Abstract
A traditional dairy product from northern Colombia is suero costeño (SC), typically handmade through artisanal processes involving the natural fermentation of raw cow’s milk (RM); it is characterized by a creamy texture and a distinctive sensory profile, with a sour/salty taste and rancid odor. This study aimed to determine the chemical identity (using GC-FID/MSD) of SC and RM samples (from eight locations in the department of Córdoba-Colombia) by analyzing volatile components (trapped by HS-SPME and SDE) and fatty acid content. Consequently, the most notable results were as follows: (a) myristic (7–12%), stearic (12–17%), oleic (13–23%), and palmitic (21–29%) acids were the most abundant constituents [without significant differences among them (p > 0.05)] in both RM and SC fats; these were also expressed as polyunsaturated (2–5%), monounsaturated (26–36%), saturated (59–69%), omega-9 (19–30%), omega-6 (0.5–1.6%), and omega-3 (0.2–1.2%) fatty acids; (b) differences in the composition (p < 0.05) of the volatile fractions were distinguished between RM and SC samples; likewise, the SC samples differed (from each other) in their volatile composition due to the preparation processes applied (processes with raw milk and natural fermentation had less variability); nonetheless, it was possible to determine the volatilome for the artisanal product; and (c) the major components responsible for the chemical identity of SC were ethyl esters (of linear saturated and unsaturated acids, short/medium chains), aliphatic alcohols (linear/branched, short/long chains), aliphatic aldehydes (long chains, >C14), alkyl methyl ketones (long chains, >C11), sesquiterpenes (caryophyllane/humulane types), monoterpenes (mono/bi-cyclics), short-chain fatty acids, and aromatic alcohol/acid, among others.
1. Introduction
“Suero costeño (SC)”, with a certain similarity in flavor and texture to sour cream, is a typical food (gastronomic heritage) of the northern region of Colombia and, as a dairy product, it is the result of the aerobic fermentation (spontaneous acidification by native microorganisms) of raw cow’s milk; its preparation method is mainly handmade by peasants in rural areas [1,2,3]. As food, some values of the proximate analysis for SC are 4.0–7.8% (protein), 65–76% (moisture), acidity (0.2–0.5%), 0.3–0.9% (crude fiber), and 3.6–5.8 (pH) [3] and its primary physical and organoleptic characteristics are that it is a colloidal fluid (gel) with little or no syneresis (no stabilizer), a white color, moderate to high viscosity, lumpy (high content of suspended solids—protein and fat), smooth texture, sour/salty taste, and acrid or rancid odor [3,4]. In Colombia, the main producing departments are Córdoba, Sucre, Cesar, Bolivar, and Magdalena [2,3,5].
Regarding the organoleptic characteristics of dairy products (related to consumer acceptance), some of the most important attributes are taste and aroma (flavor), along with mouthfeel, all of which originate through a combination of chemical components [6,7,8]. In this sense, the mouthfeel could be attributed to the fatty acid (FA) and protein contents (related to viscosity and texture) of the food, while the taste to sugars, salts, and short-chain fatty acids (SCFAs), as well as other fermentation by-products. Additionally, aromas are the result of a proper balance of volatile organic constituents, which are produced by thermal degradation, lipid oxidation, and fermentation by lactic acid bacteria (LAB) [6,9,10,11,12,13]. These odorant molecules are usually hydrophobic (some polar), with molar weights up to 300 g/mol, and become impact compounds because they impart distinctive notes to foods (e.g., esters: fruity; C4–C10 FAs and aldehydes: rancid; terpenoids: musty, earthy, and herbaceous; aromatic alcohols, aldehydes, and acids: floral; lactones: coconut-like) [9,14]. Thus, flavor is, among others, the most relevant attribute for the acceptance of SC and depends on both the fermentation and production processes, as well as on the quality and type of raw material (e.g., milk) [2,9,14].
As mentioned above, SC has a texture and taste like sour cream, which is a heavy, viscous, shiny product with a balanced, pleasant, butter-like aroma, as reported by Meunier-Goddik [15] and Shepard et al. [16]. The characteristic odor of the sour cream is due to diacetyl, acetoin, acetic and butyric acids, acetaldehyde, and/or dimethyl sulfide, but it may also contain aldehydes, ketones, alcohols, fatty acids, lactones, esters, nitrogen-containing compounds, and aromatic hydrocarbons contributed by the raw milk [17,18]. When reviewing the scientific literature on FA profiles and aroma-related volatile compounds for SC, only one record was found indirectly related to this topic (Valencia Garcia et al. [19]); however, there were other reports for SC focused on the microbiological diversity (selection, identification, and isolation), characterization, and biological effect of LAB and yeasts, starter cultures, spontaneous fermentation, physicochemical and rheological characterizations, etc. [3,4,5,20,21,22,23,24,25,26,27,28,29]. Based on these works, 22 strains of bacteria and yeasts have been isolated, e.g., Enterococcus faecium, Lactobacillus acidophilus, L. brevis, L. delbrueckii, L. lactis, L. paracasei, L. pentosus, L. plantarum, Leuconostoc lactis, Streptococcus infantarius, etc., which would be responsible for some of the physicochemical and organoleptic characteristics of SC.
Finally, according to the European Commission-Agriculture and Rural Development, when a product (food, wine, or agricultural products) is traditionally and entirely manufactured (preparation, processing, and production) in a specific region and acquires unique properties, it can be registered for a protected designation of origin (PDO). Nonetheless, this product must have unique properties (physicochemical and organoleptic, among others) identified and established, e.g., odor, taste, color, texture, viscosity, chemical constituents, etc., in addition to meeting the above criteria [30]. Colombia, through its Ministry of Industry and Commerce, has conferred seals for a denomination of origin that protects unique Colombian products with an established identity (foods (cheese and fruits), flowers, handicrafts, etc.) [31]. As a first approach to establish the identity of SC as a regional autochthonous product, this work determined the chemical composition by GC-FID/MSD of volatile fractions (by HS-SPME and SDE) and the fatty acid content (by chemical derivatization to FAMEs)—as a chemical fingerprint—of both suero costeño and raw milk from eight localities in the department of Córdoba (Colombia), where farmers produced SC from cow’s milk and five manufacturing processes, which could influence the production of SC volatiles. In addition, this study made it possible to relate the effect of the SC manufacturing process and raw material on the development of aroma-related volatile constituents.
2. Results
2.1. Fat Content and Fatty Acid Profiles
After isolation of RM and SC fats by the Rose–Gottlieb method, their yields were determined (Figure 1). Thus, 2.0 ± 0.1–4.5 ± 0.3% and 5.2 ± 0.2–8.3 ± 0.5% were the fat contents for RM and SC, respectively, and the average values for each type of samples were 3.7 ± 0.9% (RM) and 7.3 ± 0.9% (SC). Moreover, there were higher variations in fat content among the milk samples, but in the SC samples the variations were smaller; the greatest variation was found in the sample from location 8 (SC prepared from raw milk spontaneously fermented for one day in “totuma”) with the lowest yield (5.2 ± 0.2%). Nonetheless, the ratios between SC and RM yields indicated that the fat content in the SC samples was higher in most cases (1.3 to 3.6 times).
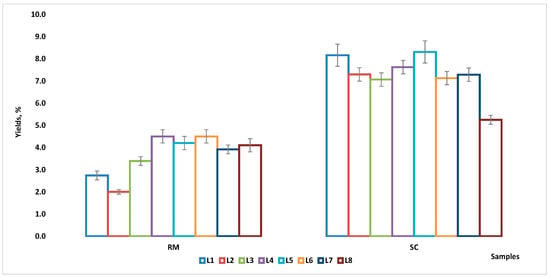
Figure 1.
Yields (%) of fat content for the RM and SC samples (measure unit: mean ± standard deviation).
The result of the Shapiro–Wilks (S-W) test for the raw data showed that both RM and SC yields had a normal distribution (p: 0.1714; p: 0.1503—Table S1), while the results of the non-parametric ANOVA showed that there were significant differences (p < 0.0001, Friedman (F) test—Table S2) between the yields of RM compared to those of SC, but within each group (RM and SC) there were no differences (p > 0.999, Kruskal–Wallis (K-W) test—Table S3).
Concerning the fatty acids of the RM- and SC-fat fractions, these were characterized as methyl esters (FAMEs) (Figure S1) and reported (>99%) in Table S4. According to the Table, the FAs of the samples were similar, with some differences in their relative amounts, with C6–C25 fatty acids (saturated and unsaturated) being representative in all samples; thus, the predominant FAs in the RM and SC samples in order of abundance were palmitic (C16:0; 21–29%), oleic (C18:1 cis-Δ9; 13–22%), stearic (C18:0; 12–17%), and myristic (C14:0; 7–12%) acids (Figure S2) and the total content of saturated (SFAs), monounsaturated (MUFAs), and polyunsaturated (PUFAs) fatty acids ranged between 59–69%, 27–36%, and 2.9–5.0%, respectively (Figure 2a). In addition, the contents of n-3, n-6, and n-9 acids were 0.2–1.2%, 0.6–1.5%, and 19–30%, in that order, for both RM and SC (Figure 2b). Another important fact is that a non-negligible percentage (7–17%) of C18:1 octadecenoic acid isomers (Z/E) was determined in all samples, as well as one n-3 pentaunsaturated fatty acid (EPA isomer) [32] and some branched and odd-numbered fatty acids (C9:0–C25:0).
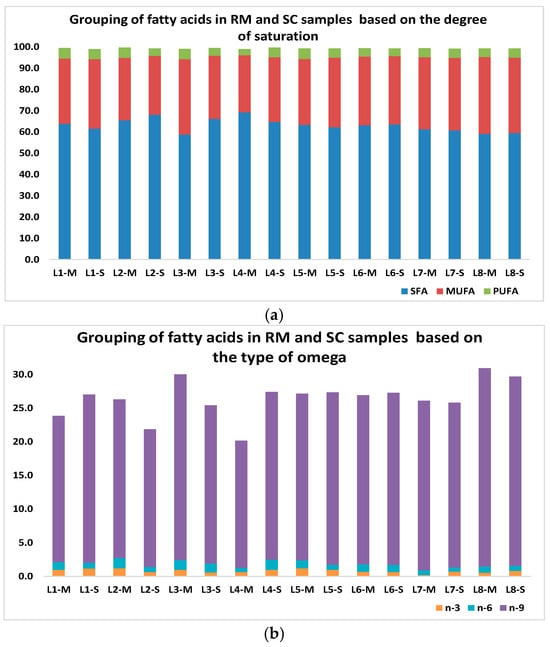
Figure 2.
Grouping of FAs in RM and SC samples based on (a) degree of saturation and (b) type of omega. SFAs: saturated fatty acids, MUFAs: monounsaturated fatty acids, and PUFAs: polyunsaturated fatty acids (n-3 acids, n-6 acids, and n-9 acids).
On the other hand, the atherogenic (AI) and thrombogenic (TI) indices, which are dietary risk values for cardiovascular disease related to the platelet aggregation inhibition (AI) and blood clot formation (TI) [33], were calculated for each sample. Thus, the AI and TI values were 1.4–2.8 and 2.0–3.3 for both RM and SC. Statistical analysis of these data using S-W, Friedman, and K-W tests (Tables S5–S13) indicated that the four main FAs, along with the classification by FA families and the AI and TI values of both types of samples (RM and SC), had a normal distribution (p: 0.3497–0.9850, p: 0.1209–0.6500, and p: 0.3186–0.5202); and there were no significant differences within and between all samples (Friedman: p: 0.9685 and 0.8546—major FA, p: 0.3251 and 0.4986—families of FAs; KW: p: > 0.9999—major FA, p: 0.4514—families of FAs, p: 0.4514—AI and TI values). However, there were significant differences for AI and TI values within the SC samples (p < 0.0001—Friedman test) but not for the RM samples (p: 0.0672—Friedman test).
2.2. Chemical Composition of Volatile Fractions
The chemical constituents positively identified (83–99%) in the volatile fractions of SC and RM samples by SDE/SPME-GC-FID/MSD (Figures S3 and S4) are listed in order of elution in Table A1 and Table A2. Thus, the volatile profiles of the RM and SC samples were, respectively, composed of 48–78 and 65–100 constituents. The common major constituents (1–46% of the relative amounts by SDE—Table A1) found in the volatile fractions of milk samples were nine fatty acids (C8:0–C16:0, including C10:1 and C14:1) together with benzoic acid. However, some minority non-acid compounds, related to the organoleptic properties of RM, were also recognized, e.g., six methyl alkyl ketones, five cyclic esters (lactones), and four aliphatic aldehydes. In addition, some miscellaneous compounds such as two sesquiterpenes (β-caryophyllene, α-humulene), 10 linear/branched hydrocarbons, one aromatic hydrocarbon, and one aromatic alcohol (benzenethanol) were present. Non-parametric ANOVA of these data indicated that there were no significant differences within each group [type of compounds (p: 0.4544, K-W test—Table S14)] for all RM samples, but there were differences (p: <0.05, Friedman test—Table S15) in the chemical composition of sample L1 compared to the other milk samples (no significant differences between them, p: > 0.05).
Furthermore, the volatile fractions by HS-SPME of the RM samples (Table A2) were mainly represented by C10:0, C8:0, C6:0, and C12:0 fatty acids together with limonene, the C2:0 and C4:0 acids, isoamyl alcohol, 2-nonanone, benzoic acid, the C14:0 and C14:1 fatty acids, acetone, and pentanol. Other types of compounds related to milk flavor were also present: five methyl alkyl ketones, four cyclic esters, two sesquiterpenes, seven linear/branched hydrocarbons, benzenethanol, and myristylaldehyde. It is important to mention that ester-type compounds (ethyl caprate, ethyl laurate, and ethyl myristate) were identified in the milk samples, as well as monoaromatic hydrocarbons (toluene and styrene), and a high content of 2-nonanone in milk sample L7. As with the SDE method, the non-parametric ANOVA of HS-SPME data showed the same trend; i.e., there were differences (p: < 0.05, Friedman test—Table S15) for the composition of sample L1 versus the remaining milk samples (no significant differences between them, p: > 0.05), but there were no differences within each group [type of compounds (p: 0.4544, K-W test—Table S14)] for all samples. Non-parametric ANOVA applied to the compositions of volatile fractions (average relative amounts per compound family for all RM samples) for both SDE and HS-SPME methods revealed no significant differences within and between all samples (Friedman: p: 0.6507; K-W: p: 0.4544—Tables S16 and S17).
Considering the chemical compositions of the volatile fractions by SDE (Figure S4A) of the SC samples, these were mainly typified by 13 fatty acids (including short-chain) [(3-Me)-C4:0 (iso-C5:0), (2-Me)-C4:0, C5:0, C6:0-C16:0, C10:1, and C14:1], six ethyl esters (e.g., ethyl caprate, ethyl 9-decenoate, etc.), three aliphatic alcohols (e.g., (2E)-tridecen-1-ol, etc.), six methyl alkyl ketones, four aliphatic aldehydes, five sesquiterpenes (e.g., selinane, cadinene isomers, etc.), eight linear/branched hydrocarbons, five lactones, one aromatic acid, and one aromatic alcohol. Other relevant molecules (volatiles) were also identified in the majority (50–75%) of the SC samples, i.e., 11 esters (e.g., ethyl caprylate, ethyl (9Z)-tetradecenoate, ethyl caproate, β-phenethyl acetate, ethyl (9Z)-heptadecenoate, etc.), two aromatic hydrocarbons, three aliphatic aldehydes, four branched/linear hydrocarbons, and three monoterpenes (camphene, fenchene, and α-pinene), along with C16:1 fatty acid, m-tolualdehyde, and octadecanol; therefore, the total constituents identified in the SC samples by SDE were 53–75 compounds. Nonetheless, if the compositions (by SDE) of SC samples are contrasted with those of the RM, and the common components are subtracted (so that those molecules that appeared after the fermentation process stand out, some of which are necessarily the result of the fermentation biochemistry (by LAB) but others are not), the new contributing composition related to the aroma profile could involve between 17–30 components, without disregarding the contribution of the inherent components of the RM. Non-parametric ANOVA of SDE data from SC samples revealed significant differences (p: < 0.05, Friedman test—Table S18) for the compositions of samples L3 vs. L6 (related to ester content) and some differences between L2 vs. L4 (related to ester and fatty acid contents); and, based on the results of the K-W test, there were no differences within each group [type of compounds (p: 0.4557—Table S19)] for all samples.
On the other hand, the volatile fractions isolated by HS-SPME (Figure S4B) from the SC samples were mostly represented by 11 fatty acids (including short-chain; C2:0, C4:0, C6:0–C16:0, C10:1, and C14:1), seven ethyl esters (e.g., ethyl caprate, ethyl caprylate, ethyl acetate, ethyl laurate, ethyl caproate, etc.), one aromatic acid, five sesquiterpenes, four methyl alkyl ketones, one aromatic alcohol, one monoterpene (limonene), one methyl alkoxyl ketone (acetoin), four aliphatic alcohols (e.g., ethanol, isoamyl alcohol, (2E)-tridecen-1-ol, etc.), seven linear/branched hydrocarbons, three lactones, and two aliphatic aldehydes (myristylaldehyde and palmitaldehyde). Lastly, a sesquiterpene [unidentified (C15H16—M+• 206, base peak 191), retention index: 1470, and non-negligible amount relative (0.6–12% by SPME; 0.4–5% by SDE)] was present in all SC sample.
The other volatile molecules identified and related to 50–75% of SC samples were 10 esters (e.g., ethyl 9-decenoate, ethyl butyrate, ethyl pelargonate, ethyl tridecanoate, ethyl (9Z)-hexadecenoate, isopropyl palmitate, etc.), four aliphatic alcohols (e.g., (2E)-undecen-1-ol, pentanol, etc.), two methyl alkyl ketones, four monoterpenes (α-pinene, p-cymene, tricyclene, and camphene), four aliphatic aldehydes, five linear/branched hydrocarbons (e.g., (7Z)-tetradecene, neophytadiene, etc.), three fatty acids (e.g., (5Z)-C12:1, C11:0, etc.), two sesquiterpenes (cyperene and trans-calamenene), and one lactone (δ-tetradecalactone), along with m-tolualdehyde and styrene. Consequently, the most frequent constituents of SC samples by HS-SPME were 48–79 compounds, but the subtraction of the common components between RM and SC would indicate that 15–42 constituents would contribute to the flavor-related composition of the SC samples, together with those corresponding to the RM. The results of non-parametric ANOVA on the composition data of SC samples showed that there were no significant differences within each group [type of compounds (p: 0.4579—Table S19)] for all samples based on the K-W test, while according to the Friedman test (Table S18), significant differences (p: 0.0075) were observed between samples L3 and L6, L1 and L6, and L8 and L3, as well as some differences between the remaining samples.
2.3. Differences and Similarities Between Constituents of Volatile Fractions by PCA and LDA
An important aspect in these results was the difference or similarity found between the SC samples (regardless of extraction method), which could be mainly attributed to the manufacturing process (including raw material and fermentation time and mode, as well as the inoculum type) of this food product (Table S20). Based on this Table, there were at least five SC preparation methods. Consequently, it was hypothesized that the chemical compositions should be similar for L2 and L8, L3 and L7, L4 and L6, with some difference for L5. For L1, it could show similarity with L2 and L8, but its fermentation was longer (for 72 h). Therefore, the comparison between the volatile fractions of the RM samples with those of SC was carried out by multivariate analysis using PCA and discriminant analysis based on the type of compounds (constituents of the volatiles fractions) and the manufacturing process of the SC. The resulting plots from the PCA and LDA are shown in Figure 3.
