Organometallic Chemistry of Propargylallenes: Syntheses, Reactivity, Molecular Rearrangements and Future Prospects
Abstract
1. Introduction
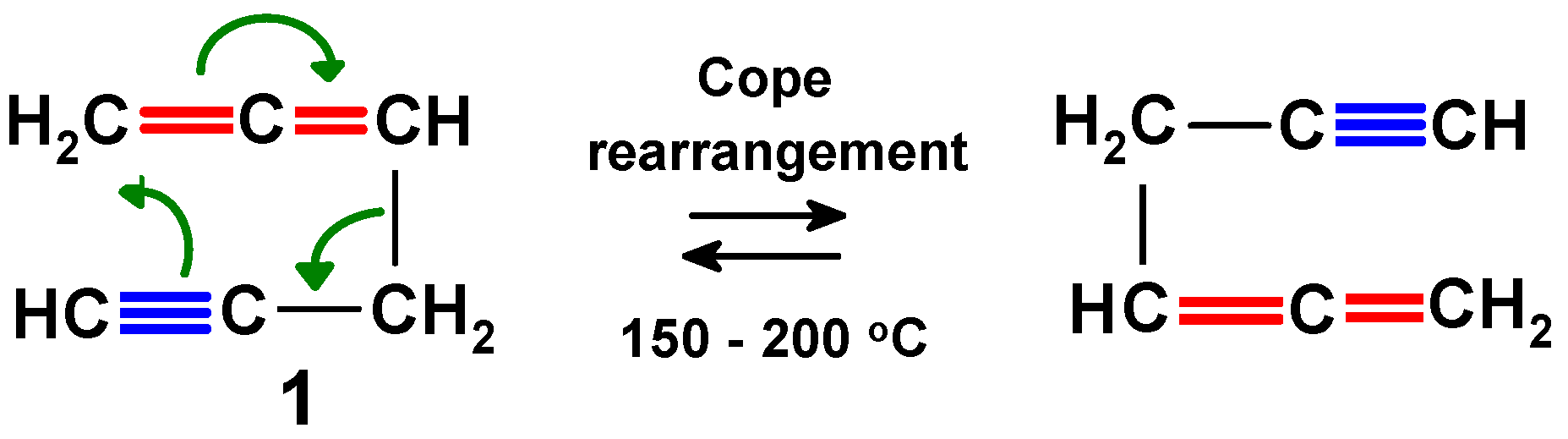
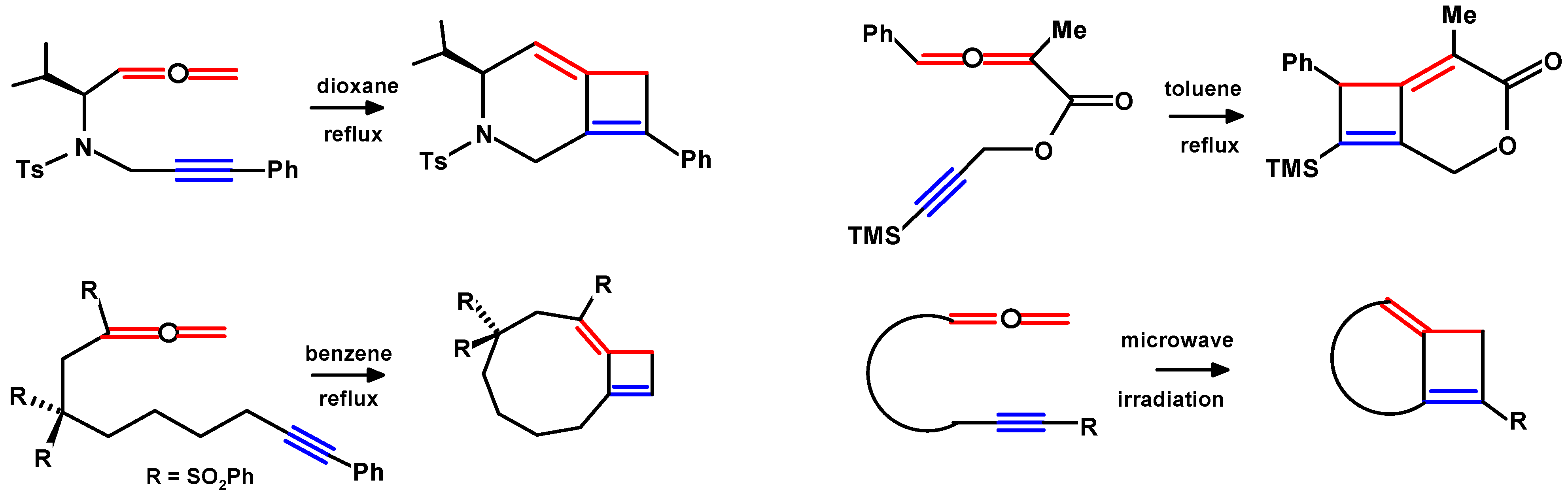
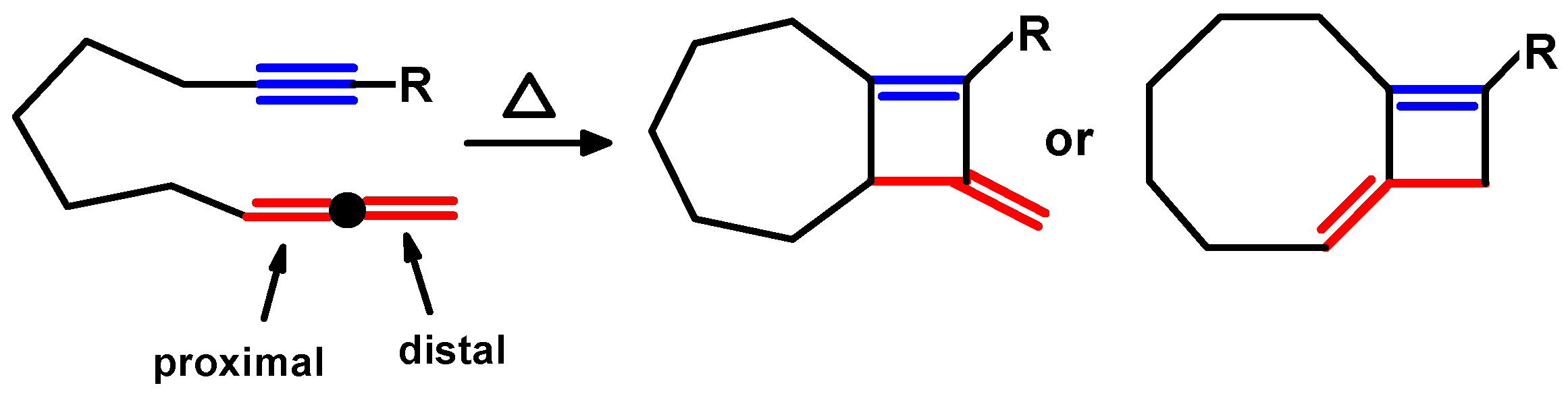
2. Thermal Cycloadditions and Rearrangements of Alkynylallenes
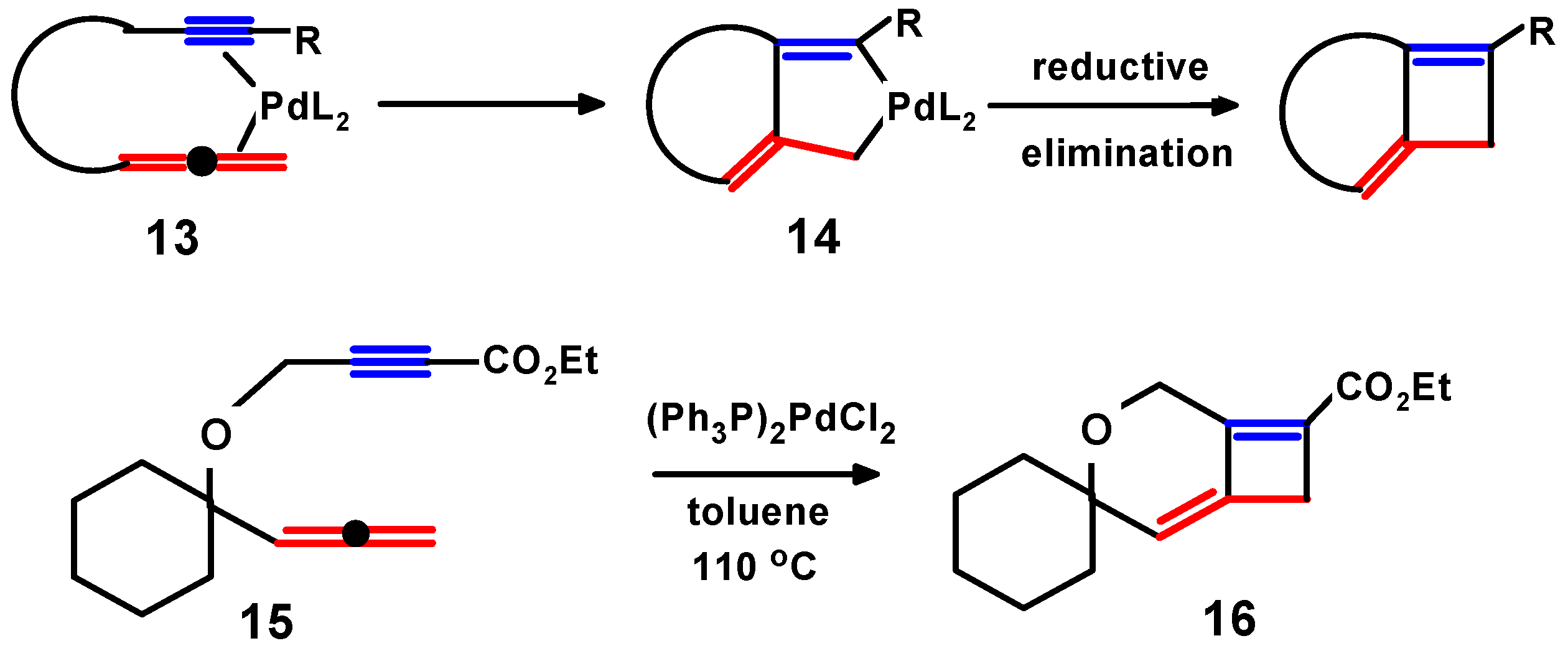
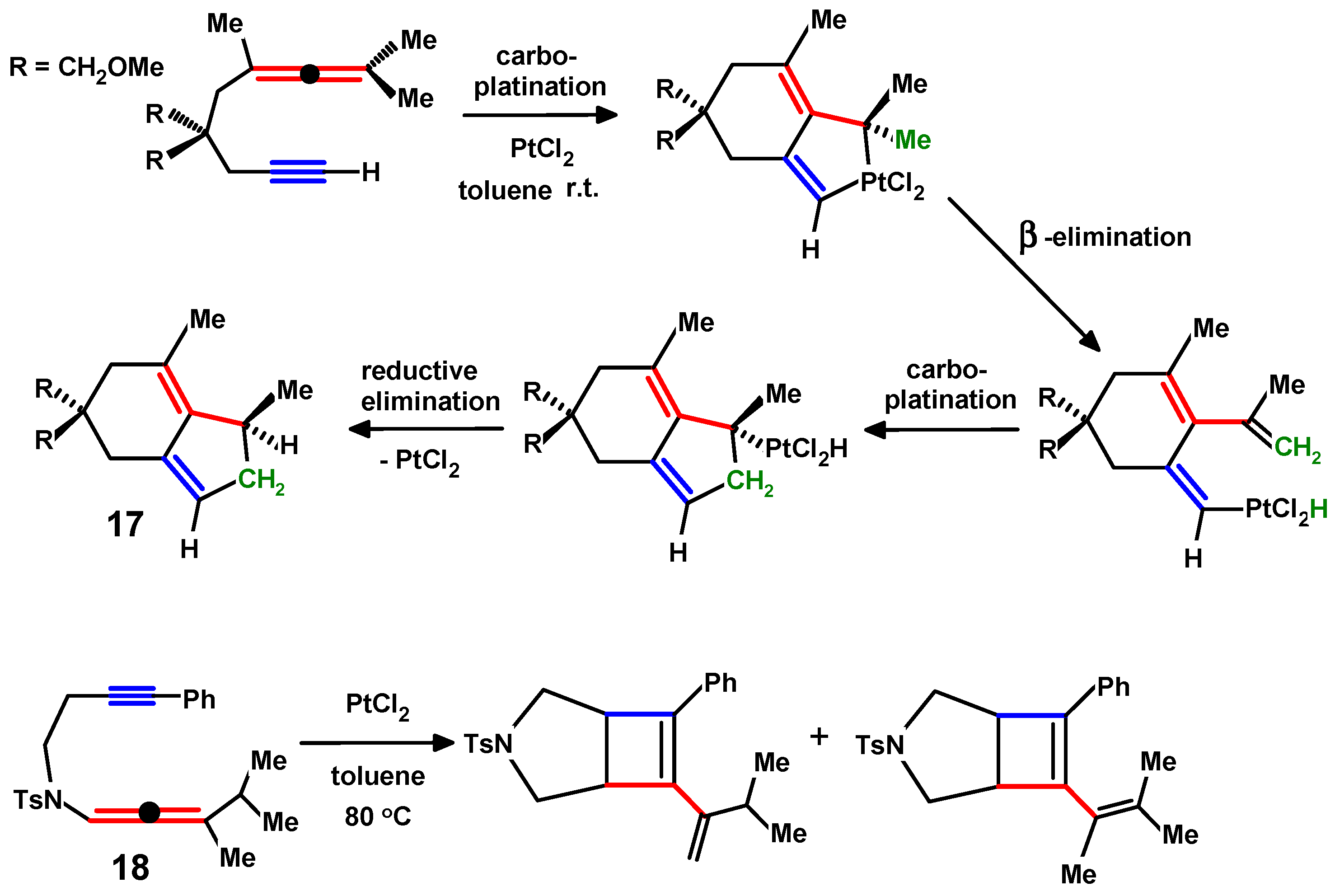
3. Metal-Mediated Cyclisations of 1,2-Dien-n-ynes
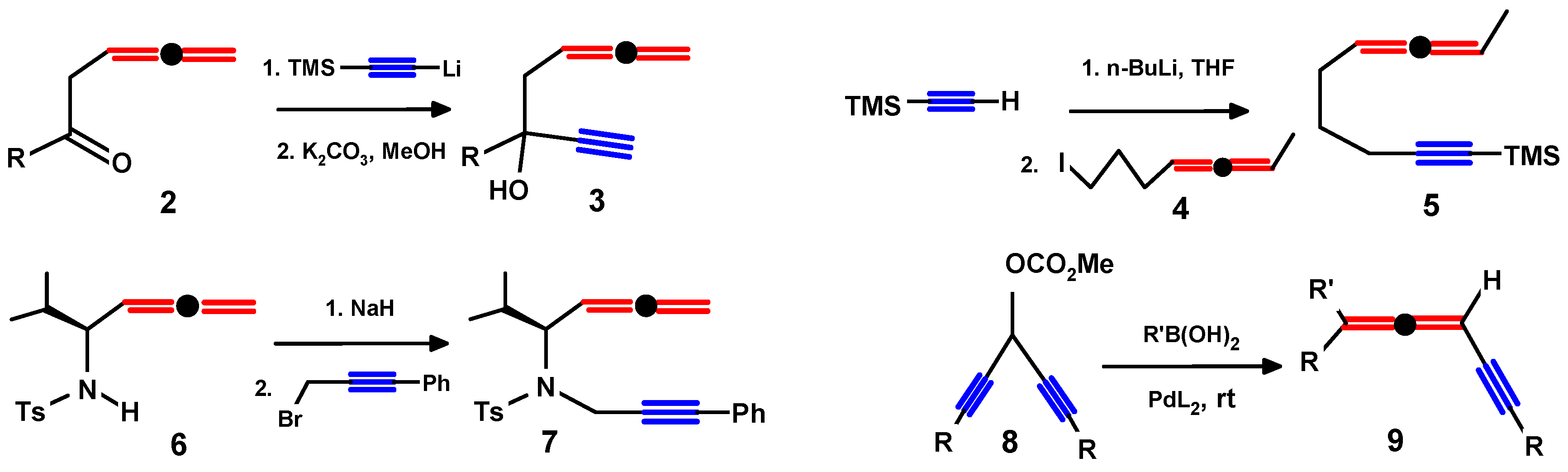
4. Propargylallenes: Syntheses and Reactivity
4.1. Propargylallene: Structure and Spectroscopy
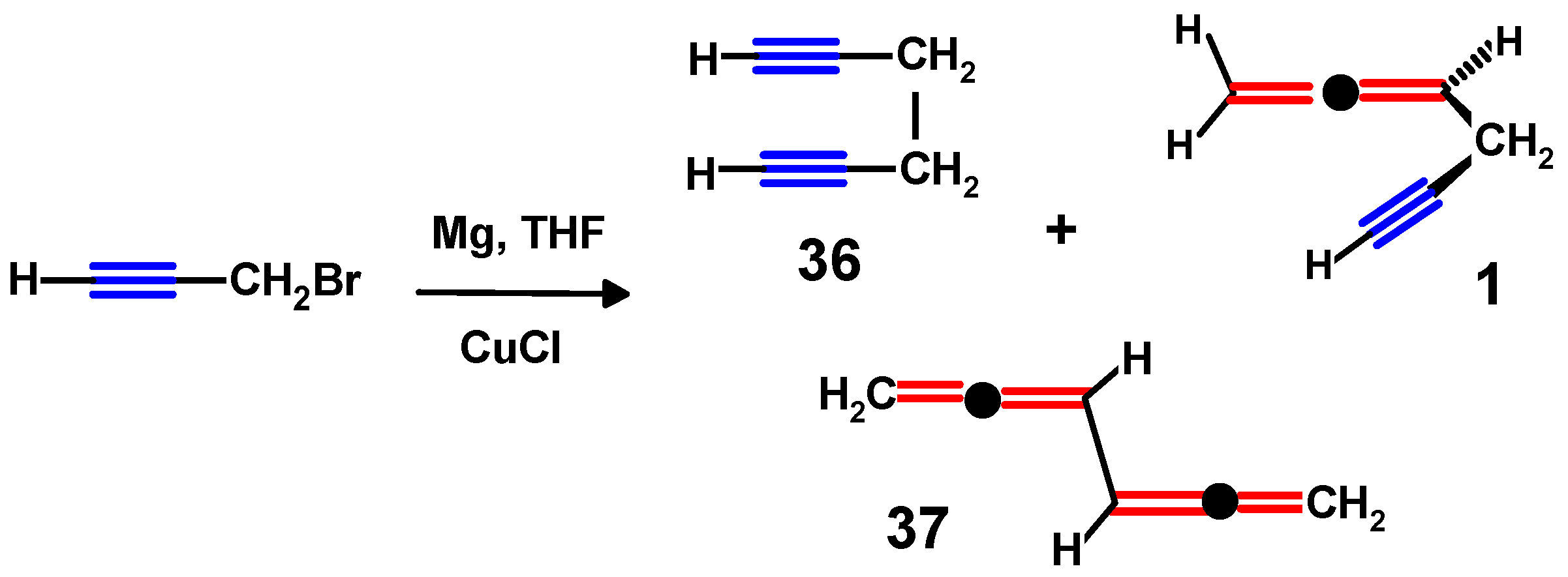
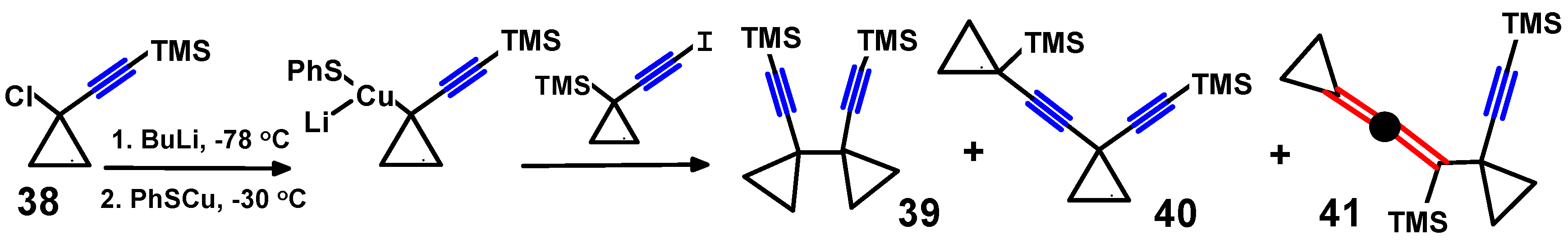
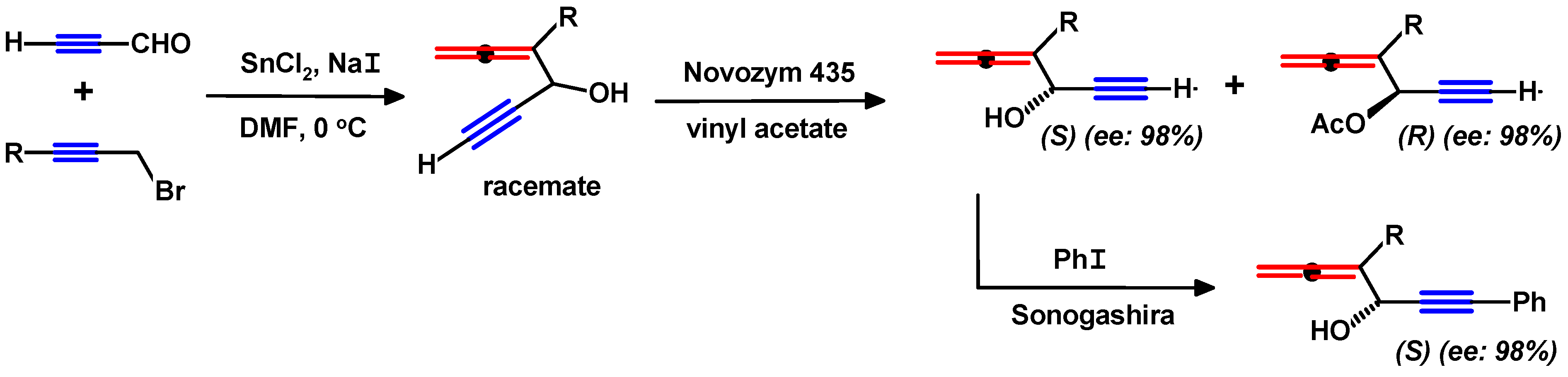
4.2. Syntheses of Propargylallenes
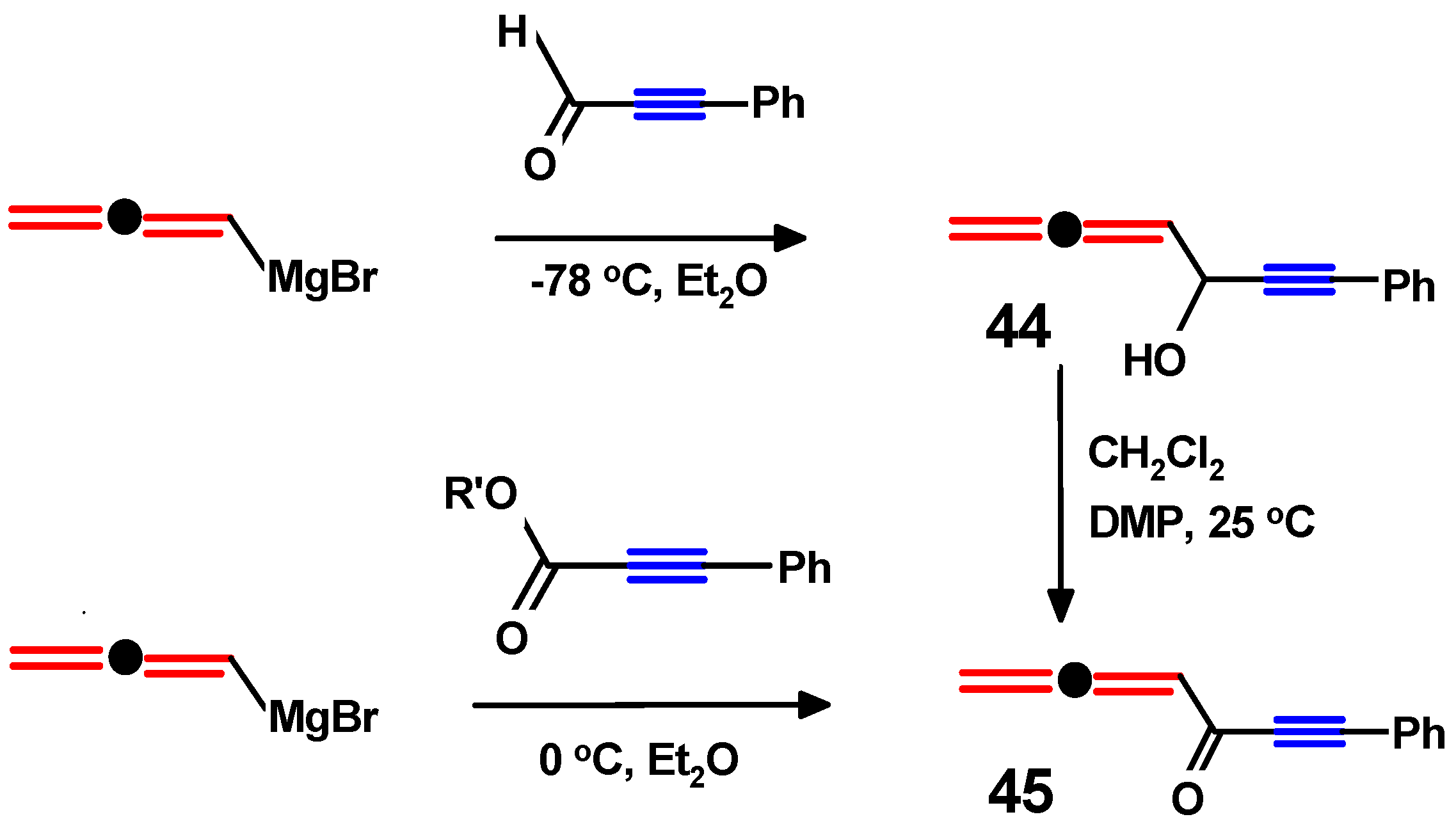
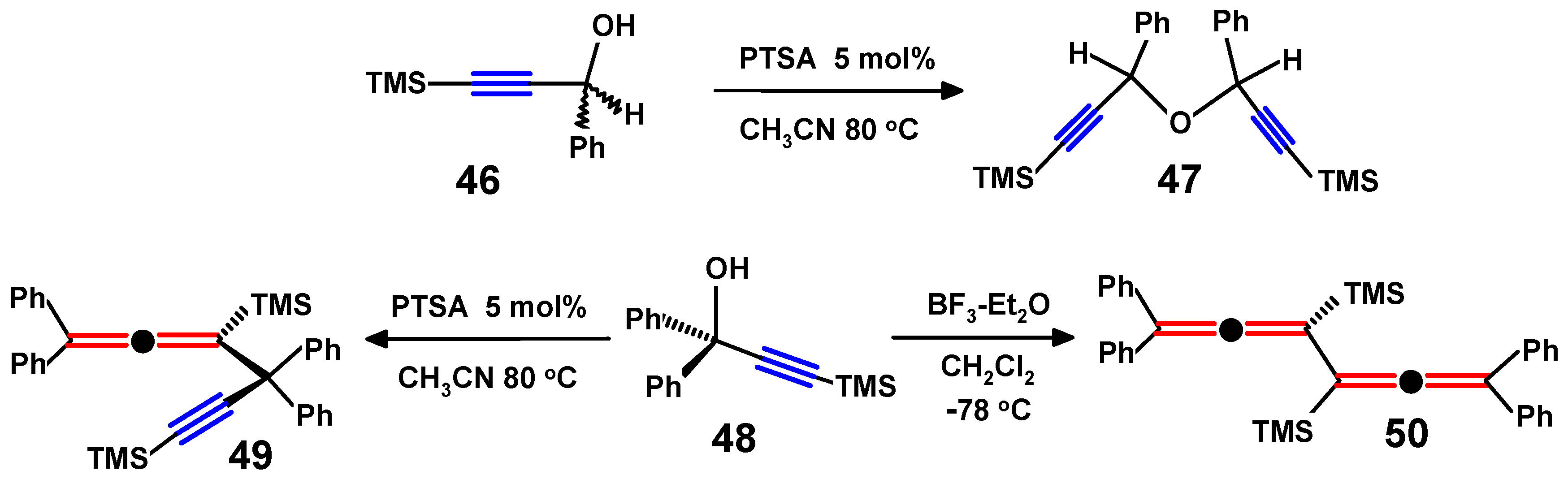
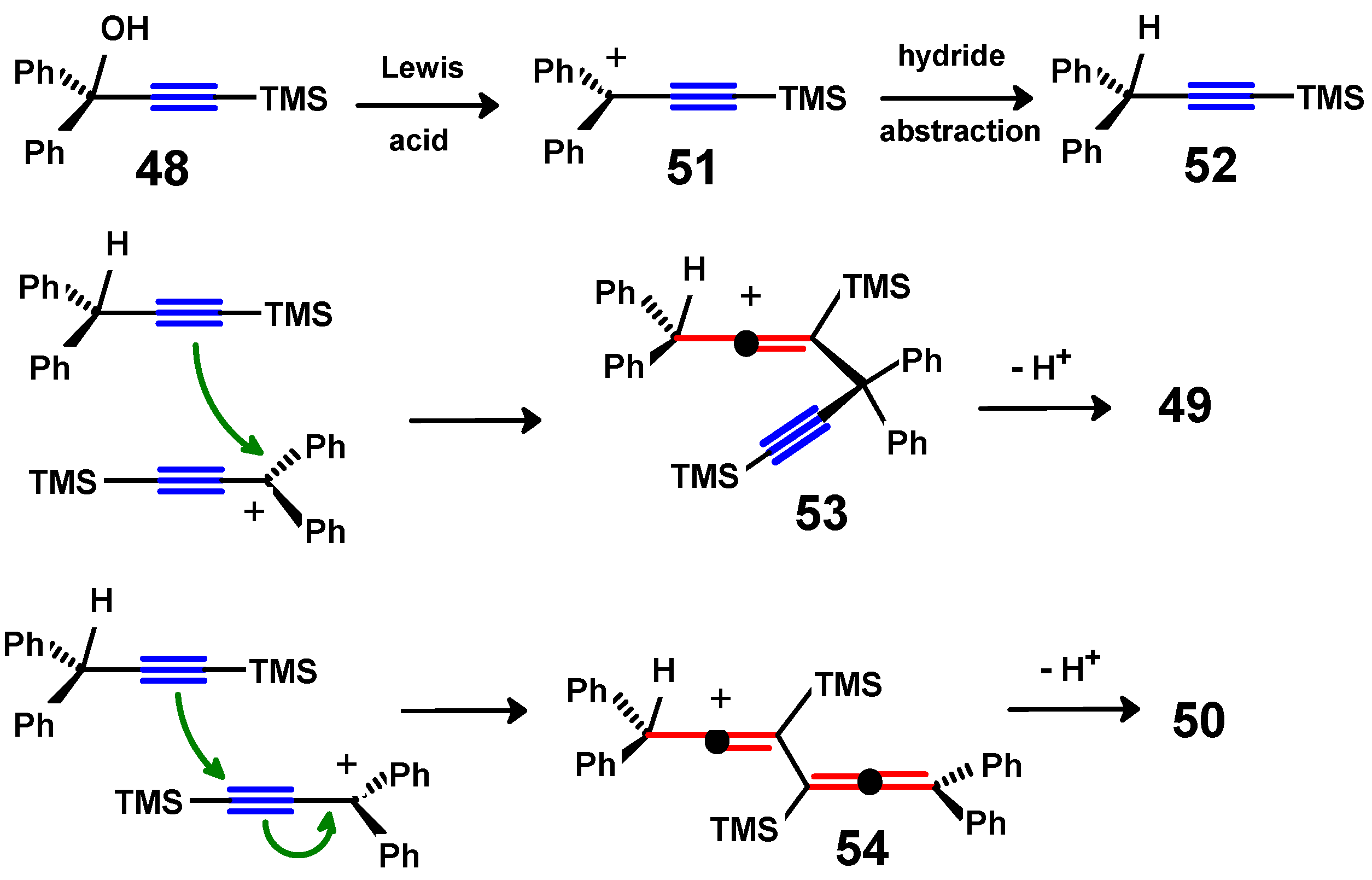
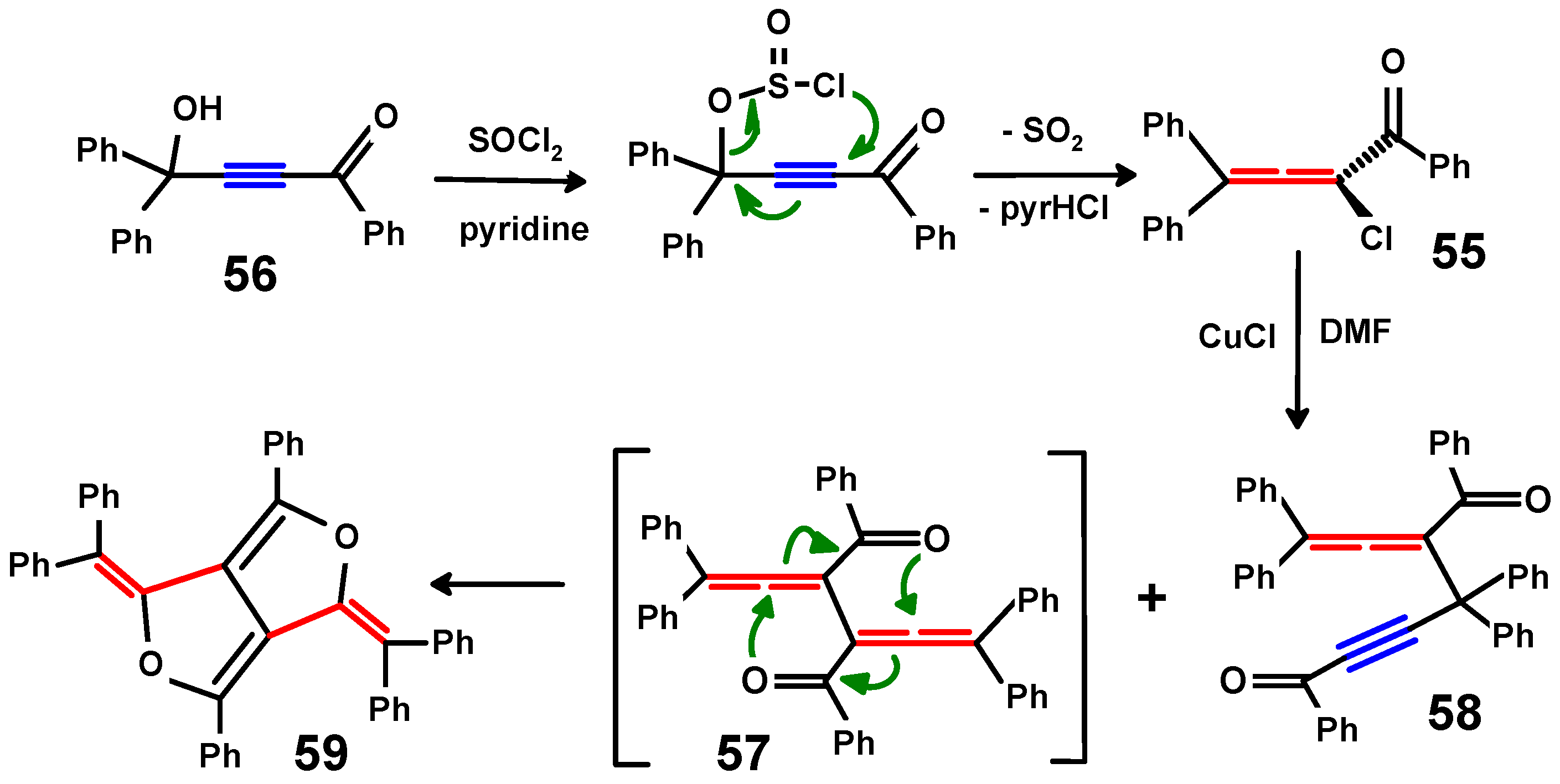
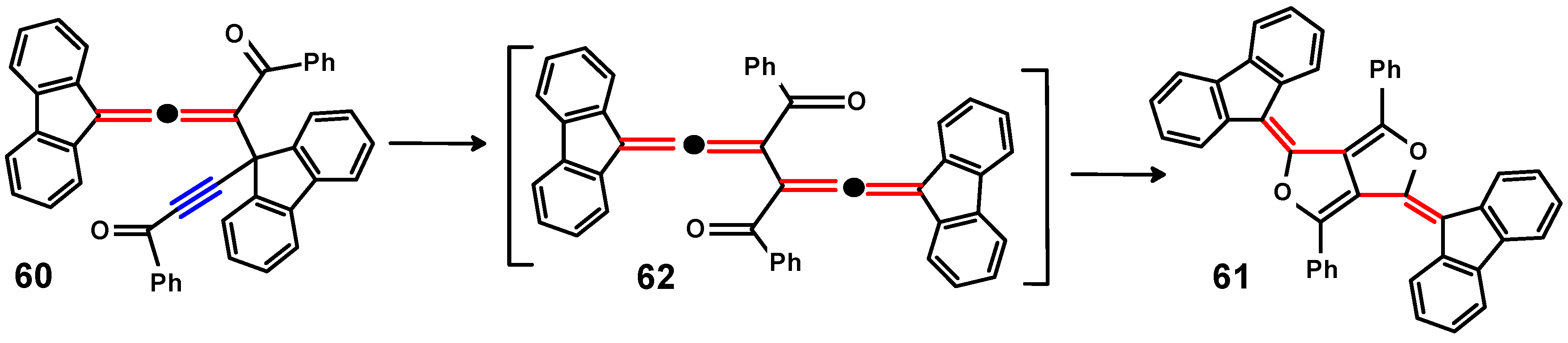
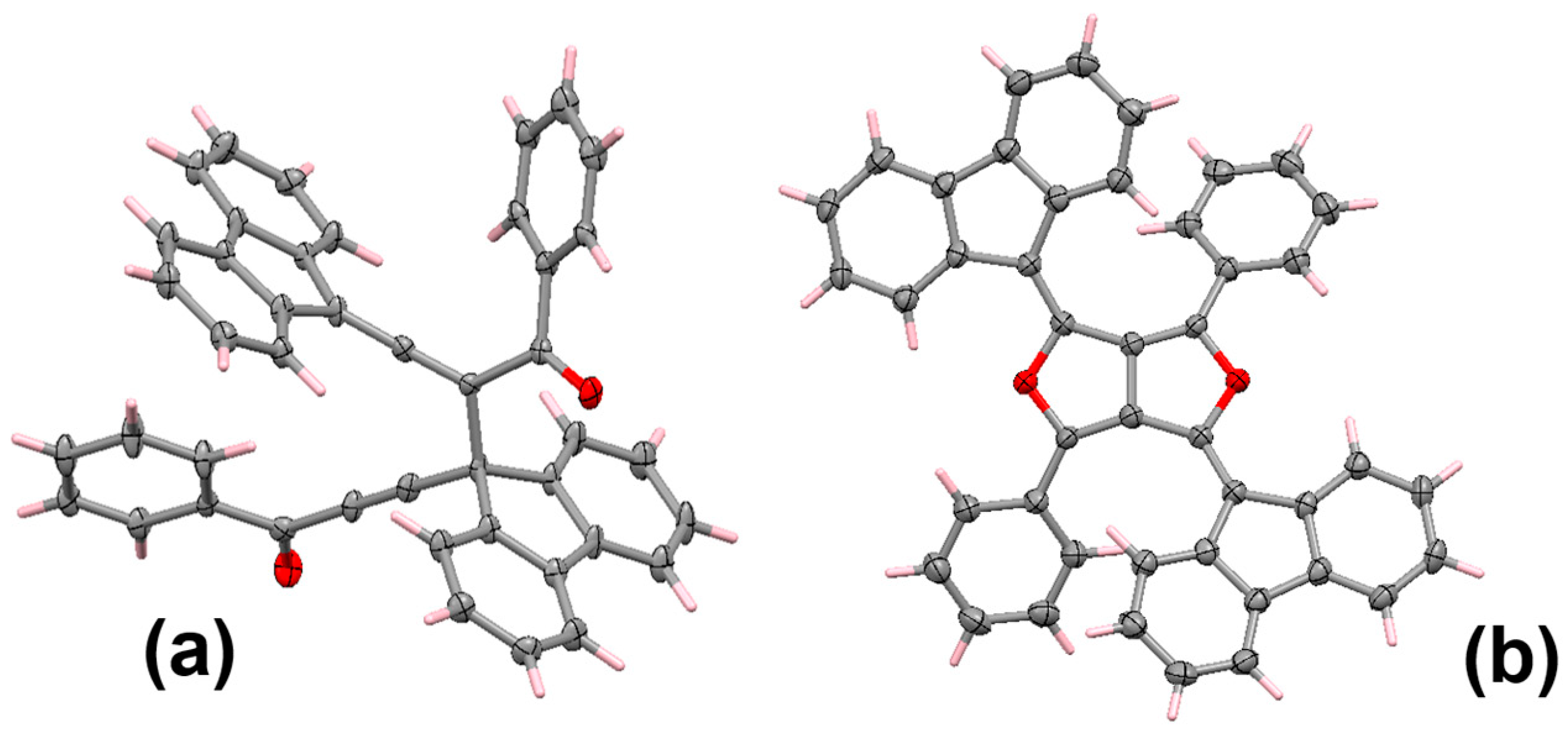
4.3. Di-Aroyl-Propargylallenes and Diallenes
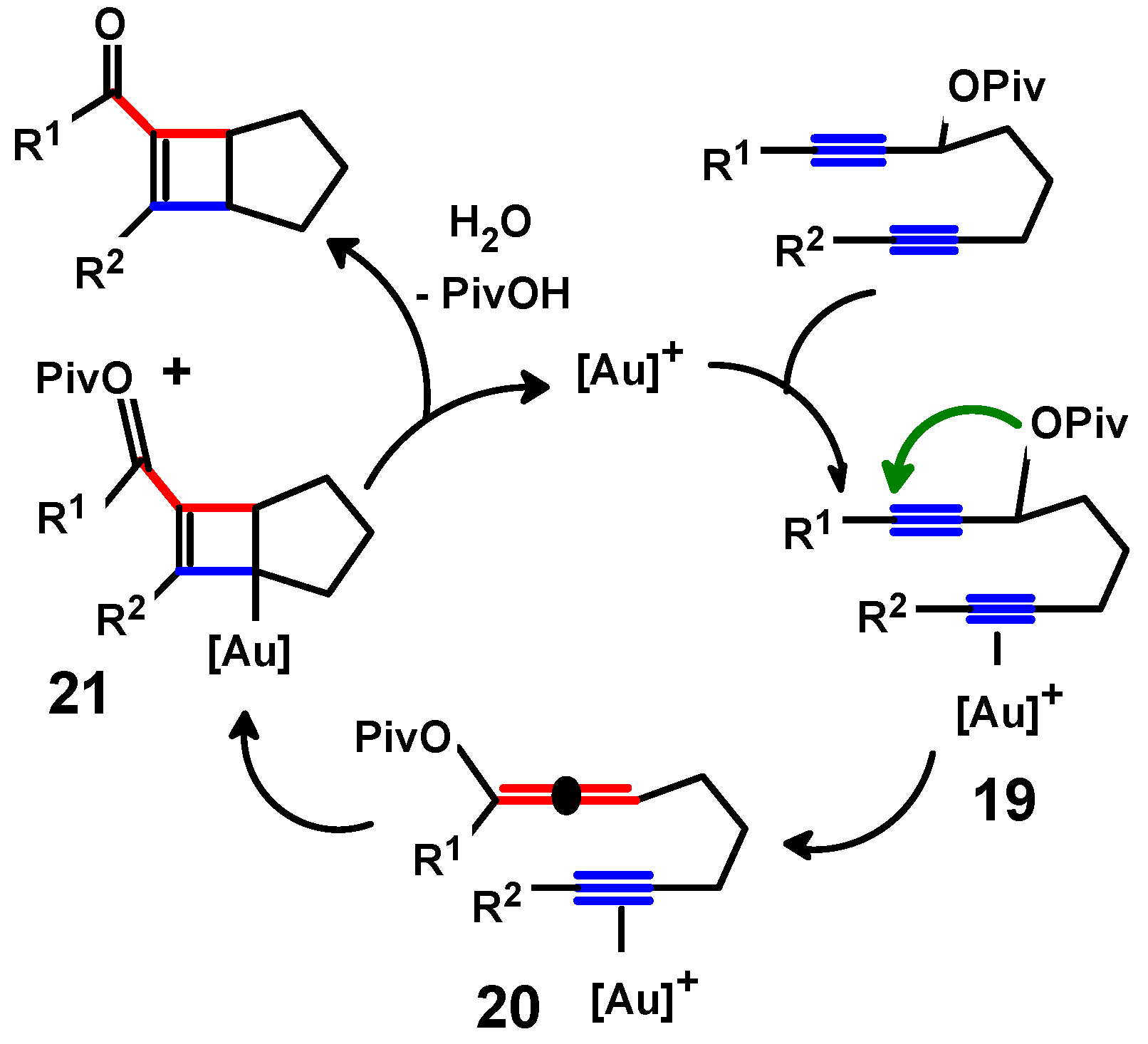
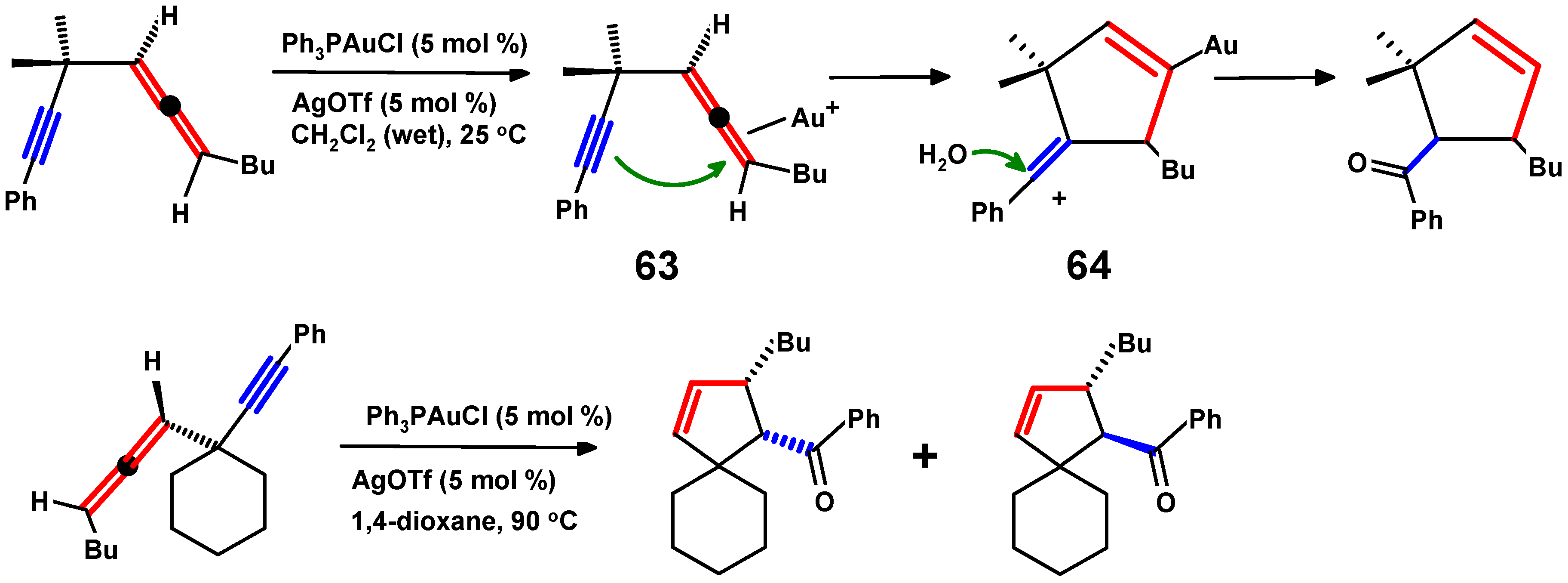
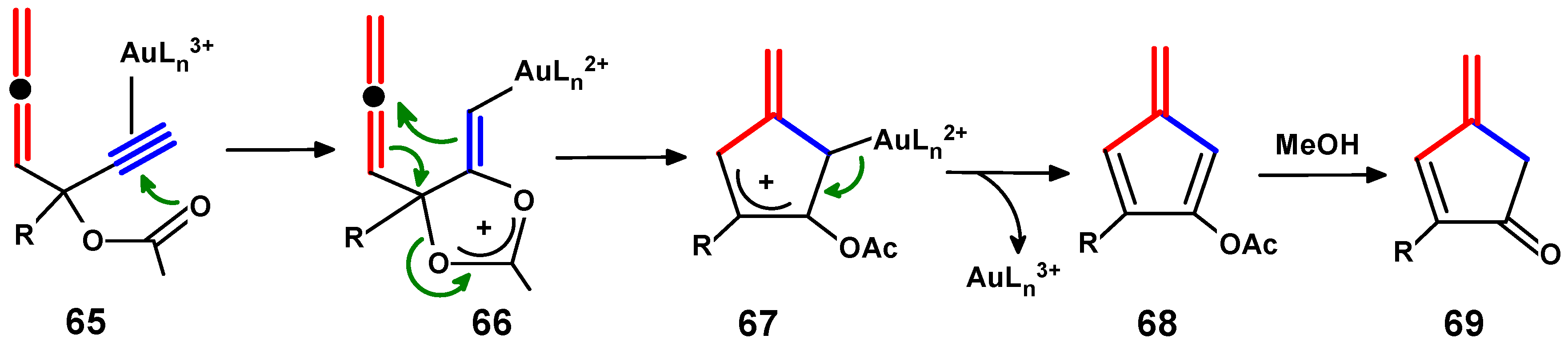
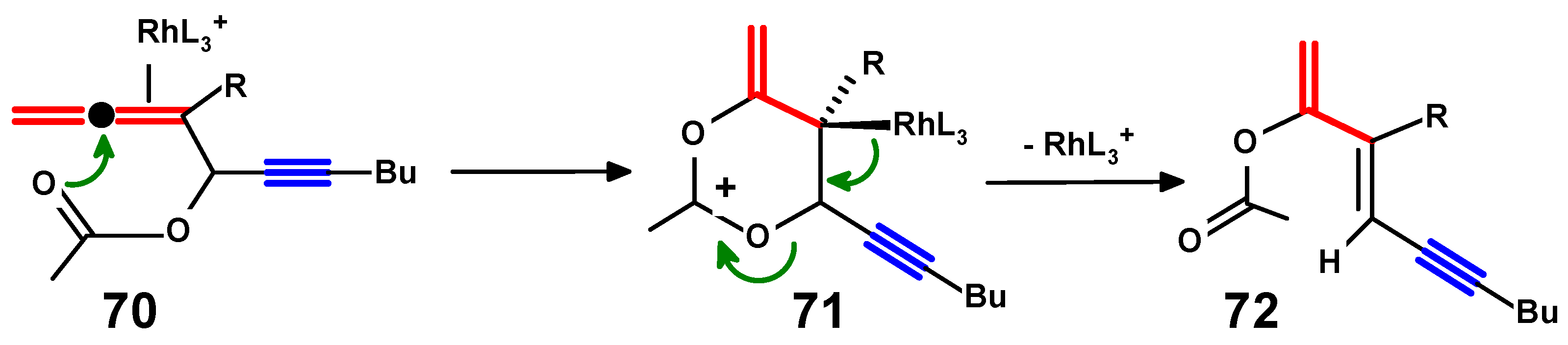
4.4. Metal-Mediated Rearrangements of Propargylallenes
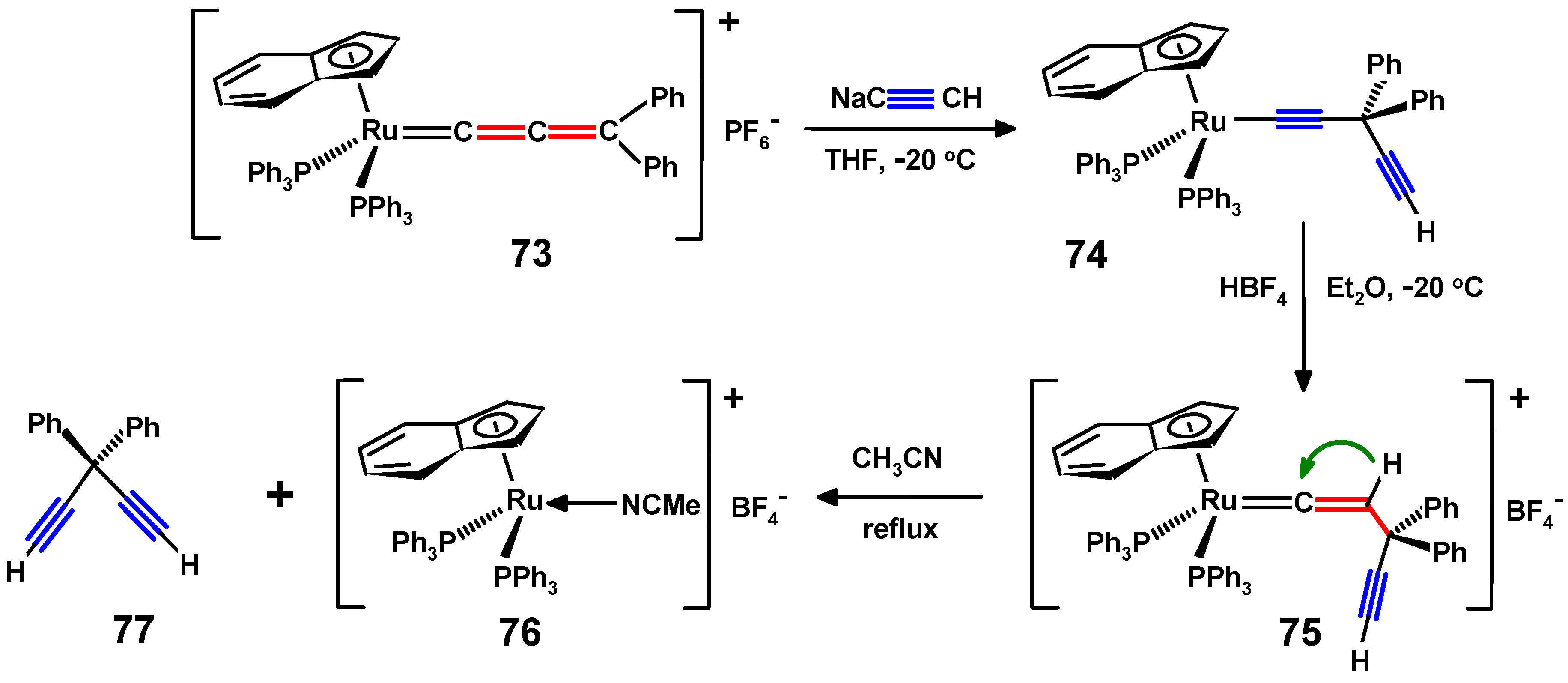
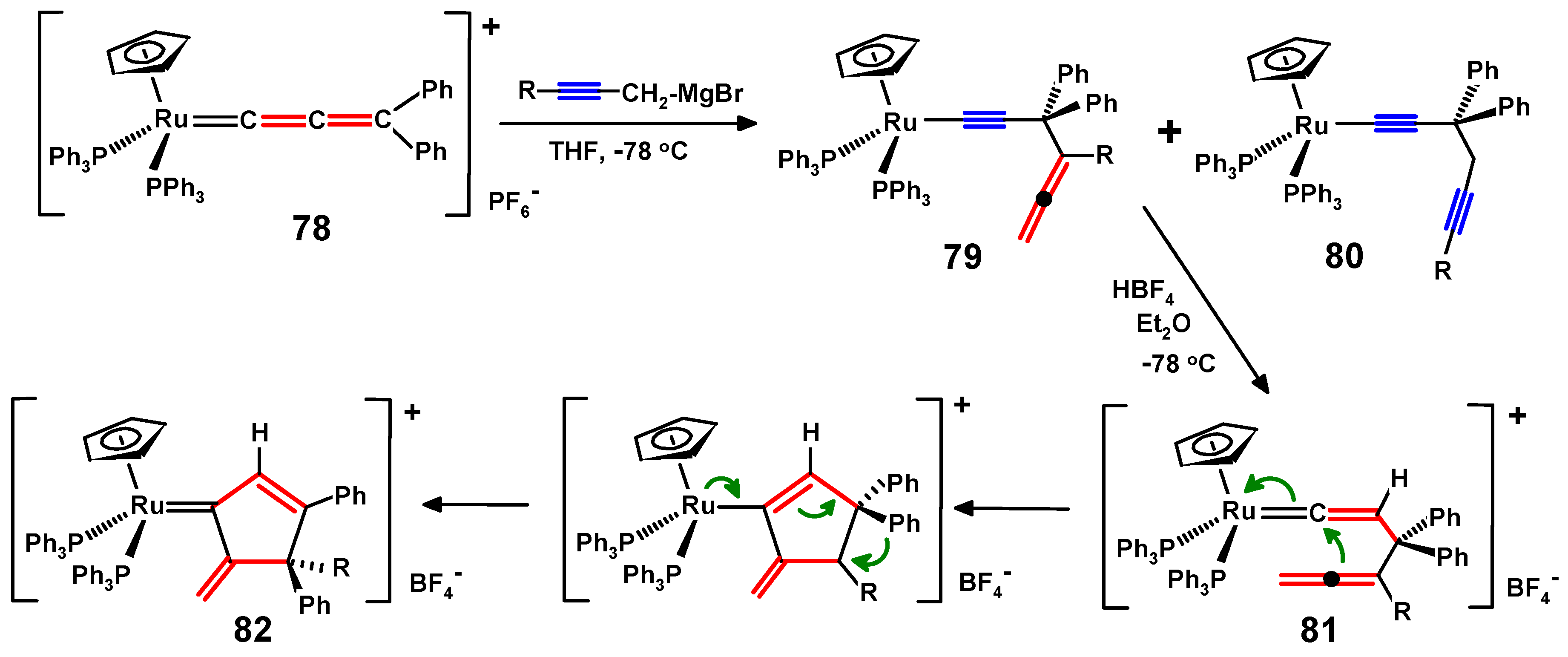
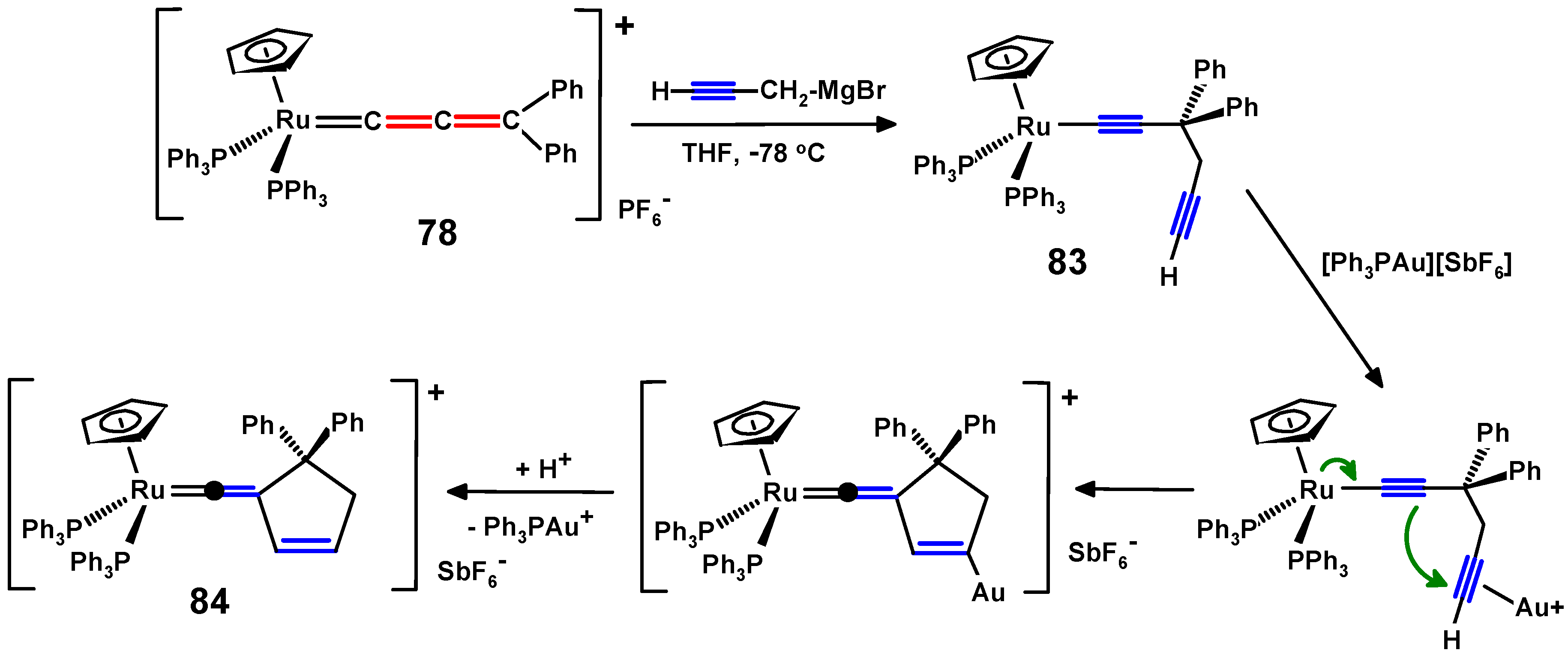
5. Propargylallenes and the Ruthenium Route to gem-Dialkynylmethanes
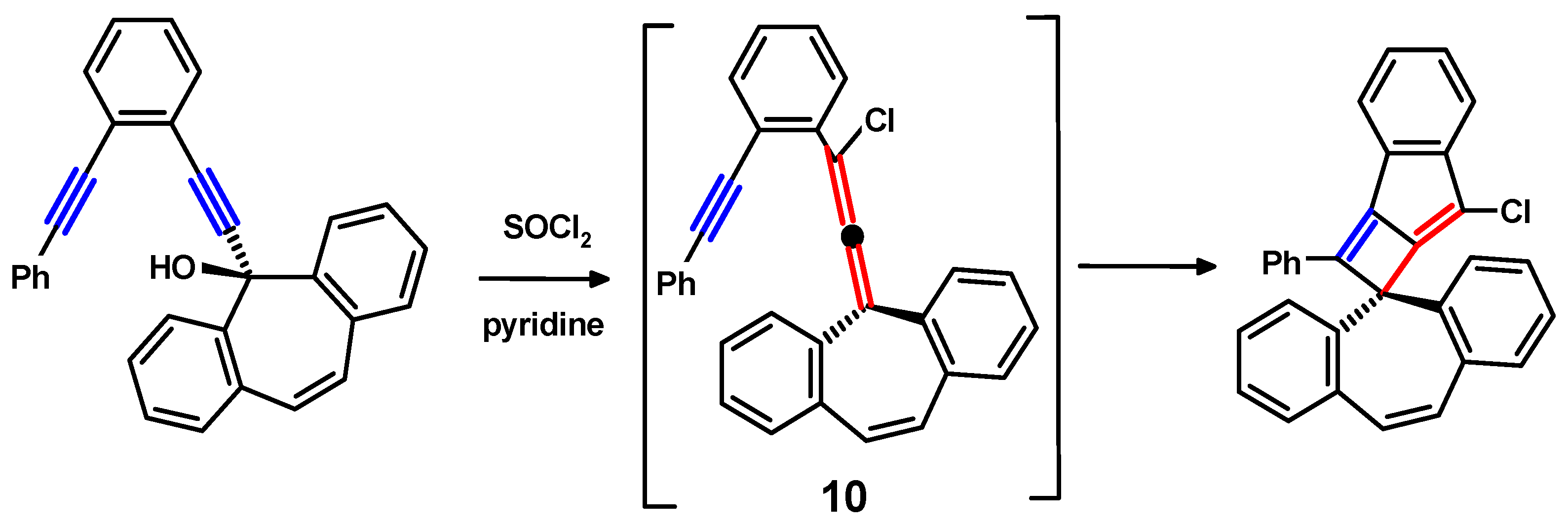
6. Propargylallenes from Alkynylfluorenols
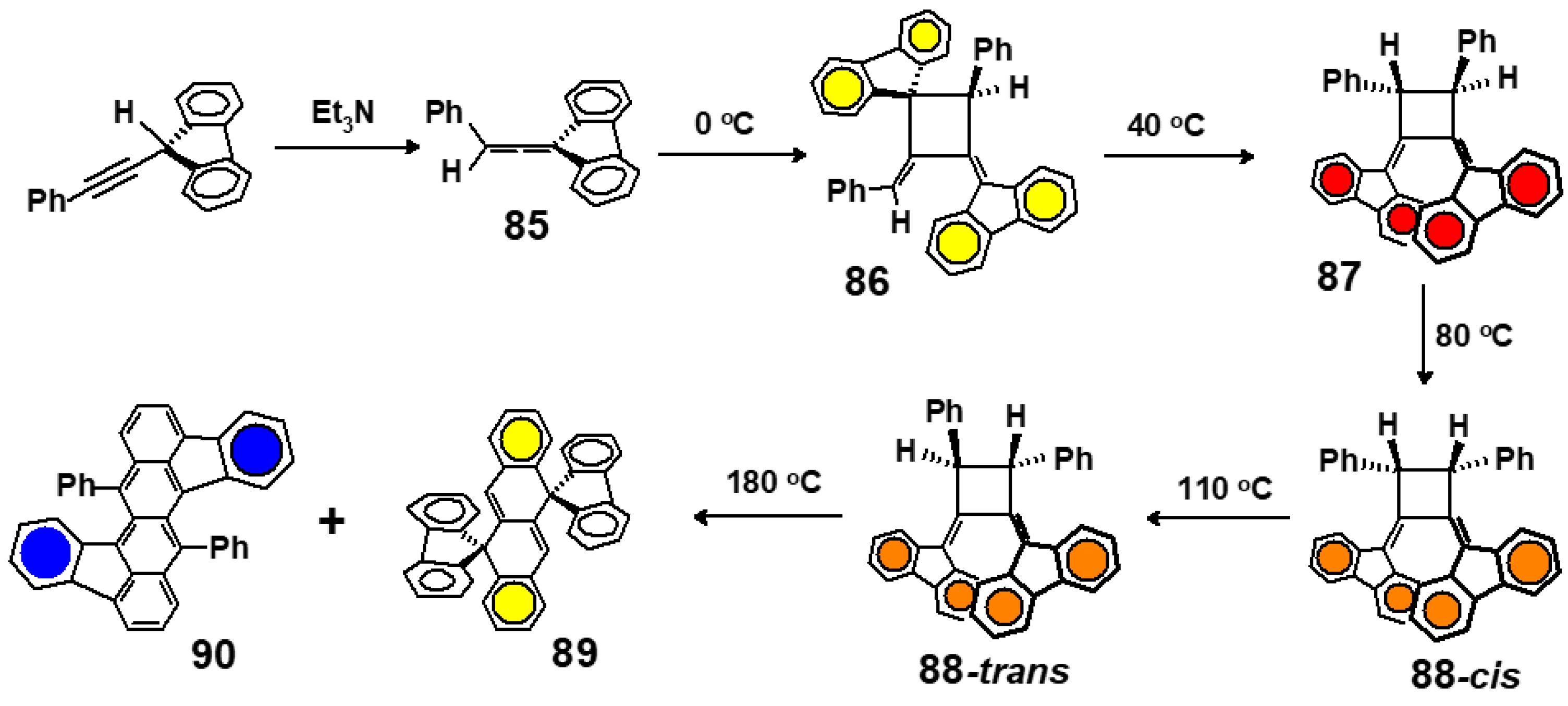
6.1. From Allenes to Tetracenes
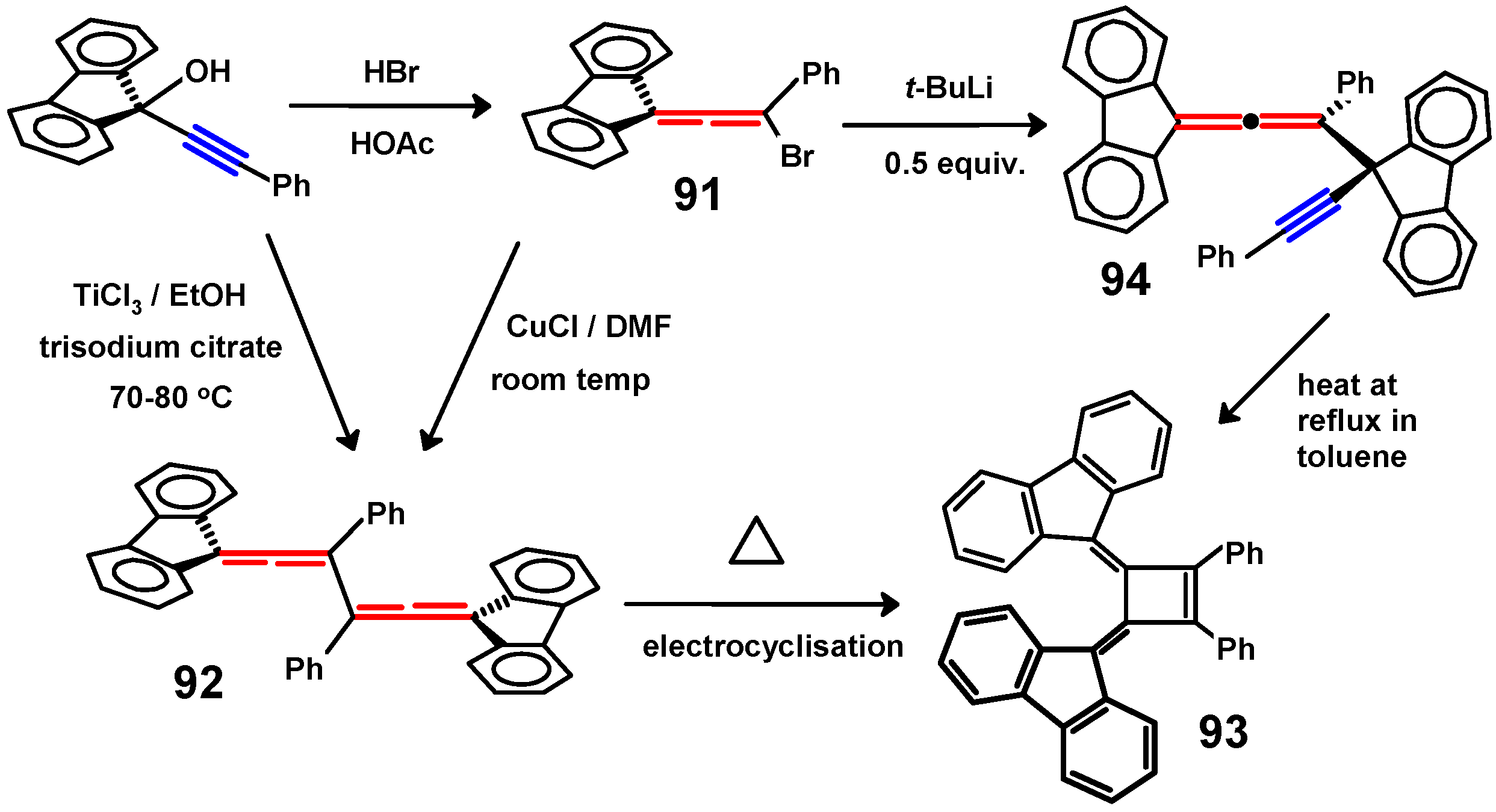
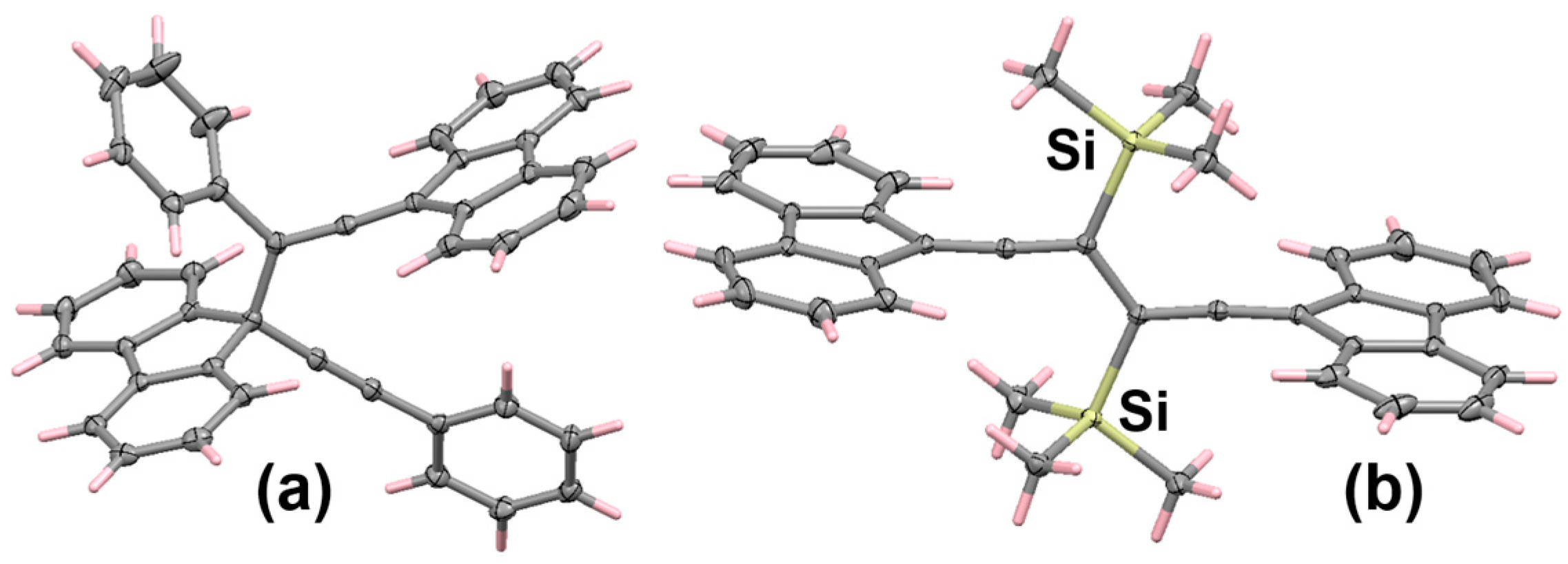
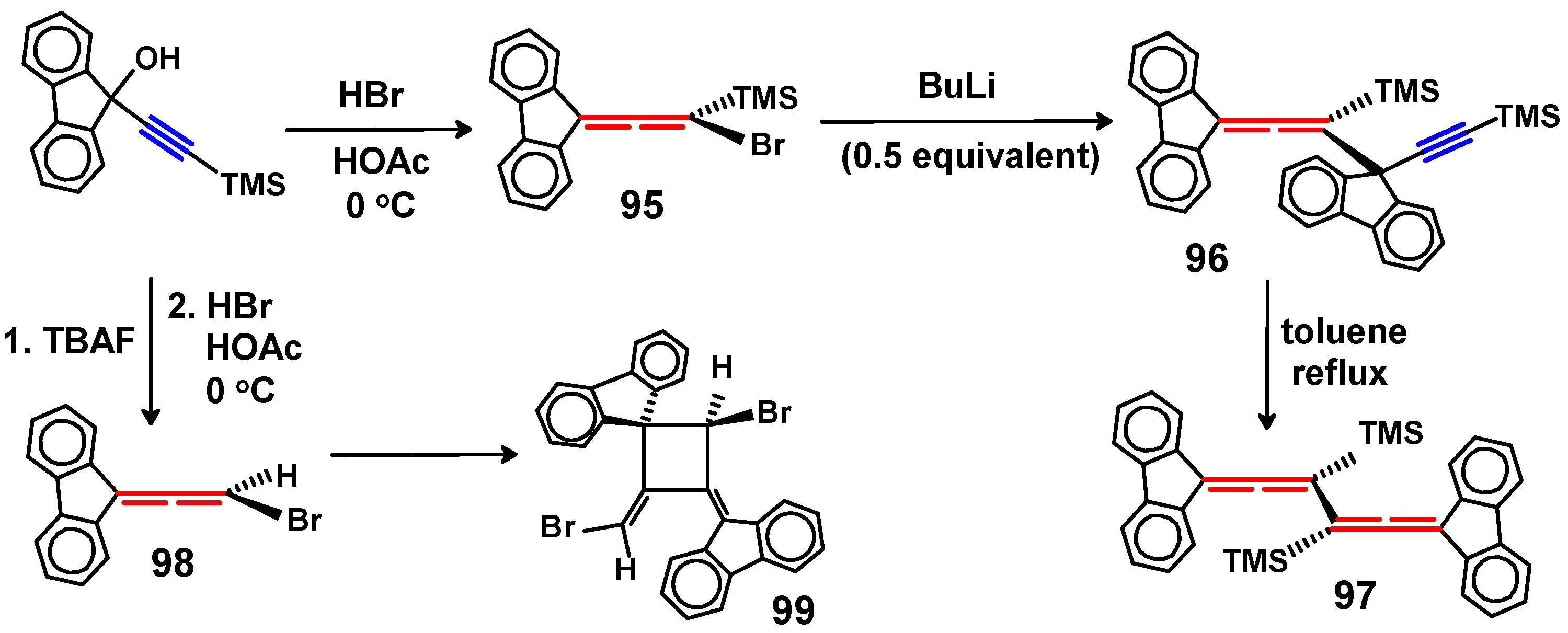
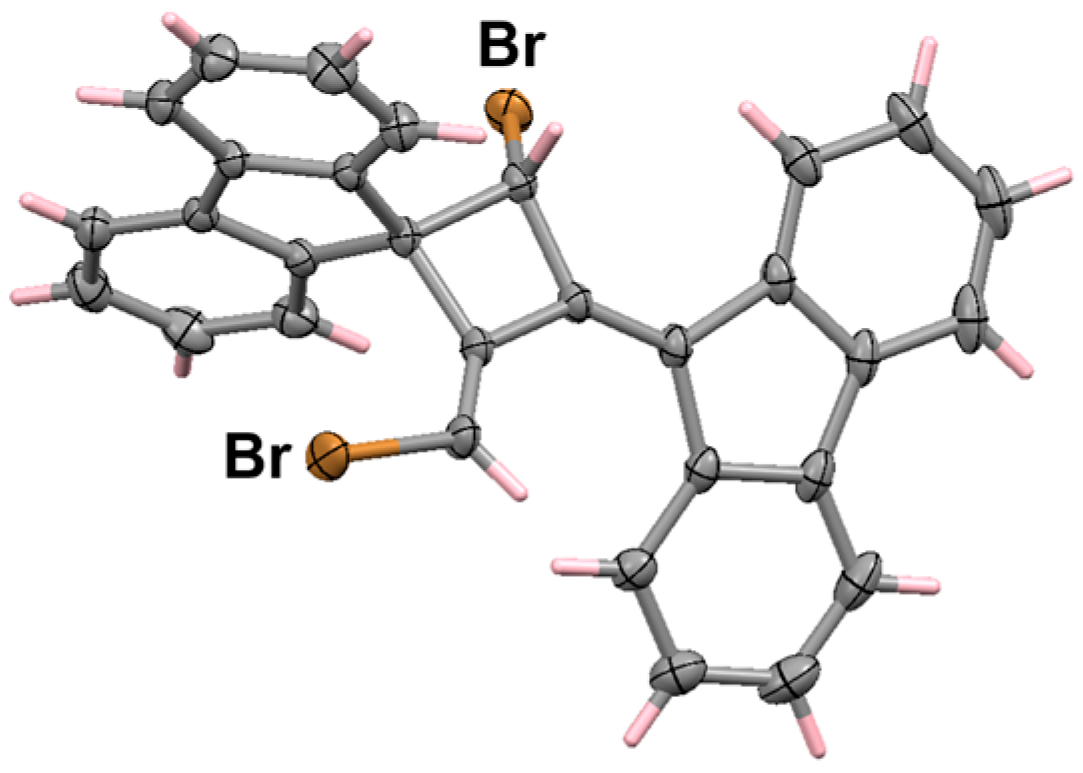
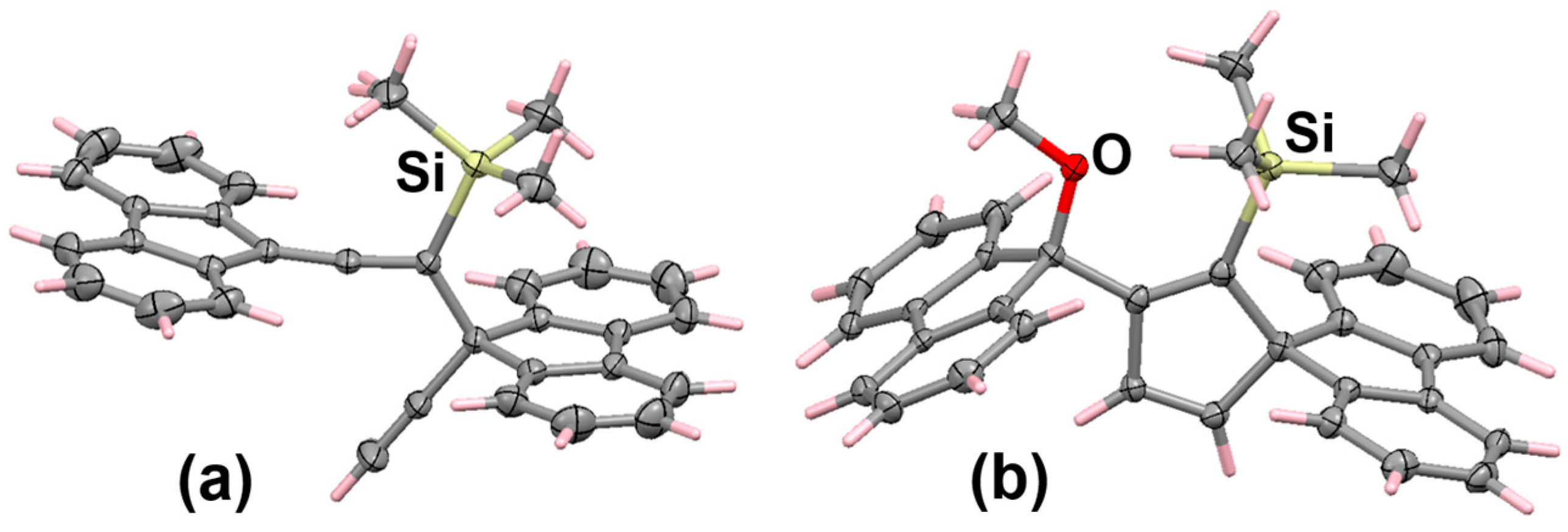
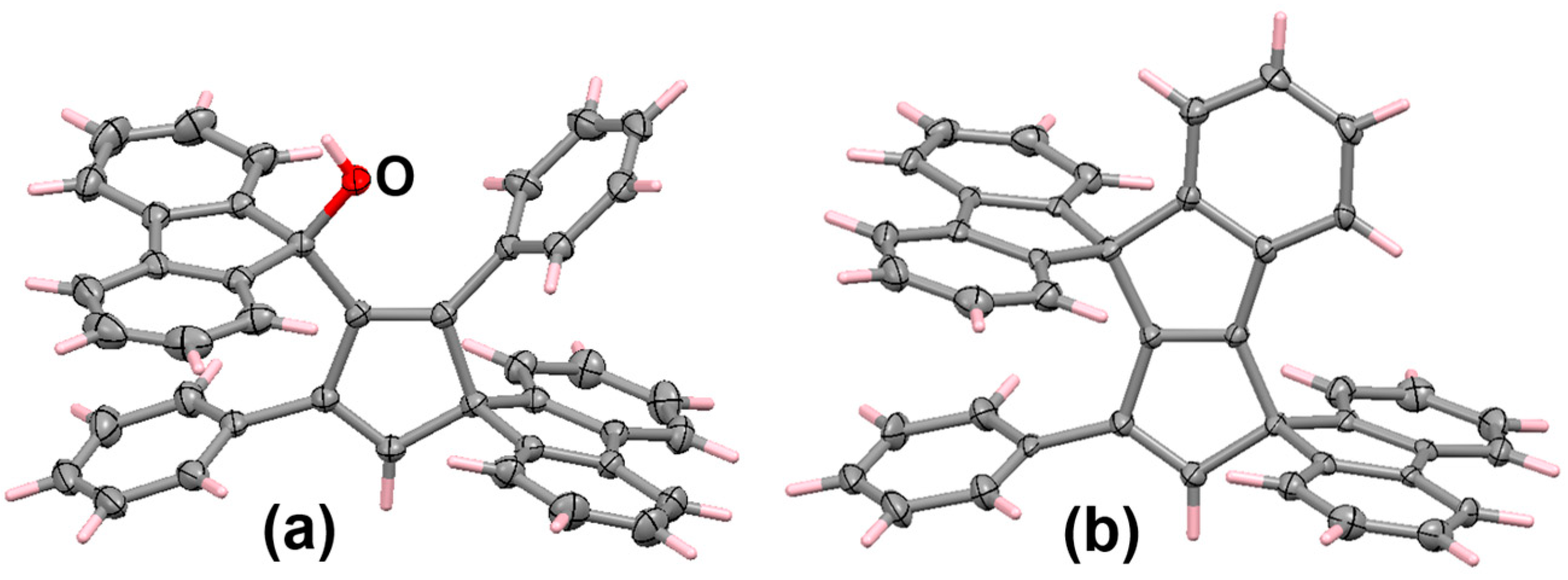
6.2. Bromo and Silyl Derivatives of Fluorenylideneallenes
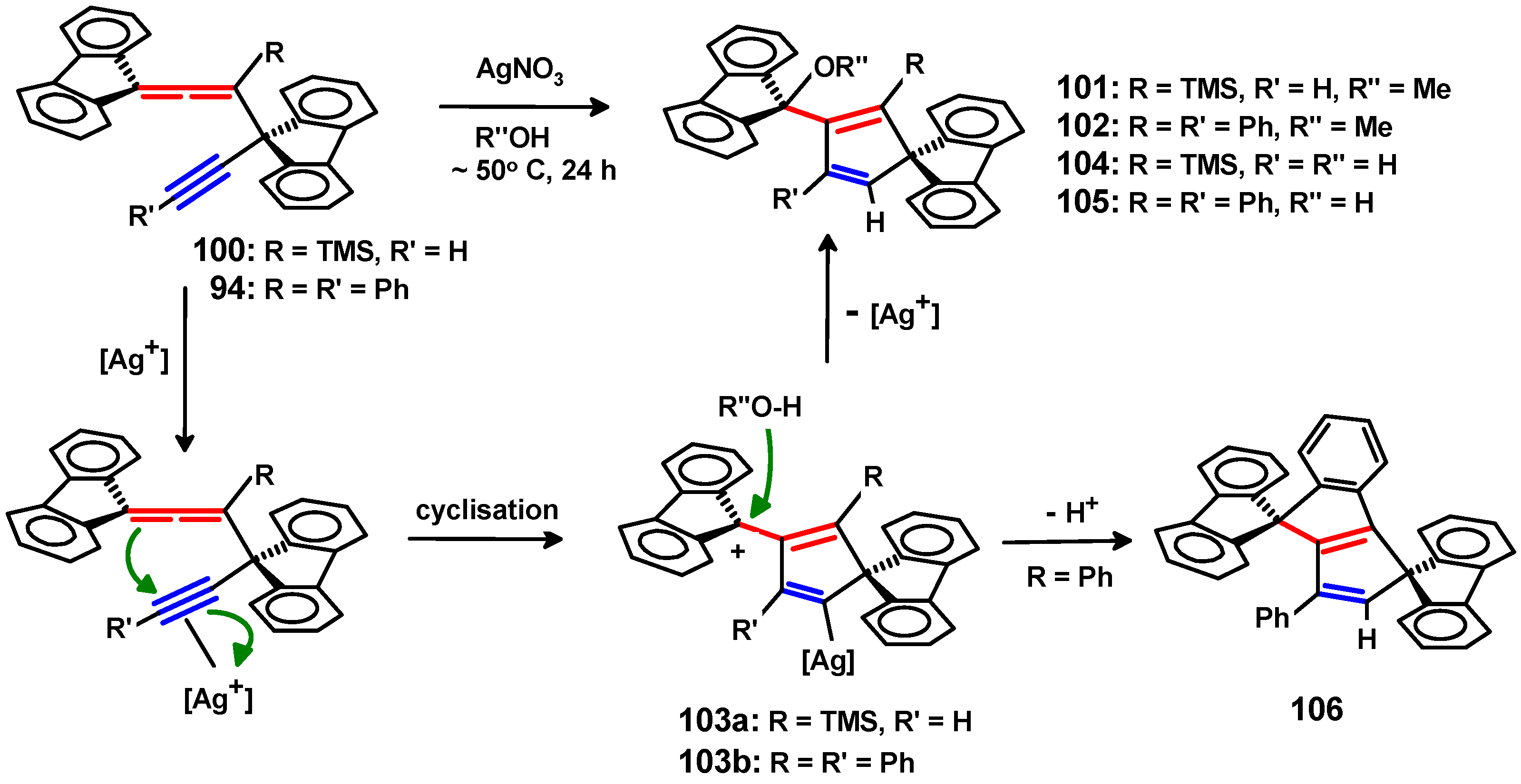
6.3. Reactions of Fluorenylidene Propargylallenes with Silver Nitrate
6.4. Ferrocenyl Derivatives of Propargylallenes
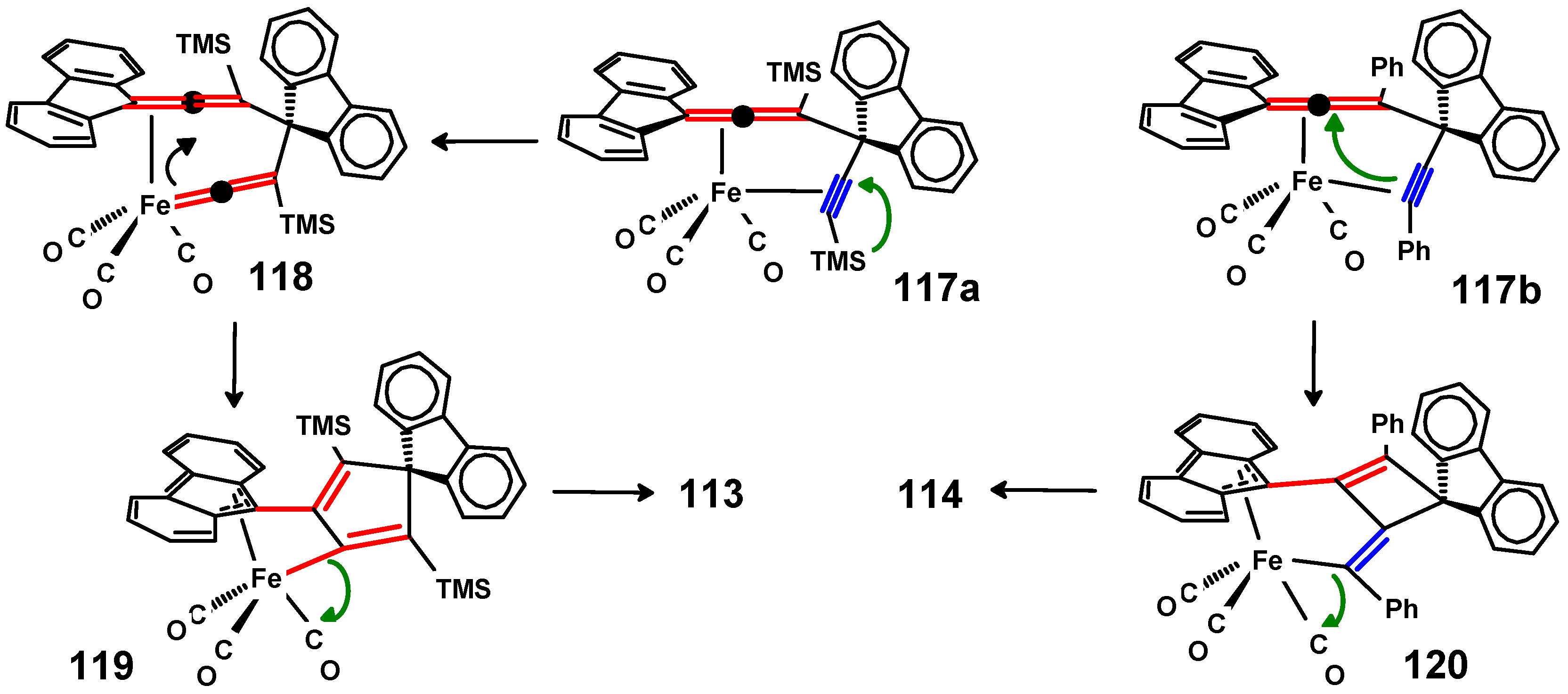
6.5. Reactions of Fluorenylideneallenes with Iron and Cobalt Carbonyls
7. Suggestions for Future Developments
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hopf, H. Thermische isomerisierungen, IV die propargyl-cope-umlagerung von 4-methyl-hexadien-(1.2)-in-(5). Tetrahedron Lett. 1972, 13, 3571–3574. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Aragoncillo, C. Exploiting [2+2] cycloaddition chemistry: Achievements with allenes. Chem. Soc. Rev. 2010, 39, 783–816. [Google Scholar] [CrossRef] [PubMed]
