Abstract
Unsymmetrical urea derivatives are essential structural motifs in a wide array of biologically significant compounds. Despite the well-established methods for synthesizing symmetrical ureas, efficient strategies for the synthesis of unsymmetrical urea derivatives remain limited. In this study, we present a novel approach for the synthesis of unsymmetrical urea derivatives through the coupling of amides and amines. Utilizing hypervalent iodine reagent PhI(OAc)2 as a coupling mediator, this method circumvents the need for metal catalysts, high temperatures, and inert atmosphere. The reaction proceeds under mild conditions and demonstrates broad substrate scope, including various primary and secondary amines and primary benzamides. This protocol not only offers a practical and versatile route for synthesizing unsymmetrical ureas but also shows significant potential for the late-stage functionalization of complex molecules in drug development.
1. Introduction
Urea derivatives are integral to a diverse range of biologically active compounds, pharmaceuticals, agrochemicals, and materials science [1,2,3,4,5,6,7,8,9]. Among these, unsymmetrical urea derivatives stand out due to their unique structural and functional properties [10]. They are prevalent in enzyme inhibitors, antiviral agents, and selective receptor modulators [11,12,13]. Accordingly, the synthesis of this class of compounds is of significance in synthetic organic chemistry, driven by the ongoing need to develop novel compounds with improved biological activities and functionalities.
Historically, the synthesis of urea derivatives has relied heavily on the reaction of amines with isocyanates [14,15]. While this approach is effective, the preparation of unsymmetrical ureas often requires additional steps and the handling of potentially hazardous reagents. Methods such as the direct carbonylation of amines and the use of phosgene or its derivatives, although effective, pose substantial challenges due to their toxicity and stringent reaction conditions [16].
The pressing need for more efficient, safer, and environmentally benign methodologies to synthesize unsymmetrical urea derivatives has motivated a number of recent studies. Rousseaux reported a metal-free synthesis of unsymmetrical ureas and carbamates from CO2 and amines via isocyanate intermediates [17], though the use of low temperature to generate the isocyanate intermediate limits its large-scale application. Transition metal-catalyzed synthesis of unsymmetrical ureas has been demonstrated, such as by Nowrouzi et al., who reported a palladium-catalyzed method using chromium hexacarbonyl as a convenient and safer alternative for introducing the carbonyl group [18]. Stahl and co-workers reported a copper-catalyzed benzylic C-H isocyanation and amine coupling sequence that enabled the high-throughput synthesis of pharmaceutically relevant ureas [19]. Furthermore, iron-catalyzed urea synthesis through dehydrogenative coupling of methanol and amines was reported by Bernskoetter [20].
In recent years, hypervalent iodine reagents have emerged as versatile tools in organic synthesis [21]. These reagents, particularly iodine(III) compounds like iodobenzene diacetate (PhI(OAc)2), have gained popularity due to their mild oxidative properties, ease of handling, and low toxicity. Hypervalent iodine reagents have been successfully employed in a variety of oxidative transformations, including C-H activation, oxidative coupling, and amination reactions [22,23,24,25,26]. Their ability to mediate bond formation under mild conditions makes them attractive for the synthesis of complex organic molecules, including urea derivatives. The use of PhI(OAc)2 as an oxidant and coupling mediator in the synthesis of unsymmetrical ureas represents a novel and promising approach by avoiding the use of toxic transition metal catalysts and reagents. Recently, Reboul [27] and Patureau [28] described the use of PhI(OAc)2 for the metal-free synthesis of unsymmetrical urea derivatives through the coupling of amines with amides. We have now exploited this development to explore the scope of this reaction through the synthesis of a wide range of primary amides and primary and secondary amines (Scheme 1). This versatility is crucial for the late-stage functionalization of complex molecules with urea moieties, particularly in pharmaceutical synthesis where functional group compatibility and the use of mild reaction conditions are paramount. The ability to introduce unsymmetrical urea functionalities into advanced intermediates or drug candidates without extensive protection–deprotection sequences or harsh conditions represents a significant advancement in this field.
Scheme 1.
I(III)-mediated urea synthesis [27,28].
2. Results and Discussion
2.1. Survey of Substrate Scope: Scope of Primary Amines
To optimize the reaction conditions, we explored various parameters, including solvents, temperature, and base selection (see Supplementary Materials Tables S1 and S2). Only inorganic bases were used, as organic bases have the potential to react with the intermediate isocyanate leading to side reactions, as reported in a previous study [29].
A comparison of solvent effects revealed that DCE provided the highest yields, while MeOH and other tested solvents resulted in lower conversions. Additionally, lowering the reaction temperature led to diminished yields, which is consistent with prior reports [27,28]. Our study demonstrates that the reaction can proceed efficiently at 80 °C, though without the need for excessive amounts of amine (e.g., 17.5 equivalents, as used in [27]).
Under the optimized reaction conditions, isolated yields were below 50% in several cases. The formation of the isocyanate appears to be the limiting step, as unreacted starting materials were observed. Increasing the amount of amine may help overcome this limitation and improve urea formation, as suggested by our comparative studies.
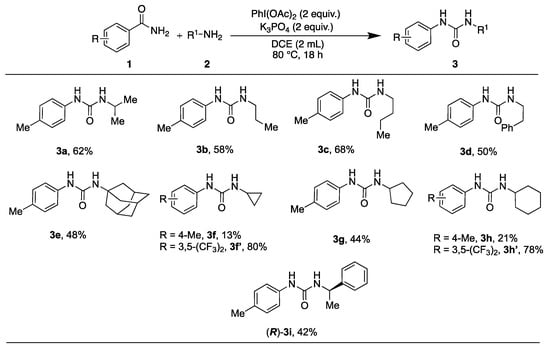
Initially, the use of aliphatic primary amines in the synthesis of unsymmetrical ureas was investigated (Scheme 2). The results indicated that primary amines with alkyl substituents, such as isopropylamine (2a), propylamine (2b), and butylamine (2c), yielded the corresponding urea derivatives (3a–3c) in relatively high yields (58–68%). However, a lower yield (50%) was observed for 2-phenylethylamine (3d). Even with the sterically demanding adamantylamine (2e), commonly used to treat dyskinesia, the expected product (3e) was isolated in a similar yield (48%). Cyclopropylamine (2f), cyclopentylamine (2g), and cyclohexylamine (2h) gave varied yields (3f–3h, 13–44%), likely due to the steric impact of these cyclic structures. Notably, the yields for cyclopropylamine (2f) and cyclohexylamine (2h) significantly improved when using the more reactive benzamide (1’), resulting in yields of 80% and 78%, respectively (3f’ and 3h’). The R-enantiomer of 1-phenylethylamine (2i) behaved as anticipated, giving the corresponding urea in 42% yield.
Scheme 2.
Scope of primary amines.
2.2. Survey of Substrate Scope: Scope of Secondary Amines
Following the use of primary amines, we expanded our study to include a variety of secondary amines for the synthesis of unsymmetrical ureas (Scheme 3). Overall, the yields were generally lower compared to the primary amines, with significant variability depending on both the secondary amine and the benzamide substituents. Although this protocol typically gave satisfactory results, there were cases with lower yields. Thin-layer chromatographic analysis revealed minimal reactivity in these instances, with unreacted amides persisting after the standard reaction time.
Scheme 3.
Scope of secondary amines.
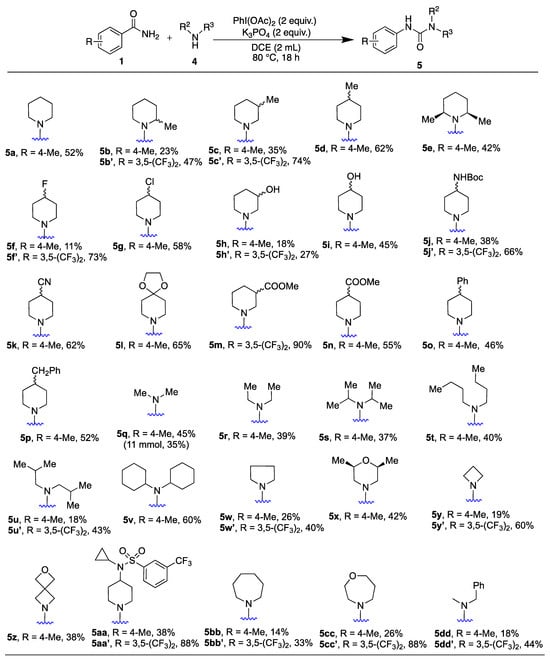
Given the prevalence of piperidine moieties in bioactive compounds and their significance in medicinal chemistry, we initially tested various C2, C3, and C4 substituted derivatives, for instance, piperidine (4a) gave a 52% yield, while 2-methylpiperidine (4b) and 3-methylpiperidine (4c) gave lower yields (5b, 23% and 5c 35%, respectively). The yields for 5b’ and 5c’ increased to 47% and 74% with the more reactive benzamide (1’). 4-methylpiperidine (4d) was well tolerated (5d, 62%); additionally, the sterically hindered 2,6-dimethylpiperidine (4e) yielded 5e in 42%, indicating the influence of steric and electronic factors on the reaction outcome. We also found that piperidines with polar functional groups (4f–4n) were generally compatible. For instance, fluoro (4f), chloro (4g), hydroxyl (4h and 4i), carbamate (4j), cyano (4r), ketal (4l), and ester (4m and 4n) functionalities yielded products in moderate yields. Enhanced yields were obtained using benzamide 1’, with fluoro (5f′, 73%), 3-OH (5h’, 27%), NHBoc (5j’, 66%), and 3-COOMe (5m, 90%) substituted piperidines. Different reactivity patterns were observed among piperidines with similar functional group substitutions, such as 3-OH (4h) versus 4-OH (4i), and 3-COOMe (4m) versus 4-COOMe (4n). However, the 3-COOMe (4m) substituted piperidine, which did not yield the product with benzamide 1, gave a remarkable 90% yield with benzamide 1’, underscoring the crucial role of the amide’s substituents in determining reactivity with the functional group in piperidine. Phenyl (4o) and benzyl (4p) substituted piperidines yielded products 5o and 5p in moderate yields (46% and 52%). Next, we screened various acyclic dialkyl secondary amines (4q–4v) and obtained moderate yields for the corresponding unsymmetrical ureas (5q–5v). Specifically, product 5u was isolated in an 18% yield, with unreacted starting material present in the reaction mixture. To improve the yields, we examined other benzamide substituents. Notably, using a 3,5-bistrifluoromethyl substituted benzamide (1′) significantly increased the yield of product 5u′ to 43%, a trend observed with other amine substrates as well. This strategy of incorporating trifluoromethyl substituted amides shows potential in medicinal chemistry [30]. It is worth noting that gaseous dimethylamine (4q) was used as a 40% aqueous solution, demonstrating the suitability of water in this reaction system and its scalability for gram-scale reactions. Dicyclohexylamine (4v) provided a higher product yield (5v, 60%). Pyrrolidine with 4-methyl substituted benzamide (1) produced 5w in a 26% yield, whereas the 3,5-bistrifluoromethyl benzamide (1′) yielded 5w′ at 40%. Both reactions showed unreacted starting material, and attempts to isolate pure starting materials resulted in only trace amounts, with the remainder decomposing. The 2,6-dimethylmorpholine (4x) moiety, significant in medicinal chemistry, yielded 5x at 42%. Incorporating small heterocycles, a common approach in medicinal chemistry, was further explored with azetidine (4y) and 2-oxa-6-azaspiro[3,3]heptane (4z), a spirocyclic bioisostere of morpholine. The highly functionalized piperidine (4aa) was successfully used, demonstrating the potential for late-stage modification of medicinally relevant compounds through this unsymmetrical urea synthesis. Other important heterocycles, such as azepane (4bb) and 1,4-oxazepane (4cc), known for their pharmacological properties, were also included, as was N-methyl benzylamine (4dd); in each case, the corresponding urea was obtained, with significantly improved yields observed with benzamide 1′ (5dd′).
2.3. Survey of Substrate Scope: Scope of Benzamides
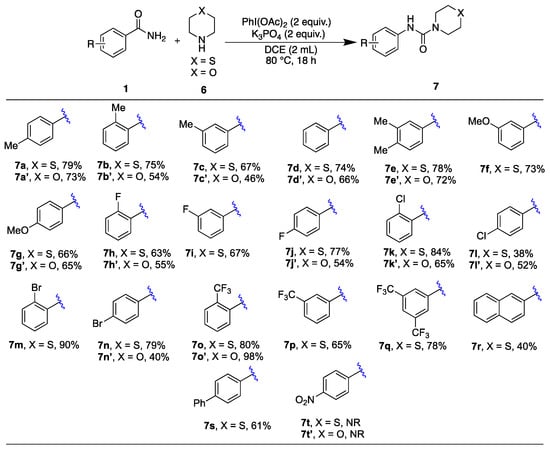
We turned our attention to examining the reaction scope and application with various benzamides (1a–1t) used with thiomorpholine (6) and morpholine (6’) (Scheme 4). We tested benzamides with different substituents (ortho, meta, and para), including methyl (1a–1c), unsubstituted (1d), 3,4-dimethyl (1e), methoxy (1f–1g), halo (1h–1n), trifluoromethyl (1o–1q), naphthyl (1r), phenyl (1s) and nitro (1t) groups. Among these, the benzamide bearing a strongly electron-withdrawing trifluoromethyl group (1o) with morpholine (6′) yielded the product with a remarkable efficiency (7o′). However, the para-nitro derivative (1t) did not react, which we attribute to the benzamide’s insolubility.
Scheme 4.
Scope of benzamides.
2.4. Survey of Substrate Scope: Scope of Late-Stage Functionalization
Late-stage functionalization is crucial in medicinal chemistry for modifying drug scaffolds to enhance their properties [31,32,33]. It enables the introduction of functional and structural diversity, which is important for exploring new chemical spaces in drug development. To illustrate the applicability of our unsymmetrical urea synthesis in late-stage functionalization, we applied our method to a range of drugs (Scheme 5): Paroxetine (8a), Memantine (8b), Bupivacaine (8c), Leelamine (8d), Sertraline (8e), Sitagliptin (8f), Fluoxetine (8g), Desloratadine (8h), Amoxapine (8i), Buspirone (8j), Troxipide (8k), Perospirone (8l), and Donepezil (8m). All the drugs showed good reactivity by furnishing the products (9a–9m) in low to moderate yields.
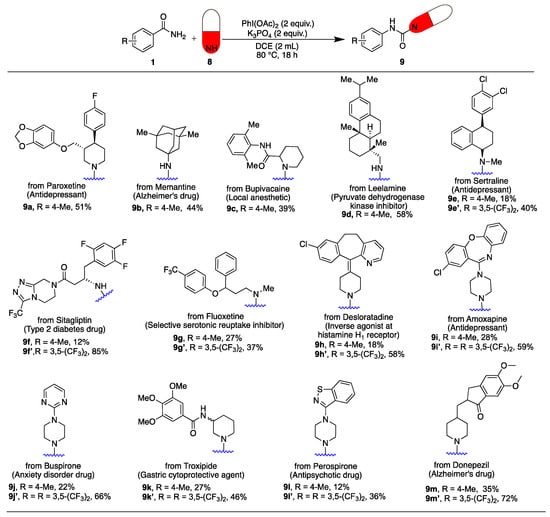
Scheme 5.
Late-stage functionalization of drugs.
2.5. Proposed Mechanism
Based on the previous reports [27,28] and the observed reactivity, we propose the following mechanism (Scheme 6): Initially, benzamide undergoes addition with PhI(OAc)2, forming an iodonium intermediate (A) and releasing acetic acid, which was confirmed by LC-MS analysis (See Supplementary Materials). The need for an excess of amine arises from its role in neutralizing the acetic acid. Following this, the iodonium intermediate (A) undergoes a Hofmann rearrangement to form an isocyanate intermediate (B), which then reacts with the amines to produce the unsymmetrical urea.
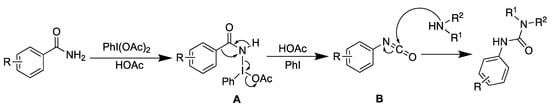
Scheme 6.
Proposed mechanism.
3. Materials and Methods
All catalytic reactions were carried out under aerobic conditions using standard procedures. Solvents and reagents were sourced from Merck (Stockholm, Sweden) and Chemtronica (Stockholm, Sweden). Moisture control measures were not implemented throughout the experiments. All glassware was dried overnight at 120 °C and further flame-dried, as necessary. Silica gel (Carlo Erba, 60 Å) was used for column chromatography. Thin-layer chromatography (TLC) was performed on silica gel-precoated aluminum sheets with a fluorescence indicator at 254 nm. Preparative TLC was conducted using plates from Aldrich (Analtech, UV254 20 × 20 cm, 500 µm). Yields represent isolated products, with purity verified by 1H NMR spectroscopy. NMR spectra were recorded on a Bruker Ascend 400 instrument at frequencies of 400 MHz (1H NMR), 101 MHz (13C NMR), and 376 MHz (19F NMR). Chemical shifts (δ) are given in ppm, with spectra referenced to residual non-deuterated solvent signals. High-resolution mass spectra (HRMS) were obtained at the Lund University Kemi Centrum Mass Spectrometry facility using a Waters XEVO-G2 QTOF instrument. The parameters for ESI+ were as follows: capillary voltage 3 kV, cone voltage 35V, extractor 4, source temperature 120 °C, desolvation temperature 300 °C, cone gas flow 50, desolvation gas flow 400. Continuum resolution mode was applied, with an m/z range of 100–1200, and manual lock mass correction using Leucine Enkephalin (m/z 556.2771). All starting materials were obtained from Aldrich or Chemtronica and used without further purification. The solvents were neither dried nor stored under an inert atmosphere.
General Procedure: In a 10 mL screw cap reaction tube, PhI(OAc)2 (2 equiv.), K3PO4 (2 equiv.), benzamide (1 equiv.) and 1,2-dichloroethane (12 M) were combined with the appropriate amine (2 equiv.) under ambient conditions. If the amine was solid, it was added at this step, whereas liquid amines were dissolved in 1,2-dichloroethane (12 M) before being added. The reaction mixture was stirred at 80 °C for 18 h, using an aluminum block heated by an oil bath. The reaction mixture was then allowed to cool to room temperature, followed by the addition of silica gel. The crude product was then purified using either column chromatography or preparative thin-layer chromatography, with an elution gradient of petroleum ether and acetone.
Spectral Data
1-isopropyl-3-(p-tolyl)urea (3a): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 2b (63 μL, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 3a (44 mg, 62%). Beige solid. mp—150–152 °C. FTIR (cm−1): 3314, 2964, 2920, 2871, 1685, 1632, 1592, 1543, 1505, 1235, 1173, 1067, 816, 776. 1H NMR (400 MHz, CDCl3) δ 1H NMR (400 MHz, Chloroform-d) δ 7.31 (s, 1H), 7.13 (d, J = 8.4 Hz, 2H), 7.02 (d, J = 8.2 Hz, 2H), 5.38 (d, J = 7.8 Hz, 1H), 3.94 (dq, J = 13.1, 6.6 Hz, 1H), 2.26 (s, 3H), 1.10 (d, J = 6.6 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 156.0, 136.4, 132.7, 129.5, 120.7, 41.9, 23.2, 20.7. HRMS (ESI): Exact mass calculated for C11H17N2O [M + H]+: 193.1336, found: 193.1337.
