Abstract
Berberis species are known for their use in traditional medicine. Here, we report the phenolic composition and bioactivity of methanolic and aqueous extracts of Berberis thunbergii DC. leaves. The phenolic profiling and the quantitation of the main compounds were performed by high-performance liquid chromatography with diode array and mass spectrometry detections. The most abundant compounds in both extracts were caffeoylquinic acids (chlorogenic acid, particularly, with a concentration of 90.1–101.3 mg g−1 dried extract), followed by caffeoylglucaric acids and quercetin glycosides. Antioxidant and radical scavenging assays (phosphomolybdenum, DPPH, ABTS, CUPRAC, FRAP, metal chelating activity), as well as enzyme inhibitory assays (acetylcholinesterase, butyrylcholinesterase, tyrosinase, amylase, glucosidase, and lipase), were carried out to evaluate the potential bioactivity of B. thunbergii. The methanolic extract presented the highest antioxidant and radical scavenging values, in agreement with its higher phenolic content. Regarding enzyme inhibitory potential, the methanolic extract was also more potent than the aqueous one. Hence, B. thunbergii leaves represent a suitable candidate for the preparation of pharmaceutical or nutraceutical products.
1. Introduction
Berberis is a large genus of the Berberidaceae family, which is represented by around 500 species distributed worldwide. This genus consists of spiny evergreen shrubs with yellow wood and flowers, yellow or orange, appear alone or in racemes (3–6 mm long). Leaves on long shoots develop into three-spine thorns and short shoots with several leaves (1–10 cm long). Fruits are small berries, red or blue after ripening [1].
The medicinal properties of Berberis have been known and appreciated for thousands of years. Many of them are due to the presence of alkaloids with different pharmacological activities [2], being berberine one of the most active compounds [3]. Some species of Berberis contain predominant phenolic compounds in their leaves, such as chlorogenic acid and rutin [4].
The plants belonging to this genus are known for their antidiabetic properties [5]. Some species have shown antibacterial and antifungal activities [6] and have been used in traditional medicine to cure heart diseases, digestive ailments, and problems with the urinary tract [4,7]. In natural medicine, Berberis leaves are used mainly for colds and common ailments of the body [4]. Commercial teas from B. buxifolia and decoctions of B. vulgaris have been traditionally used; in fact, infusions obtained from berberine-producing plants are used for their antimicrobial, anti-inflammatory, and antiseptic properties [1,8]. In addition to its curative applications, various species of this genus are commonly used in some cuisines [9].
Berberis thunbergii DC., also known as Japanese barberry, is a dense, woody shrub with deciduous nature that can reach up to 2 m high and is often used as an ornamental plant due to the bright red tones of the leaves [10]. Native to Asia, it is also present in the USA and in several European countries [3,11]. B. thunbergii is also recognized as a healing plant in Asia. It has been reported to present positive biological effects on health, such as antioxidant, anti-inflammatory, antibacterial, and antifungal activities [10,12,13,14]. Few studies have reported the phytochemical composition of B. thunbergii: The profile of alkaloids [10,15] and flavonoids contents in roots [3].
The aim of this work is to detail the composition of the phenolic content of leaves of B. thunbergii, as well as its antioxidant activity and enzyme inhibitory properties against cholinesterase, amylase, glucosidase, tyrosinase, and lipase. The results here presented may open additional ways to valorize this species as a source of bioactive compounds for the pharmaceutical or food industries.
2. Results and Discussion
First of all, MeOH and H2O were selected as the extractants, as they are the most common solvents used for the extraction of phenolics from plant materials. A comparison between ultrasound-assisted extraction using an ultrasound bath (Bandelin Sonorex Digital 10P; Sigma-Aldrich, Madrid, Spain) and an ultrasound probe (Qsonica Sonicators, Newtown, CT, USA) was performed. The optimum conditions using the ultrasound bath were 60 min at room temperature. For the ultrasound probe, 5–20 min and 25%–100% power were tested, observing that the highest recovery yields were obtained for 10 min and 50% power; different conditions resulted in lower recovery yields, approximately 20%–30%. These optimum conditions were compared with the recovery obtained with the ultrasound bath, observing similar results. Hence, we selected the ultrasound probe to minimize the time required for sample extraction: 10 min instead of 60 min.
