Isolation, Structures, and Bioactivities of Polysaccharides from Achyranthes bidentata: A Review
Abstract
1. Introduction
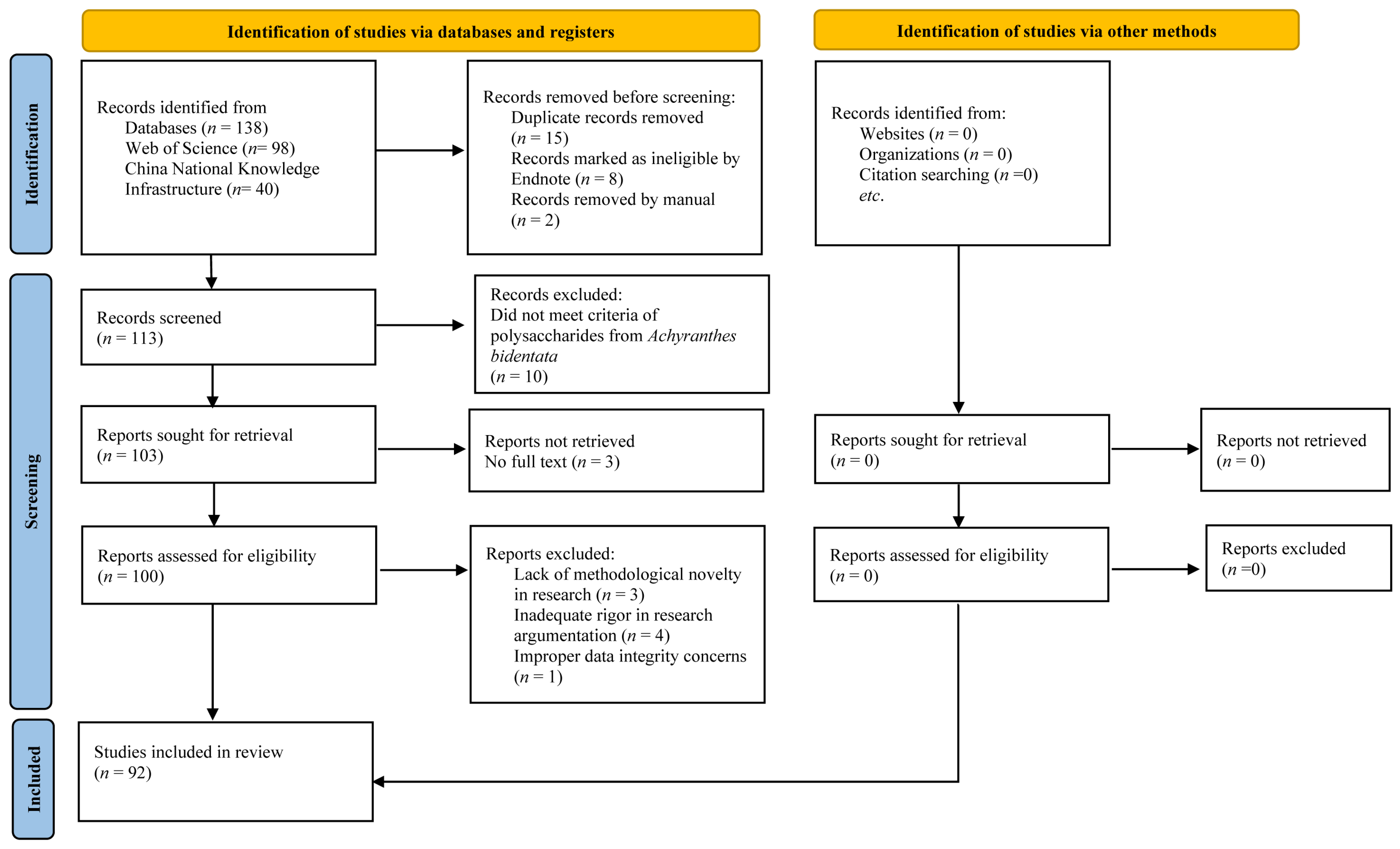
2. Data and Methods
2.1. Literature Search Strategy
2.2. Search Terminology
2.3. Inclusion/Exclusion Criteria
2.4. Data Synthesis
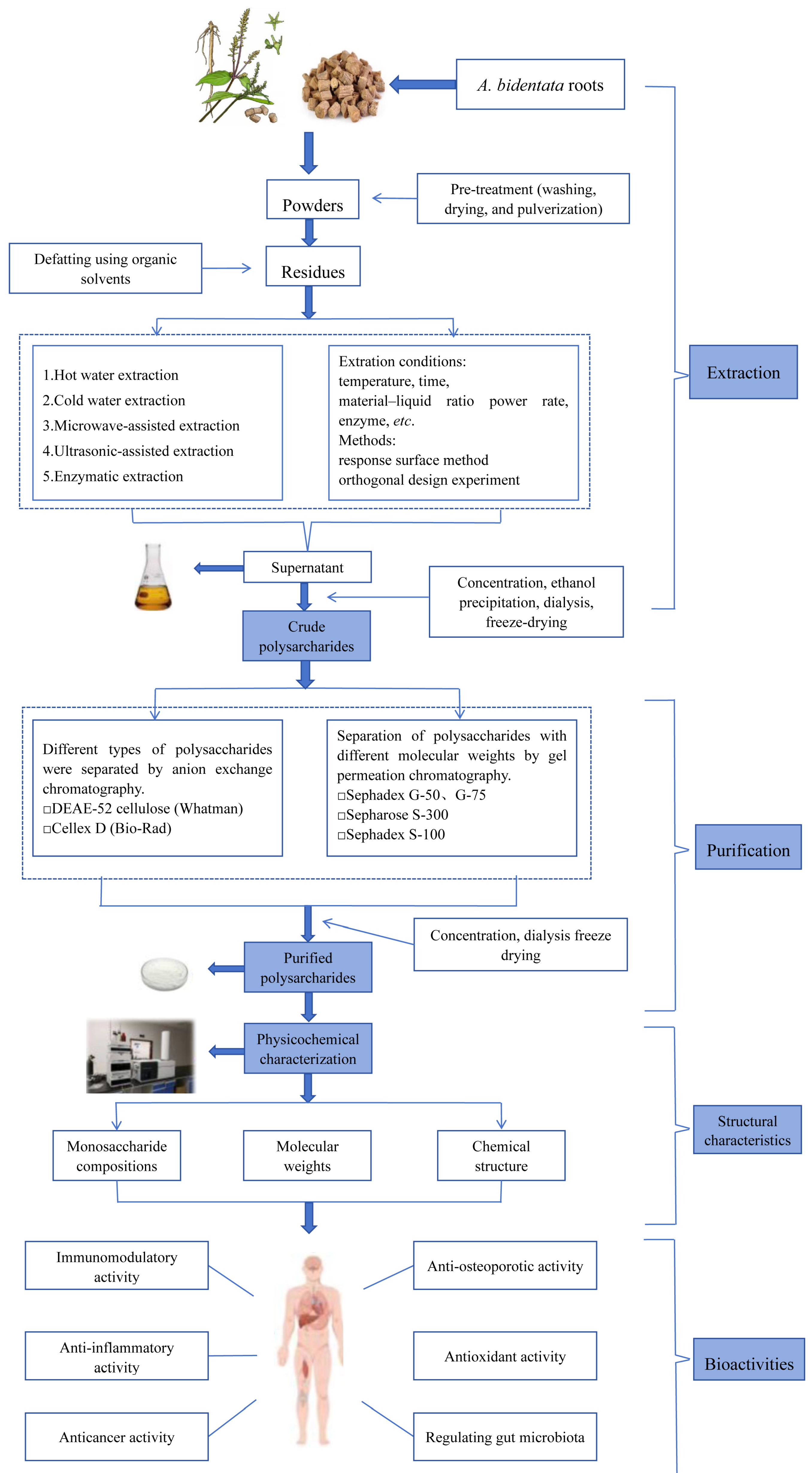
3. Extraction and Purification Methods
4. Physicochemical and Structural Features
4.1. Average Molecular Weights
4.2. Monosaccharide Compositions
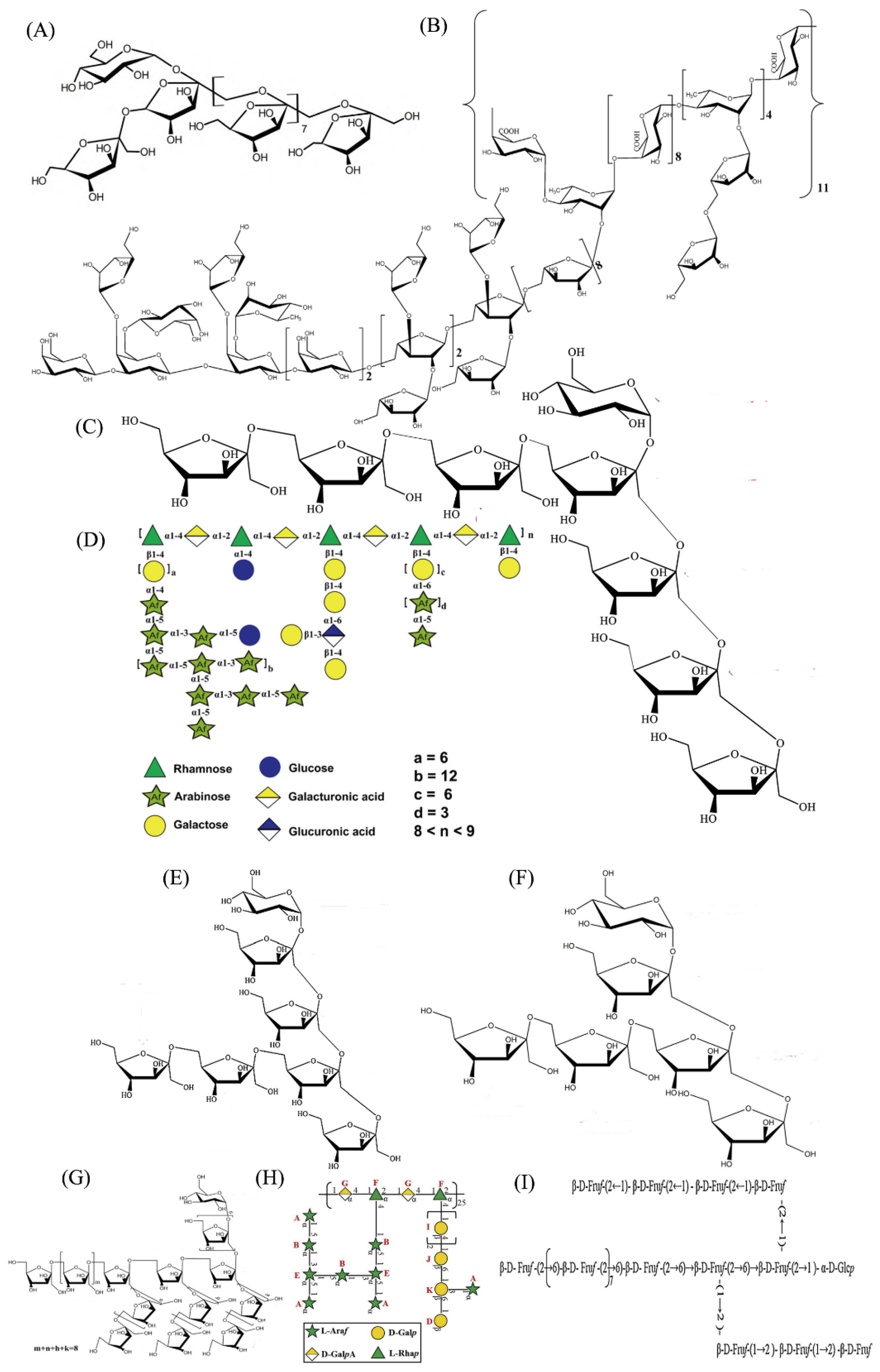
4.3. Chemical Structures
4.4. Conformational Features
4.5. Structural Modification
5. Biological Activities
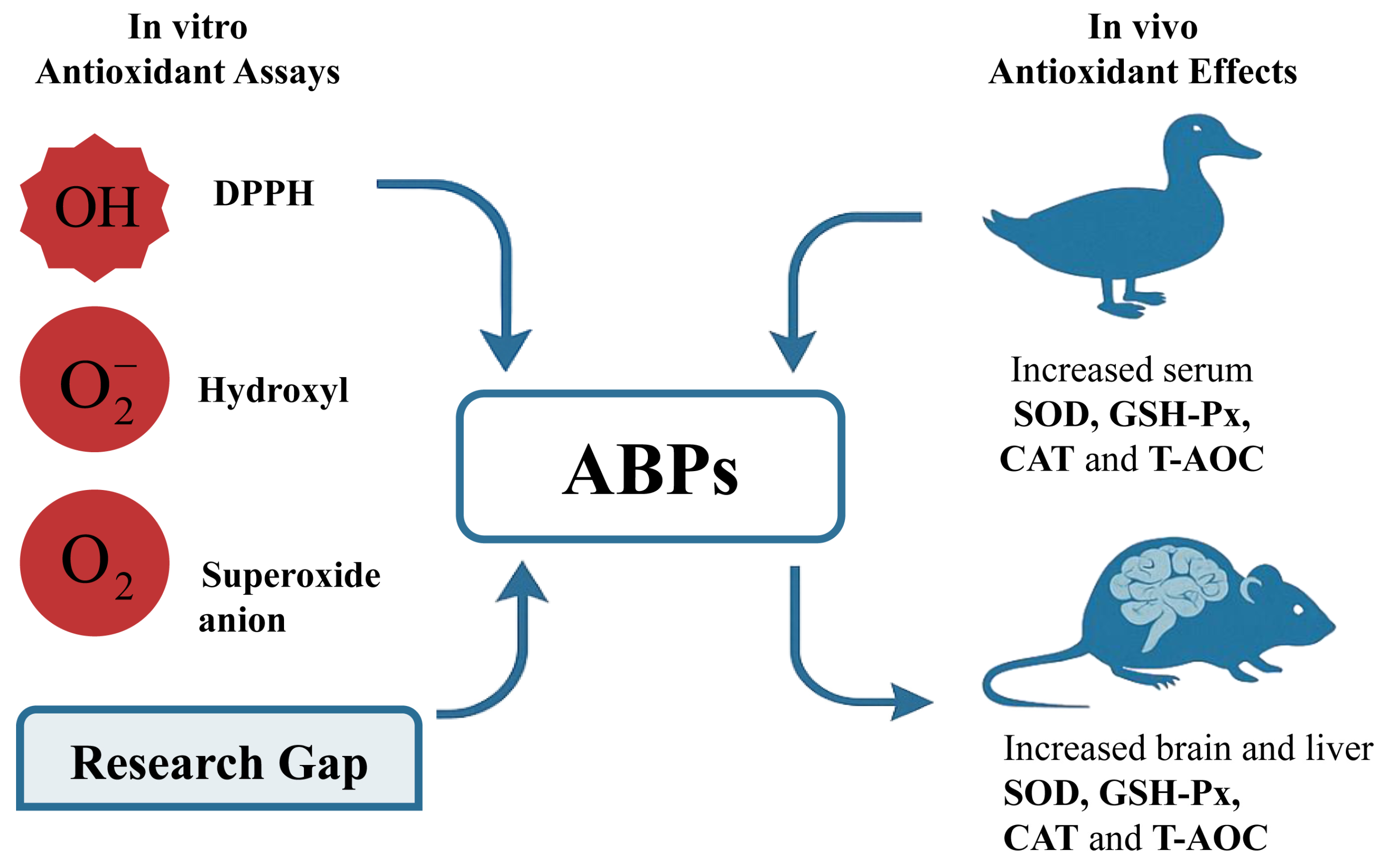
5.1. Antioxidant Activity
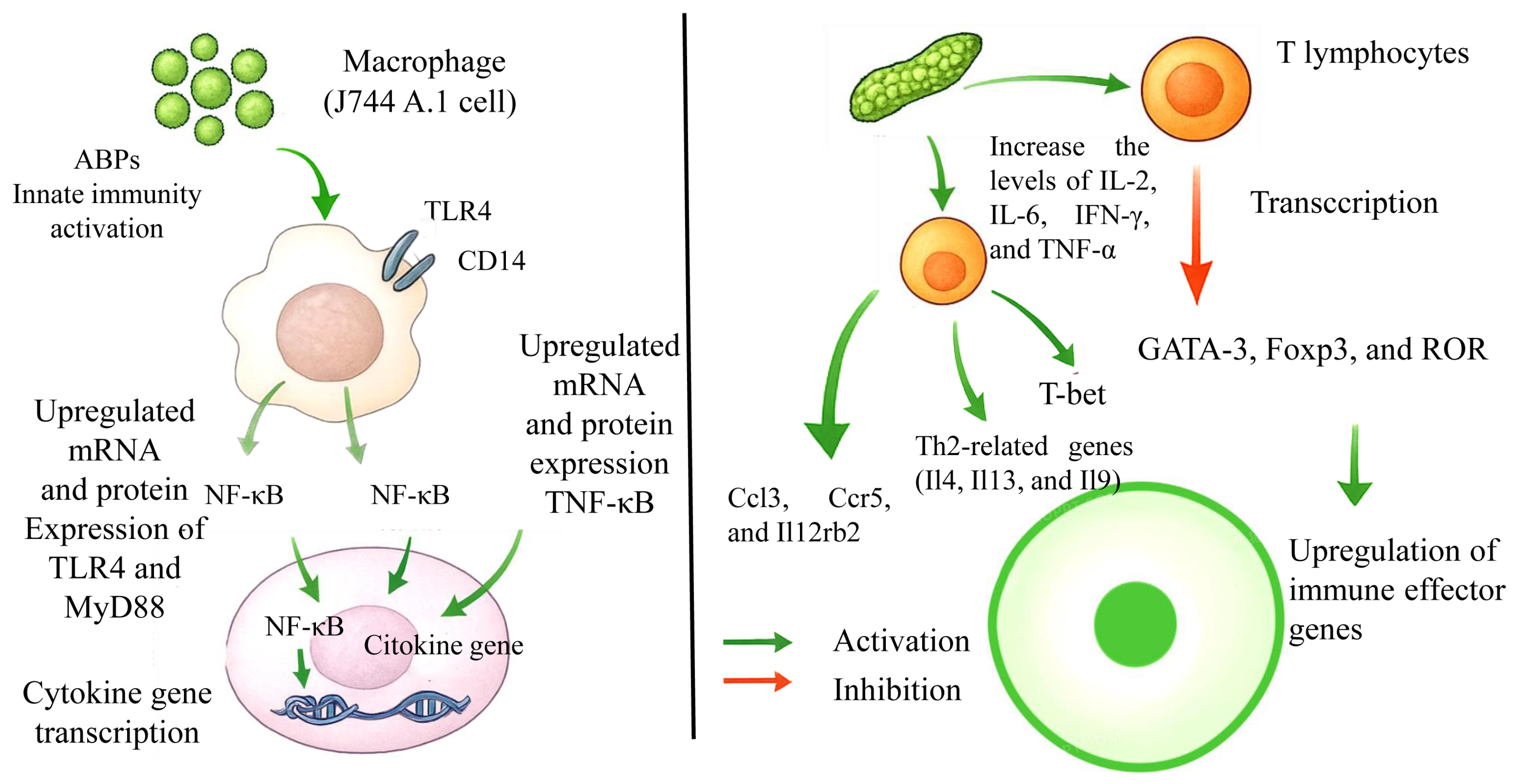
5.2. Immunomodulatory Activity
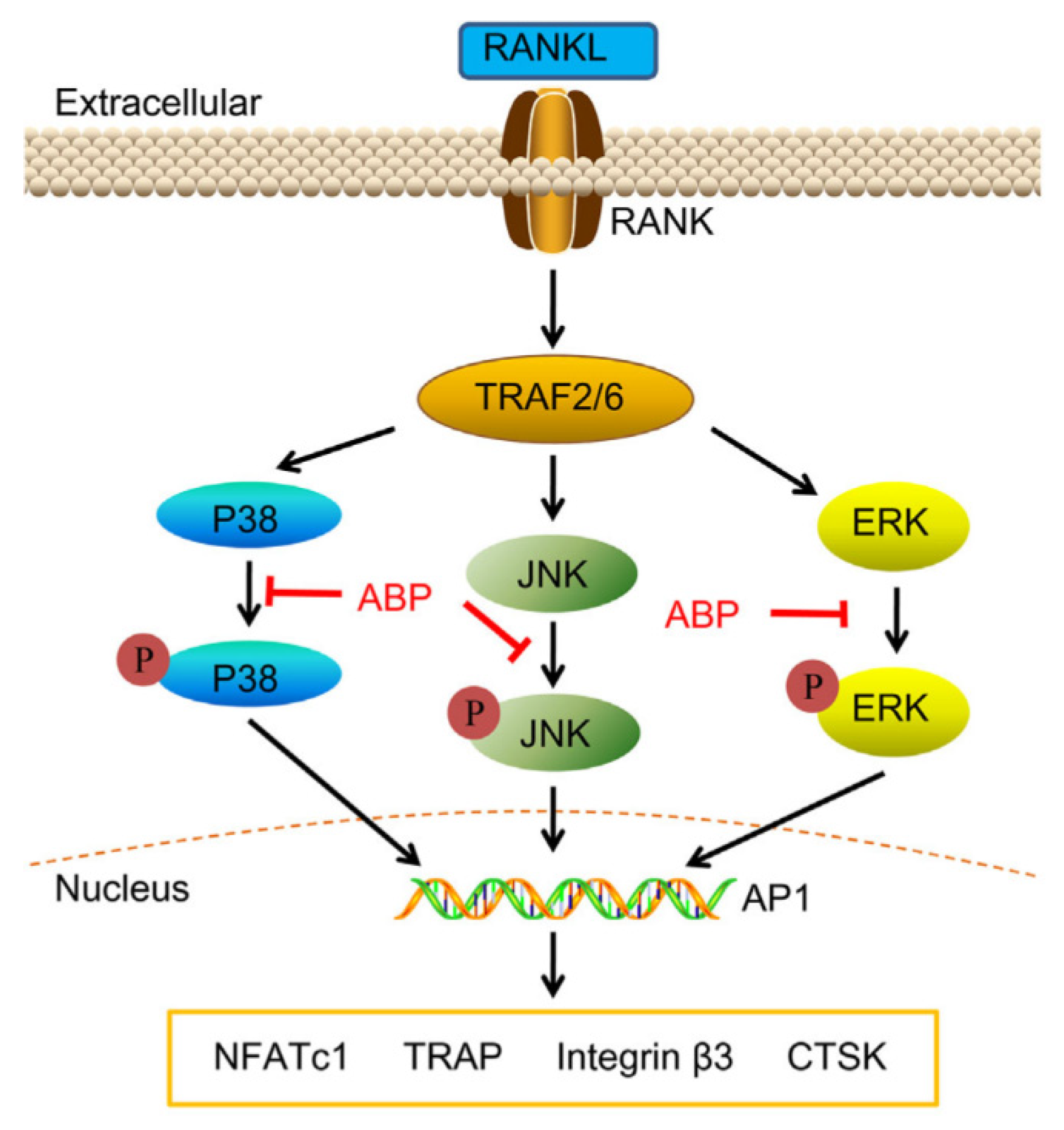
5.3. Anti-Osteoporosis Activity
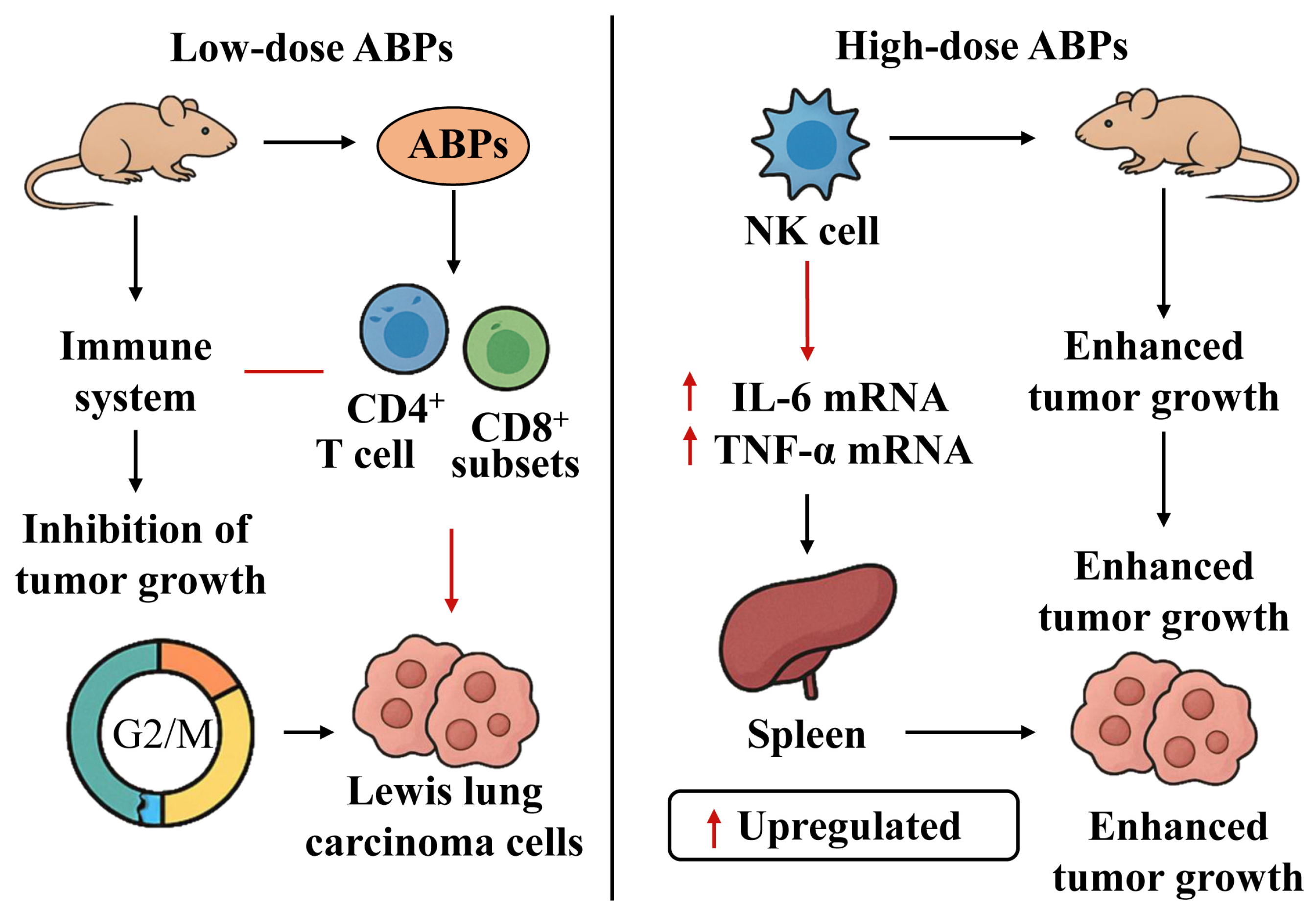
5.4. Antitumor Activity
5.5. Anti-Inflammatory Activity
5.6. Regulating Gut Microbiota
5.7. Other Bioactivities
6. Correlation of Structure, Content, and Biological Activity
7. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Tang, R.; Wu, H.L.; Li, J.H.; Zhang, C. Osteoprotective effect of Achyranthes bidentata root extract on osteoporotic rats: A systematic review and meta-analysis. Pharm. Biol. 2024, 62, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Niu, Y.S.; Zhou, H.L. Achyranthes bidentata Blume (Amaranthaceae): A review of its botany, traditional uses, phytochemistry, pharmacology, and toxicology. J. Pharm. Pharmacol. 2024, 76, 930–966. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.H.; He, T.T.; Jiang, S.L.; Zhu, X.H.; Zhang, Q.Y.; Ji, M.C.; Liang, J.; Xia, Y.G. Oligo/polysaccharides from Cyathula officinalis and Achyranthes bidentata: A review of structures and bioactivities. J. Pharm. Pharmacol. 2024, 76, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.Y.; Ye, Y.H.; Liu, Y.; Xu, J.L.; Zhang, C.L.; Lyu, C.M.; Feng, C.G.; Jiang, Y.; Yang, Y.; Ke, Y. Achyranthes bidentata polysaccharides improve cyclophosphamide-induced adverse reactions by regulating the balance of cytokines in helper T cells. Int. J. Biol. Macromol. 2024, 265, 130736. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, G.; Zheng, R. A systematic pharmacology and in vitro study to identify the role of the active compounds of Achyranthes bidentata in the treatment of osteoarthritis. Med. Sci. Monit. 2020, 26, e925545. [Google Scholar] [CrossRef]
- Yu, F.; Li, X.; Cai, L.; Li, H.; Chen, J.; Wong, X.; Xu, H.; Zheng, C.; Liu, X.; Ye, H. Achyranthes bidentata polysaccharides induce chondrocyte proliferation via the promotion of the G1/S cell cycle transition. Mol. Med. Rep. 2013, 7, 935–940. [Google Scholar] [CrossRef]
- Guo, P.; Zeng, M.; Liu, M.; Zhang, Y.; Jia, J.; Zhang, Z.; Liang, S.; Zheng, X.; Feng, W. Isolation of Calenduloside E from Achyranthes bidentata Blume and its effects on LPS/D-GalN-induced acute liver injury in mice by regulating the AMPK-SIRT3 signaling pathway. Phytomedicine 2024, 125, 155353. [Google Scholar] [CrossRef]
- Si, H.; Chen, Y.; Yang, J.; Wen, X. Characterization and comparison of polysaccharides from Achyranthes bidentata, Cyathula officinalis and Achyranthes aspera by saccharides mapping. J. Pharm. Biomed. Anal. 2023, 227, 115272. [Google Scholar] [CrossRef]
- Ji, X.; Yin, M.; Nie, H.; Liu, Y. A review of isolation, chemical properties, and bioactivities of polysaccharides from Bletilla striata. Biomed. Res. Int. 2020, 2020, 5391379. [Google Scholar] [CrossRef]