1-propyl-3-(p-tolyl)urea (3b): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 2a (61 μL, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 3b (41 mg, 58%). Green solid. mp—97–99 °C. FTIR (cm−1): 3310, 2959, 2920, 2869, 1638, 1585, 1561, 1508, 1459, 1306, 1233, 1107, 810. 1H NMR (400 MHz, CDCl3) δ 7.40 (s, 1H), 7.12 (d, J = 8.3 Hz, 2H), 7.02 (d, J = 8.3 Hz, 2H), 5.60 (t, J = 5.8 Hz, 1H), 3.15–3.05 (m, 2H), 2.26 (s, 3H), 1.43 (q, J = 7.3 Hz, 2H), 0.85 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 156.8, 136.4, 132.7, 129.5, 120.8, 41.9, 23.3, 20.7, 11.3. HRMS (ESI): Exact mass calculated for C11H17N2O [M + H]+: 193.1336, found: 193.1337.
1-butyl-3-(p-tolyl)urea (3c): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 2c (73 μL, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 3c (52 mg, 68%). Brown solid. mp—103–105 °C. FTIR (cm−1): 3312, 2959, 2931, 2857, 1625, 1585, 1556, 1282, 1226, 1107, 816, 776. 1H NMR (400 MHz, CDCl3) δ 7.46 (s, 1H), 7.13 (d, J = 8.4 Hz, 2H), 7.01 (d, J = 8.3 Hz, 2H), 5.64 (t, J = 5.7 Hz, 1H), 3.13 (td, J = 7.1, 5.6 Hz, 2H), 2.26 (s, 3H), 1.43–1.33 (m, 2H), 1.26 (dq, J = 13.9, 7.1 Hz, 2H), 0.86 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 156.9, 136.4, 132.6, 129.5, 120.8, 39.9, 32.2, 20.7, 20.0, 13.7. HRMS (ESI): Exact mass calculated for C12H19N2O [M + H]+: 207.1492, found: 207.1494.
1-phenethyl-3-(p-tolyl)urea (3d): General procedure was followed using 1a (50 mg, 37 mmol, 1.0 equiv.), 2d (93 μL, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 3d (47 mg, 50%). Yellow solid. mp—150–152 °C. FTIR (cm−1): 3322, 3057, 3026, 2920, 1631, 1592, 1561, 1450, 1406, 1306, 1227, 1079, 811, 757, 736, 701. 1H NMR (400 MHz, CDCl3) δ 7.29 (d, J = 6.9 Hz, 1H), 7.26 (s, 1H), 7.24–7.20 (m, 1H), 7.20–7.15 (m, 2H), 7.10–7.03 (m, 4H), 6.27 (s, 1H), 4.79 (s, 1H), 3.48 (td, J = 6.9, 5.8 Hz, 2H), 2.81 (t, J = 6.9 Hz, 2H), 2.30 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 156.0, 139.1, 135.4, 134.2, 129.9, 128.8, 128.6, 126.4, 122.3, 41.5, 36.2, 20.8. HRMS (ESI): Exact mass calculated for C16H19N2O [M + H]+: 255.1492, found: 255.1494.
1-((3s,5s,7s)-adamantan-1-yl)-3-(p-tolyl)urea (3e): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 2e (225 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 3e (39 mg, 48%). Brown solid. mp—231–233 °C. FTIR (cm−1): 3321, 2906, 2851, 1643, 1587, 1550, 1512, 1450, 1357, 1290, 1228, 1098, 807. 1H NMR (400 MHz, CDCl3) δ 1H NMR (400 MHz, Chloroform-d) δ 7.13 (d, J = 8.5 Hz, 2H), 7.09 (d, J = 8.5 Hz, 2H), 6.12 (s, 1H), 4.53 (s, 1H), 2.30 (s, 3H), 2.06 (d, J = 2.6 Hz, 4H), 1.98 (d, J = 2.9 Hz, 6H), 1.67 (t, J = 3.2 Hz, 8H). 13C NMR (101 MHz, CDCl3) δ 154.7, 136.1, 133.5, 129.8, 121.7, 51.2, 42.2, 36.4, 29.5, 20.8. HRMS (ESI): Exact mass calculated for C18H25N2O [M + H]+: 285.1962, found: 285.1961.
1-cyclopropyl-3-(p-tolyl)urea (3f): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 2f (51 μL, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 3f (10 mg, 13%). Yellow solid. mp—151–153 °C. FTIR (cm−1): 3301, 3090, 2984, 2915, 1632, 1592, 1556, 1503, 1302, 1282, 1235, 1018, 816, 701. 1H NMR (400 MHz, CDCl3) δ 7.30–7.23 (m, 3H), 7.11 (d, J = 8.3 Hz, 2H), 6.81 (s, 1H), 4.95 (s, 1H), 2.58 (ttd, J = 6.8, 3.6, 1.4 Hz, 1H), 2.30 (s, 3H), 0.82 (td, J = 6.8, 4.7 Hz, 2H), 0.67–0.59 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 156.6, 135.7, 133.3, 129.6, 120.6, 22.6, 20.7, 7.5. HRMS (ESI): Exact mass calculated for C11H15N2O [M + H]+: 191.1179, found: 191.1180.
1-(3,5-bis(trifluoromethyl)phenyl)-3-cyclopropylurea (3f′): General procedure was followed using 1′ (95 mg, 0.37 mmol, 1.0 equiv.), 2f (51 μL, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 3f’ (93 mg, 80%). Colorless solid. mp—175–177 °C. FTIR (cm−1): 3316, 1660, 1552, 1472, 1437, 1384, 1264, 1123, 1068, 1021, 942, 875, 678. 1H NMR (400 MHz, CDCl3) δ 7.94 (s, 2H), 7.53 (s, 1H), 7.24 (s, 1H), 5.10 (s, 1H), 2.63 (ttd, J = 6.7, 3.6, 1.2 Hz, 1H), 0.92 (td, J = 6.8, 4.8 Hz, 2H), 0.75–0.69 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 155.5, 140.0, 132.8, 132.4, 124.5, 121.8, 118.9, 116.3, 22.6, 7.7. 19F NMR (376 MHz, CDCl3) δ −63.0. HRMS (ESI): Exact mass calculated for C12H11F6N2O [M + H]+: 313.0771, found: 313.0776.
1-cyclopentyl-3-(p-tolyl)urea (3g): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 2g (73 μL, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 3g (35 mg, 44%). Brown solid. mp—186–188 °C. FTIR (cm−1): 3301, 2953, 2864, 1627, 1585, 1556, 1508, 1282, 1233, 1042, 938, 816, 778. 1H NMR (400 MHz, CDCl3) δ 7.17–7.12 (m, 2H), 7.05 (d, J = 2.1 Hz, 2H), 7.04 (d, J = 1.8 Hz, 1H), 5.29 (d, J = 7.3 Hz, 1H), 4.08 (h, J = 6.9 Hz, 1H), 2.27 (s, 3H), 1.98–1.87 (m, 2H), 1.65–1.47 (m, 4H), 1.38–1.28 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 156.1, 136.1, 133.2, 129.7, 121.2, 51.9, 33.4, 23.5, 20.7. HRMS (ESI): Exact mass calculated for C13H19N2O [M + H]+: 219.1492, found: 219.1494.
1-cyclohexyl-3-(p-tolyl)urea (3h): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 2h (84 μL, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 3h (18 mg, 21%). Brown solid. mp—181–183 °C. FTIR (cm−1): 3330, 3028, 2926, 2849, 1625, 1590, 1561, 1505, 1441, 1310, 1226, 1044, 889, 818, 776. 1H NMR (400 MHz, CDCl3) δ 7.17–7.08 (m, 5H), 6.35 (s, 1H), 4.71 (d, J = 8.1 Hz, 1H), 3.65 (dddd, J = 14.6, 10.5, 7.9, 3.9 Hz, 1H), 2.31 (s, 3H), 1.94 (dd, J = 12.5, 3.9 Hz, 3H), 1.71–1.55 (m, 6H), 1.41–1.28 (m, 4H), 1.19–1.03 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 155.4, 135.8, 133.8, 129.9, 122.0, 49.0, 33.6, 25.5, 24.9, 20.8. HRMS (ESI): Exact mass calculated for C14H21N2O [M + H]+: 233.1649, found: 233.1650.
1-(3,5-bis(trifluoromethyl)phenyl)-3-cyclohexylurea (3h′): General procedure was followed using 1′ (95 mg, 0.37 mmol, 1.0 equiv.), 2h (84 μL, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 3h′ (105 mg, 78%). Brown solid. mp—179–181 °C. FTIR (cm−1): 3342, 2936, 2854, 1656, 1543, 1474, 1439, 1386, 1269, 1127, 1041, 935, 871, 674. 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 1.6 Hz, 2H), 7.47 (s, 1H), 6.82 (s, 1H), 4.75 (d, J = 7.9 Hz, 1H), 3.66 (dtd, J = 10.7, 7.1, 3.8 Hz, 1H), 2.03–1.94 (m, 3H), 1.72 (dt, J = 13.4, 3.9 Hz, 4H), 1.68–1.55 (m, 5H), 1.44–1.31 (m, 5H), 1.21–1.09 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 153.7, 151.6, 145.8, 140.7, 132.3, 132.0, 123.5, 120.9, 118.4, 116.3, 49.3, 33.5, 29.7, 25.4, 24.8, 1.0. HRMS (ESI): Exact mass calculated for C15H17F6N2O [M + H]+: 355.1240, found: 355.1241.
(R)-1-(1-phenylethyl)-3-(p-tolyl)urea (3i): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 2i (95 μL, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 3i (39 mg, 42%). Brown solid. mp—147–149 °C. FTIR (cm−1): 3290, 3090, 3026, 2977, 2917, 1627, 1585, 1561, 1517, 1448, 1310, 1228, 1018, 829, 741. 1H NMR (400 MHz, CDCl3) δ 7.28–7.24 (m, 1H), 7.23 (d, J = 2.7 Hz, 3H), 7.21 (dt, J = 2.7, 1.4 Hz, 1H), 7.19 (s, 1H), 7.10 (s, 1H), 7.09–7.05 (m, 2H), 6.99 (d, J = 8.4 Hz, 2H), 5.67 (d, J = 7.6 Hz, 1H), 4.89 (p, J = 7.0 Hz, 1H), 2.25 (s, 3H), 1.33 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 155.8, 144.2, 136.1, 132.9, 129.6, 128.6, 127.0, 125.9, 120.8, 49.7, 22.9, 20.7. HRMS (ESI): Exact mass calculated for C16H18N2O [M + H]+: C16H19N2O [M + H]+: 255.1492, found: 255.1493.
N-(p-tolyl)piperidine-1-carboxamide (5a): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4a (63 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5a (42 mg, 52%). Yellow solid. mp—149–150 °C. FTIR (cm−1): 3274, 3124, 2936, 2847, 1627, 1594, 1508, 1421, 1240, 1070, 1019, 805, 747. 1H NMR (400 MHz, CDCl3) δ 7.27–7.23 (m, 2H), 7.09 (d, J = 8.3 Hz, 2H), 6.39 (s, 1H), 3.45 (dd, J = 6.2, 4.1 Hz, 4H), 2.31 (s, 3H), 1.71–1.57 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 155.2, 136.7, 132.3, 129.3, 120.1, 45.2, 25.7, 24.4, 20.7. HRMS (ESI): Exact mass calculated for C13H18N2O [M+Na]+: 241.1312, found: 241.1318.
2-methyl-N-(p-tolyl)piperidine-1-carboxamide (5b): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4b (73 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5b (20 mg, 23%). Brown solid. mp—153–154 °C. FTIR (cm−1): 3311, 2925, 2854, 1627, 1589, 1512, 1417, 1373, 1234, 1176, 1052, 811, 789. 1H NMR (400 MHz, CDCl3) δ 7.16 (d, J = 8.4 Hz, 2H), 7.00 (d, J = 8.3 Hz, 2H), 6.23 (s, 1H), 4.29 (pd, J = 7.0, 1.9 Hz, 1H), 3.84–3.76 (m, 1H), 2.90 (td, J = 13.0, 3.0 Hz, 1H), 2.21 (s, 3H), 1.72–1.59 (m, 3H), 1.59–1.48 (m, 3H), 1.48–1.34 (m, 1H), 1.15 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 155.1, 136.7, 132.3, 129.3, 120.1, 46.6, 39.0, 30.2, 25.6, 20.7, 18.5, 15.7. HRMS (ESI): Exact mass calculated for C14H21N2O [M + H]+: 233.1649, found: 233.1651.
N-(3,5-bis(trifluoromethyl)phenyl)-2-methylpiperidine-1-carboxamide (5b′): General procedure was followed using 1′ (95 mg, 0.37 mmol, 1.0 equiv.), 4b (73 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5b′ (61 mg, 47%). Brown solid. mp—179–181 °C. FTIR (cm−1): 3342, 2936, 2854, 1656, 1543, 1474, 1439, 1386, 1269, 1127, 1041, 935, 871, 674. 1H NMR (400 MHz, CDCl3) δ 7.87–7.83 (m, 2H), 7.45 (s, 1H), 6.99 (s, 1H), 4.42 (qd, J = 9.5, 6.1, 3.8 Hz, 1H), 3.91 (dd, J = 13.5, 2.5 Hz, 1H), 3.02 (td, J = 13.1, 3.0 Hz, 1H), 1.78–1.56 (m, 5H), 1.55–1.40 (m, 1H), 1.24 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 154.1, 141.0, 137.4, 132.3, 132.0, 131.7, 131.4, 130.2, 127.4, 127.3, 124.6, 121.9, 119.4, 119.3, 115.8, 115.7, 115.6, 46.9, 39.2, 30.1, 25.5, 18.4, 15.8. HRMS (ESI): Exact mass calculated for C15H17F6N2O [M + H]+: 355.1240, found: 355.1250.
3-methyl-N-(p-tolyl)piperidine-1-carboxamide (5c): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4c (73 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5c (30 mg, 35%). Yellow solid. mp—107–109 °C. FTIR (cm−1): 3338, 3285, 2920, 2847, 1631, 1589, 1508, 1408, 1234, 1035, 962, 807, 747. 1H NMR (400 MHz, CDCl3) δ 7.25 (d, J = 8.5 Hz, 2H), 7.10 (d, J = 8.3 Hz, 2H), 6.35 (s, 1H), 4.00–3.90 (m, 2H), 2.86 (ddd, J = 13.2, 11.8, 3.0 Hz, 1H), 2.52 (dd, J = 13.1, 10.7 Hz, 1H), 1.86 (dtd, J = 12.8, 3.7, 1.9 Hz, 1H), 1.78–1.48 (m, 5H), 1.20–1.07 (m, 1H), 0.95 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 155.1, 136.6, 132.3, 129.3, 120.0, 51.8, 44.7, 32.9, 31.0, 25.1, 20.7, 19.0. HRMS (ESI): Exact mass calculated for C14H20N2O [M + Na]+: 255.1468, found: 255.1479.
N-(3,5-bis(trifluoromethyl)phenyl)-3-methylpiperidine-1-carboxamide (5c’): General procedure was followed using 1′ (95 mg, 0.37 mmol, 1.0 equiv.), 4c (73 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5c’ (97 mg, 74%). Yellow solid. mp—108–110 °C. FTIR (cm−1): 3267, 2974, 2869, 1638, 1534, 1441, 1368, 1269, 1121, 1085, 966, 882, 838, 677. 1H NMR (400 MHz, CDCl3) δ 7.80 (s, 2H), 7.65 (s, 1H), 7.40–7.37 (m, 1H), 4.08–3.95 (m, 2H), 2.89–2.79 (m, 1H), 2.51 (dd, J = 13.1, 10.7 Hz, 1H), 1.84 (dd, J = 13.3, 3.8 Hz, 1H), 1.69 (dt, J = 13.5, 3.5 Hz, 1H), 1.60 (dt, J = 6.7, 3.9 Hz, 1H), 1.55–1.43 (m, 1H), 1.18–1.07 (m, 1H), 0.89 (d, J = 6.6 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 154.1, 141.0, 137.4, 132.3, 132.0, 131.7, 131.4, 130.2, 127.4, 127.3, 124.6, 121.9, 119.4, 119.3, 115.8, 115.7, 115.6, 46.9, 39.2, 30.1, 25.5, 18.4, 15.8. HRMS (ESI): Exact mass calculated for C15H17F6N2O [M + H]+: 355.1240, found: 355.1249.
4-methyl-N-(p-tolyl)piperidine-1-carboxamide (5d): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4d (73 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5d (53 mg, 62%). Yellow solid. mp—112–113 °C. FTIR (cm−1): 3350, 2911, 2860, 1634, 1589, 1510, 1417, 1306, 1236, 1077, 964, 805, 745. 1H NMR (400 MHz, CDCl3) δ 7.25 (d, J = 8.5 Hz, 2H), 7.09 (d, J = 8.2 Hz, 2H), 6.40 (s, 1H), 4.04 (d, J = 13.2 Hz, 2H), 2.86 (ddd, J = 13.2, 12.2, 2.7 Hz, 2H), 2.31 (s, 3H), 1.75–1.66 (m, 2H), 1.59 (ddd, J = 11.2, 8.8, 5.2 Hz, 1H), 1.20 (dddd, J = 15.4, 12.9, 9.6, 4.2 Hz, 3H), 0.99 (d, J = 6.5 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 155.2, 136.6, 132.3, 129.3, 120.1, 44.6, 33.9, 30.9, 21.8, 20.7. HRMS (ESI): Exact mass calculated for C14H20N2O [M + Na]+: 255.1468, found: 255.1476.
2,6-dimethyl-N-(p-tolyl)piperidine-1-carboxamide (5e): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4e (84 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5e (39 mg, 42%). Orange solid. mp—149–151 °C. FTIR (cm−1): 3334, 3031, 2991, 2925, 2588, 1629, 1589, 1508, 1399, 1245, 1068, 805, 787. 1H NMR (400 MHz, Chloroform-d) δ 7.21–7.17 (m, 2H), 7.00 (d, J = 8.4 Hz, 2H), 6.27 (s, 1H), 4.27–4.19 (m, 2H), 2.21 (s, 3H), 1.79–1.60 (m, 4H), 1.60–1.52 (m, 3H), 1.44 (dt, J = 12.3, 3.6 Hz, 1H), 1.22 (s, 3H), 1.20 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 154.9, 136.7, 132.4, 129.5, 129.3, 120.3, 45.6, 30.3, 20.8, 20.7, 13.7. HRMS (ESI): Exact mass calculated for C15H23N2O [M + H]+: 247.1805, found: 247.1807.
