Abstract
Due to its abundance of physiologically active ingredients, one of the oldest medicinal herbs, elderberry (EB) Sambucus nigra L., is beneficial for both therapeutic and dietary purposes. This study determined the bioaccessibility of the phenolic compounds and the prebiotic potential of the polyphenols from freeze-dried EB powder (FDEBP), along with the antioxidant and antimicrobial activities of this extract. The most significant phenolic compounds in black EB are represented by anthocyanins (41.8%), predominating cyanidin-sambubiosides and cyanidin-glucosides (90.1% of the identified anthocyanins). The FRAP assay obtained the highest antioxidant activity value (185 ± 0.18 μmol Fe2+/g DW). The most sensitive to the antimicrobial activity of the extract was proven to be Staphylococcus aureus, and Pseudomonas aeruginosa had the lowest minimum inhibitory concentration of 1.95 mg/mL. To determine the prebiotic potential of the polyphenols, the cell growth of five probiotic strains (Lactobacillus plantarum, L. casei, L. rhamnosus, L. fermentum and Saccharomyces boulardii) was tested. The influence on cell growth was positive for all five probiotic strains used. Overall, the most significant increase (p < 0.05) was recorded at 1.5% FDEBP, on L. casei with a growth index (GI) of 152.44%, very closely followed by GI at 0.5% and 1% concentrations. The stability of the total phenolic compounds through simulated gastronitestinal digestion was increased (93%), and the bioaccessibility was also elevated (75%).
1. Introduction
Since prehistoric times, plants have been used for food and as remedies [1]. In recent years, the interest in food supplements and functional foods, which contain bioactive plant-based compounds, has continuously increased. Numerous scientific studies were carried out using different methods and revealed the benefits of products rich in bioactive compounds [2,3] for preventing and treating certain diseases, including cardiovascular and metabolic diseases, pathologies responsible for millions of deaths worldwide [4,5,6,7].
An important source of bioactive compounds, relatively little studied compared to other berries, is black elderberry (EB), Sambucus nigra L.—also known as elder, black elder, European elder, European black elder, and common elder; it is part of the family Adoxaceae, genus Sambucus, being a common species. It has three subspecies: S. nigra ssp. canadensis, S. nigra ssp. Cerulea and S. nigra ssp. nigra L., the latter being the European elder [8,9,10]. Since ancient times, black EB has been used as a natural remedy in the treatment of various ailments: EB was used in the treatment of colds and flu, constipation or for its diuretic and anti-inflammatory effects [10]; elderflowers were used as a remedy for various respiratory and skin conditions, joint pain or for the diuretic effect [11]; even the elder leaves were used to treat various skin diseases [12].
The existence of phenolic constituents, which have a significant antioxidant effect and can therefore remove free radicals and combat oxidative stress, a factor contributing to the deterioration of the human body and the emergence of several diseases, is mainly responsible for the therapeutic properties of EB [13,14]. Through the wide variety of polyphenols contained, anthocyanins, flavonols, phenolic acids, and proanthocyanidins, EBs have proven their multiple beneficial effects, showing cardiovascular protection [15], antidiabetic properties [16], the ability to counteract obesity and metabolic dysfunction [17], antiviral and antibacterial activity [18], antioxidant capacity [19], antitumor potential [20], antidepressant action [21], and more recently, the prebiotic effect [22].
Phenolic compounds can deteriorate as a consequence of being exposed to light, oxygen, enzymatic activity, unfavorable pH condition, temperature, water, and metal ions; thus, their positive qualities may be modified [23]. For better comprehension and assessment of the potential biological characteristics of phenolic compounds, it is essential to confirm their stability and absorption in the digestive tract. Phenolic components are subjected to physiochemical alteration (due to pH, temperature, and digestive enzymes) in the gastrointestinal environment [22]. Yet, the bioaccessibility of dietary phenolic compounds during gastrointestinal digestion (GID) determines their therapeutic effects [24].
From this perspective, in order to evaluate the biological properties, along with the aspects regarding the chemical composition, this study determined the bioaccessibility of the phenolic compounds after GID and the prebiotic potential of the polyphenols from black EB from the spontaneous flora of Romania. Additionally, the antioxidant and antimicrobial action was tested. As far as we know, this is the first study of this type on black EB. The results provide new insights into advancing knowledge and research opportunities for the development of new nutraceutical or adjunctive strategies that use this product’s bioactive potential.
2. Results
2.1. Antioxidant Activity Analysis
The antioxidant activity of the EB extract (S. nigra L.) was analyzed with the help of four different assay methods (DPPH, ABTS, FRAP, and CUPRAC), and the results are presented in Table 1. The lowest value was obtained by ABTS, and the highest with the FRAP assay.

Table 1.
Antioxidant capacity of S. nigra L. measured by different complementary assays.
2.2. Antimicrobial Activity Assay
The antimicrobial activity of the lyophilized EB powder extract was evaluated on a total of seven strains containing Gram-positive and Gram-negative bacteria and yeasts. The lyophilized black EB powder extract shows antimicrobial activity on all tested microorganisms. The most sensitive to the activity of the extract was proven to be S. aureus, P. aeruginosa and the two yeasts, the lowest minimum inhibitory concentration (MIC) being 1.95 mg/mL. However, in the case of S. enterica and both strains of E. coli, the MIC was weaker in comparison with the other tested strains, as it presented an MIC of 3.91 mg/mL. The results are presented in Table 2.

Table 2.
Minimum inhibitory concentration against Staphylococcus aureus, Salmonella enterica, Escherichia coli (25922 and 8739), Pseudomonas aeruginosa, Candida albicans, and C. parapsilosis.
2.3. Qualitative and Quantitative Analysis of the Extracts by HPLC-DAD-ESI-MS, before and after GID
The high-performance liquid chromatography (HPLC-DAD-ESI-MS) analysis of the extract from the powder obtained from lyophilized EB revealed the presence of 12 polyphenolic compounds belonging to the subclasses: anthocyanins, flavonols, hydroxycinnamic acids, and hydroxybenzoic acid derivatives.
