Analysis of the Formation of Characteristic Aroma Compounds by Amino Acid Metabolic Pathways during Fermentation with Saccharomyces cerevisiae
Abstract
1. Introduction
2. Results and Discussion
2.1. Changes in the Content of Reducing Sugar and Pyruvic Acid
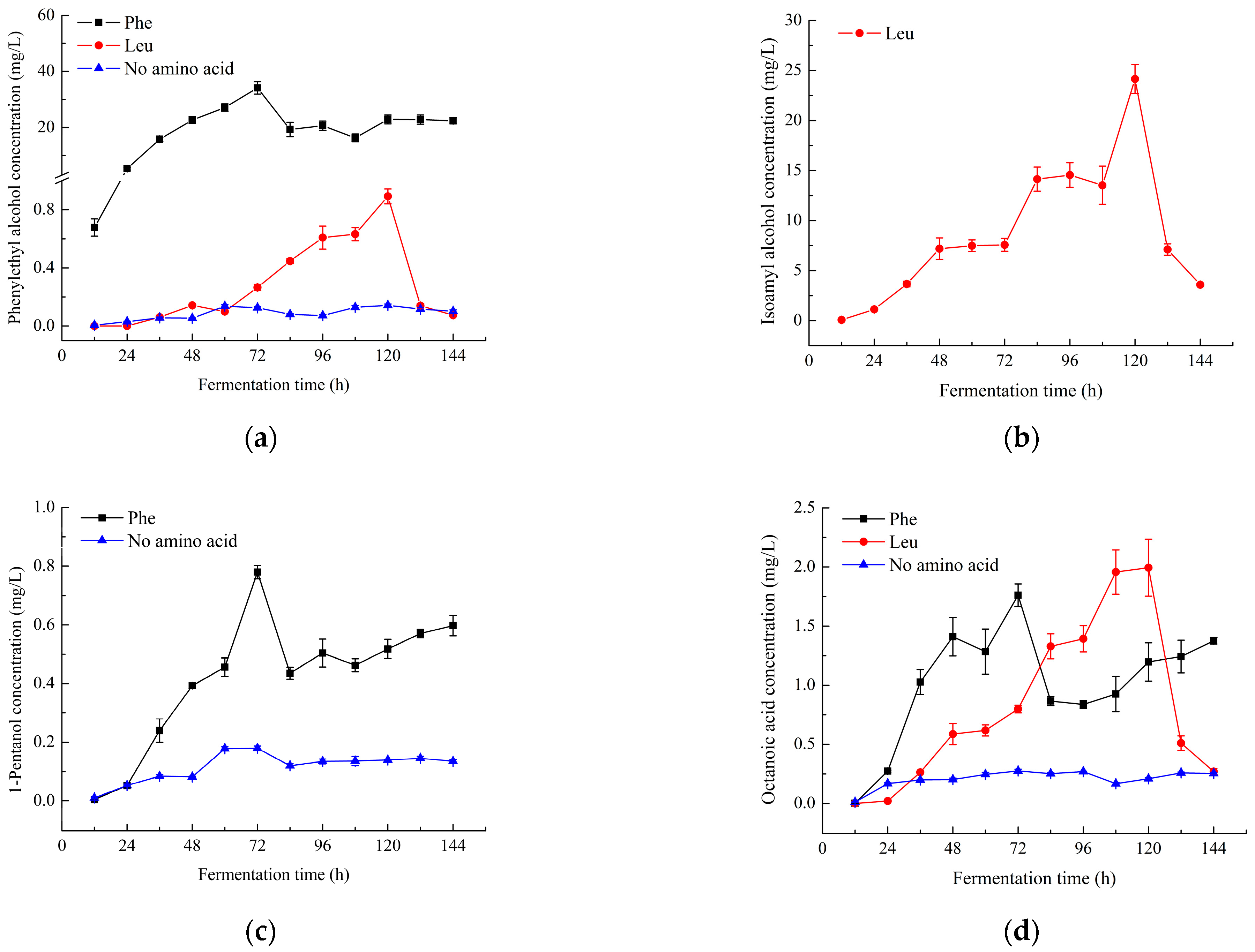
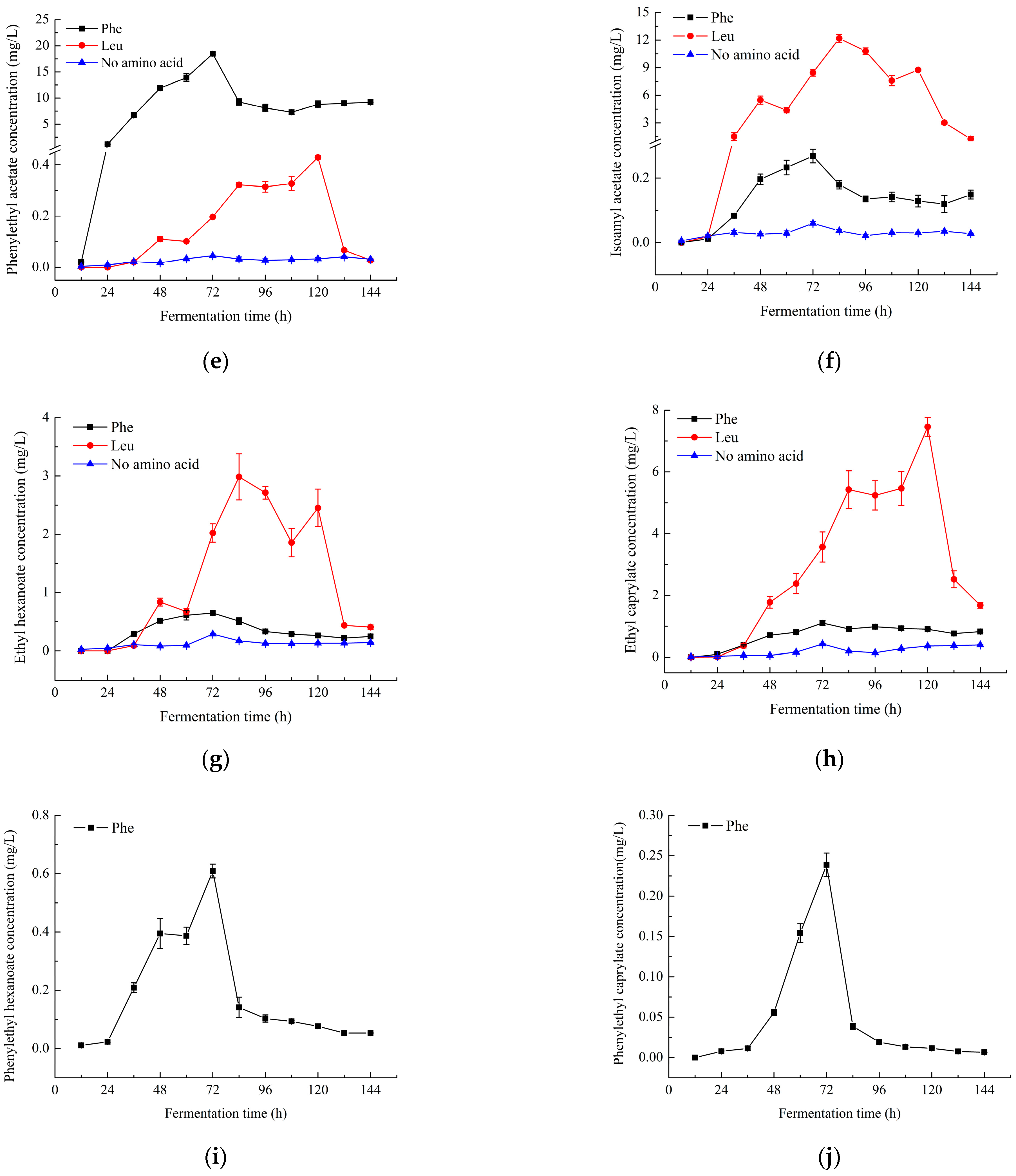
2.2. Changes in the Content of Characteristic Aroma Components
2.3. Changes in the Activities of Different Key Enzymes
2.4. Correlation Analysis of the Key Enzymes and Aroma Components
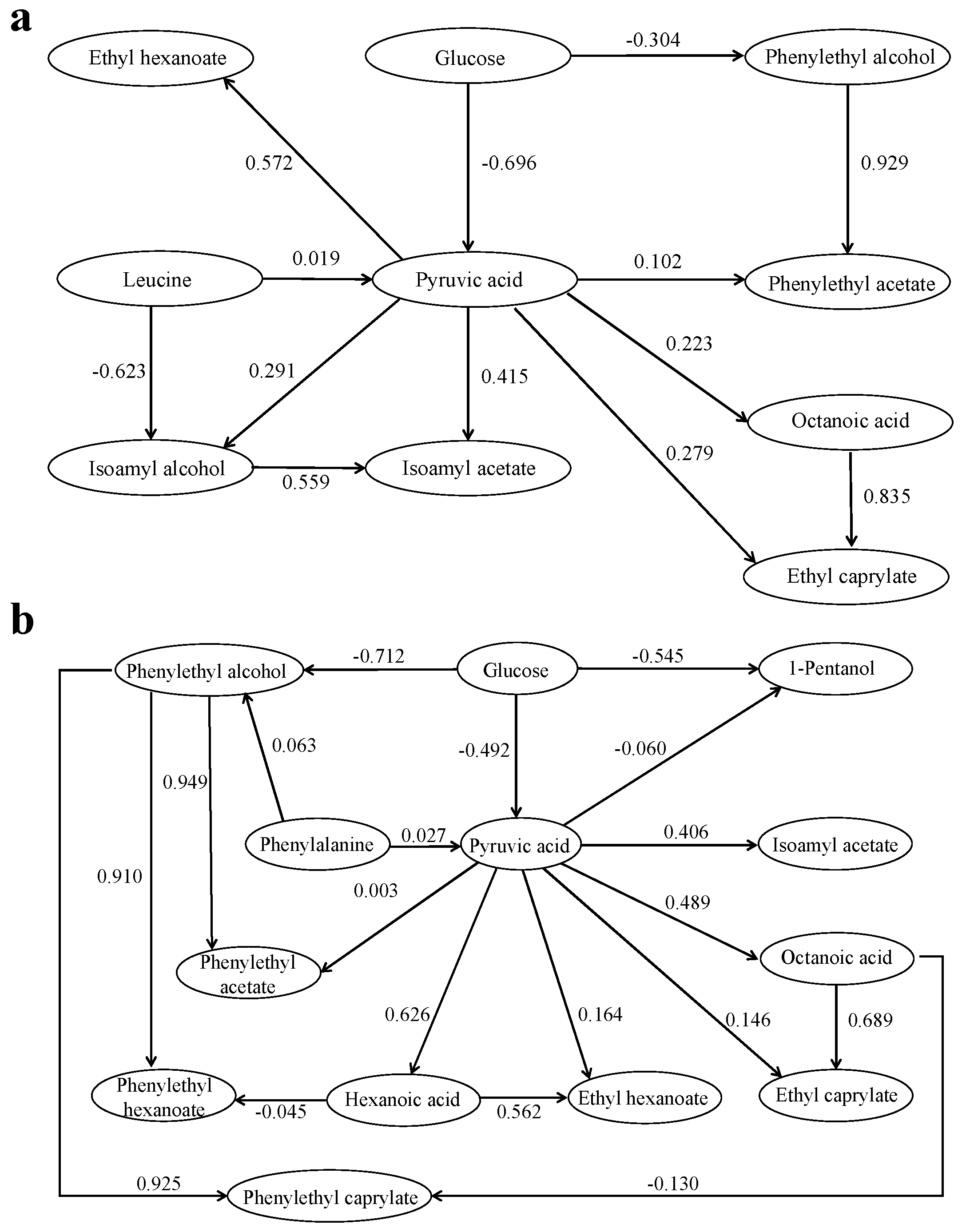
2.5. Path Analysis
3. Materials and Methods
3.1. Reagents
3.2. Preparation of Analytical Samples
3.3. Analytical Determinations
3.4. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vilanova, M.; Ugliano, M.; Varela, C.; Siebert, T.; Pretorius, I.S.; Henschke, P.A. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl. Microbiol. Biotechnol. 2007, 77, 145–157. [Google Scholar] [CrossRef]
- Bell, S.-J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Lilly, M.; Bauer, F.F.; Styger, G.; Lambrechts, M.G.; Pretorius, I.S. The effect of increased branched-chain amino acid transaminase activity in yeast on the production of higher alcohols and on the flavour profiles of wine and distillates. FEMS Yeast Res. 2006, 6, 726–743. [Google Scholar] [CrossRef]