- Buisine, O.; Gandon, V.; Fensterbank, L.; Aubert, C.; Malacria, M. Thermal intramolecular Alder-ene cycloisomerisation of 1,6-allenynes. Synlett 2008, 5, 751–754. [Google Scholar] [CrossRef]
- Ohno, H.; Mizutani, T.; Kadoh, Y.; Aso, A.; Mayamura, K.; Fujii, N.; Tanaka, T. A highly regio- and stereoselective formation of bicyclo[4.2.0]oct-5-ene derivatives through thermal intramolecular [2+2] cycloaddition of allenes. J. Org. Chem. 2007, 72, 4378–4389. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, S. Intramolecular [2+2]-allenoates for the efficient synthesis of 3-oxabicyclo[4.2.0]octa-1(8),5-dien-4-ones: A dramatic substituent effect. Tetrahedron 2007, 63, 7589–7595. [Google Scholar] [CrossRef]
- Mukai, C.; Hara, Y.; Miyashita, Y.; Inagaki, F. Thermal {2+2] cycloaddition of allenynes: Easy construction of bicyclo[6.2.0]deca-1,8-dienes, bicyclo[5.2.0]nona-1,7-dienes, and bicyclo[4.2.0]octa-1,6-dienes. J. Org. Chem. 2007, 72, 4454–4461. [Google Scholar] [CrossRef]
- Wang, G.; Qian, H.; Ma, S. Synthesis of allenynes via Pd-catalyzed coupling of 1,4-diyn-3-yl carbonates with boronic acids. Org. Chem. Front. 2024, 11, 437–441. [Google Scholar] [CrossRef]
- Oh, C.H.; Gupta, A.K.; Park, D.I.; Kim, N. Highly efficient [2+2] intramolecular cyclizations of allenynes under microwave irradiation: Construction of fused bicyclic compounds. Chem. Commun. 2005, 45, 5670–5672. [Google Scholar] [CrossRef]
- Brummond, K.M.; Chen, D. Microwave-assisted intramolecular [2+2] allenic cycloaddition reaction for the rapid assembly of bicyclo[4.2.0]octadienes and bicyclo[5.2.0]nonadienes. Org. Lett. 2005, 7, 3473–3475. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.-R.; Petersen, J.L.; Wang, K.K. Biradicals from benzoenyne-allenes. Application in the synthesis of 11H-benzo[b]fluoren-11-ols, 1H-cyclobuta[a]indenes, and related compounds. J. Org. Chem. 2001, 66, 6662–6668. [Google Scholar] [CrossRef]
- Dinadayalane, T.C.; Priyakumar, U.D.; Sastry, G.N. Exploration of the C6H6 potential energy surface: A computational effort to unravel the relative stabilities and synthetic feasibility of new benzene isomers. J. Phys. Chem. A 2004, 108, 11433–11448. [Google Scholar] [CrossRef]
- Weibel, J.-M.; Blanc, A.; Pale, P. Ag-mediated reactions: Coupling and heterocyclization reactions. Chem. Rev. 2008, 108, 3149–3173. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Núñez, H.; Echavarren, A.M. Gold-catalyzed cycloisomerizations of enynes: A mechanistic perspective. Chem. Rev. 2008, 108, 3326–3350. [Google Scholar] [CrossRef]
- Toste, E.D. Gold Catalysis for Organic Synthesis. Beilstein J. Org Chem. 2011, 7, 553–1525. [Google Scholar] [CrossRef] [PubMed]