Quantitative data show that the most significant amount of phenolic compounds in black EB is represented by anthocyanins, precisely 41.8%, predominating cyanidin-sambubiosides and cyanidin-glucosides, the two compounds constituting 90.1% of the identified anthocyanins. As for flavonols, they represented 25.5% of the total phenolic compounds in lyophilized EB. The HPLC-DAD analysis revealed the presence of quercetin derivatives (94%) and kaempferol. Rutin is the most present compound of this class, representing 75.7% of the total flavonols and 19.27% of the total phenolic compounds identified. Hydroxycinnamic acids were present in a proportion of 18.6%, and hydroxybenzoic acid derivatives in a proportion of 14.1%.
The total content of polyphenols in the analyzed extract was 41.28 mg/g of lyophilized EB powder, of which consisted 17.25 mg of anthocyanins, 10.51 mg of flavonols, 7.69 mg of hydroxycinnamic acid derivatives and 5.83 mg of hydroxybenzoic acid derivatives (Table 3).

Table 3.
The in vitro effect of gastrointestinal digestion on the phenolic content of EB mg/g.
2.4. The Bioaccessibility of Phenolic Compounds of Sambucus nigra L. Fruits during Simulated Digestion
The calculation of bioaccessibility was carried out according to the formula presented by Stefănescu et al. [25], which is:
BI (%) = (Phenolic content after gastrointestinal digestion (in vitro)/Phenolic content before digestion) × 100
Then, the individual phenolic compound content was assessed HPLC-DAD-ESI-MS in the gastric and intestinal phases. The results are presented in Table 3, and as can be seen, the anthocyanin content, such as cyanidin-diglucoside and cyanidin glucoside, decreased from 1.20 ± 0.07 and 15.56 ± 0.19 to 1.03 ± 0.09 and 8.14 ± 0.08 after SIF; cyanidin was only detected before digestion. Only hydroxybenzoic acids presented an increase throughout digestion, for instance, hydroxybenzoic acid from 3.49 ± 0.05 to 5.31 ± 0.11, and protocatechuic acid from 2.34 ± 0.11 to 7.79 ± 0.15.
The bioaccessibility (Table 4) of the bioactive compounds from EB can be perceived as the amount of the compound released inside the intestinal tract and available for assimilation. Before and after digestion, four compounds were detected, with a final bioaccessibility of 74.54 ± 5.7%.

Table 4.
The bioaccessibility of EB extract.
2.5. The Prebiotic Potential of the Phenolic Compounds of Sambucus nigra L. Fruits
The prebiotic potential of the freeze-dried EB powder (FDEBP) was tested on the following probiotic strains: L. plantarum, L. casei, L. rhamnosus, L. fermentum and S. boulardii, at three different concentrations: 0.5%, 1%, and 1.5% (w/v). The cell growth of the probiotic strains was tested after inoculation and after 24 h incubation period. The results are shown in Figure 1. For the control media, we used glucose as a carbon source and the difference of log10 CFU/mL between incubation and inoculation was expressed as 100% GI. The influence on cell growth was most prominent in the case of L. casei, which yielded significant positive growth for all tested concentrations of FDEBP, with the highest results of 152.44 % GI (p < 0.05) recorded at 1.5% FDEBP. Moreover, positive growth was also recorded for L. rhamnosus and L. plantarum, with the highest results recorded at 141.36% (p < 0.05) GI with 1.5% and GI of 133.31% (p < 0.05) at 0.5%, respectively. In the case of L. fermentum, the only tested concentration that yielded a positive GI of 115.04% (p < 0.05) was at 0.5% FDEBP concentration. However, the least growth influence was recorded by the S. boulardi strain for which the 1.5% FDEBP yielded a significant GI of 107.22%. Overall, the results showed that most of the probiotic tested strains presented a positive growth influence with FDEBP as a carbon source in comparison with glucose; thus, the tested samples exhibited a prebiotic potential.
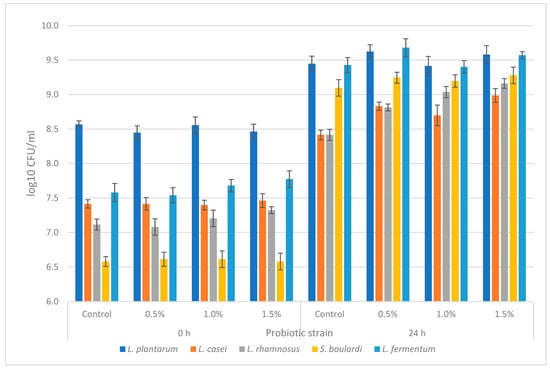
Figure 1.
Cell viability of probiotic strains after inoculation and after 24 h (n = 3).
3. Discussion
The most significant bioactive substances found in EB in relatively high concentrations are polyphenols, which are recognized for their free radical scavenging (antioxidant) action [26]—the proprieties of each active compound being strongly correlated and induced by its unique structure [27]. As a result, most of the studies carried out on elderberries have evaluated their phenolic compound content, and thus are recognized for their multiple beneficial effects on health. There are fewer studies that follow up what happens to these bioactive compounds during digestion, and the present study proposed the quantitative and qualitative evaluation of phenolic compounds through HPLC after simulating digestion. Bioaccessibility can be defined as the amount of an ingested nutrient that is available for absorption in the gut after digestion [28] and it is an essential aspect to follow—it is known that phenolic compounds are unstable under certain conditions, and GID involves pH and temperature variations, contact with digestive enzymes, etc. [25,29].