4-fluoro-N-(p-tolyl)piperidine-1-carboxamide (5f): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4f (116 mg, 0.74 mmol, 5 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5f (10 mg, 11%). Brown solid. mp—132–134 °C. FTIR (cm−1): 3318, 2953, 2920, 2854, 1733, 1634, 1594, 1508, 1419, 1222, 1015, 911, 805, 747. 1H NMR (400 MHz, CDCl3) δ 7.15 (d, J = 8.4 Hz, 2H), 7.02 (d, J = 8.4 Hz, 2H), 6.25 (s, 1H), 4.90–4.71 (m, 1H), 3.48 (dd, J = 6.4, 5.2 Hz, 5H), 2.23 (s, 4H), 1.92–1.77 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 155.0, 136.3, 132.8, 129.4, 120.2, 88.5, 86.8, 40.4 (J = 6.0 Hz), 31.1 (J = 20.2 Hz), 20.7. 19F NMR (376 MHz, CDCl3) δ −182.9, −182.9, −183.0, −183.0, −183.0, −183.0, −183.1, −183.1, −183.1, −183.2, −183.2, −183.2. HRMS (ESI): Exact mass calculated for C13H17FN2O [M+Na]+: 259.1223, found: 259.1228.
N-(3,5-bis(trifluoromethyl)phenyl)-4-fluoropiperidine-1-carboxamide (5f′): General procedure was followed using 1′ (95 mg, 0.37 mmol, 1.0 equiv.), 4f (116 mg, 0.74 mmol, 5 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5f’ (97 mg, 73%). Yellow solid. mp—140–142 °C. FTIR (cm−1): 3378, 3241, 2958, 1642, 1539, 1444, 1368, 1269, 1169, 1119, 1021, 886, 838, 754, 701, 680. 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 1.6 Hz, 2H), 7.50 (s, 1H), 6.89 (s, 1H), 5.00–4.82 (m, 1H), 3.65 (dt, J = 13.6, 5.0 Hz, 2H), 3.56 (dtd, J = 13.5, 6.6, 6.0, 2.2 Hz, 2H), 1.99–1.83 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 153.9, 140.6, 124.5, 121.8, 119.4, 119.3, 116.2, 116.1, 87.9, 86.2, 40.3 (J = 5.0 Hz), 31.0 (J = 20.2 Hz). 19F NMR (376 MHz, CDCl3) δ −63.0, −184.0, −184.1, −184.1, −184.1, −184.1, −184.1, −184.2, −184.2, −184.2, −184.3. HRMS (ESI): Exact mass calculated for C14H14F7N2O [M + H]+: 359.0989, found: 359.1001.
4-chloro-N-(p-tolyl)piperidine-1-carboxamide (5g): General procedure was followed using 1a (56 mg, 23 mmol, 1.0 equiv.), 4g (88 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5g (55 mg, 58%). Yellow solid. mp—185–186 °C. FTIR (cm−1): 3314, 2958, 2914, 1634, 1594, 1510, 1415, 1245, 1041, 1008, 802, 749. 1H NMR (400 MHz, CDCl CDCl3) δ 7.23 (d, J = 8.4 Hz, 2H), 7.10 (d, J = 8.3 Hz, 2H), 6.48 (s, 1H), 4.28 (tt, J = 7.4, 3.7 Hz, 1H), 3.74 (ddd, J = 13.5, 7.7, 3.6 Hz, 2H), 3.41 (ddd, J = 13.6, 7.4, 3.7 Hz, 2H), 2.32 (s, 3H), 2.11 (ddt, J = 14.5, 7.5, 3.7 Hz, 2H), 1.89 (dtd, J = 14.2, 7.3, 3.5 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 155.1, 136.3, 132.8, 129.4, 120.4, 56.5, 41.6, 34.7, 20.7. HRMS (ESI): Exact mass calculated for C13H17CIN2O [M + Na]+: 275.0927, found: 275.0933.
3-hydroxy-N-(p-tolyl)piperidine-1-carboxamide (5h): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4h (75 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5h (20 mg, 18%). Brown solid. mp—130–132 °C. 1H NMR (400 MHz, CDCl3) δ 7.13 (d, J = 8.4 Hz, 2H), 7.01 (d, J = 8.3 Hz, 2H), 6.43 (s, 1H), 3.79 (dt, J = 6.0, 3.4 Hz, 1H), 3.51 (dd, J = 13.4, 3.1 Hz, 1H), 3.37–3.27 (m, 4H), 2.29 (s, 1H), 2.22 (s, 3H), 1.83–1.76 (m, 2H), 1.58 (td, J = 9.2, 8.5, 4.2 Hz, 2H), 1.51–1.42 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 156.1, 136.5, 132.5, 129.3, 127.4, 120.2, 65.8, 51.1, 44.9, 32.1, 21.9, 20.7. HRMS (ESI): Exact mass calculated for C13H19N2O2 [M + H]+: 235.1442, found: 235.1441.
N-(3,5-bis(trifluoromethyl)phenyl)-3-hydroxypiperidine-1-carboxamide (5h′): General procedure was followed using 1′ (95 mg, 0.37 mmol, 1.0 equiv.), 4h (75 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5h’ (36 mg, 27%). Yellow solid. mp—188–190 °C. FTIR (cm−1): 3342, 2932, 2866, 2441, 1626, 1565, 1479, 1444, 1394, 1272, 1128, 999, 928, 883, 698, 678. 1H NMR (400 MHz, CDCl3) δ 7.94 (s, 2H), 7.41–7.39 (m, 1H), 3.80 (dd, J = 13.1, 3.8 Hz, 1H), 3.60 (ddd, J = 7.7, 4.7, 2.5 Hz, 2H), 3.21 (p, J = 1.6 Hz, 1H), 3.19–3.10 (m, 1H), 3.00 (dd, J = 13.1, 8.0 Hz, 1H), 1.94–1.82 (m, 1H), 1.75 (dq, J = 6.2, 3.2, 2.8 Hz, 1H), 1.49–1.39 (m, 2H), 1.18 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 155.6, 142.3, 131.9, 131.6, 131.3, 130.9, 124.8, 122.1, 119.2, 114.5, 114.4, 65.5, 50.4, 44.1, 32.2, 22.3. HRMS (ESI): Exact mass calculated for C14H15F6N2O2 [M + H]+: 357.1033, found: 357.1032.
4-hydroxy-N-(p-tolyl)piperidine-1-carboxamide (5i): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4i (75 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5i (38 mg, 45%). Yellow solid. mp—128–130 °C. FTIR (cm−1): 3307, 3024, 2931, 2865, 1634, 1596, 1528, 1419, 1357, 1309, 1242, 1116, 1050, 970, 809, 743. 1H NMR (400 MHz, CDCl3) δ 7.22 (d, J = 8.4 Hz, 2H), 7.09 (d, J = 8.3 Hz, 2H), 6.50 (s, 1H), 3.94–3.79 (m, 3H), 3.16 (ddd, J = 13.1, 9.3, 3.4 Hz, 2H), 2.31 (s, 4H), 1.91 (dqd, J = 13.1, 3.9, 2.1 Hz, 2H), 1.56 (dtd, J = 12.9, 8.8, 3.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 155.3, 136.4, 132.7, 129.3, 120.3, 67.2, 41.7, 33.9, 20.7. HRMS (ESI): Exact mass calculated for C13H18N2O2 [M+Na]+: 257.1261, found: 257.1270.
tert-butyl (1-(p-tolylcarbamoyl)piperidin-4-yl)carbamate (5j): General procedure was followed using 1a (56 mg, 23 mmol, 1.0 equiv.), 4j (148 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5j (46 mg, 38%). Colorless solid. mp—205–207 °C. FTIR (cm−1): 3349, 2980, 2925, 2888, 1682, 1629, 1516, 1417, 1318, 1225, 1152, 1041, 1021, 807, 743. 1H NMR (400 MHz, CDCl3) δ 7.23 (d, J = 8.4 Hz, 2H), 7.09 (d, J = 8.4 Hz, 2H), 6.49 (s, 1H), 4.53 (s, 1H), 4.00 (d, J = 13.4 Hz, 2H), 3.65 (s, 1H), 2.98 (ddd, J = 14.0, 11.6, 2.8 Hz, 2H), 2.30 (s, 4H), 2.03–1.93 (m, 2H), 1.47 (s, 11H), 1.44–1.31 (m, 3H), 0.93–0.85 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 155.1, 155.1, 136.4, 132.6, 129.3, 120.3, 79.5, 47.8, 43.2, 32.3, 28.4, 20.7. HRMS (ESI): Exact mass calculated for C18H27N3O3 [M+Na]+: 356.1950, found: 356.1959.
tert-butyl(1-((3,5-bis(trifluoromethyl)phenyl)carbamoyl)piperidin-4-yl)carbamate (5j’): General procedure was followed using 1′ (95 mg, 23 mmol, 1.0 equiv.), 4j (148 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5j’ (110 mg, 66%). Colorless solid. mp—225–227 °C. FTIR (cm−1): 3380, 3208, 1660, 1572, 1539, 1472, 1318, 1276, 1227, 1123, 1048, 871, 698. 1H NMR (400 MHz, DMSO-d6) δ 9.23 (s, 1H), 8.27 (s, 2H), 6.94 (d, J = 7.9 Hz, 1H), 4.10 (d, J = 13.9 Hz, 2H), 3.37 (d, J = 9.5 Hz, 3H), 2.99 (ddd, J = 14.0, 11.6, 2.7 Hz, 2H), 1.87–1.78 (m, 2H), 1.45 (d, J = 3.2 Hz, 9H). 13C NMR (101 MHz, DMSO-d6) δ 155.2, 154.4, 143.3, 130.9, 130.6, 122.5, 119.0, 78.0, 47.6, 43.1, 32.2, 28.7. HRMS (ESI): Exact mass calculated for C19H24F6N3O3 [M + H]+: 456.1717, found: 456.1712.
4-cyano-N-(p-tolyl)piperidine-1-carboxamide (5k): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4k (81 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5k (55 mg, 62%). Yellow solid. mp—103–109 °C. FTIR (cm−1): 3314, 2953, 2925, 2869, 2237, 1629, 1589, 1512, 1413, 1284, 1240, 1017, 975, 800, 747. 1H NMR (400 MHz, CDCl3) δ 7.22 (d, J = 8.4 Hz, 2H), 7.12 (d, J = 8.0 Hz, 2H), 6.37 (s, 1H), 3.68 (dd, J = 10.1, 4.4 Hz, 2H), 3.51–3.40 (m, 2H), 2.90 (q, J = 3.8 Hz, 1H), 2.32 (s, 3H), 2.04–1.83 (m, 5H), 1.64 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 155.0, 136.0, 136.0, 133.1, 129.4, 129.3, 127.4, 120.8, 120.4, 120.4, 42.4, 28.3, 26.2, 20.7. HRMS (ESI): Exact mass calculated for C14H17N3O [M + Na]+: 266.1269, found: 266.1274.
N-(p-tolyl)-1,4-dioxa-8-azaspiro[4,5]decane-8-carboxamide (5l): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4l (138 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5l (66 mg, 65%). Colorless solid. mp—158–160 °C. FTIR (cm−1): 3316, 2964, 2880, 1638, 1594, 1521, 1479, 1309, 1225, 1101, 1032, 944, 902, 805, 742. 1H NMR (400 MHz, CDCl3) δ 7.21 (d, J = 8.4 Hz, 2H), 7.09–7.04 (m, 2H), 6.50 (s, 1H), 3.97 (s, 4H), 3.58–3.52 (m, 4H), 2.28 (s, 3H), 1.75–1.69 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 155.0, 136.5, 132.6, 129.3, 120.3, 106.9, 64.4, 42.5, 34.9, 20.7. HRMS (ESI): Exact mass calculated for C15H21N2O3 [M + H]+: 277.1547, found: 277.1546.
Methyl-1-((3,5-bis(trifluoromethyl)phenyl)carbamoyl)piperidine-3-carboxylate (5m): General procedure was followed using 1’ (95 mg, 23 mmol, 1.0 equiv.), 4m (105 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5m (133 mg, 90%). Yellow solid. mp—82–84 °C. FTIR (cm−1): 3338, 2958, 1726, 1642, 1556, 1472, 1441, 1373, 1269, 1165, 1110, 1035, 984, 937, 879, 681. 1H NMR (400 MHz, CDCl3) δ 8.15 (s, 1H), 7.81 (s, 2H), 7.43 (s, 1H), 3.76 (s, 4H), 3.73–3.68 (m, 1H), 3.64 (dd, J = 14.1, 3.5 Hz, 1H), 3.30 (td, J = 9.0, 8.4, 4.0 Hz, 1H), 2.71–2.65 (m, 1H), 2.14–2.04 (m, 1H), 1.95 (ddt, J = 13.6, 9.0, 4.5 Hz, 1H), 1.58 (dtt, J = 17.4, 8.8, 3.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 174.6, 154.6, 141.4, 132.3, 131.9, 131.6, 131.3, 124.6, 121.9, 119.2, 119.1, 119.0, 115.4, 115.3, 52.3, 46.6, 44.2, 40.6, 26.5, 23.7. HRMS (ESI): Exact mass calculated for C16H17F6N2O [M + H]+: 399.1138, found: 399.1140.
Methyl-1-(p-tolylcarbamoyl)piperidine-4-carboxylate (5n): General procedure was followed using 1a (56 mg, 23 mmol, 1.0 equiv.), 4n (105 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5n (57 mg, 55%). Yellow solid. mp—110–112 °C. FTIR (cm−1): 3294, 2949, 2854, 1726, 1638, 1594, 1512, 1413, 1225, 1030, 970, 802,747. 1H NMR (400 MHz, CDCl3) δ 7.25–7.21 (m, 2H), 7.11–7.07 (m, 2H), 6.52 (s, 1H), 4.03–3.96 (m, 2H), 3.72 (s, 3H), 2.99 (ddd, J = 13.8, 11.2, 3.0 Hz, 2H), 2.53 (tt, J = 10.8, 4.0 Hz, 1H), 2.30 (s, 3H), 1.95 (dt, J = 12.8, 4.0 Hz, 2H), 1.78–1.68 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 174.7, 155.2, 136.4, 132.6, 129.3, 120.3, 51.8, 43.6, 40.8, 27.8, 20.7. HRMS (ESI): Exact mass calculated for C15H20N2O3 [M+Na]+: 299.1367, found: 299.1370.
4-phenyl-N-(p-tolyl)piperidine-1-carboxamide (5o): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4o (119 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5o (50 mg, 46%). Yellow solid. mp—154–155 °C. 1H NMR (400 MHz, CDCl3) δ 7.35 (t, J = 7.4 Hz, 3H), 7.31–7.20 (m, 8H), 7.12 (d, J = 8.3 Hz, 2H), 6.48 (s, 1H), 4.24 (dt, J = 13.3, 2.3 Hz, 2H), 3.00 (td, J = 13.0, 2.6 Hz, 2H), 2.74 (ddd, J = 12.1, 8.5, 3.6 Hz, 1H), 2.32 (s, 4H), 1.98–1.90 (m, 3H), 1.82–1.69 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 154.2, 144.3, 135.4, 131.5, 128.3, 127.5, 125.7, 119.2, 44.0, 41.6, 32.0, 19.7. HRMS (ESI): Exact mass calculated for C19H22N2O [M+Na]+: 317.1625, found: 317.1629.
4-benzyl-N-(p-tolyl)piperidine-1-carboxamide (5p): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4p (130 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5p (59 mg, 52%). Yellow solid. mp—148–150 °C. FTIR (cm−1): 3305, 3024, 2914, 2843, 1629, 1587, 1508, 1417, 1287, 1240, 1052, 906, 802, 745. 1H NMR (400 MHz, CDCl3) δ 7.20 (dd, J = 8.0, 6.6 Hz, 2H), 7.17–7.09 (m, 4H), 7.05 (d, J = 6.7 Hz, 2H), 6.97 (d, J = 8.3 Hz, 2H), 6.38 (s, 1H), 3.94 (d, J = 13.3 Hz, 2H), 2.68 (td, J = 12.8, 2.5 Hz, 2H), 2.46 (d, J = 6.9 Hz, 2H), 2.20 (s, 3H), 1.68–1.55 (m, 3H), 1.13 (qd, J = 12.6, 4.0 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 155.2, 140.0, 136.7, 132.3, 129.3, 129.1, 128.3, 126.0, 120.2, 44.6, 43.0, 38.1, 31.9, 20.7. HRMS (ESI): Exact mass calculated for C20H24N2O [M+Na]+: 331.1786, found: 331.1789.
1,1-dimethyl-3-(p-tolyl)urea (5q): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4q (66 mg, 74 μL, 1.48 mmol, 4 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5q (30 mg, 45%). Yellow solid. mp—152–154 °C. FTIR (cm−1): 3336, 3020, 2914, 1640, 1594, 1508, 1483, 1362, 1295, 1234, 1183, 1072, 1028, 814, 749. 1H NMR (400 MHz, CDCl3) δ 7.21–7.15 (m, 2H), 7.03–6.98 (m, 2H), 6.22 (s, 1H), 2.93 (s, 6H), 2.22 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 155.9, 136.6, 132.4, 129.3, 120.0, 36.4, 20.7. HRMS (ESI): Exact mass calculated for C10H14N2O [M+Na]+: 201.1004, found: 201.1008.
1,1-dimethyl-3-(p-tolyl)urea (5q): General procedure was followed using 1a (1.5 g, 0.37 mmol, 1.0 equiv.), 4q (3 mL, 4 equiv.), PhI(OAc)2 (7.144g, 2 equiv.), K3PO4 (4.7g, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5q (687 mg, 35%). Yellow solid. mp—152–154 °C. FTIR (cm−1): 3336, 3020, 2914, 1640, 1594, 1508, 1483, 1362, 1295, 1234, 1183, 1072, 1028, 814, 749. 1H NMR (400 MHz, CDCl3) δ 7.21–7.15 (m, 2H), 7.03–6.98 (m, 2H), 6.22 (s, 1H), 2.93 (s, 6H), 2.22 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 155.9, 136.6, 132.4, 129.3, 120.0, 36.4, 20.7. HRMS (ESI): Exact mass calculated for C10H14N2O [M+Na]+: 201.1004, found: 201.1008.
1,1-diethyl-3-(p-tolyl)urea (5r): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4r (54 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5r (29 mg, 39%). Brown solid. mp—50–51 °C. FTIR (cm−1): 3331, 2975, 2931, 2872, 1629, 1594, 1510, 1483, 1413, 1287, 1236, 1158, 1074, 807, 780, 749. 1H NMR (400 MHz, CDCl3) δ 7.22–7.16 (m, 3H), 7.04–6.97 (m, 2H), 6.15 (s, 1H), 3.29 (q, J = 7.2 Hz, 5H), 2.21 (s, 4H), 1.14 (t, J = 7.1 Hz, 7H). 13C NMR (101 MHz, CDCl3) δ 154.7, 136.7, 132.2, 129.3, 120.0, 41.6, 20.7, 13.9. HRMS (ESI): Exact mass calculated for C12H18N2O [M+Na]+: 229.1317, found: 229.1322.