- Fu, G.Q.; Li, Y.X.; He, Y.; Zhang, H.; Ma, X. Extraction, structure and bioactivity of Tremella Fuciformis polysaccharides: A review. Food Med. Homol. 2025, 2, 9420038. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, C.; Hou, X.; Yan, C. Structural characterization and osteoprotective effects of a polysaccharide purified from Achyranthes bidentata. Int. J. Biol. Macromol. 2019, 139, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.K.; Pan, X.Y.; Liang, X.R.; Zhai, K.F.; Yu, Q. Research progress on structural characterization and bioactivities of Poria cocos and Ganoderma polysaccharides. Food Med. Homol. 2025, 2, 9420040. [Google Scholar] [CrossRef]
- Hou, C.; Yin, M.; Lan, P.; Wang, H.; Nie, H.; Ji, X. Recent progress in the research of Angelica sinensis (Oliv.) Diels polysaccharides: Extraction, purification, structure and bioactivities. Chem. Biol. Technol. Agric. 2021, 8, 13. [Google Scholar] [CrossRef]
- Jing, Y.S.; Hu, J.Y.; Wang, Z.Y.; Tao, C.; Zhang, S.L.; Hu, B.B.; Li, Z.W. Research progress on extraction, structure, bioactivity, structure-activity relationship and product applications of polysaccharides from Mori Fructus. Food Med. Homol. 2025, 2, 9420067. [Google Scholar] [CrossRef]
- Wei, T.; He, P.X.; Feng, S.X.; Wei, D.Z. Study on the ultrasonic-assisted extraction technology of polysaccharides from Achyranthes bidentata Blume and its anti-oxidant activities. Food Ferment. Ind. 2011, 37, 191–194. [Google Scholar]
- Ye, X.J.; Huang, Y.X.; Li, S.F. Study on extraction of Cyathulae Radix polysaccharides by compound enzymes method. China J. Pharm. Econ. 2018, 13, 24–27. [Google Scholar]
- Chen, A.M.; Pan, J.; Hu, B.; Chen, J.W.; Wu, W. Determination of antioxidant activities of polysaccharides from Cyathulae Radixand optimization of microwave extraction. Chin. Tradit. Pat. Med. 2017, 39, 80–84. [Google Scholar]
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Tech. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Wen, C.; Li, T.; Wang, B.; Jin, C.; Li, S.; Li, Y.; Li, M.; Ding, K. A pectic polysaccharide isolated from Achyranthes bidentata is metabolized by human gut Bacteroides spp. Int. J. Biol. Macromol. 2023, 248, 125785. [Google Scholar] [CrossRef]
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef]
- Wang, C.; Hua, D.; Yan, C. Structural characterization and antioxidant activities of a novel fructan from Achyranthes bidentata Blume, a famous medicinal plant in China. Ind. Crop. Prod. 2015, 70, 427–434. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Zhang, D.; Wang, C.; Yan, C. Anti-osteoporosis activity of a novel Achyranthes bidentata polysaccharide via stimulating bone formation. Carbohyd. Polym. 2018, 184, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Peng, B.; Ding, H.; Cui, B.; Nie, H.; Yan, Y. Purification, structure and biological activity of pumpkin polysaccharides: A review. Food Rev. Int. 2021, 39, 307–319. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Shi, M.; Yan, Y.; Liu, Y. An insight into the research concerning Panax ginseng C. A. Meyer polysaccharides: A review. Food Rev. Int. 2020, 38, 1149–1165. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, S.; Wang, C.; Zhang, Q. A fructooligosaccharide from Achyranthes bidentata inhibits osteoporosis by stimulating bone formation. Carbohyd. Polym. 2019, 210, 110–118. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, D.; Zhang, M.; Jiao, Y.; Jiang, K.; Yan, C. Structural characterization of a novel oligosaccharide from Achyranthes bidentata and its anti-osteoporosis activities. Ind. Crop. Prod. 2017, 108, 458–469. [Google Scholar] [CrossRef]
- Lin, Z.; Li, T.; Yu, Q.; Chen, H.; Zhou, D.; Li, N.; Yan, C. Structural characterization and in vitro osteogenic activity of ABPB-4, a heteropolysaccharide from the rhizome of Achyranthes bidentata. Carbohyd. Polym. 2021, 259, 117553. [Google Scholar] [CrossRef]
