Identifying Glycemic Variability in Diabetes Patient Cohorts and Evaluating Disease Outcomes
Abstract
1. Background
2. Purpose of the Study
3. Methods
4. Eligibility Criteria
5. Statistical Analysis
6. Results
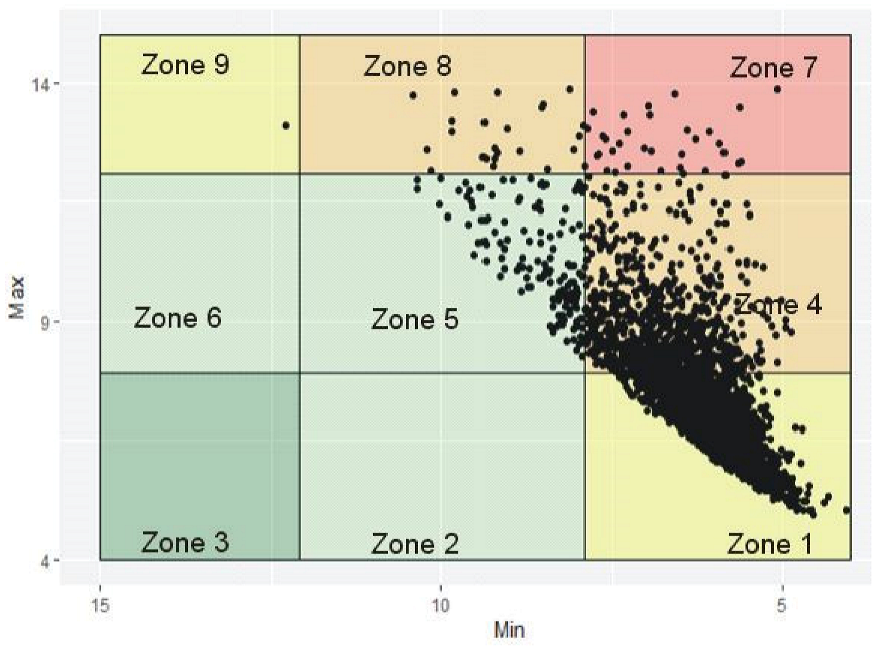
6.1. Visualizing Glycemic Control with VGA Plot
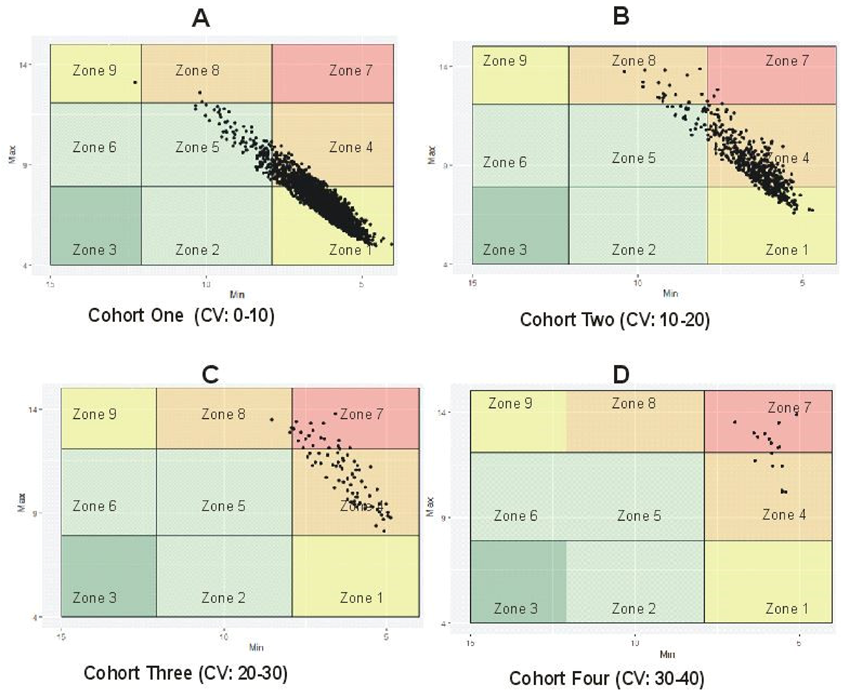
6.2. Distinguishing between Stable and Unstable GV Using %CV
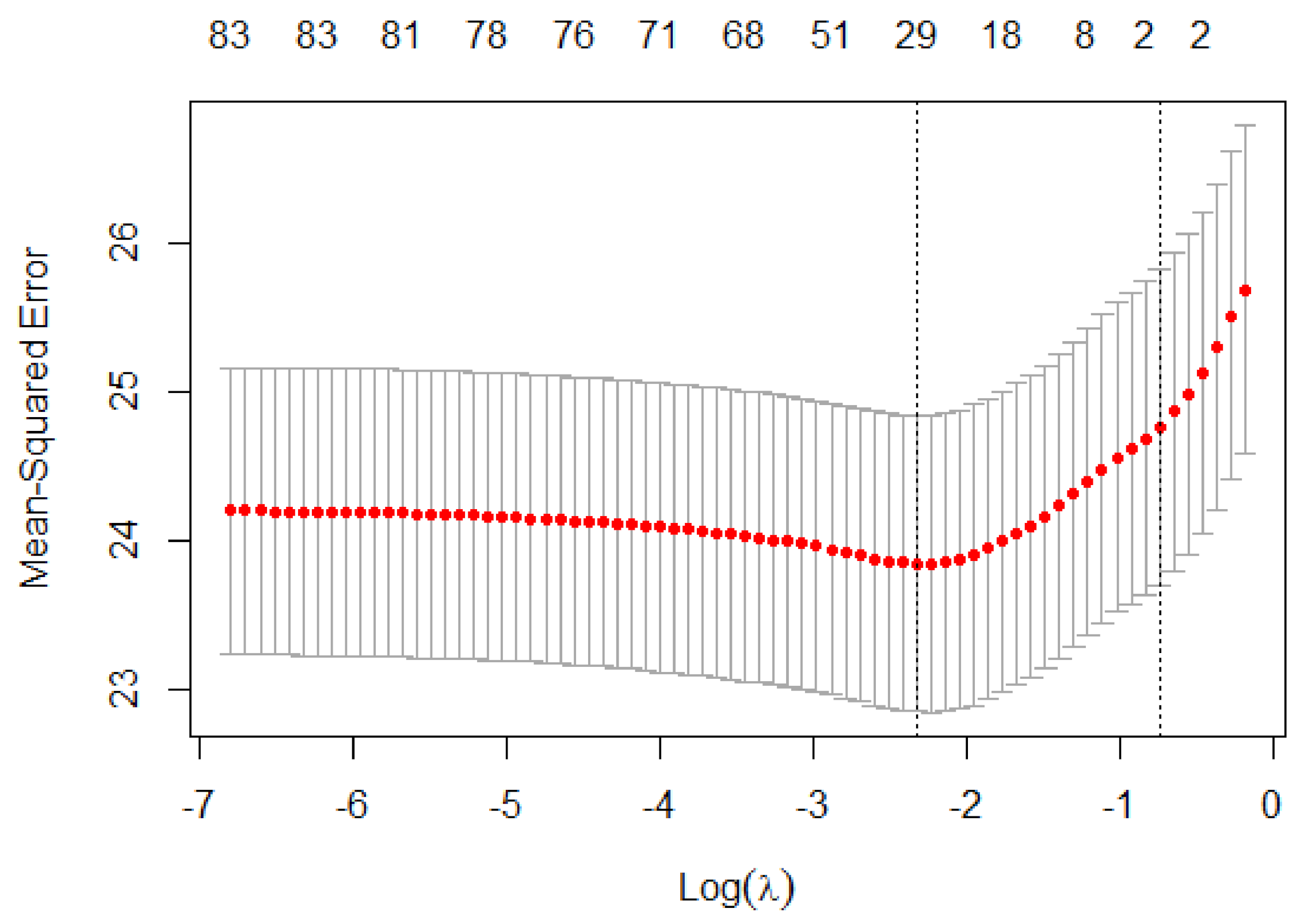
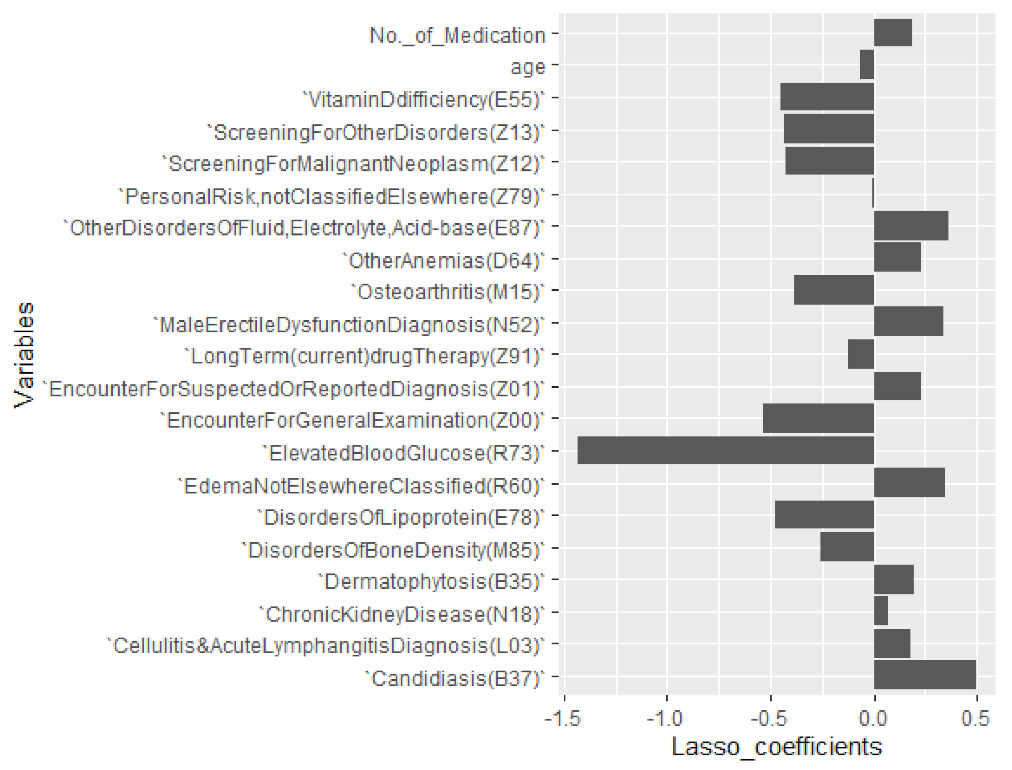
6.3. Selecting Important Diagnosis Codes and Influencers of %CV
6.4. Differences and Association of Comorbidity by Patient Cohort
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Crosstab | ICD-10 Explanations | |||||||
|---|---|---|---|---|---|---|---|---|
| ICD-10 | Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Total | Disorders of Lipoprotein | ||
| E78 | Absent | Count | 239 | 64 | 10 | 6 | 317 | |
| Expected Count | 256.3 | 51.3 | 7.9 | 1.6 | 317.0 | |||
| % within E78 | 75.4% | 19.6% | 3.2% | 1.9% | 100.0% | |||
| Adjusted Residual | −2.6 | 1.7 | 0.8 | 3.8 | ||||
| p-value | 0.0762 | 0.6901 | 0.9100 | 0.0014 | ||||
| Present | Count | 2398 | 466 | 71 | 10 | 2945 | ||
| Expected Count | 2380.7 | 476.7 | 73.1 | 14.4 | 2945.0 | |||
| % within E78 | 81.4% | 15.8% | 2.4% | 0.3% | 100.0% | |||
| Adjusted Residual | 2.6 | −1.7 | −0.8 | −3.8 | ||||
| Adj. p-value | 0.0762 | 0.6901 | 0.9100 | 0.0014 | ||||
| M15 | Absent | Count | 2342 | 491 | 77 | 16 | 2927 | |
| Expected Count | 2366.2 | 473.8 | 72.7 | 14.4 | 2927.0 | Osteoarthritis | ||
| % within M15 | 80.0% | 16.8% | 2.6% | 0.5% | 100.0% | |||
| Adjusted Residual | −3.4 | 2.7 | 1.6 | 1.4 | ||||
| Adj. p-value | 0.0054 | 0.0559 | 0.8756 | 1 | ||||
| Present | Count | 294 | 37 | 4 | 0 | 335 | ||
| Expected Count | 270.8 | 54.2 | 8.3 | 1.6 | 335.0 | |||
| % within M15 | 87.8% | 11.0% | 1.2% | 0.0% | 100.0% | |||
| Adjusted Residual | 3.4 | −2.7 | −1.6 | −1.4 | ||||
| Adj. p-value | 0.0054 | 0.0559 | 0.8756 | 1 | ||||
| E55 | Absent | Count | 2118 | 452 | 76 | 14 | 2660 | Vitamin D difficiency |
| Expected Count | 2150.3 | 430.6 | 66.1 | 13.0 | 2660.0 | |||
| % within E55 | 79.6% | 17.0% | 2.9% | 0.5% | 100.0% | |||
| Adjusted Residual | −3.7 | 2.6 | 2.9 | 0.6 | ||||
| Adj. p-value | 0.0017 | 0.0688 | 0.0313 | 1 | ||||
| Present | Count | 519 | 76 | 5 | 2 | 602 | ||
| Expected Count | 486.7 | 97.4 | 14.9 | 3.0 | 602.0 | |||
| % within E55 | 86.2% | 12.6% | 0.8% | 0.3% | 100.0% | |||