The main polyphenols found in EB, according to published data, are chlorogenic acid, cryptochlorogenic acid, neochlorogenic acid, kaempferol-3-glucoside (astragaline), kaempferol-3-rutinoside, isorhamnetin-3-rutinoside, quercetin-3-glucoside (isoquercitrin), quercetin-3-rutinoside (rutin), quercetin. Rutin is the main flavonoid found in this plant, while EB also contains minor levels of astragaline and isoquercitrin [26]. Additionally, in our study, we identified the main phenolic compounds to be cyanidin-glucoside and cyanidin-sambubioside followed by rutin and the other compounds identified cyanidin-diglucoside, cyanidin-sambubioside-glucoside, hydroxybenzoic acid, protocatechuic acid, chlorogenic acid, caffeic acid, cyaniding, kaempferol-diglucoside, feruloyquinic acid, quercetin-glucoside and quercetin.
EBs present strong anti-inflammatory characteristics that are linked to their significant antioxidant properties. Compared to similar studies, such as by Imenšek et al. [30], who also analyzed the antioxidant activity from specific hybrids of S. nigra, the DPPH (96 ± 14 μmol TE/g DW) and FRAP (208 ± 25 μmol TE/g DW) results were similar; however, the ABTS values (130 ± 14 μmol TE/g DW) were higher than in our study. This study also showed the effect of maturation on the antioxidant activity of the selected plants, which indicated a growing pattern.
Data from the specialized literature attribute the antimicrobial effect to tannins and triterpenes, as well as to peptides and oligosaccharides that are present in black EB [31]. The authors of a recent study, however, draw attention to the fact that the antimicrobial activity is due to the combination of bioactive compounds from the black EB extract, rather than to certain compounds considered individually [9]. The antimicrobial activity of EB extracts has been demonstrated in several previous studies on several Gram-positive and Gram-negative bacterial strains from the following genera: Staphylococcus, Pseudomonas, Enterococcus, Escherichia, Streptococcus, Klebsiella, Bacillus, Corynebacterium, Proteus [9,32,33,34]. Mohammadsadeghi et al. showed that EB extract had an inhibitory effect on the development of some Candida species, including Candida albicans [32]. In none of the existing examples in the scientific literature, however, were identified studies on the antimicrobial effect on Candida parapsilosis, a pathogenic agent causing fungal diseases associated with significantly increased morbidity and mortality [35,36]. The present antimicrobial activity analysis results reveal a significant antimicrobial potential of EB at a concentration between 1.95 mg/mL and 3.91 mg/mL lyophilized EB powder extract. Furthermore, to our knowledge, it is the first study to demonstrate the antimicrobial effect on C. parapsilosis.
The bioaccessibility testing of phenolic compounds was carried out using the updated in vitro static digestion method, developed by the INFOGEST working group [37]. The in vitro digestion methods have proven to be an efficient and useful solution in anticipating the effects of in vivo digestion [38]. Anthocyanins appear to be the most unstable polyphenolic compounds during GID. In the gastric phase, the amount decreases, compared to the amount found in the extract, by 13.4%, and after intestinal digestion, the bioaccessibility of anthocyanins is 53.2%. The biggest decrease was recorded for cyanidin-glucosides and cyanidin-sambubiosides, respectively, while cyanidin was not detected by HPLC. Moreover, the data from the scientific literature mention the instability of anthocyanins in an alkaline environment [39], an aspect that is also confirmed in our study. In the intestinal tract, their hydrolysis or degradation takes place, forming phenolic acids and aldehydes. Variations at the B ring level in the structure of anthocyanins determine the degradation of cyanidin and the formation of protocatechuic acid [40]. This is one of the explanations for the spectacular changes in the amount of hydroxybenzoic acid derivatives, the subclass that became the most predominant after digestion—the total amount increased by 225% in the intestinal phase compared to the amount in the extract. The bioaccessibility of protocatechuic acid was 332.34%. Hydroxybenzoic acid showed an increase of 152.23%, the hypothesis being that HA acid can be generated either as a degradation product of anthocyanins, or as a metabolite [41,42]. Derivatives of benzoic acid have demonstrated their cardiovascular protective effects, action against cancer and obesity along with the inhibition of the inflammatory response in inflammatory bowel diseases [43,44,45].
Regarding flavonols, their presence decreases in the gastric phase compared to time 0 by 23.6%, and at the end of the intestinal phase, 60.6% of the initial amount remains. Quercetin is the flavonol that was not detected by HPLC in any of the two gastrointestinal phases. The elderberry extract contained hydroxycinnamic acids in a proportion of 18.6% of the total phenolic compounds; at the end of the gastric phase, they represented 6.95%, and 6.90% in the intestinal phase. Numerous studies carried out on polyphenols have shown a higher stability of phenolic compounds in the gastric phase with their degradation in the intestinal tract [46,47,48]. In our study, the stability of the total phenolic compounds was high (93%), and the bioaccessibility in the intestinal phase was 75%.
As there is a degree of complementarity between bioaccessibility, the prebiotic effect and overall health benefits of the bioactive compounds [49], we also monitored in the study the prebiotic potential of the phenolic compounds from black elderberry.
Prebiotics are defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [50], and according to the newest definition and recent studies, polyphenols were shown as a prebiotic substrate [51]. It seems that the relationship between polyphenols-intestinal microbiota is mutual: phenolic compounds can modulate the intestinal microbiota and, at the same time, microorganisms have the ability to modulate the activity of polyphenols [52]. Various preclinical studies have shown that dietary polyphenols have a prebiotic effect, stimulating the growth of different beneficial microorganisms [53,54,55]. This effect was attributed especially to anthocyanins, proanthocyanidins and catechins [56]. A previous longitudinal intervention study followed the prebiotic properties of a purified extract from EB, observing a major change in microbial diversity immediately after initiating the administration of the extract. Furthermore, in some study participants, the relative abundance of Akkermansia spp. increased even after supplementation was completed [22].
