Abstract
Two new pentacyclic triterpene saponins, named akebiaoside K (1) and akebiaoside N (2), were isolated from the leaves of Akebia trifoliata, together with five known triterpenoids 3–7. They were all isolated from the leaves of A. trifoliata for the first time. Their structures were established by spectral and chemical means. Triterpenes 5 and 7 were found to show moderate in vitro cytotoxicity against human tumor A549, HeLa and HepG2 cell lines, with IC50 values ranging from 0.023 to 0.038 mM. Triterpenes 5–7 were further revealed to show significant in vitro α-glucosidase inhibitory activity with IC50 values from 0.040 to 0.220 mM, making them more potent than the reference compound acarbose (IC50 0.409 mM). Meanwhile, no obvious inhibitory effects were observed for the isolated triterpene saponins 1–4 in both bioactivity assays.
1. Introduction
Akebia trifoliata (Thunb.) Koidz. is a perennial, woody liana mainly distributed in the eastern part of Asia [1]. Its fresh fruit, commonly called ‘Ba-Yue-Gua’ in China, has long been consumed by the local people as a delicious food [2]. The air-dried stems and fruits of A. trifoliata have traditionally been used in China for centuries as an antiphlogistic, antineoplastic and diuretic agent [3,4]. To date, phytochemical studies have revealed structurally diverse triterpenes, triterpene saponins, phenolics and lignans from this plant, and some of them displayed significant biological activities [5,6,7,8,9,10]. However, those studies were mainly concentrated on the stems and fruits, and seldom focused on the leaves, though our thin layer chromatography (TLC) examination of the ethanol extract showed that it was highly possible that the leaves of A. trifoliata could also be a promising source of functional natural products. To examine those potential uncharacterized chemicals in the leaves of A. trifoliata, a phytochemical investigation on this plant part was carried out, whereby seven pentacyclic triterpenoids 1–7, including two new triterpene saponins (compounds 1 and 2) were obtained (Figure 1). Herein, the isolation and structure elucidation of these compounds are described. Besides, compounds 1–7 were also tested for their α-glucosidase inhibitory activity and their cytotoxic activity against three human tumor cell lines.
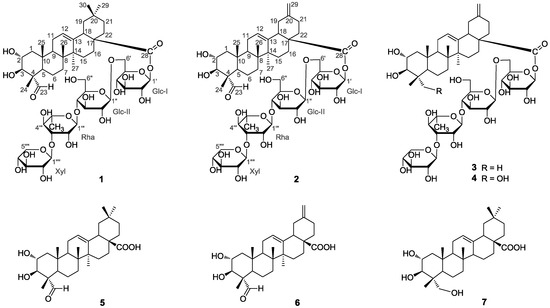
Figure 1.
Chemical structures of compounds 1–7.
2. Results and Discussion
Compound 1 was obtained as a white, amorphous powder. Its (−)- and (+)-ESI-MS displayed quasi-molecular ion peaks at m/z 1087 [M − H]− and 1111 [M + Na]+, respectively, corresponding to the molecular formula of C53H84O23, which was confirmed by further HR-ESI-MS (+) analysis ([M + Na]+ m/z 1111.5297, calcd. 