- Si, H.; Chen, Y.; Hu, D.; Yao, S.; Yang, J.; Wen, X. A graminan type fructan from Achyranthes bidentata prevents the kidney injury in diabetic mice by regulating gut microbiota. Carbohyd. Polym. 2024, 339, 122275. [Google Scholar] [CrossRef]
- Chen, X.M.; Tian, G.Y. Structural features of fructans from the root of Cyathula officinalis Kuan. Chin. J. Chem. 2003, 21, 858–863. [Google Scholar] [CrossRef]
- Li, S.F. Isolation and Identification of Hypoglycemic and Anti-Inflammatory Active Components from Achyranthes Bidentata. Master’s Thesis, Jiangnan University, Wuxi, China, 2022. [Google Scholar]
- Li, S.F.; Tang, N.C.; Xu, D.P. Isolation and identification of hypoglycemic polysaccharide from Achyranthes bidentata. J. Food Sci. Biotechnol. 2023, 42, 104–111. [Google Scholar]
- Fang, J.N.; Zhang, Z.H.; Liu, B.N. Chemical studies on polysaccharide of Achye-anthes Bidentata. Acta Pharm. Sin. 1990, 25, 526–529. [Google Scholar]
- Lin, Z.Z. Isolation, Purification, Structure Identification and Hypoglycemic Activity of Polysaccharides from Achyranthes Bidentata, Mori Fructus and Polygonatum Odoratum; Guangdong Pharmaceutical University: Guangzhou, China, 2020. [Google Scholar]
- Ji, X.; Chen, J.; Li, Z.; Meng, Y.; Li, X. Digestion characteristics of jujube polysaccharide and its regulatory effect on intestinal microbiota and metabolites during in vitro fermentation. LWT-Food Sci. Technol. 2024, 210, 116869. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, P.; Zhang, C.; Gao, Y.; Tan, J. Research progress in the preparation, structural characterization, and biological activities of polysaccharides from traditional Chinese medicine. Int. J. Biol. Macromol. 2024, 262, 129923. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zou, J.; Zhang, X.; Zhai, B.; Guo, D.; Sun, J.; Luan, F. Extraction, purification, structural features, biological activities, modifications, and applications from Taraxacum mongolicum polysaccharides: A review. Int. J. Biol. Macromol. 2024, 259, 129193. [Google Scholar] [CrossRef]
- Ji, X.L.; Guo, J.H.; Tian, J.Y.; Ma, K.; Liu, Y.Q. Research progress on degradation methods and product properties of plant polysaccharides. J. Light Ind. 2023, 38, 55–62. [Google Scholar]
- Guo, Y.; Qin, W.; Hou, Y.; Zhu, W.; Zhao, H.; Zhang, X.; Jiao, K. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Rubus L: A review. Food Chem. 2025, 478, 143711. [Google Scholar] [CrossRef]
- Zhan, K.; Ji, X.; Luo, L. Recent progress in research on Momordica charantia polysaccharides: Extraction, purification, structural characteristics and bioactivities. Chem. Biol. Technol. Agric. 2023, 10, 58. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Song, B.; Li, L.; Shi, R.; Ma, X.; Zhang, L.; Li, X. Recent advances in inulin polysaccharides research: Extraction, purification, structure, and bioactivities. Chem. Biol. Technol. Agric. 2024, 11, 136. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohyd. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Yi, J.P.; Wang, S.; Li, X.; Yang, Y.H.; Zhao, P.C.; Zhu, W.X. Optimization of steam explosion pretreatment for polysacchaides extraction from Achyranthes bidentata radix and of its antioxidant activity evaluation. Food Mach. 2018, 34, 145–151. [Google Scholar]
- Peng, Z.G.; Chen, H.S.; Guo, Z.M.; Dong, B.; Tian, G.Y.; Wang, G.Q. Anti-HIV acitivities of Achyranthes bidentata polysaccharide sulfate in vitro and in vivo. Acta Pharm. Sin. 2008, 48, 702–706. [Google Scholar]
- Deng, L.H.; Tian, G.Y. Studies on the preparation, structure and bioactivity of CM-AbPS. Acta Chim. Sin. 2002, 60, 2049–2055. [Google Scholar]