- Krause, N.; Winter, C. Gold-catalyzed nucleophilic cyclization of functionalized allenes: A powerful access to carbo- and heterocycles. Chem. Rev. 2011, 111, 1994–2009. [Google Scholar] [CrossRef]
- Collado, A.; Nelson, D.J.; Nolan, S.P. Optimizing catalyst and reaction conditions in gold(I) catalysis—Ligand development. Chem. Rev. 2021, 121, 8559–8612. [Google Scholar] [CrossRef]
- Mato, M.; Franchino, A.; Garcia-Morales, C.; Echavarren, A.M. Gold-catalyzed synthesis of small rings. Chem. Rev. 2021, 121, 8613–8684. [Google Scholar] [CrossRef]
- Campeau, D.; Rayo, D.F.L.; Mansour, A.; Muramov, K.; Gagosz, F. Gold-catalyzed reactions of specially activated alkynes, allenes and alkenes. Chem. Rev. 2021, 121, 8756–8767. [Google Scholar] [CrossRef]
- Hendrich, C.M.; Sekine, K.; Koshikawa, T.; Tanaka, K.; Hashmi, A.S.K. Homogeneous and heterogeneous gold catalysis for materials science. Chem. Rev. 2021, 121, 9113–9163. [Google Scholar] [CrossRef]
- Hashmi, A.S.K. New and selective transition metal catalyzed reactions of allenes. Angew. Chem. Int. Ed. 2000, 39, 3590–3593. [Google Scholar] [CrossRef]
- Jiménez-Núñez, E.; Raducan, M.; Lauterbach, T.; Molawi, K.; Solario, C.R.; Echavarren, A.M. Evolution of propargyl ethers into allylgold cations in the cyclization of enynes. Angew. Chem. Int. Ed. 2009, 48, 6152–6155. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-M.; Zhang, A.-B. Chemistry of 1, 2-allenyl/propargyl metal species. A personal account. Pure Appl. Chem. 2001, 73, 337–341. [Google Scholar] [CrossRef][Green Version]
- Aubert, C.; Buisine, O.; Malacria, M. The behavior of 1,n-enynes in the presence of transition metals. Chem. Rev. 2002, 102, 813–834. [Google Scholar] [CrossRef] [PubMed]
- Cadran, N.; Cariou, K.; Herve, G.; Aubert, C.; Fensterbank, L.; Malacria, M.; Marco-Contelles, J. PtCl2-catalyzed cycloisomerisations of allenynes. J. Am. Chem. Soc. 2004, 126, 3408–3409. [Google Scholar] [CrossRef]
- Matsuda, T.; Kadowaki, S.; Goya, T.; Murakami, M. A direct entry to bicyclic cyclobutenes via platinum-catalyzed cycloisomerisation of allenynes. Synlett 2006, 4, 575–578. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. Propargylic esters in gold catalysis: Access to diversity. Angew. Chem. Int. Ed. 2007, 46, 2750–2752. [Google Scholar] [CrossRef] [PubMed]
- Zriba, R.; Gandon, V.; Aubert, C.; Fensterband, L.; Malacria, M. Alkyne versus allene activation in platinum- and gold-catalyzed cycloisomerisation of hydroxyl-1,5-allenynes. Chem. Eur. J. 2008, 14, 1482–1491. [Google Scholar] [CrossRef]