- Trinh, T.T.T.; Woon, W.Y.; Yu, B.; Curran, P.; Liu, S.-Q. Effect of L-isoleucine and L-phenylalanine Addition on Aroma Compound Formation During Longan Juice Fermentation by a Co-culture of Saccharomyces cerevisiae and Williopsis saturnus. S. Afr. J. Enol. Vitic. 2010, 31, 116–124. [Google Scholar] [CrossRef]
- Kumar, K.; Venkatraman, V.; Bruheim, P. Adaptation of central metabolite pools to variations in growth rate and cultivation conditions in Saccharomyces cerevisiae. Microb. Cell Factories 2021, 20, 64. [Google Scholar] [CrossRef]
- Beltran, G.; Esteve-Zarzoso, B.; Rozès, N.; Mas, A.; Guillamón, J.M. Influence of the Timing of Nitrogen Additions during Synthetic Grape Must Fermentations on Fermentation Kinetics and Nitrogen Consumption. J. Agric. Food Chem. 2005, 53, 996–1002. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Ibarz, M.J.; Cacho, J.; Ferreira, V. Addition of amino acids to grape juice of the Merlot variety: Effect on amino acid uptake and aroma generation during alcoholic fermentation. Food Chem. 2006, 98, 300–310. [Google Scholar] [CrossRef]
- Hassing, E.-J.; de Groot, P.A.; Marquenie, V.R.; Pronk, J.T.; Daran, J.-M.G. Connecting central carbon and aromatic amino acid metabolisms to improve de novo 2-phenylethanol production in Saccharomyces cerevisiae. Metab. Eng. 2019, 56, 165–180. [Google Scholar] [CrossRef]
- Etschmann, M.M.W.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological production of 2-phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8. [Google Scholar]