- Xue, S.X.; Jin, L.Q.; Ye, F.Q.; Jia, D.M. Effect of the sulfated and phosphorylated derivative of Achyranthes bidentata polysaccharides on human lung cancer cell A549. Chin. J. Biochem. Pharm. 2007, 28, 406–409. [Google Scholar]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohyd. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef]
- Yusoff, M.H.M.; Shafie, M.H. A review of in vitro antioxidant and antidiabetic polysaccharides: Extraction methods, physicochemical and structure-activity relationships. Int. J. Biol. Macromol. 2024, 282, 137143. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Yan, Y.; Shi, M.; Liu, Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.F.; Xia, X.L. Oxidative stress and immunity of weaned piglets in response to Achyranthes bidentata polysaccharide. Jiangsu J. Agric. Sci. 2017, 33, 618–623. [Google Scholar]
- Li, J.L.; Han, X.F.; Liu, T.Q.; Zeng, X.Y. Effects of polysaccharides from the roots of Radix Cyathulae officinalis Kuan on in vivo antioxidant capacities of D-galactose-induced aging mouse model. Chin. J. Antibiot. 2014, 39, 553–559. [Google Scholar]
- Tang, J. Effects of Polysaccharides from the Roots of Cyathulaofficinalis Kuan on Antioxidative Activity in Mice; Sichuan Agricultural University: Ya’an, China, 2013. [Google Scholar]
- Hu, Y.; He, Y.; Niu, Z.; Shen, T.; Zhang, J.; Wang, X.; Hu, W.; Cho, J.Y. A review of the immunomodulatory activities of polysaccharides isolated from Panax species. J. Ginseng Res. 2022, 46, 23–32. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhuyan, P.P.; Ki, J.S. Immunomodulatory, antioxidant, anticancer, and pharmacokinetic activity of ulvan, a seaweed-derived sulfated polysaccharide: An updated comprehensive review. Mar. Drugs 2023, 21, 300. [Google Scholar] [CrossRef]
- Ji, X.; Yan, Y.; Hou, C.; Shi, M.; Liu, Y. Structural characterization of a galacturonic acid-rich polysaccharide from Ziziphus Jujuba cv. Muzao. Int. J. Biol. Macromol. 2020, 147, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Kim, I.H. Effects of Achyranthes bidentata polysaccharides on performance, immunity, antioxidant capacity and meat quality in Pekin ducks. Poult. Sci. 2020, 99, 4884–4891. [Google Scholar] [CrossRef] [PubMed]
- Ou, N.; Sun, Y.; Zhou, S.; Gu, P.; Liu, Z.; Bo, R.; Hu, Y.; Liu, J.; Wang, D. Evaluation of optimum conditions for Achyranthes bidentata polysaccharides encapsulated in cubosomes and immunological activity in vitro. Int. J. Biol. Macromol. 2018, 109, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Meng, J.; Chen, W.; Liu, J.; Li, X.; Li, W.; Lu, C.; Shan, F. Modulation of phenotypic and functional maturation of murine dendritic cells (DCs) by purified Achyranthes bidentata polysaccharide (ABP). Int. Immunopharmacol. 2011, 11, 1103–1108. [Google Scholar] [CrossRef]
- Kang, P.; Xiao, H.L.; Hou, Y.Q.; Ding, B.Y.; Liu, Y.L.; Zhu, H.L.; Hu, Q.Z.; Hu, Y.; Yin, Y.L. Effects of Astragalus polysaccharides, Achyranthes bidentata polysaccharides and Acantbepanax senticosus saponin on the performance and immunity in weaned pigs. Asian-Aust. J. Anim. Sci. 2010, 23, 750–756. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Zhang, Y.; Wu, Y.; Chen, X. Achyranthes bidentata polysaccharide activates nuclear factor-kappa B and promotes cytokine production in J774A.1 cells through TLR4/MyD88 signaling pathway. Front. Pharmacol. 2021, 12, 753599. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z.; He, J.H. Achyranthes bidentata polysaccharide enhances immune response in weaned piglets. Immunopharmacol. Immunotoxicol. 2009, 31, 253–260. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z.; He, J.; Zhao, Y.; Wu, X. Achyranthes bidentata polysaccharide enhances growth performance and health status in weaned piglets. Food Agric. Immunol. 2011, 22, 17–29. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, X.; Liang, X.; Guo, S.; Qin, B.; Liu, E.H.; Duan, J.A. Mechanisms and structure-activity relationships of natural polysaccharides as potential anti-osteoporosis agents: A review. Int. J. Biol. Macromol. 2025, 298, 139852. [Google Scholar] [CrossRef]