- Aubert, C.; Fensterbank, L.; Garcia, P.; Malacria, M.; Simmonneau, A. Transition metal catalyzed cycloisomerisations of 1,n-allenynes and -allenenes. Chem. Rev. 2011, 111, 1954–1993. [Google Scholar] [CrossRef]
- Fensterbank, L.; Malacria, M. Molecular complexity from polyunsaturates: The gold catalysis approach. Acc. Chem. Res. 2014, 47, 953–965. [Google Scholar] [CrossRef]
- Asiri, A.M.; Hashmi, A.S.K. Gold-catalysed reactions of diynes. Chem. Soc. Rev. 2016, 45, 4471–4503. [Google Scholar] [CrossRef]
- Llerena, D.; Buisine, O.; Aubert, C.; Malacria, M. Synthesis of variously substituted allenediynes and their cobalt(I)-mediated [2+2+2] cycloaddition reactions. Tetrahedron 1998, 54, 9373–9392. [Google Scholar] [CrossRef]
- Delas, C.; Urabe, H.; Sato, F. Titanium-mediated intramolecular cyclization of tethered propargyl alcohol derivatives. Access to exocyclic bis-allenes and cyclobutene derivatives. Tetrahedron Lett. 2001, 42, 4147–4150. [Google Scholar] [CrossRef]
- Oh, C.H.; Park, D.I.; Jung, S.H.; Reddy, V.R.; Gupta, A.K.; Kim, Y.M. Chemodiversity in the palladium-catalyzed cyclizations of allenynecarboxylates. Synlett 2005, 13, 2092–2094. [Google Scholar] [CrossRef]
- Oh, C.H.; Kim, A. Gold-catalyzed [2+2] cyclization of alkyne-propargylic pivaloates to fused bicyclic compounds. Synlett 2008, 5, 777–781. [Google Scholar] [CrossRef]
- Lin, G.-Y.; Yang, C.-Y.; Liu, R.-S. Gold-catalyzed synthesis of bicyclo[4.3.0]nonadiene derivatives via tandem 6-endo-dig/Nazarov cyclization of 1,6-allenynes. J. Org. Chem. 2007, 72, 6753–6757. [Google Scholar] [CrossRef]
- Brummond, K.M.; Chen, H.; Fisher, K.D.; Kerekes, A.D.; Rickards, B.; Sill, P.C.; Geib, S.J. An allenic Pauson-Khand-type reaction: A reversal in π-bond selectivity and the formation of seven-membered rings. Org. Lett. 2002, 4, 1931–1934. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, Y.; Ma, S. Rhodium-catalyzed Pauson-Khand cyclization of 1,5-allene-alkynes: A chirality transfer transfer strategy for optically active bicyclic ketones. Chem. Eur. J. 2019, 25, 9529–9533. [Google Scholar] [CrossRef]
- Xu, X.; Wang, M.; Peng, L.; Guo, C. Nickel-catalyzed asymmetric propargylation for the synthesis of axially chiral 1,3-disubstituted allenes. J. Am. Chem. Soc. 2022, 144, 21022–21029. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Flippen-Anderson, J.; Cook, J.M. The synthesis of a dicyclopenta[a,e]pentalene via a molybdenum hexacarbonyl-mediated tandem allenic Pauson-Khand reaction. J. Am. Chem. Soc. 2003, 125, 3230–3231. [Google Scholar] [CrossRef]