As far as we know, our study is among the first studies that investigated the prebiotic potential of phenolic compounds from black EB, specifically on L. plantarum, L. casei, L. rhamnosus, L. fermentum and S. boulardii. The prebiotic potential was proven on all five strains, withal, more in vitro and in vivo studies are necessary to support the findings, and to implicitly demonstrate the prebiotic potential of these compounds and their possible health-effects. However, through the results obtained regarding the prebiotic potential of the tested bacterial strains and the antimicrobial effect on C. parapsilosis, the current study offers new research perspectives unexplored until now, which can increase the use of bioactive potential of black EB fruits.
4. Materials and Methods
4.1. Plant Material
The present study used black Sambucus nigra L. fructus (elderberry) from the spontaneous flora of Romania, Bihor County (46°43′36.5″ N, 21°54′32.4″ E). The species were identified in the Pharmaceutical Botany department of the Faculty of Medicine and Pharmacy, Oradea University, Romania.
The EBs were harvested in September 2022, frozen at −20 °C and then lyophilized, using a Telstar Lyo Quest 55 plus lyophilizer (Azbil Group, Terrassa, Spain) at a temperature of −55 °C and pressure of 0.001 mbar for 72 h. Then, the lyophilized fruits were transformed by grinding and sieving into a fine powder and kept in the dark until the determinations were made.
4.2. Methanolic Extraction
The fine powder obtained from lyophilized EB (0.5 g) was extracted with 10 mL of methanol acidified with 1% hydrochloric acid of concentration 37% by vortexing (Heidolph Reax top, Heidolph Instruments, Schwabach, Germany) for 1 min, then sonication in an ultrasonic bath (Elmasonic E 15 H, Elma Schmindbauer, Singen, Germany) for 15 min and centrifugation (10,000 rpm for 10 min at 24 °C) in an Eppendorf AG 5804 centrifuge (Eppendorf, Hamburg, Germany). The extraction process was repeated until the complete decoloration of the sample was achieved. At the end of each extraction, the supernatant was filtered through a 0.45 µm Chromafil Xtra nylon filter (Macherey-Nagel, Duren, Germany) and combined in a flask. The obtained extract was brought to dryness by evaporating the solvent with a rotary evaporator (Rotavapor R-124, Buchi, Flawil, Switzerland) and brought back into a known volume of methanol (mL solvent retook). The extract solution was used for identifying and quantifying phenolic compounds from the lyophilized EB extract, using high-performance liquid chromatography (HPLC), respectively, for the determination of antioxidant and antimicrobial activity.
4.3. Qualitative and Quantitative Determinations of Phenolic Compounds Phenolic Compounds from Freeze-Dried Elderberry Extract
To identify and quantify the phenolic compounds from the lyophilized EB powder extract, an HPLC-DAD-ESI-MS system was used, consisting of an Agilent 1200 HPLC with a UV–vis detector (DAD) coupled to a mass detector (MS) with a single quadrupole Agilent 6110 (Agilent Technologies, Santa Clara, CA, USA). For the separation of phenolic compounds, the Kinetex XB C18 column (Phenomenex, Torrance, CA, USA) was used, having, as mobile phases, water + 0.1% acetic acid (solvent A), and acetonitrile + 0.1% acetic acid (solvent B), at a temperature of 25 °C, for 30 min, with a flow rate of 0.5 mL/min. The elution program was as follows: 5% B (0 min); 5% B (0–2 min); 5–40% B (2–18 min); 40–90% B; 90% B (20–24 min); 90–5% B (24–25 min); 5% B (25–30 min). For MS fragmentation, the ESI (+) ionization module was used, with a scan range between 120 and 1200 m/z, capillary voltage of 3000 V, at a temperature of 350 °C, and nitrogen flow of 7 L/min. The spectral values were recorded for all peaks in the 200–600 nm range. Phenolic compounds were identified at 280 nm, 340 nm and 520 nm. The results were analyzed using the Agilent ChemStation software (Rev B.02.01 SR2, Palo Alto, CA, USA). The phenolic compounds in the EB extract were identified considering the retention intervals; UV–vis absorption spectra and mass spectra were recorded for each peak. For the quantification of phenolic compounds, calibration curves were made with standard substances. Thus, for the quantification of the identified anthocyanins, a calibration curve was made with Cyanidin (R2 = 0.9951). For the quantification of hydroxybenzoic acids, the calibration curve was made with gallic acid (R2 = 0.9978), hydroxycinnamic acids were quantified as a chlorogenic acid equivalent (R2 = 0.9937), and flavonols as rutin equivalent (R2 = 0.9981).
4.4. Antioxidant Activity Assay
The antioxidant activity of the EB extract was tested using four complementary methods: DPPH, FRAP, ABTS and CUPRAC.
The DPPH (2,2-diphenyl-1-picrylhydrazyl) test was based on the ability of the compound to donate an electron (H+) from the structure to the DPPH radical. For the determination, the protocol previously reported by Brand-Williams et al. [57] was applied, which is the most frequently used in studies,. To summarize, lyophilized EB extract (35 μL) was mixed with 250 μL of the DPPH solution (0.02 mg/mL) and incubated for 30 min in the dark; then, the absorbance was measured at 517 nm. The resulting data were expressed as a micromole Trolox equivalent (μmol TE)/g sample.
The antioxidant method of neutralizing the ABTS radical is based on the reaction between ABTS [2,20-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] and a compound with antioxidant activity. This reaction causes a decrease in absorbance [58]. The determination was made according to the protocol described by Arnao et al. [59], adjusted to be suitable for the 96-well microplates. In short, 20 μL of the lyophilized EB extract was mixed with 170 μL of ABTS and incubated for 6 min in the dark, then the absorbance was measured at 734 nm using a BioTek microplate reader (Synerg y HT, BioTek Instruments, Winooski, VT, USA). The resulting data were expressed as a micromole μmol TE/g sample.
The FRAP (Ferric Reducing Antioxidant Power) method is a colorimetric method that quantifies the ability of compounds with antioxidant activity to reduce (Fe3+) to (Fe2+) [60]. The assay was performed according to the protocol described by Benzie and Strain [61]. A total of 20 μL of the sample extract was added to 180 μL of the FRAP reagent. After an incubation time of 3 min, the absorbance was measured at 593 nm. The antioxidant potential was expressed as the μM Fe2 equivalent/g sample.
