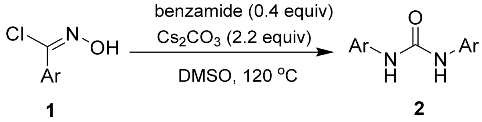
Abstract
A novel amide-assisted rearrangement reaction of hydroxybenzimidoyl chloride has been established for the efficient synthesis of 1,3-diphenylurea derivatives. A variety of electronically and sterically different 1,3-diphenylurea derivatives can be obtained in good to excellent yields, and a proposed reaction mechanism is also presented.
1. Introduction
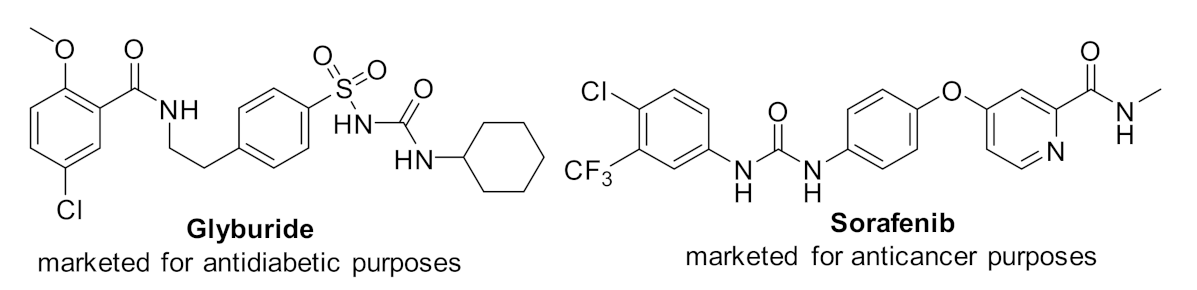
Urea derivatives have a myriad of applications in biological studies, analytical chemistry, pharmaceuticals, polymer sciences, and agrochemicals [1,2,3,4,5,6,7,8]. N, N′-disubstituted urea exhibits a wide range of potent biological properties in bioactive and pharmacologically impressive structures [9,10,11,12,13,14]. For instance, many urea-containing compounds have been used to cure human diseases (Figure 1) [15,16,17,18].
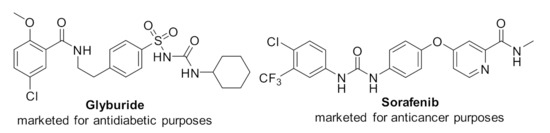
Figure 1.
Representative biological urea derivatives.
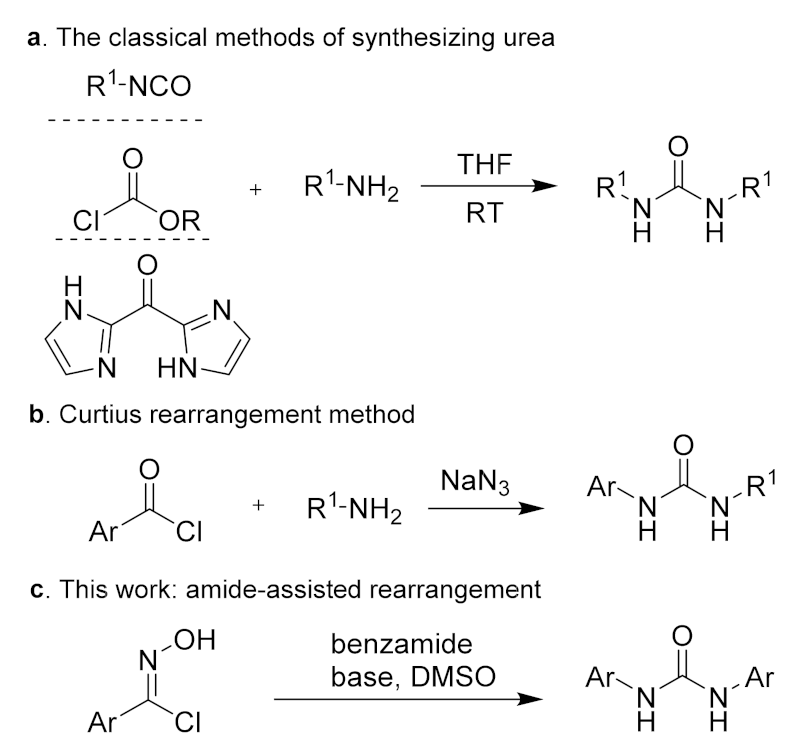
Given the medicinal and biological properties of N, N′-disubstituted urea, synthetic–organic chemists and medicinal chemists have shown considerable interest in the development of efficient methodologies for the synthesis of this structure. The traditional methods of synthesizing urea involve the condensation reaction between an amine and active carbonyl compounds, such as isocyanate [19,20,21,22], chloroformate [23], and carbonyl di-imidazole [24,25] (Scheme 1a). Also, the Curtius rearrangement provides an effective method for preparing urea from an arylformyl chloride substrate (Scheme 1b) [26,27,28]. These methods have been extensively studied and applied in actual production, but the development of novel methods for the preparation of urea is still in high demand.
