Abstract
Neurological disorders are a leading cause of death and disability worldwide. Can virtual reality (VR) based intervention, a novel technology-driven change of paradigm in rehabilitation, reduce impairments, activity limitations, and participation restrictions? This question is directly addressed here for the first time using an umbrella review that assessed the effectiveness and quality of evidence of VR interventions in the physical and cognitive rehabilitation of patients with stroke, traumatic brain injury and cerebral palsy, identified factors that can enhance rehabilitation outcomes and addressed safety concerns. Forty-one meta-analyses were included. The data synthesis found mostly low- or very low-quality evidence that supports the effectiveness of VR interventions. Only a limited number of comparisons were rated as having moderate and high quality of evidence, but overall, results highlight potential benefits of VR for improving the ambulation function of children with cerebral palsy, mobility, balance, upper limb function, and body structure/function and activity of people with stroke, and upper limb function of people with acquired brain injury. Customization of VR systems is one important factor linked with improved outcomes. Most studies do not address safety concerns, as only nine reviews reported adverse effects. The results provide critical recommendations for the design and implementation of future VR programs, trials and systematic reviews, including the need for high quality randomized controlled trials to test principles and mechanisms, in primary studies and in meta-analyses, in order to formulate evidence-based guidelines for designing VR-based rehabilitation interventions.
1. Introduction
Neurological disorders are a leading cause of death and disability worldwide with estimated annual costs of €266 billion in Europe [1]. Consequences of disabilities caused by neurological disorders can be reduced by rehabilitation programs in addition to promotion, prevention and treatment [2].
New promising interventions to improve rehabilitation outcomes such as virtual reality (VR)-based interventions have been developed. Using various technical devices (e.g., head-mounted displays, desktop computers, video capture systems, tracking systems, motion-sensing gloves), VR delivers realistic experiences by creating virtual environments (VEs) that closely resemble everyday environments [3]. Common examples of VR programs with promising results for rehabilitation of patients with non-progressive neurological conditions such as stroke or cerebral palsy (CP) are VR-based treadmill training for lower extremity [4], reaching and grasping of virtual objects exercises for the upper extremity [5], and even playing games and performing various activities using commercially available serious games platforms for upper and lower limb function: Nintendo WII or Xbox Kinect [6,7].
Significant improvements in rehabilitation outcomes for patients who underwent VR-based interventions may be explained by their ability to offer meaningful and realistic experiences, thus accommodating principles of rehabilitation [8]. Learning improves if the tasks are meaningful, specific and repetitive and if the task difficulty is increased over time [8,9]. In VR, the number of stimuli and the difficulty of tasks can be adjusted to the needs and possibilities of the patients while maintaining stimulus control and consistency [3,10,11]. Feedback is a key component for motor learning and facilitates quick self-correction [12,13] and VR systems can provide real-time, strategic and goal-directed feedback [3,11]. VR can also be viewed as a medium which offers environmental enrichment. Previous research in animal and human studies showed the positive effect of enriched environments on motor and cognitive performance [14,15].
Recently, there has been an increase in VR-based interventions for rehabilitation with mixed results. To better understand the impact of VR-based interventions on neurological rehabilitation, the first objective of this umbrella review was to summarise the evidence of published meta-analyses regarding the effects of VR-based interventions for improving physical and cognitive functions of patients with stroke, traumatic brain injury (TBI) and CP and assess the quality of the evidence. Three past umbrella reviews reviewed the effectiveness of various interventions, including VR, on upper limb outcome [16], balance outcomes [17] and activities of daily living [18] in stroke with mixed results. Pollock [16] identified moderate quality of evidence in favour of VR; Arienti [17] reported mixed results with quality of evidence ranging from low to high quality; and García-Rudolph [18] reported small to moderate effects for VR interventions, but the quality of evidence was not graded. To our best knowledge, no umbrella review has investigated the effects of VR for CP or TBI. This is the first umbrella review to comprehensively assess the effectiveness of VR-based interventions on multiple physical and cognitive domains and identify factors associated with treatment effects, for patients with stroke, CP and TBI.
Because of the increased heterogeneity of VR platforms and interventions for rehabilitation which may facilitate change via different underlying mechanisms, our second objective was to assess the effects of factors that can impact rehabilitation. Using results from subgroup comparisons, we defined two broad classes of moderator variables referring to VR technology-related variables (e.g., immersion and presence, customization of VR systems), sample and study methodology (e.g., age, clinical diagnosis, nature of the control groups used as comparators). We were also interested in safety issues related to the use of VR especially with vulnerable populations, particularly regarding adverse effects of VR exposure [19,20]. Thirdly, we assessed whether VR is safe by quantifying the number and severity of adverse effects reported in the reviews.
2. Methods
This umbrella review was conducted in accordance with the Cochrane guidelines for overview of reviews [21] and the Joanna Briggs Institute guidelines for umbrella reviews [22]. The protocol was registered on the Open Science Framework (OSF) (registration number: osf.io/w6hs8). No ethical approval was needed as we used data from published studies.
2.1. Eligibility Criteria
The eligibility criteria was: (a) studies that employed a meta-analytic method; (b) participants with a clinical diagnosis of stroke, TBI, CP and acquired brain injury (ABI), caused by either stroke, TBI or CP; (c) VR-based interventions for rehabilitation of physical and/or cognitive abilities; (d) physical functioning (e.g., upper limb function, balance, gait, motor skills) and/or cognitive functioning outcomes (e.g., attention, memory, executive functioning). We included meta-analytical reviews which used a wide range of VR platforms, such as: head-mounted displays (HMDs), television (TV) screens, desktop computers, video capture systems, tracking systems, headphones, motion-sensing gloves, joysticks, keyboards, including commercial computer games platforms such as the Nintendo WII. In addition, the meta-analysis should have employed appropriate methods. We chose to include only meta-analyses instead of having a broader approach and including systematic reviews without meta-analytical data. The reason for this is the fact that meta-analytical studies offer an effect estimate which would facilitate data synthesis, but this was not the case for systematic reviews. As recommended in the Cochrane guidelines [21] we reported our results and statistical summaries by outcomes.
We included peer reviewed articles, conference proceedings, chapters, dissertation thesis and grey literature. We restricted our focus to English language publications to ensure we had an excellent understanding of methods and data analysis reported by authors. In order to increase power and reduce selection bias, we included meta-analyses which performed subgroup analyses and reported pooled effect sizes for our variables of interest and outcomes.
2.2. Search Strategy
A comprehensive search strategy was employed and performed by two review authors to identify potentially relevant records. We searched the following databases through February, 2020 and updated in December, 2020: the Cochrane Database of Systematic Reviews, PsycINFO, EMBASE, PubMed, SCOPUS, ISI Web of Science, Database of Abstracts of Reviews of Effects, Physiotherapy Evidence Database, ACM Digital Library, IEEE Xplore Digital Library, ProQuest Dissertations & Theses A&I, Open Access Theses and Dissertations, EThOS e-theses online service. We searched for the following terms in the publication’s title, abstract, and keywords: (“virtual reality” OR “vr” OR “virtual environment” OR game OR immersive) AND (rehab* OR improv* OR train* OR intervention OR treat* OR expos* OR remediat*) AND (meta-analy* OR review). The search string was modified appropriately for the various databases and an example can be found in the Supplementary Materials. We also searched the references from the most recent systematic reviews and meta-analyses.
2.3. Data Collection and Analysis
2.3.1. Selection of Meta-Analysis Process
Two reviewers independently screened the titles and abstracts. All records deemed relevant were retrieved in full text and were reviewed by two reviewers in order to determine whether they met the selection criteria stated previously. Any disagreements were resolved through discussion with a third reviewer.
2.3.2. Data Extraction and Management
Data were extracted independently by two reviewers using a predefined extraction form. Any concerns were discussed with a third reviewer. Where any information from the reviews was unclear or missing, we contacted the review authors. Two attempts were made. We extracted: (a) meta-analysis identification data (e.g., authors, year of publication and county of origin); (b) population characteristics (e.g., age and diagnosis); (c) intervention and control group characteristics (e.g., type of intervention, VR platform, intervention time); (d) review characteristics (e.g., trial design, number of primary studies and number of participants, number of participants per intervention and control group); (e) statistical summaries (e.g., outcomes and effect measure with 95% confidence intervals, p values and heterogeneity); (f) apriori moderators (e.g., age, immersion and presence, type of VR platform).
The outcomes were categorized as: (a) lower limb activity (e.g., mobility, ambulation function, gait, walking speed); (b) balance and postural control; (c) upper limb, arm function and activity (e.g., grip strength, arm function, improvement of motor impairment and motor function, arm-hand activities); (d) activity limitation (e.g., activities of daily living, global function, independence); (e) ICF WHO Framework outcomes (e.g., participation, body structure and function, activity); (f) motor function; (g) cognitive functioning (e.g., overall cognition).