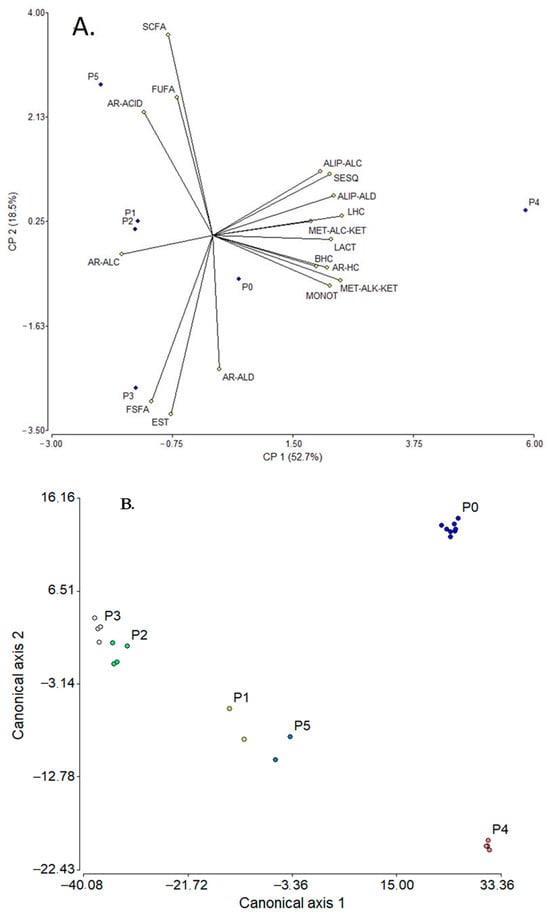
Figure 3.
PCA (A) and LDA (B) plots relating the effect of SC manufacturing method to the composition of volatile fractions. SCFAs: short-chain fatty acids (≤C6), FSFAs: free saturated fatty acids (≥C7), FUFAs: free unsaturated fatty acids, ALIP-ALDs: aliphatic aldehydes, ALIP-ALCs: aliphatic alcohols, MET-ALK-KETs: methyl alkyl ketones, AR-ALDs: aromatic aldehydes, AR-ALCs: aromatic alcohols, AR-ACIDs: aromatic acids, ESTs: esters, LACTs: lactones, MONOTs: monoterpenoids, SESQs: sesquiterpenes, LHCs: linear hydrocarbons, BHCs: branched hydrocarbons, AR-HCs: aromatic hydrocarbons, HTC-ALCs: heterocyclic alcohols, PhOHs: phenols, HTC-ETs: heterocyclic ethers, MET-ALC-KETs: methyl alcohol ketones, AL-ACIDs: aliphatic acids, P: process related to the method for obtaining suero costeño; P0: unfermented raw milk, and P1, P2, P3, P4, and P5: processes described in Table S20.
Thus, the PCA explained ca. 71% of the data variability as a function of the two main factors; the variables with the highest contribution to PC1 were linear hydrocarbons (LHCs—0.98), methyl alkyl ketones (MET-ALK-KETs—0.97), aliphatic aldehydes (ALIP-ALDs—0.92), and lactones (LACTs—0.90), while for PC2, the contributing variables were short-chain fatty acids (SCFAs—0.87), esters (EST—0.77), and free saturated fatty acids (FSFAs—0.70). These correlations allowed to discriminate each process used for SC manufacture; i.e., P4 [cooked whey residue from cheese and salting whey (or commercial milk cream)] was the process with the greatest difference (according to the chemical composition criterion), clearly observed in the graph (Figure 3A), while the most similar processes were P1 (milk fat and spontaneous fermentation, three day) and P2 [raw milk and spontaneous fermentation, one day (“totuma”)], closely grouped. It is important to mention that for this analysis the “point 0” (P0, related to unfermented RM) was included, which was used for comparison. On the other hand, Figure 3B displays the graph resulting from the LDA applied to all samples. According to this analysis, ca. 98% of the data variability could be explained considering the two canonical axes 1 (83.8%) and 2 (14.2%); the most discriminating variables were FSFAs (75.6) and ESTs (−79.0) for axis 1 and monoterpenes (MONOTs, 7.5) and methyl alkoxyl ketones (MET-ALC-KETs, −6.7) for axis 2. Additionally, it was evident that P0 differed noticeably from the other processes and three groupings could be observed: (i) P2 and P3, with greater similarity; (ii) P1 and P5, some similarity; and (iii) P4, the most dissimilar.
From another perspective, an additional multivariate analysis was performed using the same statistical tools to contrast the volatile fractions between SC samples and locations. For PCA (Figure 4A), the main findings were that the two main factors could explain ca. 71% of the data variability; the contributing variables to PC1 were LHCs and MET-ALC-KETs (0.98 for each) and branched hydrocarbons (BHCs) and ALIP-ALDs (0.96 for each), while for PC2, these were ESTs (0.86), FSFAs (0.82), and free unsaturated fatty acids [FUFAs (−0.74)]. As a result of this analysis, three groupings could be established: (i) L4 and L6, (ii) L3, and (iii) L1, L2, L5, L7, and L8; among them, L4 and L6, L2 and L8, and L1 were consistent with the hypothesis of compositional similarity based on the same manufacturing process, but L5, L7, and L3 apparently were not. Considering the LDA, the two canonical axes 1 (58.0%) and 2 (36.0%) were able to explain ca. 94% of the data variability; the most discriminating variables for both axes (1 and 2) were FSFAs (−4.70 and 7.21) and ESTs (4.42 and −7.17). In addition, four groupings were observed in the plot: (i) L3 and L7, closely related to (ii) L1, L2, and L8, (iii) L5 and L6, and (iv) L4. Considering the compositional similarity hypothesis, then, L3 and L7 and L2, L8, and L1 were consistent, but L4, L5, and L6 were not.
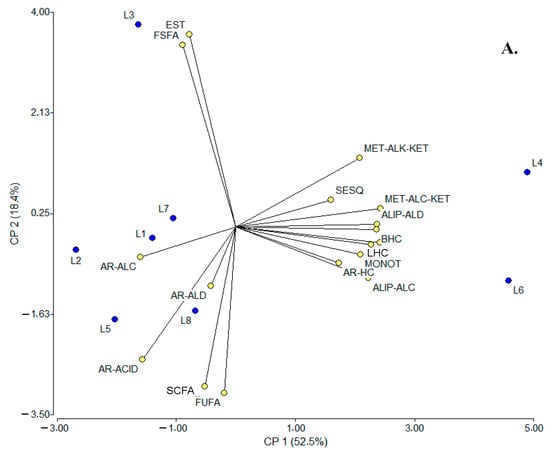
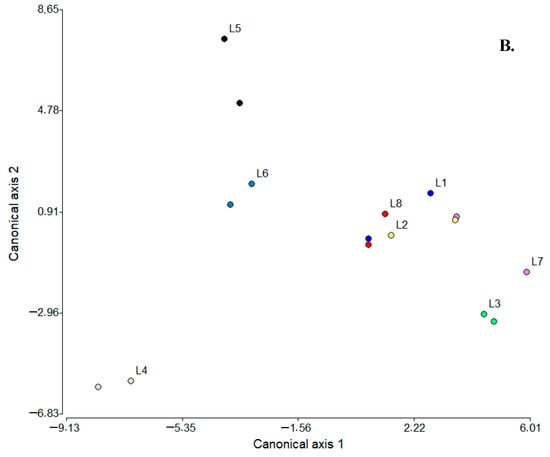
Figure 4.
PCA (A) and LDA (B) plots relating SC sampling points (locations) to the composition of volatile fractions. SCFAs: short-chain fatty acids (≤C6), FSFAs: free saturated fatty acids (≥C7), FUFAs: free unsaturated fatty acids, ALIP-ALDs: aliphatic aldehydes, ALIP-ALCs: aliphatic alcohols, MET-ALK-KETs: methyl alkyl ketones, AR-ALDs: aromatic aldehydes, AR-ALCs: aromatic alcohols, AR-ACIDs: aromatic acids, ESTs: esters, LACTs: lactones, MONOTs: monoterpenoids, SESQs: sesquiterpenes, LHCs: linear hydrocarbons, BHCs: branched hydrocarbons, AR-HCs: aromatic hydrocarbons, HTC-ALCs: heterocyclic alcohols, PhOHs: phenols, HTC-ETs: heterocyclic ethers, MET-ALC-KETs: methyl alcohol ketones, AL-ACIDs: aliphatic acids, and DIKETs: diketones. L: locations where suero costeño samples were collected; L1–L8: locations listed in Table S20.
Finally, the results of the “two-sample inference (F-test of equality of variances)” for all data, grouped by matrix types (RM and SC), are listed in Table S21. According to the table, seven types of compounds showed significant differences (p <0.05) between the samples and were, therefore, the differentiating and outstanding component types: free saturated fatty acids, esters, aliphatic aldehydes, methyl alkoxyl ketones, aromatic aldehydes and acids, and branched hydrocarbons.
Box plots were also applied to all data, comparing matrix types versus compound types (Figure 5). From these plots, it could be observed and inferred that the content (i) distinctly decreased for SFSAs, FUFAs, and BHCs, (ii) notably increased for ESTs, ALIP-ALCs, MET-ALK-KETs, MET-ALC-KETs, and SESQs, (iii) slightly decreased for SCFAs, LHCs, and AR-HCs, (iv) increased slightly for AR-ACIDs, ALIP-ALDs, AR-ALCs, AR-ALDs, and MONOTs, and (v) did not change for LACTs in SC samples compared to milk samples. This interpretation is important because it showed the formation of ESTs from SFSAs, FUFAs, and SCFAs and ALIP-ALCs; i.e., the products (esters) increased and the precursors/reagents (acids and alcohols) decreased.
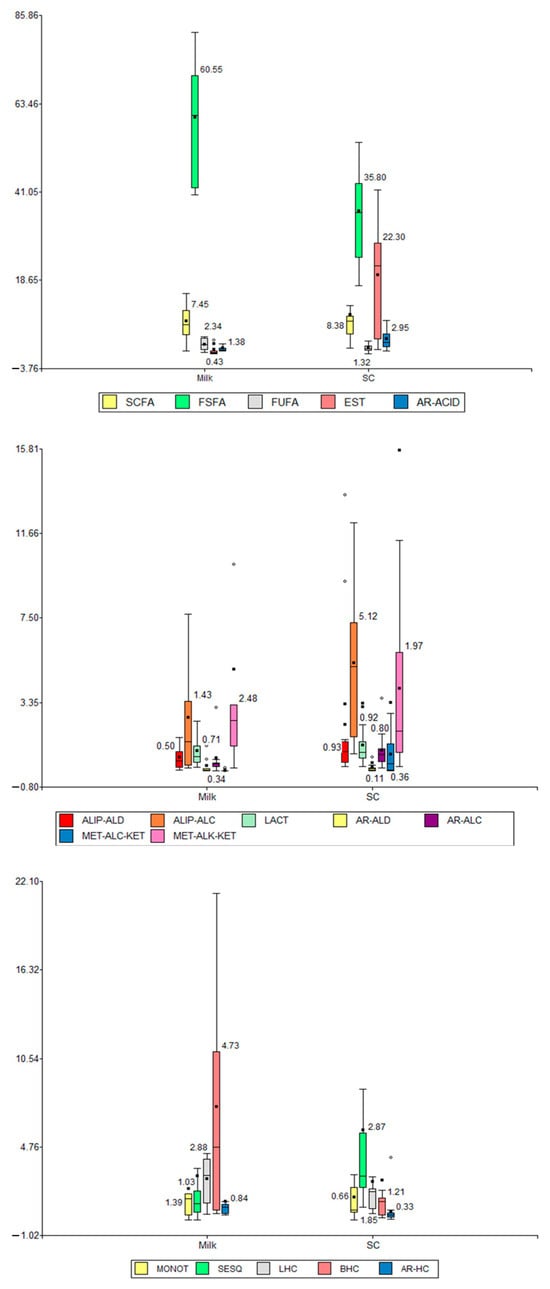
Figure 5.
Box plots relating compound types (volatile fractions) versus matrix types (milk and SC). SCFAs: short-chain fatty acids (≤C6), FSFAs: free saturated fatty acids (≥C7), FUFAs: free unsaturated fatty acids, ESTs: esters, AR-ACIDs: aromatic acids, ALIP-ALDs: aliphatic aldehydes, ALIP-ALCs: aliphatic alcohols, LACTs: lactones, AR-ALDs: aromatic aldehydes, AR-ALCs: aromatic alcohols, MET-ALC-KETs: methyl alcohol ketones, MET-ALK-KETs: methyl alkyl ketones, MONOTs: monoterpenoids, SESQs: sesquiterpenes, LHCs: linear hydrocarbons, BHCs: branched hydrocarbons, and AR-HCs: aromatic hydrocarbons.
3. Discussion
As a starting point for this discussion, when comparing the results of the yields and fat contents determined for the RM samples with the available scientific literature, some similarities and differences were found. For example, Eisenstecken et al. [34], Mehta [35], Nantapo et al. [36], O’Callaghan et al. [37], and Villeneuve et al. [38] affirmed that cow’s milk contained fat in the range of 2.3–5.3%, which was consistent with the fat content of most of the RM samples in this work, except for that at location 2 (2%), which was outside but close to the lower limit of yields. Furthermore, the FA profiles of raw cow’s milk from the literature reviewed were similar to those of this study; i.e., the main fatty acids reported by Eisenstecken et al., Mehta, Villeneuve et al., Čuboň et al. [39], Liu et al. [40], MacGibbon and Haddadian [41], Pilarczyk et al. [42], Riuzzi et al. [43], and Santos Júnior et al. [44], were palmitic (19–39%), oleic (12–34%), stearic (6–15%), and myristic (7–14%) acids.
Likewise, the contents of SFAs, MUFAs, PUFAs, and omega acids (n-3 and n-6) in this research were mostly within the ranges reported by Nantapo et al. [36], Pilarczyk et al. [42], and Butler et al. [45], which were SFAs (48–78%), MUFAs (2–30%), PUFAs (2–9%), n-3 (0.3–2%), and n-6 (1–3%); nonetheless, the values of MUFAs and n-3 and n-6 in this study were respectively higher (~27–36%) and lower (0.2–1.2% and 0.6–1.5%) than those recorded by Mollica et al. [46] (MUFAs: 2–30%, n-3: 0.3–1.8%, and n-6: 1.2–3.0%). In addition, Santos Júnior et al. [44] found values of n-3 and n-6 for milk samples (raw/pasteurized) of 2.2–3.2% and 0.4–0.5%, in that order, showing differences with this work. In contrast, Riuzzi et al. [43] reported n-3 and n-6 values for RM samples of 0.9–1.2% and 2.3–2.8%, correspondingly, which were similar and/or higher than those of this work; furthermore, SFAs (67–70%), MUFAs (26–28%), and PUFAs (4.6–5.2%) values showed some differences in SFA (higher values) and MUFA (lower values) contents.
Finally, the calculated AI and TI values were compared with the indices determined by O’Callaghan et al. and Pilarczyk et al. [37,42]; the values reported by these authors ranged between 2.4–3.6 for AI, and 2.2–4.6 for TI, which were higher than or equal to those of this research. The fundamental premise of these indices is that the lower the AI and TI values, the lower the risk of cardiovascular disease [33].
Since SC is an artisanal product from Colombia, only one report has been found (Valencia García et al. [19]) that mentions the chemical analysis of the FA profiles of SC samples manufactured at laboratory and producer scales using three different procedures; nonetheless, no chemical composition related to the FAs was included in that manuscript. Consequently, this would be the first report on yields and fatty acid composition for SC. For this reason, these contents or compositions will be compared and discussed with other artisanal products (like SC) from other countries and with commercial sour creams or fermented creams.
Thus, Yilmaz-Ersan [47] determined the FA profiles and families of commercial UHT creams fermented with three probiotic strains (Bifidobacterium lactis, L. acidophilus, and L. rhamnosus); the creams were composed of SFAs (64–65%), MUFAs (28%), and PUFAs (4%), represented by C16:0 (30–31%), C18:1 (22–23%), C18:0 (12%), and C14:0 (10%) acids. Moreover, Izsó et al. [48] reported the yield (20%) of three types of sour cream, as well as their FA contents (70%-SFAs, 23%-MUFAs, 3%-PUFAs, 0.4%-n-3, and 2.2%-n-6; characterized by C16:0-33%, C18:1-20%, C14:0-12%, and C18:0-10%). Comparisons between SC and sour creams, related to fatty acid contents and compositions, presented similarities but differed in fat yields; that is, sour creams had fat contents of 18–20% (this is a quality/manufacturing requirement ranging from 14–35% [16,49]) while SC had lower fat content values (5–8%).
In the aspects related to raw milk volatiles, the comparison between the compounds obtained by SDE and HS-SPME showed some similarities and differences both in relative amounts and in certain constituents, indicating that both techniques were complementary (Figure 6). That is, some components were trapped by SPME but not by SDE, e.g., limonene, acetone, isoamyl alcohol, pentanol, ethyl caprate/laurate/myristate, and acetic and butyric acids; and other components were obtained by SDE but not by SPME, e.g., γ-dodecalactone, octadecanal, palmitaldehyde, linear/branched/aromatic hydrocarbons, etc. Nevertheless, the most abundant constituents for both methods were similar, with differences in their relative amounts.
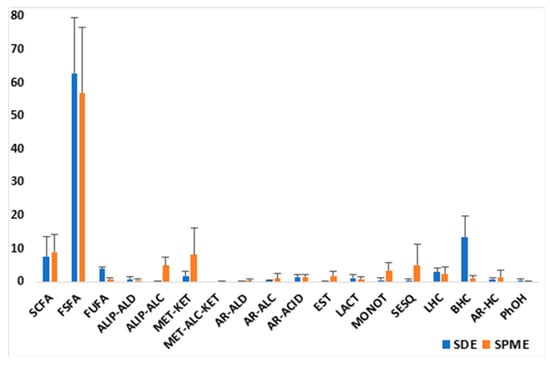
Figure 6.
Comparison of the types of compounds identified in the volatile fractions (by SDE and HS-SPME) of the RM samples (average values of the relative amounts).
The gamma of volatile constituents found in the volatile fractions (by SDE and HS-SPME) of the milk samples was consistent with the scientific literature consulted, with some differences. Thus, Yuan et al. [50] reported that Chinese raw milk mainly contained fatty acids (C15:0, C16:0, C14:0, C10:0, and C6:0), followed by styrene, benzoic and butyric acids, and 2-nonanone, while another milk sample studied by Zhang et al. [51] was constituted by C8:0, C6:0, C10:0, C2:0, and C4:0 fatty acids together with acetoin and other ketone and aldehyde compounds (low molecular weight—LMW), together with δ-decalactone/δ-dodecalactone, as the main volatile components.
Instead, in some Italian milk samples, Zacometti et al. [52] identified fatty acids (C10:0, C8:0, C7:0, and C3:0), LMW-aldehydes, 2-octanone, anisyl formate, and 2-pentanol as volatile constituents. Similarly, in another study by Genovese et al. [53], the authors reported that the volatile fractions of RM samples consisted of C8:0, C6:0, C10:0, and C4:0 fatty acids, along with hexanal, 2-ethylhexanol, nonanal, acetone, and 2-butanone.