| Adjusted Residual | 3.7 | −2.6 | −2.9 | −0.6 | ||||
| Adj. p-value | 0.0017 | 0.0688 | 0.0313 | 1 | ||||
| Z13 | Absent | Count | 1828 | 395 | 65 | 13 | 2301 | Screening for other disorders |
| Expected Count | 1860.1 | 372.4 | 57.1 | 11.3 | 2301.0 | |||
| % within Z13 | 79.4% | 17.2% | 2.8% | 0.6% | 100.0% | |||
| Adjusted Residual | −3.1 | 2.4 | 1.9 | 0.9 | ||||
| Adj. p-value | 0.0137 | 0.1496 | 0.4183 | 1 | ||||
| Present | Count | 809 | 133 | 16 | 3 | 961 | ||
| Expected Count | 776.9 | 155.6 | 23.9 | 4.7 | 961.0 | |||
| % within Z13 | 84.2% | 13.8% | 1.7% | 0.3% | 100.0% | |||
| Adjusted Residual | 3.1 | −2.4 | −1.9 | −0.9 | ||||
| Adj. p-value | 0.0137 | 0.1496 | 0.4183 | 1 | ||||
| M85 | Absent | Count | 2346 | 488 | 79 | 16 | 2929 | Disorders of bone density |
| Expected Count | 2367.8 | 474.1 | 72.7 | 14.4 | 2929.0 | |||
| % within M85 | 80.1% | 16.7% | 2.7% | 0.5% | 100.0% | |||
| Adjusted Residual | −3.2 | 2.2 | 2.3 | 1.4 | ||||
| P-value | 0.0109 | 0.2326 | 0.1586 | 1 | ||||
| Present | Count | 291 | 40 | 2 | 0 | 333 | ||
| Expected Count | 269.2 | 53.9 | 8.3 | 1.6 | 333.0 | |||
| % within M85 | 87.4% | 12.0% | 0.6% | 0.0% | 100.0% | |||
| Adjusted Residual | 3.2 | −2.2 | −2.3 | −1.4 | ||||
| Adj. p-value | 0.0109 | 0.2326 | 0.1586 | 1 | ||||
| Z91 | Absent | Count | 1592 | 354 | 62 | 11 | 2019 | Personal risk, not classified elsewhere |
| Expected Count | 1632.2 | 326.8 | 50.1 | 9.9 | 2019.0 | |||
| % within Z91 | 78.9% | 17.5% | 3.1% | 0.5% | 100.0% | |||
| Adjusted Residual | −3.7 | 2.7 | 2.7 | 0.6 | ||||
| Adj. p-value | 0.0019 | 0.0621 | 0.0478 | 1 | ||||
| Present | Count | 1045 | 174 | 19 | 5 | 1243 | ||
| Expected Count | 1004.8 | 201.2 | 30.9 | 6.1 | 1243.0 | |||
| % within Z91 | 84.1% | 14.0% | 1.5% | 0.4% | 100.0% | |||
| Adjusted Residual | 3.7 | −2.7 | −2.7 | −0.6 | ||||
| Adj. p-value | 0.0019 | 0.0621 | 0.0478 | 1 | ||||
| L03 | Absent | Count | 2263 | 421 | 67 | 14 | 2765 | Cellulitis and acute lymphangitis |
| Expected Count | 2235.2 | 447.6 | 68.7 | 13.6 | 2765.0 | |||
| % within L03 | 81.8% | 15.2% | 2.4% | 0.5% | 100.0% | |||
| Adjusted Residual | 3.4 | −3.5 | −0.5 | 0.3 | ||||
| Adj. p-value | 0.0047 | 0.0036 | 0.4828 | 0.6081 | ||||
| Present | Count | 374 | 107 | 14 | 2 | 497 | ||
| Expected Count | 401.8 | 80.4 | 12.3 | 2.4 | 497.0 | |||
| % within L03 | 75.3% | 21.5% | 2.8% | 0.4% | 100.0% | |||
| Adjusted Residual | −3.4 | 3.5 | 0.5 | −0.3 | ||||