2.3.3. Quality of Included Reviews
One review author performed quality assessment of all included meta-analysis and another two reviewers performed the assessment of a random sample of included studies and obtained good agreement. We used the AMSTAR 2 [23] to assess the methodological quality of the included reviews (see Supplementary Materials). Risk of bias (ROB) was reported as assessed by the original review authors. Quality of evidence for each outcome was judged using a modified version for systematic reviews of the GRADE approach [24] (described in the Supplementary Materials).
2.3.4. Overlapping of Studies
We calculated the corrected covered area (CCA) to account for overlapping of studies [25] (Supplementary Materials contains a spreadsheet used to calculate CCA).
2.3.5. Data Synthesis
We produced a narrative description and synthesis of the reviews. We organized the review findings by outcomes and reported all the comparisons that were provided by review authors. For each comparison, we extracted the effect size and the 95% CI (e.g., standardized mean differences, mean differences) and heterogeneity (I2) as reported by review authors. To assess the magnitude of the effect, for standardized mean difference and Hedge’s g coefficients we used Cohen’s metrics where a value of between 0.20 and 0.50 indicates a small effect, one between 0.50 and 0.80 indicates a medium effect, while a value larger than 0.80 indicates a large effect size [26]. For mean differences and weighted mean differences, we used the review authors judgements about the magnitude of results because they were in the best position to understand and evaluate the scale results and cut-off scores, given their familiarity with study-level data. For odds ratio, no estimation of the magnitude of the effect was employed because each odds ratio estimates was explained by different variables and each statistical model had a different arbitrary scaling factor [27]. We extracted I2 as a measure of heterogeneity and interpreted the heterogeneity based on the criteria provided by the Cochrane Handbook. I2 values ranging from 0 to 50% correspond to low and not important heterogeneity, values ranging from 50% to 75% correspond to moderate heterogeneity and values above 75% indicate substantial heterogeneity [28].
For moderator effects, we employed a similar approach of data extraction and reporting as we did for the overall effects. To address safety concerns, we extracted available data and reported the number of primary studies and meta-analyses that reported adverse effects and their magnitude and/or severity. Further details concerning moderator effects data synthesis are available in Supplementary Materials.
3. Results
Our search generated 30,306 records. We excluded 10,167 duplicates and screened 20,139 records. After screening the title and abstract 19,777 articles were excluded and the full text of 362 papers was assessed. We excluded 321 records because they focused on other types of interventions and populations. Thus, 41 meta-analyses met our inclusion criteria and were included in the umbrella review (see Figure 1 for the PRISMA flowchart [29]; Supplementary Materials, Table S1 contains a list of excluded studies with reasons).
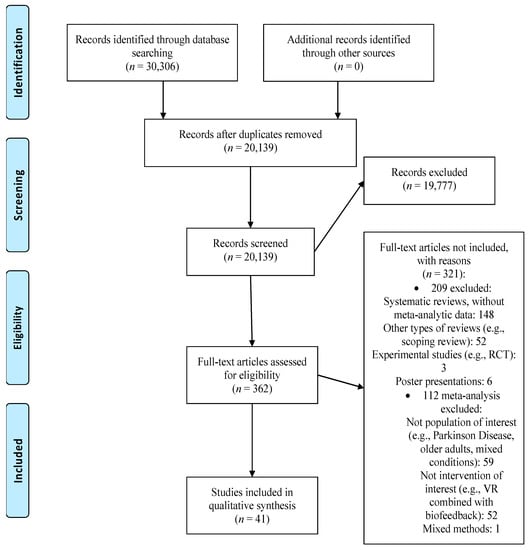
Figure 1.
PRISMA flow diagram. RCT: randomized controlled trial, VR: virtual reality.
3.1. Description and Methodological Quality of Included Reviews
3.1.1. Study Characteristics
Forty-one reviews with meta-analytical results were included in our umbrella review. Characteristics of the study, type of patient population, intervention and control conditions, type of VR platform used, and outcomes can be found in Table 1.

Table 1.
Characteristics of the included meta-analytic studies.
3.1.2. Participants
Thirty-two reviews included patients with stroke, six had samples of children with CP [34,36,45,48,55,59,65,67,68] and three had patients with ABI, including stroke and TBI [33,57,61].
3.1.3. Intervention Characteristics
All reviews focused on VR-based interventions, either delivered as standalone interventions or in combination with conventional therapy. Twenty reviews (49%) included both types of interventions in the analyses, six did not specify if VR interventions were delivered alone or in combination with conventional therapy (15%), eight included only VR interventions without conventional therapy (29%) and four reviews included VR with conventional therapy (10%). Three reviews (7%) investigated the moderator effects of VR-based interventions delivered alone versus VR-based interventions delivered in combination with conventional therapy [38,41,50].
3.1.4. Control Group Characteristics
To eliminate more sources of bias from influencing the effect of the VR-based intervention, most of the reviews (37 reviews, 90%) computed pooled effect sizes from primary studies with adequate experimental designs and adequate control groups (e.g., RCTs or quasi-RCTs) allowing comparison of effects of VR-based interventions with control conditions (passive and active conditions), the remaining four reviews included in their analysis those studies with a pre-test post-test design (10%) [34,40,45,60]. Many control interventions were active conditions (e.g., conventional therapy) (19 reviews, 51%), but a considerable number of reviews included comparisons based on heterogeneous control groups (conventional therapy and passive control groups such as waiting list included in the same analysis) (13 reviews, 35%). For some comparisons, the control group type was not specified (3 reviews, 8%) (see Supplementary Materials for Tables S4–S10).
3.1.5. Quality of Included Reviews
According to AMSTAR 2 [23] concerns regarding the methodological quality of the reviews were mainly caused by failure to: (a) report on the sources of funding for primary studies (95%); (b) perform a comprehensive literature search (93%); (c) justify the inclusion of RCTs or non-RCTs (78%); (d) to account for ROB in individual studies when interpreting and discussing results (68%) (Supplementary Materials, Table S2). Forty out of 41 reviews assessed risk of bias. Most reviews used the Physiotherapy Evidence Database (PEDro) Scale (21 reviews, 52%) and Cochrane’s “Risk of bias” tool (15 reviews, 36%). One used the Jadad scale (3%), one used the Joanna Briggs Institute Critical Appraisal tool for RCTs (3%), one used Downs-Black rating scale items (3%) and one used an adapted scoring protocol (3%). Major concerns in relation to ROB were related to performance bias as all reviews (88%) that assessed blinding of participants and personnel included primary studies at high or unclear risk of performance bias (more than 75% of the primary studies reported high or unclear risk of performance bias). Results of GRADE assessment indicated that for immediate follow-up assessment, most evidence was of very low (55 effects out of 147 effects; 37%) and low quality (76 effects out of 147 effects, 52%). Only 14 effects were of moderate quality (10%) and 2 of high quality (1%) (detailed in Supplementary Materials, Tables S4–S10).
3.1.6. Overlapping of Studies
Using the formula provided by Pieper [25] we obtained a value of CCA of 0.042 which indicates a slight overlap of studies.
3.2. Intervention Effects and Quality of Evidence
Our first goal was to investigate the effectiveness and quality of the evidence for VR-based interventions on physical and cognitive outcomes of patients with stroke, TBI and CP.
3.2.1. Intervention Effects for Lower Limb Activity
Nineteen meta-analyses assessed the effectiveness of VR interventions at immediate follow-up for lower limb activity compared with conventional therapy or no intervention. Sixteen focused on stroke and three on CP. For CP all three reviews [36,45,65] reported significant improvements in favour of VR with moderate to large effects and very low to moderate quality of evidence. Their analysis [36] included only RCTs and identified moderate heterogeneity. [65] focused only on RCTs but had substantial heterogeneity in results. [45] used a pre-post-test design with low heterogeneity in results. In the case of people with stroke, ten reviews [37,40,43,46,47,51,52,53,58,62] identified low to large significant effects in favour of VR with very low to moderate quality of evidence. Nine reviews included only RCTs in their analysis, but [40] included studies with a pre-post-test design. Heterogeneity was low for most comparisons. Four reviews which included only RCTs and used Timed Up and Go Test (TUG) as an outcome measure of mobility reported improvements for VR groups with effects ranging in magnitude from low to moderate and quality ranging from very low to moderate [42,47,52,62]. On the contrary, two reviews, one that included only RCTs [13] and one with pre-post-test design studies [44] did not identify benefits of using VR on TUG but with low quality of evidence. Heterogeneity was low. Two reviews based on RCTs analysed if effects remain at follow-up (up to 3 months) for people with stroke [38,46]. Significant effects in favour of VR but with low magnitude were reported for walking speed and gait velocity with low and very low quality of evidence. No significant improvements were obtained for functional mobility but with very low quality of evidence.