In contrast, Vagenas and Roussis [54] indicated that the volatile fraction of Greek RM (by HS-SPME) was composed of fatty acids (C8:0, C6:0, C10:0, C4:0, and C12:0) as major constituents and methyl alkyl ketones and lactones as minor components. Additionally, Villeneuve et al. [38] determined that Canadian RM volatiles were represented by fatty acids (C10:0, C6:0, C8:0, C12:0, and C2:0), followed by methyl alkyl ketones (acetone, 2-butanone, 2-heptanone, etc.), aliphatic and aromatic aldehydes, aliphatic esters (methyl- and ethyl-), lactones (δ- and γ-), terpenes (α-pinene and limonene), and alcohols. Finally, Kim Ha and Lindsay [55] determined that RM fat had C4:0, C10:0, C6:0, and C8:0 fatty acids as major volatiles.
From a general point of view on volatiles in the SC samples, some particular and unusual molecules (both in high and low relative amounts) were identified, e.g., (7Z)-tetradecene, (2E)-tridecen-1-ol, ethyl esters [of 9-C10:1, (9Z)-C14:1, (9Z)-C16:1, (9Z)-C17:1, (9Z)-C18:1, (9Z,12Z,15Z)-C18:3, (9Z,12Z)-C18:2, and C18:0], β-phenethyl acetate, m-tolualdehyde, 5-methyl-2-hexanone, 2-methylbutanoic acid, etc. Some of them [(7Z)-tetradecene, (2E)-tridecen-1-ol, ethyl 9-decenoate (C10:1), and ethyl (9Z)-tetradecenoate (C14:1)] are recorded for the first time as constituents of a dairy product (or fermented sour creams), but others have been found in other dairy products (goat cheese: ethyl 9-decenoate; goat cheese/mozzarella/camembert: β-phenethyl acetate; Spanish artisanal cheese: ethyl (9Z)-hexadecenoate, ethyl (9Z)-tetradecenoate, ethyl linoleate, and ethyl oleate) and in plants, which could be food sources for animals during grazing; likewise, sesquiterpenes (β-caryophyllene, α-humulene, etc.) and monoterpenes (limonene, α-pinene, p-cymene, etc.) found in the SC samples could come from this type of source [56,57,58,59,60].
When consulting the scientific literature on the volatiles of SC, only the report by Valencia García et al. [19] described the volatile profiles (by HS-SPME/GC-MS) of seven SC samples prepared at laboratory (four samples) and production (three samples) scales from isolated native starter cultures (L. plantarum, S. infantarius, and L. lactis). However, no report was found on the chemical composition of the volatile fraction of SC by SDE/GC-MS; therefore, this is the first report on the complete volatilome of this Colombian artisanal food product considering that the SPME technique alone is not sufficient to determine the volatile constituents of the SC, as mentioned above. Comparison between the volatiles identified in SC samples by Valencia García et al. [19] with those of this study showed differences and some similarities. That is, the authors reported for each sample 1–2 alcohols (hexanol, 1,4-butanediol), 1–2 methyl alkoxyl ketones (acetoin, acetol), 1–2 SCFAs (C2:0, C4:0), 1 FA (C10:0), 2–5 aldehydes (acetaldehyde, nonanal, etc.), and 2–3 other components (dimethylsulfide, etc.), for a total of 18 compounds for all samples, with varying relative amounts (0.01–18%); of these volatiles, only acetoin, C2:0, C4:0, C10:0, and nonanal were identified in this research. Furthermore, esters (including lactones), ethanol, fatty (iso-C4–C9 and C10:1–C16) and aromatic acids, methyl alkyl ketones, terpenes (C10 and C15), and hydrocarbons were not identified in any of the SC samples in the study by Valencia García et al. [19]; these notable compositional differences could be related to both the inoculation (strains and time) and the manufacturing processes used for SC production.
Additionally, some comparisons were made with science reports of products with similar physicochemical characteristics to those of SC and related to the fermentation process of cow’s milk (sour cream). Thus, Zhao et al. [61] characterized the profiles of volatiles produced during milk fermentation by Streptococcus thermophilus and Lactobacillus helveticus); the authors reported 56 constituents, belonging mainly to aldehyde, ketone, acid, alcohol, and ester type compounds. Among them, 2,3-butanedione, acetoin, 2,3-pentanedione, C6:0, C2:0, acetaldehyde, and C4:0 were identified as discriminating molecules between different time points and fermented milk samples.
While Tan et al. [62] identified 36 volatile compounds (14 ketones, 11 aldehydes, six alcohols, and five other compounds) in fermented milk, but as the milk fat content increased the level of ketone compounds increased; in addition, 14 constituents (eight ketones and six aldehydes) were responsible for the aroma profile. Additionally, in the research carried out by Wang et al. [63] on the release profile of flavor compounds from sour cream, the authors found that 31 components such as esters (nine), aldehydes (six), acids (three), alcohols (six), ketones (five), and other substances (two) were present in the volatile fractions of the samples. Moreover, three characteristic molecules (ethyl acetate, 1-octen-3-one, and caproic acid) increased their content as the fermentation time increased.
In contrast, Dan et al. [64] identified 53 and 43 volatile compounds from skimmed milk fermented by two LAB strains (S. thermophilus and Lactobacillus delbrueckii ssp. bulgaricus); these compounds were mainly 11 acids (e.g., C2:0, C4:0, C6:0–C10:0, benzoic acid, etc.), 10 aldehydes (e.g., nonanal, 2-methylundecanal, (E,E)-2,4-heptadienal, benzaldehyde, acetaldehyde, etc.), 13 ketones (e.g., 2-undecanone, 2-nonanone, 2-heptanone, acetoin, etc.), 13 alcohols (e.g., 1-nonanol, 2-nonanol, ethanol, etc.), seven esters (e.g., δ-nonalactone, ethyl caprate/caproate/butyrate, etc.), and 19 hydrocarbons (including terpenes, humulene, caryophyllene, C11–C13, xylene, toluene, etc.). The authors established that the volatile composition of the fermented milk product depended on the LAB strains (starter cultures) as well as whether they were used individually or in combination. Lastly, Shepard et al. [16] determined the volatile constituents (34) related to the aroma profiles of 32 commercial sour cream samples; as a result, 13 components (alkyl sulfides, ethyl(methyl)-alkenyl(alkyl)-ketones, aldehydes, SCFAs, etc.) were responsible for the flavor of 31–32 samples. Based on the concentrations of the 34 constituents, the increasing order of contribution to aroma was acetic acid > acetaldehyde > butyric acid > acetoin > octanal > 2-butanone > nonanal; furthermore, the authors stated that the profiled aroma compounds were derived (by biochemical reactions) from constituents inherent to the RM or its thermal treatment.
The comparison between the literature reports mentioned above and the results of this research showed relevant differences and some similarities; i.e., the volatile profiles of SC samples were mainly characterized by ethyl esters (as the most abundant constituents, e.g., ethyl acetate, ethyl caprate, etc.), followed by fatty (including short-chain (C2:0–C5:0 acids) and unsaturated) and aromatic acids, alcohols (ethanol, isoamyl alcohol, (2E)-tridecen-1-ol, etc.), sesquiterpenes, aliphatic aldehydes, and acetoin. Other contributing molecules were methyl alkyl ketones (2-pentadecanone, 2-tridecanone, 2-nonanone, etc.), lactones (δ-dodecalactone and δ-decalactone), and benzenethanol. This composition showed similarity with the reports by Dan et al. [64] and Zhao et al. [61] in specific constituents (C10:0, C8:0, benzoic acid, C6:0, C4:0, C2:0, nonanal, 2-methylundecanal, 2-undecanone, 2-nonanone, 2-heptanone, acetoin, 1-nonanol, 2-nonanol, ethanol, ethyl caprate, ethyl caproate, ethyl butyrate, humulene, caryophyllene, nC11–nC13 hydrocarbons, and toluene) but differences (in most chemical structures) with that reported by Wang et al. and Tan et al. [62,63]. Finally, the contrast between the composition determined by Shepard et al. [16] and this report only showed similarity in the content of acetoin and C2:0 and C4:0 acids.
All these compositional comparisons between SC and other similar dairy products based on milk fermentation (according to the available scientific literature) are necessary to support that the volatile chemical profile established for this artisanal product is original and unrepeatable, and to attribute to it one of the distinctive criteria that could give rise to its protected designation of origin.
On the other hand, it should be noted that the main metabolites identified in SC samples are probably the result of lipolysis and/or β-oxidation of fatty acids (free fatty acids, methyl alkyl ketones, alcohols, and lactones), as well as glycolysis [metabolism of lactose (ethanol and SCFAs) and citrate (ethyl acetate and acetoin)] carried out during milk fermentation by LAB [9,12,60,63]; thus, (i) the most representative constituents in the SC samples were ethyl esters (C2–C12, C10:1(Δ9)–C18:1(cis-Δ9), and C13–C16), which would contribute a floral and fruity note (or defect/off-flavor, depending on the concentration) to the SC [58,65], and (ii) according to the results on LAB isolation from the majority of SC samples (data not yet published by the coauthors, but used with permission), the predominant strain was Limosilactobacillus fermentum (along with Lacticaseibacillus rhamnosus), which could be responsible for the production of specific volatile compounds in SC; but also, as these strains were different from the LAB strains reported by the authors mentioned during the discussion, the SC volatiles could differ from the chemical profiles described by them in their fermented milk products.
Although the mechanism of ethyl ester biosynthesis by LAB in fermented milk has not been fully elucidated, it is hypothesized that the most likely route of biosynthesis could be via alcoholysis (by transferases) and/or hydrolysis (by esterases), depending on the strain involved in the fermentation, as well as the types of glycerides (acylglycerols) and alcohols [58,66,67]. In this manner, Liu et al. and Holland et al. [58,67] stated that a rate-limiting factor for ethyl ester formation in fermented products (e.g., cheeses) was the ethanol availability (>ethanol content > ethyl ester of fatty acid content), but also the type of glycerides (e.g., tri-, di-, and mono-) present in the milk, as well as the water content and activity. Thus, considering L. fermentum and L. rhamnosus (strains isolated in most of the SC samples), both strains prefer di- and mono-glycerides as substrates for alcoholysis, producing esters between C6–C14, mainly ethyl caprate (C10:0), ethyl caprylate (C8:0), and ethyl caproate (C6:0), as mentioned by Holland et al. and Liu et al.; hence, this could explain why ethyl esters (up to C14:0) were mostly identified in the SC samples.
Last but not least, L. fermentum could produce some chemical constituents in non-negligible amounts (~5%) that would confer a less-palatable perception to SC, e.g., acetic acid (vinegar-like taste) and acetoin (strong butter-like taste) [68]; the tastes [related to C2:0/acetoin, which were identified in the SC samples (1.0–3.9%/0.7–3.4%)] described above are characteristic notes of the SC product (acrid/sour and butter-like tastes, based on preliminary sensory testing—information not yet published, but used with permission).
As for the PCA and LDA applied to the data, they allowed finding and demonstrating close relationships between the volatile fractions of the SC samples as a function of the manufacturing process and locations, as well as determining those main structural groups responsible for the possible identity profile of the SC. The latter can be visualized in a general way with a box-plot representation (Figure 5), where through the comparison between matrices (RM and SC) based on volatile chemical components, for example, a decrease of FSFAs and an increase of ESTs (produced by the condensation/substitution reaction between an organic acid and an aliphatic alcohol) were observed. Nevertheless, it should be highlighted that locations 4 and 6 using process 4 (cooked whey residue from cheese + salting whey or commercial milk cream) along with location 5 using process 5 (rennet curdling) produced SC with the highest variability in the main volatile constituents identified for most SC samples, i.e., (i) the lowest and lower contents of FSFAs and esters for both L4 and L6, (ii) the highest and lowest contents of FSFAs (including SCFAs) and esters, respectively for L5, and (iii) the lowest (L4) and mid-range (L6) contents of SCFAs.
Of course, since the raw material for process 4 was whey (containing up to 14% protein and up to 4% fat [69]) from artisanal cheese making, its fat content must have been low and, consequently, lipolytic activity (or β-oxidation of fatty acids) by LAB during the fermentation process did not produce amounts of FSFAs and esters (derived from free fatty acids) comparable to the other SC samples, which would be in agreement with that reported by Asif et al. [70], who determined that during the buttermilk fermentation (by LAB) to produce cheddar cheese, the formation of free fatty acids by lipolysis depended on the fat content of the matrix, i.e., the higher the fat content, the higher the amount of free fatty acids. In addition, if there was less fat content (as well as low glyceride content), the esterification by LAB (alcoholysis) should also be low [58,67].
On the other hand, as it is well known, rennet is a complex mixture of protease-type enzymes (mainly chymosin and pectin) used industrially or domestically to curdle milk (casein coagulation) and produce fermented dairy foods (e.g., ripened and fresh cheeses), which might present desirable or undesirable sensory characteristics (taste and flavor) depending on storage time, as well as whether or not the rennet contains some lipases [71]. However, when Castillo et al. [72] studied the effect of the use of rennet (sanitized paste) on the volatile fraction of a hand-made cheese (from goat’s milk), they found the highest content of SCFAs (C4:0 >> C6:0), an increased content of mono-/di-glyceride, and a low content of ethyl esters (C8:0 and C6:0 esters being the majority) compared to other industrially processed cheeses; they also suggested that the high SCFA content was due to native lipases in raw milk. These findings showed similarities with the SC from L5: the highest content of SCFAs (differing in C6:0 >>> C4:0), an increase of monoglycerides (related to FSFA content), and a low content of ethyl esters (C8:0 > C6:0); consequently, the volatile chemical profile was different from that of the other SC samples.
Finally, Lytou et al. [73] described that (i) SPME and GC-MS are widely used techniques for food volatilome analysis, (ii) multivariate analysis (PCA and LDA) is an important tool for an accurate description of the obtained data, (iii) each food would have a particular volatile chemical fingerprint, and (iv) food volatilome profiles could be a useful and effective tool for the assessment of both quality and authenticity. According to these premises, the volatilome profile of SC could be established in this research and, therefore, this food would have its own volatile chemical fingerprint, which is described above.
4. Materials and Methods
4.1. Reagents, Standards and Materials
Dichloromethane (ACS grade, Alfa Aesar, Haverhill, MA, USA), BF3/methanol (14%, Ref. B1252, Sigma-Aldrich, Darmstadt, Germany), n-hexane (ACS grade, Merck, Billerica, MA, USA), α-pinene (98%, Ref. 147524, Sigma-Aldrich, Darmstadt, Germany), DL-limonene (for synthesis, Ref. 8.14546, Sigma-Aldrich, Darmstadt, Germany), camphor (>95% Merck), δ-decalactone (>98%, Ref. W236101, Sigma-Aldrich, Darmstadt, Germany), δ-dodecalactone (>97%, Ref. W240109, Sigma-Aldrich, Darmstadt, Germany), acetic acid glacial (>99%, Ref. 695092, Sigma-Aldrich, Darmstadt, Germany), butyric acid (>99%, Ref. B103500, Sigma-Aldrich, Darmstadt, Germany), valeric acid (>99%, Ref. 240370, Sigma-Aldrich), FAME Mix C8–C24 (Ref. 18918, Sigma Aldrich), C10–C40 alkane standard mixture (Ref. 68281, Supelco, Darmstadt, Germany), type I water (milli-Q® Integral, Merck, Billerica, MA, USA), NaCl (≥99.5%, Merck, Billerica, MA, USA), SPME device (Supelco, Darmstadt, Germany), and coated fiber of divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS, 50/30 μm) were used.
4.2. Collection of Raw Milk and Suero Costeño Samples
Eight representative samples of cow’s milk and suero costeño were collected from eight individual farms and four municipalities (mainly artisanal producers) in the department of Córdoba (northern region of Colombia—Table S20) based on convenience sampling, together with the stratified random sampling as described by Greenfield and Southgate (FAO) [74] for food composition data collection. In addition, farmers produced SC based primarily on certain factors: (i) from naturally fermented raw milk (37.5% collected samples), (ii) using boiled milk (25.0% collected samples), (iii) cooked residual whey from cheese preparation (25.0% collected samples), and (iv) rennet-fermented raw milk (12.5% collected samples). SC and RM samples were collected in the second half of 2022 (August to December) and, as current practice, the fermentation process was carried out for at least 24 h (with one exception, for 72 h). Then, 150 mL of each homogenized/representative sample was collected (in triplicate) in Falcon tubes and immediately taken to the chemical laboratory for processing and analysis. Some samples were temporarily stored at −20 °C until further processing and analysis; this storage condition was critical to decrease/prevent the biochemical activity of LAB or other microorganisms present in the SC and, therefore, did not alter their organoleptic (aroma and taste) and physicochemical properties [75,76,77].
4.3. Volatile Fractions
To isolate the volatile fractions from the samples, they were mixed with matrix modifiers, i.e., 10% NaCl (salting effect) for milk and water (1:2 H2O:sample) for SC [50,78]. Moreover, two sampling techniques were used: headspace solid-phase microextraction and simultaneous distillation-solvent extraction [79]. Each sample was extracted in triplicate by each technique.
For simultaneous distillation-solvent extraction, the volatile fractions of the dairy samples (10 g) were obtained by the Likens & Nickerson microscale apparatus, modified by Godefroot et al. [80], using CH2Cl2 (2 mL) as extraction solvent for 2 h. The extracts were dehydrated with anhydrous sodium sulfate and 1 μL of each of them (individually) was analyzed by GC-FID/MSD.
For headspace solid-phase microextraction, SC and RM vapor-phase constituents were trapped using a SPME device (Supelco) with a DVB/CAR/PDMS (50/30 μm) coated fiber, sampling the headspace. Food samples (10 g) were thermally pre-conditioned (45 °C) for 10 min and then the fiber was exposed to the headspace of each sample (separately) at 45 °C during 45 min (with magnetic stirring) as described by Gioacchini et al. [81], Zhang et al. [82], and Mounchili et al. [83]. After sampling was completed, the SPME fiber was desorbed (5 min, 250 °C) with the analytes inside the GC-FID/MS inlet port.
For GC-FID/MS analysis, each volatile fraction (SPME and SDE) was analyzed using a Trace 1310 gas chromatograph with a flame ionization detector (FID, 250 °C) and an ISQ Series mass spectrometer (Thermo Fisher Scientific), with split/splitless inlet (split ratio, 10:1—SDE; splitless—SPME), a liquid autosampler (AI/AS 1310 Series, Thermo Fisher Scientific), or manual injection (SPME). Separation of the mixtures was obtained (i) on a Rxi®-1MS column (crossbond PDMS, 30 m × 0.25 mm ID × 0.5 µm df, Restek, Bellefonte, PA, USA) and (ii) with linear temperature programming in the GC oven: 60 °C (5 min) @ 3 °C/min up to 250 °C (5 min). The separated components were reported as normalized area percentages (% relative amounts).
Mass spectra were obtained by electron ionization (EI, 70 eV) and a quadrupole mass analyzer with mass ranges m/z 40–350, in full scan mode; helium (99.999%) was used as the carrier gas (1.0 mL/min) and the temperatures of the transfer line and ion source were set to 250 °C and 230 °C, respectively. Chromatographic and spectroscopic data were processed using Thermo XcaliburTM (Version 2.2 SP1.48, Thermo Fisher Scientific) and Automated Mass Spectral Deconvolution and Identification System (AMDIS, Build 130.53, Version 2.70) software.
4.4. Isolation of Fats and Their Fatty Acids
Crude fat was determined (for RM and SC samples) using the modified Rose-Gottlieb method—AOAC official method 932.02-1945 [84] and ISO 23318 IDF 249 [85].