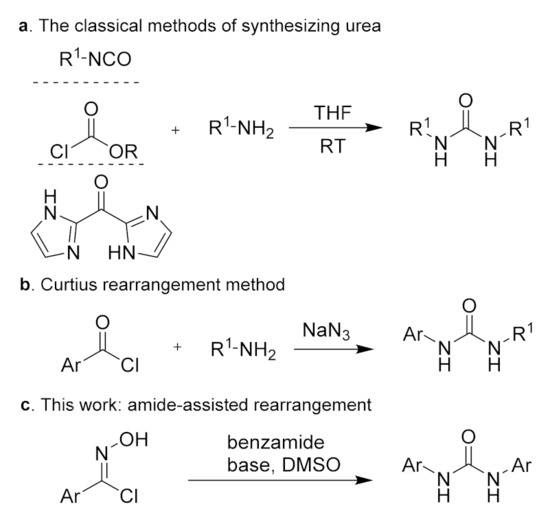
Scheme 1.
Preparation methods of N, N′-disubstituted urea.
Aromatic oxime is a vital precursor and intermediate in organic synthesis [29,30,31]. The typical Beckman rearrangement reaction can achieve the conversion of ketoxime to amide products under strong acid conditions [32,33]. Since the hydrogen-atom migration of aldoxime is difficult, metal catalysts are usually required in order to carry out the Beckmann rearrangement of aldoxime [34,35,36,37]. Therefore, it is still challenging to achieve a metal-free aldoxime rearrangement. The amides have been widely used as directing groups to activate C‒H bonds and facilitate the conversion of multiple functional groups [38,39,40,41,42]. To date, there have been no reports of amide-assisted rearrangement reactions. We envision that the amide can utilize the hydrogen bond to bind with the hydroxyl group of hydroxyarylformimidoyl chloride, thereby activating the N‒O bond, which is conducive to the departure of the hydrated positive ion and allows the rearrangement of aldoxime.
Herein, we have developed a smooth and efficient synthesis of 1,3-diphenylurea derivatives from hydroxybenzimidoyl chloride under mild conditions with amides as additives.
2. Results and Discussion
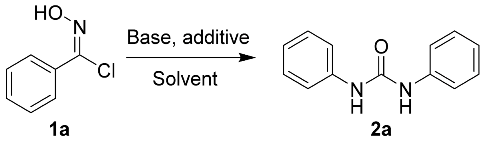
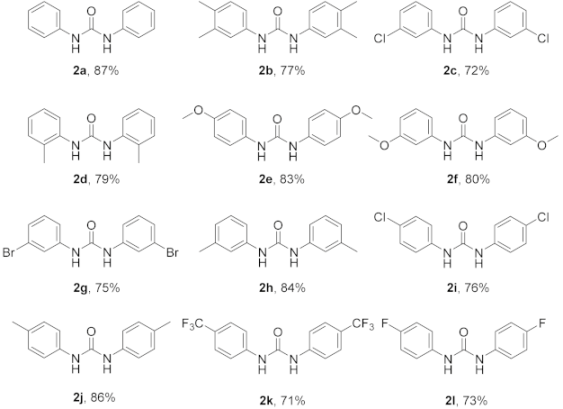
In our initial studies, N-hydroxybenzimidoyl chloride (1a) was reacted with benzamide using N, N-dimethylformamide (DMF) as the solvent, and the desired product 2a was obtained in medium yields (31%, Table 1, entry 1). Different solvents, such as methanol, ethanol, 1,4-dioxane, tetrahydrofuran (THF), dimethylsulfoxide (DMSO), and dichloromethane (DCM), were screened at room temperature. The results indicate that the most efficacious reaction occurred with DMSO (yield 42%, Table 1, entry 8). Encouraged by this result, we sought to enhance the yield of this reaction and carried out a screening of bases, such as K2CO3, Et3N, DMAP, DBU, and t-BuOK (Table 1, entries 10–14). The experimental data showed that the reaction proceeded with good yield (42%, Table 1, entry 8) when Cs2CO3 was used, while the other bases were not as effective. The reaction could not be carried out in the absence of a base (Table 1, entry 15). Additionally, increasing temperature is beneficial to the reaction, since higher yields were obtained