Abstract
The objective of this study was to establish the chromatographic fingerprints of the essential oil (EO) from Stellera chamaejasme flowers collected from various natural sites by gas chromatography (GC) combined with chemometric methods. The EO was obtained by hydrodistillation, and its chemical composition was analyzed by gas chromatography−mass spectrometry (GC−MS). Most components were identified as ketones and the relatively high-content components were fitone (38.973%), n-hentriacontane (5.807%), myristic acid (4.944%) and phytol (3.988%). In addition, the repellent activities of the EO from S. chamaejasme flowers and its four main chemical compounds were evaluated against three stored product pests (Tribolium castaneum, Lasioderma serricorne, Liposcelis bostrychophila) for the first time. In this work, the EO and the four chemical compounds showed a repellent effect against three storage pests after 2 and 4 h exposure. The experimental method and repellent activity of S. chamaejasme flower EO could provide a basis for the development of botanical pesticide and the utilization of the rich plant resources of S. chamaejasme in the future.
1. Introduction
Stellera chamaejasme (L.) is a perennial herb of the genus Stellera in the Thymelaeaceae family. Its root is commonly used for removing phlegm, relieving pain, killing pests and for external use in the treatment of scabies in traditional Chinese medicine (TCM) [1]. S. chamaejasme mainly grows on dry sunny hillsides, lawns, or riversides with an altitude range from 2600 to 4200 m above sea level, mean annual temperature of about 0 °C in the north and southwest of China, as well as in Siberian Russia. Previous studies have found that the root parts of S. chamaejasme contain flavonoids, lignans, coumarins, terpenes and other chemicals [2,3], among which coumarins are its characteristic chemical components such as daphnetin, umbelliferone, edgeworthin, etc. However, there is relatively little research into the chemical composition of the flower part of S. chamaejasme. Because S. chamaejasme has strong survivability and expansion capacity on degraded grasslands, aggravating grassland desertification, it is generally considered as a grassland desertification indicator plant [4,5,6]. In addition, studies showed the whole plant of S. chamaejasme was toxic, as a result, poultry and livestock were often found to be poisoned in pastures by misusing S. chamaejasme, which bring economic loss and a safety hazard to the urban resident and herdsmen [7,8].
Red flour beetle Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae), cigarette beetle Lasioderma serricorne (Fabricius) (Coleoptera: Anobiidae) and booklouse Liposcelis bostrychophila (Badonnel) (Psocoptera: Liposcelididae) are worldwide and destructive pests of stored products, especially found in tropical and subtropical areas [9,10,11]. Due to their strong adaption, rapid breeding ability and worldwide distribution, the above three insects have caused serious problems including agricultural economic loss, food contamination and health risks to consumers, etc. [12,13]. Therefore, pesticides such as synthetic insecticides have been widely used to control these stored product pests, in the meantime, overuse of synthetic chemical insecticides leads to environmental pollution, unexpected toxicity on the nontarget biosome and endangerment of human health [14,15]. Consequently, it is urgent to study new green and safe pesticides; natural plants have gained widespread interest in pest control on account of the insecticidal potential and easy degradation of their secondary metabolites [16,17,18]. Nowadays, the development of botanical insecticides has been recognized as an efficient and environmentally acceptable alternative strategy for pest management.
Research has suggested that many plants possess insecticidal activities. For example, Cinnamomum plants showed remarkable bioactivities against pests, and the EO of C. zeylanicum was highly poisonous to some mosquito vectors and flies [19]. The EO from roots and leaves of Asarum heterotropoides also displayed toxicity and repellency against the cigarette beetle and booklouse [20]. Additionally, some species of the Rhododendron genus, such as R. anthopogonoides, R. thymifolium, R. capitatum were all reported to have insecticidal properties, and the EO extracted from the species of Rhododendron was utilized against stored product pests [21]. According to the earliest Chinese pharmaceutical classics, the Shennong herbal scripture, the whole plant of S. chamaejasme is poisonous, and its pollen is a highly toxic part, which suggests the EO of S. chamaejasme flowers could have the potential activity of a pesticide and the development of an insecticide may also provide an approach for the reasonable utilization of S. chamaejasme plant resources [22,23,24].
As is known, TCM has been widely used in the treatment of human diseases for a long time and has played an important role in clinical practice due to its therapeutic action, low toxicity and few side effects up to now. One characteristic of TCM products which is different from synthetic drugs is their complex and unstable chemical constituents, because the chemical composition of TCM products is always affected by many factors including the producing areas, harvest time, growth environment and field management [25], resulting in a batch-to-batch variation to different degrees. In addition, the active components and the action mechanism of some TCM drugs are still unclear. Therefore, it is of great significance to establish the scientific quality control methods of TCM drugs based on their global chemical features, which could give support for identification, quality assessment and provide guidance for clinical medication. The combination of a chromatographic fingerprint and chemometrics analysis could be an effective way for the quality evaluation of TCM products, especially for judging the authenticity of TCM products by similarity evaluation [26]. By analyzing the relative retention time and peak area or intensity ratio of the common peaks, the chemical profile and fingerprint of TCM drugs are established integrally and comprehensively. According to the chromatographic fingerprints, the similarities and differences of various TCM products can be intuitively compared. On the other hand, the chemometric method has become one of the popular modern analysis methods, because it can provide more information on the samples based on the chromatographic fingerprint. The correlation coefficient in chemometrics is the common index for evaluating the similarity of different samples, which can represent the difference and similarity between a set of chromatographic fingerprints and help to classify the quality traits by comparing it with the average reference values. Consequently, the systematic clustering analysis method has been widely used in the data analysis of chromatographic fingerprints, detecting the chromatographic fingerprint information more accurately and comprehensively [27].
In this study, GC−MS was used to determine the chemical constituents of the EO from S. chamaejasme flowers for the first time, and the gas chromatographic fingerprint combined with the chemometric method was successfully developed and applied to assess the quality consistency of seven batches of EOs from S. chamaejasme flowers. By using the Chinese medicine chromatographic fingerprint similarity evaluation system (China National Pharmacopoeia Commission), the common characteristic peaks of S. chamaejasme flowers EOs were confirmed and the similarities of S. chamaejasme flowers from different areas were analyzed. In addition, the chromatographic fingerprint data of S. chamaejasme flowers EO were systematically clustered to evaluate the consistency and differences of the samples. In the past, research on the S. chamaejasme plant mainly focused on the chemical composition and pharmacological activities of the medical root part, while studies on the above-ground parts of this herb, particularly the research into the flowers of S. chamaejasme were rarely reported [28,29]. In the light of the toxicity and the reported insecticidal effects of the root [30,31], our research team extracted the EOs of S. chamaejasme flowers from various natural sites and evaluated their repellent activities against three worldwide pests (T. castaneum, L. serricorne, and L. bostrychophila). Meanwhile, the repellent activities of the four main chemical compounds of the EO against these three pests were also measured. By analyzing the chromatographic fingerprints and repellent activities of the EO from S. chamaejasme flowers, this study showed the similarities of seven batches of S. chamaejasme and could also offer enlightenment for the development of botanical pesticides.
2. Results and Discussion
2.1. Chemical Components of Essential Oil
The chemical composition of the EO from S. chamaejasme flowers was analyzed by GC−MS. Twenty chromatographic peaks were totally isolated from the EO of S. chamaejasme flowers. Sixteen chemical components were identified, accounting for 96.667% of the total peak area, after searching through the NIST 05 Standard Spectral Library data system and checking the mass spectrum fragmentation law. The analysis results are shown in Table 1. Among the identified components in the EO of S. chamaejasme flowers, the content of ketone compounds was higher in proportion, accounting for 50.894% of the identified ingredients, and the acids, alcohols and alkanes accounted for 10.811%, 9.938% and 5.870% respectively.

Table 1.
Chemical composition of essential oil from S. chamaejasme flowers.