1,1-diisopropyl-3-(p-tolyl)urea (5s): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4s (75 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5s (32 mg, 37%). Yellow solid. mp—136–138 °C. FTIR (cm−1): 3318, 2964, 2931, 2865, 1627, 1594, 1508, 1424, 1324, 1236, 1141, 1052, 800, 747. 1H NMR (400 MHz, CDCl3) δ 7.18 (d, J = 8.6 Hz, 2H), 7.00 (d, J = 8.3 Hz, 2H), 6.06 (s, 1H), 3.90 (hept, J = 6.9 Hz, 2H), 2.21 (s, 3H), 1.25 (s, 6H), 1.23 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 154.8, 136.7, 132.1, 129.3, 119.8, 45.4, 21.5, 20.7. HRMS (ESI): Exact mass calculated for C14H23N2O [M + H]+: 235.1805, found: 235.1807.
1,1-dibutyl-3-(p-tolyl)urea (5t): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4t (96 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5t (15 mg, 40%). Yellow solid. mp—76–78 °C. FTIR (cm−1): 3321, 2959, 2931, 2851, 1634, 1592, 1512, 1483, 1417, 1310, 1286, 1220, 1104, 936, 814, 750. 1H NMR (400 MHz, CDCl3) δ 7.21–7.16 (m, 3H), 7.03–6.98 (m, 2H), 6.12 (s, 1H), 3.25–3.17 (m, 4H), 2.22 (s, 3H), 1.52 (tt, J = 7.6, 6.4 Hz, 5H), 1.29 (dq, J = 14.6, 7.3 Hz, 5H), 0.89 (t, J = 7.3 Hz, 7H). 13C NMR (101 MHz, CDCl3) δ 154.0, 135.6, 131.2, 128.2, 118.8, 46.4, 29.8, 19.6, 19.2, 12.8. HRMS (ESI): Exact mass calculated for C16H27N2O [M + H]+: 263.2118, found: 263.2120.
1,1-diisobutyl-3-(p-tolyl)urea (5u): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4u (96 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5u (17 mg, 18%). Yellow solid. mp—93–95 °C. FTIR (cm−1): 3323, 2957, 2920, 2864, 1638, 1594, 1512, 1483, 1403, 1379, 1306, 1288, 1231, 1166, 1100, 1056, 810, 756. 1H NMR (400 MHz, CDCl3) δ 7.25 (d, J = 7.7 Hz, 3H), 7.08 (d, J = 8.3 Hz, 2H), 6.22 (s, 1H), 3.14 (d, J = 7.5 Hz, 4H), 2.29 (s, 3H), 2.03 (dq, J = 13.8, 6.9 Hz, 3H), 0.94 (d, J = 6.7 Hz, 12H). 13C NMR (101 MHz, CDCl3) δ 155.5, 136.6, 132.2, 129.3, 119.8, 56.0, 27.8, 20.7, 20.3. HRMS (ESI): Exact mass calculated for C16H26N2O [M + H]+: 263.2118, found: 263.2120.
3-(3,5-bis(trifluoromethyl)phenyl)-1,1-diisobutylurea (5u′): General procedure was followed using 1′ (95 mg, 0.37 mmol, 1.0 equiv.), 4u (96 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5u’ (60 mg, 43%). Yellow solid. mp—124–126 °C. FTIR (cm−1): 3316, 2969, 2876, 1638, 1534, 1468, 1444, 1373, 1271, 1119, 997, 948, 877, 838, 700, 681. 1H NMR (400 MHz, CDCl3) δ 7.88–7.83 (m, 2H), 7.45 (s, 1H), 6.85 (s, 1H), 3.18 (d, J = 7.5 Hz, 4H), 2.03 (dq, J = 13.9, 7.0 Hz, 2H), 0.95 (d, J = 6.7 Hz, 12H). 13C NMR (101 MHz, CDCl3) δ 154.7, 140.8, 132.4, 132.1, 131.7, 131.4, 127.3, 124.5, 121.8, 119.3, 115.8, 115.7, 55.9, 27.6, 20.1. 19F NMR (376 MHz, CDCl3) δ −63.0. HRMS (ESI): Exact mass calculated for C17H23F6N2O [M + H]+: 385.1710, found: 385.1713.
1,1-dicyclohexyl-3-(p-tolyl)urea (5v): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4v (134 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5v (70 mg, 60%). Brown solid. mp—157–159 °C. FTIR (cm−1): 3318, 2920, 2847, 1629, 1594, 1510, 1357, 1311, 1236, 1150, 1001, 813, 816, 745. 1H NMR (400 MHz, CDCl3) δ 7.17 (d, J = 8.4 Hz, 2H), 6.99 (d, J = 8.3 Hz, 2H), 6.13 (s, 1H), 3.39 (tt, J = 10.8, 5.0 Hz, 2H), 2.21 (s, 3H), 1.77 (d, J = 3.4 Hz, 2H), 1.74 (dd, J = 6.9, 2.9 Hz, 4H), 1.68 (dt, J = 8.1, 3.6 Hz, 7H), 1.63–1.56 (m, 3H), 1.33–1.22 (m, 5H), 1.08 (tt, J = 13.2, 3.4 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 155.0, 136.8, 132.0, 129.3, 119.7, 55.4, 31.9, 26.4, 25.5, 20.7. HRMS (ESI): Exact mass calculated for C20H31N2O [M + H]+: 315.2431, found: 315.2439.
N-(p-tolyl)pyrrolidine-1-carboxamide (5w): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4w (53 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5w (16 mg, 26%). Yellow solid. mp—147–149 °C. FTIR (cm−1): 3316, 3035, 2971, 2869, 1640, 1589, 1508, 1406, 1375, 1287, 1242, 1114, 1032, 805, 752. 1H NMR (400 MHz, CDCl3) δ 7.31 (d, J = 8.4 Hz, 2H), 7.10 (d, J = 8.3 Hz, 2H), 6.16 (s, 1H), 3.49–3.45 (m, 4H), 2.31 (s, 3H), 1.99–1.95 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 153.1, 135.5, 131.2, 128.3, 118.7, 44.7, 24.5, 19.7. HRMS (ESI): Exact mass calculated for C12H16N2O [M+Na]+: 227.1155, found: 227.1165.
N-(3,5-bis(trifluoromethyl)phenyl)pyrrolidine-1-carboxamide (5w’): General procedure was followed using 1’ (95 mg, 0.37 mmol, 1.0 equiv.), 4w(53 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5w’ (48 mg, 40%). Pale yellow solid. mp—145–147 °C. FTIR (cm−1): 3267, 2958, 2876, 1722, 1649, 1541, 1472, 1441, 1362, 1273, 1163, 1123, 1080, 875, 701, 679. 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 1.6 Hz, 2H), 7.39 (s, 1H), 6.67 (s, 1H), 3.45–3.37 (m, 4H), 1.95–1.87 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 13C NMR (101 MHz, CDCl3) δ 152.2, 139.7, 131.4, 131.1, 130.7, 130.4, 123.5, 120.8, 118.0, 117.9, 44.9, 24.5. 19F NMR (376 MHz, CDCl3) δ −63.08. HRMS (ESI): Exact mass calculated for C13H13F6N2O2 [M + H]+: 327.0927, found: 327.0930.
2,6-dimethyl-N-(p-tolyl)morpholine-4-carboxamide (5x): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4x (85 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5x (39 mg, 42%). Orange solid. mp—144–145 °C. FTIR (cm−1): 3238, 3112, 3042, 2971, 2808, 1623, 1594, 1512, 1413, 1247, 1167, 1081, 1052, 816, 725. 1H NMR (400 MHz, CDCl3) δ 7.14 (d, J = 8.4 Hz, 2H), 7.00 (d, J = 8.2 Hz, 2H), 6.41 (s, 1H), 3.76 (dd, J = 13.2, 2.1 Hz, 2H), 3.51 (dtd, J = 12.5, 6.2, 2.5 Hz, 2H), 2.50 (dd, J = 12.8, 10.6 Hz, 2H), 2.22 (s, 3H), 2.09 (s, 1H), 1.12 (d, J = 6.3 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 155.0, 136.2, 132.8, 129.3, 120.4, 71.6, 49.4, 30.9, 20.7, 18.7. HRMS (ESI): Exact mass calculated for C14H21N2O2 [M + H]+: 249.1598, found: 249.1600.
N-(p-tolyl)azetidine-1-carboxamide (5y): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4y (42 mg, 50 μL, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography using 85/15 v/v petroleum ether/acetone yielded 5y (14 mg, 19%). Brown solid. mp—211–212 °C. FTIR (cm−1): 3227, 3112, 3009, 2960, 2887, 1642, 1596, 1510, 1402, 1364, 1284, 1245, 1099, 800. 1H NMR (400 MHz, CDCl3) δ 7.23–7.16 (m, 3H), 7.00 (d, J = 8.4 H3, 2H), 5.85 (s, 1H), 3.98 (t, J = 7.5 Hz, 4H), 2.22 (s, 3H), 2.21–2.16 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 156.5, 136.0, 132.4, 129.4, 119.5, 49.2, 20.7, 15.1. HRMS (ESI): Exact mass calculated for C11H15N2O [M + H]+: 191.1179, found: 191.1180.
N-(3,5-bis(trifluoromethyl)phenyl)azetidine-1-carboxamide (5y′): General procedure was followed using 1′ (95 mg, 0.37 mmol, 1.0 equiv.), 4y (42 mg, 50 μL, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5y′ (97 mg, 60%). Colorless solid. mp—161–163 °C. FTIR (cm−1): 3278, 2958, 2887, 1654, 1545, 1441, 1362, 1271, 1165, 1119, 1001, 970, 937, 882, 701, 680. 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 0.9 Hz, 2H), 7.49–7.44 (m, 1H), 6.65 (s, 1H), 4.14–4.08 (m, 4H), 2.39–2.28 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 155.3, 140.4, 132.5, 132.2, 131.9, 131.6, 124.5, 121.8, 118.6, 115.9, 115.8, 49.3, 15.1. HRMS (ESI): Exact mass calculated for C12H11F6N2O [M + H]+: 313.0771, found: 313.0772.
N-(p-tolyl)-2-oxa-6-azaspiro[3,3]heptane-6-carboxamide (5z): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4z (73 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5z (33 mg, 38%). Colorless solid. mp—173–175 °C. FTIR (cm−1): 3287, 2928, 2862, 1647, 1596, 1532, 1508, 1406, 1368, 1333, 1244, 1087, 969, 812, 670. 1H NMR (400 MHz, CDCl3) δ 7.31–7.20 (m, 3H), 7.08 (d, J = 8.3 Hz, 2H), 6.18 (s, 1H), 4.77 (s, 4H), 4.15 (s, 4H), 2.29 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 156.57, 135.73, 133.00, 129.47, 129.28, 127.42, 119.98, 80.89, 58.89, 37.67, 21.50, 20.77. HRMS (ESI): Exact mass calculated for C13H17N2O2 [M + H]+: 233.1285, found: 233.1287.
4-((N-cyclopropyl-3-(trifluoromethyl)phenyl)sulfonamido)-N-(p-tolyl)piperidine-1-carboxamide (5aa): General procedure was followed using 1a (77 mg, 0.50 mmol, 2.0 equiv.), 4aa (100 mg, 0.28 mmol, 1 equiv.), PhI(OAc)2 (180 mg, 2 equiv.), K3PO4 (120 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5aa (67 mg, 38%). Colorless solid. mp—155–157 °C. FTIR (cm−1): 3342, 1641, 1598, 1513, 1481, 1423, 1326, 1241, 1162, 1124, 1070, 975, 928, 881, 811, 696, 647. 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 1.9 Hz, 1H), 7.98 (dt, J = 7.9, 1.5 Hz, 1H), 7.78 (d, J = 7.8 Hz, 1H), 7.61 (t, J = 7.9 Hz, 1H), 7.12 (d, J = 8.4 Hz, 2H), 6.97 (d, J = 8.4 Hz, 2H), 6.47 (s, 1H), 4.04 (d, J = 13.5 Hz, 2H), 3.95 (tt, J = 12.1, 3.8 Hz, 1H), 2.74 (td, J = 13.0, 2.4 Hz, 2H), 2.19 (s, 3H), 1.86 (dp, J = 11.8, 4.3, 3.7 Hz, 1H), 1.79 (dd, J = 12.4, 4.1 Hz, 2H), 1.52 (ddd, J = 11.8, 4.2, 2.0 Hz, 2H), 0.88–0.81 (m, 2H), 0.81–0.74 (m, 1H), 0.74–0.64 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 153.9, 139.9, 135.2, 131.7, 130.9, 130.6, 129.4, 128.9, 128.3, 128.3, 123.4, 123.3, 119.3, 57.7, 43.1, 29.8, 25.1, 19.7, 6.6. HRMS (ESI): Exact mass calculated for C23H27F3N3O3S [M + H]+: 482.1720, found: 482.1723.
N-(3,5-bis(trifluoromethyl)phenyl)-4-((N-cyclopropyl-3-(trifluoromethyl)phenyl)sulfonamido)piperidine-1-carboxamide (5aa′): General procedure was followed using 1’ (95 mg, 0.37 mmol, 1.0 equiv.), 4aa (100 mg, 0.28 mmol, 1 equiv.), PhI(OAc)2 (180 mg, 2 equiv.), K3PO4 (120 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5aa’ (200 mg, 88%). Yellow solid. mp—158–160 °C. FTIR (cm−1): 3318, 2953, 1042, 1545, 1450, 1375, 1324, 1273, 1161, 1121, 1068, 944, 873, 805, 733, 698. 1H NMR (400 MHz, CDCl3) δ 8.13 (s, 1H), 8.06 (d, J = 7.9 Hz, 1H), 7.88 (dd, J = 7.8, 0.9 Hz, 1H), 7.83 (s, 2H), 7.72 (t, J = 7.9 Hz, 1H), 7.67 (s, 1H), 7.42 (d, J = 1.6 Hz, 1H), 4.24 (dd, J = 11.1, 2.5 Hz, 2H), 4.10–4.00 (m, 1H), 2.92–2.81 (m, 2H), 1.97 (dq, J = 7.0, 3.5 Hz, 1H), 1.89 (dd, J = 12.5, 4.1 Hz, 2H), 1.69–1.60 (m, 2H), 0.90 (dt, J = 7.2, 3.2 Hz, 2H), 0.81–0.74 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 154.1, 140.9, 140.7, 132.0, 131.8, 131.6, 131.5, 130.4, 130.0, 129.5, 129.4, 124.5, 124.3, 124.2, 124.2, 121.8, 120.5, 119.5, 115.8, 112.0, 58.7, 44.0, 30.9, 30.8, 28.4, 26.2, 7.5. 19F NMR (376 MHz, CDCl3) δ -62.9, -63.1. HRMS (ESI): Exact mass calculated for C24H23F9N3O3S [M + H]+: 604.1311, found: 604.1317.
N-(p-tolyl)azepane-1-carboxamide (5bb): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4bb (73 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5bb (12 mg, 14%). Brown solid. mp—123–125 °C. FTIR (cm−1): 3280, 2931, 2854, 1629, 1524, 1510, 1474, 1413, 1289, 1236, 1189, 1099, 902, 809, 749. 1H NMR (400 MHz, CDCl3) δ 7.23–7.17 (m, 3H), 7.01 (d, J = 8.3 Hz, 2H), 6.15 (s, 1H), 3.46–3.40 (m, 4H), 2.22 (s, 3H), 1.71 (dq, J = 7.7, 2.5 Hz, 5H), 1.54 (dt, J = 7.3, 2.7 Hz, 5H). 13C NMR (101 MHz, CDCl3) δ 155.2, 136.7, 132.2, 129.3, 119.9, 46.6, 28.6, 27.2, 20.7. HRMS (ESI): Exact mass calculated for C14H20N2O [M+Na]+: 255.1468, found: 255.1476.
N-(3,5-bis(trifluoromethyl)phenyl)azepane-1-carboxamide (5bb′): General procedure was followed using 1’ (95 mg, 0.37 mmol, 1.0 equiv.), 4bb (73 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5bb’ (33 mg, 33%). Yellow solid. mp—158–160 °C. FTIR (cm−1): 3320, 2931, 2854, 1642, 1539, 1439, 1368, 1269, 1167, 1121, 997, 967, 885, 701, 681. 1H NMR (400 MHz, CDCl3) δ 8.24 (s, 2H), 7.81 (s, 1H), 7.13 (s, 1H), 3.90–3.79 (m, 4H), 2.20–2.07 (m, 4H), 2.01–1.90 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 154.4, 140.9, 132.4, 132.1, 131.8, 131.4, 127.3, 124.6, 121.9, 119.3, 119.3, 115.8, 115.8, 115.8, 46.7, 28.4, 27.1. HRMS (ESI): Exact mass calculated for C15H17F6N2O [M + H]+: 355.1240, found: 355.1249.
N-(p-tolyl)-1,4-oxazepane-4-carboxamide (5cc): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4cc (74 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5cc (22 mg, 26%). Yellow Solid. mp—112–114 °C. FTIR (cm−1): 3327, 2948, 2926, 2853, 1632, 1590, 1512, 1408, 1290, 1120, 1051, 927, 803, 750. 1H NMR (400 MHz, CDCl3) δ 7.30–7.19 (m, 2H), 7.15–7.02 (m, 2H), 6.32 (s, 1H), 3.84–3.72 (m, 4H), 3.70–3.59 (m, 4H), 2.29 (s, 3H), 1.99 (tt, J = 8.1, 5.3 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 155.2, 136.3, 132.7, 129.3, 120.3, 71.1, 70.3, 49.2, 45.2, 30.1, 20.7. HRMS (ESI): Exact mass calculated for C13H19N2O2 [M + H]+: 235.1442, found: 235.1443.
N-(3,5-bis(trifluoromethyl)phenyl)-1,4-oxazepane-4-carboxamide (5cc′): General procedure was followed using 1’ (95 mg, 0.37 mmol, 1.0 equiv.), 4cc (74 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5cc’ (115 mg, 88%). Colorless Solid. mp—109–111 °C. FTIR (cm−1): 3258, 2958, 2858, 1638, 1528, 1490, 1446, 1364, 1273, 1114, 1035, 990, 878, 750, 680. 1H NMR (400 MHz, CDCl3) δ 7.78 (d, J = 1.6 Hz, 2H), 7.43 (s, 1H), 7.29 (s, 1H), 3.78 (q, J = 5.8, 5.1 Hz, 4H), 3.72–3.64 (m, 4H), 2.02–1.93 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 154.7, 140.6, 132.2, 131.9, 131.6, 131.2, 127.2, 124.5, 121.8, 119.9, 119.8, 119.0, 116.2, 116.1, 116.0, 70.5, 70.2, 49.2, 45.3, 30.0. HRMS (ESI): Exact mass calculated for C14H15F6N2O2 [M + H]+: 357.1033, found: 357.1039.