- Shen, Q.; Hammond, G.B. Regiospecific synthesis of bicyclo- and heterobicyclo-gem-difluorocyclobutenes using functionalized fluoroallenes and a novel Mo-catalyzed intramolecular [2+2] cycloaddition reaction. J. Am. Chem. Soc. 2002, 124, 6534–6535. [Google Scholar] [CrossRef]
- McGlinchey, M.J.; Hopf, H. Organic and organometallic derivatives of propargylallene: Syntheses, structures, reactivity and rearrangements. Eur. J. Org. Chem. 2013, 2013, 4705–4728. [Google Scholar] [CrossRef]
- Klaeboe, P.; Phongsatha, A.; Cyvin, B.N.; Cyvin, S.J.; Hopf, H. The vibrational spectra and molecular structure of hexa-1,2-dien-5-yne (propargylallene). J. Mol. Struct. 1978, 43, 1–8. [Google Scholar] [CrossRef]
- Bischof, P.; Gleiter, R.; Hopf, H.; Lenich, F.T. Photoelectron spectra of open chain C6H6 isomers. J. Am. Chem. Soc. 1975, 97, 3567–3572. [Google Scholar] [CrossRef]
- Seip, R.; Bakken, P.; Traetteberg, M.; Hopf, H. The molecular structure of gaseous 1,2-hexadien-5-yne (propargylallene). Acta Chem. Scand. A 1981, 35, 365–371. [Google Scholar] [CrossRef]
- Hopf, H. Thermal isomerisations, III. Acyclic C6H6 isomers. Chem. Ber. 1971, 104, 1499–1506. [Google Scholar] [CrossRef]
- De Meijere, A.; Jaekel, F.; Simon, A.; Borrmann, H.; Köhler, J.; Johnels, D.; Scott, L.T. Cyclynes. 9. Regioselective coupling of ethynylcyclopropane units: Hexaspiro[2.0.2.4.2.0.2.4.2.0.2.4] triaconta-7,9,17,19.27,29-hexayne. J. Am. Chem. Soc. 1991, 113, 3935–3941. [Google Scholar] [CrossRef]
- Christov, V.J.; Prodanov, B. Synthesis of 1-substituted phosphorylated allenes. Phosphorus Sulfur Silicon Relat. Elem. 2000, 166, 265–273. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.; Ma, S. Novozym-435-catalyzed efficient preparation of (1S)-ethenyl and ethynyl 2,3-allenols and (1R)-ethenyl and ethynyl acetates with high enantiomeric excess. Tetrahedron Asymmetry 2003, 14, 3657–3666. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Ruppert, T.L.; Knöfel, T.; Bats, J.W. C-C-Bond formation by the palladium-catalyzed cycloisomerization/dimerization of terminal allenyl ketones: Selectivity and mechanistic aspects. J. Org. Chem. 1997, 62, 7295–7304. [Google Scholar] [CrossRef]
- Maraval, V.; Duhayon, C.; Coppel, Y.; Chauvin, R. The intricate assembling of gem-diphenylpropargylic units. Eur. J. Org. Chem. 2008, 2008, 5144–5156. [Google Scholar] [CrossRef]
- Toda, F.; Yamamoto, M.; Tanaka, K.; Mak, T.C.W. Novel copper (I) chloride-assisted coupling reaction of 1-chloro-1-aryloyl-3,3-diarylallene to afford 3,7-dioxa-2,6-diaryl-4,8-bis(diarylmethylene)bicyclo[3.3.0] octa-1,5-diene and 1,1,4,4-tetraaryl-3,6-diaryloylhexa-1,2-dien-5-yne, and thermal cyclization of the latter to a fulvene. Tetrahedron Lett. 1985, 26, 631–634. [Google Scholar] [CrossRef]