2.1. HPLC-MS Analysis of Methanolic and Aqueous Extracts
Compounds characterization was carried out by mass spectrometry, using both positive and negative ion modes. The base peak chromatograms of the methanolic and aqueous extracts of B. thunbergii leaves are shown in Figure 1 (overlapped chromatograms can be seen in Supplementary Materials). We identified or tentatively characterized 30 compounds; 50% were phenolic acids and approximately 25% flavonoids. All compounds were numbered according to their order of elution (Table 1), keeping the same numeration in both extracts. Among the identified compounds, berberine, rutin, and chlorogenic acid may be cited due to their known bioactivity.
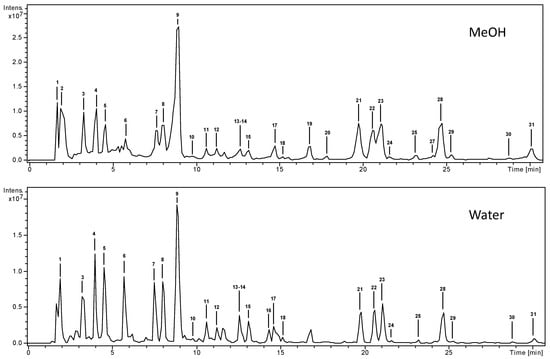
Figure 1.
High-performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI/MSn) base peak chromatograms (BPC) of the methanolic and aqueous extracts of Berberis thunbergii leaves.

Table 1.
Characterization of phytochemicals found in extracts of Berberis thunbergii by HPLC-/ESI-MSn.
2.1.1. Phenolic Acids
The HPLC profile of the extracts of B. thunbergii leaves revealed the highest peak at a retention time of 9 min, which corresponded to chlorogenic acid (compound 9; identified by comparison with an analytical standard). In other Berberis species (B. microphylla G. Forst), this compound was the main one [16]. Compounds 7, 8, 12, 13, and 17 presented deprotonated molecular ions at m/z 707 ([2M − H]−) or 353 and fragment ions characteristic of caffeoylquinic acids [17]. Several caffeoylquinic acids have been previously reported in Berberis species [16,18]. Compound 16, with [M − H]− ion at m/z 705, MS2 base peak at m/z 513 (neutral loss of 192 Da, which corresponds to quinic acid), and MS3 base peak at m/z 339 (neutral loss of 174 Da, corresponding to a dehydroquinic acid), was characterized as caffeoylquinic acid dehydrodimer, according to bibliographic data [19]; this compound has not been previously reported in Berberis species. With [M − H]− ion at m/z 515 and MSn fragment ions at m/z 353 and 191, compound 27 was identified as 3,5-dicaffeoylquinic acid [17], previously reported in B. microphylla G. Forst [16].
Compounds 2, 3, 4, 5, and 6 showed [M − H]− ions at m/z 371, and fragment ions in MS2 and MS3 at m/z 209 and 191. The fragment ion at m/z 209 was caused by the loss of a neutral fragment of a caffeoyl group. The ion at m/z 209 refers to the compound glucaric acid (compound 1). This fragmentation pattern allowed their tentative characterization as caffeoylglucaric acid isomers. A similar phytochemical profile was also found in B. microphylla G. Forst [16].
Compounds 19 and 24 displayed deprotonated molecular ions at m/z 367 with MS2 and MS3 base peaks at m/z 179 and 135, respectively. These compounds were identified as methyl-caffeoyl-quinate isomers [20].
Compounds 15 and 18 were characterized as coumaroylquinic acid isomers based on its [M − H]− ion at m/z 337 and the comparison of its fragmentation pattern with bibliographic data [17]. These compounds have been previously reported in B. darwinii [18].
2.1.2. Flavonoids
Previous studies on B. microphylla and B. darwinii [18] reported the presence of several quercetin glycosides, in agreement with the results observed in B. thunbergii. Compound 20 showed [M − H]− ion at m/z 627, with fragment ions at m/z 301 and 151 (typical of quercetin), so it was tentatively characterized as a quercetin derivative. Compounds 22 and 23 exhibited [M − H]− ion at m/z 463; they suffered the neutral loss of 162 Da (hexoside) to yield quercetin at m/z 301, so they were characterized as quercetin-O-hexoside. In a similar way, compound 28 was characterized as quercetin-O-deoxyhexoside due to the neutral loss of 146 Da to yield quercetin. Compound 25, with [M − H]− ion at m/z 505, suffered the neutral loss of 204 Da (acetylhexoside) to yield quercetin and was tentatively characterized as quercetin-O-acetylhexoside [21]. Compound 21 was identified as rutin by comparison with an analytical standard.
Compound 10, with [M − H]− ion at m/z 447, suffered the neutral loss of 162 Da, yielding the aglycone luteolin at m/z 285 (characteristic fragment ions at m/z 243 and 241), so it was characterized as luteolin-O-hexoside.