2.2. Establishment of Chromatographic Fingerprints and Selection of Reference Peaks
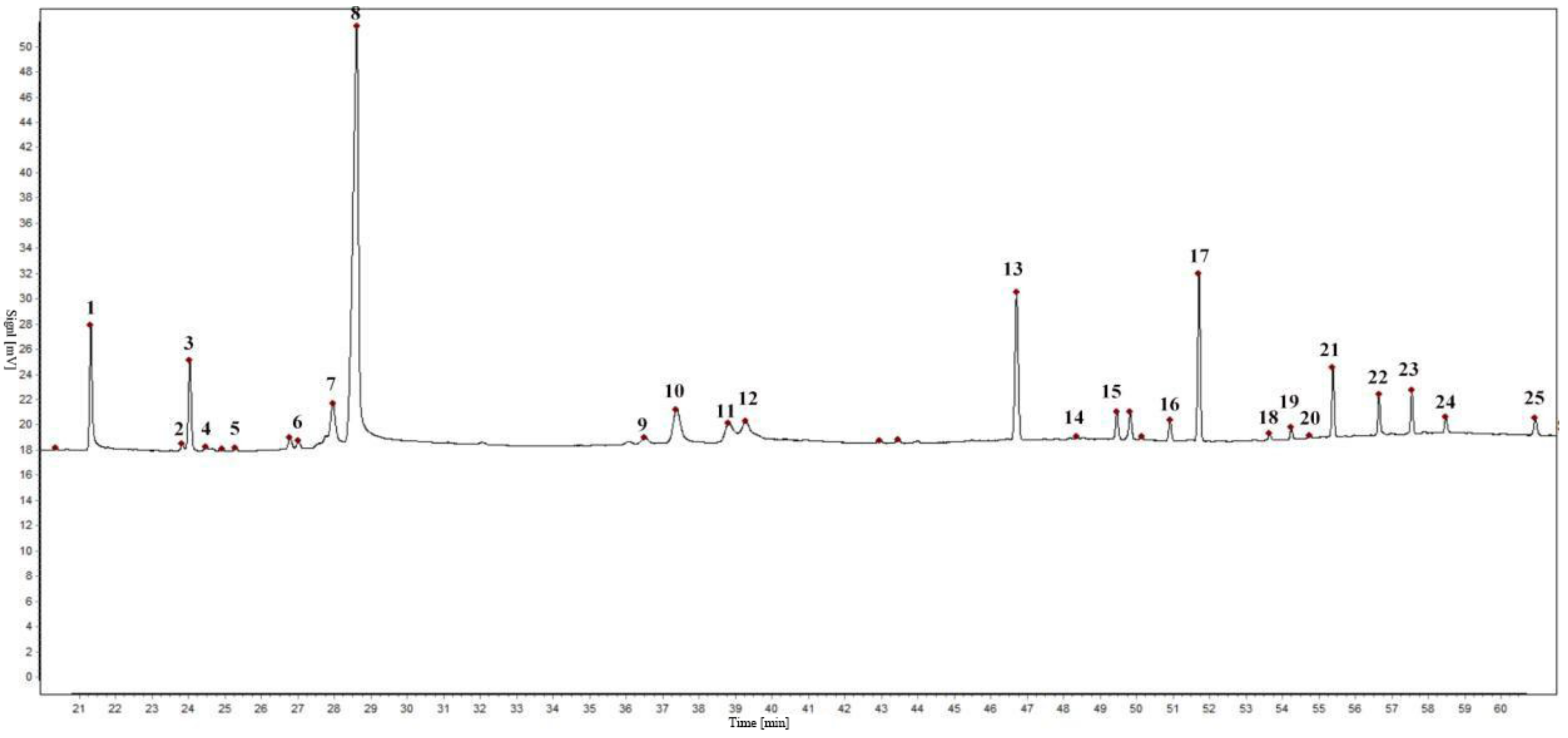
The EOs from seven batches by the establishment of chromatographic fingerprints and selection of reference peaks of S. chamaejasme flowers in different natural sites were determined by GC. The experimental results showed that 25 characteristic peaks of the EOs were confirmed as common chromatographic peaks shown in Figure 1, accounting for more than 96% of the total peak area. Among the common chromatographic peaks, three peaks respectively named characteristic peak 1, 8, and 13, labeled in Figure 1, were identified as myristic acid, fitone and n-hentriacontane by GC−MS.
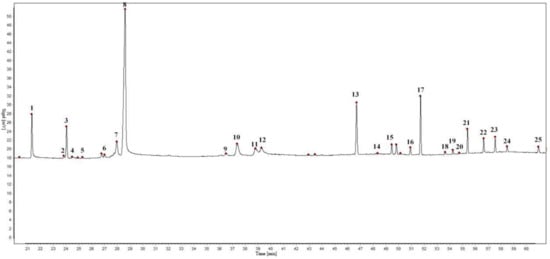
Figure 1.
Gas chromatogram of S. chamaejasme flowers.
It is necessary to select an appropriate peak as the internal reference peak in the complex chromatographic peaks of the samples, for digitizing the retention values of the chromatographic peak into relative retention values [32,33]. In the GC fingerprint of the EO from S. chamaejasme flowers, the myristic acid peak was chosen as the internal reference peak.
2.3. Calculation of Chromatographic Peak Retention Values and Similarity Analysis of Chromatographic Fingerprints
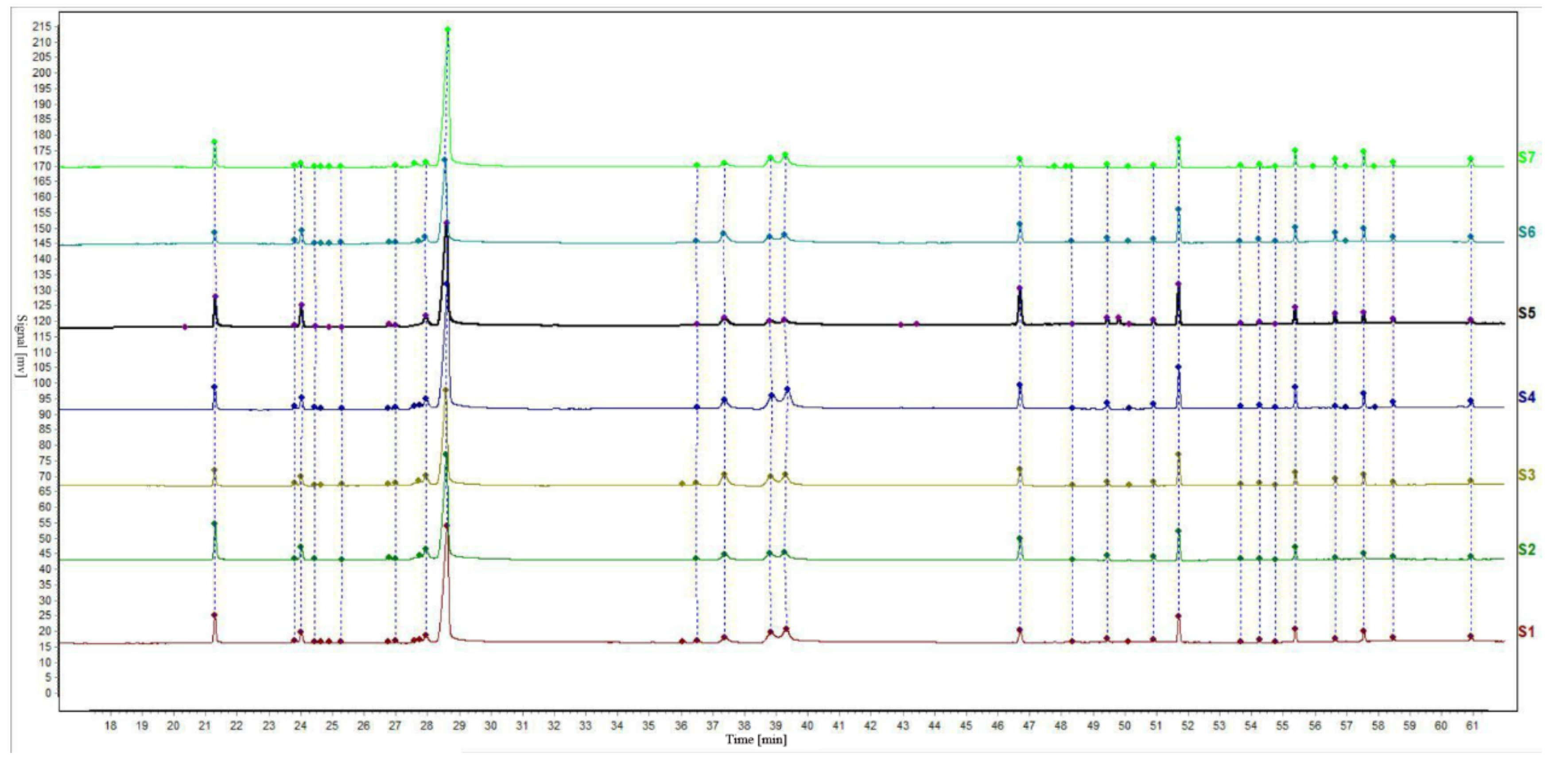
The relative retention time and relative peak area of the chromatographic peaks were calculated according to the selected internal reference peak. The relative retention time and relative peak area of the common fingerprint peaks of the EOs from seven batches of S. chamaejasme flowers are presented in Table 2 and Table 3. The relative retention time of the common peak is the ratio of the absolute retention time of the characteristic peak to that of the reference peak, and the relative peak area of the common peak is found in the same way. In addition, the gas-phase fingerprints of seven batches of S. chamaejasme flower EOs from different natural sites in Inner Mongolia were analyzed with the ‘Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System’ (Chinese Pharmacopoeia Committee) for similarity evaluation [34,35]. The chromatogram is shown in Figure 2. The internal reference peak of myristic acid was peak No. 1 and the median method was used to calculate the similarity. Sample No. 1 was taken as the reference template. According to the peak matching results, the calculated results of all similarities between the complete fingerprints of the tested samples and those of the control template are shown as Table 4. The results showed that the similarity value of the seven samples was more than 0.9. Therefore, the fingerprint maps of the seven batches of EOs were basically consistent, which indicated that the qualities of the seven batches of S. chamaejasme flowers from various natural sites of Inner Mongolia autonomous region in China were basically the same. The results can provide a reference for the qualitative identification of S. chamaejasme herbs and clinical application of S. chamaejasme flowers.