| Adj. p-value | 0.0047 | 0.0036 | 0.4828 | 0.6081 | ||||
| N52 | Absent | Count | 2311 | 444 | 67 | 12 | 2834 | Male erectile dysfunction |
| Expected Count | 2291.0 | 458.7 | 70.4 | 13.9 | 2834.0 | |||
| % within N52 | 81.5% | 15.7% | 2.4% | 0.4% | 100.0% | |||
| Adjusted Residual | 2.6 | −2.1 | −1.1 | −1.4 | ||||
| Adj. p-value | 0.0674 | 0.3055 | 0.2088 | 0.1266 | ||||
| Present | Count | 326 | 84 | 14 | 4 | 428 | ||
| Expected Count | 346.0 | 69.3 | 10.6 | 2.1 | 428.0 | |||
| % within N52 | 76.2% | 19.6% | 3.3% | 0.9% | 100.0% | |||
| Adjusted Residual | −2.6 | 2.1 | 1.1 | 1.4 | ||||
| Adj. p-value | 0.0674 | 0.3055 | 0.2088 | 0.1266 | ||||
| E87 | Absent | Count | 2309 | 438 | 74 | 10 | 2831 | Other disorders of fluid, electrolyte, acid−base |
| Expected Count | 2288.6 | 458.2 | 70.3 | 13.9 | 2831.0 | |||
| % within E87 | 81.6% | 15.5% | 2.6% | 0.4% | 100.0% | |||
| Adjusted Residual | 2.7 | −2.8 | 1.2 | −2.9 | ||||
| Adj. p-value | 0.0584 | 0.0360 | 0.1749 | 0.0322 | ||||
| Present | Count | 328 | 90 | 7 | 6 | 431 | ||
| Expected Count | 348.4 | 69.8 | 10.7 | 2.1 | 431.0 | |||
| % within E87 | 76.1% | 20.9% | 1.6% | 1.4% | 100.0% | |||
| Adjusted Residual | −2.7 | 2.8 | −1.2 | 2.9 | ||||
| Adj. p-value | 0.0584 | 0.0360 | 0.1749 | 0.0322 | ||||
| R60 | Absent | Count | 1782 | 320 | 55 | 10 | 2167 | Edema, not elsewhere classified |
| Expected Count | 1751.8 | 350.8 | 53.8 | 10.6 | 2167.0 | |||
| % within R60 | 82.2% | 14.8% | 2.5% | 0.5% | 100.0% | |||
| Adjusted Residual | 2.8 | −3.1 | 0.3 | −0.3 | ||||
| Adj. p-value | 0.0355 | 0.0157 | 0.6213 | 0.5908 | ||||
| Present | Count | 855 | 208 | 26 | 6 | 1095 | ||
| Expected Count | 885.2 | 177.2 | 27.2 | 5.4 | 1095.0 | |||
| % within R60 | 78.1% | 19.0% | 2.4% | 0.5% | 100.0% | |||
| Adjusted Residual | −2.8 | 3.1 | −0.3 | 0.3 | ||||
| Adj. p-value | 0.0355 | 0.0157 | 0.6213 | 0.5908 | ||||
| B37 | Absent | Count | 2347 | 440 | 66 | 14 | 2867 | Candidiasis |
| Expected Count | 2317.7 | 464.1 | 71.2 | 14.1 | 2867.0 | |||
| % within B37 | 81.9% | 15.3% | 2.3% | 0.5% | 100.0% | |||
| Adjusted Residual | 4.0 | −3.5 | −1.8 | 0.0 | ||||
| Adj. p-value | 0.0005 | 0.0036 | 0.5869 | 0.7693 | ||||
| Present | Count | 290 | 88 | 15 | 2 | 395 | ||
| Expected Count | 319.3 | 63.9 | 9.8 | 1.9 | 395.0 | |||
| % within B37 | 73.4% | 22.3% | 3.8% | 0.5% | 100.0% | |||
| Adjusted Residual | −4.0 | 3.5 | 1.8 | 0.0 | ||||