1111.5296). The 1H- and 13C-NMR spectra of 1 exhibited four sugar anomeric protons at δH 6.22 (Glc-I-1), 4.95 (Glc-II-1), 5.87 (Rha-1), and 5.24 (Xyl-1) (Table 1), and anomeric carbons at δC 95.5, 104.7, 102.2 and 107.1 (Table 2), suggesting the existence of four sugar moieties in the molecule. Acid hydrolysis of 1 with 2N HCl afforded d-glucose, l-rhamnose and d-xylose in the ratio of 2:1:1, which were identified by GC-MS analysis of their chiral derivatives (see experimental part). The 1H- and 13C-NMR assignments (Table 1 and Table 2) of the sugar moieties in 1 were established by interpretation of combined HSQC and HMBC data (See the Supplementary Materials). After having excluded the resonances due to the sugar moieties, the remaining signals in the 1H-NMR spectrum for the aglycone unit of 1 were readily recognized for six tertiary methyls at δH 1.42 (3H, s), 1.20 (3H, s), 1.07 (3H, s), 1.04 (3H, s), 0.90 (3H, s) and 0.88 (3H, s), an olefinic proton at δH 5.40 (1H, t), two oxymethine protons at δH 4.21 (1H, m) and 4.04 (1H, d, J = 10.1 Hz), and an aldehyde proton at δH 9.61 (1H, s). The 13C-NMR spectrum indicated, besides the signals for the sugar moieties, 30 carbons for the aglycone unit, including six methyls, nine methylenes, six methines (including an olefinic methine at δC 122.4, two oxygenated methines at δC 67.9 and 76.9 and an aldehyde carbon at δC 206.3), and nine quaternary carbons (including an olefinic carbon at δC 144.0 and a carbonyl carbon at δC 176.3). By comparison, it was found that the 1H- and 13C-NMR spectroscopic data (Table 1 and Table 2) of the aglycone of 1 were closely related to those of 2α,3β-dihydroxy-23-oxo-olean-12-en-28-oic acid [11], a known triterpene which was also obtained in this study as 5, with the major difference of the chemical shift value of the carboxyl group at C-28 was shifted from δC 180.0 in 5 to δC 176.3 in 1. These findings supported the fact that 1, as shown in Figure 1, was a monodesmoside saponin of 5 with an oligosaccharide chain linked at C-28 [12]. This deduction was consistent with the molecular formula of 1, and further in complete accordance with the 2D NMR spectroscopic data. In the HMBC spectrum, 1H-13C long-range correlation of H-1′ (δH 6.22) with C-28 (δC 176.3) was exhibited, which evidenced the glycoside linkage of Glc-I at C-1′ with the aglycone at C-28. The observation of HMBC correlations of H-1″ (δH 4.95) with C-6′ (δC 69.0), of H-1″′ (δH 5.87) with C-5″ (δC 77.0), and of H-1″″ (δH 5.24) with C-3″′ (δC 83.0) confirmed the sugar sequence as shown in Figure 2. The β-anomeric configuration of the d-glucose (Glc-I and Glc-II) and the xylose (Xyl) moieties were determined on the basis of their coupling constants of 3JH1′,H2′ (8.1 Hz), 3JH1″,H2″ (7.8 Hz) and 3JH1″″,H2″″ (7.5 Hz), respectively [12,13].

Table 1.
The 1H-NMR (500 MHz) spectral data ((ppm), J in Hz) of 1 and 2.

Table 2.
The 13C-NMR (150 MHz) spectral data (δ (ppm)) of compounds 1 and 2.
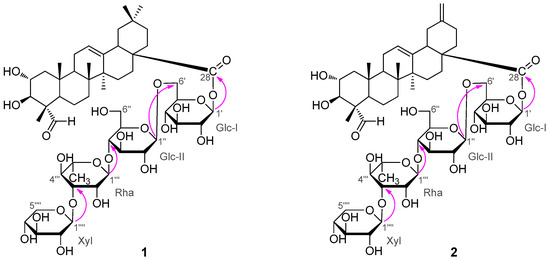
Figure 2.
Selected HMBC correlations of compounds 1 and 2.
The α-configuration of the l-rhamnose (Rha) unit was evidenced by the singlet signal δH 5.87 (br.s) observed for the anomeric proton H-1″′ [12]. Therefore, 1 was identified as 2α,3β,23,29-tetrahydroxyolean-12-en-28-oic acid-O-β-d-xylpyranosyl-(1 → 3)-O-α-l-rhamnopyranosyl-(14)-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosyl ester (1), trivially named akebiaoside K.