Fatty acid profiles were determined based on the AOAC official method 996.06 [fat (total, saturated, and unsaturated) in foods (hydrolytic extraction and GC-MS analysis)] [86], AOAC official method 969.33 (fatty acids in oils and fats preparation of methyl esters) [87], and Ackman [88]. The free fatty acids resulting from fat hydrolysis were derivatized to fatty acid methyl esters (FAMEs) using BF3/CH3OH and extracted with hexane. The FAMEs of the samples were confirmed by comparison with retention times and indices, and the mass spectra of FAME Mix C8–C24 (Table S24).
For GC-FID/MS analysis, each FAME extract was also analyzed by the same separation and detection systems (GC-FID/MS) described above, with a 1:10 split ratio. Separation of the mixtures was obtained with a GC oven temperature program that started at 100 °C (2 min) @ 2 °C/min up to 150 °C (5 min), @ 3 °C/min up to 240 °C (2 min), and @ 6 °C/min up to 280 °C (2 min). Additionally, mass spectra were obtained on a quadrupole analyzer with mass ranges of m/z 45–450. The other operating conditions of the mass detector as well as data processing were the same as described above. The separated FAMEs were reported as normalized area percentages (% relative amounts).
4.5. Identity of the Chemical Constituents Analyzed by GC-MSD
The components were identified by comparing mass spectra and calculated retention indices (RI) with available libraries (NIST11, NIST Retention Index, and Wiley9) and existing scientific literature [32,89,90,91,92,93,94]. As a complement, certified standards (lactones, terpenes, FAMEs, and linear hydrocarbons) were used to confirm some constituents. Linear retention indices were previously calculated in the usual way (C10–C40 aliphatic hydrocarbon series) for both GC-FID and GC-MSD analysis.
4.6. Design of Experiment
The selection of the SC samples (eight) was performed in a stratified random sampling (SRS), considering the most important (or common) manufacturing methods (Table S20), as well as the different localities where SC was prepared in an artisanal (non-industrial) way by small- and medium-sized farmer associations in the region. The number of samples was obtained by calculations from the Australian Bureau of Statistics website [95] considering a total of 11 samples, a 95% confidence level and a confidence intervale of 0.1; the standard and relative standard errors were, respectively, 0.051 and 5.67 (Figure S5). Subsequently, samples were also selected based on the manufacturing process using both SRS and convenience sampling (Table S25), as suggested by FAO [74]. Lastly, milk samples were selected by simple random sampling, taking four samples from the eight locations where the farms of the small- to medium-sized peasant associations were located.
4.7. Statistical Analysis
The raw data of the results were treated by non-parametric ANOVA (Kruskal–Wallis and Friedman tests), together with the Shapiro–Wilks test (normality of data (p <0.05)) and inference based on two samples (F test for equal variances). All data acquired from the samples were statistically treated and subjected to principal component analysis (PCA) and linear discriminant analysis (LDA) as tools of multivariate statistical analysis using the InfoStat software (free version 2020).
5. Conclusions
From this research, it was possible to conclude that (i) the chemical nature related to fatty acids (composition and relative amount of FAs) of RM was preserved in all SC samples analyzed; moreover, this food was a source of MUFAs, n-3, and n-6 and had acceptable values of AI and TI; (ii) the manufacturing process of SC influenced the composition of the volatile fractions as demonstrated by the variability of some of its constituents; however, the chemical volatile profiles with negligible variabilities were found in the SC prepared using raw milk and spontaneous fermentation and, in consequence, it is advisable to standardize the process (without affecting the characteristic of the artisanal product), as well as to implement good manufacturing practices; and last of all, (iii) the principal types of molecules (differentiators) that would support the possible establishment of chemical identity for this food product (for 75–88% of the SC samples) were ethyl esters [C2–C12 and C10:1(Δ9)–C18:1(cis-Δ9)], aliphatic alcohols [(2E)-tridecen-1-ol, ethanol, and isoamyl alcohol], aldehydes (palmitaldehyde and myristylaldehyde), methyl alkyl ketones (2-pentadecanone, 2-tridecanone, 2-undecanone, and 2-nonanone), sesquiterpenes (β-caryophyllene, α-humulene, and selinane), monoterpenes (limonene), benzoic acid, and benzenethanol, along with SCFAs and acetoin (resulting from the spontaneous fermentative process).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30122524/s1, Table S1: Result of Shapiro–Wilks test (modified) for fat content yields of RM and SC samples; Table S2: Result of non-parametric ANOVA using Friedman’s test for fat content yields of RM and SC samples; Table S3: Result of non-parametric ANOVA using the Kruskal–Wallis test for fat content yields of RM and SC samples; Figure S1: FAME profiles by GC-MS of a suero costeño sample (location 1); Table S4: Identification and content (>0.4%) of fatty acids in samples of raw milk and suero costeño from eight locations of Córdoba (Colombia); Figure S2: Distribution of the four main FAs that constituted the RM and SC samples from the eight locations studied; Table S5: Result of Shapiro–Wilks test (modified) for major fatty acids of RM and SC samples; Table S6: Result of non-parametric ANOVA using Friedman’s test for major fatty acids of RM and SC samples; Table S7: Result of non-parametric ANOVA using the Kruskal–Wallis test for major fatty acids of RM and SC samples; Table S8: Result of Shapiro–Wilks test (modified) for the content of fatty acids by compound families of RM and SC samples; Table S9: Result of non-parametric ANOVA using Friedman’s test for the content of fatty acids by compound families of RM and SC samples; Table S10: Result of non-parametric ANOVA using the Kruskal–Wallis test for the content of fatty acids by compound families of RM and SC samples; Table S11: Result of Shapiro–Wilks test (modified) for AI and TI values of RM and SC samples; Table S12: Result of non-parametric ANOVA using Friedman’s test for AI and TI values of RM and SC samples; Table S13: Result of non-parametric ANOVA using the Kruskal–Wallis test for AI and TI values of RM and SC samples; Figure S3: Volatile profiles by GC-MS of the raw milk (location 1) by (A) SDE and (B) HS-SPME; Figure S4: Volatile profiles by GC-MS of the suero costeño (location 1) by SDE (A) and HS-SPME (B); Table S14: Result of non-parametric ANOVA using the Kruskal–Wallis test for the volatile fractions by SDE and HS-SPME of raw milk samples; Table S15: Result of non-parametric ANOVA using Friedman’s test for the volatile fractions by SDE and HS-SPME of raw milk samples; Table S16: Result of non-parametric ANOVA using Friedman’s test comparing the chemical composition (average relative amount) of volatile fractions by SDE and HS-SPME of raw milk samples; Table S17: Result of non-parametric ANOVA using the Kruskal–Wallis test comparing the chemical composition (average relative amount) of volatile fractions by SDE and HS-SPME of raw milk samples; Table S18: Result of non-parametric ANOVA using Friedman’s test for the volatile fractions by SDE and HS-SPME of the suero costeño samples; Table S19: Result of non-parametric ANOVA using the Kruskal–Wallis test for the volatile fractions by SDE and HS-SPME of suero costeño samples; Table S20: Sampling places (eight) and manufacturing process of SC in four municipalities of Córdoba (Colombia); Table S21: F Test for equality of variances comparing volatiles between raw milk and suero costeño; Table S22: Chemical composition of volatile fractions by SDE/GC-FID/MSD of raw milk and suero costeño samples classified according to the type of compound identified; Table S23: Chemical composition of volatile fractions by HS-SPME/GC-FID/MSD of raw milk and suero costeño samples classified according to the type of compound identified; Table S24: Composition of FAME Mix C8–C24 (Ref. 18918. Sigma Aldrich) determined by GC-FID/MSD; Figure S5: Determination of sample size for SC using sample size calculator (Australian Bureau of Statistics); Table S25: Selection of samples for chemical analysis based on stratified random sampling.
Author Contributions
Conceptualization, writing—original draft preparation, review and editing, GC-FID/MS analysis, data curation, and statistical analysis, A.M.-A.; sample preparation (HS-SPME, SDE), GC-FID analysis, investigation, and data curation, O.J.C.; sample preparation (HS-SPME), review, and investigation, C.G.-C.; funding acquisition, project administration, investigation, and review, M.S.-S.; investigation, validation, and review, B.Á.-B., investigation, validation, and review, A.D.-V., investigation, validation, and review, M.A.-M., investigation, data curation, validation, and review, C.D.P., investigation, validation, and review, Y.P.-P., investigation, data curation, validation, and review, R.A.-P., investigation and review, I.B.-C.; investigation and review, R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia, Tecnología e Innovación, Código proyecto 1112-852-71092—Contrato No 80740-902-2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The most important data are included in the Supplementary Materials, as well as Appendix A.
Acknowledgments
The authors thank the Ministerio de Ciencia, Tecnología e Innovación of Colombia (Convocatoria 852 de 2019) for the financial support of the Project “Desarrollo de ruta para la vinculación al origen del suero costeño: Estrategia para el fortalecimiento de productores lácteos en el departamento de Córdoba (Colombia)” (Resolución No. 1356 de 2020)—Contrato de Financiamiento de Recuperación Contingente No 80740-902-2020 (Código proyecto 1112-852-71092).
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Table A1. Identification of constituents (>0.1%) present in the volatile fractions by SDE of the raw milk and suero costeño samples from eight locations of Córdoba (Colombia). Table A2. Identification of constituents (>0.1%) present in the volatile fractions by HS-SPME of the raw milk and suero costeño samples from eight locations of Córdoba (Colombia).

Table A1.
Identification of constituents (>0.1%) present in the volatile fractions by SDE of the raw milk and suero costeño samples from eight locations of Córdoba (Colombia).
Table A1.
Identification of constituents (>0.1%) present in the volatile fractions by SDE of the raw milk and suero costeño samples from eight locations of Córdoba (Colombia).
| Peak | Constituents | RIcal | RIlit | Raw Milk ‡ | Suero Costeño ‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L3 | L4 | L7 | a L1 | a L2 | a L3 | b L4 | c L5 | b L6 | a L7 | a L8 | ||||
| 1 | 3-Furanmethanol | 843 | 835 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | ---- | ---- | ---- |
| 2 | Isovaleric acid—C5:0 | 849 | 851 | ---- | 0.9 | ---- | ---- | 0.4 | 0.2 | 0.09 | 0.1 | 0.2 | 4.2 | 0.08 | 0.3 |
| 3 | 2-Furanmethanol | 852 | 849 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | ---- | ---- | 0.04 |
| 4 | 2-Methylbutanoic acid—C5:0 | 860 | 861 | ---- | 0.6 | ---- | ---- | 0.5 | 0.4 | ---- | 0.4 | 0.6 | 0.4 | 0.4 | |
| 5 | Xilene † | 869 | 865 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | ---- | ---- | ---- |
| 6 | 5-Methyl-2-hexanone | 870 | 865 | 0.4 | 0.6 | ---- | 0.2 | 0.2 | 0.2 | 0.1 | 0.5 | 0.2 | 1.5 | 0.2 | 0.6 |
| 7 | Valeric acid †—C5:0 | 870 | 880 | ---- | 0.5 | ---- | ---- | 0.3 | 0.3 | 0.2 | ---- | 0.5 | 0.8 | 0.3 | 0.6 |
| 8 | 2-Heptanone | 877 | 880 | 0.4 | 0.6 | ---- | 0.2 | 0.6 | 0.1 | 0.1 | 1.1 | 0.2 | 1.8 | 0.3 | 1.2 |
| 9 | Styrene | 882 | 887 | ---- | ---- | ---- | ---- | ---- | 0.06 | 0.2 | ---- | 0.1 | 0.6 | ---- | 0.1 |
| 10 | Furfuryl ethyl ether derivative * | 893 | ---- | ---- | ---- | ---- | ---- | ---- | 0.2 | 0.1 | ---- | ---- | ---- | ---- | ---- |
| 11 | Tricyclene | 919 | 920 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.2 | ---- | 0.08 | 0.2 |
| 12 | α-Pinene † | 931 | 930 | ---- | ---- | ---- | ---- | ---- | 0.03 | 0.1 | 02 | 0.06 | ---- | 0.2 | 0.3 |
| 13 | Fenchene | 940 | 942 | ---- | ---- | ---- | 0.07 | trace | 0.04 | 0.1 | 0.2 | 0.1 | ---- | 0.2 | 0.3 |
| 14 | Camphene | 945 | 947 | ---- | ---- | ---- | 0.2 | trace | 0.05 | 0.2 | 0.2 | 0.3 | ---- | 0.2 | 0.6 |
| 15 | Caproic acid—C6:0 | 981 | 983 | 9.0 | 13.3 | 5.9 | 0.8 | 8.8 | 6.2 | 4.2 | 1.4 | 27.3 | 2.0 | 5.6 | 10.9 |
| 16 | Ethyl caproate | 987 | 985 | ---- | ---- | ---- | ---- | 0.7 | 0.4 | 1.2 | ---- | ---- | ---- | 1.2 | 0.7 |
| 17 | Decane † | 1000 | 1000 | ---- | ---- | 0.1 | 0.07 | ---- | ---- | ---- | 0.09 | 0.02 | ---- | ---- | ---- |
| 18 | p-Cymene | 1016 | 1015 | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | ---- | ---- | ---- | 0.3 |
| 19 | Limonene † | 1020 | 1022 | ---- | 1.5 | 0.3 | 0.1 | ---- | 0.1 | 0.1 | ---- | ---- | 0.3 | ---- | 0.4 |
| 20 | 2,6-Dimethylnonane | 1024 | 1025 | 0.3 | 1.0 | 0.4 | 0.2 | ---- | 0.04 | 0.1 | 0.3 | ---- | ---- | 0.1 | ---- |
| 21 | m-Tolualdehyde | 1053 | 1056 | 0.6 | ---- | ---- | ---- | 0.05 | 0.1 | 0.07 | ---- | ---- | 0.4 | 0.1 | 0.1 |
| 22 | 2-Methyl-decane | 1064 | 1065 | 1.4 | 1.6 | 1.2 | 0.6 | ---- | 0.1 | 0.2 | 0.6 | 0.09 | ---- | 0.4 | 0.08 |
| 23 | 4-Methylhexanoic acid | 1065 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | ---- | ---- |
| 24 | 2,5-Dimethylethylbenzene | 1066 | 1067 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 |
| 25 | p-Cresol | 1068 | 1066 | ---- | ---- | 0.3 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 26 | 2-Nonanone | 1072 | 1070 | 0.4 | 0.5 | ---- | 0.4 | 0.4 | 0.2 | 0.2 | 1.0 | 0.2 | 1.9 | 0.4 | 1.0 |
| 27 | Enanthylic acid—C7:0 | 1074 | 1073 | ---- | ---- | 0.2 | ---- | ---- | trace | ---- | ---- | 0.06 | ---- | ---- | ---- |
| 28 | Pelargonaldehyde | 1085 | 1084 | ---- | ---- | ---- | ---- | 0.1 | ---- | ---- | 0.1 | 0.02 | ---- | ---- | ---- |
| 29 | Benzenethanol | 1093 | 1091 | 0.3 | 0.4 | 0.4 | ---- | 1 | 1.8 | 0.5 | 0.2 | 0.8 | 0.5 | 1.8 | 0.4 |
| 30 | Undecane † | 1100 | 1100 | 0.9 | 0.2 | 0.2 | 0.2 | ---- | 0.07 | ---- | 0.2 | ---- | ---- | 0.2 | ---- |
| 31 | Camphor † | 1121 | 1125 | ---- | ---- | ---- | 0.04 | ---- | trace | ---- | ---- | ---- | ---- | ---- | 0.06 |
| 32 | Benzoic acid † | 1178 | 1174 | 1.5 | 1.4 | 2.4 | 0.8 | 2.2 | 1.4 | 0.8 | 1.7 | 2.3 | 0.8 | 2.4 | 1.9 |