at 120 °C (87%, Table 1, entry 21). Under these conditions, increasing the reaction time did not affect the yield (87%, Table 1, entry 23). Subsequently, we selected acetamide, propionamide, 2-phenylacetamide, and 4-chlorobenzamide to screen the additives. The results show that the effect of using benzamide is still more potent than the other amides. Simple amides, such as acetamide and propionamide, are also effective, but 2-phenylacetamide and 4-chlorobenzamide are not as effective (Table 1, entries 24–27). Notably, without the addition of the amide reagents, the reactions described herein will not occur (Table 1, entries 28). Therefore, under optimized conditions (using DMSO as the solvent with Cs2CO3 as the base at 120 °C for 5 h), different N-hydroximoyl chlorides were selected in order to prepare products 2a–2l (yield: 71–87%, Table 2). Reducing the amount of benzamide will significantly reduce the reaction yield (Table 1, entries 29–30), although the reaction yield did not increase significantly with an increase in the amount of benzamide, (Table 1, entries 31).

Table 1.
Optimization of experimental conditions a,c.

Table 2.
Synthesis of 1,3-diphenylurea derivatives a,b.
In Table 2, the results show that N-hydroxybenzimidoyl chloride (1) substrates that bore electron-donating groups (such as methoxy or methyl) as the R substituents were well-tolerated at good yields (2e, 83%, 2f, 80%, 2h, 84%, 2j, 86%). In addition, electron-withdrawing substituents (such as chloro-, fluoro- or trifluoromethyl) are also usable in the reaction, but the reaction yield is reduced (2c, 72%, 2i, 76%, 2k, 71%, 2l, 73%). Furthermore, the yield of para-containing substituents is higher than the yield of meta-containing substituents (2c, 72%, 2i, 76%). Finally, the reaction yield of a substrate that contains two groups is not as high as a substrate that contains one group (2b, 77%, 2j, 86%). Unfortunately, no corresponding products were obtained using other heteroaromatic substrates (1), such as pyridine, furane or thiophene.
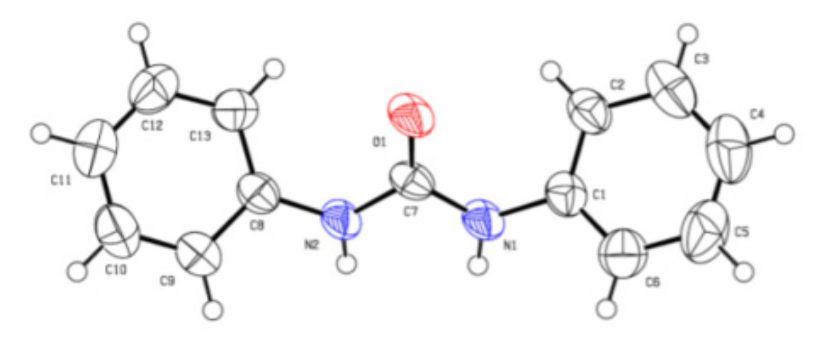
The chemical structures of the 1,3-diphenylurea derivatives were examined by 1H NMR, 13C NMR, and HRMS analyses (see Supplementary Materials). The structure of 2a was unambiguously confirmed by single-crystal X-ray analysis [43], as shown in Figure 2.
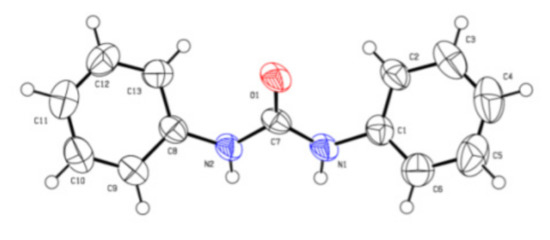
Figure 2.