1-benzyl-1-methyl-3-(p-tolyl)urea (5dd): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 4dd (90 mg, 1.15 mmol, 5 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5dd (17 mg, 18%). Yellow solid. mp—113–115 °C. FTIR (cm−1): 3311, 3064, 3026, 2916, 2865, 1629, 1594, 1510, 1373, 1245, 1021, 807, 741. 1H NMR (400 MHz, CDCl3) δ 7.31–7.26 (m, 2H), 7.22 (d, J = 6.9 Hz, 3H), 7.15 (d, J = 8.4 Hz, 2H), 7.00 (d, J = 8.3 Hz, 2H), 6.22 (s, 1H), 4.50 (s, 2H), 2.94 (s, 3H), 2.21 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 154.8, 136.5, 135.4, 131.5, 128.3, 127.8, 126.5, 126.2, 119.0, 51.3, 33.7, 19.7. HRMS (ESI): Exact mass calculated for C16H19N2O [M + H]+: 255.1492, found: 255.1494.
1-benzyl-3-(3,5-bis(trifluoromethyl)phenyl)-1-methylurea (5dd′): General procedure was followed using 1′ (95 mg, 0.37 mmol, 1.0 equiv.), 4dd (90 mg, 1.15 mmol, 5 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 5dd’ (61 mg, 44%). Yellow liquid. mp—113–115 °C. FTIR (cm−1): 2922, 2854, 1715, 1651, 1556, 1468, 1368, 1276, 1172, 1127, 1019, 878, 749, 697. 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 1.6 Hz, 2H), 7.49 (s, 1H), 7.38 (t, J = 7.2 Hz, 2H), 7.35–7.31 (m, 1H), 7.31–7.26 (m, 2H), 6.75 (s, 1H), 4.60 (s, 2H), 3.06 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 154.9, 140.6, 136.7, 132.5, 132.2, 131.8, 131.5, 129.0, 127.9, 127.2, 124.5, 121.8, 119.3, 116.2, 116.1, 116.0, 52.4, 34.8. HRMS (ESI): Exact mass calculated for C17H15F6N2O [M + H]+: 377.1084, found: 377.1080.
N-(p-tolyl)thiomorpholine-4-carboxamide (7a): General procedure was followed using 1d (50 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7d (69 mg, 79%). Orange solid. mp—179–181 °C. FTIR (cm−1): 3320, 2958, 2905, 1738, 1629, 1594, 1508, 1413, 1306, 1240, 1015, 942, 805, 743. 1H NMR (400 MHz, CDCl3) δ 7.22 (d, J = 8.4 Hz, 2H), 7.10 (d, J = 8.3 Hz, 2H), 6.45 (s, 1H), 3.81–3.75 (m, 4H), 2.68–2.62 (m, 4H), 2.32 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 154.9, 136.2, 132.9, 129.4, 120.5, 47.0, 27.0, 20.7. HRMS (ESI): Exact mass calculated for C12H16N2OS [M+Na]+: 259.0876, found: 259.0886.
N-(p-tolyl)morpholine-4-carboxamide (7a’): General procedure was followed using 1d (50 mg, 0.37 mmol, 1.0 equiv.), 6b (64 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7d′ (60 mg, 73%). Brown solid. mp—161–162 °C. FTIR (cm−1): 3320, 3035, 2958, 2909, 1631, 1594, 1510, 1472, 1417, 1309, 1289, 1236, 1207, 1017, 946, 807, 747. 1H NMR (400 MHz, CDCl3) δ 7.27–7.22 (m, 2H), 6.88–6.83 (m, 2H), 6.41 (s, 1H), 3.80 (s, 4H), 3.75–3.69 (m, 4H), 3.48–3.43 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 156.0, 155.7, 131.6, 122.6, 114.1, 66.5, 55.5, 44.2. HRMS (ESI): Exact mass calculated for C12H17N2O2 [M + H]+: 221.1285, found: 221.1289.
N-(o-tolyl)thiomorpholine-4-carboxamide (7b): General procedure was followed using 1b (50 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7b (60 mg, 75%). Orange solid. mp—190–191 °C. FTIR (cm−1): 3307, 2949, 2905, 1627, 1583, 1505, 1452, 1395, 1302, 1245, 1012, 942, 743. 1H NMR (400 MHz, CDCl3) δ 7.51 (d, J = 8.1 Hz, 1H), 7.19 (t, J = 7.3 Hz, 2H), 7.06 (td, J = 7.4, 1.3 Hz, 1H), 6.22 (s, 1H), 3.82–3.73 (m, 4H), 2.70–2.61 (m, 4H), 2.25 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 155.0, 136.8, 130.4, 129.7, 126.7, 124.5, 123.5, 47.1, 27.0, 17.8. HRMS (ESI): Exact mass calculated for C12H17N2OS [M + H]+: 237.1057, found: 237.1059.
N-(o-tolyl)morpholine-4-carboxamide (7b’): General procedure was followed using 1b (50 mg, 0.37 mmol, 1.0 equiv.), 6a (64 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7b’ (44 mg, 54%). Pink solid. mp—145–147 °C. FTIR (cm−1): 3287, 2958, 2916, 2847, 1629, 1578, 1508, 1455, 1375, 1253, 1112, 1039, 990, 858, 741. 1H NMR (400 MHz, CDCl3) δ7.59 (dd, J = 8.0, 1.3 Hz, 1H), 7.23–7.17 (m, 2H), 7.06 (td, J = 7.5, 1.3 Hz, 1H), 6.22 (s, 1H), 3.78–3.70 (m, 5H), 3.51–3.44 (m, 5H), 2.26 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 155.5, 136.7, 130.4, 129.3, 126.7, 124.4, 123.1, 66.5, 44.3, 17.8. HRMS (ESI): Exact mass calculated for C12H16N2O2 [M + H]+: 221.1285, found: 221.1287.
N-(m-tolyl)thiomorpholine-4-carboxamide (7c): General procedure was followed using 1c (50 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7c (58 mg, 67%). Yellow solid. mp—116–117 °C. FTIR (cm−1): 3300, 2960, 2909, 1631, 1583, 1530, 1421, 1291, 1209, 1039, 940, 776. 1H NMR (400 MHz, CDCl3) δ 7.24–7.12 (m, 3H), 7.10 (dt, J = 8.2, 1.5 Hz, 1H), 6.87 (d, J = 7.4 Hz, 1H), 6.62 (s, 1H), 3.82–3.73 (m, 4H), 2.67–2.58 (m, 4H), 2.32 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 154.8, 138.8, 138.7, 128.7, 128.6, 124.2, 121.1, 117.4, 47.0, 27.0, 21.4. HRMS (ESI): Exact mass calculated for C12H17N2OS [M + H]+: 237.1057, found: 237.1058.
N-(m-tolyl)morpholine-4-carboxamide (7c’): General procedure was followed using 1c (50 mg, 0.37 mmol, 1.0 equiv.), 6b (64 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7c’ (37 mg, 46%). Yellow solid. mp—126–127 °C. FTIR (cm−1): 3285, 2964, 2909, 2843, 1631, 1605, 1523, 1483, 1452, 1421, 1298, 1245, 1108, 1015, 982, 864, 776. 1H NMR (400 MHz, CDCl3) δ 7.24 (d, J = 2.0 Hz, 1H), 7.19 (t, J = 7.7 Hz, 1H), 7.12 (dt, J = 8.2, 1.6 Hz, 1H), 6.89 (d, J = 7.4 Hz, 1H), 6.46 (s, 1H), 3.77–3.69 (m, 4H), 3.52–3.42 (m, 4H), 2.34 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 155.2, 138.8, 138.6, 128.7, 124.2, 120.9, 117.2, 66.5, 44.2, 21.5. HRMS (ESI): Exact mass calculated for C12H17N2O2 [M + H]+: 221.1285, found: 221.1287.
N-phenylthiomorpholine-4-carboxamide (7d): General procedure was followed using 1a (45 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7a (56 mg, 74%). Brown solid. mp—167–168 °C. FTIR (cm−1): 3298, 2953, 2902, 1627, 1589, 1521, 1441, 1399, 1309, 1216, 1015, 942, 745. 1H NMR (400 MHz, CDCl3) δ 7.38–7.24 (m, 4H), 7.10–7.02 (m, 1H), 6.69 (s, 1H), 3.81–3.72 (m, 4H), 2.66–2.58 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 154.8, 138.9, 128.8, 123.3, 120.4, 46.9, 27.0. HRMS (ESI): Exact mass calculated for C11H15N2OS [M + H]+: 233.0900, found: 233.0901.
N-phenylmorpholine-4-carboxamide (7d’): General procedure was followed using 1a (45 mg, 0.37 mmol, 1.0 equiv.), 6b (64 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7a’ (54 mg, 66%). Colorless solid. mp—156–158 °C. FTIR (cm−1): 3250, 3124, 3046, 2953, 2854, 1627, 1592, 1523, 1497, 1439, 1402, 1298, 1242, 1108, 1074, 988, 853, 738. 1H NMR (400 MHz, CDCl3) δ 7.39–7.33 (m, 2H), 7.33–7.25 (m, 2H), 7.11–7.03 (m, 1H), 6.63 (s, 1H), 3.74–3.67 (m, 4H), 3.49–3.43 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 155.3, 138.8, 128.9, 123.3, 120.3, 66.5, 44.2. HRMS (ESI): Exact mass calculated for C11H15N2O2 [M + H]+: 207.1129, found: 207.1130.
N-(3,4-dimethylphenyl)thiomorpholine-4-carboxamide (7e): General procedure was followed using 1e (56 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7e (77 mg, 78%). Yellow solid. mp—173–174 °C. FTIR (cm-1): 3316, 2909, 1771, 1629, 1587, 1519, 1417, 1247, 1211, 1019, 942, 814, 745. 1H NMR (400 MHz, CDCl3) δ 7.06 (d, J = 1.8 Hz, 1H), 6.95 (d, J = 1.7 Hz, 2H), 6.31 (s, 1H), 3.72–3.64 (m, 4H), 2.60–2.51 (m, 4H), 2.13 (d, J = 6.8 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 154.9, 137.1, 136.4, 131.6, 129.8, 121.9, 117.9, 47.0, 27.0, 19.9, 19.0. HRMS (ESI): Exact mass calculated for C13H19N2OS [M + H]+: 251.1213, found: 251.1214.
N-(3,4-dimethylphenyl)morpholine-4-carboxamide (7e’): General procedure was followed using 1e (56 mg, 0.37 mmol, 1.0 equiv.), 6b (64 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7e’ (62 mg, 72%). Yellow solid. mp—164–165 °C. FTIR (cm-1): 3289, 2964, 2914, 2891, 2852, 1771, 1634, 1594, 1505, 1406, 1242, 1108, 1066, 988, 802. 1H NMR (400 MHz, CDCl3) δ 7.27–7.22 (m, 2H), 6.87–6.82 (m, 2H), 6.46 (s, 1H), 3.79 (s, 4H), 3.74–3.69 (m, 4H), 3.48–3.43 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 156.0, 155.7, 131.7, 122.6, 114.1, 66.5, 55.5, 44.2. HRMS (ESI): Exact mass calculated for C13H19N2O2 [M + H]+: 235.1442, found: 235.1443.
N-(3-methoxyphenyl)thiomorpholine-4-carboxamide (7f): General procedure was followed using 1f (56 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7f (68 mg, 73%). Brown solid. mp—110–112 °C. FTIR (cm−1): 3322, 3075, 3002, 2964, 2898, 1638, 1528, 1225, 1154, 1041, 946, 749. 1H NMR (400 MHz, CDCl3) δ 7.07 (t, J = 8.1 Hz, 1H), 6.98 (t, J = 2.3 Hz, 1H), 6.78–6.73 (m, 2H), 6.53–6.48 (m, 1H), 3.68 (s, 3H), 3.67–3.62 (m, 4H), 2.54–2.48 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 159.0, 153.7, 139.2, 128.4, 111.5, 107.9, 105.1, 54.2, 45.9, 26.0. HRMS (ESI): Exact mass calculated for C12H17N2O2S [M + H]+: 253.1006, found: 253.1007.
N-(4-methoxyphenyl)thiomorpholine-4-carboxamide (7g): General procedure was followed using 1g (56 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7g (60 mg, 66%). Brown solid. mp—133–134 °C. FTIR (cm-1): 3292, 2958, 2909, 2888, 1605, 1508, 1410, 1384, 1218, 1194, 1032, 946, 820, 749. 1H NMR (400 MHz, CDCl3) δ 7.21 (d, J = 9.0 Hz, 2H), 6.81 (d, J = 9.0 Hz, 2H), 6.68 (s, 1H), 3.77 (s, 3H), 3.75–3.71 (m, 4H), 2.62–2.57 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 161.2, 156.0, 155.3, 131.9, 122.8, 114.0, 55.5, 46.9, 27.0. HRMS (ESI): Exact mass calculated for C12H17N2O2S [M + H]+: 253.1006, found: 253.1008.
N-(4-methoxyphenyl)morpholine-4-carboxamide (7g’): General procedure was followed using 1g (56 mg, 0.37 mmol, 1.0 equiv.), 6b (64 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7g’ (57 mg, 65%). Pink solid. mp—117–119 °C. FTIR (cm−1): 3261, 2960, 2902, 2836, 1627, 1594, 1510, 1417, 1390, 1291, 1222, 1103, 1032, 984, 741. 1H NMR (400 MHz, CDCl3) δ 7.27–7.22 (m, 2H), 6.88–6.83 (m, 2H), 6.41 (s, 1H), 3.80 (s, 4H), 3.75–3.69 (m, 4H), 3.48–3.43 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 156.0, 155.7, 131.6, 122.6, 114.1, 66.5, 55.5, 44.2. HRMS (ESI): Exact mass calculated for C12H17N2O3 [M + H]+: 237.1234, found: 237.1237.
N-(2-fluorophenyl)thiomorpholine-4-carboxamide (7h): General procedure was followed using 1h (52 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7h (52 mg, 63%). Brown solid. mp—118–119 °C. FTIR (cm−1): 3311, 2958, 2905, 1631, 1585, 1494, 1455, 1395, 1251, 1189, 1099, 946, 745. 1H NMR (400 MHz, CDCl3) δ 8.01 (td, J = 8.2, 1.7 Hz, 1H), 7.14–7.03 (m, 2H), 6.99 (dddd, J = 8.5, 7.1, 5.3, 1.7 Hz, 1H), 6.62 (s, 1H), 3.85–3.78 (m, 4H), 2.72–2.65 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 153.9, 151.5, 127.3 (J = 11.2 Hz), 124.5, 123.2 (J = 10 Hz), 121.8, 114.7 (J = 23.7 Hz), 47.0, 27.0. 19F NMR (376 MHz, CDCl3) δ −132.1, −132.1, −132.1, −132.1, −132.2, −132.2, −132.2, −132.2. HRMS (ESI): Exact mass calculated for C11H14FN2OS [M + H]+: 241.0806, found: 241.0808.
N-(2-fluorophenyl)morpholine-4-carboxamide (7h’): General procedure was followed using 1h (52 mg, 0.37 mmol, 1.0 equiv.), 6b (64 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7h’ (44 mg, 55%). Yellow solid. mp—93–95 °C. FTIR (cm−1): 3367, 3053, 2980, 2858, 1645, 1600, 1519, 1490, 1439, 1384, 1262, 1105, 1063, 990, 745. 1H NMR (400 MHz, CDCl3) δ 8.06 (td, J = 8.2, 1.7 Hz, 1H), 7.19–6.93 (m, 3H), 6.72–6.59 (m, 1H), 3.84–3.68 (m, 4H), 3.58–3.44 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 154.4, 153.9, 151.5, 134.0, 133.9, 132.3, 132.2, 127.2 (J = 11.2 Hz), 124.5, 123.2 (J = 10 Hz), 121.7, 114.7 (J = 23.7 Hz), 66.4, 44.2. 19F NMR (376 MHz, CDCl3) δ −132.39. HRMS (ESI): Exact mass calculated for C11H14FN2O2 [M + H]+: 225.1034, found: 225.1033.
N-(3-fluorophenyl)thiomorpholine-4-carboxamide (7i): General procedure was followed using 1i (52 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography using 85/15 v/v petroleum ether/acetone yielded 7i (63 mg, 67%). Red solid. mp—128–130 °C. FTIR (cm−1): 3209, 3129, 3059, 2913, 1727, 1597, 1531, 1440, 1297, 1139, 1035, 955, 834. 1H NMR (400 MHz, CDCl3) δ 7.20–7.07 (m, 2H), 6.95–6.90 (m, 1H), 6.78 (s, 1H), 6.64 (tdd, J = 8.3, 2.6, 0.9 Hz, 1H), 3.72–3.62 (m, 4H), 2.58–2.50 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 164.2, 161.8, 154.4, 140.7 (J = 13.7 Hz), 129.9 (J = 12.5 Hz), 115.4 (J = 3.75 Hz), 109.9 (J = 26.2 Hz), 107.6 (J = 31.2 Hz), 46.9, 27.0. 19F NMR (376 MHz, CDCl3) δ −111.9, −112.0, −112.0, −112.0, −112.0, −112.0, −112.0, −112.0. HRMS (ESI): Exact mass calculated for C11H14FN2OS [M + H]+: 241.0806, found: 248.0807.
N-(4-fluorophenyl)thiomorpholine-4-carboxamide (7j): General procedure was followed using 1j (52 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7j (63 mg, 77%). Brown solid. mp—167–169 °C. FTIR (cm−1): 3201, 3126, 3042, 2900, 1616, 1532, 1505, 1417, 1302, 1205, 1024, 942, 811, 743. 1H NMR (400 MHz, CDCl3) δ 7.28 (dd, J = 9.1, 4.7 Hz, 2H), 6.99 (t, J = 8.6 Hz, 2H), 6.50 (s, 1H), 3.82–3.74 (m, 4H), 2.70–2.62 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 160.2, 157.8, 154.8, 134.7 (J = 3.7 Hz), 122.3 (J = 8.7 Hz), 115.6 (J = 28.7 Hz), 46.9, 27.0. 19F NMR (376 MHz, CDCl3) δ −119.5, −119.5, −119.5, −119.6, −119.6. HRMS (ESI): Exact mass calculated for C11H14FN2OS [M + H]+: 241.0806, found: 241.0807.
N-(4-fluorophenyl)morpholine-4-carboxamide (7j’): General procedure was followed using 1j (52 mg, 0.37 mmol, 1.0 equiv.), 6b (64 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7j’ (44 mg, 54%). Brown solid. mp—177–179 °C. FTIR (cm−1): 3254, 3141,3053, 2980, 2920, 2854, 1631, 1609, 1528, 1508, 1419, 1302, 1245, 1205, 1110, 1068, 990, 822, 741. 1H NMR (400 MHz, CDCl3) δ 7.35–7.27 (m, 3H), 7.01 (dd, J = 9.0, 8.3 Hz, 2H), 6.39 (s, 1H), 3.78–3.73 (m, 4H), 3.51–3.46 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 160.2, 157.8, 155.2, 134.6 (J = 3.7 Hz), 134.5, 122.2 (J = 10 Hz), 122.1, 115.6 (J = 27 Hz), 115.4, 66.4, 44.2. 19F NMR (376 MHz, CDCl3) δ −119.5, −119.5. HRMS (ESI): Exact mass calculated for C11H14FN2O2 [M + H]+: 225.1034, found: 225.1036.