Compound 2 was obtained as a white amorphous powder with molecular formula C52H80O23 as determined by HR-ESI-MS analysis ([M + Na]+ m/z 1095.4965, calcd. 1095.4983). Its spectral features and physicochemical properties suggested 2 was also a triterpenoid saponin. Careful comparison revealed that the 1H- and 13C-NMR spectral data of the aglycone part of 2 were quite close to those of 2α,3β-dihydroxy-23-oxo-30-norolean-12,20(29)-dien-28-oic acid [14], a known noroleanane triterpene which was also obtained in this study as compound 6, suggesting that the aglycone unit of 2 was the same as 6. This assignment was supported by the acid hydrolysis of 2 which furnished the free aglycone identified as 6, and the monosaccharide compounents concomitantly obtained from the acid hydrolysis of 2 were identified as d-glucose, l-rhamnose and d-xylose in the ratio of 2:1:1 based on GC-MS analysis of their chiral derivatives (see experimental section), supporting the presence of the same four sugar moieties as those in 1. On the basis of combined analysis of 1H-1H COSY, HMQC and HMBC spectra, all proton and carbon signals were assigned (Table 1 and Table 2). The β-anomeric configuration of the d-glucose units (Glc-I and Glc-II) and the xylose (Xyl) moiety were determined from their coupling constants of 3JH1′,H2′ (8.2 Hz), 3JH1″,H2″ (7.9 Hz) and 3JH1″″,H2″″ (7.5 Hz), respectively [12]. And the α-anomeric configuration of the l-rhamnose unit was judged by the singlet signal at δH 5.82 (br.s) for the anomeric proton H-1″′ [12]. In the HMBC spectrum, 1H-13C long-range correlation of H-1′ (δH 6.14) with C-28 (δC 175.6) was displayed, which evidenced the glycoside linkage of Glc-I at C-1′ with the aglycone at C-28. The exhibition of obvious HMBC correlations of H-1′′ (δH 4.91) with C-6′ (δC 69.1), of H-1″′ (δH 5.82) with C-5″ (δC 76.9), and of H-1″″ (δH 5.22) with C-3″′ (δC 83.0) confirmed the oligosaccharide sequence in 2 as shown in Figure 2. Based upon the above information, the strcuture of compound 2 was elucidated as 2α,3β-dihydroxy-23-oxo-29-norolean-12,20(30)-dien-28-oic acid O-β-d-xylpyranosyl-(1 → 3)-O-α-l-rhamnopy-ranosyl-(1 → 4)-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosyl ester (2), trivially named akebiaoside N.
The five known compounds were identified as akemisaponin I (3) [7], mutongsaponin B (4) [6], 2α,3β-dihydroxy-23-oxo-olean-12-en-28-oic acid (5) [11], 2α,3β-dihydroxy-23-oxo-30-norolean-12,20 (29)-dien-28-oic acid (6) [14] and arjunolic acid (7) [15], by comparison of their spectral data (1H- and 13C-NMR and MS) to those reported in the literature. They were all isolated from the leaves of A. trifoliata for the first time.
Compounds 1–7 were evaluated for their in vitro cytotoxicity against human cancer cell lines A549, HeLa and HepG2, using a MTT method as described. The resulting IC50 values are displayed in Table 3, compared to adriamycin as positive control. Compounds 5 and 7 showed moderate cytotoxicity against all the three cancer cell lines, with IC50 values ranging from 22.7 to 38.1 μM. Meanwhile, no obvious activity was detected for the four saponins 1–4. This result reveals an obvious negative effect on the cytotoxicity when the triterpenes were linked with the linear tetrasaccharide chain at C-28. Comparison of the chemical structures and their cytotoxicity of 5 versus 6 indicated that the replacement of the structure fragment C-20(Me)2 by the exocyclic double bond of C-20(29) also had a negative effect on the cytotoxicity.

Table 3.
Cytotoxicity of compounds 1–7 (IC50, µM).
These compounds were further tested for their α-glucosidase inhibitory activity with acarbose used as a reference compound. Compounds 5–7 were revealed to show strong α-glucosidase inhibitory activity, with IC50 values of 0.047, 0.220 and 0.040 mM, respectively, which were about two to ten-fold stronger than acarbose (IC50 0.409 mM). These results suggested that triterpenes 5–7 from the leaves of A. trifoliata are effective α-glucosidase inhibitors with potential value for development as effective hypoglycemic agents for diabetes chemotherapy [16]. Like in the cytotoxicity bioassay, no obvious α-glucosidase inhibitory activity was detected for the four triterpenoid saponins 1–4, indicating that a negative effect of the tetrasaccharide chain linked at C-28 on the α-glucosidase inhibitory activity was also evident.
A. trifoliata is a medicinal plant naturally widely distributed in the eastern part of Asia. Its air-dried stems and fruits have long been used in China as an antiphlogistic, antineoplastic and diuretic agent. To date phytochemical investigations have revealed a series of triterpenes and triterpene saponins from this plant species. However, those studies were mainly focused on the stems and fruits, and seldom concentrated on the leaves. Our present study revealed that the leaves of A. trifoliata is also rich in triterpenes and triterpene saponins. At the same time, however, it is also demonstrated that much future work is needed to unravel the complexity of the chemical constituents in the leaves of A. trifoliata.