Compound 14 exhibited an [M − H]− ion at m/z 449. It suffered the neutral loss of 162 Da, yielding the aglycone at m/z 287, that was characterized as dihydrokaempferol due to its fragment ion at m/z 259. Hence, this compound was identified as dihydrokaempferol-O-hexoside [22].
Compound 30, with [M − H]− ion at m/z 431, displayed the neutral loss of 146 Da (deoxyhexose), yielding kaempferol aglycone at m/z 285 (fragment ion at m/z 255), so it was tentatively characterized as kaempferol-O-deoxyhexoside [22].
2.1.3. Other Compounds
Compound 26, with M+ ion at m/z 551, suffered the neutral loss of 248 Da (malonyl + glucoside), yielding the anthocyanidin named delphinidin at m/z 303, and it was characterized as delphinidin malonyl glucose [23]. Delphinidin was also reported in B. lycium Royle and B. microphylla [24,25], being the most representative anthocyanidin in various species of Berberis [26].
Compound 32 was characterized as berberine. This alkaloid showed [M + H]+ ion at m/z 336 and MS2 fragmentation ions at m/z 321 and m/z 293 [27]. Previous studies reported this alkaloid in B. thunbergii to be at higher concentrations than in other plants of the same family [15].
Finally, compound 11, with [M − H]− ion at m/z 569, was tentatively characterized as a quinic acid derivative due to the fragments ion characteristic of quinic acid (m/z 191 and 173).
2.2. Quantification of Phenolic Compounds in All Extracts
Twenty-two compounds were quantified in the analyzed extracts of B. thunbergii by HPLC-DAD (Table 2), measuring phenolic acids at 320 nm and flavonoids at 350 nm (see Supplementary Materials).

Table 2.
Quantification of compounds in extracts of Berberis thunbergii in methanol (MeOH) and water. Values (mg g−1 dried extract; DE) are mean ± SD of three parallel measurements.
MeOH and water extracts presented similar phenolic composition, although higher concentration (total amount of phenolics) was found in the methanolic extract. For some individual compounds, higher concentrations were found in the aqueous extract. The results indicated that phenolic acids and flavonoids were the most abundant compounds. Chlorogenic acid presented the highest concentration (101.3 mg g−1 DE in MeOH and 90.1 mg g−1 DE in water), in agreement with the results reported in another species of Berberis (B. microphylla G. Forst). However, the concentration of chlorogenic acid in this species was only 0.415 mg g−1 and the total concentration of hydroxycinnamic acids was of 1.48 mg g−1 [16], lower than the ones found in our studies. In a previous study on 8 species of Berberis, the concentration of chlorogenic acid in leaves was determined, obtaining a range of concentrations of 21.8–189.4 mg g−1 [28], with only two species presenting higher concentration than the one found in the analyzed extracts of B. thunbergii. In the present research, this compound represented almost 50% of the total phenolic acids and 43% of the TIPC (total individual phenolic content, defined as the sum of all individual phenolic concentrations). Thirty per cent of the phenolic acids corresponded to caffeoylglucaric acid isomers. Caffeoylquinic acid isomers and small amounts of other phenolic acids (methyl-caffeoyl-quinate, coumaroylquinic acid isomers) accounted for the remaining 20%. The profile of the phenolic acids obtained in B. thunbergii is very similar to that of B. microphylla G. Forst, in which caffeoylquinic acids and caffeoylglucaric acids were reported [16].
Leaves were also rich in flavonoids such as quercetin derivatives and kaempferol derivatives (in minor concentrations). Quercetin derivatives represented 77% of the flavonoids quantified in the methanolic extract and around 70% in the aqueous extract. Other species of Berberis (B. lycium Royle, B. microphylla, and B. crataegina DC) also presented quercetin and rutin in their compositions [24,25,29]. TIP in MeOH (236 mg g−1 DE) was slightly higher than in the aqueous solution (213 mg g−1 DE).
2.3. Total Phenolic and Flavonoid Contents
Spectrophotometric methods are considered as an uncertain prediction for the determination of total bioactive components because there is a possibility that other phytochemicals could be participating in the reaction. However, these methods are still common and can provide valuable information for comparison purposes with other works.