| Adj. p-value | 0.0005 | 0.0036 | 0.5869 | 0.7693 | ||||
| Z12 | Absent | Count | 1057 | 249 | 38 | 9 | 1353 | Screening for malignant neoplasm |
| Expected Count | 1093.8 | 219.0 | 33.6 | 6.6 | 1353.0 | |||
| % within Z12 | 78.1% | 18.4% | 2.8% | 0.7% | 100.0% | |||
| Adjusted Residual | −3.3 | 2.9 | 1.0 | 1.2 | ||||
| Adj. p-value | 0.0072 | 0.0304 | 0.2517 | 0.1834 | ||||
| Present | Count | 1580 | 279 | 43 | 7 | 1909 | ||
| Expected Count | 1543.2 | 309.0 | 47.4 | 9.4 | 1909.0 | |||
| % within Z12 | 82.8% | 14.6% | 2.3% | 0.4% | 100.0% | |||
| Adjusted Residual | 3.3 | −2.9 | −1.0 | −1.2 | ||||
| Adj. p-value | 0.0072 | 0.0304 | 0.2517 | 0.1834 | ||||
References
- Vaisman, N.; Mordechai, K. The Medical Research Infrastructure and Health Services Fund of the Tel Aviv Medical Center. Novel Assay for Monitoring Glucose Balance and Oxidative Stress. U.S. Patent 2015/0299790 A1, 22 October 2015. [Google Scholar]
- Federation, I.D. IDF Diabetes Atlas. 2017. Available online: https://diabetesatlas.org/resources/2017-atlas.html (accessed on 4 July 2020).
- (IDF). I.D.F. IDF Key Messages. 2019. Available online: https://diabetesatlas.org/index.php (accessed on 24 March 2020).
- Hall, H.; Perelman, D.; Breschi, A.; Limcaoco, P.; Kellogg, R.; McLaughlin, T.; Snyder, M. Glucotypes reveal new patterns of glucose dysregulation. PLoS Biol. 2018, 16, e2005143. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Zhang, P.; Hoerger, T.J. Lifetime direct medical costs of treating type 2 diabetes and diabetic complications. Am. J. Prev. Med. 2013, 45, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Shaw, J.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Nwadiugwu, M.C. Multi-Morbidity in the Older Person: An Examination of Polypharmacy and Socioeconomic Status. Front. Public Health 2021, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- Alavi, N.M.; Alami, L.; Taefi, S.; Gharabagh, G.S. Factor analysis of self-treatment in diabetes mellitus: A cross-sectional study. BMC Public Health 2011, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C.; Wojtusciszyn, A.; Dejager, S.; Renard, E.; Molinari, N.; Owens, D.R. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care 2017, 40, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Dadlani, V.; Kudva, Y.C. Assessment of Interday glucose variability in type 2 diabetes. Diabetes Technol. Ther. 2017, 19, 443–445. [Google Scholar] [CrossRef]