3. Materials and Methods
3.1. General Information
Optical rotation were measured on a Perkin-Elmer 341 polarimeter (Perkin-Elmer, Waltham, MA, USA) with MeOH as solvent at the wavelength of 589 nm and 20 °C to obtain their specific optical rotation [α] values after calculation. ESI-MS data were obtained using a MDS SCIEX API 2000 LC/MS/MS system (Applied Biosystems, Foster City, CA, USA) in both positive and negative ion modes in the range of m/z 50–1000 after the test solutions were directly injected into the ESI source by a syringe pump. HR-ESI-MS mass spectra were obtained on a Bruker maXis instrument (Bruker Daltonik GmbH, Bremen, Germany) in a positive ion mode after direct injection of the test solutions. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Advance 600 instrument (Bruker, Fällanden, Switzerland) or Bruker Ascend-500 spectrometer (Bruker BioSpin GmbH, Rheistetten, Germany). Preparative HPLC was carried out on a CXTH P3000 HPLC pump and a UV 3000 UV-Vis Detector with a Fuji-C18 column (10 μm–100 A, ChuangXinTongHeng Science and Technology Co., Ltd., Beijing, China); medium pressure liquid chromatography (MPLC) was performed using a CXTH P3000 HPLC pump, a UV 3000 UV-Vis Detector and a C18 column (400 × 25 mM i.d., 50 μM, YMC Co. Ltd., Kyoto, Japan).
Column chromatography (CC) was performed with silica gel (200–300 mesh, Qingdao Haiyang Chemical Co., Qingdao, China), YMC ODS-A (50 μm, YMC Co. Ltd.), and Sephadex LH-20 (Pharmacia Fine Chemical Co., Ltd., Uppsala, Sweden). Analytical grade petroleum ether (b.p. 60–90 °C), methanol, ethyl acetate, chloroform, n-butanol, acetone were purchased from Tianjin Fuyu Fine Chemical Industry Co. (Tianjin, China); HPLC grade methanol was purchased from J & K Chemical Ltd. (Beijing, China); Fractions were monitored by pre-coated HSGF254 TLC (Yantai Jiangyou Silica Gel Co., Ltd., Yantai, China), and spot detection was performed under fluorescent light (λ = 254 nm), and then spraying 10% H2SO4 in ethanol, followed by heating. Pyridine-d5, DMSO-d6, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and α-glucosidase were purchased from Sigma Chemical Co. (Sigma-Aldrich, St. Louis, MO, USA). RPMI-1640 medium and fetal calf serum were purchased from Gibco BRL (Gaithersburg, MD, USA). Adriamycin was obtained from Pfizer Italia SRL (Roma, Italy). p-Nitrophenyl-α-d-glucopyranoside (PNPG) and acarbose were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
3.2. Plant Materials
The leaves of Akebia trifoliata were collected in August 2014, at Sangzhi, Hunan Province, China, were identified by Prof. Fu-Wu Xing at the South China Botanical Garden, the Chinese Academy of Sciences (CAS). A voucher specimen (No. 20140815) was deposited at the Laboratory of Bioorganic Chemistry of the South China Botanical Garden, Chinese Academy of Sciences.
3.3. Extraction and Isolation
Powdered air-dried leaves of A. trifoliate (dried weight 3.5 kg) were extracted three times with 95% EtOH (10 L × 3) at room temperature for 2 days each time. The extract was concentrated in vacuo to give a dark brown residue, which was suspended in 3 L of water and then sequentially extracted with petroleum ether (3 L × 3), ethyl acetate (EtOAc, 3 L × 3) and n-butanol (n-BuOH, 3 L × 3) to yield a petroleum ether-soluble fraction (31 g), an EtOAc-soluble fraction (168 g), and a n-BuOH-soluble fraction (245 g) after condensation to dryness in vacuo. The EtOAc-soluble fraction was subjected to silica gel CC (1000 mm × 105 mm i.d.) eluted with a gradient of CHCl3–MeOH (97:3–0:100, v/v) to give fractions F1–F10 after pooled according to their TLC profiles. Fraction F5 (7.1 g), obtained from the elution of CHCl3/MeOH of 85:15 (v/v), was subjected to a silica gel CC (800 × 50 mm i.d.) and successively eluted with CHCl3/MeOH (98:2–90:10, v/v) to yield sub-fractions F5-1–F5-6. Sub-fraction F5-3 (1.57 g) was separated by MPLC using MeOH/H2O (60:40–100:0, v/v) system at a flow rate of 10 mL/min, and further purified by passing through a Sephadex LH-20 column (1500 mm × 25 mm i.d.) eluted with MeOH to obtain compounds 5 (5.2 mg) and 6 (3.8 mg). Sub-fraction F5-5 (2.3 g) was separated by MPLC using a gradient of MeOH/H2O (30:70–80:20, v/v) at a flow rate of 10 mL/min to obtain six sub fractions (F5-5-1–F5-5-6), and sub fraction F5-5-5 was purified by preparative HPLC with a Fuji-C18 column (10 μm–100 A) using 73% methanol in water (v/v) as a mobile phase at flow rate of 8 mL/min to give compound 7 (15.6 mg).