The results of total phenolic content (TPC) and total flavonoid content (TFC) assays for B. thunbergii extracts are given in Table 3. In accordance with HPLC-MS results, the methanol extract possessed higher concentration of phenolics (216.30 mg GAE/g) and flavonoids (45.85 mg RE/g) than water extract (193.70 and 20.55 mg RE/g), respectively. In previous literature, total bioactive components for several Berberis species have been reported. However, most studies were conducted on Berberis fruits [30,31,32]. A recent study reported that the methanol extract (80%) of Berberis orthobotrys contained more phenolics and flavonoids than the water extract [33], in agreement with our results. Belwal et al. [34] conducted a study on leaves of Berberis asiatica, observing that the total phenolic and flavonoid contents were lower than our results. Similarly, B. vulgaris (52.54 mg GAE/g) and B. croatica (31.16 mg GAE/g) [35] presented much lower phenolic contents than the study extracts of B. thunbergii.

Table 3.
Total bioactive components, antioxidant, and enzyme inhibitory properties of the tested extracts.
2.4. Biological Activities
In the present work, antioxidant capacity and enzyme inhibitory effects of B. thunbergii extracts were evaluated for their biological effects. The results are summarized in Table 3. The evaluation of antioxidant abilities of plant extracts is of great importance in order to provide novel and safer natural antioxidants for developing functional products. However, no golden standard method has been reported. At this point, several assays could be performed to provide a full picture of the antioxidant effects of plant extracts. In this study, free radical quenching (ABTS and DPPH assays), reducing power (CUPRAC and FRAP), phosphomolybdenum, and metal chelating assays were conducted to examine antioxidant effects of B. thunbergii extracts. In all assays, the antioxidant effects in B. thunbergii extracts followed the same trend of total bioactive compounds. This was also confirmed by correlation analysis (Table 4). The methanol extract was more active on free radicals than water extract. Additionally, the methanol extract exhibited the best reduction ability in both CUPRAC and FRAP assays. The observed antioxidant abilities of B. thunbergii extracts could be explained due to the presence of chlorogenic acid. In accordance with our results, chlorogenic acid has been reported as a good radical scavenger and reductive agent [36,37,38]. In earlier studies, several Berberis species have exhibited significant antioxidant effects [30,33,39], thus, members of the genus Berberis could be considered as significant sources of natural antioxidants.

Table 4.
Correlation coefficients between total bioactive compounds and biological activities (Pearson correlation coefficient (R), p < 0.05).
Nowadays, novel and safe therapeutic strategies are required to manage several health problems, including type 2 diabetes and obesity. For example, about 425 million people were affected with type 2 diabetes in 2017 [40]. An increasing trend has been reported for obesity [41]. Based on the enzyme inhibition theory, the inhibition of key enzymes is used to alleviate the observed symptoms in diseases [42]. To this end, several compounds are chemically produced as enzyme inhibitors but most of them have undesirable side effects [43]. Hence, there is a growing interest to identify novel, more efficient and less toxic natural enzymatic inhibitors. For these reasons, we tested enzyme inhibitor effects of B. thunbergii extracts against cholinesterases (AChE and BChE), tyrosinase, amylase, glucosidase, and lipase. The results are summarized in Table 3. Similarly to the antioxidant results, the methanol extract was more active on all enzymes than water extract. For example, the methanol extract exhibited an inhibitory effect on BChE (0.19 mg GALAE/g), while the water extract was reported as inactive on this enzyme. A similar result was also found for lipase. Additionally, the methanol extract exhibited a significant inhibitory effect on enzymes linked to type 2 diabetes. The observed enzyme inhibitory effect of methanol extract can be attributed to the presence of phenolics, especially chlorogenic acid, and flavonoids such as quercetin and rutin (the main compounds in the analyzed extracts, see Table 2). This approach was also supported by several researchers. For example, Oboh et al. [44] reported that chlorogenic acid exhibited good inhibitory effects on AChE. Furthermore, the anti-amylase and anti-glucosidase effects of chlorogenic acid have been reported [45,46]. Similar observations have been also noted for rutin and quercetin [47,48,49,50]. To the best of our knowledge, the present research is the first scientific report regarding the enzyme inhibitory effect of B. thunbergii leaves. From this angle, this study could provide a scientific basis for designing further studies on B. thunbergii.
3. Materials and Methods
3.1. Plant Material
Leaves of B. thunbergii were randomly collected from different plants, manually, at the Herbarium of the University of Jaén (Jaén, southeast of Spain; 37°47’18.879”N 3°46’31.583”W, 427 m a.s.l.), in September 2018. Botanical authentication was carried out by the botanist Dr. Carlos Salazar Mendías (Department of Animal Biology, Plant Biology, and Ecology of the University of Jaén, Spain). Only the most intact and fresh leaves were selected. Leaves were washed with ultrapure water and stored in a freezer at −80 °C until use.