| 33 | Ethyl caprylate | 1184 | 1180 | ---- | ---- | ---- | ---- | 1.7 | 2.4 | 3.3 | 0.2 | ---- | ---- | 3.3 | 3.7 |
| 34 | Caprylic acid—C8:0 | 1187 | 1180 | 13.6 | 15.0 | 15.7 | 12.2 | 8.9 | 10.5 | 8.3 | 2.3 | 11.8 | 2.5 | 9.6 | 12.7 |
| 35 | 1-Dodecene | 1189 | 1187 | ---- | 0.3 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 36 | (3Z)-Dodecene | 1194 | 1195 | 0.2 | ---- | ---- | 0.2 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 37 | Dodecane † | 1200 | 1200 | ---- | 0.4 | 0.1 | 0.09 | ---- | 0.08 | ---- | ---- | ---- | ---- | 0.08 | ---- |
| 38 | 4,8-Dimethylundecane | 1211 | 1212 | 0.3 | 0.3 | 0.1 | 0.09 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 39 | 2,6-Dimethylundecane | 1218 | 1216 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | ---- | 0.09 | ---- |
| 40 | 2,8-Dimethylundecane | 1225 | 1221 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | ---- | 0.07 | ---- |
| 41 | β-Phenethyl acetate | 1235 | 1233 | ---- | ---- | ---- | ---- | 0.03 | 0.02 | ---- | 0.02 | ---- | ---- | 0.03 | 0.07 |
| 42 | Isoamyl caproate | 1239 | 1234 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.06 | ---- | ---- | ---- |
| 43 | δ-Octalactone | 1244 | 1243 | 0.04 | ---- | ---- | 0.03 | 0.04 | 0.04 | ---- | 0.09 | 0.04 | 0.08 | 0.04 | 0.05 |
| 44 | d 1,3-Di-tert-butylbenzene | 1245 | 1245 | 0.9 | 1.2 | 0.8 | 0.4 | 0.9 | ---- | ---- | 0.5 | 0.1 | 3.5 | 0.3 | 0.04 |
| 45 | (2Z)-Decenal | 1246 | 1249 | ---- | ---- | ---- | ---- | 0.02 | 0.02 | ---- | ---- | 0.01 | ---- | ---- | ---- |
| 46 | Pelargonic acid—C9:0 | 1273 | 1272 | 0.2 | 1.0 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 | ---- | 0.2 | 0.4 | 0.2 | 0.2 |
| 47 | 2-Undecanone | 1281 | 1279 | 0.6 | 0.4 | trace | 0.2 | 0.5 | 0.3 | 0.2 | 1.6 | 0.3 | 2.8 | 0.3 | 1.2 |
| 48 | Ethyl pelargonate | 1285 | 1282 | ---- | ---- | 0.1 | ---- | ---- | 0.02 | 0.04 | ---- | ---- | ---- | 0.02 | 0.04 |
| 49 | 2,6,11-Trimethyldodecane | 1286 | ---- | 1.2 | 1.4 | 0.8 | 0.5 | ---- | 0.05 | 0.2 | 0.7 | 0.09 | ---- | 0.4 | 0.06 |
| 50 | Tridecane † | 1300 | 1300 | 0.2 | 0.3 | 0.2 | 0.1 | ---- | ---- | ---- | 0.1 | ---- | ---- | 0.05 | ---- |
| 51 | 4,6-Dimethyldodecane | 1338 | ---- | 0.7 | 0.9 | 0.5 | 0.3 | ---- | 0.04 | 0.05 | 0.4 | 0.06 | 1.9 | 0.2 | ---- |
| 52 | Isobutyl caprylate | 1338 | 1334 | ---- | ---- | ---- | ---- | ---- | 0.01 | 0.03 | ---- | ---- | ---- | ---- | ---- |
| 53 | (7Z)-Tetradecene | 1362 | 1365 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.3 | 0.4 | 0.3 | ---- | 0.6 |
| 54 | Caproleic acid—C10:1 | 1376 | 1362 | 2.6 | 3.5 | 1.6 | 2.1 | 0.2 | 0.09 | 0.8 | 0.8 | 1.6 | ---- | 0.1 | 2.0 |
| 55 | Ethyl 9-decenoate | 1377 | 1370 | ---- | ---- | ---- | 0.9 | 1.6 | 1.6 | 2.2 | ---- | 0.2 | 1.5 | 2.8 | |
| 56 | Ethyl caprate | 1385 | 1381 | 0.2 | ---- | ---- | ---- | 4.8 | 6.3 | 6.8 | 0.3 | ---- | 0.2 | 7.4 | 5.7 |
| 57 | Capric acid—C10:0 | 1393 | 1388 | 25.4 | 17.1 | 32.3 | 45.7 | 16.2 | 20.8 | 17.6 | 6.6 | 20.0 | 5.0 | 16.7 | 13.7 |
| 58 | Dodecanal | 1393 | 1390 | 0.3 | 0.5 | 0.2 | ---- | 0.1 | ---- | ---- | ---- | ---- | 0.9 | ---- | ---- |
| 59 | Tetradecane † | 1400 | 1400 | 0.2 | 0.4 | 0.07 | 0.06 | 0.1 | 0.1 | ---- | 0.6 | 0.2 | 0.5 | 0.1 | 0.5 |
| 60 | Cyperene | 1402 | 1404 | ---- | ---- | ---- | ---- | 0.06 | ---- | ---- | ---- | 0.1 | 0.1 | ---- | ---- |
| 61 | β-Caryophyllene † | 1422 | 1425 | ---- | 0.2 | 0.1 | 0.4 | 0.2 | 0.4 | 0.3 | 3.4 | 0.3 | 0.7 | 0.7 | 2.3 |
| 62 | 2-Methylbutyl octanoate | 1442 | 1444 | ---- | ---- | ---- | ---- | ---- | 0.07 | 0.03 | ---- | ---- | ---- | ---- | ---- |
| 63 | α-Humulene | 1455 | 1452 | ---- | 0.2 | 0.09 | 0.2 | 0.2 | 0.3 | 0.2 | 2.6 | 0.4 | 0.5 | 0.4 | 1.6 |
| 64 | δ-Decalactone † | 1464 | 1463 | 0.7 | 0.3 | 0.1 | 0.08 | 0.4 | 0.3 | 0.20 | 0.9 | 0.02 | 0.9 | 0.3 | 0.3 |
| 65 | 2,6,10-Trimethyltridecane | 1470 | 1467 | 0.5 | 0.4 | 0.2 | 0.1 | ---- | 0.04 | 0.1 | 0.3 | ---- | 1.0 | 0.1 | 0.1 |
| 66 | Undecanoic acid—C11:0 | 1470 | 1469 | ---- | ---- | 0.1 | 0.2 | 0.08 | ---- | 0.6 | ---- | 0.06 | ---- | 0.08 | 0.1 |
| 67 | (2E)-Dodecenal | 1472 | 1472 | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | ---- | ---- | ---- | ---- | ---- |
| 68 | Propyl decanoate | 1483 | 1481 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.08 |
| 69 | 2-Tridecanone | 1485 | 1482 | 0.8 | 0.5 | 0.04 | 0.2 | 0.7 | 0.5 | 0.3 | 3.3 | 0.4 | 4.0 | 0.5 | 1.2 |
| 70 | Ethyl undecanoate | 1487 | 1483 | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | ---- | ---- | ---- | ---- |
| 71 | Selinane * | 1489 | ---- | ---- | ---- | ---- | 0.09 | ---- | 0.08 | 0.1 | 1.4 | 0.2 | ---- | 0.1 | 0.6 |
| 72 | Pentadecane † | 1500 | 1500 | 0.9 | 1.0 | 0.5 | 0.4 | 0.08 | 0.1 | 0.09 | 0.9 | 0.1 | 2.7 | 0.3 | 0.2 |
| 73 | d 2,4-Di-tert-butylphenol | 1509 | 1512 | 0.6 | 0.9 | 0.3 | 0.2 | 0.2 | 0.05 | 0.09 | 0.6 | 0.07 | 1.6 | 0.3 | 0.06 |
| 74 | γ-Cadinene | 1513 | 1512 | ---- | ---- | ---- | ---- | ---- | 0.03 | 0.08 | 0.3 | 0.02 | ---- | 0.1 | 0.07 |
| 75 | δ-Cadinene | 1521 | 1522 | ---- | ---- | ---- | ---- | ---- | 0.05 | trace | 0.5 | 0.03 | ---- | 0.1 | 0.2 |
| 76 | Isobutyl caprate | 1538 | 1533 | ---- | ---- | ---- | ---- | ---- | 0.03 | ---- | ---- | 0.01 | ---- | 0.04 | ---- |
| 77 | 2,6,10-Trimethyltetradecane | 1554 | 1557 | 0.8 | 0.8 | 0.4 | 0.2 | 0.04 | 0.05 | 0.2 | 0.5 | 0.08 | 1.8 | 0.2 | 0.07 |
| 78 | (2E)-Tridecen-1-ol | 1565 | 1564 | ---- | ---- | ---- | ---- | 1.4 | 0.7 | 1.1 | 3.1 | 1.2 | 5.5 | 0.8 | 1.9 |
| 79 | Lauric acid—C12:0 | 1573 | 1570 | 10.0 | 7.1 | 10.7 | 15.2 | 9.6 | 11.9 | 9.5 | 5.9 | 10.5 | 4.1 | 8.0 | 5.4 |
| 80 | (5Z)-Dodecenoic acid—C12:1 | 1580 | 1578 | ---- | ---- | ---- | ---- | ---- | 0.3 | ---- | ---- | ---- | ---- | 0.2 | 0.3 |
| 81 | Ethyl laurate | 1585 | 1583 | ---- | ---- | 0.1 | ---- | 2.4 | 4.5 | 6.7 | 0.5 | 0.2 | 0.3 | 3.5 | 2.7 |
| 82 | Tridecan-1-ol | 1593 | 1586 | 0.2 | 0.1 | 0.1 | 0.04 | ---- | 0.02 | ---- | 0.1 | ---- | 0.3 | 0.09 | ---- |
| 83 | Hexadecane † | 1600 | 1600 | 0.2 | 0.2 | ---- | ---- | ---- | ---- | ---- | 0.2 | 0.05 | 0.4 | 0.09 | 0.09 |
| 84 | Myristylaldehyde | 1603 | 1601 | 0.1 | 0.2 | ---- | 0.04 | 0.3 | 0.2 | 0.09 | 1.7 | 0.2 | 1.3 | 0.2 | 0.3 |
| 85 | Phenylethyl caproate | 1620 | 1619 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.04 | ---- | ---- | ---- |
| 86 | Isoamyl n-decanoate | 1637 | 1633 | ---- | ---- | ---- | ---- | ---- | 0.1 | 0.1 | ---- | ---- | ---- | ---- | ---- |
| 87 | Myristic alcohol | 1641 | 1647 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.9 | ---- | 0.1 | 0.08 | 0.1 |
| 88 | 2-Methylbutyl decanoate | 1643 | 1647 | ---- | ---- | ---- | ---- | ---- | 0.04 | 0.1 | ---- | ---- | ---- | 0.04 | ---- |
| 89 | γ-Dodecalactone | 1646 | 1645 | 0.06 | 0.06 | ---- | 0.02 | ---- | trace | trace | trace | 0.04 | 0.4 | tr | 0.06 |
| 90 | Tridecanoic acid—C13:0 | 1660 | 1659 | ---- | ---- | ---- | ---- | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | ---- | 0.1 | 0.1 |
| 91 | δ-Dodecalactone † | 1674 | 1675 | 1.1 | 0.6 | 0.1 | 0.2 | 1.1 | 0.6 | 0.4 | 1.6 | 0.06 | 1.0 | 0.5 | 0.3 |
| 92 | 13-Methyltetradecanal | 1677 | 1680 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 2.4 | 0.2 | 2.0 | 0.2 | 0.3 |
| 93 | Ethyl tridecanoate | 1688 | 1689 | ---- | ---- | 0.05 | ---- | ---- | 0.08 | 0.3 | ---- | ---- | ---- | 0.2 | 0.05 |
| 94 | 2-Pentadecanone | 1693 | 1698 | 0.6 | 0.3 | 0.1 | 0.1 | 0.7 | 0.7 | 0.4 | 3.8 | 0.4 | 3.7 | 0.5 | 0.6 |
| 95 | Heptadecane † | 1700 | 1700 | 0.4 | 0.5 | 0.2 | 0.1 | 0.1 | 0.08 | 0.2 | 0.6 | 0.08 | 1.2 | 0.2 | 0.1 |
| 96 | Pentadecanal | 1703 | 1701 | 0.1 | ---- | ---- | ---- | 0.2 | 0.05 | ---- | 1.1 | 0.09 | 0.7 | 0.08 | 0.1 |
| 97 | Myristoleic acid—C14:1 * | 1752 | ---- | 0.9 | 0.3 | 1.3 | 1.0 | 0.4 | 0.4 | 0.3 | 0.3 | 1.1 | 0.4 | 0.3 | 0.6 |
| 98 | Myristic acid—C14:0 | 1766 | 1763 | 4.7 | 1.9 | 8.5 | 6.0 | 11.7 | 7.6 | 4.6 | 4.7 | 7.8 | 5.6 | 5.1 | 4.2 |
| 99 | Ethyl (9Z)-tetradecenoate | 1772 | 1779 | ---- | ---- | ---- | ---- | ---- | 1.0 | 3.4 | 1.1 | ---- | 0.4 | 0.9 | 1.0 |
| 100 | (9Z)-Hexadecenal | 1780 | 1780 | ---- | ---- | ---- | ---- | 0.4 | ---- | ---- | 0.2 | ---- | ---- | 0.08 | ---- |
| 101 | Ethyl myristate | 1784 | 1780 | ---- | ---- | ---- | ---- | 3.6 | 4.8 | 8.6 | 0.6 | 0.3 | 0.3 | 4.1 | 2.1 |
| 102 | Octadecene | 1796 | 1797 | 0.5 | 0.7 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 1.4 | 0.2 | 1.9 | 0.4 | 0.2 |
| 103 | Octadecane † | 1800 | 1800 | ---- | ---- | ---- | ---- | ---- | 0.09 | ---- | 0.4 | ---- | ---- | ---- | ---- |
| 104 | Palmitaldehyde | 1803 | 1807 | 0.3 | 0.3 | 0.05 | 0.03 | 1.3 | 0.2 | 0.3 | 4.3 | 0.3 | 2.1 | 0.4 | 0.3 |
| 105 | Phytane | 1819 | 1816 | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | 0.3 | ---- | ---- | ---- | ---- |
| 106 | Isopentyl laurate | 1834 | 1829 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.07 | ---- | ---- | ---- |
| 107 | Neophytadiene | 1841 | 1842 | ---- | 0.1 | ---- | 0.05 | 0.06 | 0.2 | ---- | 0.6 | 0.07 | 0.2 | 0.2 | 0.09 |
| 108 | 3,7,11,15-Tetramethylhexadec-2-ene | 1851 | ---- | 0.3 | 0.6 | ---- | 0.1 | 0.2 | 0.3 | 0.1 | 1.7 | 0.2 | 2.0 | 0.4 | 0.4 |
| 109 | Pentadecanoic acid—C15:0 | 1861 | 1860 | 0.2 | ---- | 0.2 | 0.2 | 0.6 | ---- | 0.1 | ---- | 0.3 | ---- | 0.08 | 0.07 |
| 110 | Hexadecanol | 1873 | 1875 | ---- | ---- | ---- | 0.2 | 0.06 | 0.05 | ---- | 0.3 | 0.03 | 0.6 | 0.3 | ---- |
| 111 | (8Z)-14-Methyl-hexadecen-1-ol * | 1881 | ---- | ---- | ---- | 0.1 | ---- | 0.1 | 0.02 | ---- | 0.3 | 0.02 | 0.3 | 0.07 | 0.02 |
| 112 | Ethyl pentadecanoate | 1888 | 1890 | ---- | ---- | ---- | 0.02 | 0.3 | 0.3 | 0.5 | ---- | 0.02 | 0.1 | 0.3 | 0.2 |
| 113 | δ-Tetradecalactone * | 1887 | ---- | 0.4 | 0.2 | 0.2 | 0.07 | 0.3 | 0.07 | 0.1 | 0.7 | 0.04 | 0.5 | 0.08 | 0.05 |
| 114 | Nonadecane † | 1900 | 1900 | ---- | ---- | 0.1 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- |
| 115 | Heptadecanal | 1903 | 1898 | ---- | 0.2 | ---- | ---- | 0.04 | ---- | ---- | 0.3 | ---- | ---- | ---- | ---- |
| 116 | Palmitoleic acid—C16:1 | 1932 | 1933 | ---- | ---- | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | ---- | 0.1 | 0.3 | ---- | ---- |
| 118 | Methyl 2-methylpalmitate | 1946 | 1944 | ---- | ---- | ---- | ---- | 0.1 | ---- | 0.03 | ---- | ---- | 0.06 | ---- | |
| 119 | (11Z)-Hexadecenoic acid—C16:1 | 1952 | 1953 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.05 | 2.1 | ---- | ---- |
| 120 | Palmitic acid—C16:0 | 1961 | 1963 | 1.6 | 0.4 | 1.8 | 1.0 | 4.8 | 1.5 | 0.9 | 1.6 | 1.6 | ---- | 1.2 | 0.6 |
| 121 | Ethyl (9Z)-hexadecenoate | 1958 | 1955 | ---- | ---- | ---- | ---- | ---- | 0.4 | 1.8 | ---- | ---- | ---- | 0.5 | 0.2 |
| 122 | (9Z)-Octadecenal * | 1977 | ---- | ---- | ---- | ---- | ---- | 0.2 | 0.03 | 0.09 | 0.2 | 0.06 | ---- | ---- | 0.03 |
| 123 | Ethyl palmitate | 1982 | 1980 | ---- | ---- | ---- | ---- | 2.8 | 2.2 | 2.8 | 0.1 | 0.1 | 0.2 | 2.2 | 0.6 |
| 124 | Octadecanal | 2004 | 2002 | 0.2 | 0.2 | 0.1 | 0.06 | 0.3 | 0.05 | trace | 0.7 | 0.08 | 0.5 | 0.1 | 0.05 |
| 125 | Isopropyl palmitate | 2015 | 2017 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.02 | ---- | ---- | ---- |
| 126 | Ethyl (9Z)-heptadecenoate * | 2058 | ---- | ---- | ---- | ---- | ---- | 0.07 | 0.04 | 0.1 | ---- | ---- | ---- | 0.07 | 0.03 |
| 127 | Octadecanol | 2073 | 2074 | 0.2 | ---- | ---- | 0.2 | ---- | 0.05 | ---- | 0.6 | 0.03 | 0.5 | 0.3 | ---- |
| 128 | δ-Hexadecalactone * | 2100 | ---- | 0.09 | ---- | ---- | ---- | 0.07 | 0.02 | trace | ---- | ---- | ---- | ---- | ---- |
| 129 | Heneicosane | 2100 | 2100 | ---- | ---- | ---- | ---- | 0.03 | ---- | 0.1 | ---- | ---- | ---- | 0.1 | ---- |
| 130 | (9Z)-Oleic acid—C18:1 | 2123 | 2116 | 0.2 | ---- | 0.07 | 0.1 | 0.2 | 0.1 | 0.1 | ---- | 0.09 | ---- | ---- | ---- |
| 131 | Ethyl linoleate | 2142 | 2140 | ---- | ---- | ---- | ---- | 0.1 | 0.08 | 0.2 | ---- | ---- | ---- | 0.1 | 0.03 |
| 132 | Ethyl α-linolenate | 2146 | 2145 | ---- | ---- | ---- | ---- | 0.1 | 0.06 | 0.2 | ---- | ---- | ---- | 0.1 | ---- |
| 133 | Ethyl (9Z)-oleate | 2150 | 2149 | ---- | ---- | ---- | ---- | 1.2 | 0.7 | 1.4 | ---- | 0.07 | ---- | 1.1 | 0.4 |
| 134 | Ethyl elaidate | 2165 | ---- | ---- | ---- | ---- | ---- | 0.2 | 0.09 | 0.3 | ---- | ---- | ---- | 0.2 | 0.03 |
| 135 | Ethyl stearate | 2180 | 2181 | ---- | ---- | ---- | ---- | 0.3 | 0.1 | 0.2 | ---- | ---- | ---- | 0.2 | 0.03 |
| 136 | Ethyl (9Z,11E)-octadecadienoate * | 2187 | ---- | ---- | ---- | ---- | ---- | 0.08 | 0.04 | 0.1 | ---- | ---- | ---- | 0.1 | 0.02 |
| 137 | 1-Docosene | 2196 | 2195 | 0.3 | 0.3 | 0.2 | 0.08 | ---- | 0.02 | 0.09 | 0.3 | 0.04 | 1.0 | 0.2 | 0.03 |
| Parcial sum | 89.6 | 85.3 | 91.0 | 93.5 | 97.2 | 97.2 | 96.8 | 83.0 | 96.3 | 88.5 | 92.6 | 94.7 | |||
| Unidentified constituents | |||||||||||||||
| Branched hydrocarbons C10–C26 | 9.2 | 13.6 | 6.9 | 4.6 | 0.6 | 0.4 | 0.9 | 5.8 | 0.6 | 6.8 | 2.1 | 0.1 | |||
| Branched aliphatic alcohols C11 | ---- | ---- | ---- | ---- | 0.2 | ---- | ---- | ---- | 0.1 | ---- | ---- | ---- | |||
| Sesquiterpene hydrocarbons (C15H24) | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.2 | |||
| Sesquiterpene hydrocarbons (C15H26) | 0.0 | 0.3 | 0.3 | 0.5 | 0.4 | 0.6 | 0.1 | 6.5 | 1.4 | 2.0 | 0.8 | 3.7 | |||
| Branched saturated fatty acids C12–C15 | 0.6 | 0.3 | 0.5 | 0.8 | 1.0 | 0.6 | 0.5 | 0.8 | 1.3 | 0.2 | 0.4 | 0.5 | |||
| Branched unsaturated fatty acids C12–C19 | 0.1 | 0.1 | 1.3 | 0.1 | ---- | ---- | ---- | 0.2 | 0.2 | 0.3 | 0.08 | ---- | |||
| Ethyl ester of fatty acid C12–C16 | 0.2 | ---- | ---- | ---- | 0.6 | 0.8 | 1.5 | 0.1 | ---- | 0.4 | 0.8 | 0.6 | |||
| Branched aliphatic aldehydes C15 | 0.1 | 0.3 | ---- | ---- | 0.4 | 0.2 | 0.2 | 2.2 | 0.2 | 1.8 | 0.2 | 0.2 | |||
| Branched hydrocarbons C10–C26 | 9.2 | 13.6 | 6.9 | 4.6 | 0.6 | 0.4 | 0.9 | 5.8 | 0.7 | 6.8 | 2.1 | 0.1 | |||
| Parcial sum | 10.3 | 14.5 | 9.0 | 6.0 | 3.1 | 2.5 | 3.2 | 15.6 | 4.0 | 11.5 | 4.3 | 5.3 | |||
| Total (%) | 99.9 | 99.8 | 100.0 | 99.5 | 100.0 | 99.7 | 100.0 | 98.7 | 100.0 | 100.0 | 96.9 | 100.0 | |||
‡ Area normalization (%) based on GC-FID analysis and calculated from the average value (mean ± sd) of two measurements. The standard deviation of each identified component ranges between 0.01 (mean values ≤ 1%)–0.8 (mean values ≤ 45.7%); different superscript letters (a–c) show significant differences in chemical compositions (p <0.05). RI-Cal: retention index calculated; RI-it: retention index of literature; † compared to the respective certified reference standard (Rt, RI, and mass spectrum); d contaminant; * tentatively identified. L1: location 1—Cereté 5; L2: location 2—Chinú 2; L3: location 3—Chinú 3; L4: location 4—Chinú 4; L5: location 5—Ciénaga de Oro 4; L6: location 6—Ciénaga de Oro 8; L7: location 7—Sahagún 1; and L8: location 8—Sahagún 3.