The n-BuOH-soluble fraction (245 g) was dissolved in water and passed through a HP-20 column eluted with distilled water and 90% EtOH. Column chromatography of the 90% EtOH eluate portion (128 g) on silica gel (200–300 mesh) and elution with a gradient system of CHCl3/MeOH (85:15–0:100, v/v) to give six fractions (G1–G6) after pooled according to their TLC profiles. Fraction G3 (18 g), obtained from the elution of CHCl3/MeOH of 85:15 (v/v), was subjected to a silica gel CC (600 × 50 mm i.d.) and successively eluted with CHCl3/MeOH (85:15–40:60, v/v) to yield five sub-fractions (G3-1–F3-4). Sub-fraction G3-2 (2.7 g) was separated by MPLC using a gradient of MeOH/H2O (20:80–100:0, v/v) at a flow rate of 10 mL/min to obtain nine sub fractions (G3-2-1–G3-2-9), and G3-2-2 was purified by preparative HPLC with a Fuji-C18 column (10 μm–100 A) using a gradient of MeOH/H2O (50:50–70:30, v/v) as a mobile phase at flow rate of 10 mL/min to give compound 2 (6.4 mg). G3-2-6 was purified by preparative HPLC with a Fuji-C18 column (10 μm–100 A) using a gradient of methanol in water (45:55–63:37, v/v) as a mobile phase at flow rate of 10 mL/min to give compound 3 (10 mg). G3-2-7 was purified by a Sephadex LH-20 column (1500 mm × 25 mm i.d.) eluted with MeOH to afford compound 1 (12 mg). Fraction G5 was subjected to CC on silica gel (200–300 mesh) eluted with a gradient of CHCl3/MeOH (80:20–40:60) and Sephadex LH-20 with MeOH to furnish 4 (4.1 mg).
3.4. Acid Hydrolysis of 1 and 2
Each of compounds of 1 and 2 (3 mg) was heated in 2 M HCl (4 mL) at 90 °C for 2 h. The reaction mixture was extracted with EtOAc (3 × 4 mL). The EtOAc extract was purified by passing through a Sephadex LH-20 column (1500 mm × 25 mm i.d.) eluted with MeOH. By TLC comparison, the free aglycone of 1 was determined to be identical as 5, while that of 2 was identified as the same as 6, respectively. The aqueous layer was concentrated under reduced pressure to dryness to give a sugar-containing residue, which was reacted with l-cysteine methyl ester hydrochloride in pyridine at 60 °C for 2 h, then added with N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) and stirred under reflux at 60 °C for 10 h. The supernatant was then analyzed by GC–MS using a GCMS-QP2010 PLUS instrument, HP-5ms capillary column (30 m, 0.25 mm ID), Helium at constant rate of 46.5 cm/s, 1 µL injection volume, injector temperature at 230 °C, temperature program as 2 °C/min to 180 °C, then 20 °C/min to 280 °C. Electron ionization mode was used at 70 eV. In the acid hydrolysate of 1 and 2, d-glucose, l-rhamnose and d-xylose were confirmed by comparison of their retention times of their derivatives with those of authentic d-glucose (tR 11.852 min), l-rhamnose (tR 5.292 min) and d-xylose (tR 6.842 min) derivatives prepared in the same way, respectively.