3.2. Extraction
Extractions were carried out in two different media: Methanol (MeOH; HPLC grade) and water (Milli-Q waters). For the methanol extraction, leaves were lyophilized (ModulyoD/23, Thermo Savant; Waltham, MA, USA) and crushed with a grinder. Extraction was performed as follows: 2.5 g of dry material was extracted with 50 mL MeOH in an ultrasonic liquid processor (Qsonica Sonicators; Newton, CT, USA) with a power of 55 W and a frequency of 20 kHz, for 10 min (using 50% power) at room temperature. Extractions were done in triplicate. After sonication, solutions were filtered through Whatman No.1 filters. The solvent was evaporated under reduced pressure in a Hei-Vap Precision rotary evaporator (Heidolf; Schwabach; Germany) at 40 °C. Dried extracts (DE) were stored at −20 °C until analysis.
On the other hand, the extraction with water was carried out in the following way: 2.5 g of fresh leaves (crushed with a grinder) was extracted with 150 mL H2O at 100 °C in a hot plate (C-MAG HS7, IKA; Staufen, Germany) for 30 min. Extractions were done in triplicate. After that, solutions were filtered through Whatman No.1 filters. Finally, the solvent was evaporated under reduced pressure in a rotary evaporator and the dried extracts were stored at −20 °C until analysis.
3.3. HPLC Analysis
High-performance liquid chromatography with diode-array and mass spectrometry detection (HPLC-DAD-MSn) analysis was performed on an Agilent Series 1100 with a G1315B diode array detector and an ion trap mass spectrometer (Esquire 6000, Bruker Daltonics, Madrid, Spain) with an electrospray interface. A reversed-phase Luna Omega Polar C18 analytical column (150 × 3.0 mm; 5 µm particle size; Phenomenex, Madrid, Spain) and a Polar C18 Security Guard cartridge (Phenomenex) of 4 × 3.0 mm were used. Detailed conditions were previously reported [51] and given in Supplementary Materials.
5 mg of DE (MeOH) was re-dissolved in 1 mL of MeOH and 5 mg of DE (H2O) was re-dissolved in 1 mL of MeOH:H2O (10:90; v:v). After filtration through 0.45 µm nylon membrane filters, 10 μL of sample was injected.
Standards of caffeic acid, 3-O-caffeoylquinic acid (chlorogenic acid; CAS No. 327-97-9), 4-O-caffeoylquinic acid, kaempferol, quercetin, and rutin were obtained from Sigma-Aldrich (Madrid, Spain) and individual stock solutions (500–1000 mg L−1) were prepared in MeOH. LC-MS grade acetonitrile (Panreac; Barcelona, Spain) and ultrapure water (Milli-Q Waters purification system; Millipore; Milford, MA, USA) were also used. We prepared calibration curves for caffeic acid, 4-O-caffeoylquinic acid, chlorogenic acid, kaempferol, quercetin, and rutin at concentrations 0.5–100 µg mL−1 in MeOH. Chromatograms were recorded at 320 nm for caffeic acid, 4-O-caffeoylquinic acid and chlorogenic acid, and 350 nm for kaempferol, quercetin, and rutin. Peak area (at the corresponding wavelength) was plotted versus analyte concentration to construct the calibration graphs.
3.4. Assays for Total Phenolic and Flavonoid Contents
With reference to our earlier report [52], total bioactive components, namely total phenolic (TPC) and flavonoid (TFC) contents, were measured by spectrophotometric assays. The obtained results were reported as standard equivalents of gallic acid for phenolics and rutin for flavonoids. Details for the protocols are provided in Supplementary Materials.
3.5. Determination of Antioxidant and Enzyme Inhibitory Effects
The in vitro enzyme inhibitory effects of B. thunbergii extracts on five enzymes (lipase, α-amylase, α-glucosidase, cholinesterases, and tyrosinase) were evaluated as previously reported [52,53]. The enzyme inhibitory actions were assessed as kojic acid equivalents (KAE) for tyrosinase, galantamine equivalents (GALAE) for acetyl cholinesterase (AChE) and butyryl cholinesterase (BChE), acarbose equivalents (ACAE) for α-amylase and α-glucosidase, and orlistat equivalents (OE) for lipase. Details for the protocols are provided in Supplementary Materials.
Regarding the antioxidant capacity of B. thunbergii extracts, different experiments such as ferrous ion chelating, phosphomolybdenum, and radicals scavenging tests (FRAP, ABTS, CUPRAC, and DPPH) were spectrophotometrically screened. The findings are given as standard compounds equivalents of Trolox and EDTA. The assay methods were described in our earlier work [52]. Details for the protocols are provided in Supplementary Materials.