- Lei, S.S.; Su, J.; Zhang, Y.; Huang, X.W.; Wang, X.P.; Huang, M.C.; Li, B.; Shou, D. Benefits and mechanisms of polysaccharides from Chinese medicinal herbs for anti-osteoporosis therapy: A review. Int. J. Biol. Macromol. 2021, 193, 1996–2005. [Google Scholar] [CrossRef]
- He, C.C.; Hui, R.R.; Tezuka, Y.; Kadota, S.; Li, J.X. Osteoprotective effect of extract from Achyranthes bidentata in ovariectomized rats. J. Ethnopharmacol. 2010, 127, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Zhang, Q.; Wang, C.; Zhang, D.; Wan, J.B.; Yan, C. UPLC/Q-TOF-MS-based metabolomics study of the anti-osteoporosis effects of Achyranthes bidentata polysaccharides in ovariectomized rats. Int. J. Biol. Macromol. 2018, 112, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Cao, Z.; Huang, S.; Tickner, J.; Li, N.; Qiu, H.; Chen, X.; Wang, C.; Chen, K.; Sun, Y.; et al. Achyranthes bidentata polysaccharide suppresses osteoclastogenesis and bone resorption via inhibiting RANKL signaling. J. Cell. Biochem. 2018, 119, 4826–4835. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohyd. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- Li, S.W.; Zhao, Z.Y.; He, Z.Y.; Yang, J.C.; Feng, Y.J.; Xu, Y.M.; Wang, Y.N.; He, B.B.; Ma, K.S.; Zheng, Y.; et al. Effect of structural features on the antitumor activity of plant and microbial polysaccharides: A review. Food Bio. 2024, 61, 104648. [Google Scholar] [CrossRef]
- Li, J.T.; Gu, A.; Tang, N.N.; Sun, Z.Y.; Zhang, G.; Li, M.Y. Exploring anti-tumor potential of food and medicine homology substances: An in-silico evaluation of Citri Grandis Exocarpium against gallbladder cancer. Food Med. Homol. 2025, 3, 9420084. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar]
- Jin, L.Q.; Zheng, Z.J.; Peng, Y.; Li, W.X.; Chen, X.M.; Lu, J.X. Opposite effects on tumor growth depending on dose of Achyranthes bidentata polysaccharides in C57BL/6 mice. Int. Immunopharmacol. 2007, 7, 568–577. [Google Scholar] [CrossRef]
- Cao, J.; Wu, L.; Li, K. Influence of acetyltalatisamine achyranthan on tumor microenvironment T lymphocyte subpopulation and VEGF and TGF-β1 in HepG-2 bearing cancer mice. Chin. J. Mod. Drug Appl. 2014, 8, 1–4. [Google Scholar]
- Zhang, Y.Q.; Xia, Y.T.; Xiao, H.; Chen, L.; Min, L.L.; Zhang, Y.L.; Li, C.; Huang, D.; Li, Z.H. Research progress on anti-tumor effects of Achyranthes polysaccharides. Chin. Naturop. 2023, 31, 103–106. [Google Scholar]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, X.; Xia, L.; Xiong, S.; Xia, B.; Xie, J.; Lin, Y.; Lin, L.; Wu, P. Progress on the anti-inflammatory activity and structure-efficacy relationship of polysaccharides from medical and edible homologous traditional Chinese medicines. Molecules 2024, 29, 3852. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Qiu, Z.; Huang, Y.; Lin, Q.; Jin, L.; Tu, H.; Ye, J.; Zheng, C.; Zhong, W.; Ma, D. Achyranthes bidentata polysaccharides alleviate endoplasmic reticulum stress in osteoarthritis via lncRNA NEAT1/miR-377-3p pathway. Biomed. Pharmacother. 2022, 154, 113551. [Google Scholar] [CrossRef]
- Jiang, L. Experimental Study on the Intervention of Achyranthes Polysaccharides on the Proliferation and Apoptosis of Degenerated Articular Chondrocytes in Human Knee Osteoarthritis; Hunan University of Chinese Medicine: Changsha, China, 2019. [Google Scholar]
- Gao, Y. Inflammation and gut microbiota in the alcoholic liver disease. Food Med. Homol. 2024, 1, 9420020. [Google Scholar] [CrossRef]