N-(2-chlorophenyl)thiomorpholine-4-carboxamide (7k): General procedure was followed using 1k (58 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7k (79 mg, 84%). Brown solid. mp—115–116 °C. FTIR (cm−1): 3338, 2905, 1629, 1576, 1512, 1439, 1395, 1295, 1054, 944, 745. 1H NMR (400 MHz, CDCl3) δ 8.14 (dd, J = 8.3, 1.6 Hz, 1H), 7.35 (dd, J = 8.0, 1.5 Hz, 1H), 7.30–7.22 (m, 1H), 6.98 (td, J = 7.6, 1.6 Hz, 2H), 3.88–3.79 (m, 4H), 2.75–2.66 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 153.6, 135.6, 128.8, 127.7, 123.4, 122.5, 121.2, 47.1, 27.0. HRMS (ESI): Exact mass calculated for C11H14ClN2OS [M + H]+: 257.0510, found: 257.0512.
N-(2-chlorophenyl)morpholine-4-carboxamide (7k’): General procedure was followed using 1k (58 mg, 0.37 mmol, 1.0 equiv.), 6b (64 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7k’ (50 mg, 65%). Colorless solid. mp—128–130 °C. FTIR (cm−1): 3212, 2909, 2887, 2854, 1629, 1572, 1510, 1439, 1368, 1245, 1114, 1050, 1019, 993, 898, 747. 1H NMR (400 MHz, CDCl3) δ 8.21 (dd, J = 8.3, 1.6 Hz, 1H), 7.35 (dd, J = 8.0, 1.5 Hz, 1H), 7.27 (ddd, J = 8.6, 7.4, 1.6 Hz, 1H), 7.06–7.00 (m, 1H), 6.98 (dd, J = 7.7, 1.6 Hz, 1H), 3.84–3.73 (m, 4H), 3.59–3.48 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 154.2, 135.5, 128.7, 127.7, 123.3, 122.3, 120.9, 66.4, 44.1. HRMS (ESI): Exact mass calculated for C11H14ClN2O2 [M + H]+: 241.0739, found: 241.0741.
N-(4-chlorophenyl)thiomorpholine-4-carboxamide (7l): General procedure was followed using 1l (58 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7l (37 mg, 38%). Brown solid. mp—208–210 °C. FTIR (cm−1): 3298, 2958, 2894, 1631, 1512, 1587, 1490, 1413, 1298, 1240, 1220, 1194, 1085, 1008, 937, 816, 742. 1H NMR (400 MHz, CDCl3) δ 7.28 (d, J = 9.0 Hz, 2H), 7.24 (d, J = 9.0 Hz, 2H), 6.37 (s, 1H), 3.80–3.77 (m, 4H), 2.68–2.65 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 154.33, 137.4, 128.9, 128.3, 121.3, 47.0, 27.1. HRMS (ESI): Exact mass calculated for C11H13ClN2OS [M+Na]+: 279.0330, found: 279.0328.
N-(4-chlorophenyl)morpholine-4-carboxamide (7l′): General procedure was followed using 1l (56 mg, 0.37 mmol, 1.0 equiv.), 6b (64 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7l’ (39 mg, 52%). Beige solid. mp—200–202 °C. FTIR (cm−1): 3283, 3104, 3055, 2971, 2887, 2847, 1629, 1589, 1508, 1494, 1417, 1242, 1108, 1077, 988, 891, 822. 1H NMR (400 MHz, CDCl3) δ 7.33 (d, J = 8.8 Hz, 2H), 7.30–7.24 (m, 3H), 6.41 (s, 1H), 3.79–3.71 (m, 4H), 3.49 (t, J = 4.9 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 154.8, 137.3, 128.9, 128.3, 121.2, 66.4, 44.2. HRMS (ESI): Exact mass calculated for C11H14ClN2O2 [M + H]+: 241.0739, found: 241.0741.
N-(2-bromophenyl)thiomorpholine-4-carboxamide (7m): General procedure was followed using 1m (75 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7m (100 mg, 90%). Light brown solid. mp—122–123 °C. FTIR (cm−1): 3335, 2960, 1568, 1627, 1507, 1043, 1015, 949, 744. 1H NMR (400 MHz, CDCl3) δ 8.03 (dd, J = 8.3, 1.6 Hz, 1H), 7.42 (dd, J = 8.0, 1.5 Hz, 1H), 7.25–7.16 (m, 1H), 6.91 (s, 1H), 6.82 (td, J = 7.7, 1.6 Hz, 1H), 3.79–3.72 (m, 4H), 2.64–2.57 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 153.6, 136.6, 131.9, 128.3, 123.9, 121.5, 113.5, 47.1, 27.0. HRMS (ESI): Exact mass calculated for C11H14BrN2OS [M + H]+: 301.0005, found: 301.0007.
N-(4-bromophenyl)thiomorpholine-4-carboxamide (7n): General procedure was followed using 1n (75 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7n (88 mg, 79%). Brown solid. mp—212–214 °C. FTIR (cm−1): 3316, 2958, 2902, 1631, 1583, 1510, 1486, 1410, 1306, 1218, 1068, 1004, 940, 798, 746. 1H NMR (400 MHz, CDCl3) δ 7.32 (d, J = 8.3 Hz, 2H), 7.22–7.14 (m, 2H), 6.25 (s, 1H), 3.77–3.65 (m, 4H), 2.66–2.53 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 153.1, 136.9, 130.8, 120.5, 114.8, 46.0, 26.0. HRMS (ESI): Exact mass calculated for C11H14BrN2OS [M + H]+: 301.0005, found: 301.0004.
N-(4-bromophenyl)morpholine-4-carboxamide (7n′): General procedure was followed using 1n (75 mg, 0.37 mmol, 1.0 equiv.), 6b (64 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7n′ (29 mg, 40%). Colorless solid. mp—195–197 °C. FTIR (cm−1): 3289, 2969, 2887, 2847, 1631, 1585, 1510, 1488, 1413, 1300, 1242, 1110, 1063, 993, 818, 738. 1H NMR (400 MHz, CDCl3) δ 7.32 (d, J = 8.3 Hz, 2H), 7.24–7.03 (m, 3H), 6.25 (s, 1H), 3.84–3.59 (m, 4H), 2.76–2.46 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 154.7, 137.8, 131.8, 121.5, 115.8, 66.4, 44.2. HRMS (ESI): Exact mass calculated for C11H14BrN2O2 [M + H]+: 285.0234, found: 285.0236.
N-(thiomorpholinyl)-2-Trifluoromethylbenzamide (7o): General procedure was followed using 1o (70 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7o (81 mg, 80%). Brown solid. mp—115–116 °C. FTIR (cm−1): 3265, 2960, 2909, 1627, 1583, 1516, 1395, 1311, 1247, 1163, 1052, 949, 749. 1H NMR (400 MHz, CDCl3) δ 8.03 (d, J = 8.3 Hz, 1H), 7.63–7.49 (m, 2H), 7.22–7.13 (m, 1H), 6.77 (s, 1H), 3.85–3.77 (m, 4H), 2.73–2.63 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 153.8, 136.7, 136.6, 132.8, 128.5, 126.0, 125.9, 125.8, 124.3, 123.3, 123.1, 120.1, 119.8, 119.5, 119.2, 47.1, 26.9. 19F NMR (376 MHz, CDCl3) δ −60.5. HRMS (ESI): Exact mass calculated for C12H14F3N2OS [M + H]+: 291.0774, found: 291.0776.
N-(2-(trifluoromethyl)phenyl)morpholine-4-carboxamide (7o’): General procedure was followed using 1o (70 mg, 0.37 mmol, 1.0 equiv.), 6b (64 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7o’ (72 mg, 98%). Brown solid. mp—115–116 °C. FTIR (cm−1): 3265, 2960, 2909, 1627, 1583, 1516, 1395, 1311, 1247, 1163, 1052, 947, 749. 1H NMR (400 MHz, CDCl3) δ 8.11 (d, J = 8.3 Hz, 1H), 7.62–7.51 (m, 2H), 7.17 (t, J = 7.7 Hz, 1H), 6.80 (s, 1H), 3.82–3.73 (m, 4H), 3.55–3.46 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 154.3, 136.6, 136.6, 136.6, 136.6, 132.8, 126.0, 125.9, 125.8, 123.9, 123.2, 123.1, 119.8, 119.5, 119.2, 118.9, 66.4, 44.1. 19F NMR (376 MHz, CDCl3) δ −60.58. HRMS (ESI): Exact mass calculated for C12H14F3N2O2 [M + H]+: 275.1002, found: 275.1005.
N-(3-(trifluoromethyl)phenyl)thiomorpholine-4-carboxamide (7p): General procedure was followed using 1p (70 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7p (70 mg, 65%). Brown solid. mp—127–129 °C. FTIR (cm−1): 3303, 3048, 2964, 2916, 1638, 1594, 1521, 1435, 1101, 1061, 973, 787. 1H NMR (400 MHz, CDCl3) δ 7.51 (d, J = 2.1 Hz, 1H), 7.44 (dd, J = 8.1, 2.2 Hz, 1H), 7.28 (t, J = 7.9 Hz, 1H), 7.23–7.16 (m, 1H), 6.74 (s, 1H), 3.75–3.66 (m, 4H), 2.61–2.51 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 154.3, 139.5, 131.6, 131.3, 130.9, 130.6, 129.3, 123.3, 123.2, 119.8, 119.7, 116.8, 116.7, 47.0, 27.1. 19F NMR (376 MHz, CDCl3) δ −62.6. HRMS (ESI): Exact mass calculated for C12H14F3N2OS [M + H]+: 291.0774, found: 291.0776.
N-(3,5-bis(trifluoromethyl)phenyl)thiomorpholine-4-carboxamide (7q): General procedure was followed using 1’ (95 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7q (103 mg, 78%). Brwon solid. mp—140–141 °C. FTIR (cm-1): 3327, 2912, 1638, 1538, 1446, 1367, 1268, 1167, 1116, 973, 884. 1H NMR (400 MHz, CDCl3) δ 7.61 (s, 2H), 7.34 (d, J = 5.6 Hz, 2H), 3.80–3.65 (m, 4H), 2.62–2.50 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 153.1, 139.4, 131.3, 130.9, 130.6, 130.3, 126.1, 123.4, 120.7, 118.9, 118.8, 118.8, 118.7, 118.0, 115.3, 115.3, 115.2, 115.2, 115.2, 45.9, 26.1. 19F NMR (376 MHz, CDCl3) δ −63.1. HRMS (ESI): Exact mass calculated for C13H13F6N2OS [M + H]+: 359.0648, found: 359.0650.
N-(naphthalen-2-yl)thiomorpholine-4-carboxamide (7r): General procedure was followed using 1r (64 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7r (40 mg, 40%). Brown solid. mp—187–188 °C. FTIR (cm−1): 3298, 3057, 2961, 2911, 1628, 1599, 1535, 1392, 1016, 943, 786, 759. 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 13.4 Hz, 2H), 7.60 (d, J = 8.1 Hz, 1H), 7.40 (m, 4H), 6.63 (s, 1H), 3.76–3.58 (m, 4H), 2.52 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 155.7, 134.2, 133.9, 128.6, 128.4, 126.1, 125.9, 125.7, 125.5, 121.4, 121.3, 47.1, 27.0. HRMS (ESI): Exact mass calculated for C15H17N2O2S [M + H]+: 273.1057, found: 273.1058.
N-([1,1’-biphenyl]-4-yl)thiomorpholine-4-carboxamide (7s): General procedure was followed using 1s (64 mg, 0.37 mmol, 1.0 equiv.), 6a (76 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 7s (40 mg, 40%). Beige solid. mp—202–204 °C. FTIR (cm−1): 3318, 2958, 2909, 1627, 1516, 1399, 1306, 1245, 1220, 1196, 1169, 1085, 942, 811, 760, 693. 1H NMR (400 MHz, CDCl3) δ 7.58–7.50 (m, 5H), 7.41 (ddd, J = 8.0, 4.4, 2.0 Hz, 4H), 7.34–7.28 (m, 1H), 6.53 (s, 1H), 3.82–3.77 (m, 4H), 2.70–2.64 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 154.61, 140.59, 138.19, 136.18, 128.74, 127.53, 126.94, 126.77, 120.40, 47.05, 27.10. HRMS (ESI): Exact mass calculated for C17H19N2OS [M + H]+: 299.1213, found: 299.1210.
(3R,4S)-3-((benzo[d][1,3]dioxol-5-yloxy)methyl)-4-(4-fluorophenyl)-N-(p-tolyl)piperidine-1-carboxamide (9a): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 8a (182 mg, 0.55 mmol, 1.5 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9a (87 mg, 51%). Brown oily liquid. FTIR (cm−1): 3301, 2915, 1632, 1592, 1505, 1483, 1217, 1175, 1031, 929, 810, 723. 1H NMR (400 MHz, CDCl3) δ 7.32–7.26 (m, 2H), 7.19–7.08 (m, 4H), 7.01 (t, J = 8.7 Hz, 2H), 6.70–6.63 (m, 2H), 6.38 (d, J = 2.5 Hz, 1H), 6.17 (dd, J = 8.5, 2.5 Hz, 1H), 5.90 (s, 2H), 4.43–4.21 (m, 2H), 3.64 (dd, J = 9.5, 2.9 Hz, 1H), 3.50 (dd, J = 9.5, 6.7 Hz, 1H), 2.98 (dd, J = 13.5, 11.0 Hz, 2H), 2.79–2.68 (m, 1H), 2.32 (s, 3H), 2.19 (s, 2H), 2.13 (tq, J = 7.7, 4.0 Hz, 1H), 2.08 (s, 1H), 1.92–1.72 (m, 2H), 1.38–1.26 (m, 1H), 0.95–0.85 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 162.8, 160.4, 155.3, 154.1, 148.2, 141.8, 138.7 (J = 3.7 Hz), 136.5, 132.6, 129.4, 128.8 (J = 8.7 Hz), 120.3, 115.7 (J = 26.2 Hz), 107.9, 105.6, 101.1, 98.0, 68.7, 47.9, 44.9, 44.0, 41.9, 33.8, 30.9, 20.7. 19F NMR (376 MHz, CDCl3) δ −115.8, −115.8, −115.8, −115.9, −115.9. HRMS (ESI): Exact mass calculated for C27H28FN2O4 [M + H]+: 463.2028, found: 463.2030.
1-((1R,3R,5S)-3,5-dimethyladamantan-1-yl)-3-(p-tolyl)urea (9b): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 8b (132 mg, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9b (50 mg, 44%). Brown solid. mp—152–154 °C. FTIR (cm−1): 3323, 2891, 2838, 1643, 1594, 1556, 1512, 1452, 1310, 1295, 1224, 1031, 816. 1H NMR (400 MHz, CDCl3) δ 7.15 (d, J = 8.4 Hz, 2H), 7.06 (d, J = 8.3 Hz, 2H), 6.74 (s, 1H), 4.95 (s, 1H), 2.28 (s, 3H), 2.13–2.07 (m, 1H), 1.80 (d, J = 3.2 Hz, 2H), 1.66–1.54 (m, 5H), 1.34 (dt, J = 12.1, 2.5 Hz, 3H), 1.30–1.23 (m, 3H), 1.16–1.08 (m, 3H), 0.82 (s, 7H). 13C NMR (101 MHz, CDCl3) δ 155.2, 136.4, 132.8, 129.6, 121.0, 52.7, 50.6, 48.2, 42.7, 40.7, 32.4, 30.2, 30.1, 20.7. HRMS (ESI): Exact mass calculated for C20H29N2O [M + H]+: 313.2275, found: 313.2274.
N2-(2,6-dimethylphenyl)-N1-(p-tolyl)piperidine-1,2-dicarboxamide (9c): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 8c (171 mg, 0,74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9c (52 mg, 39%). Colorless solid. mp—218–220 °C. FTIR (cm−1): 3219, 2935, 2857, 1638, 1592, 1525, 1512, 1446, 1406, 1379, 1344, 1297, 1224, 1036, 821, 767. 1H NMR (400 MHz, CDCl3) δ 7.91 (s, 1H), 7.16 (d, J = 8.2 Hz, 3H), 7.04–6.98 (m, 8H), 6.47 (s, 1H), 5.07–5.03 (m, 1H), 3.70 (dt, J = 13.5, 3.6 Hz, 2H), 3.23 (td, J = 12.6, 2.6 Hz, 1H), 2.29 (dd, J = 8.9, 5.7 Hz, 2H), 2.23 (s, 3H), 2.17 (d, J = 2.1 Hz, 1H), 2.13 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 170.1, 156.5, 135.8, 135.0, 133.8, 133.4, 129.6, 129.5, 128.3, 128.1, 127.0, 126.0, 120.6, 53.7, 43.5, 25.4, 25.0, 20.7, 20.3, 18.8, 18.6, 18.4, 14.0. HRMS (ESI): Exact mass calculated for C22H28N3O2 [M + H]+: 366.2177, found: 366.2178.
1-(((1R,4aS,10aR)-7-isopropyl-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydrophenanthren-1-yl)methyl)-3-(p-tolyl)urea (9d): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 8d (211 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9d (89 mg, 58%). Colorless solid. mp—209–211 °C. FTIR (cm−1): 3325, 2948, 2913, 1687, 1641, 1592, 1512, 1308, 1295, 1235, 1082, 1036, 815, 667. 1H NMR (400 MHz, CDCl3) δ 7.12 (dd, J = 12.3, 8.3 Hz, 3H), 7.01 (d, J = 8.1 Hz, 2H), 6.97 (dd, J = 8.1, 2.1 Hz, 1H), 6.85–6.81 (m, 2H), 5.14 (t, J = 6.4 Hz, 1H), 3.08 (t, J = 6.4 Hz, 2H), 2.88–2.68 (m, 4H), 2.25 (s, 3H), 1.86–1.76 (m, 2H), 1.76–1.52 (m, 5H), 1.44–1.25 (m, 5H), 1.21 (s, 4H), 1.19 (s, 3H), 1.17 (s, 3H), 0.87 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 156.5, 147.2, 145.5, 136.0, 134.8, 133.4, 129.7, 126.8, 124.2, 123.7, 121.5, 50.6, 45.4, 38.4, 37.5, 37.4, 36.1, 33.4, 30.2, 25.2, 24.0, 20.8, 18.9, 18.6, 18.5. HRMS (ESI): Exact mass calculated for C28H39N2O [M + H]+: 419.3057, found: 419.3056.