Crystal structure of 2a.
3. Conclusions
In summary, an amide-assisted rearrangement reaction of hydroxybenzimidoyl chloride has been developed for the preparation of 1,3-diphenylurea derivatives. This highly effective reaction proceeds well to afford 1,3-diphenylurea derivatives without metal catalysts under mild conditions and shows good functional-group tolerance. A proposed reaction mechanism has been presented, suggesting that the reaction went through a novel rearrangement process. We believe that the findings of this study promote the rapid synthesis of novel diphenylurea compounds exhibiting crucial biological activity.
Supplementary Materials
The following are available online, X-ray crystallography data of compounds: 2a, Characterization data and copies of 1H and 13C NMR spectra for all new compounds.
Author Contributions
Writing—original draft, X.S. and X.L.; writing—review and editing, W.Y. and Y.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSFC, grant number 22067020 and Yunnan Fundamental Research Projects (202101AS070034 and 202001BB050009) and the Program for Excellent Young Talents, Yunnan University, China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated during the present study are available from the corresponding author on reasonable request.
Acknowledgments
This research was funded by NSFC, grant number 22345323 and Yunnan Fundamental Research Projects (202101AS070034 and 202001BB050009) and the Program for Excellent Young Talents, Yunnan University, China.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of all the compounds are available from the authors.
References
- Gallou, I. Unsymmetrical ureas. Synthetic methodologies and application in drug design. Org. Prep. Proceed. Int. 2007, 39, 355–383. [Google Scholar] [CrossRef]
- Regan, J.; Breitfelder, S.; Cirillo, P.; Gilmore, T.; Graham, A.G.; Hickey, E.; Klaus, B.; Madwed, J.; Moriak, M.; Moss, N.; et al. Pyrazole urea-based inhibitors of p38 MAP kinase: From lead compound to clinical candidate. J. Med. Chem. 2002, 45, 2994–3008. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Gemma, S. Structure-Based Design of Drugs and Other Bioactive Molecules: Tools and Strategies; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Hubbard, R.E. Structure-Based Drug Discovery: An Overview; RSC Publishing: London, UK, 2006. [Google Scholar]
- Steverding, D. The development of drugs for treatment of sleeping sickness: A historical review. Parasites Vectors 2010, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Nowick, J.S.; Mahrus, S.; Smith, E.M.; Ziller, J.W. Triurea Derivatives of Diethylenetriamine as Potential Templates for the Formation of Artificial β-Sheets. J. Am. Chem. Soc. 1996, 118, 1066–1072. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Arachchige, D.M.B.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Guo, Z.L.; Song, H.J.; Liu, Y.X.; Wang, L.Z.; Wang, Q.M. Design, synthesis, and biological activity of β-carboline analogues containing hydantoin, thiohydantoin, and urea moieties. J. Agric. Food Chem. 2018, 66, 8253–8261. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Morisseau, C.; Watanabe, T.; Hammock, B.D. Design, synthesis, and biological activity of 1, 3-disubstituted ureas as potent inhibitors of the soluble epoxide hydrolase of increased water solubility. J. Med. Chem. 2004, 47, 2110–2122. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Lin, L.; Ruiz, C.; Khan, S.; Cameron, M.D.; Grant, W.; Pocas, J.; Eid, N.; Park, H.J.; Schröter, T.; et al. Synthesis and biological evaluation of urea derivatives as highly potent and selective rho kinase inhibitors. J. Med. Chem. 2013, 56, 3568–3581. [Google Scholar] [CrossRef]