The CUPRAC test (cupric ion reducing antioxidant capacity) is a spectrophotometric technique which measures the antioxidant capacity of a compound, based on the ability of antioxidants to reduce (Cu2+) to (Cu+) [62]. The determination was performed based on the protocol described by Apak et al. [63]. The results were expressed as the μmol TE/g sample.
4.5. Antimicrobial Capacities
The following seven standard strains were tested: Escherichia coli ATCC 25922, E. coli ATCC 8739, Staphylococcus aureus ATCC 29213, Pseudomonas aeruginosa ATCC 27853, Salmonella enterica NCTC 6017, Candida albicans ATCC 10231 and C. parapsilosis ATCC 22019. They were all acquired from American Type Culture Collection (ATCC), VA, USA. The microorganisms were grown on a specific medium, Tryptic Soy agar (M1968, HiMedia Laboratories, Pvt. Ltd., Thane, India) for E. coli ATCC 8739, P. aeruginosa and both Candida strains, and on Mueller–Hinton agar (Oxoid Ltd., Basingstoke, Hampshire, England) for the others, within the Food Biotechnology Laboratory of the University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca, Romania. The plates were incubated for 24 h at 37 °C for bacteria and 30 °C for yeasts, respectively. Bacterial and yeast morphology were confirmed by optical microscopy [64].
For each tested strain, several colonies cultivated on agar plates (Oxoid Ltd., Basingstoke, Hampshire, UK) were transferred in a sterile saline solution (8.5 g/L NaCl) and adjusted to match the turbidity of McFarland 0.5 standard which corresponded to 1.5–3 × 108 CFU/mL. Then, bacterial suspensions were serially diluted 10-fold in a ratio of 1:9 in sterile serum, and 105 CFU/mL solutions were added to each microplate well.
The minimum inhibitory concentration (MIC) was determined using the resazurin microtiter plate-based antibacterial assay [65,66,67]. A total of 100 µL of sterile specific growth broth medium was added to the wells of a 96-well microplate. Then, 100 µL of lyophilized EB methanolic extract (FDEBME) was added in the first well, and serial 11-fold dilutions were made in the subsequent wells of each row by transferring 100 µL from well to well. The surplus of 100 µL in the last well of the row was discarded. Then, 10 µL of appropriate inoculum was added to all wells. The positive control was gentamicin (0.4 mg/mL in saline solution). The extracts’ solvent solution (methanol: H2O 1:1) was added as a negative control. The microplates were incubated for 20–22 h at 37 °C or 30 °C, respectively, and then 20 µL of the 0.2 mg/mL resazurin aqueous solution was added in all wells. The microplates were subjected to a subsequent two-hour incubation. After this period, resazurin (a blue non-fluorescent dye) was oxidized to resorufin (fluorescent pink) wherever the wells contained viable bacterial cells. Thus, the concentration in the last well on each row that remained blue was considered to completely inhibit bacterial growth, the MIC. The assay was run in triplicate. The results are expressed as the mean ± standard deviation.
4.6. Static In Vitro Digestion of the S. nigra Samples
The updated static in vitro digestion method, developed by the INFOGEST working group, was used to simulate the GID of the samples. The protocol extensively described by Brodkorb et al. [37] is based on sequential oral, gastric and intestinal digestion. In contrast, parameters such as electrolytes, enzymes, bile, pH, dilution, and digestion time are established on available physiological data. The samples (microcapsules and lyophilized powder of S. nigra fruits) were subjected to a three-stage in vitro digestion process, mimicking the conditions of the mouth, stomach and small intestine. Due to the absence of starch in the matrix, the oral phase was conducted without amylase.
The samples (2 g) were diluted with 3 mL of water to achieve the proper consistency and were further diluted 1:1 (wt/wt) with simulated oral fluid (SOF) to achieve a swallowable bolus with a paste-like consistency. The SOF was composed of electrolyte solutions KCl, KH2PO4, NaHCO3, NaCl, MgCl2·6H2O, (NH4)2CO3, alongside CaCl2(H2O)2 and water. Further, the oral bolus was mixed with 10 mL of the simulated gastric fluid (SGF). The SGF was composed of electrolyte solutions KCl, KH2PO4, NaHCO3, MgCl2·6H2O, (NH4)2CO3, alongside CaCl2(H2O)2 solution (0.3 M), porcine pepsin (2000 U/mL in the final digestion mixture), and water. The pH of the samples was adjusted to 3 by adding HCl (1 M), and the mixture was homogenized and incubated for 2 h in a shaking incubator (New Brunswick Innova 44, Eppendorf AG, Hamburg, Germany). For the intestinal phase, the samples were mixed with 20 mL of pre-warmed simulated intestinal fluid (SIF) to achieve a final ratio of 1:1 (v/v). The SIF was composed of electrolyte solutions KCl, KH2PO4, NaHCO3, NaCl, MgCl2·6H2O, alongside CaCl2(H2O)2, the bile extract solution (10 mM in total digesta) and pancreatic enzymes (100 U/mL). The pH was set to 7 using NaOH (1 M), and the mixture was homogenized and incubated at 37 °C for 2 h in a shaking incubator (95 rpm). After the process was complete, 1 mL of the samples was filtered and further analyzed by HPLC to establish the bioaccessibility index.
4.7. The Prebiotic Potential of Phenolic Compounds of Sambucus nigra L. Fruits
To determine the prebiotic potential of the FDEBP, we tested its influence on the cell growth for the following probiotic strains: L. plantarum ATCC 14917, L. casei ATCC 393, L. rhamnosus LMG 25626, L. fermentum CECT5716 Lc40 and S. boulardii MYA 796, which were all acquired from the American Type Culture Collection (ATCC, Manassas, VA, USA).