Table 2.
Relative retention time of common peaks in seven batches of S. chamaejasme flowers chromatograms.

Table 3.
Relative peak area of common peaks in seven batches of S. chamaejasme flowers chromatograms.
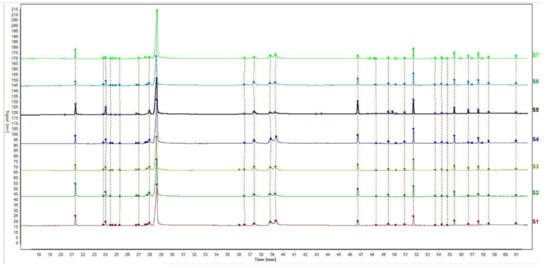
Figure 2.
Gas phase fingerprint of essential oil from S. chamaejasme flowers.

Table 4.
Evaluation results of fingerprint similarities of essential oil from seven batches of S. chamaejasme flowers.
The percentage values of the peak area of the common and noncommon peaks of the seven tested samples were shown in Table 5. The results indicated that the common peak area of the seven samples accounted for more than 95% of the total peak area, and the noncommon peak area account for less than 5%. Hence, the above experimental results were officially valid and also meet the requirements of fingerprinting [36,37].

Table 5.
Percentage of common peaks and noncommon peaks of the seven batches of S. chamaejasme flowers samples to the total peak area.
2.4. Multivariate Data Processing and Analysis
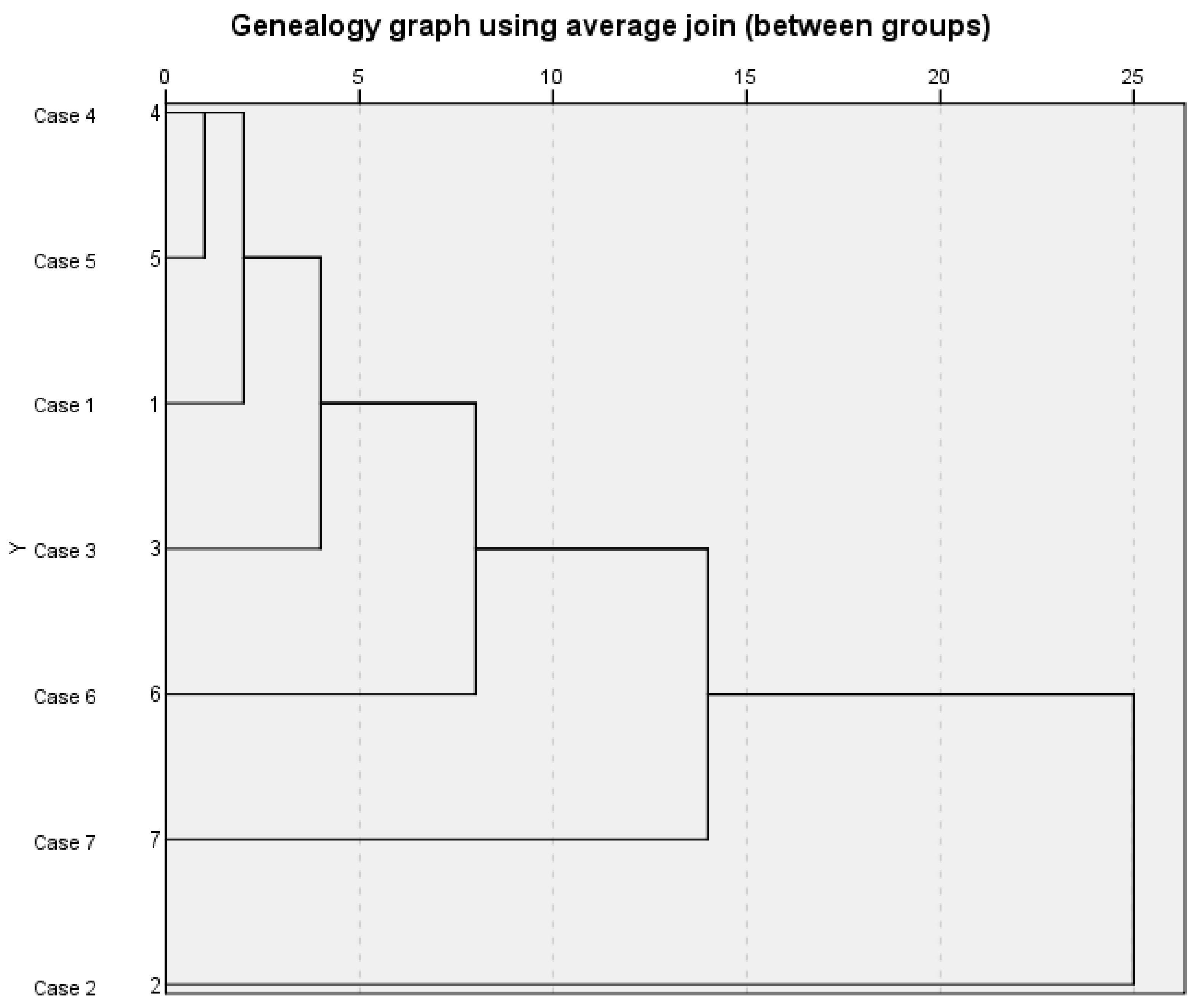
Systematic clustering analysis was carried out on seven samples of S. chamaejasme flowers. After standardized processing of the peak areas of 25 common peaks in the fingerprint, SPSS 26.0 software was used to perform systematic clustering analysis with the average connection of groups and the squared Euclidean distance as metric standard [38,39,40]. The results are shown in Figure 3. The abscissa of the system cluster analysis pedigree diagram represents the relative distance between groups, and the ordinate represents the sample numbers. The pedigree diagram of system cluster analysis can visually show the process of the samples merging gradually [41,42]. Among them, when the distance between groups was 5, the seven batches of samples could be divided into four types, sample numbers 2, 6, and 7 belonged to their respective type, while Nos. 1, 3, 4 and 5 were one class which indicated that the quality of S. chamaejasme flowers in these four natural sites was relatively consistent.
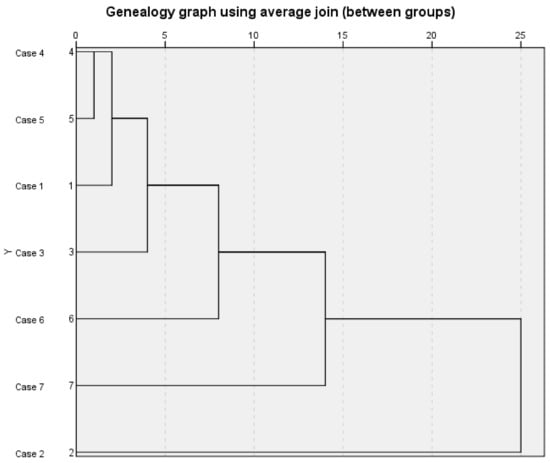
Figure 3.