Table A2.
Identification of constituents (>0.1%) present in the volatile fractions by HS-SPME of the raw milk and suero costeño samples from eight locations of Córdoba (Colombia).
Table A2.
Identification of constituents (>0.1%) present in the volatile fractions by HS-SPME of the raw milk and suero costeño samples from eight locations of Córdoba (Colombia).
| Peak | Constituents | RIcal | RIlit | Raw Milk ‡ | Suero Costeño ‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L3 | L4 | L7 | a L1 | a L2 | a L3 | b L4 | c L5 | b L6 | a L7 | a L8 | ||||
| 1 | Acetone † | ---- | 471 | 2.5 | 0.7 | 0.3 | 2.3 | ---- | ---- | ---- | ---- | 0.07 | ---- | ---- | ---- |
| 2 | Ethanol † | ---- | 482 | ---- | ---- | ---- | ---- | 0.9 | 3.3 | 2.2 | 0.6 | 0.2 | 1.1 | 2.4 | 2.5 |
| 3 | Ethyl acetate † | ---- | 615 | ---- | ---- | ---- | ---- | 0.8 | 3.8 | 7.2 | 0.6 | 3.0 | 1.8 | 3.5 | |
| 4 | Diacetyl | ---- | 616 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 2.0 | ---- | ---- |
| 5 | Acetic acid †—C2:0 | ---- | 646 | 8.2 | 0.7 | 0.6 | ---- | 2.2 | 3.9 | 1 | 1.1 | ---- | 1.0 | 2.4 | 2.4 |
| 6 | Acetoin | 727 | 717 | ---- | 0.1 | 0.2 | ---- | 1.4 | 0.7 | 1.2 | 3.4 | 0.8 | 2.8 | 1.3 | 1.4 |
| 7 | Isoamyl alcohol | 749 | 741 | 1.3 | 5.0 | 0.5 | ---- | 2.5 | 2.9 | 2.7 | 1.9 | 1.5 | 2.8 | 1.4 | 1.4 |
| 8 | Isobutyric acid—C4:0 | 754 | 752 | ---- | ---- | ---- | ---- | ---- | ---- | 0.2 | ---- | ---- | 1.5 | ---- | ---- |
| 9 | Pentanol | 757 | 752 | 1.3 | 0.7 | 0.7 | ---- | ---- | 1.0 | ---- | 2.7 | ---- | 2.7 | ---- | 2.1 |
| 10 | Butyric acid—C4:0 | 776 | 775 | 1.4 | 0.8 | 0.3 | 0.6 | 0.8 | 0.4 | 0.2 | ---- | 1.6 | 1.3 | 0.5 | 0.6 |
| 11 | Toluene | 778 | 771 | 1.3 | 0.04 | 0.3 | 0.3 | ---- | ---- | ---- | ---- | ---- | ---- | 0.4 | ---- |
| 12 | Ethyl butyrate | 784 | 785 | ---- | ---- | ---- | ---- | ---- | 1.1 | 0.7 | ---- | ---- | 0.7 | ---- | 0.7 |
| 13 | Isovaleric acid—C5:0 | 849 | 851 | ---- | 0.3 | ---- | ---- | ---- | 0.2 | trace | ---- | ---- | 0.5 | ---- | 0.3 |
| 14 | 2-Furanmethanol | 852 | 849 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.4 | ---- | ---- |
| 15 | 2-Methylbutanoic acid—C5:0 | 860 | 861 | ---- | 0.2 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.4 | ---- | 0.2 |
| 16 | 5-Methyl-2-hexanone | 870 | 865 | 1.4 | 0.3 | 0.07 | 0.4 | 0.2 | ---- | 0.2 | 0.8 | 0.1 | 1.3 | ---- | 0.04 |
| 17 | Valeric acid †—C5:0 | 870 | 880 | ---- | 0.3 | ---- | ---- | ---- | 0.07 | ---- | ---- | ---- | 0.4 | ---- | ---- |
| 18 | 2-Heptanol | 873 | 877 | ---- | ---- | ---- | ---- | ---- | ---- | 0.4 | ---- | ---- | 1.6 | ---- | ---- |
| 19 | 2-Heptanone | 877 | 880 | 2.0 | 0.3 | 0.08 | 0.8 | 0.2 | ---- | ---- | 1.3 | 0.09 | 0.4 | ---- | ---- |
| 20 | Styrene | 882 | 887 | 3.1 | 0.3 | 0.07 | 0.7 | 0.2 | 0.4 | 0.4 | 0.4 | ---- | ---- | ---- | 0.05 |
| 21 | 2,6-Dimethyl-4-heptanol | 913 | ---- | ---- | 1.8 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 22 | Tricyclene | 919 | 920 | ---- | ---- | ---- | 0.1 | 0.02 | ---- | ---- | 0.4 | 0.05 | ---- | ---- | 0.05 |
| 23 | Benzaldehyde | 920 | 923 | ---- | ---- | ---- | ---- | 0.03 | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 24 | α-Pinene † | 931 | 930 | ---- | ---- | ---- | 0.2 | ---- | ---- | 0.04 | 1.2 | 0.2 | 0.3 | 0.1 | 0.2 |
| 25 | Fenchene | 940 | 942 | ---- | ---- | ---- | 0.4 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 26 | Camphene | 945 | 947 | ---- | ---- | ---- | 0.4 | 0.06 | 0.07 | 0.1 | 0.1 | ---- | ---- | ---- | ---- |
| 27 | 6-Methyl-2-heptanone | 961 | 957 | ---- | ---- | ---- | 0.07 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 28 | Caproic acid—C6:0 | 981 | 983 | 5.8 | 8.6 | 3.7 | 4.2 | 6.6 | 4.6 | 1.8 | 2.9 | 16.5 | 14.1 | 4.2 | 5.9 |
| 29 | Ethyl caproate | 987 | 985 | ---- | ---- | 0.7 | ---- | 0.7 | 0.7 | 0.9 | ---- | 1.0 | 0.8 | 1.6 | 2.4 |
| 30 | Decane † | 1000 | 1000 | ---- | ---- | 0.1 | 0.07 | ---- | ---- | ---- | 0.09 | 0.02 | ---- | ---- | ---- |
| 31 | α-Tolualdehyde | 1014 | 1012 | ---- | 1.2 | ---- | ---- | ---- | 0.2 | ---- | ---- | ---- | ---- | ---- | ---- |
| 32 | p-Cymene | 1016 | 1015 | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | ---- | ---- | ---- | 0.3 |
| 33 | Limonene † | 1020 | 1022 | ---- | 1.5 | 0.3 | 0.1 | ---- | 0.1 | 0.1 | ---- | ---- | 0.3 | ---- | 0.4 |
| 34 | Isoamyl butyrate | 1041 | 1040 | 0.3 | 1.0 | 0.4 | 0.2 | ---- | 0.04 | 0.1 | 0.3 | ---- | ---- | 0.1 | ---- |
| 35 | m-Tolualdehyde | 1053 | 1056 | 0.6 | ---- | ---- | ---- | 0.05 | 0.1 | 0.07 | ---- | ---- | 0.4 | 0.1 | 0.1 |
| 36 | 8-Nonen-2-one | 1057 | 1055 | ---- | ---- | ---- | 1.3 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 37 | Octan-1-ol | 1058 | 1060 | 0.2 | ---- | ---- | 0.1 | 0.09 | ---- | ---- | ---- | ---- | 0.3 | ---- | ---- |
| 38 | 2-Methyl-decane | 1064 | 1065 | 0.4 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.03 | ---- | ---- | ---- |
| 39 | p-Cresol | 1068 | 1066 | ---- | 0.09 | 0.02 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 40 | 2-Nonanone | 1072 | 1070 | 3.1 | 0.5 | 0.2 | 12.8 | 0.4 | ---- | 0.1 | 2.7 | 0.3 | 4.7 | 0.09 | 0.08 |
| 41 | p-Cymenene | 1073 | 1072 | ---- | ---- | ---- | ---- | ---- | ---- | 0.07 | ---- | ---- | ---- | 0.03 | 0.1 |
| 42 | Enanthylic acid—C7:0 | 1074 | 1073 | ---- | ---- | 0.09 | ---- | ---- | ---- | ---- | ---- | 0.3 | ---- | ---- | ---- |
| 43 | Pelargonaldehyde | 1085 | 1084 | 0.4 | ---- | ---- | 0.5 | ---- | ---- | ---- | 0.2 | ---- | 0.4 | ---- | ---- |
| 44 | 2-Nonanol | 1089 | 1087 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.7 | ---- | ---- |
| 45 | Benzenethanol | 1093 | 1091 | 0.6 | 3.1 | 0.1 | ---- | 1.6 | 3.6 | 0.9 | 0.4 | 0.8 | 0.3 | 0.7 | 1.1 |
| 46 | Undecane † | 1100 | 1100 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.2 | ---- | 0.2 | 0.09 | 0.04 |
| 47 | Hexahydrobenzoic acid | 1110 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.4 | ---- | ---- |
| 48 | 4-Methyl-benzenemethanol | 1117 | 1122 | ---- | ---- | 0.07 | 0.2 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 49 | Camphor † | 1121 | 1125 | ---- | ---- | ---- | 0.4 | ---- | ---- | 0.03 | ---- | ---- | ---- | 0.03 | 0.1 |
| 50 | (2E)-Nonen-1-al | 1136 | 1136 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | 0.2 | ---- | 0.06 |
| 51 | 1-Nonanol | 1164 | 1162 | 0.6 | ---- | ---- | 0.03 | 0.08 | 0.06 | 0.1 | 0.6 | ---- | 0.6 | 0.05 | 0.07 |
| 52 | p-Cymen-8-ol | 1165 | 1163 | ---- | ---- | 0.02 | 0.07 | ---- | ---- | 0.08 | ---- | ---- | 0.08 | ---- | 0.06 |
| 53 | Benzoic acid † | 1178 | 1174 | 1.4 | 2.5 | 1.2 | 0.8 | 6.0 | 8.2 | 5.0 | 3.7 | 7.1 | 4.7 | 3.5 | 8.5 |
| 54 | Ethyl caprylate | 1184 | 1180 | 1.5 | ---- | ---- | ---- | 4.1 | 6.0 | 7.3 | 0.7 | 1.3 | 0.9 | 7.6 | 7.0 |
| 55 | Caprylic acid—C8:0 | 1187 | 1180 | 19.5 | 34.3 | 23.6 | 19.8 | 15.6 | 12.2 | 10.7 | 5.2 | 19.6 | 10.5 | 11.1 | 12.4 |
| 56 | 1-Dodecene | 1189 | 1187 | 0.4 | ---- | ---- | ---- | ---- | ---- | ---- | 0.4 | ---- | ---- | ---- | ---- |
| 57 | (3Z)-Dodecene | 1194 | 1195 | 0.3 | ---- | ---- | 0.4 | ---- | ---- | ---- | ---- | ---- | ---- | 0.03 | ---- |
| 58 | Dodecane † | 1200 | 1200 | 0.7 | 0.3 | ---- | 0.6 | ---- | ---- | 0.04 | 0.6 | ---- | 1.1 | 0.6 | 0.3 |
| 59 | β-Phenethyl acetate | 1235 | 1233 | ---- | ---- | ---- | ---- | 0.2 | 0.2 | 0.06 | ---- | ---- | ---- | 0.08 | 0.3 |
| 60 | Isoamyl caproate | 1239 | 1234 | ---- | ---- | ---- | ---- | 0.03 | ---- | ---- | ---- | 0.06 | ---- | ---- | ---- |
| 61 | δ-Octalactone | 1244 | 1243 | 0.3 | 0.07 | ---- | 0.09 | 0.07 | 0.09 | 0.06 | 0.2 | 0.07 | 0.2 | 0.09 | 0.2 |
| 62 | d 1,3-Di-tert-butylbenzene | 1245 | 1245 | 0.1 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.05 | 0.4 | ---- | ---- |
| 63 | (2Z)-Decenal | 1246 | 1249 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.2 | ---- | ---- |
| 64 | Decanol | 1264 | 1259 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.2 | ---- | 0.08 | ---- | ---- |
| 65 | (6Z)-Undecen-2-one | 1273 | 1274 | ---- | ---- | ---- | 0.1 | ---- | ---- | 0.2 | ---- | ---- | ---- | ---- | ---- |
| 66 | Pelargonic acid—C9:0 | 1273 | 1272 | ---- | 0.2 | 0.4 | 0.2 | 0.3 | 0.3 | 0.3 | 0.4 | 0.3 | 0.3 | 0.1 | 0.3 |
| 67 | Bornyl acetate | 1275 | 1277 | 0.4 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 68 | Propyl octanoate | 1280 | 1277 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.05 | 0.1 |
| 69 | 2-Undecanone | 1281 | 1279 | 0.9 | 0.2 | 0.2 | 0.9 | 0.4 | 0.07 | 0.1 | 1.3 | 0.5 | 2.5 | 0.07 | 0.06 |
| 70 | Ethyl pelargonate | 1285 | 1282 | ---- | ---- | 0.3 | ---- | 0.04 | 0.05 | 0.1 | ---- | ---- | ---- | 0.09 | 0.05 |
| 71 | 2,6,11-Trimethyldodecane | 1286 | ---- | 0.1 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.07 | ---- | ---- |
| 72 | 2-Undecanol | 1297 | 1298 | ---- | ---- | ---- | ---- | ---- | ---- | 0.02 | ---- | ---- | 0.05 | ---- | ---- |
| 73 | Tridecane † | 1300 | 1300 | 0.1 | ---- | ---- | ---- | ---- | ---- | ---- | 0.08 | ---- | 0.1 | 0.08 | 0.03 |
| 74 | 4,6-Dimethyldodecane | 1336 | ---- | 0.2 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.04 | ---- | ---- |
| 75 | Isobutyl caprylate | 1338 | 1334 | ---- | ---- | ---- | ---- | ---- | 0.02 | 0.03 | ---- | ---- | ---- | ---- | ---- |
| 76 | (2Z)-Undecenal | 1341 | 1335 | ---- | ---- | ---- | ---- | ---- | 0.02 | ---- | ---- | 0.03 | 0.04 | ---- | 0.04 |
| 77 | 2-Methylundecanal | 1358 | 1350 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.06 | ---- |
| 78 | Undecanol | 1362 | 1360 | ---- | ---- | ---- | ---- | ---- | ---- | 0.3 | ---- | ---- | ---- | ---- | ---- |
| 79 | (7Z)-Tetradecene | 1362 | 1365 | ---- | ---- | 0.2 | 1.0 | 0.3 | ---- | ---- | 4.1 | 1.0 | ---- | 0.4 | 0.9 |
| 80 | (2E)-Undecen-1-ol * | 1374 | ---- | ---- | ---- | 1.2 | 5.6 | ---- | ---- | ---- | 5.0 | 3.8 | 0.5 | ---- | 3.7 |
| 81 | Caproleic acid—C10:1 | 1376 | 1362 | 1.2 | 0.8 | ---- | 0.8 | 1.5 | 0.2 | 0.1 | ---- | 0.7 | 0.7 | 1.5 | 2.0 |
| 82 | Ethyl 9-decenoate | 1377 | 1370 | ---- | ---- | ---- | ---- | 2.3 | 2.7 | 4.9 | ---- | ---- | ---- | 4.9 | 2.3 |
| 83 | α-Copaene | 1381 | 1379 | 0.5 | ---- | ---- | ---- | ---- | ---- | ---- | 0.2 | ---- | ---- | ---- | ---- |
| 84 | Ethyl caprate | 1385 | 1381 | 0.6 | 0.8 | 2.1 | 0.3 | 8.6 | 11.1 | 11.5 | 0.7 | ---- | 0.4 | 14.6 | 4.5 |
| 85 | Capric acid—C10:0 | 1393 | 1388 | 19.7 | 24.5 | 45.6 | 16.1 | 18.4 | 13.1 | 16.4 | 9.4 | 22.6 | 9.5 | 12.2 | 11.4 |
| 86 | Dodecanal | 1393 | 1390 | 0.5 | 0.2 | ---- | ---- | 0.06 | ---- | 0.05 | 0.1 | ---- | 0.17 | 0.07 | 0.03 |
| 87 | 1-Tetradecene | 1395 | 1391 | ---- | ---- | ---- | 0.4 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 88 | Tetradecane † | 1400 | 1400 | 0.4 | 0.1 | ---- | ---- | 0.2 | 0.09 | 0.3 | 2.2 | 0.5 | 0.4 | 0.3 | 0.3 |
| 89 | Cyperene | 1402 | 1404 | 1.1 | ---- | ---- | ---- | 0.04 | ---- | 0.02 | 0.2 | 0.07 | 0.1 | ---- | ---- |
| 90 | (3Z)-Tetradecene | 1419 | 1421 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.1 | ---- | ---- | ---- | ---- |
