± 6.9 | 16.33 ± 8.0 | 22.36 ± 4.9 | 25.89 ± 10.7 |
| Glucose | 0.7 ± 1.0 | 0.1 ± 0.5 | 1.5 ± 0.7 | 2.2 ± 2.2 | 3.4 ± 3.1 | 2.0 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agabo-Martínez, A.; Gomez-Chang, E.; Hernández-Hipólito, E.; Estrada-Muñiz, E.; Escobedo-Martínez, C.; Obregón-Mendoza, M.A.; Enríquez, R.G.; Vega, L.; Romero, I. Anti-Helicobacter pylori Activity and Gastroprotective Effects of Diacetylcurcumin and Four Metal Derivatives. Molecules 2025, 30, 3849. https://doi.org/10.3390/molecules30193849
Agabo-Martínez A, Gomez-Chang E, Hernández-Hipólito E, Estrada-Muñiz E, Escobedo-Martínez C, Obregón-Mendoza MA, Enríquez RG, Vega L, Romero I. Anti-Helicobacter pylori Activity and Gastroprotective Effects of Diacetylcurcumin and Four Metal Derivatives. Molecules. 2025; 30(19):3849. https://doi.org/10.3390/molecules30193849
Chicago/Turabian StyleAgabo-Martínez, Almanelly, Erika Gomez-Chang, Erick Hernández-Hipólito, Elizabet Estrada-Muñiz, Carolina Escobedo-Martínez, Marco A. Obregón-Mendoza, Raúl G. Enríquez, Libia Vega, and Irma Romero. 2025. "Anti-Helicobacter pylori Activity and Gastroprotective Effects of Diacetylcurcumin and Four Metal Derivatives" Molecules 30, no. 19: 3849. https://doi.org/10.3390/molecules30193849
APA StyleAgabo-Martínez, A., Gomez-Chang, E., Hernández-Hipólito, E., Estrada-Muñiz, E., Escobedo-Martínez, C., Obregón-Mendoza, M. A., Enríquez, R. G., Vega, L., & Romero, I. (2025). Anti-Helicobacter pylori Activity and Gastroprotective Effects of Diacetylcurcumin and Four Metal Derivatives. Molecules, 30(19), 3849. https://doi.org/10.3390/molecules30193849