Systematic cluster analysis pedigree of 7 batches of S. chamaejasme flowers samples.
2.5. Repellent Activity
The chemical composition of EO from S. chamaejasme flowers was identified and analyzed by GC−MS, the content of compound fitone (38.978%) was the highest, followed by myristic acid (4.944%), methyl palmitate (4.854%), and phytol (3.988%). The results of the tested samples are presented in Table 6. Additionally, in this study, the insecticidal activity of the EO of S. chamaejasme flowers and its main chemical components were evaluated by using the target insects of the T. castaneum, L. serricorne, and L. bostrychophila.

Table 6.
Test samples for experiment.
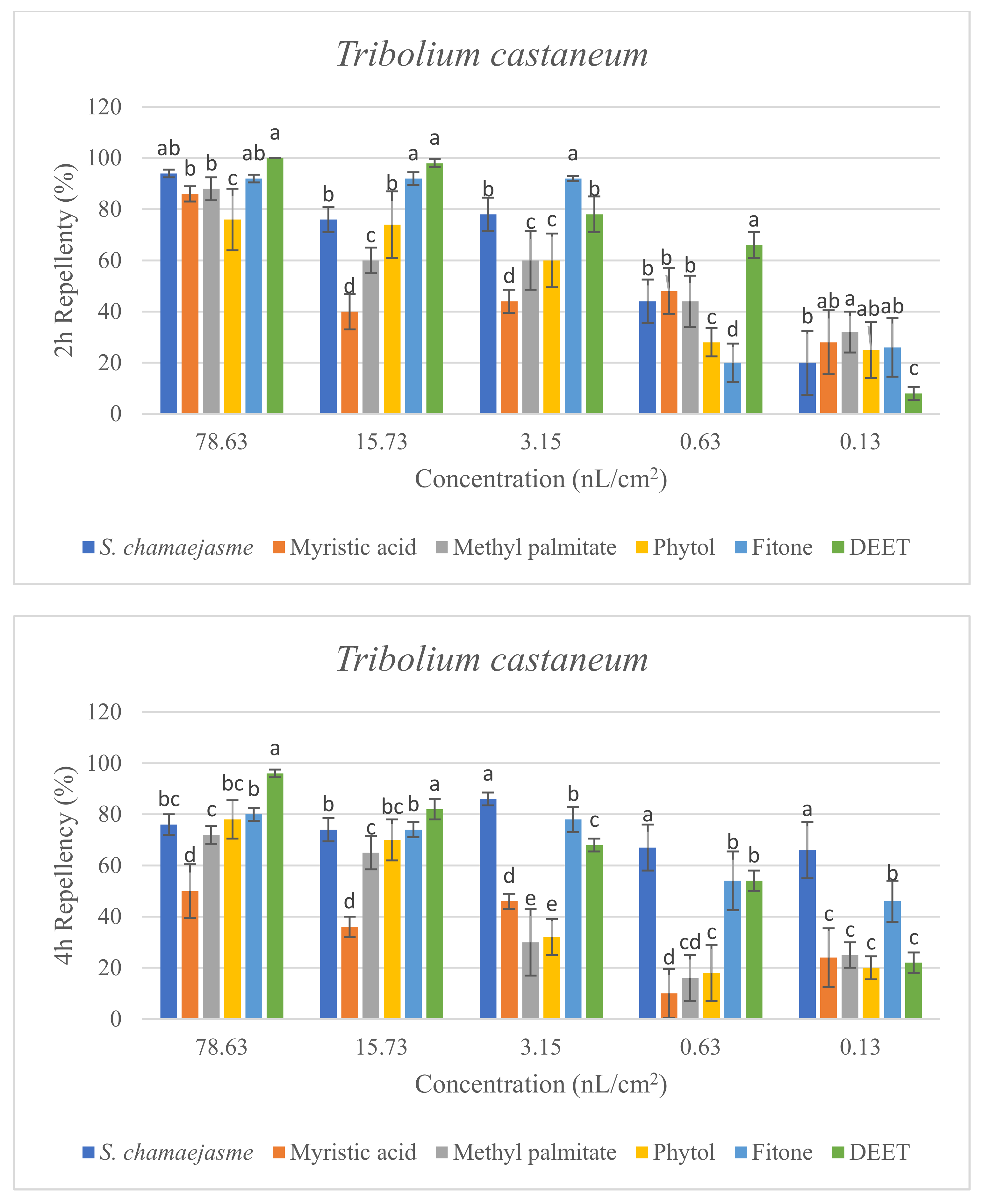
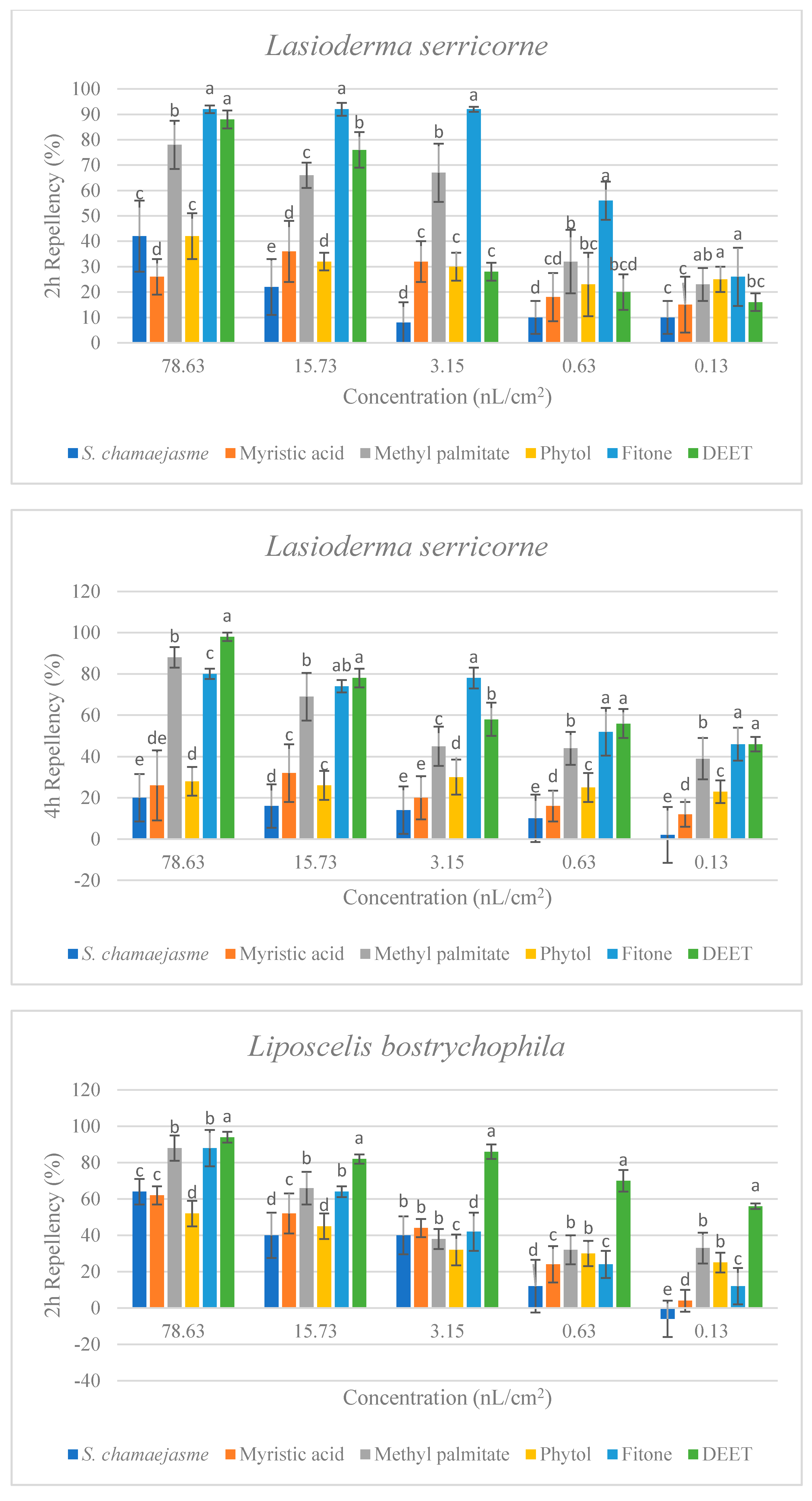
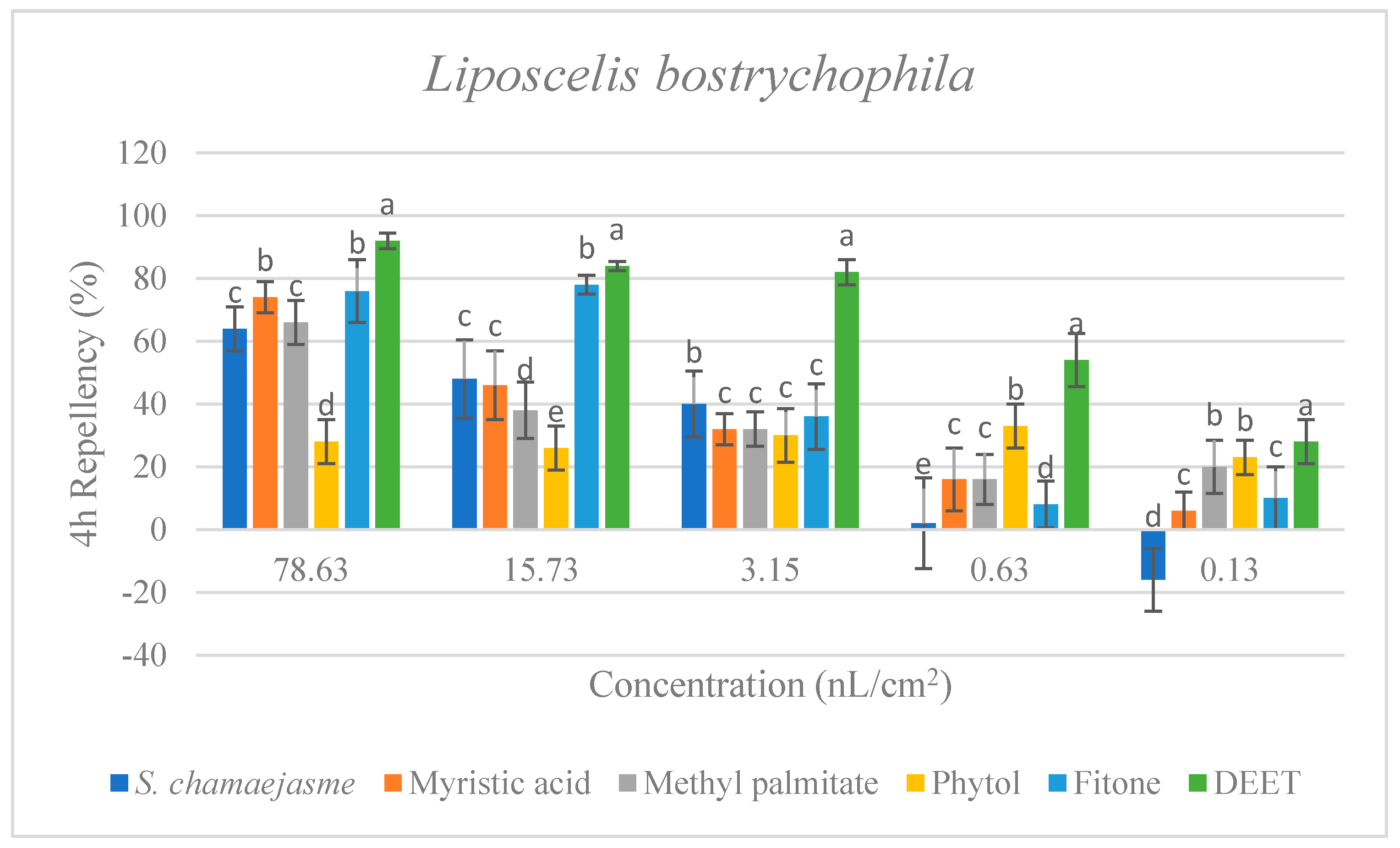
In the repellent activity experiment against these three target insects, the repellent rate (%) at 2 h and 4 h was determined. The results are shown in Figure 4. The results of the repellent experiment showed that the EO from S. chamaejasme flowers had significant repellent effect on these three target insects at the different concentrations tested, and the repellent activity was reduced as the dose decreased. The EO exhibited higher repellency against the red flour beetle and the booklouse than the cigarette beetle after 2 and 4 h exposure. In addition, at the concentration of 3.15, 0.63 and 0.13 nL/cm2, the EO exhibited higher repellency against the red flour beetle at 4 h than that at 2 h, which indicated that EO could have a long-term effect on the red flour beetle. At the tested concentration of 3.15 nL/cm2, the EO showed higher repellency than the positive control of DEET against the red flour beetle after 2 and 4 h exposure. In addition, the results suggested the four chemical