| 91 | β-Caryophyllene † | 1422 | 1425 | 0.3 | 0.2 | 0.5 | 3.7 | 0.4 | 0.5 | 0.9 | 7.5 | 1.3 | 0.6 | 0.7 | 1.3 |
| 92 | γ-Decalactone | 1433 | 1429 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.03 | ---- | 0.01 |
| 93 | α-Guaiene | 1440 | 1441 | ---- | ---- | ---- | 0.1 | ---- | ---- | ---- | 0.5 | 0.2 | ---- | ---- | ---- |
| 94 | 2-Methylbutyl octanoate | 1442 | 1444 | ---- | ---- | ---- | ---- | ---- | 0.03 | 0.09 | ---- | ---- | ---- | ---- | ---- |
| 95 | α-Humulene | 1455 | 1452 | 0.2 | 0.2 | 0.3 | 2.9 | 0.3 | 0.5 | 0.7 | 5.6 | 1.1 | 0.4 | 0.6 | 1.0 |
| 96 | δ-Decalactone † | 1464 | 1463 | 1.0 | 0.5 | 0.09 | 0.3 | 0.4 | 0.5 | 0.3 | 0.8 | 0.2 | 1.3 | 0.3 | 0.7 |
| 97 | Rotundene | 1467 | 1466 | 0.7 | ---- | ---- | 0.08 | 0.1 | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 98 | 2,6,10-Trimethyltridecane | 1470 | 1467 | 0.4 | ---- | ---- | 0.2 | 0.08 | ---- | 0.06 | 0.3 | 0.1 | 0.1 | ---- | 0.09 |
| 99 | Undecanoic acid—C11:0 | 1470 | 1469 | ---- | 0.09 | 0.3 | ---- | 0.09 | 0.07 | 0.06 | ---- | 0.06 | ---- | 0.05 | 0.07 |
| 100 | γ-Muurolene | 1477 | 1478 | 0.2 | ---- | ---- | 0.04 | ---- | ---- | ---- | 0.09 | ---- | ---- | ---- | ---- |
| 101 | Propyl decanoate | 1483 | 1481 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.06 |
| 102 | 2-Tridecanone | 1485 | 1482 | 0.2 | ---- | 0.5 | 0.1 | 0.4 | 0.07 | 0.2 | 0.5 | 0.3 | 0.7 | 0.06 | ---- |
| 103 | Ethyl undecanoate | 1487 | 1494 | ---- | ---- | ---- | ---- | 0.06 | 0.07 | ---- | ---- | ---- | ---- | ---- | ---- |
| 104 | Selinane * | 1489 | ---- | ---- | ---- | 0.1 | 1.0 | 0.06 | 0.07 | 0.4 | 3.0 | 0.5 | 0.05 | 0.3 | 0.5 |
| 105 | Pentadecane † | 1500 | 1500 | 0.7 | 0.2 | 0.06 | 0.4 | 0.1 | 0.1 | 0.1 | 0.3 | 0.2 | 0.3 | 0.2 | 0.2 |
| 106 | d BHT | 1500 | 1501 | ---- | 0.07 | 0.03 | 0.03 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 107 | d 2,4-Di-tert-butylphenol | 1509 | 1512 | 0.1 | ---- | 0.02 | 0.09 | 0.03 | 0.01 | 0.02 | 0.05 | 0.03 | 0.05 | 0.1 | 0.04 |
| 108 | γ-Cadinene | 1513 | 1512 | ---- | ---- | 0.02 | 0.09 | trace | 0.02 | 0.04 | 0.2 | 0.04 | 0.02 | 0.02 | 0.04 |
| 109 | trans-Calamenene | 1517 | 1515 | 0.03 | ---- | 0.01 | 0.03 | ---- | 0.01 | 0.02 | 0.1 | 0.01 | 0.02 | ---- | 0.03 |
| 110 | δ-Cadinene | 1521 | 1522 | 0.05 | ---- | 0.03 | 0.1 | trace | 0.04 | 0.06 | 0.5 | 0.07 | 0.03 | 0.05 | 0.08 |
| 111 | Selina-3,7(11)-diene | 1534 | 1535 | ---- | ---- | ---- | ---- | ---- | ---- | 0.01 | 0.06 | ---- | ---- | ---- | ---- |
| 112 | α-Calacorene | 1536 | 1535 | ---- | ---- | 0.01 | 0.04 | ---- | ---- | 0.01 | 0.05 | ---- | ---- | ---- | ---- |
| 113 | α-Cadinene | 1536 | 1534 | ---- | ---- | 0.01 | 0.03 | ---- | ---- | ---- | 0.03 | ---- | ---- | ---- | ---- |
| 114 | Isobutyl caprate | 1538 | 1533 | ---- | ---- | 0.01 | ---- | ---- | 0.03 | 0.04 | ---- | ---- | ---- | ---- | ---- |
| 115 | 2,6,10-Trimethyltetradecane | 1554 | 1557 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.06 | 0.07 | 0.07 | ---- | 0.1 |
| 116 | (2E)-Tridecen-1-ol | 1565 | 1564 | ---- | ---- | ---- | ---- | 1.2 | 0.2 | ---- | 0.4 | 1.2 | 0.5 | ---- | 0.8 |
| 117 | Lauric acid—C12:0 | 1573 | 1570 | 2.2 | 4.5 | 8.5 | 3.1 | 5.0 | 4.4 | 4.7 | 1.1 | 2.7 | 2.3 | 4.1 | 2.5 |
| 118 | (5Z)-Dodecenoic acid—C12:1 | 1580 | 1578 | ---- | ---- | ---- | ---- | 0.1 | 0.2 | ---- | ---- | ---- | ---- | 0.4 | 0.2 |
| 119 | Ethyl (5Z)-dodecenoate | 1583 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.3 | ---- | ---- | ---- | ---- | ---- |
| 120 | Ethyl laurate | 1585 | 1583 | 0.1 | ---- | 0.1 | 0.08 | 3.1 | 3.5 | 5.1 | 0.2 | 0.1 | 0.2 | 5.4 | 1.5 |
| 121 | Cetene | 1596 | 1595 | 0.4 | ---- | ---- | 0.4 | ---- | ---- | ---- | 0.03 | 0.04 | 0.09 | ---- | ---- |
| 122 | Hexadecane † | 1600 | 1600 | 0.2 | ---- | 0.03 | 0.09 | 0.04 | 0.04 | 0.04 | 0.08 | 0.04 | 0.08 | 0.05 | 0.05 |
| 123 | Myristylaldehyde | 1603 | 1601 | 0.06 | ---- | 0.04 | 0.03 | 0.1 | 0.05 | 0.04 | 0.1 | 0.09 | 0.1 | 0.04 | 0.06 |
| 124 | Phenylethyl caproate | 1620 | 1619 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.04 | ---- | ---- | ---- |
| 125 | Isoamyl n-decanoate | 1637 | 1633 | ---- | ---- | ---- | ---- | ---- | 0.08 | 0.07 | ---- | ---- | ---- | ---- | ---- |
| 126 | Myristic alcohol | 1641 | 1647 | ---- | ---- | 0.03 | 0.1 | 0.06 | ---- | ---- | 0.3 | ---- | ---- | 0.09 | 0.07 |
| 127 | 2-Methylbutyl decanoate | 1643 | 1647 | ---- | ---- | ---- | ---- | ---- | ---- | 0.06 | ---- | ---- | ---- | ---- | ---- |
| 128 | γ-Dodecalactone | 1646 | 1645 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.03 | ---- | 0.05 |
| 129 | Tridecanoic acid—C13:0 | 1660 | 1659 | ---- | ---- | 0.05 | ---- | 0.05 | ---- | 0.03 | ---- | 0.04 | ---- | 0.06 | 0.03 |
| 130 | δ-Dodecalactone † | 1674 | 1675 | 0.5 | 0.3 | 0.07 | 0.2 | 0.4 | 0.3 | ---- | 0.4 | 0.2 | 0.6 | 0.2 | 0.3 |
| 131 | 13-Methyltetradecanal | 1677 | 1680 | ---- | ---- | ---- | ---- | 0.08 | ---- | ---- | 0.1 | 0.06 | 0.1 | 0.04 | 0.07 |
| 132 | Ethyl tridecanoate | 1688 | 1689 | ---- | ---- | ---- | ---- | 0.04 | 0.04 | 0.04 | ---- | ---- | ---- | 0.1 | 0.03 |
| 133 | 2-Pentadecanone | 1693 | 1698 | 0.09 | ---- | 0.1 | 0.01 | 0.1 | 0.07 | 0.1 | 0.1 | 0.08 | 0.2 | 0.03 | 0.03 |
| 134 | Heptadecane † | 1700 | 1700 | 0.4 | 0.1 | 0.03 | 0.1 | 0.07 | 0.04 | 0.04 | 0.07 | 0.04 | 0.09 | 0.06 | 0.05 |
| 135 | Pentadecanal | 1703 | 1701 | ---- | ---- | ---- | ---- | 0.04 | ---- | 0.02 | 0.03 | 0.03 | 0.04 | ---- | 0.02 |
| 136 | Myristoleic acid—C14:1 * | 1752 | ---- | ---- | 0.2 | 0.4 | 0.08 | 0.2 | 0.1 | 0.1 | ---- | 0.2 | 0.06 | 0.2 | 0.2 |
| 137 | Myristic acid—C14:0 | 1766 | 1763 | 0.4 | 1.0 | 2.2 | 0.8 | 2.7 | 1.8 | 1.5 | 0.5 | 1.6 | 1.1 | 1.5 | 1.4 |
| 138 | Ethyl (9Z)-tetradecenoate | 1772 | 1779 | ---- | ---- | 0.1 | ---- | 0.3 | 0.3 | 0.5 | 0.07 | ---- | ---- | 1.0 | 0.4 |
| 139 | Ethyl myristate | 1784 | 1780 | 0.05 | ---- | 0.04 | 0.06 | 1.8 | 1.6 | 1.6 | 0.2 | 0.1 | 0.2 | 2.1 | 0.7 |
| 140 | Ethylhexyl salicylate | 1790 | 1805 | ---- | ---- | ---- | ---- | 0.09 | 0.03 | ---- | ---- | ---- | ---- | ---- | ---- |
| 141 | Octadecene | 1796 | 1797 | 0.2 | 0.2 | 0.04 | 0.2 | 0.05 | 0.07 | 0.05 | 0.2 | 0.08 | 0.1 | 0.07 | 0.08 |
| 142 | Octadecane † | 1800 | 1800 | 0.3 | 0.1 | 0.03 | 0.1 | 0.05 | 0.06 | 0.04 | 0.07 | 0.04 | 0.09 | 0.07 | 0.05 |
| 143 | Palmitaldehyde | 1803 | 1807 | ---- | ---- | ---- | ---- | 0.1 | 0.07 | 0.05 | 0.1 | 0.08 | 0.1 | 0.03 | 0.08 |
| 144 | Phytane | 1819 | 1816 | 0.09 | ---- | ---- | ---- | ---- | ---- | ---- | 0.06 | ---- | ---- | ---- | ---- |
| 145 | Neophytadiene | 1841 | 1842 | ---- | ---- | ---- | 0.04 | ---- | 0.03 | ---- | 0.05 | 0.02 | 0.03 | ---- | 0.02 |
| 146 | 3,7,11,15-Tetramethylhexadec-2-ene | 1851 | ---- | 0.8 | 0.5 | 0.07 | 0.3 | 0.1 | 0.2 | 0.09 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 |
| 147 | Pentadecanoic acid—C15:0 | 1861 | 1860 | ---- | ---- | ---- | ---- | 0.07 | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 148 | Hexadecanol | 1873 | 1875 | ---- | 0.09 | 0.02 | 0.3 | 0.02 | ---- | 0.05 | 0.08 | 0.06 | 0.2 | 0.06 | ---- |
| 149 | Ethyl pentadecanoate | 1888 | 1890 | ---- | ---- | ---- | ---- | 0.08 | 0.09 | 0.05 | ---- | ---- | ---- | 0.1 | 0.06 |
| 150 | δ-Tetradecalactone * | 1887 | ---- | 0.07 | ---- | 0.01 | 0.03 | 0.05 | ---- | 0.02 | 0.06 | 0.04 | 0.1 | ---- | 0.02 |
| 151 | Palmitoleic acid—C16:1 | 1932 | 1933 | ---- | ---- | 0.04 | ---- | 0.03 | ---- | ---- | ---- | 0.03 | ---- | ---- | ---- |
| 152 | (11Z)-Hexadecenoic acid—C16:1 | 1952 | 1953 | ---- | ---- | ---- | ---- | 0.07 | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 153 | Palmitic acid—C16:0 | 1961 | 1963 | 0.2 | 0.2 | 0.4 | 0.2 | 0.6 | 0.4 | 0.3 | 0.2 | 0.8 | 0.4 | 0.3 | 0.4 |
| 154 | Ethyl (9Z)-hexadecenoate | 1958 | 1955 | ---- | ---- | ---- | ---- | ---- | 0.08 | 0.09 | ---- | ---- | ---- | 0.2 | 0.09 |
| 155 | Ethyl palmitate | 1982 | 1980 | ---- | ---- | 0.02 | 0.02 | 0.4 | 0.4 | 0.2 | 0.07 | 0.05 | 0.08 | 0.2 | 0.2 |
| 156 | Isopropyl palmitate | 2015 | 2017 | ---- | ---- | 0.01 | 0.05 | 0.07 | 0.05 | ---- | ---- | 0.03 | 0.04 | 0.06 | 0.05 |
| 157 | Octadecanol | 2073 | 2074 | ---- | ---- | ---- | 0.03 | ---- | ---- | ---- | ---- | 0.02 | 0.04 | ---- | ---- |
| 158 | Methyl elaidate | 2080 | 2081 | ---- | ---- | ---- | ---- | 0.06 | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 159 | Methyl stearate | 2110 | 2113 | ---- | ---- | ---- | ---- | 0.2 | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 160 | (9Z)-Oleic acid—C18:1 | 2123 | 2116 | ---- | ---- | 0.04 | ---- | 0.06 | ---- | ---- | ---- | 0.06 | ---- | ---- | ---- |
| 161 | Ethyl (9Z)-oleate | 2150 | 2149 | ---- | ---- | 0.03 | ---- | 0.1 | 0.09 | 0.06 | ---- | 0.09 | ---- | 0.1 | 0.1 |
| 162 | Ethyl stearate | 2180 | 2181 | ---- | ---- | ---- | ---- | 0.02 | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| Parcial sum | 99.0 | 99.3 | 98.4 | 92.6 | 97.7 | 98.2 | 96.9 | 84.1 | 94.2 | 96.2 | 96.7 | 96.7 | |||
| Unidentified constituents | |||||||||||||||
| Branched hydrocarbons C11-C25 | 0.5 | 0.1 | 0.4 | 0.7 | ---- | ---- | ---- | 0.6 | 0.7 | 0.8 | 0.09 | 0.04 | |||
| Branched aliphatic alcohols C9-C11 | ---- | ---- | ---- | 0.07 | ---- | ---- | ---- | 0.2 | 1.0 | ---- | ---- | ||||
| Sesquiterpene hydrocarbons (C15H24) | ---- | ---- | ---- | 0.04 | ---- | ---- | 0.1 | 0.5 | ---- | ---- | ---- | 0.03 | |||
| Sesquiterpene hydrocarbons (C15H26) | 0.4 | 0.6 | 0.9 | 6.0 | 1.2 | 1.1 | 2.1 | 14.0 | 3.4 | 1 | 1.4 | 2.7 | |||
| Branched saturated fatty acids C9-C15 | ---- | ---- | 0.2 | 0.2 | 0.3 | 0 | 0.1 | 0.5 | 0.3 | 0.1 | 0.1 | 0.2 | |||
| Branched unsaturated fatty acids C12 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.9 | ---- | ---- | ---- | |||
| Ethyl ester of unsaturated fatty acids C12 | ---- | ---- | ---- | ---- | 0.1 | 0.1 | 0.2 | ---- | ---- | ---- | 0.3 | 0.06 | |||
| Ethyl ester of branched fatty acids C13-C15 | ---- | ---- | ---- | ---- | 0.2 | 0.2 | 0.2 | ---- | ---- | ---- | 0.6 | 0.2 | |||
| Branched aliphatic aldehydes C15 | ---- | ---- | ---- | ---- | 0.1 | 0.07 | 0.1 | 0.1 | 0.1 | 0.09 | 0.04 | 0.05 | |||
| Parcial sum | 0.9 | 0.6 | 1.4 | 7.0 | 2.1 | 1.6 | 2.9 | 15.7 | 5.7 | 3.1 | 2.5 | 3.3 | |||
| Total (%) | 99.9 | 99.9 | 99.8 | 99.5 | 99.8 | 99.8 | 99.8 | 99.8 | 99.9 | 99.3 | 99.2 | 100.0 | |||
‡ Area normalization based on GC-FID analysis and calculated from the average value (mean ± sd) of two measurements. The standard deviation of each identified component ranges between 0.01 (mean values ≤ 1%)–0.5 (mean values ≤ 46.5%); different superscript letters (a–c) show significant differences in chemical compositions (p < 0.05). RI-Cal: retention index calculated; RI-it: retention index of literature; † compared to the respective certified reference standard (Rt, RI, and mass spectrum); d contaminant; * tentatively identified. L1: location 1—Cereté 5; L2: location 2—Chinú 2; L3: location 3—Chinú 3; L4: location 4—Chinú 4; L5: location 5—Ciénaga de Oro 4; L6: location 6—Ciénaga de Oro 8; L7: location 7—Sahagún 1; and L8: location 8—Sahagún 3.