3.2.2. Intervention Effects for Balance and Postural Control
Nineteen reviews investigated the effectiveness of VR interventions at immediate follow-up for balance and postural control compared with conventional therapy or no intervention. Three meta-analyses included children with CP [36,65,68] and three included people with Acquired Brain Injury (ABI) (e.g., stroke, TBI) [33,57,61]. Thirteen reviews focused on the effect of VR on people with stroke [13,35,37,38,40,42,44,46,47,52,56,62,64]. All included only RCTs except for one that included studies with a pre-post-test design [40]. For CP all reviews reported significant improvements on balance and postural control measures for VR interventions. The magnitude of effects ranged from small to large effects, but with low quality of evidence. For ABI results from three reviews with low and very low quality of evidence did not support better rehabilitation outcomes on measures such as Sit to Stand Test [33] and multiple measures of balance including Berg Balance Scale (BBS) [57,61]. In the case of people with stroke, results reported in the reviews were mixed, depending on the outcome measure used. For BBS [13,38,40,42,47,52,56,62] reported significant improvements for VR, but with effects ranging from low to large in magnitude and quality ranging from low to moderate. Using the same BBS as outcome [35,37,44] identified no effects for VR, but with very low and low quality of evidence. Reviews that used measures such as anteroposterior and mediolateral deviations of the centre of gravity [44] and postural sway measures (e.g., centre of pressure sway/path length) [37,46] did not identify significant improvements for VR with very low and low quality of evidence. Non-significant effects were also reported for the Functional Reach Test (FRT) [42,44,52] and Balance Confidence Scale (BCS) [52,64] with very low and low quality of evidence. A pooled effect based on balance measures such as: BBS, FRT, TUG and Four Step Square Test (FSTQ) significantly favoured VR but was low in magnitude and low in quality [57]. Heterogeneity was low for most comparisons. At up to three months follow-up, only one review [46] reported effects, and in this case they were non significant for VR for people with stroke on balance outcomes, but with very low study quality.
3.2.3. Intervention Effects for Upper Limb, Arm Function and Activity
Eighteen reviews assessed the effectiveness of VR interventions in improving upper limb, arm function and activity for people with stroke, ABI, and CP. Three included children with CP [34,36,48] and reported significant and large effects for VR, but low quality of evidence. Two reviews [36,48] included in their analysis only RCTs, and one [34] reported an analysis based on studies that used a pre-post-test design. One review focused on people with ABI [61] and reported a small but significant effect on the Fugl Myer (FM) Assessment scale with moderate quality of evidence based on RCTs. However, the same study did not identify a significant effect for VR for upper limb function measured using various scales such as the Wolf Motor Test, 9-hole peg test for example, but with low quality of evidence. For people with stroke, five reviews that used FM reported significant improvements for VR [43,50,55,59,62]. The effects were based on RCTs and were moderate to large with very low to high quality. Two reviews with low quality of evidence reported no significant improvements for the VR groups [35,66], noting that both reviews included only RCTs in their analysis. Some reviews that included comparisons between VR and controls on scales such as the Wolf Motor Function Test [60] and Box and Block Test [43,60,62] did not identify any significant improvements for VR interventions, but with very low quality of evidence. To the contrary, one review identified a small but significant effect for upper limb function measured using the Box and Block Test or the Motor Activity Log but with low quality of evidence [55]. Mixed evidence comes from studies which used various upper limb, arm function and activity measures to pool effects. For example, [48,49,51,60] identified significant effects for VR ranging from low to large in magnitude, but with low quality of evidence. Two reviews [49,60] included in their analysis studies that used a pre-post test design. Other reviews that included only RCTs [39,50,53,59,63,66] did not identify any improvements for VR, though the study quality ranged from low to high. In general heterogeneity was low. Only one review [50] reported follow up effects (up to three months) for upper limb function, but the effect was not significant with high quality of evidence.
3.2.4. Intervention Effects for Activity Limitation
Six reviews focused on the effectiveness of VR interventions compared with control interventions for people with stroke and one review on people with ABI. All of them included only RCTs. No review focused on activity limitation of children with CP. For people with ABI Saywell [61] identified a medium and significant effect of VR but with low quality of evidence for independence outcome. For people who had had a stroke two reviews [30,63] identified small and large effects in favour of VR on activities of daily living, but with very low and low quality of evidence. Reported heterogeneity was low. Two reviews [35,43] reported no improvements for VR compared with controls for daily living activities measured using the Barthel Index Scale. Heterogeneity was low for one comparison [35], but substantial in the case of the other [43]. Again, the quality of evidence ranged from very low to low. Two reviews assessed global functioning using the Functional Independence Measure. Domingue-Tellez [43] reported a moderate effect with very low quality of evidence and substantial heterogeneity. Cheok [37] did not identify improvements for VR with low heterogeneity but the quality of evidence was low. Da-Silva [39] reported a significant effect for VR in the case of perceived quality of use of the stroke arm, but no significant results for the perceived amount of use of the stroke arm. For both outcomes, the quality of evidence was rated as very low. None of the reviews included follow up effects for this outcome.
3.2.5. Intervention Effects for ICF WHO Framework: Body Structures/Function, Activity, and Participation
Five reviews investigated the effectiveness of VR for body structures/function, activity, and participation. Two reviews focused on children with CP and three on people with stroke. One review included studies with a pre-post-test design [34] and the rest of the reviews included RCTs. Chen [34] identified significant effects in favour of VR for children with CP for participation and body structure/function. The effects were large and moderate in magnitude, and the quality of evidence was very low and moderate. Noting that the estimates of effects were based on studies which used a pre-post-test design. Chen [36] reported significant improvements in favour of VR for all outcomes for children with CP. Large effects were reported for activity outcome with low quality of evidence. For body function the effect was moderate and the quality was low and for participation the effect was low in magnitude with very low quality of evidence. Results from three reviews suggest significant effects for body structures/functions and activity for people with stroke [10,31,54]. However, the effects were mostly small in magnitude and the quality of evidence ranged from low to moderate. For participation outcome results from two reviews suggested contradictory results. Aminov [31] reported non-significant results with low quality of evidence and [54] reported a moderate effect for VR but with very low quality of evidence for people with stroke. Overall, heterogeneity was mostly low, with a few cases of moderate heterogeneity. None of the reviews included follow up effects for this outcome.
3.2.6. Intervention Effects for Motor Function
Three reviews assessed the effectiveness of VR for motor function. One included children with CP [45] and two included people with stroke [46,50]. Ghai [45] reported a moderate significant effect for gross motor function with low quality of evidence. Noting that the evidence comes from studies with a pre-post design and not RCTs which can lessen the quality of evidence with moderate heterogeneity. For people with stroke, neither of the two reviews which included only RCTs identified significant improvements for the VR groups with quality of evidence ranging from very low to moderate [46,50]. Heterogeneity ranged from low to substantial. There were no reviews that included follow up effects for this outcome.
3.2.7. Intervention Effects for Cognitive Functioning
Only two reviews which included RCTs investigated the effectiveness of VR in improving cognitive functioning for people with stroke [31,67]. Aminov [31] reported a significant small to medium effect size with very low quality of evidence for overall cognition. Heterogeneity was low. While Wiley [67] did not identify any significant results which favour VR on cognitive outcomes such as: global cognition, attention, memory, and language with very low quality of evidence and small to moderate heterogeneity. None of the reviews included follow up effects for this outcome.
3.3. Moderator Effects
For our second objective that aimed to identify factors that can enhance rehabilitation outcomes we detected four moderator variables that were reported in reviews (Supplementary Materials, Tables S12–S15).
3.3.1. Mode of Delivery
The first moderator aimed to identify differences in effects between VR standalone interventions and VR interventions delivered in combination with conventional therapy. Three reviews investigated this moderator and all focused on people with stroke [38,41,50]. None of the reviews investigated other conditions. For lower limb outcomes such as gait speed or mobility (measured with TUG) three reviews pointed out no significant differences between the effects of VR interventions delivered alone vs. those combined with conventional therapy [38,41,50]. Similar results emerged for activity limitation [50]. Balance (measured with BBS) results in two reviews were inconclusive as one review [41] indicated positive effects only for VR interventions delivered alone and not for VR combined with conventional therapy. However, another review [50] reported significant effects for VR interventions combined with conventional therapy, but not for VR standalone interventions. A slight benefit reported in one review suggested significant improvements for VR interventions delivered with conventional therapy for upper limb outcomes. Such improvements were not significant for VR interventions delivered alone [50].