- Thomas, V.; Kumari, T.V.; Jayabalan, M. In vitro studies on the effect of physical cross-linking on the biological performance of aliphatic poly (urethane urea) for blood contact applications. Biomacromolecules 2001, 2, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Chayah, M.; Carrión, M.D.; Gallo, M.A.; Jiménez, R.; Duarte, J.; Camacho, M.E. Development of urea and thiourea kynurenamine derivatives: Synthesis, molecular modeling, and biological evaluation as nitric oxide synthase inhibitors. ChemMedChem 2015, 10, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Mounetou, E.; Legault, J.; Lacroix, J.; C-Gaudreault, R. Antimitotic Antitumor Agents: Synthesis, Structure−Activity Relationships, and Biological Characterization of N-Aryl-N‘-(2-chloroethyl)ureas as New Selective Alkylating Agents. J. Med. Chem. 2001, 44, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Brindisi, M. Urea derivatives in modern drug discovery and medicinal chemistry. J. Med. Chem. 2020, 63, 2751–2788. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.A.; Biggs, W.H.; Treiber, D.K.; Atteridge, C.E.; Azimioara, M.D.; Benedetti, M.G.; Carter, T.A.; Ciceri, P.; Edeen, P.T.; Floyd, M.; et al. A small molecule–kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 2005, 23, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Cortes, J.; Kantarjian, H.; Zhang, W.; Andreef, M.; Ravandi, F. Potential role of sorafenib in the treatment of acute myeloid leukemia. Leuk. Lymphoma. 2008, 49, 2246–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malcolm, B.A.; Liu, R.; Lahser, F.; Agrawal, S.; Belanger, B.; Butkiewicz, N.; Chase, R.; Gheyas, F.; Hart, A.; Hesk, D.; et al. SCH 503034, a Mechanism-Based Inhibitor of Hepatitis C Virus NS3 Protease, Suppresses Polyprotein Maturation and Enhances the Antiviral Activity of Alpha Interferon in Replicon Cells. Antimicrob. Agents Chemother. 2006, 50, 1013–1020. [Google Scholar] [CrossRef] [Green Version]
- Jensen, D.M. A new era of hepatitis C therapy begins. N. Engl. J. Med. 2011, 364, 1272–1274. [Google Scholar] [CrossRef] [PubMed]
- Busschaert, N.; Kirby, I.L.; Young, S.; Coles, S.J.; Horton, P.N.; Light, M.E.; Gale, P.A. Squaramides as potent transmembrane anion transporters. Angew. Chem. Int. Ed. 2012, 51, 4426–4430. [Google Scholar] [CrossRef]
- Lin, B.; Waymouth, R.M. Urea anions: Simple, fast, and selective catalysts for ring-opening polymerizations. J. Am. Chem. Soc. 2017, 139, 1645–1652. [Google Scholar] [CrossRef]
- Siddique, M.U.M.; McCann, G.J.P.; Sonawane, V.; Horley, N.; Williams, I.S.; Joshi, P.; Bharate, S.B.; Jayaprakash, V.; Sinha, B.N.; Chaudhuri, B. Biphenyl urea derivatives as selective CYP1B1 inhibitors. Org. Biomol. Chem. 2016, 14, 8931–8936. [Google Scholar] [CrossRef]
- Harinath, A.; Bano, K.; Ahmed, S.; Panda, T.K. 2-Picolylamino(diphenylphosphinoselenoic)amide supported zinc complexes: Efficient catalyst for insertion of N–H bond into carbodiimides, isocyanates, and isothiocyanate. Phosphorus Sulfur Silicon Relat Elem. 2018, 193, 23–32. [Google Scholar] [CrossRef]
- Enein, M.N.A.; Azzouny, A.A.E.; Attia, M.I.; Maklad, Y.A.; Amin, K.M.; Rehim, M.A.; Behairy, M.F.E. Design and synthesis of novel stiripentol analogues as potential anticonvulsants. Eur. J. Med. Chem. 2012, 47, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.L.; Soeiro, P.F.; Reis, M.A.; Boto, R.E.F.; Silvestre, S.; Almeida, P. Synthesis and process optimization of symmetric and unsymmetric barbiturates C5-coupled with 2, 1-benzisoxazoles. Mol. Divers. 2020, 24, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, A.; Jędrzejewska, B.; Józefowicz, M.; Grela, I.; Osmiałowski, B. The trans/cis photoisomerization in hydrogen bonded complexes with stability controlled by substituent effects: 3-(6-aminopyridin-3-yl) acrylate case study. RSC Adv. 2018, 8, 23698–23710. [Google Scholar] [CrossRef] [Green Version]