- Dickinson, J.R.; Lanterman, M.M.; Danner, D.J.; Pearson, B.M.; Sanz, P.; Harrison, S.J.; Hewlins, M.J.E. A 13C Nuclear Magnetic Resonance Investigation of the Metabolism of Leucine to Isoamyl Alcohol in Saccharomyces cerevisiae. J. Biol. Chem. 1997, 272, 26871–26878. [Google Scholar] [CrossRef]
- Liu, P.; Ivanova-Petropulos, V.; Duan, C.; Yan, G. Effect of Unsaturated Fatty Acids on Intra-Metabolites and Aroma Compounds of Saccharomyces cerevisiae in Wine Fermentation. Foods 2021, 10, 277. [Google Scholar] [CrossRef]
- Nikolaou, E.; Soufleros, E.H.; Bouloumpasi, E.; Tzanetakis, N. Selection of indigenous Saccharomyces cerevisiae strains according to their oenological characteristics and vinification results. Food Microbiol. 2006, 23, 205–211. [Google Scholar] [CrossRef]
- Yuan, J.; Mishra, P.; Ching, C.B. Engineering the leucine biosynthetic pathway for isoamyl alcohol overproduction in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2017, 44, 107–117. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Álvarez-Fernández, M.A.; Fernández-Cruz, E.; Garcia-Parrilla, M.C.; Troncoso, A.M.; Mattivi, F.; Vrhovsek, U.; Arapitsas, P. Saccharomyces cerevisiae and Torulaspora delbrueckii Intra- and Extra-Cellular Aromatic Amino Acids Metabolism. J. Agric. Food Chem. 2019, 67, 7942–7953. [Google Scholar] [CrossRef]
- Englezos, V.; Cocolin, L.; Rantsiou, K.; Ortiz-Julien, A.; Bloem, A.; Seguinot, P.; Camarasa, C. Influence of Single Nitrogen Compounds on Growth and Fermentation Performance of Starmerella bacillaris and Saccharomyces cerevisiae during Alcoholic Fermentation. Appl. Environ. Microbiol. 