In conclusion, the summary of evidence suggests that adding conventional therapy to VR training does not significantly improve lower limb activity, balance and activity limitation outcomes compared to only delivering VR interventions alone. For upper limb function, results suggest better rehabilitation outcomes in the case of VR interventions combined with conventional therapy.
3.3.2. Timed Match Interventions
A second moderator reported in one review compared differences in effects between time dose matched interventions and time non-dose matched interventions for people with ABI [61]. There were no significant effects reported for non-dose matched interventions on any of the outcomes: lower limb gait, upper limb, or FM. Non-significant effects were also identified for dose-matched interventions on lower limb and upper extremity. A small significant effect was reported for FM [61]. For all the comparisons the heterogeneity was low. Based on the above results we might conclude that there is limited evidence to support any differences between interventions that are dose matched and those that are not on physical functioning.
3.3.3. Intervention Length
Two reviews assessed the effect of intervention length (using meta-regression and categorical variables) and reported non-significant effects on upper limb activity for children with CP and people with stroke [34,49]. One review identified that interventions with a total duration greater than 15 h positively impacted upper limb function [55]. Taken together, evidence that supports the significant effect of intervention length on rehabilitation outcomes is mixed.
3.3.4. Technological Features of the VR Platforms
Two moderators focused on identifying if technological features of the VR platforms used produced different effects. Comparisons concerned potential differences between commercially available systems and customized systems [10,31,34,36,50,54] and between VE-based interventions and interactive gaming (IG)-based interventions [47]. Overall, results highlighted the importance of the technological components that underlie VR interventions and stress that specially designed and customized VR interventions were more effective for: upper extremity, ambulation and postural control [36]; arm function [36]; upper limb body function and activity [10]; overall body function and activity [54] with small to large effects and low heterogeneity. VEs -based interventions showed significant improvements with small to moderate effects for functional mobility and balance [47].
3.4. Safety Concerns in VR-Adverse Effects
Our third objective aimed to investigate whether VR is safe. Ten out of 41 meta-analysis included in our umbrella review reported adverse effects (see Table 2). Six reviews reported no major adverse effects [35,38,52,56,63,65]. Four reviews reported a few cases of mild adverse effects linked with study participation: transient dizziness and headache, pain, dizziness, increase in hypertonicity, loss of control, increased spasticity, back ache and fatigue [33,37,50,62] (see Table 2).

Table 2.
Adverse effects. Summary of findings.
4. Discussion
The current umbrella review assessed if VR based interventions could aid rehabilitation in patients with stroke, ABI and CP. The meta-analyses in this umbrella review identified some beneficial effects of VR-based interventions on physical and cognitive functioning. We included 41 eligible meta-analyses which increased the statistical power. This umbrella review included separate data synthesis for several outcomes of interest: lower limb activity; balance and postural control; upper limb, arm function and activity; activity limitation; ICF WHO Framework (body structures/function, activity, and participation); motor function; cognitive functioning. This allowed us to conduct an in-depth data synthesis to identify for which functional outcome VR works best. Additionally, we quantified the ROB reported in the reviews and assessed the quality of evidence for each outcome to clearly inform researchers and practitioners about the evidence that supports the use of VR interventions. We chose to focus the discussion mostly on evidence that comes from moderate or high quality of evidence [69]. The certainty of the evidence that comes from moderate quality studies suggests that the true effect is probably close to the estimated effect and high quality indicates that the true effect is similar to the estimated effect. To the contrary, evidence of very low and low quality suggests that it is probable that the true effect is different than the estimated effect [69,70]. The data synthesis found mostly low- or very low-quality evidence that supports the effectiveness of VR interventions. Most reviews focused on people with stroke, and only six on children with CP and three on people with ABI. Only a limited number of effects were rated as having moderate and high quality of evidence, but overall, results of moderate and high quality of evidence highlighted potential benefits of VR for improving ambulation function of children with CP, mobility, balance, upper limb function, and body structure/function and activity of people with stroke, and upper limb function of people with ABI. Our results are in line with other studies that investigated the efficacy of VR interventions in various vulnerable populations. For example, significant improvements in VR-based rehabilitation interventions compared with control interventions were also obtained for older healthy adults and older adults with other neurological conditions such as dementia, Parkinson’s Disease, Multiple Sclerosis [71,72,73,74,75].
Mixed evidence of very low quality emerged for cognitive functioning for people with stroke, but no data was available for this outcome in the case of people with ABI, including TBI and children with CP. A lack of reviews that included samples of people with ABI was also identifed in the case of lower limb function, ICF WHO framework (body function, activity, and participation), and motor function. The quality of evidence for most effects was downgraded mainly due to small sample sizes, high ROB of primary studies and failure to include grey literature and conduct a comprehensive literature search (as assessed by four items from AMSTAR) according to the criteria proposed by Pollock [24]. Regarding the ROB, the main weakness was caused by the lack of participants and personnel blinding. We agree with Laver [50] that this domain is more strongly related to the type and intrinsic characteristics of the intervention and less to the study quality. Even if the blinding of participants and personnel might be more difficult for VR-based studies, adding an active control group that can undergo equivalent less immersive VR interventions (e.g., training using interactive gaming or interventions delivered via PCs) may reduce the likelihood of performance bias.
An important question is whether the effects were maintained at follow up. Two reviews identified small effects with small 95% CIs at follow-up (up to three months) for people with stroke on walking speed and gait velocity [38,46]. Effects were not significant for mobility, but the 95% CIs were wide [46]. Because all these effects were rated as having low and very low quality, this restricts our confidence in the estimate of effects. Only one review reported effects at three months follow-up for people with stroke which were not significant with narrow 95% CIs, but with low quality of evidence [46]. In the case of upper limb function one review reported no significant improvements for the VR group, but with high quality of evidence and narrow 95% CIs which reflects enough precision in the effect estimates [50]. Regarding children with CP and people with ABI, including TBI no review assessed VR-based interventions at follow up. Taken together, results suggest that there is currently a lack of reporting of follow up data to assess if the benefits of using VR were sustained in the long run.
Another key point concerns the clinical relevance of the results. Support in favour of VR on the TUG mobility outcome for people with stroke comes from two reviews with moderate study quality of evidence [47,52]. The 95% CIs reported by [47] were small, but those reported by [52] were wide which might limit our confidence in the results. It is important to notice that even if the two reviews pointed out statistical significance for TUG outcomes, the results showed that the effect reflects minimal clinically important changes. In previous studies [76] reported 95% CIs of the smallest real difference (SRD) for TUG between −3.75 to 2.59 s. SRD was proposed as a measure of sensitivity to change. Values that fall outside this range indicate real or clinical changes. Both reviews reported values within these ranges, which limit our ability to conclude that the improvements were real or of practical significance. For people with stroke, two reviews rated as having moderate quality of evidence suggested that VR was more effective than control groups in improving balance as measured with BBS with moderate magnitude of effects and narrow 95% CIs [47,56]. Taking into account the practical significance of these results, [47] calculated coefficients (95% minimal detectable changes) and reported that the effects observed for BBS indicated that the improvements reflect clinically meaningful changes. Such a result strengthens our ability to conclude that the effects reflected real improvements. For upper limb function measured with FM evidence of high-quality pointed out that VR was effective for people with stroke with relatively narrow 95% CI which could indicate that despite some uncertainty there still can be enough precision to highlight the utility of the intervention. However, the mean difference reported by [50] was lower than the minimum value of 7.2 or 9 reported in previous studies for SRD to reflect real or clinical changes [77,78].
In the case of children with CP no data was reported for outcomes measured with individual scales such as TUG mobility, balance measured with BBS, or upper limb function assessed with FM. In these cases outcomes resulted from composite scores from multiple measurement instruments. In terms of magnitude of effects, large effects which suggest meaningful improvements, were obtained for balance and upper limb function, although the quality of evidence remains of very low and low quality. For people with ABI most of the reported effects for balance measured with BBS and Sit to Stand Test were small in magnitude and non significant though with very low and low quality of evidence. For FM outcome results indicated a significant small to moderate effect with moderate quality of evidence.
4.1. Factors That Can Enhance Rehabilitation Outcomes
4.1.1. Factors Identified via Moderator Analysis
Our second objective aimed to identify factors that can enhance rehabilitation outcomes and highlight the underlying mechanisms that can explain their effect. Overall, results offer support in favour of customized VR systems compared to commercially available VR systems (e.g., Nintendo Wii, Microsoft Kinect), especially for upper limb extremity, body function and activity. Bespoke VR systems are more likely to follow rehabilitation principles compared to commercial VR by adjusting to user needs and abilities, supporting feedback, task-specific practice and usage of affected limb, and increasing difficulty [10,30,31]. Research using these environments is also more likely to design and conduct usability evaluations with users to select the type of tasks and activities to reach specific rehabilitation goals [73,74]. Even if customized VR systems may require more intensive time for development than off-the-shelf commercial VR systems, they may also be more effective in rehabilitation. Moderators assessing the impact of delivering VR interventions alone or in combination with conventional therapy, and those assessing the length of VR intervention did not have any clinical significance.