- Hopf, H.; Markopolous, G. The chemistry of bisallenes. Beilstein J. Org. Chem. 2012, 8, 1936–1998. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tomomori, A.; Scott, J.L. Novel thermally induced rearrangement of a propargylallene to a furofuran derivative in the solid state. Eur. J. Org. Chem. 2003, 2003, 2035–2038. [Google Scholar] [CrossRef]
- Scott, J.L.; Tanaka, K. Photochromic crystals: Toward an understanding of color development in the solid state. Cryst. Growth Des. 2005, 5, 1209–1213. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Lin, G.-Y.; Liao, H.-Y.; Datta, S.; Liu, R.-S. Gold-catalyzed hydrative carbocyclization of 1,5- and 1,7-allenynes mediated by π-allene transfer: Mechanistic evidence supported by the chirality transfer of allenyne substrates. J. Org. Chem. 2008, 73, 4907–4914. [Google Scholar] [CrossRef]
- Kato, K.; Kobayashi, T.; Fujinami, T.; Motodate, S.; Kusakabe, T.; Mochida, T.; Akita, H. New cationic bisoxazoline-Au(III) complex catalyzed cycloisomerisation of 1-allenyl-1-ethynyl acetate. Synlett 2008, 7, 1081–1085. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, C.; Ma, S. Highly selective facile synthesis of 2-acetoxy-1,3(E)-alkadienes via a Rh(I)-catalyzed isomerization of 2,3-allenyl carboxylates. Org. Lett. 2011, 13, 1920–1923. [Google Scholar] [CrossRef]
- Chen, K.-H.; Feng, Y.J.; Ma, H.-W.; Lin, Y.-C.; Liu, Y.-H.; Kuo, T.-S. Cyclization accompanied with 1,2-phenyl migration in the protonation of ruthenium acetylide complex containing an allenyl group. Organometallics 2010, 29, 6829–6836. [Google Scholar] [CrossRef]
- Kuhn, R.; Rewicki, D. Concerning cumulenes, XX: 1-phenyl-3,3-biphenylene-allene. Chem. Ber. 1965, 98, 2611–2618. [Google Scholar] [CrossRef]
- Banide, E.V.; Ortin, Y.; Seward, C.M.; Müller-Bunz, H.; Harrington, L.E.; McGlinchey, M.J. Sequential formation of yellow, red, and orange 1-phenyl-3,3-biphenylene-allene dimers prior to blue tetracene formation: Helicity reversal in trans-3,4-diphenyl-1,2-bis(fluorenylidene)cyclobutane. Chem. Eur. J. 2006, 12, 3275–3286. [Google Scholar] [CrossRef]
- Banide, E.V.; Oulié, P.; McGlinchey, M.J. From allenes to tetracenes: Syntheses, structures and reactivity of the intermediates. Pure Appl. Chem. 2009, 81, 1–17. [Google Scholar] [CrossRef]
- Toda, F.; Takehira, Y. New synthetic routes to conjugated diallenes from bromoallenes and prop-2-ynyl acetates. Novel C–C coupling with copper(I) chloride. J. Chem. Soc. Chem. Commun. 1975, 174. [Google Scholar] [CrossRef]