compounds in the EO from S. chamaejasme flowers played a certain role in repelling three target insects. Among them, myristic acid and phytol possessed higher repellency against the red flour beetle than against the cigarette and booklouse, whereas, methyl palmitate and fitone exhibited a significantly higher repellent effect than myristic acid and phytol against three insects, especially against the cigarette beetle. Additionally, at the concentration of 3.15 and 0.63 nL/cm2, the repellent effect of fitone and methyl palmitate was better than the positive control of DEET against the cigarette beetle after 2 h exposure, particularly fitone showed higher repellency than methyl palmitate against the cigarette beetle. To sum up, the volatile oil of S. chamaejasme flowers showed remarkable repellent activity against the red flour beetle, and also had a varying degree of repellent effect against the cigarette beetle and booklouse. The four monomeric compounds also have certain repellent activity against three insects, and the repellent effect of methyl palmitate and fitone were relatively higher than the other two compounds. In addition, the EO showed lower repellency than fitone against the cigarette beetle, which may be due to the synergistic or antagonistic effects of different components in the EO.
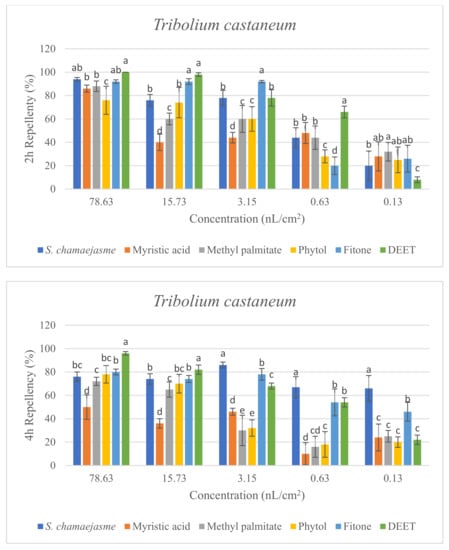
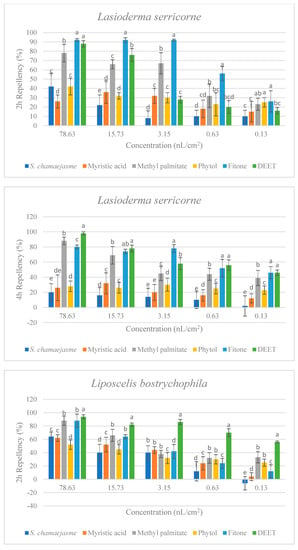
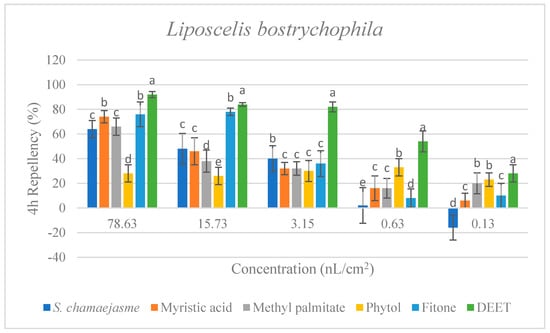
Figure 4.
Percentage repellency (PR) of EO from S. chamaejasme and main monomeric compounds against T. castaneum, L. serricorne, and L. bostrychophila at 2 and 4 h after exposure. The meaning of the letters a, b, c, d, e in figures means in the same column followed by the same letters do not differ significantly in the tests of ANOVA and Tukey (p > 0.05).