- Kovatchev, B.; Cobelli, C. Glucose variability: Timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care 2016, 39, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Rodbard, D. Glycemic variability: Measurement and utility in clinical medicine and research—One viewpoint. Diabetes Technol. Ther. 2011, 13, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- DeVries, J.H. Glucose variability: Where it is important and how to measure it. Diabetes 2013, 62, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Acciaroli, G.; Sparacino, G.; Hakaste, L.; Facchinetti, A.; Di Nunzio, G.M.; Palombit, A.; Tuomi, T.; Gabriel, R.; Aranda, J.; Vega, S. Diabetes and prediabetes classification using glycemic variability indices from continuous glucose monitoring data. J. Diabetes Sci. Technol. 2018, 12, 105–113. [Google Scholar] [CrossRef]
- Magni, L.; Raimondo, D.M.; Man, C.D.; Breton, M.; Patek, S.; De Nicolao, G.; Cobelli, C.; Kovatchev, B.P. Evaluating the efficacy of closed-loop glucose regulation via control-variability grid analysis. J. Diabetes Sci. Technol. 2008, 2, 630–635. [Google Scholar] [CrossRef]
- Breton, M.D.; Kovatchev, P.B. Method, System, and Computer Program Product for tracking of Blood Glucose Variability in Diabetes. U.S. Patent No. 9,317,657, 19 April 2011. [Google Scholar]
- Kan, H.J.; Kharrazi, H.; Chang, H.-Y.; Bodycombe, D.; Lemke, K.; Weiner, J.P. Exploring the use of machine learning for risk adjustment: A comparison of standard and penalized linear regression models in predicting health care costs in older adults. PLoS ONE 2019, 14, e0213258. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Brownlee, M.; Hirsch, I.B. Glycemic variability: A hemoglobin A1c–independent risk factor for diabetic complications. JAMA 2006, 295, 1707–1708. [Google Scholar] [CrossRef]
- Hirsch, I.B. Glycemic variability and diabetes complications: Does it matter? Of course it does! Diabetes Care 2015, 38, 1610–1614. [Google Scholar] [CrossRef]
- Yu, J.H.; Han, K.; Park, S.; Lee, D.Y.; Nam, G.E.; Seo, J.A.; Kim, S.G.; Baik, S.H.; Park, Y.G.; Kim, S.M. Effects of long-term glycemic variability on incident cardiovascular disease and mortality in subjects without diabetes: A nationwide population-based study. Medicine 2019, 98, e16317. [Google Scholar] [CrossRef]
- Think Whole Person Health Care (TWPH). Think Ahead. 2020. Available online: https://thinkhealthcare.org/about-think/ (accessed on 4 July 2020).