3.5. Cytotoxic Assay
Compounds 1–7 were testeded for their cytotoxity against A549 (human lung adenocarcinoma), HeLa (human cervical carcinoma) and HepG2 (human liver hepatocellular carcinoma) cell lines. The three tumor cell lines were obtained from Kunming Institute of Zoology, Chinese Academy of Sciences. The cytotoxic activity of tested chemicals were assayed according to the MTT method using 96 well plates [17]. Briefly, the cells were cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum in a humidified atmosphere with 5% CO2 at 37 °C. 100 μL adherent cells at the density of 5 × 104 cell/mL was seeded into each well of 96-well cell culture plates and incubated in 5% CO2 at 37 °C for 24 h to form a monolayer on the flat bottoms. Then, the supernatant per well was removed and subsequently added with 100 μL fresh medium and 100 μL medium containing a test compound. The plate was then incubated in 5% CO2 at 37 °C. After 72 h, 20 μL of 5 mg/mL MTT in DMSO was added into each well and incubated for 4 h. The supernatant per well was carefully removed and 150 μL DMSO was added. The plate was then vortex shaken for 15 min to dissolve blue formazan crystals. The optical density (OD) of each well was measured on a Genois microplate reader (Tecan GENios, Männedorf, Switzerland) at the wavelength of 570 nm. All experiments were performed in triplicate and adriamycin was used as a positive control. In each experiment, each of the tumor cell lines was exposed to the test compound at concentrations 50, 25, 12.5, 6.25, 3.125, 1.5625 µg/mL. The inhibitory rate of cell growth was calculated according to the following formula: Inhibition rate (%) = (ODcontrol − ODtreated)/ODcontrol × 100%. IC50 values were calculated by SPSS 16.0 statistic software. The values were based on three individual experiments and expressed as means ± standard deviation (SD).
3.6. α-Glucosidase Inhibition Assay
The a-glucosidase inhibitory activity of 1–7 were determined spectrophotometrically in a 96-well microtiter plate based on p-nitrophenyl-α-d-glucopyranoside (PNPG) as a substrate following the method described in literature with slight modifications [18,19]. In brief, α-glucosidase (20 μL, 0.8 U/mL) and various concentrations (500, 250, 125, 62.5, 31.25, 15.625 µg/mL) of tested compounds (120 μL) in 67 mM phosphate buffer (pH 6.8) were mixed at room temperature for 10 min. Reactions were initiated by addition of 5.0 mM PNPG (20 μL). The reaction mixture was incubated for 15 min at 37 °C in a final volume of 160 μL. Then, 0.2 M Na2CO3 (80 μL) was added to the incubation solution to stop the reaction. The activities were detected in a 96-well plate, and the absorbance was determined at 405 nm (for p-nitrophenol). The negative blank was set by adding phosphate buffer instead of the sample via the same way as the test. Acarbose was utilized as positive control. The blank was set by adding phosphate buffer instead of the α-glucosidase using the same method. Inhibition rate (%) = [(ODnegative control − ODblank) − (ODtest − ODtest blank)]/(ODnegative blank − ODblank) × 100%. IC50 values of the samples were calculated using the Microsoft Office Excel soft.
4. Conclusions
Seven pentacyclic triterpenoids, including two new triterpene saponins 1 and 2, were isolated from the leaves of A. trifoliata. Their structures were identified by spectroscopic and chemical means, including NMR and HRESIMS. All the compounds were isolated from the leaves of A. trifoliata for the first time. Triterpenes 5 and 7 were found to show moderate in vitro cytotoxicity against human tumor A549, HeLa and HepG2 cell lines. Triterpenes 5–7 were further revealed to show significant in vitro α-glucosidase inhibitory activity, much more potent than the reference compound acarbose. No obvious inhibitory effects were displayed by the triterpene saponins 1–4 in either bioactivity assay. The present study indicate that the leaves of A. trifoliata are rich in triterpenoids which are potential functional chemicals worthy of further investigation.
Supplementary Materials
The following are available online as supporting information at: http://www.mdpi.com/1420-3049/21/7/962/s1, HR-ESI-MS and NMR spectra data of compounds 1 and 2.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31470422, 31270406 and 31500291), the Natural Science Foundation of Guangdong Province (2014A030313742), and the Science and Technology Project of Guangdong Province (2014B020206003).