4.1.2. Proposed Factors
While performing our literature review and data synthetises, we noticed that the existing literature concerning moderator factors for VR intervention effects was missing some important variables. To cover this gap, informed by a literature review, we propose other factors that might impact VR treatment outcomes such as: type of interaction in VR, components of the VR intervention (e.g., tasks, activities, gaming elements), immersion, presence and participant enjoyment and motivation.
Interaction is achieved mainly via technical capabilities of the VR system (hardware) that allows the user to explore and manipulate the environment, ultimately changing the events [79]. Many primary studies used a form of VR interaction (e.g., motion capture technology to capture patient’s movement) that accommodates neurorehabilitation principles and creates enriched environments to facilitate neuroplasticity by helping patients practice and learn in VR real life tasks and activities. Previous studies showed that interaction in VR improves performance. For example, medical students who manipulated directly and in real-time virtual 3D anatomical structures had better learning outcomes than students who passively viewed the interaction in the same stereoscopic 3D environment [80]. We speculate that environments in which interaction takes place in real time such as the situation in which the VR system responds to the user’s actions and sends feedback can improve rehabilitation outcomes. An example of such real-time interaction is when the participant walks on a treadmill and the speed of the treadmill is adjusted according to user’s movements and the projected VR environment changes the direction while the user moves throughout the environment. Immersion is an objective feature related more to the technology being used to deliver virtual experiences and the ability to simulate the real world and create authentic experiences [3,79]. Some VR systems are more immersive than others. For example, those that use body and head tracking technology coupled with a large field of view displays (e.g., HMDs) to generate a 360° “first person” view of the scenario are highly immersive [3]. Less immersive VR systems use desktop computer screens without motion tracking technology. Presence is a subjective state of consciousness and describes the extent to which people can actually feel they are “there” in the VR [79] and is often measured using questionnaires [81,82]. It is commonly accepted that technological features of the VR systems (e.g., motion tracking technology, field of view and stereoscopy) which make the experience highly immersive increase presence [83]. Adequate immersion and presence help the user to behave in VR as they normally do in real life situations [79] and might contribute to the successful transference of skills and knowledge acquired in VR to the real world [84] though the role of immersion and presence in rehabilitation should further be explored in meta-analyses. Increased interaction in VR was also suggested to be positively related to task enjoyment which can lead to a higher level of programme enjoyment. Research has shown that enjoyment of VR interventions for rehabilitation elevated adherence to therapy [85]. Another mechanism proposed by Howard [71] to explain positive rehabilitation outcomes of VR that is closely related to user needs was participants’ increased excitement which contributes to increased motivation. Adding gaming elements to the application can also boost motivation, engagement and adherence to intervention because people will be less focused on the physical impairment and focus their attention on the experience [75,86]. There is need for further empirical studies to test these proposed factors in order to identify mechanisms that can enhance VR rehabilitation outcomes.
Less emphasis in the stroke, TBI and CP literature was placed on differentiating the methodology used to deliver the intervention than in other domains [87] such as tasks, activities or games. VR tasks refer to specific actions, activities are broader and target high level functions and games follow specific rules [87]. Various tasks, activities and games were used for VR rehabilitation ranging from less complex (e.g., grasping and reaching objects) to more complex (e.g., playing games which require interacting within the game, following rules and keeping score.). In line with rehabilitation principles that stress the importance of task specific practice and gradually increasing task difficulty [9], we suggest designing interventions which start at a low level of complexity with tasks and continue at a higher level with activities and games.
4.2. Safety Concerns in VR-Adverse Effects
The few meta-analyses that reported adverse effects did not identify an increased number of adverse effects and none reported severe adverse effects. However, adverse effects in VR should be documented to allow for an informed decision about the safety and feasibility of using VR with vulnerable populations.
4.3. Implications for Neurorehabilitation
A main question is whether improvements observed in VR can translate to real life improvements and the underlying clinical impact. Most effects that were expressed via standardized mean differences were of moderate and large magnitude, which suggests that VR-based interventions have clinical significance. Major clinical improvements based on large effects were reported for lower limb activity, balance and postural control. Small improvements were observed for motor function. Some studies computed effect sizes using mean differences for well-established scales such as lower limb activity measured with TUG, balance measured with BBS and upper limb function measured with FM. In the case of these studies we were able to benchmark the results reported from these meta-analyses with SRD values published in other studies that can indicate whether the changes had clinical relevance. For TUG and FM the reported effect sizes were of small magnitude and limited clinical relevance. For BBS the values were large and likely to reflect clinically significant changes.
The most investigated condition was stroke and only a limited number of reviews included children with CP and people with ABI. When it comes to the target population, compelling evidence of moderate and high quality of evidence emerged for people with stroke on most outcomes: mobility, balance, upper limb function, and body structure/function and activity. Evidence of moderate quality in favour of VR for improving upper limb function was reported for people with ABI, including TBI. For children with CP, evidence of moderate quality supports the use of VR interventions for rehabilitation of lower limb activity such as ambulation function.
Larger effects were reported for VR interventions which consisted of various rehabilitation activities (e.g., treadmill walking, gait training for lower limb activity; balance training exercises, postural control exercises for balance and postural control) delivered via commercially available systems and engineer-built systems resulted in greater improvements. VR interventions designated to improve upper limb functions resulted in smaller improvements. Such interventions consisted mostly of VR programs in which people had to perform motor tasks by moving or manipulating virtual objects. Some VR devices were coupled with data gloves to allow for real-time feedback. Several explanations can account for larger effects for lower limb activity and balance versus upper limb function. First, treadmill training and postural VR interventions usually use larger screens or HMDs which allow for increased immersion compared to reaching and grasping tasks that can be delivered on smaller screens which can be less immersive [47]. It was also argued that VR interventions for upper limb rehabilitation should include high intensity training with many repetitions [8,88]. However, two meta-analyses showed that the duration of the intervention does not impact treatment effects for children with CP and people with stroke for upper limb function [34,49]. Based on the synthesis of evidence we could not identify any superiority effects of age e.g., young people outcomes such as those of children with CP compared to older adults such as people with stroke. We also mention that stroke was the most studied condition with increased data availability which could also contribute to the quality and number of trials included in the meta-analysis.
There is a general agreement that VR can provide meaningful and realistic experiences which can facilitate rehabilitation outcomes [8,89]. For example, by being able to repetitively deliver the intervention while gradually increasing the level of difficulty VR can be an efficient means to apply principles of experience-dependent plasticity for rehabilitation of patients with brain damage [9] and principles of motor learning which are known to improve rehabilitation outcomes [8]. Main advantages of VR are accessibility of practice repetition, multisensory feedback, increasing task difficulty, task specificity [8,89]. All the VR interventions included in the meta-analyses included to a degree rehabilitation tasks that allowed for repetition, multisensory and immediate feedback, variability and adaptation of task difficulty to particular user needs. Additionally, evidence from moderation analysis suggests that customizing the VR systems and adapting them to patients’ needs can improve rehabilitation outcomes by implementing rehabilitation principles (e.g., supporting feedback, task-specific practice and usage of affected limb, adjusting for task difficulty).
Despite promising results concerning the effectiveness of VR-based interventions in rehabilitation, there is still inconclusive evidence concerning the successful transference of skills from VR to real life settings [89]. Examples of rehabilitation tasks that follow motor learning principles in VR are reaching movements while wearing an HMD, virtually rotating a hand held virtual object, arm or joint motions to play various sports in VR [89]. In short, the repetitive practice of specific motor skills improves the ability to perform the task. Rehabilitation outcomes are improved if the practice of the motor task takes place in realistic and meaningful environments where multisensory information can modulate performance [90]. It was suggested that successful implementation depends on the software and hardware capabilities [91]. For example, a mismatch in sensory and motor information between the virtual and real environment can lead to a failure of successful skill transfer. Key features of the VR environment such as fidelity (multisensory stimuli: haptic, visual and auditory) and dimensionality lead to successful rendering of the real world tasks to VR, which in turn impacts motor learning and motor execution [89]. Main challenges concern barriers of transfer of learning issues that relate to reduced ecological validity and task specificity. Major limitations can be caused by system delays (e.g., delays in the visual display of stimuli or system latency between participants actions via controllers and the VR system responses) that can reduce the realism of the experience or failure to correctly estimate the perceived distance in virtual environments compared to real situations which can prevent optimal transfer of skills acquired in VR to real life [91]. Addressing technical limitations such as these can improve the ecological validity of the intervention effects.
