Challenges and Opportunities in Drug Delivery Research
Topic Information
Dear Colleagues,
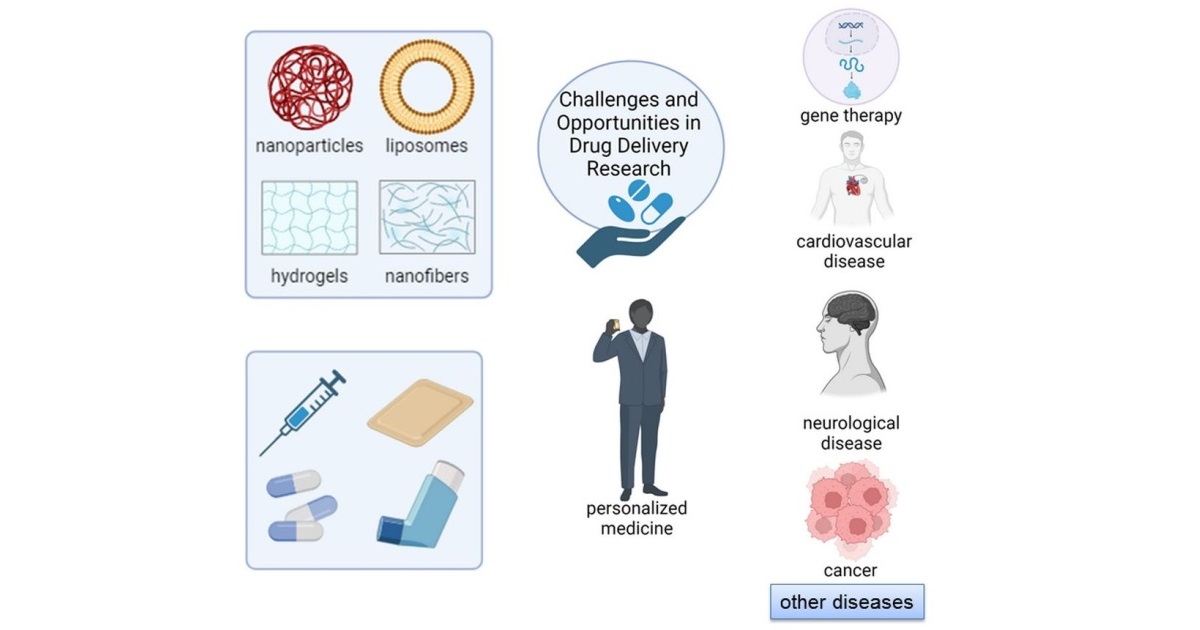
The topic “Challenges and Opportunities in Drug Delivery Research” encompasses innovative drug delivery and release science research with significant implications in medical fields such as cancer therapy, neurology, and cardiovascular medicine. This pioneering research aims to transform treatment methods by developing advanced drug delivery systems that improve precision, reduce side effects, and enhance patient outcomes. The scope extends to gene therapies, personalized medicine, and novel approaches addressing complex health challenges, potentially reshaping healthcare by providing tailored and effective solutions for various diseases and conditions.
Key focus areas involve a mechanistic comprehension of drug delivery systems based on biological and physicochemical principles and mathematical modeling, predictive analytics, drug delivery systems, and cellular interactions tailored for particular therapeutic objectives.
Potential topics of interest for discussion cover various drug delivery technologies, nanomedicine, different entry pathways into the human body, technology evaluations, drug delivery strategies, precise delivery and targeting at various levels and disease indications, and preclinical and clinical findings data related to drug delivery systems. Additionally, in-depth discussions could be extended to detailed explorations of the methods involved in delivering and releasing genes, vaccines, and antibodies.
Prof. Dr. Lenuta Profire
Dr. Ioana Mirela Vasincu
Topic Editors
Keywords
- drug delivery technologies
- release profile
- nanotechnology
- smart drug delivery
- targeted delivery