The probiotic strains were obtained in freeze-dried powder form. They were activated in test tubes containing 10 mL of Man–Rogosa–Sharpe (MRS) broth (1.10661, Merck, Rahway, NJ, USA) for Lactobacillus strains and Potato Dextrose (PD) broth (GM403, HIMEDIA) for probiotic yeast S. boulardii. The tubes were incubated aerobically for 18–24 h at 37 °C in case of bacteria and at 30 °C in case of the yeast. The grown microorganisms were propagated further (10% v/v) in 100 mL flasks with 45 mL of fresh sterile media, that were used as inoculum after incubation in same conditions [68].
For the prebiotic potential assay, freeze-dried EB powder was used as the carbon source in the formulation of MRS broth and Potato Dextrose broth in three different concentrations: 0.5%, 1% and 1.5% (w/v). The control media consisted of MRS or Potato Dextrose broth with glucose as a carbon source. For the experiment, 50 mL of media in 100 mL flasks, containing either 0.5%, 1% or 1.5% of FDEBP or glucose as a carbon source, was autoclaved at 121 °C for 15 min. After that, the media was cooled to room temperature and inoculated in sterile conditions with 10% (v/v) inoculum. The inoculum used in the assay was obtained by overnight culturing in MRS broth or PD broth, respectively, for each strain. The flasks were incubated in the Heidolph 1000 shaking incubator (Heidolph Instruments, Schwabach, Germany) with 150 rpm for 24 h at 37 °C or 30 °C, respectively, in aerobic conditions.
The cell growth of the probiotic strains was tested after inoculation and after a 24 h incubation period by using the pour plate method for Lactobacillus strains and the spread plate method for the yeast of the serially diluted samples. Roughly 1 mL of the sample was serially diluted in 9 mL of the sterile serum solutions (0.85% w/v NaCl solution), and 1 mL of the tested dilution was pipetted in a sterile Petri dish over which 15–20 mL of semi-molten agar was poured, followed by mixing and cooling in the case of the pour plate method. For the spread plate method, 100 µL of the tested dilution was pipetted on a solidified agar plate and spread with a Driglaski spatula until complete absorption. The plates were then incubated at appropriate temperatures for 24–48 h in aerobic conditions, after which the grown colonies were counted. The experiments were run in triplicate, and the results were expressed as the mean colony-forming units CFU/mL ± standard deviation (n = 3). The prebiotic potential was calculated as follows: growth index GI (%) = (sample log10 CFU/mL 24 h − 0 h)/(control log10 CFU/mL 24 h − 0 h) × 100 for each strain. The difference between 24 h incubation and inoculation viability of each control was expressed as 100% GI.
5. Conclusions
The results show that the black elder, from the spontaneous flora of Romania, presents a high antioxidant and antimicrobial potential. Polyphenols showed a significant bioaccessibility index. During gastrointestinal digestion, anthocyanins were the most unstable polyphenolic compounds, while hydroxybenzoic acid derivatives increased significantly in the intestinal phase, compared to the amount in the extract. Moreover, this study provided, for the first time, results regarding the prebiotic potential of elderberries on L. plantarum, L. casei, L. rhamnosus, L. fermentum and S. boulardii, opening new directions for research and exploration of these berries. For future perspectives, in vivo studies should also be carried out in order to confirm the health benefits of elderberry.
Author Contributions
Conceptualization, I.M.H. and D.M.T.; methodology, I.M.H., B.-E.T., K.S., E.S., F.R. and Z.M.D.; software, F.R. and D.-C.V.; validation, B.-E.T., K.S., D.M.T. and D.-C.V.; formal analysis, B.-E.T.; investigation, E.S., K.S., M.N., A.L.P. and F.R.; resources, I.M.H., M.N. and D.-C.V.; data curation, B.-E.T., D.M.T. and K.S.; writing—original draft preparation, I.M.H., A.L.P. and B.-E.T.; writing—review and editing, I.M.H., D.M.T. and D.-C.V.; visualization, D.M.T. and D.-C.V.; supervision, D.M.T. and D.-C.V.; project administration, D.M.T. and D.-C.V.; funding acquisition, D.M.T. and D.-C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 101007783—FRIETS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the whole team from the Department of Food Science, University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca for their continued support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Martău, G.A.; Teleky, B.-E.; Odocheanu, R.; Soporan, D.A.; Bochis, M.; Simon, E.; Vodnar, D.C. Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules 2023, 28, 1533. [Google Scholar] [CrossRef]
- Khuntia, A.; Martorell, M.; Ilango, K.; Bungau, S.G.; Radu, A.F.; Behl, T.; Sharifi-Rad, J. Theoretical evaluation of Cleome species’ bioactive compounds and therapeutic potential: A literature review. Biomed. Pharmacother. 2022, 151, 113161. [Google Scholar] [CrossRef]
- Pallag, A.; Bungau, S.; Tit, D.M.; Jurca, T.; Sirbu, V.; Honiges, A.; Horhogea, C. Comparative study of polyphenols, flavonoids and chlorophylls in Equisetum arvense L. populations. Rev. Chim. 2016, 67, 530–533. [Google Scholar]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.-E.; Ranga, F.; Roman, I.; Khaoula, H.; Boudaya, E.; Ltaief, A.B.; Aouani, W.; Thiamrat, M.; Vodnar, D.C. Carotenoid Recovery from Tomato Processing By-Products through Green Chemistry. Molecules 2022, 27, 3771. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, N.; Tian, J.; Xin, G.; Liu, L.; Sun, X.; Li, B. Advanced approaches for improving bioavailability and controlled release of anthocyanins. J. Control. Release 2022, 341, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Teleky, B.E.; Martău, G.A.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Biofunctional soy-based sourdough for improved rheological properties during storage. Sci. Rep. 2022, 12, 17535. [Google Scholar] [CrossRef] [PubMed]
- Sambucus, L. Taxonomic Serial No.: 35315. Integrated Taxonomic Information System. Available online: http://www.itis.gov (accessed on 14 February 2023).
