Advanced Oxidation Process: Applications and Prospects
Topic Information
Dear Colleagues,
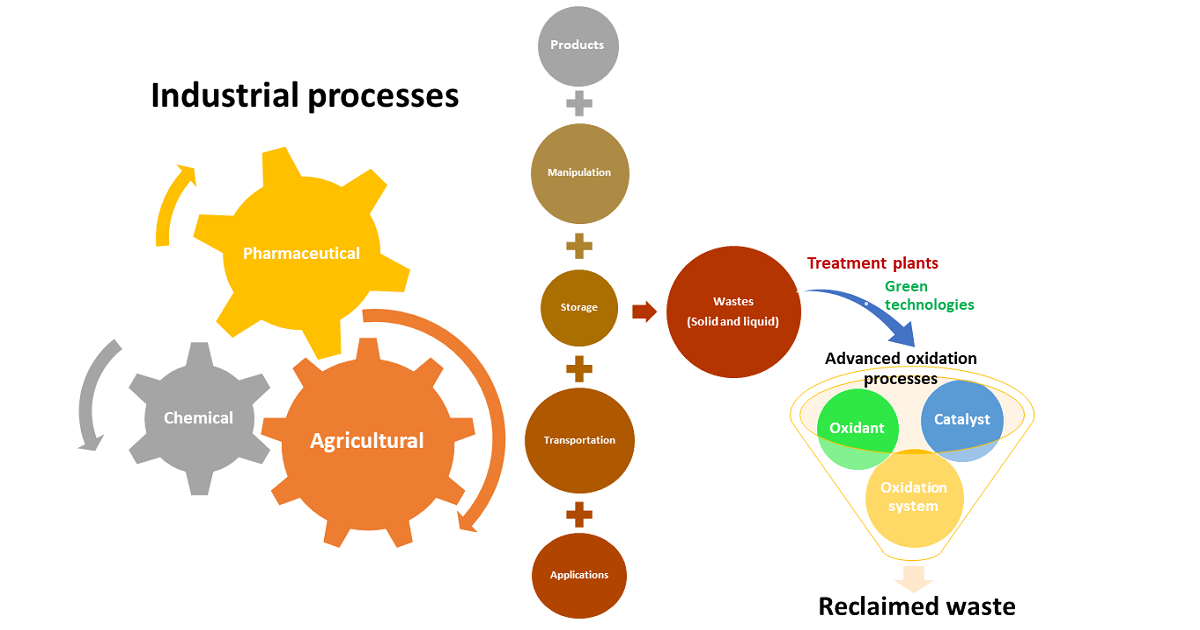
Within the last twenty years, advanced oxidation technologies have begun to be taken seriously as green technologies and are highly efficient from the point of view of final yields since they are chemical reactions that can be controlled and directed according to the end goals in each case. Thus far, they have considered emerging technologies to be of little application at the industrial level as they are commonly considered relatively expensive technologies compared with conventional technologies. This consideration previously indicated is not entirely true since, in many cases, the costs of these technologies can be low as they are simple technologies and the costs of the chemical reagents used depend on each geographical area. Without forgetting to mention the degree of previous optimization of these technologies in each case, which usually implies a notable reduction in costs, especially when considering the circular economy of the processes in which these technologies are applied.
Advanced oxidation technologies are technologies that can be used individually or incorporated into more complete processes or bioprocesses. In this sense and as a non-limiting example, they can be applied in wastewater treatment as a main technology/for pretreatment or as a final operation for the adjustment of the final percentages required by current legislation.
This Special Volume is characterized by being a multidisciplinary volume of the journals Catalysts, Processes, Sci, International Journal of Environmental Research and Public Health, and Water, in which the aim is to extend our knowledge of the current state-of-the-art related to current and possible future applications of advanced oxidation processes.
Prof. Dr. Gassan Hodaifa
Prof. Dr. Antonio Zuorro
Dr. Joaquín R. Dominguez
Prof. Dr. Juan García Rodríguez
Prof. Dr. José A. Peres
Dr. Zacharias Frontistis
Dr. Mha Albqmi
Topic Editors
Keywords
- advanced oxidation processes
- chemical oxidation
- Fenton reaction
- photolysis
- photo-Fenton
- nanoparticles for oxidation