- Oulié, P.; Altes, L.; Milosevic, S.; Bouteille, R.; Müller-Bunz, H.; McGlinchey, M.J. Different reactivity patterns of trimethylsilyl- and phenyl-substituted propargylallenes: Fe2(CO)9 and [Ag]+-promoted cyclizations. Organometallics 2010, 29, 676–686. [Google Scholar] [CrossRef]
- Banide, E.V.; Molloy, B.C.; Ortin, Y.; Müller-Bunz, H.; McGlinchey, M.J. From allenes to tetracenes: A synthetic and structural study of silyl- and halo-allenes and their dimers. Eur. J. Org. Chem. 2007, 2007, 2611–2622. [Google Scholar] [CrossRef]
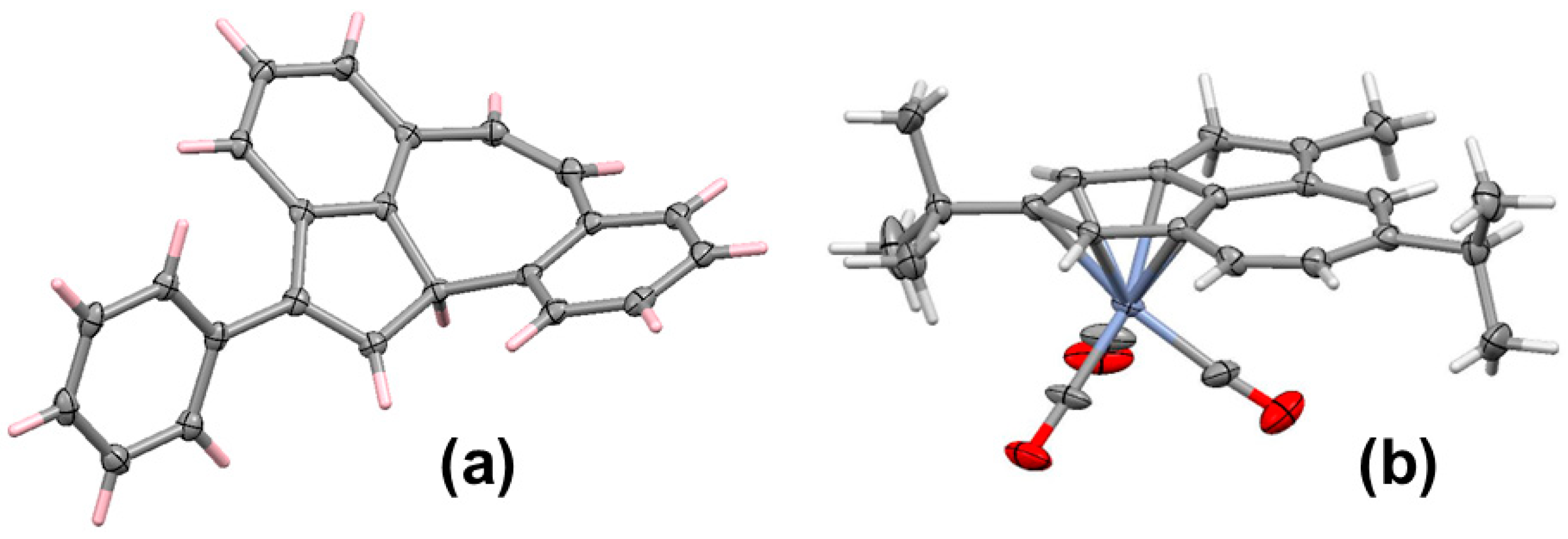
- Banide, E.V.; Oulié, P.; Müller-Bunz, H.; McGlinchey, M.J. Unexpected dimerization of a bis(fluorenyl)-bis(trimethylsilyl)-diallene to a dihydroquatercyclopentadiene, an octacyclic C36 fragment corresponding to 60% of C60: Reaction of the diallene with Fe2(CO)9. Organometallics 2008, 27, 5657–5664. [Google Scholar] [CrossRef]
- Salisbury, L. Synthesis of (fluoren-9-ylidene)(dibenzo[a,d]cycloheptene-5-ylidene)methane-allenes as ground-state carbenes. J. Org. Chem. 1972, 37, 4075–4077. [Google Scholar] [CrossRef]
- Saalfrank, R.W. Strongly thermostable “push-pull”-substituted allene. Tetrahedron Lett. 1975, 16, 4405–4408. [Google Scholar] [CrossRef]
- Sestrick, M.R.; Miller, M.; Hegedus, L.S. Photochemical-reactions of chromium alkoxycarbene complexes with stabilized ylides to produce push-pull captodative allenes. J. Am. Chem. Soc. 1992, 114, 4079–4088. [Google Scholar] [CrossRef]
- McGlinchey, M.J. Ferrocenyl migrations and molecular rearrangements: The significance of electronic charge delocalization. Inorganics 2020, 8, 68. [Google Scholar] [CrossRef]
- Casper, L.A.; Osswald, S.; Anders, P.; Rosenbaum, L.C.; Winter, R.F. Extremely electron-poor bis(diarylmethylium)-substituted ferrocenes and the first peroxoferrocenophane. Z. Anorg. Allg. Chem. 2020, 646, 712–715. [Google Scholar] [CrossRef]
- Banide, E.V.; Ortin, Y.; Chamiot, B.; Cassidy, A.; Niehaus, J.; Moore, A.; Seward, C.M.; Müller-Bunz, H.; McGlinchey, M.J. Syntheses, structures, and dimerizations of ferrocenyl- and fluorenylideneallenes: Push-pull multiple bonds. Organometallics 2008, 27, 4173–4182. [Google Scholar] [CrossRef]
- Toda, F. Naphthocyclobutenes and benzodicyclobutadienes: Synthesis in the solid state and anomalies in the bond lengths. Eur. J. Org. Chem. 2000, 2000, 1377–1386. [Google Scholar] [CrossRef]
- Bildstein, B.; Schweiger, M.; Kopacka, H.; Ongania, K.-H.; Wurst, K. Cationic and neutral [4]-cumulenes C=C=C=C=C with five cumulated carbons and three to four ferrocenyl termini. Organometallics 1998, 17, 2414–2424. [Google Scholar] [CrossRef]