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The A1C Test & Diabetes. 2018. Available online: https://www.niddk.nih.gov/health-information/diagnostic-tests/a1c-test (accessed on 5 July 2020).
- Sacks, D.B. A1C versus glucose testing: A comparison. Diabetes Care 2011, 34, 518–523. [Google Scholar] [CrossRef]
- Service, F.J. Glucose variability. Diabetes 2013, 62, 1398–1404. [Google Scholar] [CrossRef]
- Fabris, C.; Facchinetti, A.; Sparacino, G.; Zanon, M.; Guerra, S.; Maran, A.; Cobelli, C. Glucose variability indices in type 1 diabetes: Parsimonious set of indices revealed by sparse principal component analysis. Diabetes Technol. Ther. 2014, 16, 644–652. [Google Scholar] [CrossRef]
- International Organization for Standardization. In Vitro Diagnostic Test Systems: Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus; ISO: Geneva, Switzerland, 2003. [Google Scholar]
- Wilmoth, D.R. The relationships between common measures of glucose meter performance. J. Diabetes Sci. Technol. 2012, 6, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Faria Filho, D.; Dias, A.; Veloso, A.; Bueno, C.; Couto, F.; Matos Júnior, J.; Barreto, K.; Rodrigues, P.; Carneiro, W. Classification of coefficients of variation in experiments with commercial layers. Braz. J. Poult. Sci. 2010, 12, 255–257. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- McHugh, M.L. The chi-square test of independence. Biochem. Med. 2013, 23, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Gerstenberger, S. Fisher’s exact approach for post hoc analysis of a chi-squared test. PLoS ONE 2017, 12, e0188709. [Google Scholar] [CrossRef]
- Diabetes UK. Guide to HbA1c. 2020. Available online: https://www.diabetes.co.uk/what-is-hba1c.html (accessed on 4 June 2020).
- Pfeiffer, A.F.; Klein, H.H. Therapie des diabetes mellitus typ 2. Dtsch. Arztebl. Int. 2014, 111, 69–82. [Google Scholar]
- Cryer, P.E. Glycemic goals in diabetes: Trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 2014, 63, 2188–2195. [Google Scholar] [CrossRef]
- Dai D, H.S.; Szapiro, N. Hba1c Variability, Absolute Changes in Hba1c, and the Risk of Poor Glycemic Control Among Older Adults with Diabetes Enrolled in Medicare Advantage Plans. Int. J. Diabetes Clin. Res. 2016, 3, 063. [Google Scholar] [CrossRef]
- Lanspa, M.J.; Dickerson, J.; Morris, A.H.; Orme, J.F.; Holmen, J.; Hirshberg, E.L. Coefficient of glucose variation is independently associated with mortality in critically ill patients receiving intravenous insulin. Crit. Care 2014, 18, 1–8. [Google Scholar] [CrossRef]
- Dandona, P. Minimizing glycemic fluctuations in patients with type 2 diabetes: Approaches and importance. Diabetes Technol. Ther. 2017, 19, 498–506. [Google Scholar] [CrossRef]
- Farese, R.V.; Sajan, M.P. Metabolic Functions of Atypical Protein Kinase C: “Good and Bad”. Am. J. Physiol. Endocrinol. Metab. 2009, 298, E385–E394. [Google Scholar] [CrossRef]
- Schofield, J.D.; Liu, Y.; Rao-Balakrishna, P.; Malik, R.A.; Soran, H. Diabetes dyslipidemia. Diabetes Ther. 2016, 7, 203–219. [Google Scholar] [CrossRef]
- Hasona, N.A.; Elasbali, A. Evaluation of electrolytes imbalance and dyslipidemia in diabetic patients. Med. Sci. 2016, 4, 7. [Google Scholar] [CrossRef]
- Valsesia, A.; Saris, W.H.; Astrup, A.; Hager, J.; Masoodi, M. Distinct lipid profiles predict improved glycemic control in obese, nondiabetic patients after a low-caloric diet intervention: The Diet, Obesity and Genes randomized trial. Am. J. Clin. Nutr. 2016, 104, 566–575. [Google Scholar] [CrossRef]