1-((1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydronaphthalen-1-yl)-1-methyl-3-(p-tolyl)urea (9e): General procedure was followed using 1a (44 mg, 0.32 mmol, 2.0 equiv.), 8e (50 mg, 0.16 mmol, 1 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9e (32 mg, 18%). Orange solid. mp—151–153 °C. FTIR (cm−1): 3290, 3028, 2942, 2855, 1632, 1590, 1512, 1408, 1290, 1120, 1051, 927, 803, 750. 1H NMR (400 MHz, CDCl3) δ 7.36–7.30 (m, 3H), 7.30–7.24 (m, 2H), 7.22–7.17 (m, 1H), 7.13–7.07 (m, 3H), 6.97 (dd, J = 7.6, 1.2 Hz, 1H), 6.85 (dd, J = 8.3, 2.1 Hz, 1H), 6.40 (s, 1H), 5.70 (dd, J = 10.9, 6.1 Hz, 1H), 4.20 (dd, J = 5.7, 2.9 Hz, 1H), 2.78 (s, 3H), 2.30 (s, 3H), 2.07–1.96 (m, 1H), 1.81 (dt, J = 5.7, 3.8 Hz, 1H), 1.73 (ddd, J = 13.3, 10.7, 2.8 Hz, 1H), 0.92–0.79 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 156.1, 147.1, 138.2, 136.7, 136.4, 132.7, 132.3, 130.8, 130.6, 130.1, 129.4, 128.0, 127.5, 127.4, 120.1, 54.1, 43.0, 30.4, 30.1, 22.6, 22.2, 20.7. HRMS (ESI): Exact mass calculated for C25H25Cl2N2O [M + H]+: 439.1339, found: 439.1341.
3-(3,5-bis(trifluoromethyl)phenyl)-1-((1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydronaphthalen-1-yl)-1-methylurea (9e’): General procedure was followed using 1′ (95 mg, 0.32 mmol, 2.0 equiv.), 8e (46 mg, 0.16 mmol, 1 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9e’ (84 mg, 40%). Orange sticky solid. FTIR (cm−1): 3320, 2936, 1642, 1539, 1466, 1446, 1368, 1273, 1119, 1028, 953, 874, 731, 699, 679. 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 2H), 7.53 (s, 1H), 7.35 (d, J = 8.3 Hz, 1H), 7.31–7.20 (m, 4H), 7.10 (d, J = 2.1 Hz, 1H), 7.00 (d, J = 7.3 Hz, 1H), 6.86 (dd, J = 8.4, 2.1 Hz, 2H), 2.84 (s, 3H), 2.32 (tdd, J = 13.4, 5.7, 2.9 Hz, 1H), 2.17 (s, 4H), 2.05 (dt, J = 13.7, 2.7 Hz, 1H), 1.87–1.68 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 155.3, 146.9, 140.6, 138.3, 135.9, 132.6, 132.4, 132.3, 131.9, 131.6, 131.0, 130.6, 130.2, 130.2, 127.9, 127.7, 127.6, 127.2, 124.5, 121.8, 119.4, 116.3, 42.9, 30.9, 30.5, 30.0, 22.2. 19F NMR (376 MHz, CDCl3) δ −62.99. HRMS (ESI): Exact mass calculated for C26H21Cl2F6N2O [M + H]+: 561.0930, found: 561.0933.
(S)-1-(4-oxo-4-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-1-(2,4,5-trifluorophenyl)butan-2-yl)-3-(p-tolyl)urea (9f): General procedure was followed using 1a (30 mg, 0.22 mmol, 1.0 equiv.), 8f (90 mg, 0.22 mmol, 1 equiv.), PhI(OAc)2 (141 mg, 2 equiv.), K3PO4 (93 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 100/4 v/v dichloromethane/methanol as eluent and yielded 9f (30 mg, 12%). Brown solid. mp—122–124 °C. FTIR (cm−1): 3325, 1654, 1512, 1419, 1268, 1250, 1138, 1013, 725. 1H NMR (400 MHz, DMSO-d6) δ 8.44 (d, J = 23.1 Hz, 1H), 7.55–7.39 (m, 2H), 7.21 (d, J = 8.7 Hz, 2H), 7.03 (d, J = 8.1 Hz, 2H), 6.24 (dd, J = 21.9, 9.0 Hz, 1H), 5.07 (d, J = 7.2 Hz, 1H), 4.96 (s, 1H), 4.31 (td, J = 18.1, 14.8, 9.5 Hz, 3H), 4.14 (d, J = 5.8 Hz, 1H), 4.05 (t, J = 6.0 Hz, 2H), 2.93 (t, J = 6.7 Hz, 2H), 2.86–2.76 (m, 2H), 2.25 (s, 3H), 2.15 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 170.2, 170.1, 155.1, 151.4, 138.2, 130.2, 129.4, 120.2, 118.1, 106.2, 79.7, 79.4, 79.1, 47.0, 44.0, 42.4, 41.5, 38.7, 37.8, 31.1, 20.7. 19F NMR (376 MHz, DMSO-d6) δ −61.87, −61.88, −118.50, −118.51, −118.53, −118.55, −118.56, −118.57, −137.30, −137.31, −137.33, −137.35, −137.36, −137.39, −144.04, −144.05, −144.07, −144.08, −144.10. HRMS (ESI): Exact mass calculated for C24H23F6N6O2 [M + H]+: 541.1782, found: 541.1781.
(S)-1-(3,5-bis(trifluoromethyl)phenyl)-3-(4-oxo-4-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-1-(2,4,5-trifluorophenyl)butan-2-yl)urea (9f′): General procedure was followed using 1’ (95 mg, 0.22 mmol, 1.0 equiv.), 8f (226 mg, 0.22 mmol, 1 equiv.), PhI(OAc)2 (141 mg, 2 equiv.), K3PO4 (93 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 100/4 v/v dichloromethane/methanol as eluent and yielded 9f’ (207 mg, 85%). Brown solid. mp—122–124 °C. FTIR (cm−1): 3325, 1654, 1512, 1419, 1268, 1250, 1138, 1013, 725. 1H NMR (400 MHz, DMSO-d6) δ 8.54 (s, 1H), 8.04 (s, 2H), 7.82 (s, 1H), 7.41 (s, 1H), 7.35 (d, J = 9.2 Hz, 1H), 7.16–7.06 (m, 2H), 6.87 (td, J = 9.5, 6.4 Hz, 2H), 6.08 (d, J = 17.8 Hz, 1H), 5.09 (t, J = 15.1 Hz, 1H), 5.00–4.88 (m, 1H), 4.78 (d, J = 18.0 Hz, 1H), 4.51 (s, 1H), 4.41–4.08 (m, 7H), 3.90 (ddd, J = 13.9, 8.8, 4.5 Hz, 2H), 3.25 (dd, J = 13.6, 8.4 Hz, 1H), 3.05 (dd, J = 13.9, 6.8 Hz, 1H), 3.00–2.85 (m, 2H), 2.82–2.70 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 170.0, 156.0, 155.9, 154.7, 151.1, 141.4, 132.0, 131.7, 129.0, 128.0, 124.6, 121.9, 119.2 (J = 10 Hz), 118.1, 117.7, 116.8, 114.3, 109.9, 105.7, 105.4, 105.2, 103.6, 47.4, 43.8, 41.5, 38.8, 35.3, 32.6, 30.9. 19F NMR (376 MHz, DMSO-d6) δ −63.14, −119.76, −119.78, −119.81, −119.83, −134.88, −135.21, −135.25, −135.27, −142.49, −142.52, −142.54, −142.57. HRMS (ESI): Exact mass calculated for C25H19F12N6O2 [M + H]+: 663.1372, found: 663.1373.
1-methyl-1-(3-phenyl-3-(4-(trifluoromethyl)phenoxy)propyl)-3-(p-tolyl)urea (9g): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 8g (121 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9g (26 mg, 27%). Yellow oily liquid. FTIR (cm−1): 3321, 2922, 1641, 1610, 1512, 1317, 1239, 1153, 1107, 1064, 832, 810, 707. 1H NMR (400 MHz, CDCl3) δ 7.36–7.31 (m, 3H), 7.26 (d, J = 4.4 Hz, 5H), 7.22–7.17 (m, 2H), 6.99–6.90 (m, 4H), 6.83 (d, J = 8.3 Hz, 2H), 6.20 (s, 1H), 5.20 (dd, J = 8.5, 4.3 Hz, 1H), 3.59–3.42 (m, 2H), 2.92 (s, 3H), 2.19 (s, 4H), 2.17–2.08 (m, 2H), 0.89–0.75 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 160.0, 155.5, 140.3, 136.2, 132.5, 129.2, 128.9, 128.0, 126.9, 125.7, 123.3, 123.0, 122.9, 120.0, 115.8, 77.6, 45.9, 37.1, 34.7, 23.8, 22.6, 20.7. 19F NMR (376 MHz, CDCl3) δ -61.6. HRMS (ESI): Exact mass calculated for C25H26F3N2O2 [M + H]+: 443.1941, found: 443.1941.
3-(3,5-bis(trifluoromethyl)phenyl)-1-methyl-1-(3-phenyl-3-(4-(trifluoromethyl)phenoxy)propyl)urea (9g′): General procedure was followed using 1′ (95 mg, 0.37 mmol, 1.0 equiv.), 8g (0,19 mg, 0.74 mmol, 1.5 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9g’ (208 mg, 37%). Yellow oily liquid. FTIR (cm−1): 3318, 2936, 1649, 1616, 1539, 1472, 1364, 1324, 1273, 1108, 1066, 833, 697, 680. 1H NMR (400 MHz, CDCl3) δ 7.68 (s, 2H), 7.43 (s, 1H), 7.41–7.37 (m, 2H), 7.36–7.31 (m, 4H), 7.30–7.25 (m, 1H), 6.93–6.85 (m, 3H), 5.30 (dd, J = 8.4, 4.3 Hz, 1H), 3.69 (dt, J = 15.0, 7.5 Hz, 1H), 3.61–3.52 (m, 1H), 3.05 (s, 3H), 2.30–2.19 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 159.5, 154.7, 140.6, 139.9, 132.4, 132.0, 131.7, 131.4, 129.0, 128.3, 127.0, 126.9, 125.7, 124.5, 123.7, 123.4, 122.7, 121.8, 119.0, 119.0, 115.8, 45.9, 36.8, 34.6, 30.9. 19F NMR (376 MHz, CDCl3) δ −61.84, −63.06. HRMS (ESI): Exact mass calculated for C26H22F9N2O2 [M + H]+: 565.1533, found: 565.1534.
4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-N-(p-tolyl)piperidine-1-carboxamide (9h): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 8h (172 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9h (29 mg, 18%). Beige solid. mp—203–205 °C. FTIR (cm−1): 3301, 2915, 1709, 1630, 1592, 1514, 1477, 1417, 1239, 1080, 993, 810. 1H NMR (400 MHz, CDCl3) δ 8.34 (dd, J = 4.8, 1.7 Hz, 1H), 7.37 (dd, J = 7.7, 1.7 Hz, 1H), 7.20–7.08 (m, 4H), 7.08–7.03 (m, 3H), 7.03–6.97 (m, 2H), 6.27 (s, 1H), 3.74–3.64 (m, 2H), 3.30 (dtd, J = 16.1, 8.7, 7.7, 3.9 Hz, 2H), 3.17 (ddt, J = 12.8, 9.2, 3.8 Hz, 2H), 2.85–2.68 (m, 2H), 2.55 (ddd, J = 14.2, 9.1, 4.5 Hz, 1H), 2.40 (ddd, J = 13.9, 9.1, 4.6 Hz, 1H), 2.37–2.26 (m, 3H), 2.21 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 156.9, 155.0, 146.7, 139.5, 137.6, 136.9, 136.4, 134.5, 133.3, 133.0, 132.6, 130.5, 129.4, 129.2, 129.0, 127.4, 126.2, 122.3, 120.1, 60.4, 44.9, 44.8, 31.7, 31.5, 30.5, 30.3, 20.7, 14.2. HRMS (ESI): Exact mass calculated for C27H27ClN3O [M + H]+: 444.1843, found: 444.1847.
N-(3,5-bis(trifluoromethyl)phenyl)-4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidine-1-carboxamide (9h′): General procedure was followed using 1’ (95 mg, 0.37 mmol, 1.0 equiv.), 8h (172 mg, 0.74 mmol, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9h′ (121 mg, 58%). Brown sticky solid. FTIR (cm−1): 3314, 1645, 1550, 1439, 1368, 1273, 1220, 1167, 1121, 990, 880, 827, 726, 699, 680. 1H NMR (400 MHz, CDCl3) δ 8.40 (dd, J = 4.9, 1.7 Hz, 1H), 7.81 (d, J = 1.6 Hz, 2H), 7.49 (dd, J = 7.7, 1.7 Hz, 1H), 7.42 (d, J = 3.4 Hz, 2H), 7.18–7.07 (m, 4H), 3.80 (dq, J = 11.2, 5.3, 4.8 Hz, 2H), 3.43–3.22 (m, 4H), 2.92–2.76 (m, 2H), 2.58 (ddd, J = 13.9, 9.1, 4.5 Hz, 1H), 2.50–2.29 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 156.6, 154.3, 146.2, 141.0, 139.4, 138.0, 137.1, 136.6, 134.2, 133.7, 133.1, 132.2, 131.9, 131.6, 131.3, 130.4, 129.1, 126.2, 124.5, 122.6, 121.8, 119.4, 115.7, 115.6, 44.8, 44.7, 31.6, 31.3, 30.9, 30.4, 30.2. HRMS (ESI): Exact mass calculated for C28H23ClF6N3O [M + H]+: 566.1429, found: 566.1425.
4-(2-chlorodibenzo[b,f][1,4]oxazepin-11-yl)-N-(p-tolyl)piperazine-1-carboxamide (9i): General procedure was followed using 1a (85 mg, 0.62 mmol, 2.0 equiv.), 8i (100 mg, 0.31 mmol, 1 equiv.), PhI(OAc)2 (256 mg, 2 equiv.), K3PO4 (135 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9i (46 mg, 28%). Orange solid. Mp—205–207 °C. FTIR (cm−1): 3292, 2917, 2849, 1638, 1585, 1508, 1459, 1397, 1233, 1100, 1013, 987, 807, 770. 1H NMR (400 MHz, CDCl3) δ 7.34 (dd, J = 8.6, 2.6 Hz, 1H), 7.25 (d, J = 2.6 Hz, 1H), 7.16 (dd, J = 12.9, 4.5 Hz, 3H), 7.13–7.06 (m, 3H), 7.06–6.98 (m, 6H), 6.94 (td, J = 7.5, 1.8 Hz, 2H), 6.35 (s, 1H), 3.61–3.42 (m, 8H), 2.22 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 158.3, 157.6, 154.3, 150.7, 138.8, 135.0, 132.0, 131.7, 129.4, 128.6, 128.4, 127.8, 126.0, 124.8, 123.9, 123.7, 121.8, 119.4, 119.1, 46.1, 42.7, 19.7. HRMS (ESI): Exact mass calculated for C25H24ClN4O2 [M + H]+: 447.1583, found: 447.1588.
N-(3,5-bis(trifluoromethyl)phenyl)-4-(2-chlorodibenzo[b,f][1,4]oxazepin-11-yl)piperazine-1-carboxamide (9i′): General procedure was followed using 1′ (95 mg, 0.62 mmol, 2.0 equiv.), 8i (174 mg, 0.31 mmol, 2 equiv.), PhI(OAc)2 (256 mg, 2 equiv.), K3PO4 (135 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9i’ (210 mg, 59%). Yellow sticky solid. FTIR (cm−1): 3320, 1654, 1598, 1552, 1468, 1360, 1273, 1169, 1123, 1015, 878, 827, 775, 727, 702, 679. 1H NMR (400 MHz, CDCl3) δ 7.99 (s, 1H), 7.89 (s, 1H), 7.53–7.46 (m, 2H), 7.44–7.39 (m, 1H), 7.34 (dd, J = 17.1, 2.6 Hz, 1H), 7.20 (dd, J = 8.6, 1.9 Hz, 1H), 7.18–7.07 (m, 3H), 7.03 (dt, J = 7.7, 1.8 Hz, 1H), 5.11 (s, 1H), 3.82 (s, 1H), 3.63 (d, J = 35.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 159.3, 159.1, 158.7, 156.1, 154.6, 152.5, 151.8, 151.7, 140.7, 140.4, 139.7, 139.5, 133.0, 132.9, 132.1, 131.8, 130.5, 130.5, 128.8, 128.4, 127.1, 127.0, 126.0, 125.9, 125.2, 125.1, 124.6, 124.5, 122.8, 122.6, 120.3, 120.2, 119.6, 119.4, 119.3, 62.6, 47.2, 44.3, 43.7. HRMS (ESI): Exact mass calculated for C26H20ClF6N4O2 [M + H]+: 569.1174, found: 569.1178.
4-(pyrimidin-2-yl)-N-(p-tolyl)piperazine-1-carboxamide (9j): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 8j (91 mg, 0.74 mmol, 5 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9j (24 mg, 22%). Yellow solid. mp—167–169 °C. FTIR (cm−1): 3305, 2902, 2862, 1632, 1581, 1512, 1470, 1412, 1355, 1306, 1288, 1239, 1080, 978, 792. 1H NMR (400 MHz, CDCl3) δ 8.26 (d, J = 4.7 Hz, 2H), 7.20–7.14 (m, 2H), 7.02 (d, J = 8.3 Hz, 2H), 6.47 (t, J = 4.7 Hz, 1H), 6.37 (s, 1H), 3.86–3.78 (m, 4H), 3.55–3.46 (m, 4H), 2.22 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 160.4, 156.7, 154.3, 135.1, 131.8, 128.4, 119.3, 109.3, 42.7, 42.3, 19.7. HRMS (ESI): Exact mass calculated for C16H19N5O [M+Na]+: 320.1482, found: 320.1490.
N-(3,5-bis(trifluoromethyl)phenyl)-4-(pyrimidin-2-yl)piperazine-1-carboxamide (9j′): General procedure was followed using 1’ (95 mg, 0.37 mmol, 1.0 equiv.), 8j (91 mg, 0.74 mmol, 1.5 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9j’ (83 mg, 66%). Yellow solid. mp—159–161 °C. FTIR (cm−1): 3327, 2925, 2858, 1642, 1583, 1550, 1466, 1439, 1373, 1271, 1240, 1165, 1116, 997, 979, 944, 886, 796, 701, 680. 1H NMR (400 MHz, CDCl3) δ 8.39 (d, J = 4.8 Hz, 2H), 7.98–7.95 (m, 2H), 7.53 (s, 1H), 6.71 (s, 1H), 6.67 (t, J = 4.8 Hz, 1H), 5.11 (s, 2H), 4.01–3.88 (m, 4H), 1.25 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 159.2, 158.0, 152.0, 140.1, 132.7, 132.4, 132.0, 131.7, 124.5, 121.8, 119.1, 116.5, 111.3, 61.1, 44.3. 19F NMR (376 MHz, CDCl3) δ −63.04. HRMS (ESI): Exact mass calculated for C17H16F6N5O [M + H]+: 420.1254, found: 420.1264.