- Han, D.; Yang, L.; Liang, Q.C.; Sun, H.; Sun, Y.; Yan, G.L.; Zhang, X.W.; Han, Y.; Wang, X.Y.; Wang, X.J. Natural resourced polysaccharides: Preparation, purification, structural elucidation, structure-activity relationships and regulating intestinal flora, a system review. Int. J. Biol. Macromol. 2024, 280, 135956. [Google Scholar] [CrossRef]
- Zhou, Y.; Sheng, Y.J.; Li, C.Y.; Zou, L.; Tong, C.Y.; Zhang, Y.; Cao, G.; Shou, D. Beneficial effect and mechanism of natural resourced polysaccharides on regulating bone metabolism through intestinal flora: A review. Int. J. Biol. Macromol. 2025, 253, 127428. [Google Scholar] [CrossRef]
- Xia, T.; He, W.; Luo, Z.; Wang, K.; Tan, X. Achyranthes bidentata polysaccharide ameliorates type 2 diabetes mellitus by gut microbiota-derived short-chain fatty acids-induced activation of the GLP-1/GLP-1R/cAMP/PKA/CREB/INS pathway. Int. J. Biol. Macromol. 2024, 270, 132256. [Google Scholar] [CrossRef]
- Ma, W. Study on Digestive Characteristics of Achyranthes Bidentata Polysaccharide In Vitro and Its Intervention Effect on Lung Cancer; Jiangnan University: Wuxi, China, 2022. [Google Scholar]
- Liu, Z.; Wang, X.; Ou, S.; Arowolo, M.A.; Hou, D.X.; He, J. Effects of Achyranthes bidentata polysaccharides on intestinal morphology, immune response, and gut microbiome in yellow broiler chickens challenged with Escherichia coli K88. Polymers 2018, 10, 1233. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Chen, K.; Gao, Y.; Gao, S.; Liu, X.; Li, J. Sulfated modification can enhance antiviral activities of Achyranthes bidentata polysaccharide against porcine reproductive and respiratory syndrome virus (PRRSV) in vitro. Int. J. Biol. Macromol. 2013, 52, 21–24. [Google Scholar] [CrossRef]
- Zhu, X.T.; Pan, Y.Y.; Zheng, L.; Cui, L.W.; Cao, Y.M. Polysaccharides from the Chinese medicinal herb Achyranthes bidentata enhance anti-malarial immunity during Plasmodium yoelii 17XL infection in mice. Malaria J. 2012, 11, 49. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Lin, J.Y. Effects of Achyranthes bidentata polysaccharides on physical fatigue. Asian J. Anim. Vet. Adv. 2012, 7, 726–732. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhang, Z.G.; Xin, X.M.; Wang, H. The protection of achyrantata polysaccharides on immunological liverinjury mice. J. Taishan Med. Coll. 2010, 31, 97–99. [Google Scholar]
- Jin, Y.Z. Protective effects of Achyranthes bidentata polysacchariedes on acute liver injury induced by carbon tetrachloride in rats. J. Med. Sci. Yanbian Univ. 2018, 41, 248–251. [Google Scholar]
- Li, C.C.; Hu, X.G.; Zhang, W.X.; Xie, L.W.; Zhang, H.Y.; Dong, L.; Cai, X.H.; Wu, R.X.; Zhang, Z.X.; He, Q.X. Eosinophils apoptosis, fas mRNA and bc-l2mRNA expressions in asthma model of young rat and effects of Achyranthes bidentata polysaccharides. Chin. J. Pediatr. 2003, 41, 657–660. [Google Scholar]
- Shi, L.; He, Q.; Li, J.; Liu, Y.; Cao, Y.; Liu, Y.; Sun, C.; Pan, Y.; Li, X.; Zhao, X. Polysaccharides in fruits: Biological activities, structures, and structure-activity relationships and influencing factors: A review. Food Chem. 2024, 451, 139408. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Cao, T.; Zhang, T.; Liu, Y.; Yan, Y. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Well. 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Huang, J.; Ou, Y.; Yew, T.W.; Liu, J.; Leng, B.; Lin, Z.; Su, Y.; Zhuang, Y.; Lin, J.; Li, X.; et al. Hepatoprotective effects of polysaccharide isolated from Agaricus bisporus industrial wastewater against CCl4-induced hepatic injury in mice. Int. J. Biol. Macromol. 2016, 82, 678–686. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| No. | Method | Advantages | Disadvantages | Extraction Method | Yield | Ref. |