- Kashi, Z.; Mahrooz, A.; Kianmehr, A.; Alizadeh, A. The role of metformin response in lipid metabolism in patients with recent-onset type 2 diabetes: HbA1c level as a criterion for designating patients as responders or nonresponders to metformin. PLoS ONE 2016, 11, e0151543. [Google Scholar] [CrossRef]
- Nwadiugwu, M. Inflammatory activities in type 2 diabetes patients with co-morbid angiopathies and exploring beneficial interventions: A Systematic Review. Front. Public Health 2021, 8, 1024. [Google Scholar] [CrossRef]
- Peron, E.P.; Ogbonna, K.C.; Donohoe, K.L. Antidiabetic medications and polypharmacy. Clin. Geriatr. Med. 2015, 31, 17–27. [Google Scholar] [CrossRef]
- Nwadiugwu, M.C. Frailty and the Risk of Polypharmacy in the Older Person: Enabling and Preventative Approaches. J. Aging Res. 2020, 2020, 6759521. [Google Scholar] [CrossRef]
- Kasim, K.; Amar, M.; El Sadek, A.A.; Gawad, S.A. Peripheral neuropathy in type-II diabetic patients attending diabetic clinics in Al-Azhar University Hospitals, Egypt. Int. J. Diabetes Mellit. 2010, 2, 20–23. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Egan, J.M. Diabetes and altered glucose metabolism with aging. Endocrinol. Metab. Clin. 2013, 42, 333–347. [Google Scholar] [CrossRef]
- Higgins, T.; Saw, S.; Sikaris, K.; Wiley, C.L.; Cembrowski, G.C.; Lyon, A.W.; Khajuria, A.; Tran, D. Seasonal variation in hemoglobin A1c: Is it the same in both hemispheres? J. Diabetes Sci. Technol. 2009, 3. [Google Scholar] [CrossRef]
- Radin, M.S. Pitfalls in hemoglobin A1c measurement: When results may be misleading. J. Gen. Intern. Med. 2014, 29, 388–394. [Google Scholar] [CrossRef]
- Nitin, S. HbA1c and factors other than diabetes mellitus affecting it. Singap. Med. J. 2010, 51, 616–622. [Google Scholar]
- Coban, E.; Ozdogan, M.; Timuragaoglu, A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004, 112, 126–128. [Google Scholar] [CrossRef] [PubMed]
| Statistics of %CV, Age, and Medication | |||||||
|---|---|---|---|---|---|---|---|
| %CV | Age | No. of Medications | |||||
| Number of Patients | Valid | 3262 | 3262 | 3262 | |||
| Missing | 0 | 0 | 0 | ||||
| Mean | 6.90 | 74.36 | 0.69 | ||||
| Std. Error of Mean | 0.09 | 0.2 | 0.022 | ||||
| Std. Deviation | 5.07 | 13.052 | 1.271 | ||||
| Minimum | 0.72 | 21 | 0 | ||||
| Maximum | 44.51 | 107 | 9 | ||||
| Antidiabetic drug class for control of glucose for each cohort | |||||||
| Antidiabetic drug class | |||||||
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | ||||
|
Metformin, Insulins, Sulfonylurea, Insulins, Thiazolidinediones(TZDs), Dipeptidyl peptidase 4 (DPP IV) inhibitors, Combination products, glucagon-like peptide1 (GLP) agonist, DGLT V inhibitors |
Metformin, Sulfonylurea, Insulins, GLP agonist, DPP IV inhibitors, Combination Products, TZDs, DGLT V inhibitors |
Sulfonylurea, GLP agonist, Metformin, DPP IV inhibitors |
Combination products, Insulin, Metformin | ||||
| Diagnostic Codes and ICD 10 Explanation | Coefficients |
|---|---|
| Elevated Blood Glucose (R73) | −1.426739684 |
| Encounter for General Examination (Z00) | −0.537150731 |
| Disorders of Lipoprotein (E78) | −0.478726309 |
| Vitamin D Deficiency (E55) | −0.451607901 |
| Screening for Other Disorders (Z13) | −0.428622075 |
| Screening for Malignant Neoplasm (Z12) | −0.423327639 |
| Osteoarthritis (M15) | −0.383649241 |
| Disorders of Bone Density (M85) | −0.259501973 |
| Long-Term (current) Drug Therapy (Z91) | −0.124074972 |
| Age | −0.063355789 |
| Personal Risk, Not Classified Elsewhere (Z79) | −0.001640932 |
| Chronic Kidney Disease (N18) | 0.074005080 |
| Cellulitis and Acute Lymphangitis Diagnosis (L03) | 0.181943299 |
| No. of Medications by Antidiabetic Drug Class | 0.189978300 |
| Dermatophytosis (B35) | 0.192212136 |
| Other Anemias (D64) | 0.227729951 |