5. Limitations and Future Directions
Our study raises several points of interest for future work. First, we included both RCTs and studies with a pre-test post-test design in order to increase statistical power. However, only four reviews included studies with a pre-test post-test design and 37 reviews included only RCTs. To account for this, we have signposted throughout our review where evidence came for studies with a pre-post-test design which consequently reduced our confidence in the results that came from those reviews.
Our moderation synthetises may be limited by subgroup comparisons performed in reviews. Even though we identified important apriori moderators (e.g., immersion), we were not able to assess directly their contribution to VR effectiveness because the reviews did not account for these variables. In future reviews, it would be useful to identify the effectiveness or superiority of VR interventions by comparing the intervention groups with passive and active control groups. Similarly, identifying whether highly immersive VR environments are more effective than low immersive VR environments will allow for better design of VR protocols for intervention. Even though stroke, TBI and CP have negative impacts on cognitive functions, there is currently a lack of reviews that focus on cognitive rehabilitation. Future reviews should investigate the effect and quality of evidence of VR interventions on cognitive functioning. Currently there is limited data on the cost-effectiveness of VR interventions compared to traditional neurorehabilitation, as none of the reviews provided such data.
6. Conclusions
Our umbrella review synthesised a large body of literature on the effects and quality of the evidence of VR-based interventions for physical and cognitive rehabilitation of patients with stroke, TBI and CP. Overall, there is evidence of a benefit of VR in improving physical functioning in people with stroke, TBI and CP, however, most results are based on very low- and low-quality studies. There is a need for high quality RCTs to further investigate the effects of VR interventions.
Our results suggest that the effectiveness of VR interventions is boosted by variables that relate to the technological features of the VR environment, such as customization of VR environments and, possibly, by immersive and interactive VR. We highlight the need to identify and test potential mechanisms that are responsible for effective VR-based rehabilitation, in order to formulate evidence-based guidelines for the design of VR-based rehabilitation interventions.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10071478/s1, Table S1: Excluded meta-analysis with reasons, Table S2: AMSTAR 2 quality assessment of meta-analyses of randomized and non-randomized studies, Table S3: Risk of bias for the included studies, Table S4: Details of included reviews. Reported effects of interventions and quality of evidence for reported outcomes: Lower limb activity, Table S5: Details of included reviews. Reported effects of interventions and quality of evidence for reported outcomes: Balance and postural control, Table S6: Details of included reviews. Reported effects of interventions and quality of evidence for reported outcomes: Upper limb, arm function and activity, Table S7: Details of included reviews. Reported effects of interventions and quality of evidence for reported outcomes: Activity limitation, Table S8: Details of included reviews. Reported effects of interventions and quality of evidence for reported outcomes: ICF WHO Framework: body structures/function, activity and participation, Table S9: Details of included reviews. Reported effects of interventions and quality of evidence for reported outcomes: Motor function, Table S10: Details of included reviews. Reported effects of interventions and quality of evidence for reported outcomes: Cognitive functioning, Table S11: Reported effects for follow-up, Table S12: Subgroup comparisons. Moderator effects for comparisons between VR interventions alone versus VR interventions with conventional therapy, Table S13: Subgroup comparisons. Moderator effects for comparisons between time dose matched interventions versus time non-dose matched interventions, Table S14: Subgroup comparisons. Moderator effects for comparisons between commercially available systems versus customized systems, Table S15: Subgroup comparisons. Moderator effects for comparisons between VE-based interventions versus IG-based interventions
Author Contributions
Conceptualization, A.V; methodology, A.V, J.S. and D.S.F.; validation, J.S.; formal analysis, A.V., J.S. and D.S.F.; writing—original draft preparation, A.V.; writing—review and editing, A.V., J.S. and D.S.F.; supervision, D.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gustavsson, A.; Svensson, M.; Jacobi, F.; Allgulander, C.; Alonso, J.; Beghi, E.; Dodel, R.; Ekman, M.; Faravelli, C.; Fratiglioni, L.; et al. Cost of disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 718–779. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Neurological Disorders: Public Health Challenges; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Rizzo, A.S.; Koenig, S.T. Is clinical virtual reality ready for primetime? Neuropsychology 2017, 31, 877–899. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, D.L.; Brown, D.A.; Pierson-Carey, C.D.; Buckley, E.L.; Lew, H.L. Stepping over obstacles to improve walking in individuals with poststroke hemiplegia. J. Rehabil. Res. Dev. 2004, 41, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Piron, L.; Turolla, A.; Agostini, M.; Zucconi, C.; Cortese, F.; Zampolini, M.; Zannini, M.; Dam, M.; Ventura, L.; Battauz, M.; et al. Exercises for paretic upper limb after stroke: A combined virtual-reality and telemedicine approach. J. Rehabil. Med. 2009, 41, 1016–1102. [Google Scholar] [CrossRef]
- Acar, G.; Altun, G.P.; Yurdalan, S.; Polat, M.G. Efficacy of neurodevelopmental treatment combined with the Nintendo® Wii in patients with cerebral palsy. J. Phys. Ther. Sci. 2016, 28, 774–780. [Google Scholar] [CrossRef]
- Askin, A.; Atar, E.; Kocyigit, H.; Tosun, A. Effects of Kinect-based virtual reality game training on upper extremity motor recovery in chronic stroke. Somatosens. Mot. Res. 2018, 35, 25–32. [Google Scholar] [CrossRef]
- Levin, M.F. Can virtual reality offer enriched environments for rehabilitation? Expert Rev. Neurother. 2011, 11, 153–155. [Google Scholar] [CrossRef]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech. Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef]
- Maier, M.; Rubio Ballester, B.; Duff, A.; Duarte Oller, E.; Verschure, P. Effect of Specific Over Nonspecific VR-Based Rehabilitation on Poststroke Motor Recovery: A Systematic Meta-analysis. Neurorehabil. Neural Repair 2019, 33, 112–129. [Google Scholar] [CrossRef]
- Rizzo, A.S.; Kim, G.J. A SWOT Analysis of the Field of Virtual Reality Rehabilitation and Therapy. Presence Teleoperators Virtual Environ. 2005, 14, 119–146. [Google Scholar] [CrossRef]
- Pedreira da Fonseca, E.; da Silva Ribeiro, N.M.; Pinto, E.B. Therapeutic Effect of Virtual Reality on Post-Stroke Patients: Randomized Clinical Trial. J. Stroke Cerebrovasc. Dis. 2017, 26, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Carvas, N., Jr.; Artilheiro, M.C.; Pompeu, J.E.; Hassan, S.A.; Kasawara, K.T. Interactive Video Gaming Improves Functional Balance in Poststroke Individuals: Meta-Analysis of Randomized Controlled Trials. Eval. Health Prof. 2020, 43, 23–32. [Google Scholar] [CrossRef]
- Clemenson, G.D.; Deng, W.; Gage, F.H. Environmental enrichment and neurogenesis: From mice to humans. Curr. Opin. Behav. Sci. 2015, 4, 56–62. [Google Scholar] [CrossRef]
- Morgan, C.; Novak, I.; Badawi, N. Enriched environments and motor outcomes in cerebral palsy: Systematic review and meta-analysis. Pediatrics 2013, 132, e735–e746. [Google Scholar] [CrossRef]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; van Wijck, F. Interventions for improving upper limb function after stroke. Stroke 2015, 46, e57–e58. [Google Scholar] [CrossRef][Green Version]
- Arienti, C.; Lazzarini, S.G.; Pollock, A.; Negrini, S. Rehabilitation interventions for improving balance following stroke: An overview of systematic reviews. PLoS ONE 2019, 14, e0219781. [Google Scholar] [CrossRef]
- Garcia-Rudolph, A.; Sanchez-Pinsach, D.; Salleras, E.O.; Tormos, J.M. Subacute stroke physical rehabilitation evidence in activities of daily living outcomes: A systematic review of meta-analyses of randomized controlled trials. Medicine (Baltimore) 2019, 98, e14501. [Google Scholar] [CrossRef]
- Mousavi, M.; Jen, Y.H.; Musa, S.N.B. A review on cybersickness and usability in virtual environments. Adv. Eng. Forum 2013, 10, 34–39. [Google Scholar] [CrossRef]
- Kim, A.; Darakjian, N.; Finley, J.M. Walking in fully immersive virtual environments: An evaluation of potential adverse effects in older adults and individuals with Parkinson’s disease. J. Neuroeng. Rehabil. 2017, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.A.; Oxman, A.D. Overviews of reviews. In Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0); The Cochrane Collaboration: London, UK, 2011; pp. 1–21. [Google Scholar]
- Aromataris, E.; Fernandez, R.S.; Godfrey, C.; Holly, C.; Khalil, H.; Tungpunkom, P. Methodology for JBI Umbrella Reviews. 2014. Available online: https://nursing.lsuhsc.edu/JBI/docs/ReviewersManuals/Umbrella%20Reviews.pdf (accessed on 26 March 2021).