As we all know, the pests will gradually increase their own resistance when a single insecticide such as DEET has been used for a long time. Researchers have long been interested in the insecticidal activities of individual compounds, but gradually found that complete extracts were more active than their individual components [43,44]. Consequently, the complex mixture of secondary metabolites (mostly terpenoids) or plant extracts should be studied in future because they may provide multiple insecticidal mechanisms and ultimately reduce insect resistance [45,46]. In addition, the mixed EOs were made into microemulsions that still have the original insecticidal effect, which would improve the practicability of the mixtures of EOs [47]. Because the EO and some chemical compounds from S. chamaejasme flowers showed repellent bioactivity against stored product pests, the S. chamaejasme plant should be studied in future and considered for the preparation of a nanosized microemulsion, which may provide a good prospect for the control of storage insects.
3. Experiment
3.1. Plant Materials and Extraction
The raw materials were collected from 7 wild-growing populations of chamaejasme, identified in the region of Inner Mongolia (Table 7), China. The flowers of S. chamaejasme as experimental raw materials were used in this experiment. All medicinal herbs were identified as Stellera chamaejasme plants in Stellera genus of Thymelaeaceae family by the Plant Resources Appraisal Office of the Inner Mongolia Autonomous Region Food and Drug Inspection Institute. All samples had been identified as shown in Table 7. The fresh flowers of S. chamaejasme were selected and air-dried at room temperature. The flowers were subjected to hydrodistillation for a period of 6 h in a Clevenger (São Paulo, SP, Brazil) apparatus to extract the essential oil by using steam distillation equipment. The yellow-green EO was finally obtained and stored in an airtight container at 4 °C in refrigerator.

Table 7.
Collected information of S. chamaejasme flowers.
3.2. Experimental Insects
The three species of insects (T. castaneum, L. serricorne, and L. bostrychophila) used in the experiment were sampled from laboratory reproduction and verified by Professor Z. L. Liu (College of Plant Protection, China Agricultural University, Beijing, China). These three insect species were cultivated in a constant temperature and humidity chamber with temperature controlled at 30 ± 1 °C and humidity maintained at 70–80%. The booklouse was raised in conical bottles (50 mL) containing a mixture of milk powder, yeast, and flour (1:1:10, w/w). The red flour beetle and the cigarette beetle were bred in glass containers (0.5 L) with a proportional mixture of yeast and flour (1:10, w/w). All insects used in this experiment were 1–2 weeks old.
3.3. GC-FID and GC−MS Analysis
GC instrument (Agilent 7890A/5975C, Santa Clara, CA, USA) equipped with flame ionization detector (FID) was used in this experiment to establish the chromatographic fingerprints of 7 batches of EOs of S. chamaejasme flowers from different natural sites. The chromatographic column was HP–5MS (30 m × 0.32 mm × 0.25 μm). Nitrogen gas was used as the carrier gas at flow rate of 1.0 mL/min and the volume injection was 3.0 μL of 0.4% solution (diluted in n-hexane). The following measurement procedure was finally adopted after screening. The starting temperature of the column was set at 80 °C for 4 min, firstly rising to 160 °C at a speed of 10 °C/min, then rising to 184 °C at a speed of 2 °C/min, rising to 186 °C at a speed of 0.2 °C/min for 5 min, lastly rising to 280 °C at a speed of 5 °C/min for 25 min. The temperature of 280 °C was determined as the final detection temperature and 82.8 min was the total time of the chromatographic fingerprints.
In order to determine the chemical composition of the EO from S. chamaejasme flowers, a GC instrument (Agilent 6890N, Wilmington, DE, USA) combined with a mass spectrometer (Bruker 320, Folsom, CA, USA) was used in this experiment. The capillary column was DB–5MS (30 m × 0.25 mm × 0.25 μm) and the standard mass spectra was NIST 05 (Standard Reference Data, Gaithersburg, MD, USA). The starting temperature was set as 50 °C for 5 min, then rising to 290 °C at the speed of 10 °C/min for 11 min. Forty minutes chromatography in total was recorded and the volume injected was 1 μL of 1% solution (diluted in n-hexane). Each of the EO’s chemical constituents’ relative percentages was calculated by averaging peak area reports.
3.4. Experimental Data Analysis Methods
The chemical composition of EO from S. chamaejasme flowers was determined by GC−MS, identified by computer mass spectrometry (NIST 05) standard spectrometry database, and checked by reference to relevant literatures and according to the law of mass spectrometry lysis. Chromatographic fingerprint data were valued by the ‘Similarity Valuation system of TCM chromatographic fingerprints’ (China National Pharmacopoeia Commission) to analyze the similarity of S. chamaejasme flowers in different natural sites. The common characteristic peaks of the chromatographic fingerprints of samples from different areas could be confirmed by this system.
3.5. Repellent Test
The four compounds, fitone, methyl palmitate, myristic acid and phytol, are the main components in the EO of S. chamaejasme flowers, accounting for 52.764% of the total EO. Except phytol, the other three compounds are all terpenoids, a group of natural products usually possessing potential insecticidal activities. The repellent tests of EO from S. chamaejasme flowers and the 4 compounds (fitone, methyl palmitate, myristic acid, and phytol) against red flour beetle and cigarette beetle were carried out in 9 cm diameter petri dishes, while the repellent tests for the booklouse were implemented in 5.5 cm diameter petri dishes [48]. The EO and chemical compounds of S. chamaejasme flowers were dissolved in n-hexane to make a series of concentrations (78.63, 15.73, 3.15, 0.63, and 0.13 nL/cm2) to test the repellent rate of the red flour beetle and the cigarette beetle [49,50,51]. The specific experimental steps were to cut the filter paper with 9 cm diameter into two pieces on average. One half of paper was given 500 μL of n-hexane solvent as a blank control and the other half was treated with testing medicine in same volume. Both pieces of paper were stuck to the bottom of the petri dish with a glue stick after drying in the air for 30 s. Twenty insects were placed in the center of petri dish and covered. As for the booklouse, the EO and compounds were dissolved in n-hexane to prepare five different concentrations (63.17, 12.63, 2.53, 0.51, and 0.10 nL/cm2) to test the repellent rate of the booklouse [52,53]. The specific methods were the same as above, but the difference was that the dose was 150 μL. The testing insects were kept in a constant temperature and humidity box with temperature of 29 °C ± 1 °C and relative humidity of 70−80% [54,55]. At 2 and 4 h, the number of insects present on both sides of paper in each petri dish was recorded. Each concentration test was repeated five times. Meanwhile, the commercial insect repellent N, N-diethyl-3-methylbenzamide (DEET) was used as positive control [56,57]. The formula for calculating the percent repellency (PR) was as follows:
Nc represented the number of insects in the control groups, and Nt represented the number of insects in the testing group. The average repellent rate and standard deviation values were evaluated using Tukey’s test and variance analysis (ANOVA) by SPSS 26.0 software (IBM SPSS, Armonk, NY, USA).
4. Conclusions
In this research, the GC fingerprints of seven samples of EOs from S. chamaejasme flowers of different regions were established. Twenty-five common peaks were confirmed by the similarity evaluation system of Chinese medicine fingerprints and the similarity of the seven samples was between 0.9–1.0, indicating that the qualities of the seven samples of S. chamaejasme from various natural sites in the Inner Mongolia autonomous region were basically the same. In addition, the study evaluated the repellent activity of EO from S. chamaejasme flowers and its four chemical compounds against three common stored product insects for the first time. The experimental results showed that the volatile oil and its four chemical compounds from S. chamaejasme flowers had varying degrees of repellent effects against three target pests, particularly against the red flour beetle. The above results provided a basis for the development of plant-derived insecticides from S. chamaejasme, and also showed a good prospect for the prevention and control of stored product pests. However, the insecticidal mechanism of action of these active chemical components and the EO have not been clarified completely. The mutual synergistic or antagonistic relationships between the individual chemical compounds remain to be discovered by future research.
Author Contributions
Y.S. designed the study. L.B., L.S., Y.X. and J.L. performed all the experiments. J.L. wrote the manuscript with the input from other authors. Y.S. and X.W. checked the manuscript. Y.H. and Y.S. provided guidance throughout experiment. All authors have read and agreed to the published version of the manuscript.