N-(p-tolyl)-3-(3,4,5-trimethoxybenzamido)piperidine-1-carboxamide (9k): General procedure was followed using 1a (91 mg, 0.68 mmol, 2.0 equiv.), 8k (100 mg, 0.34 mmol, 1 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9k (42 mg, 27%). Colorless solid. mp—129–131 °C. FTIR (cm−1): 3312, 2937, 1627, 1579, 1523, 1499, 1408, 1337, 1233, 1126, 1084, 989, 838, 807. 1H NMR (400 MHz, CDCl3) δ 7.20–7.15 (m, 3H), 6.97 (d, J = 7.4 Hz, 4H), 6.85 (s, 1H), 4.03–3.97 (m, 1H), 3.83–3.79 (m, 2H), 3.79 (s, 3H), 3.74 (s, 6H), 3.59–3.47 (m, 1H), 3.41–3.27 (m, 2H), 2.21 (s, 3H), 1.84–1.63 (m, 2H), 1.56–1.43 (m, 1H), 0.88–0.75 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 174.5, 167.3, 156.8, 153.1, 140.8, 136.2, 132.9, 129.4, 129.2, 120.3, 104.3, 60.8, 56.1, 48.8, 47.0, 46.2, 28.9, 22.2, 20.7. HRMS (ESI): Exact mass calculated for C23H29N3O5 [M+Na]+: 450.2000, found: 450.2005.
N-(3,5-bis(trifluoromethyl)phenyl)-3-(3,4,5-trimethoxybenzamido)piperidine-1-carboxamide (9k’): General procedure was followed using 1’ (95 mg, 0.68 mmol, 2.0 equiv.), 8k (100 mg, 0.34 mmol, 1 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9k’ (203 mg, 46%). Beige solid. mp—163–165 °C. FTIR (cm−1): 3312, 2937, 1627, 1579, 1523, 1499, 1408, 1337, 1233, 1126, 1084, 989, 838, 807. 1H NMR (400 MHz, CDCl3) δ 1H NMR (400 MHz, CDCl3) δ 7.95 (s, 2H), 7.70 (s, 1H), 7.45 (s, 1H), 6.94 (s, 2H), 6.47 (d, J = 5.1 Hz, 1H), 4.02 (s, 1H), 3.85 (s, 3H), 3.84 (s, 6H), 3.71–3.50 (m, 4H), 2.06–1.88 (m, 3H), 1.82 (dd, J = 8.8, 4.3 Hz, 2H), 1.76–1.62 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 168.0, 155.3, 153.2, 141.2, 141.1, 132.3, 131.9, 131.6, 131.3, 129.0, 127.3, 124.6, 121.8, 119.1, 119.0, 115.7, 115.7, 115.6, 104.3, 60.8, 56.1, 48.9, 47.3, 45.2, 30.9, 29.3, 22.5. 19F NMR (376 MHz, CDCl3) δ -63.02. HRMS (ESI): Exact mass calculated for C24H26F6N3O5 [M + H]+: 550.1772, found: 550.1780.
4-(benzo[d]isothiazol-3-yl)-N-(p-tolyl)piperazine-1-carboxamide (9l): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 8l (121 mg, 0.55 mmol, 1.5 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9l (16 mg, 12%). Yellow solid. mp—172–174 °C. FTIR (cm−1): 3354, 2842, 1636, 1592, 1517, 1481, 1412, 1370, 1235, 1018, 996, 807, 730. 1H NMR (400 MHz, CDCl3) δ 7.84 (dt, J = 8.2, 1.0 Hz, 1H), 7.76 (dd, J = 8.1, 1.0 Hz, 1H), 7.42 (ddd, J = 8.2, 6.9, 1.1 Hz, 1H), 7.32 (ddd, J = 8.0, 6.9, 1.0 Hz, 1H), 7.21–7.16 (m, 2H), 7.04 (d, J = 8.3 Hz, 2H), 6.33 (s, 1H), 3.70–3.62 (m, 4H), 3.57–3.49 (m, 4H), 2.23 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 163.4, 155.3, 152.8, 136.1, 132.9, 129.4, 127.8, 127.7, 124.1, 123.6, 120.6, 120.3, 49.7, 43.8, 20.7. HRMS (ESI): Exact mass calculated for C19H20N4OS [M+Na]+: 375.1251, found: 375.1255.
4-(benzo[d]isothiazol-3-yl)-N-(3,5-bis(trifluoromethyl)phenyl)piperazine-1-carboxamide (9l′): General procedure was followed using 1’ (95 mg, 0.37 mmol, 1.0 equiv.), 8l (121 mg, 0.55 mmol, 1.5 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9l′ (63 mg, 36%). Yellow sticky solid. FTIR (cm−1): 3309, 1654, 1561, 1505, 1472, 1439, 1357, 1273, 1167, 1116, 1000, 880, 729, 700, 678. 1H NMR (400 MHz, CDCl3) δ 7.99 (d, J = 8.3 Hz, 1H), 7.97 (s, 2H), 7.80 (d, J = 8.2 Hz, 1H), 7.51–7.46 (m, 2H), 7.38–7.33 (m, 1H), 7.24 (s, 1H), 5.27 (s, 2H), 4.14 (dd, J = 7.2, 6.0 Hz, 2H), 3.86 (t, J = 6.6 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 159.2, 153.2, 152.5, 140.3, 132.5, 132.2, 131.9, 131.5, 128.0, 126.9, 124.5, 124.4, 123.5, 121.8, 120.5, 119.3, 119.3, 116.3, 116.3, 116.3, 64.1, 48.4, 44.0, 30.9. 19F NMR (376 MHz, CDCl3) δ -63.08. HRMS (ESI): Exact mass calculated for C20H17F6N4OS [M + H]+: 475.1022, found: 475.1024.
4-((5,6-dimethoxy-1-oxo-2,3-dihydro-1H-inden-2-yl)methyl)-N-(p-tolyl)piperidine-1-carboxamide (9m): General procedure was followed using 1a (50 mg, 0.37 mmol, 1.0 equiv.), 8m (214 mg, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9m (55 mg, 35%). Yellow solid. mp—160–162 °C. FTIR (cm−1): 3330, 2913, 2842, 1683, 1638, 1590, 1497, 1308, 1259, 1239, 1111, 1031, 962, 807, 723. 1H NMR (400 MHz, CDCl3) δ 7.24 (d, J = 8.4 Hz, 2H), 7.16 (s, 1H), 7.07 (d, J = 8.3 Hz, 2H), 6.87 (s, 1H), 6.50 (s, 1H), 4.14–4.05 (m, 2H), 3.97 (s, 3H), 3.90 (s, 3H), 3.27 (dd, J = 17.6, 8.2 Hz, 1H), 2.93–2.82 (m, 2H), 2.71 (dt, J = 12.8, 3.7 Hz, 2H), 2.28 (s, 3H), 2.17 (s, 1H), 1.97–1.88 (m, 1H), 1.85–1.69 (m, 3H), 1.42–1.32 (m, 1H), 1.27 (tdt, J = 9.5, 4.1, 1.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 207.4, 155.6, 155.2, 149.5, 148.7, 136.6, 132.4, 129.3, 129.1, 120.1, 107.3, 104.3, 56.2, 56.1, 45.1, 44.5, 38.6, 34.5, 33.4, 32.6, 31.6, 20.7. HRMS (ESI): Exact mass calculated for C25H31N2O4 [M + H]+: 423.2279, found: 423.2279.
N-(3,5-bis(trifluoromethyl)phenyl)-4-((5,6-dimethoxy-1-oxo-2,3-dihydro-1H-inden-2-yl)methyl)piperidine-1-carboxamide (9m’): General procedure was followed using 1’ (50 mg, 0.37 mmol, 1.0 equiv.), 8m (214 mg, 2 equiv.), PhI(OAc)2 (238 mg, 2 equiv.), K3PO4 (157 mg, 2 equiv.), 1,2-DCE (2 mL), at 80 °C for 18 h. Column chromatography was performed using 85/15 v/v petroleum ether/acetone as eluent and yielded 9m’ (144 mg, 72%). Colorless solid. mp—202–204 °C. FTIR (cm−1): 3300, 1684, 1638, 1539, 1497, 1472, 1439, 1375, 1304, 1276, 1169, 1121, 1068, 1024, 861, 836, 703, 680. 1H NMR (400 MHz, CDCl3) δ 7.92 (s, 2H), 7.74 (s, 1H), 7.43 (s, 1H), 7.13 (s, 1H), 6.88 (s, 1H), 4.25–4.15 (m, 2H), 3.97 (s, 3H), 3.88 (s, 3H), 3.28 (dd, J = 17.6, 8.0 Hz, 1H), 2.91 (td, J = 13.3, 12.7, 2.4 Hz, 2H), 2.77–2.67 (m, 2H), 1.95–1.72 (m, 4H), 1.43–1.16 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 207.8, 155.8, 154.4, 149.6, 149.0, 141.3, 132.2, 131.9, 131.6, 131.2, 128.9, 124.6, 121.9, 119.4, 119.3, 115.5, 107.4, 104.2, 56.2, 56.0, 45.0, 44.5, 44.5, 38.5, 34.3, 33.3, 32.6, 31.6. 19F NMR (376 MHz, CDCl3) δ −62.99. HRMS (ESI): Exact mass calculated for C26H27F6N2O4 [M + H]+: 545.1870, found: 545.1873.
4. Conclusions
In summary, we have developed a highly efficient method for synthesizing unsymmetrical ureas from benzamides and primary and secondary amines via a hypervalent iodine-mediated Hofmann-type rearrangement. This method exhibits broad functional group tolerance on both coupling partners and demonstrates compatibility with water, making it a valuable tool for generating a wide variety of unsymmetrical ureas. Additionally, this protocol can be effectively applied to the late-stage modification of various important drug structures, underscoring its potential utility in drug development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29235669/s1, Optimization Table S1: NMR spectra (1H NMR, 13C NMR, & 19F NMR), LC-MS study of crude reaction mixture revealing acetic acid release. Table S2: Optimization of base.
Author Contributions
Conceptualization, S.K.; methodology, S.K., P.D. and T.Z.; formal analysis, S.K. and P.D.; writing—original draft preparation, S.K.; writing—review and editing, S.K., P.D. and I.A.N.; supervision, S.K. and I.A.N.; funding acquisition, S.K. and I.A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swedish Research Council, grant numbers 2014-4573 and 2023-03406, the Helge Axon Johnsons Foundation, grant numbers 2019-0318 and 2022-0317, and the Crafoord Foundation, grant numbers 2019-0925 and 2020-0775.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data associated with this article can be obtained from Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ghosh, A.K.; Brindisi, M. Urea derivatives in modern drug discovery and medicinal chemistry. J. Med. Chem. 2020, 63, 2751–2788. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Q.; Lv, P.-C.; Yan, T.; Zhu, H.-L. Urea derivatives as anticancer agents. Anti-Cancer Agents Med. Chem. 2009, 9, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit-Kaymakcioglu, B.; Clene, A.O.; Tabanca, N.; Ali, A.; Khan, S.I.; Khan, I.A.; Wedge, D.E. Synthesis and biological activity of substituted urea and thiourea derivatives containing 1,2,4-triazoles. Molecules 2013, 18, 3562–3576. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P.M.S.; Dhar, K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014, 77, 422–487. [Google Scholar] [CrossRef]
- Manickam, M.; Pillaiyar, T.; Boggu, P.; Venkateswararao, E.; Jalani, H.B.; Kim, N.-D.; Lee, S.K.; Jeon, J.S.; Kim, S.K.; Jung, S.-H. Discovery of enantioselectivity of urea inhibitors of soluble epoxide hydrolase. Eur. J. Med. Chem. 2016, 117, 113–124. [Google Scholar] [CrossRef]
- Jagtap, A.D.; Kondekar, N.B.; Sadani, A.A.; Chern, J.W. Ureas: Application in drug design. Curr. Med. Chem. 2017, 24, 622–651. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.; Liu, C.; Yang, X.; Dkeyser, M. Application of the intermediate derivatization approach in agrochemical discovery. Chem. Rev. 2014, 114, 7079–7107. [Google Scholar] [CrossRef]
- Kishida, T.; Fujita, N.; Sada, K.; Shinkai, S. Phorphyrin gels reinforced by sol-gel reaction via the organogel phase. Langmuir 2005, 21, 9432–9439. [Google Scholar] [CrossRef]
- Boije af Gennas, G.; Mologni, L.; Ahmed, S.; Rajaratnam, M.; Marin, O.; Lindholm, N.; Viltadi, M.; Gambacorti-Passerini, C.; Scapozza, L.; Yli-Kauhaluoma, J. Design, synthesis and biological activity of urea derivatives as anaplastic lymphoma kinase inhibitors. ChemMedChem 2011, 6, 1680–1692. [Google Scholar] [CrossRef]
- Lam, P.Y.; Jadhav, P.K.; Dyermann, C.J.; Hodge, C.N.; Ru, Y.; Bacheler, L.T.; Meek, J.L.; Otto, M.J.; Rayner, M.M.; Wong, Y.N.; et al. Rational design of potent, bioavailable, nonpeptide cyclic ureas as HIV protease inhibitors. Science 1994, 263, 380–384. [Google Scholar] [CrossRef]
- Batisan, A.A.; Marcozzi, A.; Herrmann, A. Selective transformations of complex molecules are enabled by aptameric protective groups. Nat. Chem. 2012, 11, 789–793. [Google Scholar]
- Santella, J.B.; Gardner, D.S.; Duncia, J.V.; Wu, H.; Dhar, M.; Cavallaro, C.; Tebben, A.J.; Carter, P.H.; Barrish, J.C.; Yarde, M.; et al. Discovery of the CCR1 antagonist, BMS-817399 for the treatment of Rheumatoid arthritis. J. Med. Chem. 2014, 57, 7550–7564. [Google Scholar] [CrossRef] [PubMed]
- Fotsch, C.; Sonnenberg, J.D.; Chen, N.; Hale, C.; Karbon, W.; Norman, M.H. Synthesis and structure activity relationships of trisubstituted phenyl urea derivatives as neuro peptide Y5 receptor antagonists. J. Med. Chem. 2001, 44, 2344–2356. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, S.; Tan, Q.; Gao, M.; Li, M.; Xu, B. A copper-mediated tandem reaction through isocyanide insertion into N-H bonds: Efficient access to unsymmetrical tetrasubstituted ureas. Chem. Commun. 2014, 50, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Angyal, A.; Demjen, A.; Wolfling, J.; Puskas, L.G.; Kanizsai, I. A green, isocyanide-based three-component reaction approach for the synthesis of multisubstituted ureas and thioureas. Tetrahedron Lett. 2018, 59, 54–57. [Google Scholar] [CrossRef]
- Hegarty, A.F.; Drennan, L.J. Comprehensive Organic Functional Group Transformations; Katritzky, A.R., Meth-Cohn, O., Rees, C.W., Eds.; Pergamon: Oxford, UK, 1995; Volume 6, pp. 499–526. [Google Scholar]
- Ren, Y.; Rousseaux, S. Metal-free synthesis of unsymmetrical ureas and carbamates from CO2 and amines via isocyanate intermediates. J. Org. Chem. 2018, 83, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, M.; Nowrouzi, N. Palladium-catalyzed synthesis of symmetrical and unsymmetrical ureas using chromium hexacarbonyl as a convenient and safe alternative carbonyl source. Eur. J. Org. Chem. 2019, 2019, 7541–7544. [Google Scholar] [CrossRef]
- Suh, S.-E.; Nkulu, L.E.; Lin, S.; Krska, S.W.; Stahl, S.S. Benzylic C-H isocyanation/amine coupling sequence enabling high throughput synthesis of pharmaceutically relevant ureas. Chem. Sci. 2021, 12, 10380–10387. [Google Scholar] [CrossRef]
- Lane, E.M.; Hazari, N.; Bernskoetter, W.H. Iron-catalyzed urea synthesis: Dehydrogenative coupling of methanol and amines. Chem. Sci. 2018, 9, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Zhdankin, V.V. Recent progress in synthetic applications of hypervalent iodine(III) reagents. Chem. Rev. 2024, 124, 11108–11186. [Google Scholar] [CrossRef]
- Kiyokawa, K.; Okumatsu, D.; Minakata, S. Synthesis of hypervalent iodine(III) reagents containing a transferable (Diarylmethylene)amino group and their use in the oxidative amination of silyl ketene acetals. Angew. Chem. Int. Ed. 2019, 58, 8907–8911. [Google Scholar] [CrossRef] [PubMed]
- Allouche, E.M.D.; Grinhagena, E.; Waser, J. Hypervalent iodine-mediated late-stage peptide and protein functionalization. Angew. Chem. Int. Ed. 2022, 61, e202112287. [Google Scholar] [CrossRef] [PubMed]
- Souto, J.A.; Martinez, C.; Velilla, I.; Muniz, K. Defined hypervalent iodine(III) reagents incorporating transferable nitrogen groups: Nucleophilic amination through electrophilic activation. Angew. Chem. Int. Ed. 2013, 52, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, J.; Cho, S.H.; Chang, S. Intermolecular oxidative C-N bond formation under metal-free conditions: Control of chemoselectivity between aryl sp2 and benzylic sp3 C-H bond imidation. J. Am. Chem. Soc. 2011, 133, 16382–16385. [Google Scholar] [CrossRef] [PubMed]
- Souto, J.A.; Becker, P.; Iglesias, A.; Muniz, K. Meta-free iodine(III) promoted direct intermolecular C-H amination reactions of acetylenes. J. Am. Chem. Soc. 2012, 134, 15505–15511. [Google Scholar] [CrossRef] [PubMed]
- Rosa, N.S.; Glachet, T.; Ibert, Q.; Lohier, J.-F.; Franck, X.; Reboul, V. A straightforward synthesis of N-substituted ureas from primary amides. Synthesis 2020, 52, 2099–2105. [Google Scholar]
- Hohenadel, M.; Ebel, B.; Oppel, I.M.; Patureau, F.W. Oxidative N-N bond formation versus the Curtius rearrangement. Chem. Eur. J. 2024, 30, e202402355. [Google Scholar] [CrossRef] [PubMed]
- Polenz, I.; Laue, A.; Uhrin, T.; Ruffer, T.; Lang, H.; Schmidt, F.G.; Spange, S. Thermally cleavable imine base/isocyanate adducts and oligomers suitable as initiators for radical homo and copolymerization. Poly. Chem. 2014, 5, 6678–6686. [Google Scholar] [CrossRef]
- Nair, A.S.; Singh, A.K.; Kumra, A.; Kumar, S.; Sukumaran, S.; Koyiparambath, V.P.; Pappachen, L.K.; Rangarajan, T.M.; Kim, H.; Mathew, B. FDA-approved trifluoromethyl group-containing drugs: A review of 20 years. Processes 2022, 10, 2054. [Google Scholar] [CrossRef]
- Castellino, N.J.; Montgomery, A.P.; Danon, J.J.; Kassiou, M. Late-stage functionalization for improving drug-like molecular properties. Chem. Rev. 2023, 123, 8127–8153. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Sang, R.; Ham, W.S.; Plutschack, M.B.; Berger, F.; Chabbara, S.; Schnegg, A.; Genicot, C.; Ritter, T. Photoredox catalysis with aryl sulfonium salts enables site-selective lage-stage fluorination. Nat. Chem. 2019, 12, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Guillemard, L.; Kaplaneris, N.; Ackermann, L.; Johansson, M.J. Late-stage C-H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 2021, 5, 522–545. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).