- Lebel, H.; Leogane, O. Curtius rearrangement of aromatic carboxylic acids to access protected anilines and aromatic ureas. Org. Lett. 2006, 8, 5717–5720. [Google Scholar] [CrossRef] [PubMed]
- Dubé, P.; Nathel, N.F.F.; Vetelino, M.; Couturier, M.; Aboussafy, C.L.; Pichette, S.; Jorgensen, M.L.; Hardink, M. Carbonyldiimidazole-mediated Lossen rearrangement. Org. Lett. 2009, 11, 5622–5625. [Google Scholar] [CrossRef]
- Singh, A.S.; Agrahari, A.K.; Singh, S.K.; Yadav, M.S.; Tiwari, V.K. An Improved Synthesis of Urea Derivatives from N-Acylbenzotriazole via Curtius Rearrangement. Synthesis 2019, 51, 3443–3450. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yin, Z.; Wang, H.; Wu, X.F. Iron-catalyzed carbonylative cyclization of γ, δ-unsaturated aromatic oxime esters to functionalized pyrrolines. Chem. Commun. 2020, 56, 7045–7048. [Google Scholar] [CrossRef]
- Forrester, A.R.; Gill, M.; Thomson, R.H. Cyclic amination onto aromatic ring of sulfonamides with (diacetoxyiodo) arenes: Effect of sulfonyl group. J. Chem. Soc. Chem. Commun. 1976, 37, 677–678. [Google Scholar] [CrossRef]
- Zhang, J.J.; Duan, X.H.; Wu, Y.; Yang, J.C.; Guo, L.N. Transition-metal free C–C bond cleavage/borylation of cycloketone oxime esters. Chem. Sci. 2019, 10, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Beckmann, E. Zur Kenntniss der Isonitrosoverbindungen. Ber. Dtsch. Chem. Ges. 1886, 19, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Kumar, P.; Khatana, A.K.; Chandra, D.; Yadav, A.K.; Tiwari, B.; Jat, J.L. Zinc (II)-Catalyzed Synthesis of Secondary Amides from Ketones via Beckmann Rearrangement Using Hydroxylamine-O-sulfonic Acid in Aqueous Media. Synthesis 2020, 52, 3272–3276. [Google Scholar]
- Paul, B.; Maji, M.; Panja, D.; Kundu, S. Tandem Transformation of Aldoximes to N-Methylated Amides Using Methanol. Adv. Synth. Catal. 2019, 361, 5357–5362. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Lee, H.Y.; Chang, S. Significant Self-Acceleration Effects of Nitrile Additives in the Rhodium-Catalyzed Conversion of Aldoximes to Amides: A New Mechanistic Aspect. Adv. Synth. Catal. 2009, 351, 1807–1812. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ogasawara, Y.; Kotani, M.; Yamaguchi, K.; Mizuno, N. A supported rhodium hydroxide catalyst: Preparation, characterization, and scope of the synthesis of primary amides from aldoximes or aldehydes. Chem. Asian J. 2008, 3, 1715–1721. [Google Scholar] [CrossRef]
- Gnanamgari, D.; Crabtree, R.H. Terpyridine ruthenium-catalyzed one-pot conversion of aldehydes into amides. Organometallics 2009, 28, 922–924. [Google Scholar] [CrossRef]
- Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Joanna, W.D.; Besset, T.; et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauf, W.; Brown, J.M. Catalytic Amide-Mediated Methyl Transfer from Silanes to Alkenes in Fujiwara–Moritani Oxidative Coupling. Angew. Chem. Int. Ed. 2008, 47, 4228–4230. [Google Scholar] [CrossRef]
- Liang, D.; Li, Y.; Gao, S.; Li, R.; Li, X.; Wang, B.; Yang, H. Amide-assisted radical strategy: Metal-free direct fluorination of arenes in aqueous media. Green Chem. 2017, 19, 3344–3349. [Google Scholar] [CrossRef]
- Li, D.D.; Yuan, T.T.; Wang, G.W. Palladium-Catalyzed Ortho-Arylation of Benzamides via Direct sp2 C–H Bond Activation. J. Org. Chem. 2012, 77, 3341–3347. [Google Scholar] [CrossRef]
- Kianmehr, E.; Tanbakouchian, A. Palladium-catalyzed regio- and chemoselective direct desulfitative arylation of anilides with arylsulfonyl chlorides. Tetrahedron 2017, 73, 5337–5343. [Google Scholar] [CrossRef]
- CCDC 2015673 (2a) contain the Supplementary Crystallographic Data for this Paper. These Data can Be Obtained Free of Charge from the Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 21 August 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).