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Swierk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska; Stuper-Szablewska, K. Sambucus nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—a review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Ho, G.T.T.; Zou, Y.F.; Aslaksen, T.H.; Wangensteen, H.; Barsett, H. Structural characterization of bioactive pectic polysaccharides from elderflowers (Sambuci flos). Carbohydr. Polym. 2016, 135, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.; Plastina, P.; Meijerink, J.; Witkamp, R.F.; Gabriele, B. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from Southern Italy: Fatty acid composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chem. 2013, 140, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Kashi, D.S.; Shabir, A.; Da Boit, M.; Bailey, S.J.; Higgins, M.F. The Efficacy of Administering Fruit-Derived Polyphenols to Improve Health Biomarkers, Exercise Performance and Related Physiological Responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef] [PubMed]
- Pascuta, M.S.; Vodnar, D.C. Nanocarriers for sustainable active packaging: An overview during and post COVID-19. Coatings 2022, 12, 102. [Google Scholar] [CrossRef]
- Festa, J.; Singh, H.; Hussain, A.; Da Boit, M. Elderberry extract inhibits tumour necrosis factor induced monocyte adhesion to endothelial cells via modulation of the NF-κB pathway. Cardiovasc. Res. 2022, 118, cvac066-172. [Google Scholar] [CrossRef]
- Salvador, Â.C.; Król, E.; Lemos, V.C.; Santos, S.A.O.; Bento, F.P.M.S.; Costa, C.P.; Almeida, A.; Szczepankiewicz, D.; Kulczyński, B.; Krejpcio, Z.; et al. Effect of elderberry (Sambucus nigra L.) extract supplementation in STZ-induced diabetic rats fed with a high-fat diet. Int. J. Mol. Sci. 2017, 18, 13. [Google Scholar] [CrossRef]
- Zielińska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczyński, R. Elderberry (Sambucus nigra L.) fruit extract alleviates oxidative stress, insulin resistance, and inflammation in hypertrophied 3T3-L1 adipocytes and activated RAW 264.7 macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef]
- Mocanu, M.L.; Amariei, S. Elderberries—A Source of Bioactive Compounds with Antiviral Action. Plants 2022, 11, 740. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Ma, X.; Ning, S. Cyanidin-3-glucoside attenuates the angiogenesis of breast cancer via inhibiting STAT3/VEGF pathway. Phyther. Res. 2019, 33, 81–89. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Ebrahimzadeh, M.A.; Dooshan, A.; Arimi, A.; Ghasemi, N.; Fathiazad, F. Antidepressant activities of Sambucus ebulus and Sambucus nigra. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3350–3353. [Google Scholar] [PubMed]
- Reider, S.; Watschinger, C.; Längle, J.; Pachmann, U.; Przysiecki, N.; Pfister, A.; Zollner, A.; Tilg, H.; Plattner, S.; Moschen, A.R. Short- and Long-Term Effects of a Prebiotic Intervention with Polyphenols Extracted from European Black Elderberry—Sustained Expansion of Akkermansia spp. J. Pers. Med. 2022, 12, 1479. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Mulero, M.; Cuevas-Rodríguez, E.O.; Mondor, M.; Arcand, Y.; Hernández-Álvarez, A.J. In vitro gastrointestinal digestion impact on stability, bioaccessibility and antioxidant activity of polyphenols from wild and commercial blackberries (Rubus spp.). Food Funct. 2021, 12, 7358–7378. [Google Scholar] [CrossRef]
- Ștefănescu, B.E.; Nemes, S.A.; Teleky, B.E.; Călinoiu, L.F.; Mitrea, L.; Martău, G.A.; Szabo, K.; Mihai, M.; Vodnar, D.C.; Crișan, G. Microencapsulation and Bioaccessibility of Phenolic Compounds of Vaccinium Leaf Extracts. Antioxidants 2022, 11, 674. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. As a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otrisal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Chen, C.L.; Jeng, T.L.; Lin, T.C.; Sung, J.M. Bioavailability of cranberry bean hydroalcoholic extract and its inhibitory effect against starch hydrolysis following in vitro gastrointestinal digestion. Food Res. Int. 2014, 64, 939–945. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.-E.; Ranga, F.; Simon, E.; Pop, O.L.; Babalau-Fuss, V.; Kapsalis, N.; Vodnar, D.C. Bioaccessibility of microencapsulated carotenoids, recovered from tomato processing industrial by-products, using in vitro digestion model. LWT-Food Sci. Technol. 2021, 152, 112285. [Google Scholar] [CrossRef]
- Imenšek, N.; Kristl, J.; Šumenjak, T.K.; Ivančič, A. Antioxidant activity of elderberry fruits during maturation. Agriculture 2021, 11, 555. [Google Scholar] [CrossRef]
- Hearst, C.; Mccollum, G.; Nelson, D.; Ballard, L.M.; Millar, B.C.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Moore, J.E.; Rao, J.R. Antibacterial activity of elder (Sambucus nigra L.) flower or berry against hospital pathogens. J. Med. Plants Res. 2010, 4, 1805–1809. [Google Scholar] [CrossRef]
- Mohammadsadeghi, S.; Malekpour, A.; Zahedi, S.; Eskandari, F. The antimicrobial activity of elderberry (Sambucus nigra L.) extract against gram positive bacteria, gram negative bacteria and yeast. Res. J. Appl. Sci. 2013, 8, 240–243. [Google Scholar]
- Krawitz, C.; Mraheil, M.A.; Stein, M.; Imirzalioglu, C.; Domann, E.; Pleschka, S.; Hain, T. Inhibitory activity of a standardized elderberry liquid extract against clinically-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Complement. Altern. Med. 2011, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Konečná, M.; Sedlák, V.; Tkáčiková, L.; Kšonžeková, P.; Mydlárová-Blaščáková, M.; Gruľová, D.; Gaľová, J.; Gogaľová, Z.; Babejová, A.; Vašková, H.; et al. Inhibition of the growth of gram-negative bacteria by anthocyanins of berries fruits. Sci. Bull. Uzhhorod Univ. Biol. Ser. 2019, 42–47. [Google Scholar] [CrossRef]