2021, 87, e02485-20. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zhang, Z.; Peng, B. Correlation analysis of key enzyme activities and aroma compounds during fermentation of simulated juice system with Saccharomyces cerevisiae. LWT-Food Sci. Technol. 2019, 108, 214–220. [Google Scholar] [CrossRef]
- Gai, B.; Ji, B.; Zhang, H.; Zhou, F.; Jiang, H. Study on the Utilization of Amino Acids during the Cider Making. Food Ferment. Ind. 2005, 31, 34–38. [Google Scholar]
- Benito, A.; Calderon, F.; Benito, S. Combined use. of S. pombe and L. thermotolerans in winemaking. Benificial effects determined through the study of wines’ analytical characteristics. Molecules 2016, 21, 1744. [Google Scholar] [CrossRef]
- Tang, Y. The Building of Metabolic Flow in the Process of Saccharomyces Cerevisiae Fermentation; Dalian Polytechnic University: Dalian, China, 2014. [Google Scholar]
- Hu, Z.; Li, L.; Yuan, Y.; Yue, T. Ultrasensitive and simultaneous determination of twenty-one amino acids and amines in culture media, red wine and beer. Food Chem. 2014, 158, 56–65. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Chen, Y.; He, D.; Ni, L. Synthesis pathway of phenethyl acetate in Wickerhamomyces anomalus. J. Chin. Inst. Food Sci. Technol. 2015, 15, 48–53. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
 ) and L-phenylalanine (
) and L-phenylalanine ( ), respectively, during fermentation.