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; van Wijck, F.; Wiffen, P.J. An algorithm was developed to assign GRADE levels of evidence to comparisons within systematic reviews. J. Clin. Epidemiol. 2016, 70, 106–110. [Google Scholar] [CrossRef]
- Pieper, D.; Antoine, S.-L.; Mathes, T.; Neugebauer, E.A.; Eikermann, M. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014, 67, 368–375. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; La Wrence Erlbaum Associates, Publishers: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Norton, E.C.; Dowd, B.E.; Maciejewski, M.L. Odds ratios—current best practice and use. JAMA 2018, 320, 84–85. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; 2019; Available online: https://training.cochrane.org/handbook/current/chapter-10 (accessed on 26 March 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Ahn, S.; Hwang, S. Virtual rehabilitation of upper extremity function and independence for stoke: A meta-analysis. J. Exerc. Rehabil. 2019, 15, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Aminov, A.; Rogers, J.M.; Middleton, S.; Caeyenberghs, K.; Wilson, P.H. What do randomized controlled trials say about virtual rehabilitation in stroke? A systematic literature review and meta-analysis of upper-limb and cognitive outcomes. J. Neuroeng. Rehabil. 2018, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Barclay, R.E.; Stevenson, T.J.; Poluha, W.; Ripat, J.; Nett, C.; Srikesavan, C.S. Interventions for improving community ambulation in individuals with stroke. Cochrane Database Syst. Rev. 2015, CD010200. [Google Scholar] [CrossRef]
- Booth, V.; Masud, T.; Connell, L.; Bath-Hextall, F. The effectiveness of virtual reality interventions in improving balance in adults with impaired balance compared with standard or no treatment: A systematic review and meta-analysis. Clin. Rehabil. 2014, 28, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Lee, S.Y.; Howard, A.M. Effect of virtual reality on upper extremity function in children with cerebral palsy: A meta-analysis. Pediatr. Phys. Ther. 2014, 26, 289–300. [Google Scholar] [CrossRef]
- Chen, J.; Jin, W.; Zhang, X.X.; Xu, W.; Liu, X.N.; Ren, C.C. Telerehabilitation Approaches for Stroke Patients: Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Stroke Cerebrovasc. Dis. 2015, 24, 2660–2668. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fanchiang, H.D.; Howard, A. Effectiveness of Virtual Reality in Children With Cerebral Palsy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phys. Ther. 2018, 98, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Cheok, G.; Tan, D.; Low, A.; Hewitt, J. Is Nintendo Wii an Effective Intervention for Individuals With Stroke? A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015, 16, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, D.; Imeri, F.; Gatti, R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: A systematic review. J. Physiother. 2015, 61, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Da-Silva, R.H.; Moore, S.A.; Price, C.I. Self-directed therapy programmes for arm rehabilitation after stroke: A systematic review. Clin. Rehabil. 2018, 32, 1022–1036. [Google Scholar] [CrossRef]
- De Keersmaecker, E.; Lefeber, N.; Geys, M.; Jespers, E.; Kerckhofs, E.; Swinnen, E. Virtual reality during gait training: Does it improve gait function in persons with central nervous system movement disorders? A systematic review and meta-analysis. NeuroRehabilitation 2019, 44, 43–66. [Google Scholar] [CrossRef]
- de Rooij, I.J.; van de Port, I.G.; Meijer, J.G. Effect of Virtual Reality Training on Balance and Gait Ability in Patients With Stroke: Systematic Review and Meta-Analysis. Phys. Ther. 2016, 96, 1905–1918. [Google Scholar] [CrossRef]
- Dominguez-Tellez, P.; Moral-Munoz, J.A.; Casado-Fernandez, E.; Salazar, A.; Lucena-Anton, D. Effects of virtual reality on balance and gait in stroke: A systematic review and meta-analysis. Rev. Neurol. 2019, 69, 223–234. [Google Scholar] [CrossRef]
- Dominguez-Tellez, P.; Moral-Munoz, J.A.; Salazar, A.; Casado-Fernandez, E.; Lucena-Anton, D. Game-Based Virtual Reality Interventions to Improve Upper Limb Motor Function and Quality of Life After Stroke: Systematic Review and Meta-analysis. Games Health J. 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Garcia-Munoz, C.; Casuso-Holgado, M.J. Effectiveness of Wii Fit Balance board in comparison with other interventions for post-stroke balance rehabilitation. Systematic review and meta-analysis. Rev. Neurol. 2019, 69, 271–279. [Google Scholar] [CrossRef]
- Ghai, S.; Ghai, I. Virtual Reality Enhances Gait in Cerebral Palsy: A Training Dose-Response Meta-Analysis. Front. Neurol. 2019, 10, 236. [Google Scholar] [CrossRef]
- Gibbons, E.M.; Thomson, A.N.; de Noronha, M.; Joseph, S. Are virtual reality technologies effective in improving lower limb outcomes for patients following stroke—A systematic review with meta-analysis. Top. Stroke Rehabil. 2016, 23, 440–457. [Google Scholar] [CrossRef]
- Iruthayarajah, J.; McIntyre, A.; Cotoi, A.; Macaluso, S.; Teasell, R. The use of virtual reality for balance among individuals with chronic stroke: A systematic review and meta-analysis. Top. Stroke Rehabil. 2017, 24, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Johansen, T.; Strom, V.; Simic, J.; Rike, P.O. Effectiveness of training with motion-controlled commercial video games on hand and arm function in young people with cerebral palsy: A systematic review and meta-analysis. J. Rehabil. Med. 2019, 52, jrm00012. [Google Scholar] [CrossRef] [PubMed]
- Karamians, R.; Proffitt, R.; Kline, D.; Gauthier, L.V. Effectiveness of Virtual Reality- and Gaming-Based Interventions for Upper Extremity Rehabilitation Poststroke: A Meta-analysis. Arch. Phys. Med. Rehabil. 2020, 101, 885–896. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, Y.J.; Park, S.W. The Effects of Virtual Reality Training on Function in Chronic Stroke Patients: A Systematic Review and Meta-Analysis. Biomed Res. Int. 2019, 2019, 7595639. [Google Scholar] [CrossRef]
- Li, Z.; Han, X.G.; Sheng, J.; Ma, S.J. Virtual reality for improving balance in patients after stroke: A systematic review and meta-analysis. Clin. Rehabil. 2016, 30, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.H.; Tsai, H.T.; Wang, C.Y.; Hsu, C.Y.; Liou, T.H.; Lin, Y.N. Effectiveness and Superiority of Rehabilitative Treatments in Enhancing Motor Recovery Within 6 Months Poststroke: A Systemic Review. Arch. Phys. Med. Rehabil. 2019, 100, 366–378. [Google Scholar] [CrossRef]
- Lohse, K.R.; Hilderman, C.G.; Cheung, K.L.; Tatla, S.; Van der Loos, H.F. Virtual reality therapy for adults post-stroke: A systematic review and meta-analysis exploring virtual environments and commercial games in therapy. PLoS ONE 2014, 9, e93318. [Google Scholar] [CrossRef]
- Mekbib, D.B.; Han, J.; Zhang, L.; Fang, S.; Jiang, H.; Zhu, J.; Roe, A.W.; Xu, D. Virtual reality therapy for upper limb rehabilitation in patients with stroke: A meta-analysis of randomized clinical trials. Brain Inj. 2020, 34, 456–465. [Google Scholar] [CrossRef]
- Mohammadi, R.; Semnani, A.V.; Mirmohammadkhani, M.; Grampurohit, N. Effects of Virtual Reality Compared to Conventional Therapy on Balance Poststroke: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2019, 28, 1787–1798. [Google Scholar] [CrossRef]
- Prosperini, L.; Tomassini, V.; Castelli, L.; Tacchino, A.; Brichetto, G.; Cattaneo, D.; Solaro, C.M. Exergames for balance dysfunction in neurological disability: A meta-analysis with meta-regression. J. Neurol. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Baroni, J.M.; Nascimento, L.R.; Ada, L.; Teixeira-Salmela, L.F. Walking training associated with virtual reality-based training increases walking speed of individuals with chronic stroke: Systematic review with meta-analysis. Braz. J. Phys. Ther. 2014, 18, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, S.; Kiper, P.; Cacciante, L.; Cieślik, B.; Mazurek, J.; Turolla, A.; Szczepańska-Gieracha, J. Use of virtual reality-based training in different fields of rehabilitation: A systematic review and meta-analysis. J. Rehabil. Med. 2020, 52, jrm00121. [Google Scholar] [CrossRef]