3.6. Statistical Analysis
The analysis were performed in triplicate. The results were given as mean and standard deviation. The differences in the extracts were investigated by using student t-test (p < 0.05) and this test was performed in Xlstat 2018. The relationship between biological activities and total bioactive compounds based on the estimation of Pearson’s correlation coefficients were conducted. The correlation analysis was performed by using R software v. 3.6.1.
4. Conclusions
We have analyzed the phenolic profile of B. thunbergii DC. leaves and quantified the most abundant compounds. Two extracts (MeOH and water) were prepared, observing a higher recovery yield in the MeOH extract. The most abundant compounds in both extracts were chlorogenic acid (101.3 mg g−1 DE in MeOH extract), followed by other caffeoylquinic acids and caffeoylglucaric acids; quercetin glycosides were also quantified. Although both extracts exhibited potent antioxidant activity, the MeOH extract was the most potent, probably due to the highest TIPC, although all compounds may interact in a synergetic way. The enzyme inhibitory effect of the extracts was tested, observing that the MeOH extract was active against acetylcholinesterase, butyrylcholinesterase, tyrosinase, amylase, glucosidase, and lipase. However, the aqueous extract was inactive against butyrylcholinesterase and lipase, showing also less inhibition than the MeOH extract towards the other enzymes. In general, MeOH extract presented a high concentration of phenolic compounds and potent bioactivity, making it a suitable candidate for further analysis (isolation and bioactivity of the main compounds) with potential applications in the pharmaceutical and food industry (preparation of novel supplements or nutraceuticals).
Supplementary Materials
The following are available online. Detailed protocols for chromatographic analysis, total phenolic and flavonoid contents, antioxidant and enzyme inhibitory assays; HPLC-MS and HPLC-UV chromatograms of Berberis extracts.
Author Contributions
Conceptualization, E.J.L.-M. and A.R.-M.; methodology, E.J.L.-M. and G.Z.; software, E.J.L.-M. and G.Z.; validation, M.d.P.F.-P.; formal analysis, M.d.P.F.-P. and G.Z.; investigation, E.J.L.-M., M.d.P.F.-P., A.R. and G.Z.; writing, E.J.L.-M., M.d.P.F.-P., A.R. and G.Z.; supervision, E.J.L.-M.; project administration, A.R.-M.; funding acquisition, A.R.-M.
Funding
This study was funded by the Ministerio de Economía y Competitividad (grant number CTQ2016-7511-R).
Acknowledgments
Technical and human support provided by CICT of Universidad de Jaén (UJA, MINECO, Junta de Andalucía, FEDER) is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mokhber-Dezfuli, N.; Saeidnia, S.; Gohari, A.R.; Kurepaz-Mahmoodabadi, M. Phytochemistry and pharmacology of berberis species. Pharm. Rev. 2014, 8, 8–15. [Google Scholar]
- Bhardwaj, D.; Kaushik, N. Phytochemical and pharmacological studies in genus Berberis. Phytochem. Rev. 2012, 11, 523–542. [Google Scholar] [CrossRef]
- Hussain, N.; Adhikari, A.; Ahmad, M.S.; Wahab, A.-; Ali, M.; Choudhary, M.I. Two new prenylated flavonoids from the roots of Berberis thunbergii DC. Nat. Prod. Res. 2017, 31, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Bober, Z.; Stępień, A.; Aebisher, D.; Ożóg, Ł.; Bartusik-Aebisher, D. Fundamentals of the use of Berberis as a medicinal plant. Eur. J. Clin. Exp. Med. 2018, 16, 41–46. [Google Scholar] [CrossRef]
- Khan, I.; Najeebullah, S.; Ali, M.; Shinwari, Z.K. Phytopharmacological and ethnomedicinal uses of the Genus Berberis (Berberidaceae): A review. Trop. J. Pharm. Res. 2016, 15, 2047–2057. [Google Scholar] [CrossRef]
- Singh, M.; Srivastava, S.; Rawat, A. Antimicrobial activities of Indian Berberis species. Fitoterapia 2007, 78, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Fatehi-Hassanabad, Z.; Jafarzadeh, M.; Tarhini, A.; Fatehi, M. The antihypertensive and vasodilator effects of aqueous extract from Berberis vulgaris fruit on hypertensive rats. Phyther. Res. 2005, 19, 222–225. [Google Scholar] [CrossRef]
- Pitta-Alvarez, S.I.; Medina-Bolivar, F.; Alvarez, M.A.; Scambatto, A.A.; Marconi, P.L. In vitro shoot culture and antimicrobial activity of Berberis buxifolia Lam. In Vitro Cell. Dev. Biol.-Plant 2008, 44, 502–507. [Google Scholar] [CrossRef]