| Encounter for Suspected or Reported Diagnosis (Z01) | 0.229726767 |
| Male Erectile Dysfunction Diagnosis (N52) | 0.336851533 |
| Edema Not Elsewhere Classified (R60) | 0.349784394 |
| Other Disorders of Fluid, Electrolyte, Acid–base (E87) | 0.361342201 |
| Candidiasis (B37) | 0.495985527 |
| (Intercept) | 12.46255677 |
| Diagnosis | Χ2 | df | Asymptotic Significance (Two Sided) | Exact Significance (Two Sided) | ICD 10 Explanation |
|---|---|---|---|---|---|
| Z12 | 11.557 | 3 | 0.0090 | Screening for malignant neoplasm | |
| Z91 | 16.221 | 3 | 0.0010 | Personal risk, not classified elsewhere | |
| R60 | 9.7755 | 3 | 0.0205 | Edema, not elsewhere classified | |
| Z00 | 3 | 9.29 × | Encounter for general examination | ||
| R73 | 3 | 0.0009 | Elevated blood glucose | ||
| E78 | 3 | 0.0019 | Disorders of lipoprotein | ||
| M15 | 3 | 0.0052 | Osteoarthritis | ||
| E55 | 3 | 0.0003 | Vitamin D deficiency | ||
| Z13 | 3 | 0.0108 | Screening for other disorders | ||
| M85 | 3 | 0.0030 | Disorders of bone density | ||
| L03 | 3 | 0.0050 | Cellulitis and acute lymphangitis | ||
| N52 | 3 | 0.0324 | Male erectile dysfunction | ||
| E87 | 3 | 0.0009 | Other disorders of fluid, electrolyte, acid–base | ||
| Z79 | 5.0793 | 3 | 0.1661 | Long term (current) drug therapy | |
| Z01 | 2.8959 | 3 | 0.4080 | Encounter for suspected or reported diagnosis | |
| Z51 | 3 | 0.5930 | Encounter for other outer, medical care | ||
| N18 | 3 | 0.5002 | Chronic kidney disease | ||
| B35 | 3 | 0.2162 | Dermatophytosis | ||
| D64 | 3 | 0.2373 | Other anemias |
| Pairwise Comparisons of %CV and Age | |||||
|---|---|---|---|---|---|
| Sample 1–Sample 2 | Test Statistic | Std. Error | Std. Test Statistic | Significance | Adjusted Significance |
| cohort 3–cohort 4 | −57.841 | 257.569 | −0.225 | 0.8220 | 1.0000 |
| cohort 3–cohort 2 | 280.031 | 112.346 | 2.493 | 0.0130 | 0.0760 |
| cohort 3–cohort 1 | 537.612 | 106.203 | 5.062 | 0.0000 | 0.0000 |
| cohort 4–cohort 2 | 222.190 | 238.909 | 0.930 | 0.3520 | 1.0000 |
| cohort 4–cohort 1 | 479.771 | 236.082 | 2.032 | 0.0420 | 0.2530 |
| cohort 2–cohort 1 | 257.581 | 44.887 | 5.738 | 0.0000 | 0.0000 |
| Pairwise Comparisons of %CV and Number of Medications | |||||
| cohort 1–cohort 3 | −127.472 | 88.261 | −1.444 | 0.1490 | 0.8920 |
| cohort 1–cohort 2 | −152.083 | 37.304 | −4.077 | 0.0000 | 0.0000 |
| cohort 1–cohort 4 | −224.917 | 196.198 | −1.146 | 0.2520 | 1.0000 |
| cohort 3–cohort 2 | 24.611 | 93.366 | 0.264 | 0.7920 | 1.0000 |
| cohort 3–cohort 4 | −97.445 | 214.055 | −0.455 | 0.6490 | 1.0000 |
| cohort 2–cohort 4 | −72.833 | 198.547 | −0.367 | 0.7140 | 1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwadiugwu, M.C.; Bastola, D.R.; Haas, C.; Russell, D. Identifying Glycemic Variability in Diabetes Patient Cohorts and Evaluating Disease Outcomes. J. Clin. Med. 2021, 10, 1477. https://doi.org/10.3390/jcm10071477
Nwadiugwu MC, Bastola DR, Haas C, Russell D. Identifying Glycemic Variability in Diabetes Patient Cohorts and Evaluating Disease Outcomes. Journal of Clinical Medicine. 2021; 10(7):1477. https://doi.org/10.3390/jcm10071477
Chicago/Turabian StyleNwadiugwu, Martin C., Dhundy R. Bastola, Christian Haas, and Doug Russell. 2021. "Identifying Glycemic Variability in Diabetes Patient Cohorts and Evaluating Disease Outcomes" Journal of Clinical Medicine 10, no. 7: 1477. https://doi.org/10.3390/jcm10071477
APA StyleNwadiugwu, M. C., Bastola, D. R., Haas, C., & Russell, D. (2021). Identifying Glycemic Variability in Diabetes Patient Cohorts and Evaluating Disease Outcomes. Journal of Clinical Medicine, 10(7), 1477. https://doi.org/10.3390/jcm10071477