), respectively, during fermentation.
 ) and L-phenylalanine (
) and L-phenylalanine ( ), respectively, during fermentation.
), respectively, during fermentation.

| Isoamyl Alcohol | Isoamyl Acetate | Ethyl Hexanoate | Phenylethyl Alcohol | Phenylethyl Acetate | Octanoic Acid | Ethyl Caprylate | Ethyl Caprate | Pyruvic Acid | Glucose | Leucine | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LTR | 0.452 | 0.752 ** | 0.669 * | 0.321 | 0.511 | 0.4 | 0.494 | 0.152 | 0.758 ** | −0.395 | −0.376 |
| PK | 0.687 * | 0.623 * | 0.669 * | 0.798 ** | 0.759 ** | 0.834 ** | 0.783 ** | 0.599 * | 0.444 | −0.732 ** | −0.765 ** |
| Acetyl-CoA | 0.610 * | 0.238 | 0.321 | 0.685 * | 0.564 | 0.688 * | 0.613 * | 0.749 ** | 0.254 | −0.53 | −0.592 * |
| 1-Pentanol | Isoamyl Acetate | Hexanoic Acid | Ethyl Hexanoate | Phenylethyl Hexanoate | Phenylethyl Alcohol | Phenylethyl Acetate | Octanoic Acid | Ethyl Caprylate | Phenylethyl Caprylate | Pyruvic Acid | Glucose | Phenylalanine | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAL | 0.377 | 0.791 ** | 0.610 * | 0.897 ** | 0.773 ** | 0.599 * | 0.718 ** | 0.503 | 0.515 | 0.690 * | 0.817 ** | −0.384 | −0.389 |
| PK | 0.777 ** | 0.915 ** | 0.730 ** | 0.864 ** | 0.851 ** | 0.908 ** | 0.957 ** | 0.845 ** | 0.704 * | 0.882 ** | 0.488 | −0.611 * | −0.613 * |
| Acetyl-CoA | −0.09 | 0.163 | 0.355 | 0.278 | 0.321 | 0.093 | 0.153 | 0.252 | −0.004 | −0.075 | 0.722 ** | 0.082 | 0.065 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Yang, C.; Yang, Y.; Peng, B. Analysis of the Formation of Characteristic Aroma Compounds by Amino Acid Metabolic Pathways during Fermentation with Saccharomyces cerevisiae. Molecules 2023, 28, 3100. https://doi.org/10.3390/molecules28073100
Lu X, Yang C, Yang Y, Peng B. Analysis of the Formation of Characteristic Aroma Compounds by Amino Acid Metabolic Pathways during Fermentation with Saccharomyces cerevisiae. Molecules. 2023; 28(7):3100. https://doi.org/10.3390/molecules28073100
Chicago/Turabian StyleLu, Xingjun, Chao Yang, Yingdi Yang, and Bangzhu Peng. 2023. "Analysis of the Formation of Characteristic Aroma Compounds by Amino Acid Metabolic Pathways during Fermentation with Saccharomyces cerevisiae" Molecules 28, no. 7: 3100. https://doi.org/10.3390/molecules28073100
APA StyleLu, X., Yang, C., Yang, Y., & Peng, B. (2023). Analysis of the Formation of Characteristic Aroma Compounds by Amino Acid Metabolic Pathways during Fermentation with Saccharomyces cerevisiae. Molecules, 28(7), 3100. https://doi.org/10.3390/molecules28073100