- Siow, Y.L.; Sarna, L.; Karmin, O. Redox regulation in health and disease—Therapeutic potential of berberine. Food Res. Int. 2011, 44, 2409–2417. [Google Scholar] [CrossRef]
- Villinski, J.; Dumas, E.; Chai, H.-B.; Pezzuto, J.; Angerhofer, C.; Gafner, S. Antibacterial activity and alkaloid content of Berberis thunbergii, Berberis vulgaris and Hydrastis canadensis. Pharm. Biol. 2003, 41, 551–557. [Google Scholar] [CrossRef]
- Gudzinskas, Z.; Petrulaitis, L.; Zalneravicius, E. New woody alien plant species recorded in Lithuania. Bot. Lith. 2017, 23, 153–168. [Google Scholar] [CrossRef][Green Version]
- Li, A.-R.; Zhu, Y.; Li, X.-N.; Tian, X.-J. Antimicrobial activity of four species of Berberidaceae. Fitoterapia 2007, 78, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Potdar, D.; Hirwani, R.R.; Dhulap, S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia 2012, 83, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-R.; Schutzki, R.E.; Nair, M.G. Antioxidant and anti-inflammatory compounds in the popular landscape plant Berberis thunbergii var. atropurpurea. Nat. Prod. Commun. 2013, 8, 165–168. [Google Scholar] [CrossRef]
- Och, A.; Szewczyk, K.; Pecio, Ł.; Stochmal, A.; Załuski, D.; Bogucka-Kocka, A. UPLC-MS/MS profile of alkaloids with cytotoxic properties of selected medicinal plants of the Berberidaceae and Papaveraceae families. Oxid. Med. Cell. Longev. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Ruiz, A.; Mardones, C.; Vergara, C.; Hermosín-Gutiérrez, I.; von Baer, D.; Hinrichsen, P.; Rodriguez, R.; Arribillaga, D.; Dominguez, E. Analysis of hydroxycinnamic acids derivatives in calafate (Berberis microphylla G. Forst) berries by liquid chromatography with photodiode array and mass spectrometry detection. J. Chromatogr. A 2013, 1281, 38–45. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Reiner, G.; Theoduloz, C.; Ladio, A.; Schmeda-Hirschmann, G.; Gómez-Alonso, S.; Jiménez-Aspee, F. Polyphenol composition and (bio)activity of Berberis species and wild strawberries from the Argentinean Patagonia. Molecules 2019, 24, 3331. [Google Scholar] [CrossRef]
- Castillo-Fraire, C.M.; Poupard, P.; Guilois-Dubois, S.; Salas, E.; Guyot, S. Preparative fractionation of 5′-O-caffeoylquinic acid oxidation products using centrifugal partition chromatography and their investigation by mass spectrometry. J. Chromatogr. A 2019, 1592, 19–30. [Google Scholar] [CrossRef]
- Simirgiotis, M.J. Antioxidant capacity and HPLC-DAD-MS profiling of Chilean peumo (Cryptocarya alba) fruits and comparison with German peumo (Crataegus monogyna) from southern Chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef]
- Spínola, V.; Llorent-Martínez, E.J.; Gouveia-Figueira, S.; Castilho, P.C. Ulex europaeus: From noxious weed to source of valuable isoflavones and flavanones. Ind. Crop. Prod. 2016, 90, 9–27. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Spínola, V.; Gouveia, S.; Castilho, P.C. HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Ind. Crop. Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, Z.; Yao, S.; Li, S.; Yang, W.; Jiang, B.; Liu, X.; Wu, W.; Qv, H.; Guo, D. Neutral loss ion mapping experiment combined with precursor mass list and dynamic exclusion for screening unstable malonyl glucoside conjugates. J. Am. Soc. Mass Spectrom. 2016, 27, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Chandra Pradhan, P.; Saha, S. Anthocyanin profiling of Berberis lycium Royle berry and its bioactivity evaluation for its nutraceutical potential. J. Food Sci. Tecnhology 2016, 53, 1205–1213. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfeld, A.; Obando, L.; Mardones, C. Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Zhang, Y.; Zhang, X.; Zhang, Z.; Liao, Y.; Zhang, B. A new method for simultaneous determination of phenolic acids, alkaloids and limonoids in Phellodendri Amurensis Cortex. Molecules 2019, 24, 709. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Arya, K.R.; Sharma, K.R.; Kumar, B. Quantitative determination of isoquinoline alkaloids and chlorogenic acid in Berberis species using ultra high performance liquid chromatography with hybrid triple quadrupole linear ion trap mass spectrometry. J. Sep. Sci. 2015, 38, 2007–2013. [Google Scholar] [CrossRef]