|---|---|---|---|---|---|---|
| 1 | Hot water extraction | cost-effective, economically viable, minimal impact on polysaccharide structure | considerable impurity content, susceptibility to degradation, prolonged extraction duration, and limited extraction efficiency | three successive extraction cycles with a 10:1 solvent-to-solid ratio at 80 °C for 3 h per cycle | 49.18% /29.4% | [11] |
| 2 | Cold water extraction | solvent-free, thermal stability, bioactivity retention, suitable for heat-sensitive compounds | low extraction efficiency, time-consuming, limited solubility, poor selectivity | a duration of 5 h, solid-to-liquid ratio of 1:40 (mL/g) | - | [12] |
| 3 | Microwave-assisted extraction | elevated yield, short processing duration, minimal impurity introduction | economically unfavorable, challenging for industrial application | a solid-to-liquid ratio of 1:20 (g/mL), microwave power of 300 W, extraction time of 10 min, and temperature of 50 °C | 8.2% | [14] |
| 4 | Ultrasonic-assisted extraction | capable of preserving structural integrity, user-friendly and low-risk process, enhanced extraction performance | depolymerization of soluble polysaccharides | ultrasonic power of 1000 W, extraction temperature of 60 °C, extraction time of 60 min, a solid-to-liquid ratio of 1:30 (g/mL) | 6.1% | [15] |
| 5 | Enzyme-assisted extraction | high affinity for target analytes, time-efficient extraction, impurities can be readily eliminated | enzyme deactivation, equipment-intensive process | solid-to-liquid ratio of 1:20 (g/mL), 0.7% compound enzyme addition, pH 6.0, temperature of 45 °C | - | [16] |
| No. | Name | Purification | Molecular Weight (Da) | Monosaccharide Composition | Structural Features | Bioactivities | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | A23-1 | DEAE-Cellulose 52, Sepharose S-300 column | 93,085 | Rha, GlcA, GalA, Glc, Gal, Ara in a molar ratio of 7.26:0.76:5.12:2.54:23.51:60.81 | Backbone of 1,2,4-Rhap and 1,4-GlapA, and different branches composed of galactose, arabinose, glucose which were linked at C-4 of rhamnose | Regulating gut microbiota | [19] |
| 2 | ABW50-1 | DEAE-Cellulose 52, Sepharose S-100 column | 1260 | Fru, Glc in the ratio of 6:1 | Backbone of →2)-β-d-Fruf-(1→, →2)-β-d-Fruf-(1,6→ and →2)-β-d-Fruf-(6→, terminated with Glcp and Fruf residues | Stimulating bone formation | [25] |
| 3 | ABW70-1 | DEAE-Cellulose 52, Sepharose S-100 column | 1316 | Fru, Glc in the ratio of 7:1 | Backbone of (2→1)-linked-β-d-Fruf, (2→6)-linked-β-d-Fruf and (1→1,6)-linked -β-d-Fruf residues, and terminated with fructose and glucose residues | Anti-osteoporosis | [11] |
| 4 | ABW90-1 | DEAE-Cellulose 52, Sepharose S-100 column | 1074 | Fru, Glc in the ratio of 5:1 | Backbone of (2→6)-linked β-d-Fruf, (2→1)-linked β-d-Fruf residues and terminated with Glcp and Fruf residues | Anti-osteoporosis | [26] |
| 5 | ABPB-3 | DEAE-Cellulose 52, Sephadex G-75 column | 77,230 | Ara:Gal:Rha:GalA in the ratio of 18.4:9.3:2.5:1.0 | Backbone of →4)-α-d-GalpA-(1→, →2,4)-α-l-Rhap-(1→, →5)-α-l-Araf-(1→, →2,3,5)-α-l-Araf-(1→, →3)-β-d-Galp-(1→, →3,4,6)-β-d-Galp-(1→, terminated with α-l-Araf, α-l-Rhap and β-d-Galp | Anti-osteoporosis | [22] |
| 6 | ABPB-4 | DEAE-Cellulose 52, Sephadex G-75 column | 63,500 | Rha, Gal, GalA, Ara | Backbone of and →2,4)-α-l-Rhap-(1→, and the branch chains →4)-β-d-Galp -(1→, →6)-β-d-Galp-(1→, →3,6)-β-d-Galp-(1→, →5)-α-l-Araf-(1→ and →3,5)-α-l-Araf-(1→, and terminated with α-l-Araf-(1→ and β-d-Galp-(1→ | Osteogenic activity | [27] |
| 7 | ABPW1 | DEAE Bestarose FF, Sephadex G25 column | 3998 | Fru, Glc in the ratio of 24:1 | Backbone of →1)-β-d-Fruf-(2→, →2)-β-d-Fruf-(6→, →1,6)-β-d-Fruf-(2→ connected fructose and terminated with T-α-d-Glcp and β-d-Fruf-(2→ | Regulating gut microbiota | [28] |