- Saposnik, G.; Levin, M.; Outcome Research Canada Working Group. Virtual reality in stroke rehabilitation: A meta-analysis and implications for clinicians. Stroke 2011, 42, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Saywell, N.; Taylor, N.; Rodgers, E.; Skinner, L.; Boocock, M. Play-based interventions improve physical function for people with adult-acquired brain injury: A systematic review and meta-analysis of randomised controlled trials. Clin. Rehabil. 2017, 31, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Tay, E.L.; Lee, S.W.H.; Yong, G.H.; Wong, C.P. A systematic review and meta-analysis of the efficacy of custom game based virtual rehabilitation in improving physical functioning of patients with acquired brain injury. Technol. Disabil. 2018, 30, 1–23. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; van Wegen, E.; van Peppen, R.; van der Wees, P.J.; Hendriks, E.; Rietberg, M.; Kwakkel, G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE 2014, 9, e87987. [Google Scholar] [CrossRef]
- Wang, X.Q.; Pi, Y.L.; Chen, B.L.; Chen, P.J.; Liu, Y.; Wang, R.; Li, X.; Waddington, G. Cognitive motor interference for gait and balance in stroke: A systematic review and meta-analysis. Eur. J. Neurol. 2015, 22, 555-e37. [Google Scholar] [CrossRef]
- Warnier, N.; Lambregts, S.; Port, I.V. Effect of Virtual Reality Therapy on Balance and Walking in Children with Cerebral Palsy: A Systematic Review. Dev. Neurorehabil. 2019, 1–17. [Google Scholar] [CrossRef]
- Wattchow, K.A.; McDonnell, M.N.; Hillier, S.L. Rehabilitation Interventions for Upper Limb Function in the First Four Weeks Following Stroke: A Systematic Review and Meta-Analysis of the Evidence. Arch. Phys. Med. Rehabil. 2018, 99, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Wiley, E.; Khattab, S.; Tang, A. Examining the effect of virtual reality therapy on cognition post-stroke: A systematic review and meta-analysis. Disabil. Rehabil. Assist. Technol. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Loprinzi, P.D.; Ren, Z. The Rehabilitative Effects of Virtual Reality Games on Balance Performance among Children with Cerebral Palsy: A Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2019, 16, 4161. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Mustafa, R.A.; Brozek, J.; Steingart, K.R.; Leeflang, M.; Murad, M.H.; Bossuyt, P.; Glasziou, P.; Jaeschke, R.; Lange, S.; et al. GRADE guidelines: 21 part 2. Test accuracy: Inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2020, 122, 142–152. [Google Scholar] [CrossRef]
- Ryan, R.; Hill, S. How to GRADE the Quality of the Evidence. Cochrane Consumers and Communication, 2016. Available online: https://colorectal.cochrane.org/sites/colorectal.cochrane.org/files/public/uploads/how_to_grade.pdf (accessed on 26 March 2021).
- Howard, M.C. A meta-analysis and systematic literature review of virtual reality rehabilitation programs. Comput. Human Behav. 2017, 70, 317–327. [Google Scholar] [CrossRef]
- Maggio, M.G.; Russo, M.; Cuzzola, M.F.; Destro, M.; La Rosa, G.; Molonia, F.; Bramanti, P.; Lombardo, G.; De Luca, R.; Calabrò, R.S. Virtual reality in multiple sclerosis rehabilitation: A review on cognitive and motor outcomes. J. Clin. Neurosci. 2019, 65, 106–111. [Google Scholar] [CrossRef]
- Kim, O.; Pang, Y.; Kim, J.H. The effectiveness of virtual reality for people with mild cognitive impairment or dementia: A meta-analysis. BMC Psychiatry 2019, 19, 219. [Google Scholar] [CrossRef]
- Bevilacqua, R.; Maranesi, E.; Riccardi, G.R.; Di Donna, V.; Pelliccioni, P.; Luzi, R.; Lattanzio, F.; Pelliccioni, G. Non-Immersive Virtual Reality for Rehabilitation of the Older People: A Systematic Review into Efficacy and Effectiveness. J. Clin. Med. 2019, 8, 1882. [Google Scholar] [CrossRef]
- Neri, S.G.; Cardoso, J.R.; Cruz, L.; Lima, R.M.; de Oliveira, R.J.; Iversen, M.D.; Carregaro, R.L. Do virtual reality games improve mobility skills and balance measurements in community-dwelling older adults? Systematic review and meta-analysis. Clin. Rehabil. 2017, 31, 1292–1304. [Google Scholar] [CrossRef]
- Flansbjer, U.B.; Holmbäck, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Her, J.; Ko, J.; Park, D.-s.; Woo, J.-H.; You, Y.; Choi, Y. Reliability, Concurrent Validity, and Responsiveness of the Fugl-Meyer Assessment (FMA) for Hemiplegic Patients. J. Phys. Ther. Sci. 2012, 24, 893–899. [Google Scholar] [CrossRef]
- Hsueh, I.-P.; Hsu, M.-J.; Sheu, C.-F.; Lee, S.; Hsieh, C.-L.; Lin, J.-H. Psychometric Comparisons of 2 Versions of the Fugl-Meyer Motor Scale and 2 Versions of the Stroke Rehabilitation Assessment of Movement. Neurorehabil. Neural Repair 2008, 22, 737–744. [Google Scholar] [CrossRef]
- Slater, M.; Wilbur, S. A framework for immersive virtual environments (FIVE): Speculations on the role of presence in virtual environments. Presence Teleoperators Virtual Environ. 1997, 6, 603–616. [Google Scholar] [CrossRef]
- Jang, S.; Vitale, J.M.; Jyung, R.W.; Black, J.B. Direct manipulation is better than passive viewing for learning anatomy in a three-dimensional virtual reality environment. Comput. Educ. 2017, 106, 150–165. [Google Scholar] [CrossRef]
- Witmer, B.G.; Singer, M.J. Measuring presence in virtual environments: A presence questionnaire. Presence 1998, 7, 225–240. [Google Scholar] [CrossRef]
- Usoh, M.; Catena, E.; Arman, S.; Slater, M. Using presence questionnaires in reality. Presence Teleoperators Virtual Environ. 2000, 9, 497–503. [Google Scholar] [CrossRef]
- Cummings, J.J.; Bailenson, J.N. How immersive is enough? A meta-analysis of the effect of immersive technology on user presence. Media Psychol. 2016, 19, 272–309. [Google Scholar] [CrossRef]
- Slater, M.; Linakis, V.; Usoh, M.; Kooper, R. Immersion, Presence and Performance in Virtual Environments: An Experiment with Tri-Dimensional Chess. Available online: https://dl.acm.org/doi/abs/10.1145/3304181.3304216 (accessed on 26 March 2021).
- Rose, T.; Nam, C.S.; Chen, K.B. Immersion of virtual reality for rehabilitation—Review. Appl. Ergon. 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Lange, B.S.; Requejo, P.; Flynn, S.M.; Rizzo, A.A.; Valero-Cuevas, F.J.; Baker, L.; Winstein, C. The Potential of Virtual Reality and Gaming to Assist Successful Aging with Disability. Phys. Med. Rehabil. Clin. N. Am. 2010, 21, 339–356. [Google Scholar] [CrossRef]
- Garcia-Betances, R.I.; Arredondo Waldmeyer, M.T.; Fico, G.; Cabrera-Umpierrez, M.F. A succinct overview of virtual reality technology use in Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Brunner, I.; Skouen, J.S.; Hofstad, H.; Aßmus, J.; Becker, F.; Sanders, A.-M.; Pallesen, H.; Qvist Kristensen, L.; Michielsen, M.; Thijs, L.; et al. Virtual Reality Training for Upper Extremity in Subacute Stroke (VIRTUES). Multicent. RCT 2017, 89, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Levac, D.E.; Huber, M.E.; Sternad, D. Learning and transfer of complex motor skills in virtual reality: A perspective review. J. Neuroeng. Rehabil. 2019, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Sveistrup, H. Motor rehabilitation using virtual reality. J. Neuroeng. Rehabil. 2004, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Morel, M.; Bideau, B.; Lardy, J.; Kulpa, R. Advantages and limitations of virtual reality for balance assessment and rehabilitation. Neurophysiol. Clin. 2015, 45, 315–326. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).