References
- Chaves-López, C.; Serio, A.; Grande-Tovar, C.D.; Cuervo-Mulet, R.; Delgado-Ospina, J.; Paparella, A. Traditional fermented foods and beverages from a microbiological and nutritional perspective: The Colombian heritage. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1031–1048. [Google Scholar] [CrossRef]
- Rodríguez Calderón, A. Manual de Elaboración de Productos Lácteos Fermentados; Instituto de Ciencia y Tecnología de Alimentos (ICTA), Universidad Nacional de Colombia—Programa Andino de Desarrollo Tecnológico para el Medio Rural (PADT-RURAL): Bogotá, Colombia, 1988. [Google Scholar]
- Ruiz-Petro, M.; Torres-Ruiz, M.; Sequeda-Goez, D.; Ricardo-Polo, C.; Simanca-Sotelo, M.; Álvarez-Badel, B.; Durango-Villadiego, A.; Arteaga-Márquez, M.; De Paula, C.; Pastrana-Puche, Y.; et al. Physicochemical, microbiological, and rheological characteristics of fermented sour cream (“suero costeño”) produced in Colombia. Appl. Sci. 2025, 15, 4598. [Google Scholar] [CrossRef]
- Acevedo, D.; Granados, C.; Torres, R. Caracterización reológica del suero costeño de Turbaco, Arjona, El Carmen de Bolívar y uno Comercial (Colombia). Inform. Tecnol. 2014, 25, 3–10. [Google Scholar] [CrossRef]
- Motato, K.E.; Milani, C.; Ventura, M.; Valencia, F.E.; Ruas-Madiedo, P.; Delgado, S. Bacterial diversity of the Colombian fermented milk “Suero Costeño” assessed by culturing and high-throughput sequencing and DGGE analysis of 16S rRNA gene amplicons. Food Microbiol. 2017, 68, 129–136. [Google Scholar] [CrossRef]
- Bart, J.C.J. Organoleptic product quality. In Polymer Additive Analytics Industrial Practice and Case Studies; Firenze University Press: Firenze, Italy, 2006; pp. 219–248. [Google Scholar]
- De Rovira, D.A. The Dictionary of Flavors and General Guide for Those Training in the Art and Science of Flavor Chemistry; Food & Nutrition Press: Trumbull, CT, USA, 1999. [Google Scholar]
- Dixon, M.P. Organoleptic qualities. Int. J. Food Sci. Technol. 1968, 3, 423–429. [Google Scholar] [CrossRef]
- Cadwallader, K.R.; Sing, T.K. Flavours and off-flavours in milk and dairy products. In Advanced Dairy Chemistry: Lactose, Water, Salts and Minor Constituents; McSweeney, P.L.H., Fox, P.F., Eds.; Springer: New York, NY, USA, 2009; Volume 3, pp. 631–690. [Google Scholar]
- Mei, J.; Reineccius, G. The influence of texture on aroma release and perception related to dairy products. In Flavor of Dairy Products; Cadwallader, K.R., Drake, M., McGorrin, R.J., Eds.; ACS Symposium Series 971; American Chemical Society: Washington, DC, USA, 2007; pp. 93–110. [Google Scholar]
- Richardson, N.J.; Booth, D.A. Multiple physical patterns in judgements of the creamy texture of milks and creams. Acta Psychol. 1993, 84, 93–101. [Google Scholar] [CrossRef]
- Sing, T.K.; Cadwallader, K.R.; Drake, M.A. Biochemical processes in the production of flavor in milk and milk products. In Handbook of Food Products Manufacturing. Health, Meat, Milk, Poultry, Seafood, and Vegetables; Hui, Y.H., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 715–748. [Google Scholar]
- Urbach, G. Contribution of lactic acid bacteria to flavour compound formation in dairy products. Int. Dairy J. 1995, 5, 877–903. [Google Scholar] [CrossRef]
- Schieberle, P.; Gassenmeier, K.; Guth, H.; Sen, A.; Grosch, W. Character impact odour compounds of different kinds of butter. Leb. Wiss. Technol. 1993, 26, 347–356. [Google Scholar] [CrossRef]
- Meunier-Goddik, L. Sour cream and crème fraîche. In Handbook of Food and Beverage Fermentation Technology; Hui, Y.H., Meunier-Goddik, L., Hansen, Å.S., Josephsen, J., Nip, W.-K., Stanfield, P.S., Toldrá, F., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 171–183. [Google Scholar]
- Shepard, L.; Miracle, R.E.; Leksrisompong, P.; Drake, M.A. Relating sensory and chemical properties of sour cream to consumer acceptance. J. Dairy Sci. 2013, 96, 5435–5454. [Google Scholar] [CrossRef]
- Cadwallader, K.R.; Sing, T.K.; Jerrell, J.P. Streamlined analysis of short-, medium-, and long-chain free fatty acids in dairy products. In Flavor of Dairy Products; Cadwallader, K.R., Drake, M., McGorrin, R.J., Eds.; ACS Symposium Series 971; American Chemical Society: Washington, DC, USA, 2007; pp. 111–122. [Google Scholar]
- Marsili, R. Flavors and off-flavors in dairy foods. In Encyclopedia of Dairy Sciences; McSweeney, P.L.H., McNamara, J.P., Eds.; Elsevier: San Diego, CA, USA, 2022; pp. 1069–1081. [Google Scholar]
- Valencia García, F.E.; Vela-Caicedo, A.; Rojas, L.F. Indigenous microorganisms for the elaboration of lactic cultures for specific use in “suero costeño”. South Fla. J. Dev. 2022, 3, 2775–2793. [Google Scholar] [CrossRef]
- Yacub, B.; Cajal, J.; Acevedo, D.; Murillo, L.; Leal, R.; Tirado, D.F. Diseño de un prototipo fermentador de leche semiautomático para la elaboración de suero costeño. Rev. Bio Agro 2016, 14, 95–102. [Google Scholar] [CrossRef]
- Acevedo, D.; Guzmán, L.; Rodríguez, A. Cinética de la fermentación en la producción de suero costeño. Rev. UDCA Actual. Divulg. Cient. 2013, 16, 427–433. [Google Scholar]
- Acevedo, D.; Guzmán, L.E.; Rodríguez, A. Cultivo iniciador para la producción del suero costeño. Rev. Bio Agro 2012, 10, 16–20. [Google Scholar]
- Acevedo, D.; Rodríguez, A.; Fernández, A. Low-amplitude oscilatory determinations for suero costeño. Rev. UDCA Actual. Divulg. Cient. 2012, 15, 219–225. [Google Scholar]
- Batista Cañate, K.Y. Evaluación y Caracterización Fisicoquímica y Microbiológica del Suero Costeño Elaborado en el Municipio de Turbaco, Arjona y el Carmen de Bolívar. Bachelor’s Thesis, Universidad de Cartagena, Cartagena, Colombia, 2011. [Google Scholar]
- Acuña-Monsalve, Y. Selección e Identificación de BAL Con Potencial Probiótico Aisladas del Suero Costeño. Master’s Thesis, Universidad de la Sabana, Bogotá, Colombia, 2009. [Google Scholar]
- Cueto, C.; García, D.; Garcés, F.; Cruz, J. Preliminary studies on the microbiological characterization of lactic acid bacteria in suero costeño, a Colombian traditional fermented milk product. Rev. Latinoam. Microbiol. 2007, 49, 12–18. [Google Scholar]
- Rodríguez-Peña, J.M.; Torres-Lozano, L.M. Evaluación de Sustancias Antimicrobianas Presentes en los Extractos Obtenidos de Bacterias Ácido Lácticas Aisladas de Suero Costeño. Bachelor’s Thesis, Universidad de la Sabana, Bogotá, Colombia, 2006. [Google Scholar]
- Farelo De La Hoz, H.D. Caracterización Microbiológica y de los Parámetros Fisicoquímicos Relacionados Con el Proceso de Fermentación del “Suero Costeño” Como Producto Final Elaborado en el Difícil, Ariguani. Bachelor’s Thesis, Universidad de la Sabana, Bogotá, Colombia, 2002. [Google Scholar]
- Cruz-Martínez, J.G. Aislamiento y Caracterización Bioquímica de Levaduras y Bacterias Acidolácticas Productoras de Sustancias Antimicrobianas de Interés Industrial Presentes en el “Suero Costeño”. Bachelor’s Thesis, Universidad de la Sabana, Bogotá, Colombia, 2006. [Google Scholar]
- Berard, J.; Bianchi, F.; Careri, M.; Chatel, A.; Mangia, A.; Musci, M. Characterization of the volatile fraction and of free fatty acids of “Fontina Valle d’Aosta”, a protected designation of origin Italian cheese. Food Chem. 2007, 105, 293–300. [Google Scholar] [CrossRef]
- Superintendencia de Industria y Comercio. Denominaciones de Origen. Ministerio de Comercio, Industria y Turismo. Gobierno de Colombia. Available online: https://www.sic.gov.co/marcas/denominaciones-de-origen (accessed on 30 September 2024).
- Christie, W.W. Mass Spectrometry of Methyl Ester Derivatives of Fatty Acid. The Lipid Web. Available online: https://www.lipidmaps.org/resources/lipidweb/lipidweb_html/ms/methylesters.htm (accessed on 15 January 2024).
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Eisenstecken, D.; Stanstrup, J.; Robatscher, P.; Huck, C.W.; Oberhuber, M. Fatty acid profiling of bovine milk and cheese from six European areas by GC-FID and GC-MS. Int. J. Dairy Technol. 2021, 74, 215–224. [Google Scholar] [CrossRef]
- Mehta, B.M. Chemical composition of milk and milk products. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Heidelberg, Germany, 2015. [Google Scholar]
- Nantapo, C.T.; Muchenje, V.; Hugo, A. Atherogenicity index and health-related fatty acids in different stages of lactation from Friesian, Jersey and Friesian x Jersey cross cow milk under a pasture-based dairy system. Food Chem. 2014, 146, 127–133. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Hennessy, D.; McAuliffe, S.; Kilcawley, K.N.; O’Donovan, M.; Dillon, P.; Ross, R.P.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016, 99, 9424–9440. [Google Scholar] [CrossRef]
- Villeneuve, M.-P.; Lebeuf, Y.; Gervais, R.; Tremblay, G.F.; Vuillemard, J.C.; Fortin, J.; Chouinard, P.Y. Milk volatile organic compounds and fatty acid profile in cows fed timothy as hay, pasture, or silage. J. Dairy Sci. 2013, 96, 7181–7194. [Google Scholar] [CrossRef] [PubMed]
- Čuboň, J.; Vavrišínová, K.; Henrieta, A.; Simona, K.; Kačániová, M.; Adriana, P.; Andrea, B.; Lukáš, H.; Marek, B. Analysis of fatty acid content of raw milk. J. Microbiol. Biotechnol. Food Sci. 2013, 2, 1138–1146. [Google Scholar]
- Liu, S.; Zhang, R.; Kang, R.; Meng, J.; Ao, C. Milk fatty acids profiles and milk production from dairy cows fed different forage quality diets. Anim. Nutr. 2016, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- MacGibbon, A.K.H.; Haddadian, Z. Milk lipids. Fatty acids. In Encyclopedia of Dairy Sciences; McSweeney, P.L.H., McNamara, J.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 3. [Google Scholar]
- Pilarczyk, R.; Wójcik, J.; Sablik, P.; Czerniak, P. Fatty acid profile and health lipid indices in the raw milk of Simmental and Holstein-Friesian cows from an organic farm. S. Afr. J. Anim. Sci. 2015, 45, 30–38. [Google Scholar] [CrossRef]
- Riuzzi, G.; Davis, H.; Lanza, I.; Butler, G.; Contiero, B.; Gottardo, F.; Segato, S. Multivariate modelling of milk fatty acid profile to discriminate the forages in dairy cows’ ration. Sci. Rep. 2021, 11, 23201. [Google Scholar] [CrossRef]
- Santos Júnior, O.O.; Pedrão, M.R.; Dias, L.F.; Paula, L.N.; Coró, F.A.G.; De Souza, N.E. Fatty acid content of bovine milkfat from raw milk to yoghurt. Am. J. Appl. Sci. 2012, 9, 1300–1306. [Google Scholar]
- Butler, G.; Stergiadis, S.; Seal, C.; Eyre, M.; Leifert, C. Fat composition of organic and conventional retail milk in northeast England. J. Dairy Sci. 2011, 94, 24–36. [Google Scholar] [CrossRef]
- Mollica, M.P.; Trinchese, G.; Cimmino, F.; Penna, E.; Cavaliere, G.; Tudisco, R.; Musco, N.; Manca, C.; Catapano, A.; Monda, M.; et al. Milk fatty acid profiles in different animal species: Focus on the potential effect of selected PUFAs on metabolism and brain functions. Nutrients 2021, 13, 1111. [Google Scholar] [CrossRef]
- Yilmaz-Ersan, L. Fatty acid composition of cream. Mljekarstvo 2013, 63, 132–139. [Google Scholar]
- Izsó, T.; Kasza, G.; Somogyi, L. Differences between fat-related characteristics of sour cream and sour cream analogues. Acta Aliment. 2020, 49, 390–397. [Google Scholar] [CrossRef]
- Rieder, W. Science and technology of sour cream. In Handbook of Food Products Manufacturing; Hui, Y.H., Ed.; John Wiley & Sons: London, UK, 2007. [Google Scholar]
- Yuan, N.; Chi, X.; Ye, Q.; Liu, H.; Zheng, N. Analysis of volatile organic compounds in milk during heat treatment based on E-nose, E-tongue and HS-SPME-GC-MS. Foods 2023, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, R.; Zhao, W.; Hua, X.; Zhang, W.; Zhang, Z. Influence of pulsed electric field treatments on the volatile compounds of milk in comparison with pasteurized processing. J. Food Sci. 2011, 76, C127–C132. [Google Scholar] [CrossRef] [PubMed]
- Zacometti, C.; Tata, A.; Massaro, A.; Riuzzi, G.; Bragolusi, M.; Cozzi, G.; Piro, R.; Khazzar, S.; Gerardi, G.; Gottardo, F.; et al. Seasonal variation in raw milk VOC profile within intensive feeding systems. Foods 2023, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Marrazzo, A.; De Luca, L.; Romano, R.; Manzo, N.; Masucci, F.; Di Francia, A.; Sacchi, R. Volatile organic compound and fatty acid profile of milk from cows and buffaloes fed mycorrhizal or nonmycorrhizal ensiled forage. Molecules 2019, 24, 1616. [Google Scholar] [CrossRef]
- Vagenas, G.; Roussis, I.G. Fat-derived volatiles of various products of cows’, ewes’, and goats’ milk. Int. J. Food Prop. 2012, 15, 665–682. [Google Scholar] [CrossRef]
- Kim Ha, J.; Lindsay, R.C. Method for the quantitative analysis of volatile free and total branched-chain fatty acids in cheese and milk fat. J. Dairy Sci. 1990, 73, 1988–1999. [Google Scholar] [CrossRef]
- Koczura, M.; Martin, B.; Musci, M.; Massimo, M.D.; Bouchon, M.; Turille, G.; Kreuzer, M.; Berard, J.; Coppa, M. Little difference in milk fatty acid and terpene composition among three contrasting dairy breeds when grazing a biodiverse Mountain pasture. Front. Vet. Sci. 2021, 7, 612504. [Google Scholar] [CrossRef]
- Tornambé, G.; Cornu, A.; Pradel, P.; Kondjoyan, N.; Carnat, A.P.; Petit, M.; Martin, B. Changes in terpene content in milk from pasture-fed cows. J. Dairy Sci 2006, 89, 2309–2319. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Holland, R.; Crow, V.L. Esters and their biosynthesis in fermented dairy products: A review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]
- de Frutos, M.D.; Sanz, J.; Martinez-Castro, I. Characterization of artisanal cheeses by GC and GC/MS analysis of their medium volatility (SDE) fraction. J. Agric. Food Chem. 1991, 39, 524–530. [Google Scholar] [CrossRef]
- Forss, D.A. Mechanisms of formation of aroma compounds in milk and milk products. J. Dairy Res. 1979, 46, 691–706. [Google Scholar] [CrossRef]
- Zhao, X.; Ge, Y.; Yu, X.; Liu, C.; Li, H.; Wang, X.; Yao, S. Fermentation characteristics of fermented milk with Streptococcus thermophilus CICC 6063 and Lactobacillus helveticus CICC 6064 and volatile compound dynamic profiles during fermentation and storage. Molecules 2024, 29, 1257. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Tian, Y.; Tao, L.; Xie, J.; Wang, M.; Zhang, F.; Yu, Z.; Sheng, J.; Zhao, C. Exploring the effect of milk fat on fermented milk flavor based on gas chromatography–ion mobility spectrometry (GC-IMS) and multivariate statistical analysis. Molecules 2024, 29, 1099. [Google Scholar] [CrossRef]
- Wang, M.S.; Fan, M.; Zheng, A.R.; Wei, C.K.; Liu, D.H.; Thaku, K.; Wei, Z.J. Characterization of a fermented dairy, sour cream: Lipolysis and the release profile of flavor compounds. Food Chem. 2023, 423, 136299. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Jin, R.L.; Zhang, H.P.; Zhou, T.T.; Sun, T.S. Characterization of volatile compounds in fermented milk using solid-phase microextraction methods coupled with gas chromatography-mass spectrometry. J. Dairy Sci. 2017, 100, 2488–2500. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Crow, V.L.; Holland, R. Production of natural fruity flavour in dairy foods. Nutr. Food Sci. 2009, 39, 483–489. [Google Scholar] [CrossRef]
- Abeijon Mukdsi, M.C.; Maillard, M.B.; Medina, R.B.; Thierry, A. Ethyl butanoate is synthesised both by alcoholysis and esterification by dairy Lactobacilli and Propionibacteria. LWT—Food Sci. Technol. 2018, 89, 38–43. [Google Scholar] [CrossRef]
- Holland, R.; Liu, S.Q.; Crow, V.L.; Delabre, M.L.; Lubbers, M.; Bennett, M.; Norris, G. Esterases of lactic acid bacteria and cheese flavour: Milk fat hydrolysis, alcoholysis and esterification. Int. Dairy J. 2005, 15, 711–718. [Google Scholar] [CrossRef]
- Ahansaz, N.; Tarrah, A.; Pakroo, S.; Corich, V.; Giacomini, A. Lactic acid bacteria in dairy foods: Prime sources of antimicrobial compounds. Fermentation 2023, 9, 964. [Google Scholar] [CrossRef]
- Zandona, E.; Blažić, M.; Režek Jambrak, A. Whey utilization: Sustainable uses and environmental approach. Food Technol. Biotechnol. 2021, 59, 147–161. [Google Scholar] [CrossRef]
- Asif, M.; Nadeem, M.; Imran, M.; Ullah, R.; Tayyab, M.; Khan, F.A.; Al-Asmari, F.; Rahim, M.A.; Rocha, J.M.; Korma, S.A.; et al. Effect of fat contents of buttermilk on fatty acid composition, lipolysis, vitamins and sensory properties of cheddar-type cheese. Front. Microbiol. 2023, 14, 1209509. [Google Scholar] [CrossRef] [PubMed]
- Moschopoulou, E. Characteristics of rennet and other enzymes from small ruminants used in cheese production. Small Rumin. Res. 2011, 101, 188–195. [Google Scholar] [CrossRef]
- Castillo, I.; Calvo, M.V.; Alonso, L.; Juárez, M.; Fontecha, J. Changes in lipolysis and volatile fraction of a goat cheese manufactured employing a hygienized rennet paste and a defined strain starter. Food Chem. 2007, 100, 590–598. [Google Scholar] [CrossRef]
- Lytou, A.E.; Panagou, E.Z.; Nychas, G.-J.E. Volatilomics for food quality and authentication. Curr. Opin. Food Sci. 2019, 28, 88–95. [Google Scholar] [CrossRef]
- Greenfield, H.; Southgate, D.A.T. Food Composition Data. Production, Management and Use; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; pp. 63–82. [Google Scholar]
- De Angelis, M.; Gobbetti, M. Environmental stress responses in Lactobacillus: A review. Proteomics 2004, 4, 106–122. [Google Scholar] [CrossRef]
- Alonso, L.; Juárez, M.; Ramos, M.; Martín-Alvarez, P.J. Effects of changes during ripening and frozen storage on the physicochemical and sensory characteristics of Cabrales cheeses. Int. J. Food Sci. Technol. 1987, 22, 525–534. [Google Scholar] [CrossRef]
- Griffiths, M.W.; Phillips, J.D.; Muir, D.D. Development of flavour defects in pasteurized double cream during storage at 6 °C and at 10 °C. Int. J. Dairy Technol. 1981, 34, 142–146. [Google Scholar] [CrossRef]
- Salum, P.; Erbay, Z. Optimization of headspace solid-phase microextraction for the analysis of volatile compounds of high-fat dairy powders. Food Anal. Methods 2019, 12, 2216–2225. [Google Scholar] [CrossRef]
- d’Acampora Zellner, B.; Dugo, P.; Dugo, G.; Mondello, L. Flavor. In Handbook of Food Analysis; Nollet, L.M.L., Toldrá, F., Eds.; CRC Press: Boca Ratón, FL, USA, 2015; pp. 47–64. [Google Scholar]
- Godefroot, M.; Sandra, P.; Verzele, M. New method for quantitative essential oil analysis. J. Chromatogr. A 1981, 203, 325–335. [Google Scholar] [CrossRef]
- Gioacchini, A.M.; De Santi, M.; Guescini, M.; Brandi, G.; Stocchi, V. Characterization of the volatile organic compounds of Italian ‘Fossa’cheese by solid-phase microextraction gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 3405–3412. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Wang, L.; Chen, S.; Xu, Y. Optimization and validation of a head space solid-phase microextraction-arrow gas chromatography-mass spectrometry method using central composite design for determination of aroma compounds in Chinese liquor (Baijiu). J. Chromatogr. A 2020, 1610, 460584. [Google Scholar] [CrossRef] [PubMed]
- Mounchili, A.; Wichtel, J.J.; Bosset, J.O.; Dohoo, I.R.; Imhof, M.; Altieri, D.; Mallia, S.; Stryhn, H. HS-SPME gas chromatographic characterization of volatile compounds in milk tainted with off-flavour. Int. Dairy J. 2005, 15, 1203–1215. [Google Scholar] [CrossRef]
- AOAC Official Method 932.02-1945; Fat (Crude) or Ether Extract in Dried Milk Products. AOAC International: Rockville, MD, USA, 2000.
- ISO 23318 IDF 249; Milk, Dried Milk Products and Cream—Determination of Fat Content—Gravimetric Method. International Organization for Standardization: Geneva, Switzerland, 2022.
- AOAC Official Method 996.06; Fat (Total, Saturated, and Unsaturated) in Foods. AOAC International: Rockville, MD, USA, 2002.
- AOAC Official Method 969.33; Fatty Acids in Oils and Fats Preparation of Methyl Esters. AOAC International: Rockville, MD, USA, 2000.
- Ackman, R.G. Remarks on official methods employing boron trifluoride in the preparation of methyl esters of the fatty acids of fish oils. J. Am. Oil Chem. Soc. 1998, 75, 541–545. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017; 804p. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Christie, W.W.; Han, X. Lipid Analysis—Isolation, Separation, Identification and Lipidomic Analysis; Woodhead Publishing Ltd.: Sawston, UK, 2010. [Google Scholar]
- Joulian, D.; König, W.A. The Atlas of Spectral Data of Sesquiterpenes Hydrocarbons; E.B.-Verlag: Hamburg, Germany, 1998; 658p. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook; NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA. Available online: http://webbook.nist.gov/chemistry/ (accessed on 1 January 2024).
- Australian Bureau of Statistics. Sample Size Calculator. Available online: https://www.abs.gov.au/websitedbs/D3310114.nsf/home/Sample+Size+Calculator?opendocument (accessed on 15 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).