Author Contributions
Q.-L.X. and J.W. performed the isolation and structure elucidation of the chemicals and participated in the bioassay experiments. Q.-L.X. also contributed the preparation of the manuscript. L.-M.D. carried out the ethanol extraction and contribute part of the structural identification. Q.Z. and B.L. partially contribute the bioassays. Y.-X.J. carried out the GC-MS analysis. H.-F.W. contribute the collection of the plant material. J.-W.T. planned, designed and organized the whole research of this study. All authors approved the final version manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, L.; Yao, X.; Zhong, C.; Chen, X.; Huang, H. Akebia: A potential new fruit crop in China. HortScience 2010, 45, 4–10. [Google Scholar]
- Wang, Z.; Zhong, C.; Bu, F.; Peng, D.; Peng, J.; Yuan, F. Akebia—A valuable wild fruit under domestication. Agric. Sci. Technol. 2005, 6, 12–18. [Google Scholar]
- Li, L.; Chen, X.Z.; Yao, X.H.; Tian, H.; Huang, H.W. Geographic distribution and resources status of three important Akebia species. J. Wuhan Bot. Res. 2010, 28, 497–506. [Google Scholar]
- Jiangsu New Medical College. Dictionary of Chinese Herbal Medicines; Shanghai Science and Technology Press: Shanghai, China, 1995; p. 169. [Google Scholar]
- Mimaki, Y.; Kuroda, M.; Yokosuka, A.; Harada, H.; Fukushima, M.; Sashida, Y. Triterpenes and triterpene saponins from the stems of Akebia trifoliata. Chem. Pharm. Bull. 2003, 51, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Wang, Z. Triterpenoid saponins and phenylethanoid glycosides from stem of Akebia trifoliata var. australis. Phytochemistry 2006, 67, 2697–2705. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, S.; Warashina, T.; Miyase, T. Triterpene saponins from the pericarps of Akebia trifoliata. Chem. Pharm. Bull. 2012, 60, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, J.; Lin, R.C. The chemical constituents from the stem of Akebia trifoliata. Chin. Tradit. Herb. Drugs 2004, 35, 495–498. [Google Scholar]
- Guan, S.G.; Yu, W.B.; Guan, S.H. The phenolic acid and phenoglycoside of Akebia trifoliata. Lishizhen Med. Mater. Med. Res. 2010, 21, 905–906. [Google Scholar]
- Guan, S.H.; Xia, J.M.; Lu, Z.Q.; Chen, G.T.; Jiang, B.H.; Liu, X.; Guo, D.A. Structure elucidation and NMR spectral assignments of three new lignan glycosides from Akebia trifoliata. Magn. Reson. Chem. 2008, 46, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, H.; Xu, Q.L.; Zhou, Z.Y.; Wu, P.; Wei, X.Y.; Cao, Y.; Chen, X.X.; Tan, J.W. Antibacterial oleanane-type triterpenoids from pericarps of Akebia trifoliata. Food Chem. 2015, 168, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.G.; Ma, L.; Kong, L.Y. New triterpenoid saponins with strong a-glucosidase inhibitory activity from the roots of Gypsophila oldhamiana. Bioorg. Med. Chem. 2008, 16, 2912–2920. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, G.P.; Yang, Y.S.; Su, Y.L.; Ji, T.F. A new bidesmoside triterpenoid saponin from Stauntonia chinensis. Chin. Chem. Lett. 2009, 20, 833–835. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Q.L.; Zheng, M.F.; Ren, H.; Lei, T.; Wu, P.; Zhou, Z.Y.; Wei, X.Y.; Tan, J.W. Bioactive 30-Noroleanane triterpenes from the pericarps of Akebia trifoliata. Molecules 2014, 19, 4301–4312. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.Y.; Jin, Y.R.; Wang, B.Z. Chemical constituents of Rabdosia japonica. Chin. J. Pharm. 1999, 34, 516–518. [Google Scholar]
- Ieyama, T.; Gunawan-Puteri, M.D.; Kawabata, J. α-Glucosidase inhibitors from the bulb of Eleutherine americana. Food Chem. 2011, 128, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Xie, H.H.; Hao, J.; Jiang, Y.M.; Wei, X.Y. Eudesmane sesquiterpene glucosides from lychee seed and their cytotoxic activity. Food Chem. 2010, 123, 1123–1126. [Google Scholar] [CrossRef]
- Feng, J.; Yang, X.W.; Wang, R.F. Bio-assay guided isolation and identification of α-glucosidase inhibitors from the leaves of Aquilaria sinensis. Phytochemistry 2011, 72, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fu, H.W.; Bai, H.; Sasaki, T.; Kato, H.; Koike, K. Triterpenoid saponins from Rubus ellipticus var. obcordatus. J. Nat. Prod. 2009, 72, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–7 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).