- Banide, E.V.; Müller-Bunz, H.; Manning, A.R.; Evans, P.; McGlinchey, M.J. X-ray crystal structure of an alkene-pentacarbonyldicobalt-alkyne complex: Isolation of a stable Magnus-type Pauson-Khand reaction intermediate. Angew. Chem. Int. Ed. 2007, 46, 2907–2910. [Google Scholar] [CrossRef]
- Brusey, S.A.; Banide, E.V.; Dörrich, S.; O’Donohue, P.; Ortin, Y.; Müller-Bunz, H.; Long, C.; Evans, P.; McGlinchey, M.J. X-ray crystallographic and NMR spectroscopic study of (η2-alkene)(µ-alkyne)-pentacarbonyldicobalt complexes: Arrested Pauson-Khand reaction intermediates. Organometallics 2009, 28, 6308–6319. [Google Scholar] [CrossRef]
- Hartline, D.R.; Zeller, M.; Uyeda, C. Well-defined models for the elusive dinuclear intermediates for the Pauson-Khand reaction. Angew. Chem. Int. Ed. 2016, 55, 6084–6087. [Google Scholar] [CrossRef]
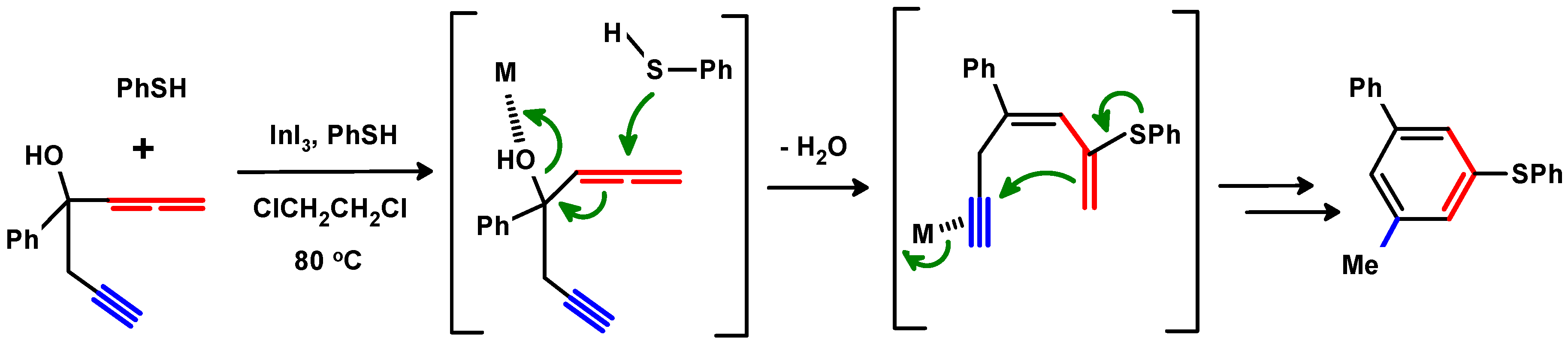
- Ma, J.; Peng, L.; Zhang, X.; Zhang, Z.; Campbell, M.; Wang, J. InI3 or ZnI2-catalyzed reaction of hydroxylated 1,5-allenynes with thiols: A new access to 3,5-disubstituted toluene derivatives. Chem. Asian J. 2010, 5, 2214–2220. [Google Scholar] [CrossRef]
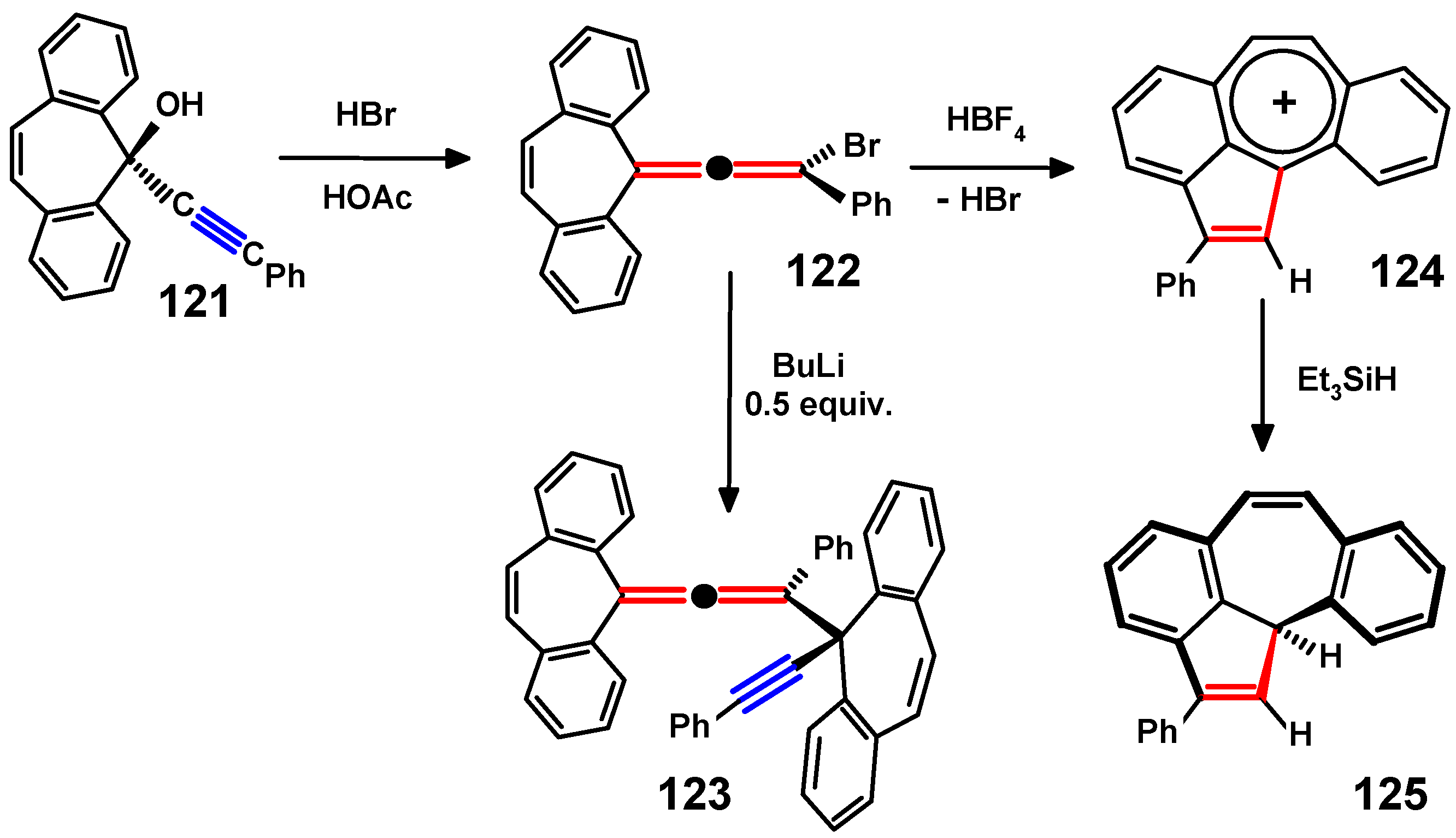
- Banide, E.V.; O’Connor, C.; Fortune, N.; Ortin, Y.; Milosevic, S.; Müller-Bunz, H.; McGlinchey, M.J. Syntheses, X-ray structures and reactivity of fluorenylidene- and dibenzosuberenylidene-allenes: Convenient precursors to dispirotetracenes, di-indenotetracenes and 2-phenyl-11bH-dibenz[cd,h]azulene. Org. Biomol. Chem. 2010, 8, 3997–4010. [Google Scholar] [CrossRef]
- Balduzzi, S.; Müller-Bunz, H.; McGlinchey, M.J. A convenient synthetic route to benz[cd]azulenes: Versatile ligands with the potential to bind metals in an η5, η6, or η7 fashion. Chem. Eur. J. 2004, 10, 5398–5405. [Google Scholar] [CrossRef]

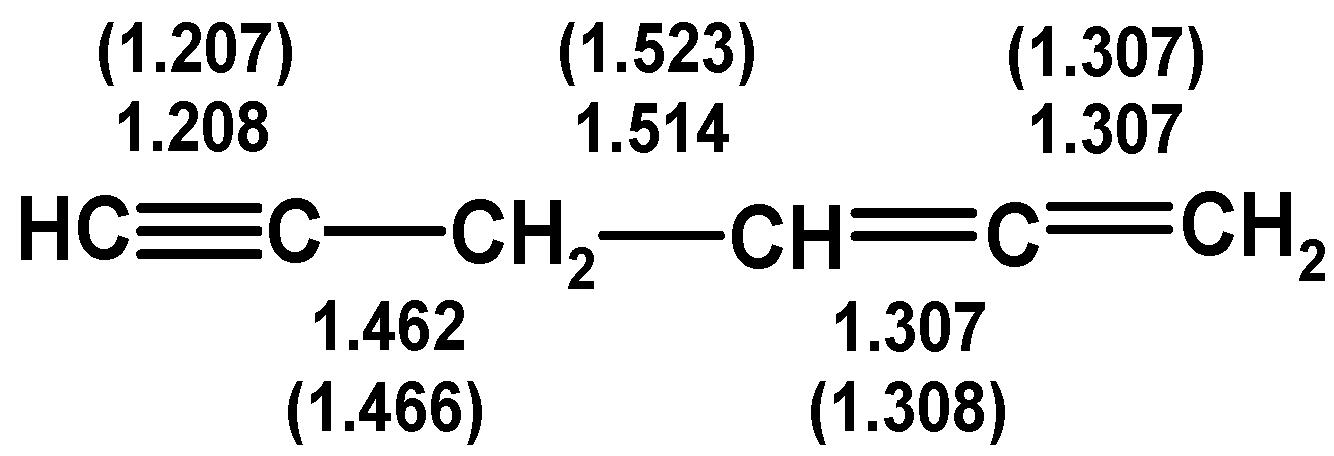



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGlinchey, M.J. Organometallic Chemistry of Propargylallenes: Syntheses, Reactivity, Molecular Rearrangements and Future Prospects. Molecules 2024, 29, 5670. https://doi.org/10.3390/molecules29235670
McGlinchey MJ. Organometallic Chemistry of Propargylallenes: Syntheses, Reactivity, Molecular Rearrangements and Future Prospects. Molecules. 2024; 29(23):5670. https://doi.org/10.3390/molecules29235670
Chicago/Turabian StyleMcGlinchey, Michael J. 2024. "Organometallic Chemistry of Propargylallenes: Syntheses, Reactivity, Molecular Rearrangements and Future Prospects" Molecules 29, no. 23: 5670. https://doi.org/10.3390/molecules29235670
APA StyleMcGlinchey, M. J. (2024). Organometallic Chemistry of Propargylallenes: Syntheses, Reactivity, Molecular Rearrangements and Future Prospects. Molecules, 29(23), 5670. https://doi.org/10.3390/molecules29235670