| 8 | ABP70-2 | DEAE-Cellulose 52, Sephacryl S-100HR column | 3406 | Fru, Glc in the ratio of 18:1 | Backbone of (2→6)-linked β-d-Fruf, with (2→1)- Linked β-d-Fruf branched chains, and terminated with Glcp and Fruf residues | Antioxidant | [21] |
| 9 | CoPS1 | Sephadex G-50, CM-Sephadex C-50 | 5200 | Fru, Glc in the ratio of 39:1 | →2)-Fruf-(6→, →1)-Fruf-(2→, Fruf-(2→, →1,6)- Fruf-(2→, α-d-Glcp-(1→ | [29] | |
| 10 | CoPS2 | Sephadex G-50, CM-Sephadex C-50 | 3000 | Fru, Glc in the ratio of 36:1 | →2)-Fruf-(6→, →1)-Fruf-(2→, Fruf-(2→, →1,6)- Fruf-(2→, α-d-Glcp-(1→ | [29] | |
| 11 | CoPS3 | Sephadex G-50, CM-Sephadex C-50 | 1400 | Fru, Glc in the ratio of 21:1 | →2)-Fruf-(6→, →1)-Fruf-(2→, Fruf-(2→, →1,6)- Fruf-(2→, α-d-Glcp-(1→ | [29] | |
| 12 | M2 | DEAE-Cellulose, HW-55F, Sephacryl S-400 | 1800.5 | Fru, Glc in the ratio of 10:1 | →1,6)- Fruf-(2→, α-d-Glcp-(1→ | Hypoglycemia | [30] |
| 13 | Abs | Sephadex G-50 column | 1400 | Fru, Glc | 2→6 linked and 2→1 linkedβ-d-Fruf residues | [31] | |
| 14 | ABAB | Cellex D, Sephadex G-150 column | 23,000 | GlcA, Gal, GalA, Ara, Rha in the ratio of 12:2:3:1:1 | →1)-d-GlcpA-(4→, →4)-d-GalpA-(1→ | [32] | |
| 15 | ABP90-2 | DEAE-Cellulose 52 Sepharose S-100 column | 2976 | Fru, Glc in the ratio of 16:1 | α-d-Glcp-(1→, β-d-Fruf-(2→, →1,6)-β-d-Fruf -(2→, →1)-β-d-Fruf-(2→, →2)-β-d-Fruf-(6→ | [21] | |
| 16 | ABP50-2 | DEAE-Cellulose 52 Sepharose S-100 column | 5118 | Fru, Glc in the ratio of 27:1 | α-d-Glcp-(1→, β-d-Fruf-(2→, →1,6)-β-d-Fruf- (2→, →1)-β-d-Fruf-(2→, →2)-β-d-Fruf-(6→ | [21] | |
| 17 | FTN-5 | DEAE-Cellulose 52 | 10,000 | Man:Rha:Rib:GlcA:GalA:Glc:Gal:Xyl:Ara:Fuc in the ratio of 1.21:2.13:0.38:0.79:19.38:2.32:2.42:1.00:0.97:0.36 | [33] | ||
| 18 | OTN-6 | DEAE-Cellulose 52 | 22,400 | Man:Rha:Rib:Glc:Gal:Ara:Xyl in the ratio of 1.27:0.73:0.71:1.84:2.06:0.60: 1.01 | [3,34] | ||
| 19 | OTN-5 | DEAE-Cellulose 52 | 30,800 | Man:Rha:Rib:GalA:Glc:Gal:Xyl:Ara:Fuc in the ratio of 2.99:4.20:0.48:4.85:5.28:4.89:1.64:1.87:0.43 | [3] | ||
| 20 | TTN-5 | DEAE-Cellulose 52 | 97,700 | Man:Rha:Rib:GalA:Glc:Gal:Ara:Xyl:Fuc in the ratio of 0.67:3.22:0.33:10.73:1.73:4.53:2.44:1.78:0.36 | [3] | ||
| 21 | TTN-6 | DEAE-Cellulose 52 | 46,800 | Man:Rha:Rib:GlcA:GalA:Glc:Gal:Ara:Xyl:Fuc in the ratio of 1.53:1.24:0.37:2.96:1.43:8.32:4.00:0.86:1.33:0.29 | [3] | ||
| 22 | TTN-7 | DEAE-Cellulose 52 | 57,100 | Man:Rha:Rib:GlcA:GalA:Glc:Gal:Ara:Xyl:Fuc in the ratio of 1.96:1.98:0.39:1.27:2.30:6.60:4.71:1.68:1.85:0.30 | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wang, Z.; Zhang, W.; Chen, L.; Yang, Y.; Yang, W.; Li, M.; Yuan, C.; Zhang, L.; Wang, L. Isolation, Structures, and Bioactivities of Polysaccharides from Achyranthes bidentata: A Review. Molecules 2025, 30, 2523. https://doi.org/10.3390/molecules30122523
Li L, Wang Z, Zhang W, Chen L, Yang Y, Yang W, Li M, Yuan C, Zhang L, Wang L. Isolation, Structures, and Bioactivities of Polysaccharides from Achyranthes bidentata: A Review. Molecules. 2025; 30(12):2523. https://doi.org/10.3390/molecules30122523
Chicago/Turabian StyleLi, Lin, Zhihong Wang, Wei Zhang, Longxin Chen, Yingying Yang, Wan Yang, Mengkun Li, Chunhuan Yuan, Limeng Zhang, and Linqing Wang. 2025. "Isolation, Structures, and Bioactivities of Polysaccharides from Achyranthes bidentata: A Review" Molecules 30, no. 12: 2523. https://doi.org/10.3390/molecules30122523
APA StyleLi, L., Wang, Z., Zhang, W., Chen, L., Yang, Y., Yang, W., Li, M., Yuan, C., Zhang, L., & Wang, L. (2025). Isolation, Structures, and Bioactivities of Polysaccharides from Achyranthes bidentata: A Review. Molecules, 30(12), 2523. https://doi.org/10.3390/molecules30122523