Funding
The 2021 Intercollegiate Cooperation Project of the Education Department of Liaoning Province. The 2021 Ministry of Education Industry−University Cooperation Collaborative Education Project. The establishment of the technical system for the preparation of classic Mongolian medicine Zhachong Shisanwei Pills and Anshen Buxin Liuwei Pills and the formulation of high-quality preparation standards (2018YFC1708205).
Institutional Review Board Statement
The pests used in this experiment are reproduced in the laboratory and do not involve human or animal ethical issues, so it is not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All research data has been presented in this article and available from authors.
Acknowledgments
We thank Du from Beijing Normal University for providing insects, and Professor Wang from Inner Mongolia University for Nationalities for providing medicinal plants.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Li, X.-Q.; Rahman, K.; Zhu, J.-Y.; Zhang, H. Chemical constituents and pharmacological activities of Stellera chamaejasme. Curr. Pharm. Des. 2018, 24, 2825–2838. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.H.; Tanaka, T.; Sakamoto, T.; Kouno, I.; Duan, J.A.; Zhou, R.H. Biflavanones, diterpenes, and coumarins from the roots of Stellera chamaejasme L. Chem. Pharm. Bull. 2002, 50, 137–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.H.; Qin, G.W.; Li, X.Y.; Xu, R.S. New biflavanones and bioactive compounds from Stellera chamaejasme L. Yao Xue Xue Bao = Acta Pharm. Sin. 2001, 36, 669–671. [Google Scholar]
- Guo, L.; Li, J.; He, W.; Liu, L.; Huang, D.; Wang, K. High nutrient uptake efficiency and high water use efficiency facilitate the spread of Stellera chamaejasme L. in degraded grasslands. BMC Ecol. 2019, 19, 50. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Detheridge, A.; Liu, Y.; Wang, L.; Wei, H.; Griffith, G.W.; Scullion, J.; Wei, Y. Variation in soil fungal composition associated with the invasion of Stellera chamaejasme L. in Qinghai–Tibet Plateau Grassland. Microorganisms 2019, 7, 587. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Li, K.; Zhao, C.; Ren, H.; Nie, X.; Jia, D.; Li, L.; Li, Q. The expansion process of a Stellera chamaejasme population in a degraded alpine meadow of Northwest China. Environ. Sci. Pollut. Res. 2019, 26, 20469–20474. [Google Scholar] [CrossRef]
- Liu, X.; Guan, H.; Song, M.; Fu, Y.; Han, X.; Lei, M.; Ren, J.; Guo, B.; He, W.; Wei, Y. Reference gene selection for qRT-PCR assays in Stellera chamaejasme subjected to abiotic stresses and hormone treatments based on transcriptome datasets. PeerJ 2018, 6, e4535. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Zhao, H.; Zhai, X.; Wang, K.; Liu, L.; Wang, K.; Huang, D. Study on life histroy traits of Stellera chamaejasme provide insights into its control on degraded typical steppe. J. Environ. Manag. 2021, 291, 112716. [Google Scholar] [CrossRef]
- Wellmeyer, B.; Merzendorfer, H. Tribolium castaneum: A model for investigating the mode of action of insecticides and mechanisms of resistance. Curr. Pharm. Des. 2020, 26, 3554–3568. [Google Scholar] [CrossRef]
- Edde, P. Biology, ecology, and control of Lasioderma serricorne (F.) (Coleoptera: Anobiidae): A review. J. Econ. Èntomol. 2019, 112, 1011–1031. [Google Scholar] [CrossRef]
- Feng, S.; Yang, Q.; Li, H.; Song, F.; Stejskal, V.; Opit, G.P.; Cai, W.; Li, Z.; Shao, R. The highly divergent mitochondrial genomes indicate that the booklouse, Liposcelis bostrychophila (Psocoptera: Liposcelididae) is a cryptic species. G3 Genes Genomes Genet. 2018, 8, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Skourti, A.; Kavallieratos, N.G.; Papanikolaou, N.E. How is fitness of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) affected when different developmental stages are exposed to chlorfenapyr? Insects 2020, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Blackie, H.M.; Mackay, J.W.; Allen, W.J.; Smith, D.H.V.; Barrett, B.; Whyte, B.I.; Murphy, E.C.; Ross, J.; Shapiro, L.; Ogilvie, S.; et al. Innovative developments for long-term mammalian pest control. Pest Manag. Sci. 2013, 70, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benelli, G.; Caselli, A.; Di Giuseppe, G.; Canale, A. Control of biting lice, Mallophaga—A review. Acta Trop. 2018, 177, 211–219. [Google Scholar] [CrossRef]
- Martínez-Hidalgo, P.; Maymon, M.; Pule-Meulenberg, F.; Hirsch, A.M. Engineering root microbiomes for healthier crops and soils using beneficial, environmentally safe bacteria. Can. J. Microbiol. 2019, 65, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.-J.; Pang, X.; Cao, J.-Q.; Du, S.-S. Comparative analysis on bioactivity against three stored insects of Ligusticum pteridophyllum Franch. rhizomes essential oil and supercritical fluid (SFE-CO2) extract. Environ. Sci. Pollut. Res. 2020, 27, 15584–15591. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Münzbergová, Z.; Santino, A. Plant volatile aldehydes as natural insecticides against stored-product beetles. Pest Manag. Sci. 2007, 64, 57–64. [Google Scholar] [CrossRef]
- Guo, S.-S.; Pang, X.; Wang, Y.; Geng, Z.-F.; Cao, J.-Q.; Liang, J.-Y.; Deng, Z.-W.; Du, S.-S. Chemical constituents isolated from stems of Schisandra chinensis and their antifeedant activity against Tribolium castaneum. Nat. Prod. Res. 2019, 34, 2595–2601. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, P.-P.; Guo, S.-S.; Cao, J.-Q.; Pang, X.; Geng, Z.-F.; Sang, Y.-L.; Du, S.-S. Supercritical carbon dioxide extract of Cinnamomum cassia bark: Toxicity and repellency against two stored-product beetle species. Environ. Sci. Pollut. Res. 2018, 25, 22236–22243. [Google Scholar] [CrossRef] [PubMed]
- Perumalsamy, H.; Chang, K.S.; Park, C.; Ahn, Y.J. Larvicidal activity of Asarum heterotropoides root constituents against insecticide-susceptible and resistant Culex pipiens pallens and Aedes aegypti and Ochlerotatus togoi. J. Agric. Food Chem. 2010, 58, 10001–10006. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Jiao, M.-L.; Zang, H.-Y.; Guo, S.-S.; Wang, Y.; Sang, Y.-L.; Du, S.-S. Chemical composition of essential oils from four Rhododendron species and their repellent activity against three stored-product insects. Environ. Sci. Pollut. Res. 2019, 26, 23198–23205. [Google Scholar] [CrossRef]
- Zhang, N.; He, J.; Xia, C.-Y.; Lian, W.-W.; Yan, Y.; Ding, K.; Zhang, Y.-Y.; Xu, J.-K.; Zhang, W.-K. Ethnopharmacology, phytochemistry, pharmacology, clinical applications and toxicology of the genus Stellera Linn.: A review. J. Ethnopharmacol. 2020, 264, 112915. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, S.; Wang, L. Purification and identification of carvacrol from the root of Stellera chamaejasme and research on its insecticidal activity. Nat. Prod. Res. 2011, 25, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Hou, T. Isolation and identification of 2-isopropyl-5-methylphenol from Stellera chamaejasme and its insecticidal activity against Aphis craccivora and Pieris rapae. Nat. Prod. Res. 2011, 25, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.-Y.; Chi, Y.-Y.; Han, J.-X.; Xiang, H.; Xie, Q. The toxicity and attenuation methods of toxic Chinese materia medica for its reasonable application: A review. Am. J. Chin. Med. 2021, 49, 41–67. [Google Scholar] [CrossRef]
- Shen, T.; Yu, H.; Wang, Y.Z. Assessing geographical origin of Gentiana Rigescens using untargeted chromatographic fingerprint, data fusion and chemometrics. Molecules 2019, 24, 2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Zhang, S.; Shen, Q.; Hu, G.; Lu, J. Multivariate similarity clustering analysis: A new method regarding biogeography and its application in global insects. Integr. Zool. 2020, 16, 390–403. [Google Scholar] [CrossRef]