- Mitrea, L.; Ranga, F.; Fetea, F.; Dulf, F.V.; Rusu, A.; Trif, M.; Vodnar, D.C. Biodiesel-derived glycerol obtained from renewable biomass-A suitable substrate for the growth of Candida zeylanoides yeast strain ATCC 20367. Microorganisms 2019, 7, 265. [Google Scholar] [CrossRef]
- Trofa, D.; Gácser, A.; Nosanchuk, J.D. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 2008, 21, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Del Bò, C.; Ciappellano, S.; Klimis-Zacas, D.; Daniela, M.; Claudio, G.; Riso, P.; Porrini, M. Anthocyanin absorption, metabolism, and distribution from a wild blueberry-enriched diet (Vaccinium angustifolium) is affected by diet duration in the sprague-dawley rat. J. Agric. Food Chem. 2010, 58, 2491–2497. [Google Scholar] [CrossRef]
- Woodward, G.; Kroon, P.; Cassidy, A.; Kay, C. Anthocyanin stability and recovery: Implications for the analysis of clinical and experimental samples. J. Agric. Food Chem. 2009, 57, 5271–5278. [Google Scholar] [CrossRef]
- Kay, C.D.; Mazza, G.; Holub, B.J. Anthocyanins exist in the circulation primarily as metabolites in adult men. J. Nutr. 2005, 135, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, H.J.; Kim, Y.S.; Yeo, S.H.; Kim, S. Effects of Maclura tricuspidata (Carr.) Bur fruits and its phytophenolics on obesity-related enzymes. J. Food Biochem. 2020, 44, e13110. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, M.; Sun, L.; Liu, X.; Yin, Y.; Hao, J.; Zhang, W. p-Hydroxybenzoic Acid Ameliorates Colitis by Improving the Mucosal Barrier in a Gut Microbiota-Dependent Manner. Nutrients 2022, 14, 5383. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Wang, K.Y.; Zhang, X.S.; Yang, C.; Li, X.Y. 4-Hydroxybenzoic acid (4-HBA) enhances the sensitivity of human breast cancer cells to adriamycin as a specific HDAC6 inhibitor by promoting HIPK2/p53 pathway. Biochem. Biophys. Res. Commun. 2018, 504, 812–819. [Google Scholar] [CrossRef]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Zafrilla, P.; Tomás-Barberán, F.A. An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Eur. Food Res. Technol. 2002, 214, 155–159. [Google Scholar] [CrossRef]
- Liu, G.; Ying, D.; Guo, B.; Cheng, L.J.; May, B.; Bird, T.; Sanguansri, L.; Cao, Y.; Augustin, M. Extrusion of apple pomace increases antioxidant activity upon: In vitro digestion. Food Funct. 2019, 10, 951–963. [Google Scholar] [CrossRef]
- Precup, G.; Pocol, C.B.; Teleky, B.-E.; Vodnar, D.C. Awareness, Knowledge, and Interest about Prebiotics–A Study among Romanian Consumers. Int. J. Environ. Res. Public Health 2022, 19, 1208. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef]
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of gut microbiota and metabolic endotoxemia with dietary factors. Nutrients 2019, 11, 2277. [Google Scholar] [CrossRef]
- Mitrea, L.; Nemes, S.-A.; Szabo, K.; Teleky, B.-E.; Vodnar, D.-C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association With Neurological and Psychiatric Disorders. Front. Med. 2022, 9, 81324. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, prebiotics, and synbiotics: Implications and beneficial effects against irritable bowel syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, T.; Li, N.; Wang, X.; Chen, G.; Lyu, X. Bilberry anthocyanin extract promotes intestinal barrier function and inhibits digestive enzyme activity by regulating the gut microbiota in aging rats. Food Funct. 2019, 10, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Teleky, B.-E.; Mitrea, L.; Plamada, D.; Nemes, S.A.; Călinoiu, L.-F.; Pascuta, M.S.; Varvara, R.-A.; Szabo, K.; Vajda, P.; Szekely, C.; et al. Development of Pectin and Poly(vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants 2022, 11, 1729. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Gerasimenko, I.; Sheludko, Y.; Unger, M.; Stöckigt, J.; Arnao, M.B. Estimation of free radical-quenching activity of leaf pigment extracts. Phytochem. Anal. 2001, 12, 138–143. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Stefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crisan, G. The Chemical and Biological Profiles of Leaves from Commercial Blueberry Varieties. Plants 2020, 9, 1193. [Google Scholar] [CrossRef] [PubMed]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef]
- Bogdan, M.A.; Bungau, S.; Tit, D.M.; Zaha, D.C.; Nechifor, A.C.; Behl, T.; Chambre, D.; Lupitu, A.I.; Copolovici, L.; Copolovici, D.M. Chemical profile, antioxidant capacity, and antimicrobial activity of essential oils extracted from three different varieties (Moldoveanca 4, vis magic 10, and alba 7) of Lavandula angustifolia. Molecules 2021, 26, 4381. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, M.; Tit, D.M.; Behl, T.; Heghedűş-Mîndru, R.C.; Zaha, D.C.; Ursu, F.; Popa, M.; Glevitzky, I.; Bungău, S. The antimicrobial activity of honey and propolis extracts from the central region of Romania. Food Biosci. 2021, 41, 101014. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.-F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Ferenczi, L.-J.; Ştefănescu, B.-E.; vodnar, D.C. Inhibitory Potential of Lactobacillus plantarum on Escherichia coli. Bull. Univ. Agric. Sci. Veter-Med. Cluj-Napoca. Food Sci. Technol. 2017, 74, 99–101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).