- Gulsoy, S.; Ozkan, G.; Ozkan, K. Mineral elements, phenolics and organic acids of leaves and fruits from Berberis crataegina DC. Asian J. Chem. 2011, 23, 3071–3074. [Google Scholar]
- Shan, S.; Huang, X.; Shah, M.H.; Abbasi, A.M. Evaluation of polyphenolics content and antioxidant activity in edible wild fruits. Biomed. Res. Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Bustamante, L.; Pastene, E.; Duran-Sandoval, D.; Vergara, C.; Von Baer, D.; Mardones, C. Pharmacokinetics of low molecular weight phenolic compounds in gerbil plasma after the consumption of calafate berry (Berberis microphylla) extract. Food Chem. 2018, 268, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Lemoui, R.; Benyahia, S.; Noman, L.; Bencherchar, I.; Oke-Altuntas, F.; Rebbas, K.; Benayache, S.; Benayache, F.; Demirtas, I. Isolation of phytoconstituents and evaluation of biological potentials of Berberis hispanica from Algeria. Bangladesh J. Pharm. 2018, 13, 179–186. [Google Scholar] [CrossRef]
- Karimkhani, M.M.; Salarbashi, D.; Sanjari Sefidy, S.; Mohammadzadeh, A. Effect of extraction solvents on lipid peroxidation, antioxidant, antibacterial and antifungal activities of Berberis orthobotrys Bienerat ex C.K. Schneider. J. Food Meas. Charact. 2019, 13, 357–367. [Google Scholar] [CrossRef]
- Belwal, T.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Microwave-assisted extraction (MAE) conditions using polynomial design for improving antioxidant phytochemicals in Berberis asiatica Roxb. ex DC. leaves. Ind. Crop. Prod. 2017, 95, 393–403. [Google Scholar] [CrossRef]
- Zovko Končic, M.; Kremer, D.; Karlovic, K.; Kosalec, I. Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food Chem. Toxicol. 2010, 48, 2176–2180. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzyńska, K.; De Peña, M.P. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
- Xiang, Z.; Ning, Z. Scavenging and antioxidant properties of compound derived from chlorogenic acid in South-China honeysuckle. Lwt-Food Sci. Technol. 2008, 41, 1189–1203. [Google Scholar] [CrossRef]
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef]
- Sabahi, Z.; Farmani, F.; Soltani, F.; Moein, M. DNA protection, antioxidant and xanthin oxidase inhibition activities of polyphenol-enriched fraction of Berberis integerrima Bunge fruits. Iran. J. Basic Med. Sci. 2018, 21, 411–416. [Google Scholar]
- IDF Diabetes Atlas Eigth Edition, International Diabetes Federation. Available online: https://www.idf.org/ (accessed on 24 April 2019).
- WHO, World Health Organization, Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 April 2019).
- Rauf, A.; Jehan, N. Natural products as a potential enzyme inhibitors from medicinal plants. In Enzyme Inhibitors and Activators; Senturk, M., Ed.; InTech: Londond, UK, 2017; pp. 165–177. [Google Scholar]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharm. 2015, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.; Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.-X.; Yin, S.-J.; Oh, S.; Wang, Z.-J.; Ye, S.; Yan, L.; Yang, J.-M.; Park, Y.-D.; Lee, J.; Qian, G.-Y. An integrated study of tyrosinase inhibition by rutin: Progress using a computational simulation. J. Biomol. Struct. Dyn. 2012, 29, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative properties and effect of quercetin and its glycosylated form (rutin) on acetylcholinesterase and butyrylcholinesterase activities. J. Evid. Based. Complementary Altern. Med. 2016, 21, NP11–NP17. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Núñez-Gastélum, J.A.; Vazquez-Flores, A.A.; Gonzalez-Aguilar, G.A. In vitro inhibition of pancreatic lipase by polyphenols: A kinetic, fluorescence spectroscopy and molecular docking study. Food Technol. Biotechnol. 2017, 55, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of α-amylase by flavonoids: Structure activity relationship (SAR). Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Zengin, G.; Lobine, D.; Molina-García, L.; Mollica, A.; Mahomoodally, M.F. Phytochemical characterization, in vitro and in silico approaches for three Hypericum species. New J. Chem. 2018, 42, 5204–5214. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharm. 2017, 8, 290. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).