- El-Desoky, A.H.; Eguchi, K.; Kato, H.; Kishimoto, N.; Misumi, S.; Watanabe, T.; Tsukamoto, S. Chamaejasmins, cytotoxic guaiane sesquiterpenes from the root of Stellera chamaejasme L. Fitoterapia 2020, 146, 104714. [Google Scholar] [CrossRef]
- Yu, L.; Pu, J.; Zuo, M.; Zhang, X.; Cao, Y.; Chen, S.; Lou, Y.; Zhou, Q.; Hu, H.; Jiang, H.; et al. Hepatic Glucuronidation of Isoneochamaejasmin A from the Traditional Chinese Medicine Stellera Chamaejasme L. Root. Drug Metab. Dispos. 2014, 42, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiaorong, T.; Taiping, H. Separation and identification of botanical insecticide 7-hydroxycoumarin and its biological activity against Aphis craccivora and Culex pipiens pallens. Nat. Prod. Res. 2008, 22, 365–370. [Google Scholar] [CrossRef]
- Yan, Z.; Guo, H.; Yang, J.; Liu, Q.; Jin, H.; Xu, R.; Cui, H.; Qin, B. Phytotoxic flavonoids from roots of Stellera chamaejasme L. (Thymelaeaceae). Phytochemistry 2014, 106, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.C.; Yan, H.Y. Research progress on chromatographic fingerprint similarity evaluation method for traditional chinses medicine in the past 30 years (1988–2017) and its prospect. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2018, 43, 1969–1977. (In Chinese) [Google Scholar]
- Hu, X.; Wang, D.; Pang, Y.; Wu, Z.; Huan, H.; Chen, Z.; Li, W. Development of chromatographic fingerprint for quality analysis of diploid and tetraploid Lonicera japonica. J. Tradit. Chin. Med. 2020, 40, 73–82. [Google Scholar]
- Zhu, L.; Fang, L.; Li, Z.; Xie, X.; Zhang, L. A HPLC fingerprint study on Chaenomelis Fructus. BMC Chem. 2019, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, C.; Wang, X.; Liang, Y.; Zeng, Y.; Wu, H.; Xu, Q.; Lv, H. Chromatographic fingerprint investigation for quality evaluation and control of Shengui hair-growth tincture. Planta Med. 2009, 76, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Zhu, L.; Yi, T. Fingerprint analysis of Resina Draconis by ultra-performance liquid chromatography. Chem. Cent. J. 2017, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, X.-W.; Marriott, P.J. Evaluation of Coptidis Rhizoma–Euodiae Fructus couple and Zuojin products based on HPLC fingerprint chromatogram and simultaneous determination of main bioactive constituents. Pharm. Biol. 2013, 51, 1384–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhang, L.F.; Deng, Y.; Qin, J.; Zhang, S.B.; Hu, J.M. Chemical constituents of species in the Genus Pleione (Orchidaceae) and the implications from molecular phylogeny. Chem. Biodivers. 2021, 18, e2000870. [Google Scholar] [CrossRef]
- Tang, T.-X.; Guo, W.-Y.; Xu, Y.; Zhang, S.-M.; Xu, X.-J.; Wang, N.-M.; Zhao, Z.-M.; Zhu, L.-P.; Yang, D.-P. Thin-layer chromatographic identification of Chinese propolis using chemometric fingerprinting. Phytochem. Anal. 2014, 25, 266–272. [Google Scholar] [CrossRef]
- Song, X.; Liu, G.; Duan, W.; Liu, T.; Huang, Z.; Ren, J.; Li, Y.; Hou, X. Genome-wide identification, classification and expression analysis of the heat shock transcription factor family in Chinese cabbage. Mol. Genet. Genom. 2014, 289, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Yu, H.; Li, P. Analysis of Chinese drug beimu and its fake species with clustering analysis and FTIR spectra. Zhong Yao Cai = Zhongyaocai = J. Chin. Med. Mater. 2005, 28, 89–91. (In Chinese) [Google Scholar]
- Zaslavsky, L.; Ciufo, S.; Fedorov, B.; Tatusova, T. Clustering analysis of proteins from microbial genomes at multiple levels of resolution. BMC Bioinform. 2016, 17, 276. [Google Scholar] [CrossRef] [Green Version]
- Tak, J.-H.; Jovel, E.; Isman, M.B. Comparative and synergistic activity of Rosmarinus officinalis L. essential oil constituents against the larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Pest Manag. Sci. 2015, 72, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Scalerandi, E.; Flores, G.A.; Palacio, M.; Defagó, M.T.; Carpinella, M.C.; Valladares, G.; Bertoni, A.; Palacios, S.M. Understanding synergistic toxicity of terpenes as insecticides: Contribution of metabolic detoxification in Musca domestica. Front. Plant Sci. 2018, 9, 1579. [Google Scholar] [CrossRef] [Green Version]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Feng, Y.-X.; Wang, Y.; Geng, Z.-F.; Zhang, D.; Almaz, B.; Du, S.-S. Contact toxicity and repellent efficacy of Valerianaceae spp. to three stored-product insects and synergistic interactions between two major compounds camphene and bornyl acetate. Ecotoxicol. Environ. Saf. 2019, 190, 110106. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Cianfaglione, K.; Bajalan, I.; Morshedloo, M.R.; Lupidi, G.; Romano, D.; et al. Microemulsions for delivery of Apiaceae essential oils—Towards highly effective and eco-friendly mosquito larvicides? Ind. Crop. Prod. 2019, 129, 631–640. [Google Scholar] [CrossRef]
- Cao, J.Q.; Guo, S.S.; Wang, Y.; Pang, X.; Geng, Z.F.; Du, S.S. Toxicity and repellency of essential oil from Evodia lenticellata Huang fruits and its major monoterpenes against three stored-product insects. Ecotoxicol. Environ. Saf. 2018, 160, 342–348. [Google Scholar] [CrossRef]
- Saad, M.M.G.; El-Deeb, D.A.; Abdelgaleil, S.A.M. Insecticidal potential and repellent and biochemical effects of phenylpropenes and monoterpenes on the red flour beetle, Tribolium castaneum Herbst. Environ. Sci. Pollut. Res. 2019, 26, 6801–6810. [Google Scholar] [CrossRef]
- Lü, J.; Ma, D. Repellent and contact toxicity of Alpinia officinarum rhizome extract against Lasioderma serricorne adults. PLoS ONE 2015, 10, e0135631. [Google Scholar] [CrossRef]
- You, C.-X.; Guo, S.-S.; Zhang, W.-J.; Geng, Z.-F.; Liang, J.-Y.; Lei, N.; Du, S.-S.; Deng, Z.-W. Chemical constituents of Murraya tetramera Huang and their repellent activity against Tribolium castaneum. Molecules 2017, 22, 1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.C.; Li, Y.P.; Li, H.Q.; Deng, Z.W.; Zhou, L.; Liu, Z.L.; Du, S.S. Identification of Repellent and Insecticidal Constituents of the Essential Oil of Artemisia rupestris L. Aerial Parts against Liposcelis bostrychophila Badonnel. Molecules 2013, 18, 10733–10746. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.C.; Shi, W.P.; Liang, Y.; Zhou, L. Repellent and insecticidal effects of the essential oil of Kaempferia galanga rhizomes to Liposcelis bostrychophila (Psocoptera: Liposcelidae). J. Econ. Èntomol. 2014, 107, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Feng, Y.-X.; Qi, X.-J.; Wang, Y.; Almaz, B.; Yixi, F.; Du, S.-S. Toxicity and repellent activity of essential oil from Mentha piperita Linn. leaves and its major monoterpenoids against three stored product insects. Environ. Sci. Pollut. Res. 2019, 27, 7618–7627. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Stashenko, E.E. Repellent activity of essential oils and some of their individual constituents against Tribolium castaneum Herbst. J. Agric. Food. Chem. 2011, 59, 1690–1696. [Google Scholar] [CrossRef]
- Legeay, S.; Clere, N.; Apaire-Marchais, V.; Faure, S.; Lapied, B. Unusual modes of action of the repellent DEET in insects highlight some human side effects. Eur. J. Pharmacol. 2018, 825, 92–98. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Chandu, A.G.S.; Devi, S.S.; Quirin, K.-W.; Pasha, A.; Vijayendra, S.V.N. In-vitro evaluation of antimicrobial and insect repellent potential of supercritical-carbon dioxide (SCF-CO2) extracts of selected botanicals against stored product pests and foodborne pathogens. J. Food Sci. Technol. 2019, 57, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).